Abstract
Regenerative medicine is continuously facing new challenges and it is searching for new biocompatible, green/natural polymer materials, possibly biodegradable and non-immunogenic. Moreover, the critical importance of the nano/microstructuring of surfaces is overall accepted for their full biocompatibility and in vitro/in vivo performances. Chitosan is emerging as a promising biopolymer for tissue engineering and its application can be further improved by exploiting its nano/microstructuration. Here, we report the state of the art of chitosan films and scaffolds nano/micro-structuration. We show that it is possible to obtain, by solvent casting, chitosan thin films with good mechanical properties and to structure them at the microscale and even nanoscale level, with resolutions down to 100 nm.
Keywords: Chitosan, Microgratings, Nanostructured surfaces, Solvent casting, Nerve regeneration
Introduction
Tissue engineering is an interdisciplinary field that aims to use an implantation (i.e., scaffold) as (temporary) support to repair, replace, or enhance the function of a particular tissue. The ultimate aim is to develop a scaffold that can interact with the living tissue and stimulate and support its spontaneous regeneration (Shafiee and Atala 2016). Scaffolds can be optimized in chemistry, geometry, and functionalization (i.e., with biological factors, such as growth factors, or drugs) in order to better interact with patient’s cells (Lee et al. 2011; Almeida and Bártolo 2013). The scaffold acts as a temporary extracellular matrix (ECM), thus guiding cell behavior and tissue progression, until it is completely regrown (Ma 2004).
Typically, a scaffold is described as a three-dimensional solid support made of biomaterials. An ideal biomaterial is commonly defined as biocompatible, biodegradable, non-cytotoxic/non-mutagenic with respect to its degradation products. Accordingly, a biomaterial can promote cellular interaction, cell adhesion, and extracellular membrane deposition, all necessary steps to improve the subsequent cell proliferation on the surface and the final tissue regeneration (Ghassemi et al. 2018; Zhang et al. 2018). A key feature of any scaffold is its mechanical stability: it should physically sustain the tissue regrowth before biodegradation occurs (Bitar and Zakhem 2014).
Consequently, new materials, suitable for tissue engineering, are the object of continuous scientific research (Chan and Mooney 2008). Recent trends have placed the focus on natural biomaterials that do not have a high footprint on the environment and, at the same time, are not expensive and easy to be molded (Jahangirian et al. 2018). Biopolymers are a wide category of materials whose main sources are living organisms, not only plants and animals but also microorganisms (Rao et al. 2014).
One of the main reasons behind the choice of nature-derived materials employed as scaffolds is their high biodegradability. In addition, their biological origin often makes them favorable to interact with the biological systems (Le Bao Ha et al. 2013). Natural polymers can be further classified for their chemical composition: polysaccharides (cellulose, starch, chitin, and glycosaminoglycans) or proteins (keratin, collagen, silk, elastin, and fibrin); polynucleotides are less used in this field (Ratner et al. 2004). Natural polymers, such as collagen or gelatin, are the first biodegradable materials employed in human clinical practice (Nair and Laurencin 2007). Compared with synthetic materials, they tend to perform a greater biological interaction with cells and to have fewer side effects, such as toxic biodegradation products (Barua et al. 2018). For instance, collagen has a good biological interaction with cells: being the main fibrous structural protein in our body, it is non-immunogenic and resembles the cell’s native environment (Dong and Lv 2016). However, its mechanical properties and fast biodegradability are strong limitations (Ma et al. 2003).
Chitosan as a promising material for regenerative medicine
Chitin is one of the most abundant polymers present in nature, second only to cellulose (Elieh-Ali-Komi and Hamblin 2016). It is a natural homopolymer of N-acetyl-D-glucosamine widely found in the exoskeletons of arthropods and insects and in crustacean shells as well as in fungi cell wall. From the controlled deacetylation of chitin, it is possible to obtain a copolymer of β(1-4)-linked N-acetyl-D-glucosamine and D-glucosamine subunits, called chitosan (Islam et al. 2017). In nature, the extracted chitin is usually bound to proteins and minerals, which can be removed through processes of acidification and alkalization (Tapan Kumar and Bijaya 2018). The purified chitin is then converted into chitosan through controlled chemical processes, with the tuning of parameters such as concentration, ratio of chitin to alkali and temperature, in order to obtain a precise deacetylation degree in the final product (Sorlier et al. 2001). The degree of deacetylation impacts on the biological properties of chitosan, such as cell adhesion, healing capacity, and breakdown processes. Another important parameter is the molecular weight that depends on chitosan preparation procedures. It correlates with viscosity and it is inversely proportional to swelling capacity (Rodríguez-Vázquez et al. 2015).
The use of chitosan as a biomaterial is approved by the Food and Drug Administration (FDA) for application in biomedical devices, in particular, in drug delivery and in tissue engineering, with the final goal to restore the functionality of defective or lost tissues. As already hinted, chitosan is a completely biodegradable material; through an enzymatic transformation, it is broken down to its basic, non-toxic building blocks. In vivo, there are several enzymes that promote its degradation: the predominant one is lysozyme, a non-specific protease found in all mammalian tissues (Szymańska and Winnicka 2015). Importantly, chitosan is a hypoallergenic and bio-tolerated material: it does not routinely stimulate inflammation when implanted (Rodríguez-Vázquez et al. 2015). Moreover, it has shown interesting antimicrobial and antifungal properties and, for this reason, it is intensely studied for food packaging (Fernandez-Saiz 2011; Gutiérrez 2017) and tissue engineering applications (Rodríguez-Vázquez et al. 2015). The physico-chemical properties of scaffolds based on chitosan depend mainly on two parameters: the degree of deacetylation and the molecular weight of the starting material. For biomedical applications, a high degree of deacetylation is preferred, because this parameter has also an impact on the biological properties of the biomaterial, such as the degradation time in vitro and in vivo (Wei Wang et al. 2006).
Thanks to its biodegradability, chitosan has been extensively employed in medicine not only as scaffold material but also as a material of choice for the synthesis of nanoparticles for non-parenteral drug delivery of many drugs and vaccines, via several routes of administration (Mohammed et al. 2017). In particular, the ability to open the tight junctions in the epithelia makes it ideal for mucosal delivery, increasing the paracellular permeation and, as a consequence, the adsorption of the nanoparticles (Sonaje et al. 2012). This polymer can be also variously modified to finely tune the degradation pH and time and so modify the pharmacokinetic profile of drug release (Yuan et al. 2013; Miladi et al. 2015; Fonseca-Santos and Chorilli 2017).
Since the main topic of this review is the use of chitosan for regenerative medicine, we will focus on the chitosan employment for the fabrication of scaffolds for tissue engineering. In fact, it was already shown in literature that addressed tissue can be various (skin, bone and cartilage, nerve tissue, liver, heart, or cornea), as in the case of applications (Dutta, Rinki, and Dutta et al. 2011).
Chitosan has been used alone or in combination with other materials, in order to enhance the mechanical properties and degradation time for scaffolds. For instance, employment of chitosan would not be useful for skin tissue repair, but it can be an effective modifier for scaffolds made of polymers that, like collagen, have limitations in terms of rapid biodegradation and poor mechanical properties (Romanova et al. 2015). In order to avoid the short-time degradability and to enhance its mechanical properties, collagen was combined with chitosan, enhancing the scaffold stability over time (Tangsadthakun et al. 2006).
Bone tissue engineering aims for the construction of scaffolds that are mechanically strong enough to sustain bone regrowth. Usually, scaffolds for bone tissue are made of combinations of polymers and ceramic materials, such as calcium phosphate (Saravanan et al. 2016). Chitosan, thanks to its biodegradability and biocompatibility, is a good candidate for this medical application. Chitosan has been mixed with hydroxyapatite to create an ideal matrix for osteoblast proliferation and mineral deposition (Zo et al. 2012). Another possibility is to complex chitosan with whitlockite (an unusual form of calcium phosphate). Comparing the whitlockite/chitosan with the hydroxyapatite/chitosan composites, the first composite material shows better biocompatibility and enhances osteoblast proliferation (Zhou et al. 2017).
Peripheral nerve regeneration is one of the research fields in which chitosan, even when employed alone, shows the best results in terms of regeneration performances. One of the main options for the repair of short (below 3 cm) nerve gaps is becoming the implantation of a nerve guidance conduit, a tubular scaffold that connects the two ends of the injured nerve and sustains the regeneration process (Lundborg 2000; Ijpma et al. 2008; Sachanandani et al. 2014; Subramanian et al. 2009; Zeugolis et al. 2011). Chitosan-based nerve conduits, alone or in combination with other biomaterials, have been found to bridge efficiently peripheral nerve defects (Gnavi et al. 2013). Apart from rats (Gonzalez-Perez et al. 2015; Fregnan et al. 2016), chitosan conduits have been tested in several animal models for nerve regeneration, such as dogs (Tanaka et al. 2015) and goats (Muheremu et al. 2017). To provide an example, chitosan nerve conduits having an internal longitudinal chitosan membrane were used on a 10-mm sciatic nerve defects in adult healthy and diabetic rats and provide an enhancement in functional and morphological nerve regeneration (Meyer et al. 2016). In another work, chitosan flat membranes, crosslinked with dibasic sodium phosphate, were fabricated with a solvent casting technique (Fregnan et al. 2016). In vitro, the membranes allowed Schwann and DRGs’ cell proliferation and in vivo promoted nerve functional recovery, but leading only to an outcome comparable to median nerve repaired by autograft. Again, for the repair of long-gap peripheral nerve injury in the rat, the results with chitosan tubes (with varying degree of acetylation) were significantly better compared with silicon tubes, but lower than those with autografting (Gonzalez-Perez et al. 2015).
In fact, despite recent developments in biomaterial-based artificial scaffolds (Daly et al. 2012), autografting (with the related donor-site morbidity) still remains the gold standard in the clinical practice for nerve reconstruction (Raimondo et al. 2011), in particular for large nerve gaps. Chitosan has been already approved for clinical use in Europe. Reaxon® Nerve Guide conduits are smooth chitosan conduits, sold with different diameters (from 2.1 to 6 mm) and 3 cm long, to bridge gaps up to 26 mm. They are promoted as biocompatible, antibacterial, and antiadhesive, limiting scar tissue formation (Neubrech et al. 2016). When compared with autologous nerve grafts, the classical gold standard treatment for nerve injuries, Reaxon conduits gave similar results, with no statistically significant difference in the healing process (Shapira et al. 2016). The use of nerve guidance conduits for small diameter nerves has shown promising results, with most of the human studies describing neuronal recoveries between 74 and 100% (Braga Silva et al. 2017). However, there are still problems in repairing large-diameter nerves and wider gaps (Rebowe et al. 2018) and enhancing the regenerative potential of conduits could help in facing these limitations.
Chitosan topography modifications
Nowadays, chitosan films and conduits have been mainly modified in their chemical composition, by adding other materials (e.g., synthetic polymers, nanofillers) or cells (Gnavi et al. 2013), with less efforts in tuning their physical features.
Cells in vivo are embedded in a complex textured environment, composed of ECM meshed nano/microfibers (Tuzlakoglu et al. 2005; Wade and Burdick 2012; Andalib et al. 2016). It is a 3D physical environment composed by factors secreted by cells, mainly proteoglycans and fibrous proteins (Frantz et al. 2010). The ECM conveys not only biochemical but also physical cues to cells, triggering then an intracellular signaling cascade: this phenomenon is called mechanotransduction (Shih et al. 2011; Steward and Kelly 2015; Smith et al. 2017; Wolfenson et al. 2018). Hence, cells can respond to topography, at microscale and even nanoscale levels.
It was recently demonstrated that, by changing the surface topography at the nano/microscale, it is possible to control and guide the behavior and differentiation of a cell to a particular phenotype, changing its fate (Ferrari et al. 2010a, 2010b, Ferrari et al. 2011; Ankam et al. 2013; Franco et al. 2013). Not only the differentiation but also other processes involved in tissue regeneration can be regulated by substrate topography, such as cell polarization, neurite growth, and migration. Cell migration can be tuned by nano-microstructured surfaces and in particular significantly directed/enhanced with anisotropic topographies, such as nano/microgratings (Cecchini et al. 2008; Ferrari et al. 2010b; Jacchetti et al. 2014; Tonazzini et al. 2014a). These topographies (i.e., alternating lines of ridges and grooves with (sub) micrometric dimensions) have been designed and optimized in dimensions accordingly to the cell type and application (Tonazzini et al. 2014b), to promote neurite growth, cell polarization, and cell migration in the desired direction. Human endothelial cell migration is enhanced on gratings with a 2-μm period (Antonini et al. 2015). It has been shown that primary rat Schwann cells migrate faster on gratings with a 20-μm period (period = ridge width + groove width), analyzed as single cells, while their collective migration (i.e., in a monolayer, simulating a tissue wound healing situation) is enhanced on gratings with a 4-μm period (Tonazzini et al. 2015).
As already stated, chitosan is one of the major candidates as a suitable material for regeneration applications (reviewed in Rodríguez-Vázquez et al. 2015). Overall, chitosan, although an environmentally friendly promising biopolymer itself for regenerative medicine (Jahangirian et al. 2018), could be further improved in its regeneration potential by the introduction of topographical cues for cells, in order to direct their migration/differentiation and speed up the healing process.
There are a few fabrication techniques that have already been used to create nano/microstructures on chitosan films or scaffolds, but not all of them can be used for a precise and directional nano/micro-topography structuration. (i) Electrospinning is a useful manufacturing technique to obtain micro/nanofibers and mimic the texture of the ECM (e.g., collagen fibers). In fact, nanofibers have been effective in improving Schwann cells’ healing (Tonazzini et al. 2017). Wang and coworkers successfully created a chitosan nano/microfiber mesh tube (Wang et al. 2006). The chitosan solution (5%, in trifluoroacetic acid and methylene chloride) was electrospun on a negatively charged steel use stainless bar, which was intermittently compressed during chitosan deposition, compacting the fibers into a tube. Depending on the degree of chitosan deacetylation (DAc), the obtained fibers were 200 nm (for DAc 93%) or 400–600 nm (for DAc 78%) in diameter, with 10.98 MPa and 5.30 MPa Young’s moduli respectively. The chitosan tube made of 200-nm fiber mesh (DAc 93%) performed as the best conduit for nerve regeneration; however, its regenerative outcome was lower or comparable than for the iso-grafting control group. Additionally, 3D nanofibrillar chitosan scaffolds have been developed by electrospinning for skin regeneration and demonstrated to induce a faster regeneration of both the epidermis and dermis compartments, both in vitro and in vivo (while 3D chitosan sponges developed by freeze-drying induced granuloma formation) (Tchemtchoua et al. 2011). (ii) Nanosphere lithography (NSL) was used to generate surfaces of chitosan that mimic the nanostructures found on the surface of certain insect wings (Chandran et al. 2018). In NLS, the substrate is firstly covered with a close packed nanosphere layer and then processed by material deposition or etching. Here, chitosan, such as chitosan/nanosilver particles, was also able to self-assemble in a self-masking thin film, thus enabling a novel tool for the NLS. Both with classic NLS and the self-masking techniques, it was possible to obtain nano-cone patterns of about 250 nm in diameter. Regrettably, this is the only type of topography that is possible to obtain with these techniques and was not tested further in vitro or in vivo. (iii) Freeze-drying has been used to create three-dimensional chitosan-based–structured scaffolds. Yin and coworkers built a chitosan nerve conduit with highly aligned, double-layered porosity, having an overall control on pore size and orientation through the materials used to shape the scaffold (Yin et al. 2018). The solution (3.5 w/v% chitosan in 1.5 v/v% acetic acid) was injected into a coaxial tube, having the external part in aluminum and the internal one in brass. The system was frozen and subsequently lyophilized. This resulted in the formation of two distinct structural chitosan layers, with differently oriented and shaped pores, due to the two opposite thermal gradients during freezing generated by the two materials. The conduits were tested for compression showed a fully recoverable behavior, with an initial low stiffness for compression and a higher resistance due to compaction of the wall. These conduits were evaluated in bridging 10-mm Lewis rat sciatic nerve gap at 12 weeks post-implantation and qualitatively showed good regenerative efficacy.
Even though there are several reports about 2D and 3D chitosan biomaterials, chitosan has been mainly exploited for nanoparticles and nanocarriers and chitosan-based films or scaffolds have been mainly fabricated with no surface structuration, into tubular forms or at most into nanofiber scaffolds (Elieh-Ali-Komi and Hamblin 2016). There are very few studies about the precise micro/nanopatterning of chitosan films. The above-mentioned techniques cannot transfer a defined geometrical pattern on a chitosan membrane with micrometer or nanometric dimensionality. At the moment, chitosan has been patterned using only two techniques: (1) low-pressure low-temperature nanoimprinting and (2) solvent casting.
Nanoimprinting is a process that enables the imprinting of a pattern onto a thin film of a second (usually thermoplastic) material (Truskett and Watts 2006). The film is pressed on a silicon or polymeric mold with the desired pattern, with controlled high pressure and temperature. After the removal of the mold, the pattern is reproduced, inversely, on the film. A modified version of the nanoimprinting technique was used to produce chitosan films: it is slightly different from the classical nanoimprint process (carried at high temperatures), since it takes advantage of the relatively low temperature solidification of chitosan (Park et al. 2007). Medium molecular weight chitosan was dissolved in a solution of acetic acid 50% and heated at 40 °C to form a hydrogel, without using any additional plasticizer to lower the viscosity of the chitosan solution. Polydimethylsiloxane (PDMS) molds with micrometric and nanometric patterns were created as follows: microstructures consisted of microwells (3.5 μm diameter and 220 nm depth), microposts (2.2 μm width and 350 nm height), and checkerboards (2.2 μm width and 280 nm height); nanostructures consisted of nanowires (150 nm width and 500 nm pitch) and nanodots (150 nm width and 400 nm pitch). Molds were pressed on drops of chitosan solution, applying moderate heating (90 °C) and pressure (5–25 psi). Overall, the smallest features replicated had the resolution of 150 nm in width. This method successfully conveyed nano- and microfeatures on chitosan films, for bionanodevice applications. However, this method required several passages, careful control of the viscosity, and the use of a nanoimprinter and therefore of clean room facilities.
Solvent casting is probably the easiest method to fabricate plain or structured chitosan substrates. With this technique, a polymer solution is poured on a patterned mold, previously created with lithography techniques (Siemann 2005). Then, the solvent is allowed to evaporate, leaving a solid chitosan film that can be peeled off the mold. This method has been recently used to structure chitosan from silicon molds, creating micropatterned substrates (Sung et al. 2015). A 1% chitosan solution was poured on micropatterned silicon molds created by photolithography and left overnight at 60 °C. The molds had squared (50 μm wide and 5 or 15 μm deep), line, and hexagonal-like geometric patterns, with flat area surfaces printed on nanotextured regions created by Ag nanoparticle-assisted etching. Here, the solvent casting technique achieved good results in replicating both the micrometric features and nanotexturization. Neuro-2a cells preferred to adhere to the flat chitosan surfaces rather than the nanotextured areas and the hexagonal-like micropattern provided the most suitable surface for promoting neural cell network formation on these chitosan substrates in vitro. Though promising, Sung’s work is the only example of chitosan micropatterning with this technique, at the best of our knowledge.
Towards the nanoscale: fabrication of nano/micropatterns on chitosan membranes
Bio-based chitosan biopolymer scaffolds reinforced with nanostructures are emerging therefore as an interesting, but not yet deeply investigated, area of research. The easiest technique to obtain thin chitosan films is solvent casting. This technique does not involve complex instrumentations, high temperature, or harsh chemicals; it preserves the biocompatibility of the material and avoids its thermal degradation. We therefore set up a protocol to develop micro- and nanostructured chitosan thin films with topographical patterns of gratings (GRs; i.e., alternating lines of ridge and grooves) that can induce directional stimuli to cells (Tonazzini et al. 2015). The aim was to assess to which extent solvent casting was able to replicate nanoscopic features on chitosan films.
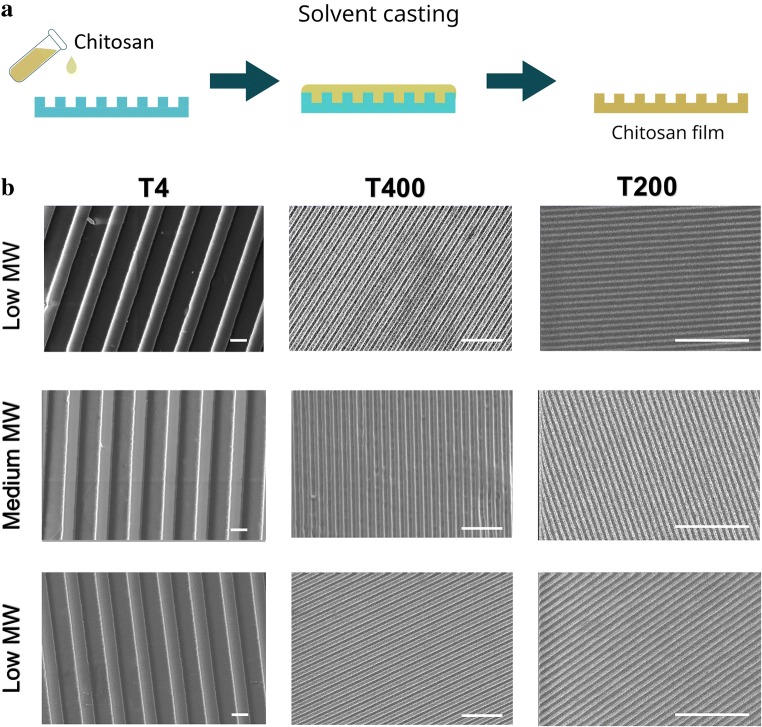
Chitosan was purchased in three different molecular weights, classified as low, medium, or high (Sigma-Aldrich). A 2% w/v chitosan solution (in distilled water + acetic acid 1% v/v) was filtered with a filter paper having a 10-μm cut-off (Superfiltro, Milano, IT) and poured on different pre-fabricated (methods in Masciullo et al. 2018, Masciullo et al. 2017) cyclic olefin copolymer molds having GR patterns of decreasing period: (1) a mold having 4 μm of period and 370 nm of depth (T4); (2) a mold with GRs with a 400-nm period and 200 nm of depth (T400); (3) a mold with GRs of a 200-nm period and 90 nm of depth (T200). Chitosan solution was baked at 37 °C until complete evaporation of the solvent. After evaporation, thin (~ 300 μm) chitosan films were peeled off of the molds (Fig. 1a) and evaluated by scanning electron microscopy (SEM) with a LEO 1525 field-emission scanning electron microscope (Zeiss).
Fig. 1.
a Schematic representation of the chitosan patterning process. b SEM images of microstructured and nanostructured films made of chitosan with low, medium, and high molecular weights; scale bars = 2 μm
As demonstrated by the SEM images reported in Fig. 1b, all of the three different molecular weights (low, medium, and high) of chitosan were able to replicate the GR features, from T4 down to T200 with no differences in the replication effectiveness. It was possible to distinguish well-defined ridges and grooves, with the expected period imposed by the mold. Even the smallest GR pattern (T200) was finely replicated on the chitosan surfaces. Altogether, this solvent casting technique allowed us to replicate nanostructured directional features on chitosan films with an overall resolution down to 100 nm, for the first time. This result is an important achievement: it demonstrates that it is possible to obtain chitosan films with the desired nano-GR pattern with a solvent casting technique that does not impact on the biological and mechanical properties of the material; it is simple (e.g., no clean room facilities need for the process itself, while any original mold can also be easily purchased on the market) and cost-effective (e.g., high mold reuse). This protocol represents an easy process to create nanostructured scaffolds for tissue engineering.
Mechanical characterization of chitosan membranes
The regeneration process is also influenced by the mechanical properties of the scaffold. An artificial substrate conveys to cells’ physical signals (e.g., stiffness) that regulate many processes in regeneration, such as cell proliferation and migration. For this reason, the mechanical compatibility of the material is fundamental in determining the outcome of the regeneration process and the scaffold would rather resemble the mechanical properties of the native tissue.
Chitosan films were mechanically characterized by uniaxial tensile tests at a constant cross-head speed using an Instron 5564 Testing System (Instron, Norwood, USA) equipped with a 2-kN load cell (Puppi et al. 2016). These measurements were performed again on the three different molecular weights of chitosan (low, medium, and high). Thin films of chitosan were prepared by solvent casting on silanized silicon wafers. Chitosan scaffolds are brittle when dry (such as before implantation), while their mechanical properties change when soaked in a liquid (such as in body fluids). For this reason, the mechanical properties of both dry and wet films were measured, in order to determine the different behavior of chitosan films in the two conditions. For the preparation of wet samples, dry chitosan films were first neutralized with NaOH 1% w/v for 30 min and then rinsed with deionized water.
Dog-bone–shaped (21.1 × 4.75 × 0.90 mm) samples were tested at a strain rate of 10 mm/min until specimen failure. The test was conducted at room temperature on 7 replicates for each molecular weight and dry/wet test condition. By analyzing the obtained stress-strain curves, the Young’s modulus (MPa) was calculated as the slope of the initial linear region, while the stress (MPa) and strain (%) at break were obtained at the sample break point. The measured values are reported in Table 1.
Table 1.
Mechanical properties of low, medium, and high molecular weights (MWs) chitosan films in dry and wet conditions. All values are reported as mean ± standard deviation
| Young’s modulus (MPa) | Stress at break (Mpa) | Strain at break (%) | |
|---|---|---|---|
| Low Mw-dry | 1644 ± 400 | 35 ± 9 | 18 ± 5 |
| Medium MW-dry | 2782 ± 500 | 56 ± 10 | 19 ± 4 |
| High Mw-dry | 430 ± 60 | 8.6 ± 1.5 | 15 ± 7 |
| Low Mw-wet | 2.9 ± 0.5 | 3.9 ± 1.5 | 123 ± 35 |
| Medium Mw-wet | 5 ± 1 | 5 ± 1 | 130 ± 11 |
| High MW-wet | 1.8 ± 0.4 | 1.6 ± 0.3 | 108 ± 13 |
As expected, the Young’s modulus and stress at break of wet films were consistently lower than those of dry films, for all molecular weights. On the other hand, the strain at break was significantly larger when samples were tested in wet conditions. The explanation for this behavior is that water acted as a plasticizer and enhanced the elasticity of the material.
For optimal tissue regeneration, there should be a match between the mechanical properties of the native tissue and the ones of the material. The stiffness of the material should be as close as possible to the natural environment of the cells we are trying to regenerate. For instance, the nerve Young’s modulus is about 0.58 MPa (Borschel et al. 2003), roughly an order of magnitude lower than medium Mw chitosan films (5 MPa) and only one-third that of high Mw chitosan films (1.8 MPa) tested in wet conditions.
Several factors other than material mechanical properties need to be considered, first of all the biocompatibility, but also the swelling due to degradation/fluid absorption, and the implantation procedure/suturability (if needed). An effective compromise between all these parameters should be optimized.
Conclusions
Regenerative medicine is facing new challenges and it is continuously searching for biocompatible and green/natural polymeric materials that are possibly biodegradable, non-immunogenic and having a good interaction with biological systems. Moreover, it is overall accepted that the nano/microstructure of (chitosan) devices deeply affects their regenerative performances. Chitosan is emerging as a promising biopolymer for tissue engineering and its application can be further improved by exploiting its nano/microstructuration, creating topographical features in chitosan membranes and conduits. Here, we reported the state of the art of chitosan films and scaffolds nano/micro-structuration and showed that it is possible to structure chitosan films at the microscale and even nanoscale levels, with resolutions down to 100 nm. Chitosan mechanical properties have been also characterized, as preliminary information on the possible use of this material in tissue regeneration. Further studies will confirm the enhanced regenerative potential of micro/nanostructured chitosan as scaffolds for nerve regeneration.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Almeida HA, Bártolo PJ (2013) Topological optimisation of scaffolds for tissue engineering. In: Procedia Engineering [DOI] [PubMed]
- Andalib MN, Dzenis Y, Donahue HJ, Lim JY (2016) Biomimetic substrate control of cellular mechanotransduction. Biomater. Res [DOI] [PMC free article] [PubMed]
- Ankam Soneela, Suryana Mona, Chan Lesley Y., Moe Aung Aung Kywe, Teo Benjamin K.K., Law Jaslyn B.K., Sheetz Michael P., Low Hong Yee, Yim Evelyn K.F. Substrate topography and size determine the fate of human embryonic stem cells to neuronal or glial lineage. Acta Biomaterialia. 2013;9(1):4535–4545. doi: 10.1016/j.actbio.2012.08.018. [DOI] [PubMed] [Google Scholar]
- Antonini S, Meucci S, Jacchetti E, Klingauf M, Beltram F, Poulikakos D, Cecchini M, Ferrari A. Sub-micron lateral topography affects endothelial migration by modulation of focal adhesion dynamics. Biomedical Materials. 2015;10(3):035010. doi: 10.1088/1748-6041/10/3/035010. [DOI] [PubMed] [Google Scholar]
- Barua E, Deoghare AB, Deb P, Lala S Das (2018) Naturally derived biomaterials for development of composite bone scaffold: a review. In: IOP Conference Series: Materials Science and Engineering
- Bitar Khalil N., Zakhem Elie. Design Strategies of Biodegradable Scaffolds for Tissue Regeneration. Biomedical Engineering and Computational Biology. 2014;6:BECB.S10961. doi: 10.4137/BECB.S10961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borschel Gregory H, Kia Kevin F, Kuzon William M, Dennis Robert G. Mechanical properties of acellular peripheral nerve. Journal of Surgical Research. 2003;114(2):133–139. doi: 10.1016/S0022-4804(03)00255-5. [DOI] [PubMed] [Google Scholar]
- Braga Silva J, Marchese GM, Cauduro CG, Debiasi M (2017) Nerve conduits for treating peripheral nerve injuries: a systematic literature review. Hand Surg. Rehabil [DOI] [PubMed]
- Cecchini M, Ferrari A, Beltram F. PC12 polarity on biopolymer nanogratings. Journal of Physics: Conference Series. 2008;100(1):012003. [Google Scholar]
- Chan G, Mooney DJ (2008) New materials for tissue engineering: towards greater control over the biological response. Trends Biotechnol [DOI] [PubMed]
- Chandran R, Nowlin K, LaJeunesse DR (2018) Nanosphere lithography of chitin and chitosan with colloidal and self-masking patterning. Polymers (Basel) 10. 10.3390/polym10020218 [DOI] [PMC free article] [PubMed]
- Daly William T., Yao Li, Abu-rub Mohammad T., O'Connell Claire, Zeugolis Dimitrios I., Windebank Anthony J., Pandit Abhay S. The effect of intraluminal contact mediated guidance signals on axonal mismatch during peripheral nerve repair. Biomaterials. 2012;33(28):6660–6671. doi: 10.1016/j.biomaterials.2012.06.002. [DOI] [PubMed] [Google Scholar]
- Dong C, Lv Y (2016) Application of collagen scaffold in tissue engineering: recent advances and new perspectives. Polymers (Basel) [DOI] [PMC free article] [PubMed]
- Dutta P. K., Rinki Kumari, Dutta Joydeep. Advances in Polymer Science. Berlin, Heidelberg: Springer Berlin Heidelberg; 2011. Chitosan: A Promising Biomaterial for Tissue Engineering Scaffolds; pp. 45–79. [Google Scholar]
- Elieh-Ali-Komi D, Hamblin MR (2016) Chitin and chitosan: production and application of versatile biomedical nanomaterials. Int J Adv Res [PMC free article] [PubMed]
- Fernandez-Saiz P (2011) Chitosan polysaccharide in food packaging applications. In: Multifunctional and Nanoreinforced Polymers for Food Packaging
- Ferrari Aldo, Cecchini Marco, Serresi Michela, Faraci Paolo, Pisignano Dario, Beltram Fabio. Neuronal polarity selection by topography-induced focal adhesion control. Biomaterials. 2010;31(17):4682–4694. doi: 10.1016/j.biomaterials.2010.02.032. [DOI] [PubMed] [Google Scholar]
- Ferrari Aldo, Faraci Paolo, Cecchini Marco, Beltram Fabio. The effect of alternative neuronal differentiation pathways on PC12 cell adhesion and neurite alignment to nanogratings. Biomaterials. 2010;31(9):2565–2573. doi: 10.1016/j.biomaterials.2009.12.010. [DOI] [PubMed] [Google Scholar]
- Ferrari Aldo, Cecchini Marco, Dhawan Akshay, Micera Silvestro, Tonazzini Ilaria, Stabile Ripalta, Pisignano Dario, Beltram Fabio. Nanotopographic Control of Neuronal Polarity. Nano Letters. 2011;11(2):505–511. doi: 10.1021/nl103349s. [DOI] [PubMed] [Google Scholar]
- Fonseca-Santos B, Chorilli M (2017) An overview of carboxymethyl derivatives of chitosan: their use as biomaterials and drug delivery systems. Mater Sci Eng C [DOI] [PubMed]
- Franco Davide, Klingauf Mirko, Cecchini Marco, Falk Volkmar, Starck Christoph, Poulikakos Dimos, Ferrari Aldo. On cell separation with topographically engineered surfaces. Biointerphases. 2013;8(1):34. doi: 10.1186/1559-4106-8-34. [DOI] [PubMed] [Google Scholar]
- Frantz C., Stewart K. M., Weaver V. M. The extracellular matrix at a glance. Journal of Cell Science. 2010;123(24):4195–4200. doi: 10.1242/jcs.023820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fregnan F, Ciglieri E, Tos P, Crosio A, Ciardelli G, Ruini F, Tonda-Turo C, Geuna S, Raimondo S. Chitosan crosslinked flat scaffolds for peripheral nerve regeneration. Biomedical Materials. 2016;11(4):045010. doi: 10.1088/1748-6041/11/4/045010. [DOI] [PubMed] [Google Scholar]
- Ghassemi T, Shahroodi A, Ebrahimzadeh MH, et al (2018) Current concepts in scaffolding for bone tissue engineering. Arch bone Jt Surg [PMC free article] [PubMed]
- Gnavi S, Barwig C, Freier T, et al (2013) The use of chitosan-based scaffolds to enhance regeneration in the nervous system. In: International Review of Neurobiology [DOI] [PubMed]
- Gonzalez-Perez F., Cobianchi S., Geuna S., Barwig C., Freier T., Udina E., Navarro X. Tubulization with chitosan guides for the repair of long gap peripheral nerve injury in the rat. Microsurgery. 2014;35(4):300–308. doi: 10.1002/micr.22362. [DOI] [PubMed] [Google Scholar]
- Gutiérrez TJ (2017) Chitosan applications for the food industry. In: Chitosan
- IJPMA F. F. A., VAN DE GRAAF R. C., MEEK M. F. The Early History of Tubulation in Nerve Repair. Journal of Hand Surgery (European Volume) 2008;33(5):581–586. doi: 10.1177/1753193408091349. [DOI] [PubMed] [Google Scholar]
- Islam SZ, Khan M, Alam AKM Nowsad. Production of chitin and chitosan from shrimp shell wastes. Journal of the Bangladesh Agricultural University. 2017;14(2):253–259. doi: 10.3329/jbau.v14i2.32701. [DOI] [Google Scholar]
- Jacchetti E, Tonazzini I, Meucci S, et al. Microstructured polydimethylsiloxane membranes for peripheral nerve regeneration. Microelectron Eng. 2014;124:26–29. doi: 10.1016/j.mee.2014.04.014. [DOI] [Google Scholar]
- Jahangirian H, Lemraski EG, Rafiee-Moghaddam R, Webster TJ (2018) A review of using green chemistry methods for biomaterials in tissue engineering. Int. J. Nanomedicine [DOI] [PMC free article] [PubMed]
- Bao Ha T Le, Minh T, Nguyen D, Minh D (2013) Naturally derived biomaterials: preparation and application. In: Regenerative Medicine and Tissue Engineering
- Lee K, Silva EA, Mooney DJ (2011) Growth factor delivery-based tissue engineering: general approaches and a review of recent developments. J R Soc Interface [DOI] [PMC free article] [PubMed]
- Lundborg Göran. A 25-year perspective of peripheral nerve surgery: Evolving neuroscientific concepts and clinical significance. The Journal of Hand Surgery. 2000;25(3):391–414. doi: 10.1053/jhsu.2000.4165. [DOI] [PubMed] [Google Scholar]
- Ma Peter X. Scaffolds for tissue fabrication. Materials Today. 2004;7(5):30–40. doi: 10.1016/S1369-7021(04)00233-0. [DOI] [Google Scholar]
- Ma L, Gao C, Mao Z et al (2003) Collagen/chitosan porous scaffolds with improved biostability for skin tissue engineering. Biomaterials [DOI] [PubMed]
- Masciullo Cecilia, Dell'Anna Rossana, Tonazzini Ilaria, Böettger Roman, Pepponi Giancarlo, Cecchini Marco. Hierarchical thermoplastic rippled nanostructures regulate Schwann cell adhesion, morphology and spatial organization. Nanoscale. 2017;9(39):14861–14874. doi: 10.1039/C7NR02822A. [DOI] [PubMed] [Google Scholar]
- Masciullo Cecilia, Sonato Agnese, Romanato Filippo, Cecchini Marco. Perfluoropolyether (PFPE) Intermediate Molds for High-Resolution Thermal Nanoimprint Lithography. Nanomaterials. 2018;8(8):609. doi: 10.3390/nano8080609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer C, Stenberg L, Gonzalez-Perez F, et al. Chitosan-film enhanced chitosan nerve guides for long-distance regeneration of peripheral nerves. Biomaterials. 2016;76:33–51. doi: 10.1016/j.biomaterials.2015.10.040. [DOI] [PubMed] [Google Scholar]
- Miladi K., Sfar S., Fessi H., Elaissari A. Enhancement of alendronate encapsulation in chitosan nanoparticles. Journal of Drug Delivery Science and Technology. 2015;30:391–396. doi: 10.1016/j.jddst.2015.04.007. [DOI] [Google Scholar]
- Mohammed MA, Syeda JTM, Wasan KM, Wasan EK (2017) An overview of chitosan nanoparticles and its application in non-parenteral drug delivery. Pharmaceutics [DOI] [PMC free article] [PubMed]
- Muheremu A, Chen L, Wang X, et al. Chitosan nerve conduits seeded with autologous bone marrow mononuclear cells for 30 mm goat peroneal nerve defect. Sci Rep. 2017;7:1–12. doi: 10.1038/srep44002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair LS, Laurencin CT (2007) Biodegradable polymers as biomaterials. Prog Polym Sci
- Neubrech F, Heider S, Harhaus L et al (2016) Chitosan nerve tube for primary repair of traumatic sensory nerve lesions of the hand without a gap: study protocol for a randomized controlled trial. Trials. 10.1186/s13063-015-1148-5 [DOI] [PMC free article] [PubMed]
- Park I, Cheng J, Pisano AP, et al. Low temperature, low pressure nanoimprinting of chitosan as a biomaterial for bionanotechnology applications. Appl Phys Lett. 2007;90:1–4. doi: 10.1063/1.2709914. [DOI] [Google Scholar]
- Puppi D, Piras AM, Pirosa A et al (2016) Levofloxacin-loaded star poly(ε-caprolactone) scaffolds by additive manufacturing. J Mater Sci Mater Med. 10.1007/s10856-015-5658-1 [DOI] [PubMed]
- Raimondo Stefania, Fornaro Michele, Tos Pierluigi, Battiston Bruno, Giacobini-Robecchi Maria G., Geuna Stefano. Perspectives in regeneration and tissue engineering of peripheral nerves. Annals of Anatomy - Anatomischer Anzeiger. 2011;193(4):334–340. doi: 10.1016/j.aanat.2011.03.001. [DOI] [PubMed] [Google Scholar]
- Rao M, Bharathi P, Akila RM (2014) A comprehensive review on biopolymers. Sci Revs Chem Commun
- Ratner BD, Hoffman AS, Schoen FJ, Lemons JE (2004) Biomaterials science: an introduction to materials in medicine
- Rebowe Ryan, Rogers Ashley, Yang Xuebin, Kundu S., Smith Thomas, Li Zhongyu. Nerve Repair with Nerve Conduits: Problems, Solutions, and Future Directions. Journal of Hand and Microsurgery. 2018;10(02):61–65. doi: 10.1055/s-0038-1626687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez-Vázquez M, Vega-Ruiz B, Ramos-Zúñiga R, et al. Chitosan and its potential use as a scaffold for tissue engineering in regenerative medicine. Biomed Res Int. 2015;2015:1–15. doi: 10.1155/2015/821279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanova O. A., Grigor’ev T. E., Goncharov M. E., Rudyak S. G., Solov’yova E. V., Krasheninnikov S. T., Saprykin V. P., Sytina E. V., Chvalun S. N., Pal’tsev M. A., Panteleev A. A. Chitosan as a Modifying Component of Artificial Scaffold for Human Skin Tissue Engineering. Bulletin of Experimental Biology and Medicine. 2015;159(4):557–566. doi: 10.1007/s10517-015-3014-6. [DOI] [PubMed] [Google Scholar]
- Sachanandani NF, Pothula A, Tung TH. Nerve gaps. Plast Reconstr Surg. 2014;133:313–319. doi: 10.1097/01.prs.0000436856.55398.0f. [DOI] [PubMed] [Google Scholar]
- Saravanan S., Leena R.S., Selvamurugan N. Chitosan based biocomposite scaffolds for bone tissue engineering. International Journal of Biological Macromolecules. 2016;93:1354–1365. doi: 10.1016/j.ijbiomac.2016.01.112. [DOI] [PubMed] [Google Scholar]
- Shafiee Ashkan, Atala Anthony. Tissue Engineering: Toward a New Era of Medicine. Annual Review of Medicine. 2017;68(1):29–40. doi: 10.1146/annurev-med-102715-092331. [DOI] [PubMed] [Google Scholar]
- Shapira Yuval, Tolmasov Michael, Nissan Moshe, Reider Evgeniy, Koren Akiva, Biron Tali, Bitan Yifat, Livnat Mira, Ronchi Giulia, Geuna Stefano, Rochkind Shimon. Comparison of results between chitosan hollow tube and autologous nerve graft in reconstruction of peripheral nerve defect: An experimental study. Microsurgery. 2015;36(8):664–671. doi: 10.1002/micr.22418. [DOI] [PubMed] [Google Scholar]
- Shih Yu-Ru V, Tseng Kuo-Fung, Lai Hsiu-Yu, Lin Chi-Hung, Lee Oscar K. Matrix stiffness regulation of integrin-mediated mechanotransduction during osteogenic differentiation of human mesenchymal stem cells. Journal of Bone and Mineral Research. 2011;26(4):730–738. doi: 10.1002/jbmr.278. [DOI] [PubMed] [Google Scholar]
- Siemann Ulrich. Scattering Methods and the Properties of Polymer Materials. Berlin, Heidelberg: Springer Berlin Heidelberg; 2005. Solvent cast technology – a versatile tool for thin film production; pp. 1–14. [Google Scholar]
- Smith Lucas R., Cho Sangkyun, Discher Dennis E. Stem Cell Differentiation is Regulated by Extracellular Matrix Mechanics. Physiology. 2018;33(1):16–25. doi: 10.1152/physiol.00026.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonaje K, Chuang EY, Lin KJ, et al. Opening of epithelial tight junctions and enhancement of paracellular permeation by chitosan: microscopic, ultrastructural, and computed-tomographic observations. Mol Pharm. 2012;9:1271–1279. doi: 10.1021/mp200572t. [DOI] [PubMed] [Google Scholar]
- Sorlier P, Denuzière A, Viton C, Domard A (2001) Relation between the degree of acetylation and the electrostatic properties of chitin and chitosan. Biomacromolecules. 10.1021/bm015531+ [DOI] [PubMed]
- Steward AJ, Kelly DJ (2015) Mechanical regulation of mesenchymal stem cell differentiation. J Anat [DOI] [PMC free article] [PubMed]
- Subramanian A, Krishnan UM, Sethuraman S. Development of biomaterial scaffold for nerve tissue engineering: biomaterial mediated neural regeneration. J Biomed Sci. 2009;16:108. doi: 10.1186/1423-0127-16-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung Chun-Yen, Yang Chung-Yao, Chen Wen-Shiang, Wang Yang-Kao, Yeh J Andrew, Cheng Chao-Min. Probing neural cell behaviors through micro-/nano-patterned chitosan substrates. Biofabrication. 2015;7(4):045007. doi: 10.1088/1758-5090/7/4/045007. [DOI] [PubMed] [Google Scholar]
- Szymańska E, Winnicka K. Stability of chitosan - a challenge for pharmaceutical and biomedical applications. Mar Drugs. 2015;13:1819–1846. doi: 10.3390/md13041819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka N, Matsumoto I, Suzuki M, et al. Chitosan tubes can restore the function of resected phrenic nerves. Interact Cardiovasc Thorac Surg. 2015;21:8–13. doi: 10.1093/icvts/ivv091. [DOI] [PubMed] [Google Scholar]
- Tangsadthakun C, Kanokpanont S, Sanchavanakit N, et al (2006) Properties of collagen/chitosan scaffolds for skin tissue engineering. J Met Mater Miner
- Tapan Kumar G, Bijaya G (2018) Polysaccharide-based nano-biocarrier in drug delivery
- Tchemtchoua Victor T., Atanasova Ganka, Aqil Abdel, Filée Patrice, Garbacki Nancy, Vanhooteghem Olivier, Deroanne Christophe, Noël Agnès, Jérome Christine, Nusgens Betty, Poumay Yves, Colige Alain. Development of a Chitosan Nanofibrillar Scaffold for Skin Repair and Regeneration. Biomacromolecules. 2011;12(9):3194–3204. doi: 10.1021/bm200680q. [DOI] [PubMed] [Google Scholar]
- Tonazzini Ilaria, Cecchini Alessandra, Elgersma Ype, Cecchini Marco. Interaction of SH-SY5Y Cells with Nanogratings During Neuronal Differentiation: Comparison with Primary Neurons. Advanced Healthcare Materials. 2013;3(4):581–587. doi: 10.1002/adhm.201300216. [DOI] [PubMed] [Google Scholar]
- Tonazzini Ilaria, Pellegrini Monica, Pellegrino Mario, Cecchini Marco. Interaction of leech neurons with topographical gratings: comparison with rodent and human neuronal lines and primary cells. Interface Focus. 2014;4(1):20130047. doi: 10.1098/rsfs.2013.0047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonazzini I, Jacchetti E, Meucci S, et al. Schwann cell contact guidance versus boundary -interaction in functional wound healing along nano and microstructured membranes. Adv Healthc Mater. 2015;4:1849–1860. doi: 10.1002/adhm.201500268. [DOI] [PubMed] [Google Scholar]
- Tonazzini Ilaria, Moffa Maria, Pisignano Dario, Cecchini Marco. Neuregulin 1 functionalization of organic fibers for Schwann cell guidance. Nanotechnology. 2017;28(15):155303. doi: 10.1088/1361-6528/aa6316. [DOI] [PubMed] [Google Scholar]
- Truskett VN, Watts MPC (2006) Trends in imprint lithography for biological applications. Trends Biotechnol [DOI] [PubMed]
- Tuzlakoglu K, Bolgen N, Salgado AJ, et al (2005) Nano- and micro-fiber combined scaffolds: a new architecture for bone tissue engineering. In: journal of materials science: materials in medicine [DOI] [PubMed]
- Wade RJ, Burdick JA (2012) Engineering ECM signals into biomaterials. Mater Today
- Wei Wang, Soichiro Itoh, Atsushi Matsuda, Shizuko Ichinose, Kenichi Shinomiya, Yuiro Hata JT (2006) Influences of mechanical properties and permeability on chitosan nano/microfiber mesh tubes as a scaffold for nerve regeneration. 557–566. doi: 10.1002/jbm.a [DOI] [PubMed]
- Wolfenson Haguy, Yang Bo, Sheetz Michael P. Steps in Mechanotransduction Pathways that Control Cell Morphology. Annual Review of Physiology. 2019;81(1):585–605. doi: 10.1146/annurev-physiol-021317-121245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin K, Divakar P, Hong J, et al. Freeze-cast porous chitosan conduit for peripheral nerve repair. MRS Adv. 2018;3:1–7. doi: 10.1557/adv.2018.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Zeting, Ye Yajing, Gao Feng, Yuan Huihui, Lan Minbo, Lou Kaiyan, Wang Wei. Chitosan-graft-β-cyclodextrin nanoparticles as a carrier for controlled drug release. International Journal of Pharmaceutics. 2013;446(1-2):191–198. doi: 10.1016/j.ijpharm.2013.02.024. [DOI] [PubMed] [Google Scholar]
- Zeugolis D, Daly W, Windebank A, et al. A biomaterials approach to peripheral nerve regeneration: bridging the peripheral nerve gap and enhancing functional recovery. J R Soc Interface. 2011;9:202–221. doi: 10.1098/rsif.2011.0438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K, Wang S, Zhou C, et al (2018) Advanced smart biomaterials and constructs for hard tissue engineering and regeneration. Bone Res [DOI] [PMC free article] [PubMed]
- Zhou Ding, Qi Chao, Chen Yi-Xuan, Zhu Ying-Jie, Sun Tuan-Wei, Chen Feng, Zhang Chang-Qing. Comparative study of porous hydroxyapatite/chitosan and whitlockite/chitosan scaffolds for bone regeneration in calvarial defects. International Journal of Nanomedicine. 2017;Volume 12:2673–2687. doi: 10.2147/IJN.S131251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zo SM, Singh D, Kumar A et al (2012) Chitosan-hydroxyapatite macroporous matrix for bone tissue engineering. Curr Sci