Abstract
Caloric restriction (CR) increases the preservation of the ovarian primordial follicular reserve, which can potentially delay menopause. Rapamycin also increases preservation on the ovarian reserve, with similar mechanism to CR. Therefore, the aim of our study was to evaluate the effects of rapamycin and CR on metabolism, ovarian reserve, and gene expression in mice. Thirty-six female mice were allocated into three groups: control, rapamycin-treated (4 mg/kg body weight every other day), and 30% CR. Caloric restricted females had lower body weight (P < 0.05) and increased insulin sensitivity (P = 0.003), while rapamycin injection did not change body weight (P > 0.05) and induced insulin resistance (P < 0.05). Both CR and rapamycin females displayed a higher number of primordial follicles (P = 0.02 and 0.04, respectively), fewer primary, secondary, and tertiary follicles (P < 0.05) and displayed increased ovarian Foxo3a gene expression (P < 0.05). Despite the divergent metabolic effects of the CR and rapamycin treatments, females from both groups displayed a similar increase in ovarian reserve, which was associated with higher expression of ovarian Foxo3a.
Keywords: Rapamycin, Ovarian reserve, mTOR, FOXO3a
Introduction
Ovarian aging is associated with depletion of the ovarian follicular reserve. The reserve is most often measured by basal follicle stimulating hormone (FSH) or anti-Mullerian hormone (AMH) levels, but most accurately by ovarian biopsy primordial follicle count. Primordial follicle levels are defined during fetal life in women (Te Velde et al. 1998) and soon after birth in mice (Peters 1969). The primordial follicles are continuously recruited from the reserve in an irreversible process and may undergo ovulation or become atretic (Baker 1963). The recruitment and activation of primordial follicles leads to the depletion of the ovarian reserve, culminating in the onset of menopause in women (Liu et al. 2006). Thus, activation of the otherwise quiescent primordial follicles is a fundamental step in the depletion of the ovarian reserve. An understanding of this process is crucial to improving female fertility and health in older ages. The transplant of young ovaries to older females can prolong lifespan and suggest the pivotal role of the gonads to the health decline in postmenopausal women (Habermehl et al. 2019). Some studies also suggest that genetic parameters are determinant for age at menopause (Shi et al. 2016), which can be implicated in overall lifespan and healthspan in women.
Activation of the primordial follicles is a process regulated by several pathways. One of the major signaling pathways implicated in this process is phosphoinositide-3-kinase (Pi3k)/protein kinase B (Akt1) and its effector, the Forkhead Box Transcription Factor O3a (Foxo3a) (Castrillon et al. 2003). In primordial follicles, the phosphorylation of Foxo3a is associated with oocyte growth and irreversible activation (Castrillon et al. 2003). The hyperactivation of the Pi3k/Akt pathway results in hyperphosphorylation of Foxo3a, which facilitates exportation of FoxO3a from the nucleus to the cytoplasm, culminating in the activation of multiple primordial follicles and ultimately leading to premature depletion of the ovarian reserve (Kalich-Philosoph et al. 2013). Growth hormone (GH)-deficient Ames dwarf (df/df) mice display decreased activation of primordial follicles associated with increased Foxo3a gene expression and decreased Foxo3a protein phosphorylation (Schneider et al. 2014b; Saccon et al. 2016). Similar characteristics are found in df/df mice subjected to caloric restriction (CR) (Bartke 2005), including increased longevity, very low levels of insulin like growth factor 1 (IGF-1) and insulin (Bartke 2008), and reduced phosphorylation of Foxo3a (Barzilai et al. 2012).
A level of CR between 20 and 40% without compromising the ingestion of essential nutrients (Genaro et al. 2009; Cantó and Auwerx 2009) can preserve the ovarian reserve in female mice and rats (Shi et al. 2013; Li et al. 2015). Eight week old female mice on CR maintain an increased proportion of primordial follicles, indicating decreased follicle activation and a threefold increase in expression of AMH (Shi et al. 2013), a common marker of ovarian reserve (Broer et al. 2014). Mice under CR from 3 to 15 months of age have prolonged fertility once returned to ad libitum feeding and were able to generate more pups in older ages when ad libitum fed mice had already became infertile (Selesniemi et al. 2008).
Rapamycin is an immunosuppressive drug (Wullschleger et al. 2006) and is recognized as a mimetic of CR (Powers et al. 2006). Rapamycin inhibits the mechanistic target pathway of rapamycin kinase (mTOR; Sarbassov et al. 2006), more specifically the mTORC1 complex, which is sensitive to rapamycin (Blagosklonny, 2010). Studies suggest that mTOR may also be involved in the activation of primordial follicles along with Foxo3a and that suppression of mTOR signaling in oocytes could preserve primordial follicles in quiescent state (Adhikari et al. 2009). In rats under CR, there was also suppression of mTOR signaling activity and decreased primordial follicle activation (Li et al. 2015). In contrast, treatment of mice with activators of the AKT and mTOR pathways increased the number of antral follicles accompanied by a decrease in primordial follicles (Cheng et al. 2015). This further suggests a role for the PI3K/AKT and mTOR pathways in primordial follicle activation and for the modulatory effects of CR and rapamycin in this process. Short-term treatment of young and middle-aged mice with rapamycin decreased primordial follicle activation and was able to improve fertility rates after the end of treatment (Dou et al. 2017).
The decreasing number of follicles with advancing age is accompanied by decreased oocyte quality (Broekmans et al. 2009) due to accumulation of DNA damage and is also associated with decreased embryo quality and consequent higher frequency of abortions and increased chromosomal abnormalities (Fujimoto et al. 2011). A delay in oocyte depletion of the ovarian reserve as well as maintenance of oocyte quality would be essential to prolong female fertility (Treff et al. 2016). Therefore, the aim of this study was to evaluate the effects of rapamycin and CR on metabolism, ovarian reserve, and ovarian gene expression in mice.
Materials and methods
Animals and treatment
This study was approved by the Committee on Ethics in Animal Experimentation of the Universidade Federal de Pelotas (UFPel), number 23110.009349/2016-31. Thirty-six 30-day-old female C57BL/6 mice, maintained under controlled conditions (22 ± 2 °C, cycles of 12 h light/12 h dark and 40–60% humidity), were used. The mice were housed in groups of four animals per cage. The 36 mice were randomly allocated into three groups: control group (n = 12), rapamycin (n = 12), and 30% CR (n = 12) and treatments lasted for 93 days. All mice were fed a standard ration for rodents (Nuvilab® CR-1 with 50 g/kg of fat and 220 g/kg of protein) and water ad libitum. Control and rapamycin groups received ad libitum feed. In the CR group, a 30% lower amount was offered, based on the average weight of the food consumed during the previous week in the control group. All mice were allowed a week of adaptation before beginning of treatments. CR females started the week after the adaptation period with a CR of 10%, the next week 20% CR, and from the third week forward, a 30% CR level (Ribeiro et al. 2009). Micronutrient enrichment was not performed for the CR group along the study. The food consumption data were collected daily during the experiment. Body weight data were collected once a week.
Mice in the rapamycin group received rapamycin (4 mg/kg bw) intraperitoneally (InLab, Brazil), every other day for 93 days (Fang et al. 2013; Wilkinson et al. 2012). Rapamycin, diluted in dimethyl sulfoxide (DMSO), was added to a solution containing sorbitan monolaurate (PEG) and polysorbate (Tween 80%) (Fang et al. 2013). A placebo solution without rapamycin made with the same vehicles as the rapamycin diluent was administered intraperitoneally every other day for 93 days in mice from the control and CR groups.
At the end of the 93-day period, all mice were euthanized. After a four hour fast, mice were anesthetized with isoflurane and submitted to cardiac puncture exsanguination followed by cervical dislocation. The mice were dissected, and the ovaries collected. One ovary was kept at − 80 °C for RNA extraction and another in 10% formalin solution for histological analysis. During dissection, abdominal visceral fat was removed and weighed.
Insulin tolerance test and glucose tolerance test
Insulin tolerance test (ITT) and glucose tolerance test (GTT) were performed 85 days after the beginning of treatments. Six mice from each group were used for each test. For the ITT, 1 IU/kg body weight of insulin was administered intraperitoneally after two hour of fasting. The blood was collected through a small incision at the tip of the tail at 0, 5, and 20 min after insulin injection, and glucose levels were measured with a glucose meter (AccuChek Activ, Roche Diagnostics®, USA) (Fang et al. 2013). The constant rate for glucose disappearance (KITT) was calculated by converting glucose values to percentage, considering glucose level at 5 min as 100% for each animal, then applying the formula (T20 − T5)/15 (De Barros et al. 2012). KITT was expressed as percent per minute. For the GTT, 2 g glucose/kg body weight was administered intraperitoneally after 12 h of fasting. Blood was also collected through a small incision at the tip of the tail at 0, 15, 30, 60, and 120 min after glucose injection, and glucose levels were measured with a glucose meter (AccuChek Activ, Roche Diagnostics®, USA) (Bennis et al. 2017).
Histological analysis
The ovaries were removed from the 10% buffered formaldehyde, dehydrated in alcohol, cleared in xylol, and embedded in paraffin. The paraffin-embedded ovaries were sequentially cut into a 5-μm thickness in a semi-automatic microtome (RM2245, Leica Biosystems Newcastle Ltd., Newcastle Upon Tyne, UK). All ovaries were serially cut, and one of every six sections was selected and placed on a standard histological slide Myers et al. (2004). The slides, after drying at 56 °C for 24 h, were stained with hematoxylin-eosin and mounted with coverslips and synthetic resin (Sigma Chemical Company®, St. Louis, USA). Images of the ovarian sections were captured by a Moticam 5.0 digital camera (Motic®, Hong Kong, China) coupled to a Nikon Eclipse E200 microscope (Nikon Corporation, Tokyo, Japan) using 4, 10, and 40× lenses. Follicular classification and counting were performed using images from the 40× lenses.
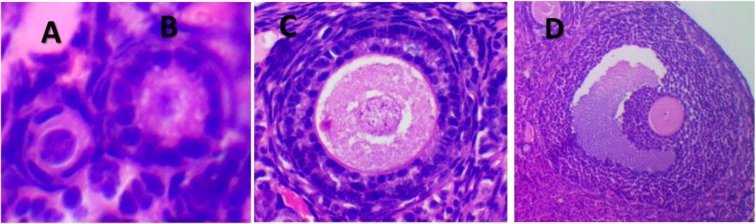
Five ovaries from each group were used for follicle counting. Only follicles with a clearly visible oocyte nucleus were counted. The final number of follicles was multiplied by two to mimic the number of follicles in two ovaries and multiplied by six to account for the sampling by section. The protocol used for follicle classification was based on Myers et al. (2004). Briefly, a primordial follicle contains an oocyte surrounded by a single layer of squamous granulosa cells. The transition follicle has at least one cuboid granulosa cell. The primary follicle has an oocyte surrounded by a single layer of cuboid granulosa cells. A follicle was determined to be a secondary when surrounded by more than one layer of granulosa cuboid cells without visible antrum and as tertiary if an antral space was clearly defined and a follicular layer of granulosa cells surrounded the oocyte. For measurement of oocyte and follicle size, three images of primordial follicles, transition follicles, and primary follicles per mouse were selected using Motic 2.0 software for measuring the diameter of the oocyte nucleus, oocyte, and whole follicle. Additionally, the number of granulosa cells around these follicles was counted. Representative images of each follicle type are shown in Fig. 1.
Fig. 1.
Representative hematoxylin eosin stained images of primordial (a), primary (b), secondary (c), and tertiary follicles (d) under 40× lenses
RNA extraction and gene expression analysis
Total ovarian RNA extraction was performed using the guanidine isothiocyanate reagent protocol (Trizol, Invitrogen, Carlsbad, USA) associated with a column purification kit (Quiagen, Hilden, Germany) according to the manufacturer protocols. RNA concentration was measured using spectrophotometer (NanoDrop Lite, Thermo Fisher, Waltham, USA), and all samples were adjusted to a final concentration of 200 ng/μL. In addition, the purity of the samples was checked by the absorbance rate 260/280 nm.
Complementary DNA (cDNA) was synthesized from 1 μg of total RNA using commercial kit (iScript cDNA Synthesis Kit, Bio-Rad, Hercules, CA, USA), following the manufacturer’s recommendations. Real-time quantitative PCR (RTq-PCR) was performed using GoTaq® qPCR Master Mix (Promega, Madison, WI, USA) on a StepOne 7500 RT-PCR equipment (Applied Biosystems, Foster City, CA, USA). For each assay, 45 cycles (95 °C for 15 s and 60 °C for 60 s) were run and a dissociation curve was included at the end of the reaction to verify the amplification of a single PCR product. β-2 Microglobulin (β2m) and beta actin (Actb) genes were used as endogenous controls. The following target genes were evaluated, for ovarian reserve: growth differentiation factor 9 (Gdf9), anti-Mullerian hormone (Amh), and bone morphogenetic protein 15 (Bmp-15), and for activation of primordial follicles: protein kinase B (Akt1), phosphoinositide-3-kinase (Pi3k), mechanistic target of rapamycin kinase (Mtor), Forkhead box protein O3 (Foxo3a), phosphatase and tensin homolog (Pten), proto-oncogene receptor tyrosine kinase (Kit), and Kit Ligand (Kitl). The primer sequences are shown in Table 1.
Table 1.
Primer pairs (forward and reverse) used in the experiment
| Gene | Primers | Length | Code |
|---|---|---|---|
| β-2-Microglobulin (β2m) |
F: AAGTATACTCACGCCACCCA R: CAGGCGTATGTATCAGTCTC |
217 | NM_009735.3 |
| Beta actin (Actb) |
F: GAGACCTTCAACACCCCAGC R: ATGTCACGCACGATTTCCC |
263 | NM_007393.5 |
| Protein kinase B (Akt1) |
F: CCGGTTCTTTGCCAACATCG R: ACACACTCCATGCTGTCATCTT |
168 | NM_001331107.1 |
| Phosphoinositide-3-kinase (Pi3k) |
F: TAGCTGCATTGGAGCTCCTT R: TACGAACTGTGGGAGCAGAT |
119 | XM_006507441.3 |
| Mechanistic target of rapamycin kinase (Mtor) |
F: CGGCAACTTGACCATCCTCT R: TGCTGGAAGGCGTCAATCTT |
101 | XM_006539077.2 |
| Forkhead box protein O3 (Foxo3a) |
F: CGGCTCACTTTGTCCCAGAT R: GCCGGATGGAGTTCTTCCA |
106 | XM_006512806.1 |
| Growth differentiation factor 9 (Gdf9) |
F: AATACCGTCCGGCTCTTCAG R: GGTTAAACAGCAGGTCCACCAT |
97 | XM_006532220.3 |
| Anti-Mullerian hormone (Amh) |
F: TCCTACATCTGGCTGAAGTGATATG R: CAGGTGGAGGCTCTTGGAACT |
66 | XM_006513119.3 |
| Bone morphogenetic protein 15 (Bmp-15) |
F: GAGCGAAAATGGTGAGGCTG R: GGCGAAGAACACTCCGTCC |
342 | NM_009757.5 |
| Proto-oncogene receptor tyrosine kinase (Kit) |
F: CTCCCCCAACAGTGTATTCAC R: TAGCCCGAAATCGCAAATCTT |
90 | NC_000071.6 |
| Kit Ligand (Kitl) |
F: TTCGCACAGTGGCTGGTAAC R: TTCACAGCGAAGCACTCTGC |
163 | NM_013598.3 |
| Phosphatase and tensin homolog (Pten) |
F: TGGATTCGACTTAGACTTGACCT R: GCGGTGTCATAATGTCTCTCAG |
180 | NM_008960.2 |
The relative expression was calculated from the equation 2A-B/2C-D (where A is the threshold value of the cycle [Ct] for the gene of interest in the first control sample; B, the Ct value for the gene of Ct; C, the geometric mean of the Ct values for the β2m and Actb genes in the first control sample; and D, the Ct mean for the β2m and Actb genes in the sample analyzed) (Masternak et al. 2005).
Statistical analysis
The results are presented as mean ± standard error of the mean. Statistical analysis was performed using GraphPad Prism 6.0 software. The one-way ANOVA statistical test was used for analysis of continuous variables (follicle count, follicle diameter, oocyte diameter, oocyte nucleus diameter, KITT, and gene expression) with the Tukey post-hoc test to compare individual means. The repeated measures ANOVA test was used for the analysis of food consumption, body weight, and glucose levels during TTI and TTG also using the Tukey post-hoc test to compare individual means. Values of P lower than 0.05 were considered as significant and tendency between 0.05 and 0.10.
Results
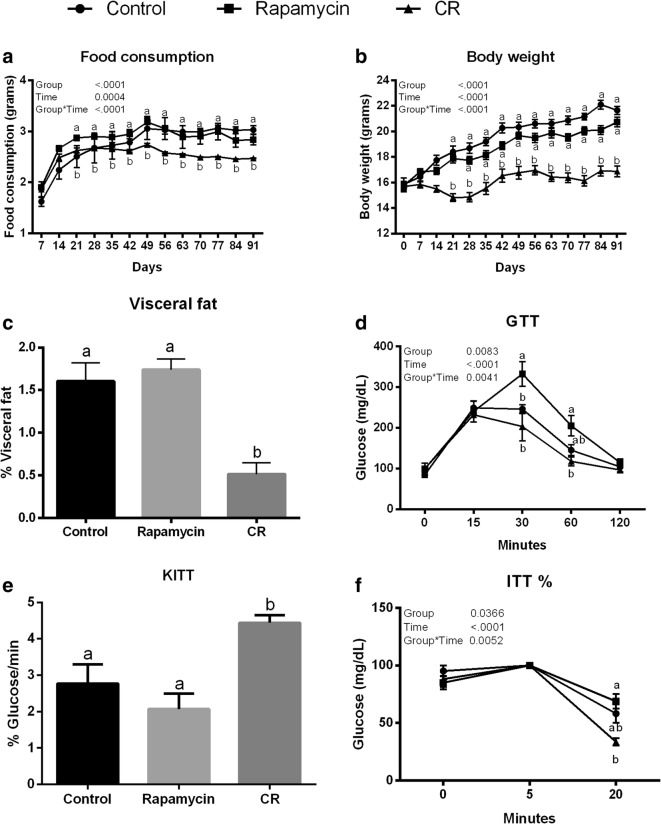
There was no difference in food consumption between rapamycin and control groups (Fig. 2a; P > 0.05). Food consumption for the CR group was about 30% lower than for rapamycin and control groups (Fig. 2a; P < 0.05). Body weight was lower in the CR than control and rapamycin groups (Fig. 2b; P < 0.05), and no difference between rapamycin and control groups was observed (Fig. 2b; P > 0.05). Abdominal visceral fat depot weight was lower in CR than control and rapamycin groups (P < 0.05; Fig. 2c). No difference in fat depots between control and rapamycin groups was observed (P > 0.05; Fig. 2c).
Fig. 2.
Analysis of food consumption (a), body weight (b), visceral fat (c), glucose tolerance test (GTT) (d), constant rate for glucose disappearance (KITT) (e), and insulin tolerance test (ITT) (f) for caloric restriction (CR), rapamycin, and control groups. Different letters indicate difference between groups (P < 0.05). Values were plotted as mean ± standard error of the mean. Twelve mice/group were used for this analysis
In the GTT, the highest glycemic levels were observed in the rapamycin group compared to control and CR groups (P > 0.05; Fig. 2d). In the ITT, CR mice had increased insulin sensitivity, indicated by the lower glycemic levels observed for the CR group compared with rapamycin (P = 0.001). Additionally, the CR group had a lower glycemic level 20 min after insulin injection than the rapamycin group (Fig. 2f) and the KITT was twice as high in the CR than rapamycin group and 1.5 times higher than in the control group (Fig. 2e; P = 0.0006 and P = 0.01, respectively).
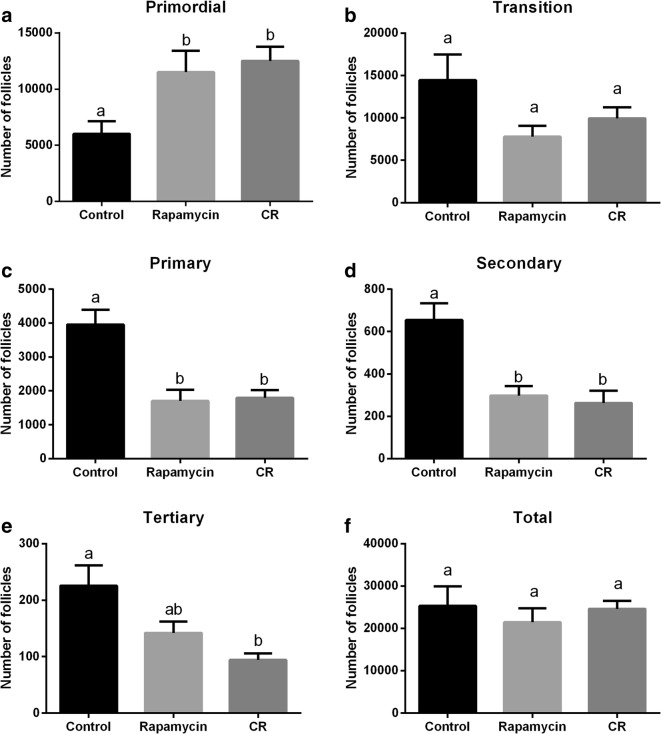
The analysis of the ovarian follicular population is shown in Fig. 3. Mice from the CR and rapamycin group had twice the number primordial follicles as in the control group (P = 0.02 and 0.04, respectively; Fig. 3a). For the transition follicles, there was no difference among groups (P = 0.08); however, the rapamycin group tended to have fewer transition follicles than the control group (P = 0.06; Fig. 3b). The number of primary and secondary follicles was lower in the CR and rapamycin groups than in the control group (P < 0.05; Fig. 3c and d). The CR group had fewer tertiary follicles than the control group (P = 0.005), although the rapamycin group only had a tendency to have fewer tertiary follicles than the control group (P = 0.06; Fig. 3e). Also, a tendency towards fewer tertiary follicles in CR than rapamycin group was observed (P = 0.06; Fig. 3e). For the total number of follicles (Fig. 3f), there was no difference among control, CR, and rapamycin groups (P > 0.05).
Fig. 3.
Analysis of the mean number of follicles at different stages of growth. Primordial (a), transition (b), primary (c), secondary (d), tertiary (e), and total follicles (f) for control, rapamycin, and caloric restriction (CR) groups. Different letters indicate statistical difference (P < 0.05). Values were plotted as mean ± standard error of the mean. Six mice/group were used for this analysis
Primordial follicle oocyte and nucleus diameter were greater in CR than control or rapamycin groups (Table 2; P < 0.05). Transition follicle diameter was reduced in rapamycin-treated mice, compared with controls (P = 0.005). For primary follicles, oocyte and follicle diameter were reduced in CR (Table 2; P < 0.05), as was the number of granulosa cells (Table 2; P = 0.002).
Table 2.
Mean ± standard error of the diameter of oocyte nucleus, oocyte, and follicle and number of granulosa cells from primordial, transition, and primary follicles from the control, rapamycin, and caloric restriction (CR) groups
| Control | Rapamycin | CR | P | |
|---|---|---|---|---|
| Primordial | ||||
| Nucleus1 | 3.2 ± 0.1a | 3.1 ± 0.2a | 3.8 ± 0.2b | 0.0054* |
| Oocyte1 | 5.9 ± 0.4ab | 5.4 ± 0.4a | 7.0 ± 0.4b | 0.0228* |
| Follicle1 | 10.8 ± 0.6a | 9.8 ± 0.7a | 11.7 ± 0.5a | 0.0787 |
| Granulosa2 | 4.1 ± 0.2a | 4.2 ± 0.2a | 4.4 ± 0.2a | 0.5516 |
| Transition | ||||
| Nucleus1 | 3.9 ± 0.1a | 3.7 ± 0.1a | 4.1 ± 0.2a | 0.1871 |
| Oocyte1 | 7.9 ± 0.3a | 7.0 ± 0.3a | 7.5 ± 0.2a | 0.1088 |
| Follicle1 | 15.7 ± 0.5a | 13.3 ± 0.6b | 14.0 ± 0.3ab | 0.0053* |
| Granulosa2 | 7.0 ± 0.3a | 6.3 ± 0.3a | 6.4 ± 0.2a | 0.1572 |
| Primary | ||||
| Nucleus1 | 5.7 ± 0.2a | 5.1 ± 0.2a | 5.4 ± 0.3a | 0.2062 |
| Oocyte1 | 17.0 ± 1.8a | 15.3 ± 1.1ab | 11.5 ± 0.8b | 0.0094* |
| Follicle1 | 29.8 ± 2.6a | 27.2 ± 1.9ab | 21.7 ± 1.3b | 0.0159* |
| Granulosa2 | 15.7 ± 1.5ab | 17.6 ± 1.2a | 11.8 ± 0.8b | 0.0027* |
Different letters represent statistical difference in the row
*Statistical difference at P < 0.05
1Measured in micrometers
2Number of granulosa cells
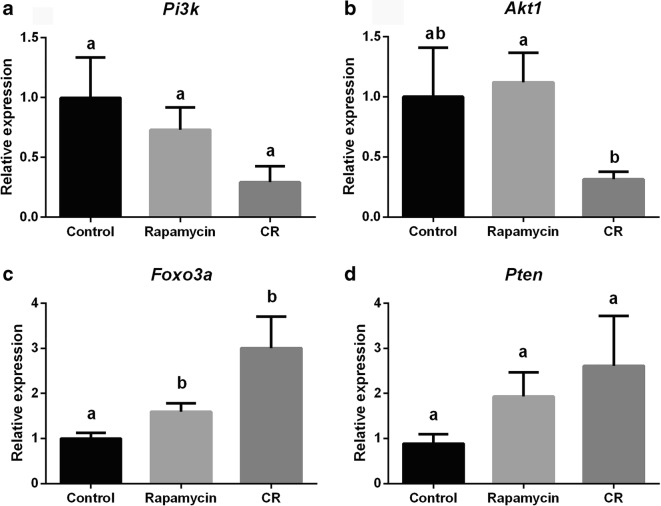
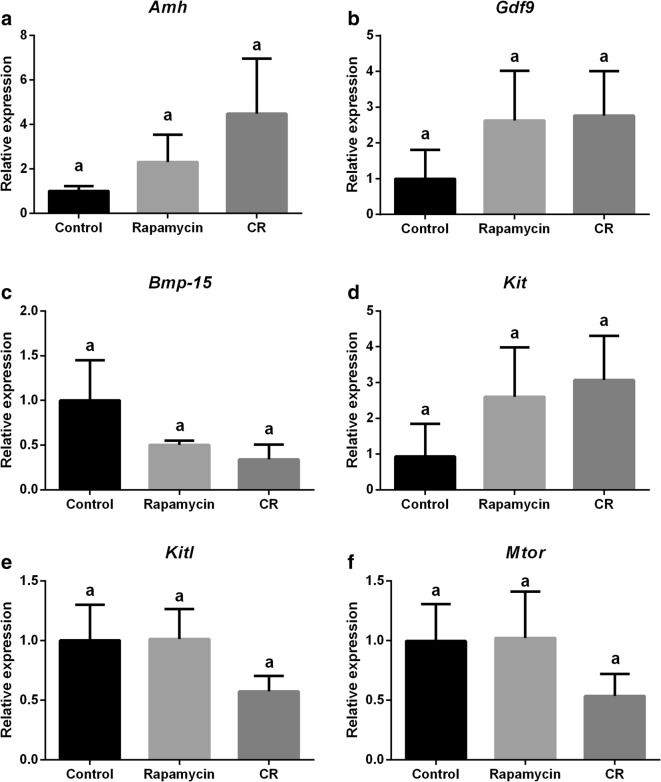
Relative expression of genes related to the ovarian reserve and mTOR signaling pathway is represented in Fig. 5. Relative expression of genes related to the PI3k signaling pathway is represented in Fig. 5. Foxo3a expression was higher in CR and rapamycin groups compared with the control group (Fig. 5c; P = 0.02 and 0.015, respectively). Akt1 gene expression (Fig. 5b) was lower (P = 0.009) in CR mice compared with the rapamycin group. There were no changes in Amh expression (Fig. 4a; P = 0.33), Gdf9 (Fig. 4b; P = 0.51), Bmp-15 (Fig. 4c; P = 0.25), Kit (Fig. 4d; P = 0.47), Kitl (Fig. 4e; P = 0.35), Mtor (Fig. 4f; P = 0.51), Pi3k (Fig. 5a; P = 0.14), and Pten (Fig. 5d; P = 0.26) among groups.
Fig. 5.
Analysis of relative gene expression of ovarian genes of control, rapamycin, and caloric restriction (CR) mice. a Phosphininositide-3-kinase (Pi3k). b Protein kinase B (Akt1). c Forkhead Box class O3a (Foxo3a). d Phosphatase and tensin homolog (Pten). Different letters indicate difference between groups (P < 0.05). Values were plotted as mean ± standard error of the mean. Six mice/group were used for this analysis
Fig. 4.
Analysis of the relative gene expression of ovarian genes of control, rapamycin, and caloric restriction (CR) mice. a Anti-mullerian hormone (Amh). b Growth factor and differentiation 9 (Gdf9). c Bone morphogenic protein 15 (Bmp-15). d Proto-oncogene receptor tyrosine kinase (Kit). e Kit Ligand (Kitl). f Mammalian target of Rapamycin (Mtor). Different letters indicate difference between groups (P < 0.05). Values were plotted as mean ± standard error of the mean. Six mice/group were used for this analysis
Discussion
Our study compared the effects of CR and rapamycin in the ovarian aging of mice. Both CR and rapamycin reduced primordial follicle activation in a very similar magnitude and lead to increased ovarian Foxo3a expression. Despite the similar effects on ovarian aging, CR was associated with reduced body weight, abdominal visceral fat depot, and increased insulin sensitivity. Rapamycin did not alter body weight or visceral fat and decreased insulin sensitivity. These results are interesting as there are no previous studies comparing both treatments and the similarity in the ovarian effects in this short-term treatment. Despite the divergence in systemic metabolic effects, these observations demand more detailed studies to understand the mechanisms of action.
Our results showed that, while CR and rapamycin groups had twice as many primordial follicles, the number of primary and secondary follicles was reduced to half in these groups, compared with controls. This suggests a very similar decrease in the rate of primordial follicle activation in both the CR and rapamycin groups. Zhang et al. (2013) reported an increased number of primordial follicles in rats after rapamycin treatment at a similar dose and duration as in our study. However, different from our study, females treated with rapamycin had lower food consumption and lower body weight when compared to the control group (Zhang et al. 2013). Similarly, other research groups have shown an increase in the number of primordial follicles in CR females (Li et al. 2015, 2011; Wang et al. 2014). It is interesting to note that the extent of primordial follicle preservation was very similar between the 30% CR and 4 mg/day of rapamycin. We are not aware of previous studies comparing both treatments directly. Therefore, CR and rapamycin treatment had a beneficial effect on preserving the ovarian reserve despite divergent effects on the metabolism. Overall, there was no difference in the total number of follicles among groups as treatments were not long term, demonstrating that both treatments had no overall effects in the number of follicles. Rather, our study suggests that treatments affected only the proportion of follicles in each stage of development, reflecting reduced primordial follicle activation. Besides to preserving the ovarian reserve, rapamycin is known to reduce oxidative stress (Nacarelli et al. 2018). Additionally, even short-term treatments can have positive effects on healthspan (Urfer et al. 2017) and even reverse aging–related diseases in older mice (An et al. 2017). Therefore, these short-term treatments used in our study can have long-lasting effects on somatic and gonadal aging. As demonstrated before, short-term treatment of young and middle-aged mice with rapamycin improved fertility rates in later in life (Dou et al. 2017), similarly to the observed for mice under CR with preserved fertility later in life when ad libitum fed mice were already infertile (Selesniemi et al. 2008).
Along with the lower rate of primordial follicle activation, we observed that expression of Foxo3a was higher in the CR and rapamycin groups compared to the control group. The nuclear presence of Foxo3a in its non-phosphorylated form is essential to maintain primordial follicles in their quiescent stage, preserving the ovarian reserve (John et al. 2009). The increase in Foxo3a expression can explain the reduced activation of primordial follicles observed in the present study for both CR and rapamycin groups. The same results were also observed in GH-deficient df/df mice (Schneider et al. 2014b) that have a series of physical and metabolic characteristics that resemble mice submitted to CR (Bartke and Brown-Borg 2004). It has previously been shown that df/df mice have three times more primordial follicles than normal littermates (Saccon et al. 2016; Schneider et al. 2017). The increased ovarian reserve is accompanied by increased ovarian Foxo3a gene expression and decreased phosphorylation of Foxo3a protein (Schneider et al. 2014b). Phosphorylation of Foxo3a leads to its removal from the nucleus and the irreversible activation of the primordial follicles (Liu et al. 2014). Therefore, these three models of delayed ovarian aging, GH-deficient df/df, CR, and rapamycin treated mice, have an increased ovarian Foxo3a expression, although through different metabolic systemic effects and intracellular signaling pathways, further reinforcing the essential role of Foxo3a in the preservation of primordial follicles in the quiescent stage. Further studies modulating ovarian Foxo3a may confirm its central role on fertility preservation.
In the present study, the oocyte and follicle size were reduced, as well as the number of granulosa cells was lower in primary follicles of CR mice. No previous studies measuring oocyte and follicle size after treatment with CR or rapamycin were found in the literature. However, in a similar way, a previous paper from our group indicated that transgenic bGH mice had a reduced primordial follicle reserve associated with increased primary oocyte and follicle size (Saccon et al. 2016). Zhang and Liu (2015) suggested that an increased number of granulosa cells in primordial and primary follicles could indicate a greater tendency of the follicles to become active leading to oocyte growth and consequent exit from the ovarian reserve. Stimulation of mTORC1 signaling in granulosa cells of primordial follicles leads to the differentiation and proliferation of these surrounding cells and, consequently, stimulates growth of quiescent oocytes (Zhang and Liu 2015). Primordial follicle activation is associated to transition from flattened to cuboidal cells (Lintern-Moore and Moore 1979). The transition to cuboidal cells is associated to increased number of metabolically active granulosa cells surrounding the oocyte, where at four or five cells, the majority of oocytes were quiescent, and at around nine cells, the majority were activated (Lintern-Moore and Moore 1979). Therefore, the fact that primary follicles from CR mice have fewer granulosa cells and oocytes are smaller suggests that an effect on surrounding granulosa cell growth could be responsible for the lower activation of quiescent primordial follicles.
There was no difference in the regulation of Kit, Kitl, Mtor, Amh, Gdf9, and Bmp-15 gene expression among groups in the present study. It is known that blocking of the Kit gene resulted in disturbances in the activation of primordial follicles (Yoshida et al. 1997), showing the essential role of the KIT/KITL system in follicle transition. A lower expression of Kitl as well as of Mtor could be expected in the rapamycin group, since the mTOR/KITL/AKT/FOXO3a pathway is less activated after inhibiting Mtor. These genes are activated differently depending on the stage of oocyte development (Celestino et al. 2009). Therefore, the use of the entire ovary for the gene expression analysis could also have an influence, not allowing us to understand the regulation specifically in primordial follicles. For Amh expression, the use of the whole ovary may also have been a factor, as it is known that AMH is a hormone released not only by primordial follicles but also by primary and secondary follicles (Skałba et al. 2008). Therefore, as we observed increased primordial follicles, but decreased numbers of primary and secondary follicles and the number of small and total follicles did not change in the ovary of these mice, AMH may not be a very specific marker for the reserve of primordial follicles. The same factor could account for the dilution in the effect for other tested genes specific in primordial follicles versus the whole ovarian tissue. Therefore, more functional studies are needed to understand the activation of the Pi3/Akt and mTOR pathways in primordial follicles.
Finally, as expected, weight gain and food consumption were lower in the CR group, since there was an intentional reduction in food supply. Rapamycin did not affect food consumption or weight gain. Previous studies have demonstrated an effect of rapamycin on body weight and food consumption dependent on the duration of treatment (Fang et al. 2013). Additionally, the rate of weight gain decreases proportionally to the dose of rapamycin (up to 8 mg/kg) (Johnson et al. 2015). However, the dose and duration of treatment with rapamycin in the current experiment did not change these parameters. Regarding insulin and glucose sensitivity, we could observe that CR mice were more sensitive to insulin as showed before (Wiesenborn et al. 2014). CR mice had reduced visceral fat content in our study, and it is suggested that increased plasma levels of adiponectin, due to decreased body fat, may contribute to this increased insulin sensitivity (Nikolai et al. 2015). On the other hand, females treated with rapamycin had reduced glucose tolerance. The effect of rapamycin on glucose metabolism is dependent on the length of the treatment. Fang et al. (2013) noted improvement in glucose metabolism only after 20 weeks of rapamycin treatment, while our study treatment lasted 13 weeks and detected some changes in glucose metabolism. The effect of rapamycin on insulin resistance has been associated with inhibition of insulin-dependent signaling pathway (Deblon et al. 2012). It is interesting to notice that although rapamycin treatment had divergent effects from CR regarding insulin sensitivity, the overall effect on the activation of primordial follicles was very similar. This is interesting also considering that both CR and rapamycin mice had increased expression of ovarian Foxo3a expression, suggesting it is an insulin-independent effect. Therefore, more studies are needed in order to understand the mechanisms that can contribute to preservation the primordial follicle reserve without affecting whole body metabolism.
In conclusion, mice submitted to CR or rapamycin treatment for 3 months had decreased activation of primordial follicles, despite divergent effects on systemic metabolism. Female mice treated with rapamycin did not change food consumption, visceral fat, or body weight gain and became intolerant to glucose, but CR mice had lower weight gain, reduced visceral fat, and increased sensitivity to insulin. Both treatments increased ovarian expression of Foxo3a, a known regulator of primordial follicle activation, suggesting a consistent effect between treatments. Our results with CR and rapamycin, along with previous findings in GH-deficient Ames dwarf (Saccon et al. 2016) and GH receptor knockout mice (Schneider et al. 2014a), show a strong relationship between longevity and ovarian aging. This suggests that interventions that extend lifespan might work through improvement of reproductive organs. Further studies are needed to investigate other dietary or pharmacological strategies involved in the longevity and delay of ovarian aging, as well as the intracellular signaling pathways involved in this process.
Authors’ contribution
CCB, MMM, AB, and AS designed research; DNG, TDS, JP, JAAR, and KRSA performed experiments; MTR, RGM, LAXC, MMM, and AS analyzed and interpreted data; DNG and AS wrote the manuscript, TDS, JP, JAAR, KRSA, MTR, RGM, LAXC, CCB, MMM, and AB revised the article.
Funding
This work was supported by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) through a scholarship for DNG, TDS, JAAR, and JP; Fundação de Amparo a Pesquisa do Estado do Rio Grande do Sul (FAPERGS); and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq). This work was also supported by the National Institute on Aging (NIA) R15 AG059190 and R03 AG059846.
Compliance with ethical standards
This study was approved by the Committee on Ethics in Animal Experimentation of the Universidade Federal de Pelotas (UFPel), number 23110.009349/2016-31.
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Adhikari D, Zheng W, Shen Y, et al. Tsc/Mtorc1 signaling in oocytes governs the quiescence and activation of primordial follicles. Hum Mol Genet. 2009;19:397–410. doi: 10.1093/hmg/ddp483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An JY, Quarles EK, Mekvanich S, Kang A, Liu A, Santos D, Miller RA, Rabinovitch PS, Cox TC, Kaeberlein M. Rapamycin treatment attenuates age-associated periodontitis in mice. GeroScience. 2017;39:457–463. doi: 10.1007/s11357-017-9994-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker TG. A quantitative and cytological study of germ cells in human ovaries. Proc R Soc Lond B Biol Sci. 1963;158:417–4133. doi: 10.1098/rspb.1963.0055. [DOI] [PubMed] [Google Scholar]
- Bartke A. Role of the growth hormone/insulin-like growth factor system in mammalian aging. Endocrinology. 2005;146:3718–3723. doi: 10.1210/en.2005-0411. [DOI] [PubMed] [Google Scholar]
- Bartke A. Impact of reduced insulin-like growth factor-1/insulin signaling on aging in mammals: novel findings. Aging Cell. 2008;7:285–290. doi: 10.1111/j.1474-9726.2008.00387.x. [DOI] [PubMed] [Google Scholar]
- Bartke A, Brown-Borg H. Life extension in the Dwarf mouse. Curr Top Dev Biol. 2004;63:189–225. doi: 10.1016/S0070-2153(04)63006-7. [DOI] [PubMed] [Google Scholar]
- Barzilai N, Huffman DM, Muzumdar RH, Bartke A. The critical role of metabolic pathways in aging. Diabetes. 2012;61:1315–1322. doi: 10.2337/db11-1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennis MT, Schneider A, Victoria B, Do A, Wiesenborn DS, Spinel L, Gesing A, Kopchick JJ, Siddiqi SA, Masternak MM. The role of transplanted visceral fat from the long-lived growth hormone receptor knockout mice on insulin signaling. Geroscience. 2017;39:51–59. doi: 10.1007/s11357-017-9957-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blagosklonny MV. Calorie restriction: decelerating Mtor-driven aging from cells to organisms (including humans) Cell Cycle. 2010;9:683–688. doi: 10.4161/cc.9.4.10766. [DOI] [PubMed] [Google Scholar]
- Broekmans FJ, Soules MR, Fauser BC. Ovarian aging: mechanisms and clinical consequences. Endocr Rev. 2009;30:465–493. doi: 10.1210/er.2009-0006. [DOI] [PubMed] [Google Scholar]
- Broer SL, Broekmans FJ, Laven JS, Fauser BC. Anti-Müllerian hormone: ovarian reserve testing and its potential clinical implications. Hum Reprod Update. 2014;20:688–701. doi: 10.1093/humupd/dmu020. [DOI] [PubMed] [Google Scholar]
- Cantó C, Auwerx J. Caloric restriction, SIRT1 and longevity. Trends Endocrinol Metab. 2009;20:325–331. doi: 10.1016/j.tem.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castrillon DH, Miao L, Kollipara R, et al. Suppression of ovarian follicle activation in mice by the transcription factor Foxo3a. Science. 2003;301:215–218. doi: 10.1126/science.1086336. [DOI] [PubMed] [Google Scholar]
- Celestino JJH, Matos MHT, Saraiva MVA, Figueiredo JR. Regulation of ovarian folliculogenesis by Kit Ligand and the c-Kit system in mammals. Anim Reprod. 2009;6:431–439. [Google Scholar]
- Cheng Y, Kim J, Li XX, Hsueh AJ. Promotion of ovarian follicle growth following Mtor activation: synergistic effects of AKT stimulators. PLoS One. 2015;10:1–9. doi: 10.1371/journal.pone.0117769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Barros CC, Haro A, Russo FJVP, et al. Altered glucose homeostasis and hepatic function in obese mice deficient for both Kinin receptor genes. PLoS One. 2012;7:40573. doi: 10.1371/journal.pone.0040573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deblon N, Bourgoin L, Veyrat-Durebex C, Peyrou M, Vinciguerra M, Caillon A, Maeder C, Fournier M, Montet X, Rohner-Jeanrenaud F, Foti M. Chronic Mtor inhibition by rapamycin induces muscle insulin resistance despite weight loss in rats. Br J Pharmacol. 2012;165:2325–2340. doi: 10.1111/j.1476-5381.2011.01716.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dou X, Sun Y, Li J, Zhang J, Hao D, Liu W, Wu R, Kong F, Peng X, Li J. Short-term rapamycin treatment increases ovarian lifespan in young and middle-aged female mice. Aging Cell. 2017;16:825–836. doi: 10.1111/acel.12617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang Y, Westbrook R, Hill C, Boparai RK, Arum O, Spong A, Wang F, Javors MA, Chen J, Sun LY, Bartke A. Duration of rapamycin treatment has differential effects on metabolism in mice. Cell Metab. 2013;17:456–462. doi: 10.1016/j.cmet.2013.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto VY, Bloom MS, Huddleston HG, Shelley WB, Ocque AJ, Browne RW. Correlations of follicular fluid oxidative stress biomarkers and enzyme activities with embryo morphology parameters during in vitro fertilization. Fertil Steril. 2011;96:1357–1361. doi: 10.1016/j.fertnstert.2011.09.032. [DOI] [PubMed] [Google Scholar]
- Genaro PDS, Sarkis KS, Martini LA. O efeito da restrição calórica na longevidade. Arq Bras Endocrinol Metabol. 2009;53:667–672. doi: 10.1590/s0004-27302009000500019. [DOI] [PubMed] [Google Scholar]
- Habermehl TL, Parkinson KC, Hubbard GB, Ikeno Y, Engelmeyer JI, Schumacher B, Mason JB. Extension of longevity and reduction of inflammation is ovarian-dependent, but germ cell-independent in post-reproductive female mice. GeroScience. 2019;41:25–38. doi: 10.1007/s11357-018-0049-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John GB, Gallardo TD, Shirley LJ, Castrillon DH. Foxo3 is a Pi3k-dependent molecular switch controlling the initiation of oocyte growth. Cancer. 2009;321:197–204. doi: 10.1016/j.ydbio.2008.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SC, Yanos ME, Bitto A, et al. Dose-dependent effects of Mtor inhibition on weight and mitochondrial disease in mice. Front Genet. 2015;6:1–8. doi: 10.3389/fgene.2015.00247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalich-Philosoph L, Roness H, Carmely A, et al. Cyclophosphamide triggers follicle activation and “burnout”; AS101 prevents follicle loss and preserves fertility. Sci Transl Med. 2013;5:162–185. doi: 10.1126/scitranslmed.3005402. [DOI] [PubMed] [Google Scholar]
- Li L, Fu YC, Xu JJ, Chen XC, Lin XH, Luo LL. Caloric restriction promotes the reproductive capacity of female rats via modulating the level of insulin-like growth factor-1 (IGF-1) Gen Comp Endocrinol. 2011;174:232–237. doi: 10.1016/j.ygcen.2011.09.005. [DOI] [PubMed] [Google Scholar]
- Li L, Fu YC, Xu JJ, Lin XH, Chen XC, Zhang XM, Luo LL. Caloric restriction promotes the reserve of follicle pool in adult female rats by inhibiting the activation of mammalian target of rapamycin signaling. Reprod Sci. 2015;22:60–67. doi: 10.1177/1933719114542016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lintern-Moore S, Moore GPM. The initiation of follicle and oocyte growth in the mouse ovary. Biol Reprod. 1979;20:773–778. doi: 10.1095/biolreprod20.4.773. [DOI] [PubMed] [Google Scholar]
- Liu K, Rajareddy S, Liu L, Jagarlamudi K, Boman K, Selstam G, Reddy P. Control of mammalian oocyte growth and early follicular development by the oocyte PI3 kinase pathway: new roles for an old timer. Dev Biol. 2006;299:1–11. doi: 10.1016/j.ydbio.2006.07.038. [DOI] [PubMed] [Google Scholar]
- Liu K, Zhang H, Risal S, et al. Somatic cells initiate primordial follicle activation and govern the development of dormant oocytes in mice. Curr Biol. 2014;24:2501–2508. doi: 10.1016/j.cub.2014.09.023. [DOI] [PubMed] [Google Scholar]
- Masternak MM, Al-Regaiey KA, Del Rosario LMM, et al. Caloric restriction results in decreased expression of peroxisome proliferator-activated receptor superfamily in muscle of normal and long-lived growth hormone receptor/binding protein knockout mice. J Gerontol A Biol Sci Med Sci. 2005;60:1238–1245. doi: 10.1093/gerona/60.10.1238. [DOI] [PubMed] [Google Scholar]
- Myers M, Britt KL, Wreford NG, et al. Methods for quantifying follicular numbers within the mouse ovary. Reproduction. 2004;127:569–580. doi: 10.1530/rep.1.00095. [DOI] [PubMed] [Google Scholar]
- Nacarelli T, Azar A, Altinok O, Orynbayeva Z, Sell C. Rapamycin increases oxidative metabolism and enhances metabolic flexibility in human cardiac fibroblasts. GeroScience. 2018;40:243–256. doi: 10.1007/s11357-018-0030-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolai S, Pallauf K, Huebbe P, Rimbach G. Energy restriction and potential energy restriction mimetics. Nutr Res Rev. 2015;28:100–120. doi: 10.1017/S0954422415000062. [DOI] [PubMed] [Google Scholar]
- Peters H. The development of the mouse ovary from birth to maturity. Acta Endocrinol. 1969;62:98–116. doi: 10.1530/acta.0.0620098. [DOI] [PubMed] [Google Scholar]
- Powers RWR, Kaeberlein M, Caldwell SD, et al. Extension of chronological life span in yeast by decreased TOR pathway signaling. Genes Dev. 2006;20:174–184. doi: 10.1101/gad.1381406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro LC, Quincozes-Santos A, Leite MC, Abib RT, Kleinkauf-Rocha J, Biasibetti R, Rotta LN, Wofchuk ST, Perry MLS, Gonçalves CA, Gottfried C. Caloric restriction increases hippocampal glutamate uptake and glutamine synthetase activity in Wistar rats. Neurosci Res. 2009;64:330–334. doi: 10.1016/j.neures.2009.04.004. [DOI] [PubMed] [Google Scholar]
- Saccon TD, Moreira F, Cruz LA, et al. Ovarian aging and the activation of the primordial follicle reserve in the long-lived Ames dwarf and the short-lived bGH transgenic mice. Mol Cell Endocrinol. 2016;455:23–32. doi: 10.1016/j.mce.2016.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarbassov DD, Ali SM, Sengupta S, Sheen JH, Hsu PP, Bagley AF, Markhard AL, Sabatini DM. Prolonged rapamycin treatment inhibits Mtorc2 assembly and Akt/PKB. Mol Cell. 2006;22:159–168. doi: 10.1016/j.molcel.2006.03.029. [DOI] [PubMed] [Google Scholar]
- Schneider A, Zhi X, Bartke A, Kopchick JJ, Masternak MM. Effect of growth hormone receptor gene disruption and PMA treatment on the expression of genes involved in primordial follicle activation in mice ovaries. Age (Dordr) 2014;36(4):9701. doi: 10.1007/s11357-014-9701-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider A, Zhi X, Moreira F, et al. Primordial follicle activation in the ovary of Ames dwarf mice. J Ovarian Res. 2014;7:1–9. doi: 10.1186/s13048-014-0120-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider A, Matkovich SJ, Saccon T, Victoria B, Spinel L, Lavasani M, Bartke A, Golusinski P, Masternak MM. Ovarian transcriptome associated with reproductive senescence in the long-living Ames dwarf mice. Mol Cell Endocrinol. 2017;439:328–336. doi: 10.1016/j.mce.2016.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selesniemi K, Lee H-J, Tilly JL. Moderate caloric restriction initiated in rodents during adulthood sustains function of the female reproductive axis into advanced chronological age. Aging Cell. 2008;7:622–629. doi: 10.1111/j.1474-9726.2008.00409.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi LY, Luo AY, Tian Y, Lai ZW, Zhang JJ, Wang SX. Protective effects of caloric restriction on ovarian function. Zhonghua Fu Chan Ke Za Zhi. 2013;48:745–749. [PubMed] [Google Scholar]
- Shi J, Zhang B, Choi J-Y, Gao YT, Li H, Lu W, Long J, Kang D, Xiang YB, Wen W, Park SK, Ye X, Noh DY, Zheng Y, Wang Y, Chung S, Lin X, Cai Q, Shu XO. Age at menarche and age at natural menopause in East Asian women: a genome-wide association study. Age (Dordr) 2016;38:513–523. doi: 10.1007/s11357-016-9939-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skałba P, Cygal A, Dabkowska-Huć A. The influence of anti-Mullerian hormone on folliculogenesis. Ginekol Pol. 2008;79:137–140. [PubMed] [Google Scholar]
- Te Velde ER, Scheffer GJ, Dorland M, et al. Developmental and endocrine aspects of normal ovarian aging. Mol Cell Endocrinol. 1998;145:67–73. doi: 10.1016/s0303-7207(98)00171-3. [DOI] [PubMed] [Google Scholar]
- Treff NR, Krisher RL, Tao X, et al. Next generation sequencing-based comprehensive chromosome screening in mouse polar bodies, oocytes, and embryos. Biol Reprod. 2016;94:76. doi: 10.1095/biolreprod.115.135483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urfer SR, Kaeberlein TL, Mailheau S, Bergman PJ, Creevy KE, Promislow DEL, Kaeberlein M. A randomized controlled trial to establish effects of short-term rapamycin treatment in 24 middle-aged companion dogs. GeroScience. 2017;39:117–127. doi: 10.1007/s11357-017-9972-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang N, Luo LL, Xu JJ, Xu MY, Zhang XM, Zhou XL, Liu WJ, Fu YC. Obesity accelerates ovarian follicle development and follicle loss in rats. Metabolism. 2014;63:94–103. doi: 10.1016/j.metabol.2013.09.001. [DOI] [PubMed] [Google Scholar]
- Wiesenborn DS, Menon V, Zhi X, Do A, Gesing A, Wang Z, Bartke A, Altomare DA, Masternak MM. The effect of calorie restriction on insulin signaling in skeletal muscle and adipose tissue of Ames dwarf mice. Aging. 2014;6:900–912. doi: 10.18632/aging.100700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson JE, Burmeister L, Brooks SV, Chan CC, Friedline S, Harrison DE, Hejtmancik JF, Nadon N, Strong R, Wood LK, Woodward MA, Miller RA. Rapamycin slows aging in mice. Aging Cell. 2012;11:675–682. doi: 10.1111/j.1474-9726.2012.00832.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell. 2006;124:471–484. doi: 10.1016/j.cell.2006.01.016. [DOI] [PubMed] [Google Scholar]
- Yoshida H, Takakura N, Kataoka H, Kunisada T, Okamura H, Nishikawa SI. Stepwise requirement of c-kit tyrosine kinase in mouse ovarian follicle development. Dev Biol. 1997;184:122–137. doi: 10.1006/dbio.1997.8503. [DOI] [PubMed] [Google Scholar]
- Zhang H, Liu K. Cellular and molecular regulation of the activation of mammalian primordial follicles: somatic cells initiate follicle activation in adulthood. Hum Reprod Update. 2015;21:779–786. doi: 10.1093/humupd/dmv037. [DOI] [PubMed] [Google Scholar]
- Zhang XM, Li L, Xu JJ, Wang N, Liu WJ, Lin XH, Fu YC, Luo LL. Rapamycin preserves the follicle pool reserve and prolongs the ovarian lifespan of female rats via modulating Mtor activation and sirtuin expression. Gene. 2013;523:82–87. doi: 10.1016/j.gene.2013.03.039. [DOI] [PubMed] [Google Scholar]