Abstract
Motoric cognitive risk syndrome (MCR), anxio-depressive disorders (ADD), and depression are associated with cognitive complaint and slow gait speed. The study aims to examine (1) the association of ADD and depression with MCR, and (2) the influence of the type and the severity of ADD and age on this association in older adults. A total of 29,569 participants free from cognitive impairment with walking speed measure recruited at baseline in the Canadian Longitudinal Study on Aging (CLSA) Comprehensive were selected in this cross-sectional study. They were separated into different sub-groups based on their age groups (i.e., 45–54, 55–64, 65–74, and ≥ 75) and the presence of MCR. Anxiety, mood, and depressive disorders (ADD) were assessed. Depression was defined by the Center for Epidemiological Studies Depression Scale (CES-D) score ≥ 10. The overall prevalence of MCR was 7.0 % and was greater in the youngest age group (8.9 %) as compared to the other age groups (P < 0.05). There was a higher prevalence of ADD and depression in individuals with MCR compared to those without MCR for all age groups (P ≤ 0.001). Depression was significantly associated with MCR regardless of age group (odds ratio ≥ 3.65 with P ≤ 0.001). The association of ADD with MCR depended on the accumulation of disorders and not their type, and was weaker and more inconstant in the oldest age group as compared to younger age groups. MCR is associated with ADD and depression in both younger and older individuals. This association is stronger for depression in younger individuals.
Keywords: Epidemiology, Walking speed, Cognitive complaint, Depression, Older adults, CLSA
Introduction
Motoric cognitive risk syndrome (MCR) is a new clinical syndrome, defined by subjective cognitive complaint and objective slow gait speed, with a high prevalence around 10 % in the population aged 60 and above (Verghese et al. 2012, 2014a, b). MCR predicts mild and major neurocognitive disorders (Verghese et al. 2014a, b). MCR does not rely on complex time-consuming assessments, making it applicable to screen individuals at risk for mild and major neurocognitive disorders in any type of healthcare setting (Verghese et al. 2014a, b).
Slow gait speed and cognitive complaints are unspecific symptoms that can be found to a wide variety of morbidities (i.e., having a disease or a symptom of the disease), thus causing overlap with other syndromes, which may influence the value of MCR risk for major neurocognitive disorders. For instance, there is an overlap between MCR and mild neurocognitive disorders also referred to mild cognitive impairment (MCI), which is a pre-dementia syndrome similar to MCR (Sekhon et al. 2017). Patients diagnosed with MCR can also be diagnosed with MCI, although this is not always the case. It has been suggested that patients cumulating both syndromes are more at risk for mild and major neurocognitive disorders compared to patients with MCR or MCI alone (Sekhon et al. 2017). Similarly, anxio-depressive disorders (ADD) defined by anxiety, mood, or depressive disorders as well as depression are associated with slow gait speed and cognitive complaints (Callisaya et al. 2016; Canada S. Table 13-10-0096-18 n.d.; Knoll and MacLennan 2017). Prevalence of ADD and depression similar to MCR is high and estimated to be around 9 % with some age variation; younger adults being at higher prevalence compared to older adults (Canada S. Table 13-10-0096-18 n.d.; Knoll and MacLennan 2017). Due to the high prevalence of MCR, ADD, and depression, there is a high probability of overlap. This probability is particularly high as subjective cognitive complaint used in MCR is often extracted from an item of depressive scales (Verghese et al. 2014a; Doi et al. 2015; Allali et al. 2015).
Few studies have examined the association of ADD and depression with MCR, those that have reported diverging results, as studies have found both significant and not significant associations (Verghese et al. 2012, 2014a, b; Callisaya et al. 2016; Doi et al. 2015; Allali et al. 2015; Beauchet et al. 2016a; Maguire et al. 2018; Wang et al. 2016; Winter et al. 2011). These mixed results may be explained by the definition used for ADD and depression which may be based on standardized questionnaires or antidepressant use (Verghese et al. 2014b; Callisaya et al. 2016; Doi et al. 2015; Beauchet et al. 2016a). Moreover, this divergence may also be related to the type of the ADD symptomology (e.g., anxiety mood, depressive disorders), and age, because of age-related changes in ADD prevalence (Canada S. Table 13-10-0096-18 n.d.; Knoll and MacLennan 2017; Canada S. Table 13-10-0465-01 n.d.). Thus, to better understand the relationship of ADD and depression with MCR, it seems important to examine this association taking into consideration parameters that may influence it.
We have the opportunity with the baseline assessment of a large population-based observational, prospective study in Canada—the Canadian Longitudinal Study on Aging (CLSA)—to better understand the parameters influencing the association of ADD and depression with MCR (Canadian longitudinal study on aging – Etude longitudinale canadienne sur le vieillissement n.d.). We hypothesized that there was an association of ADD and depression with MCR in the Canadian population and that this association may be influenced by the type of ADD and age. The study aims to examine (1) the association of ADD and depression with MCR and (2) the influence of the type and the severity of ADD and age on this association in the participants of the CLSA.
Material and methods
Population and study design
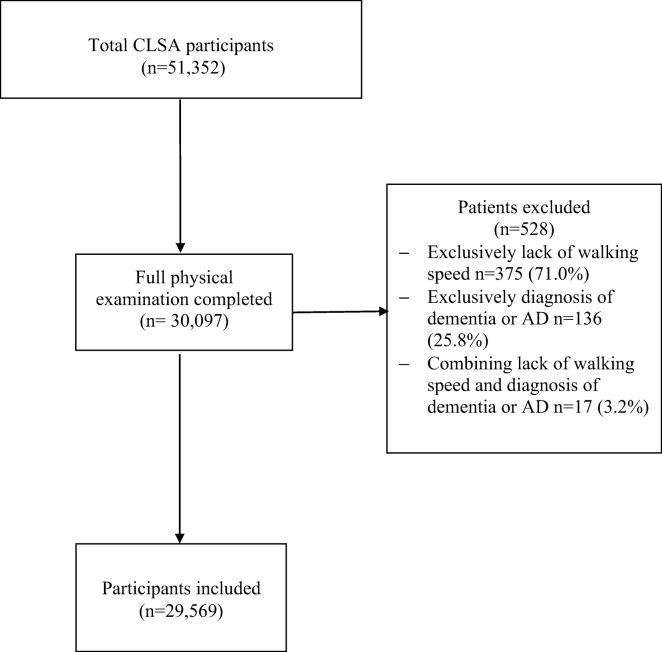
Data for this cross-sectional study are a subset of individuals recruited in the CLSA, which is a population-based, 20-year prospective cohort study (Canadian longitudinal study on aging – Etude longitudinale canadienne sur le vieillissement n.d.; Raina et al. 2009). This observational cohort is composed of over 50,000 Canadians, 45-85 years of age at the time of recuitment. The CLSA is accessible upon approval from the CLSA Data and Sample Access Committee (Canadian longitudinal study on aging – Etude longitudinale canadienne sur le vieillissement n.d.; Raina et al. 2009). The data collection methods have been previously described in detail (Raina et al. 2009). For this study, we selected a subset of the CLSA Comprehensive cohort. The inclusion criteria for this study were available walking speed measure and no diagnosis of dementia or Alzheimer’s disease (AD) (defined as “Has a doctor ever told you that you have dementia or Alzheimer’s disease?”). A total of 30,097 participants in the CLSA Comprehensive have a comprehensive physical examination with information about these two criteria and, thus, were considered for selection for this study (Canadian longitudinal study on aging – Etude longitudinale canadienne sur le vieillissement n.d.; Raina et al. 2009). After applying the inclusion criteria, 98.2 % (n = 29,569) participants were selected. One hundred thirty-six participants (0.5 %) were excluded because of lack of walking speed measure, 375 participants (1.3 %) were excluded because of having dementia or AD, and 17 participants (0.1 %) due to the accumulation of both exclusion criterion. Figure 1 shows the flow diagram for the selection of recruited participants. Selected participants were separated into four age groups based on their age at the time of the first baseline data collection: 45–54, 55–64, 65–74, and ≥ 75.
Fig. 1.
Flow diagram of selection of participants
Clinical assessment
All participants selected for this study had comprehensive full clinical examinations recording the following variables: age, sex, aboriginal identity (self-identifying as Aboriginal), country of birth (Canada versus other), independent place of living (i.e., not residing in an assisted living dwelling/institution), living alone (i.e., single, separated, divorced or widowed), low household income (i.e., CAD, < $50,000 annual), high education level (i.e., grade 9 or higher), total number of medications taken daily, mean value of body mass index (BMI) in kg/m2 and category overweight/obese defined with—BMI ≥ 25 kg/m2, and walking speed. Mean walking speed was considered using the time taken to complete the 4-meter walking test (seconds and milliseconds). The participants were instructed to stand behind the start line with their toes touching the start line and to walk (once instructed) until past the finish line (Raina et al. 2009). The participants were also allowed to practice once (Raina et al. 2009). The stopwatch was started after the instructions were given. The research staff member then said (ready, set) “go” to indicate that the participant should start walking and the stopwatch was stopped once the participant had completely passed the finish line (Raina et al. 2009). In addition, participants were considered to have a subjective cognitive complaint if they self-reported “sometimes, occasionally or always” for the variable trouble concentrating or “yes” for the variable memory problem on standardized questionnaires. Trouble concentrating was determined by asking the participants “How often did you have trouble keeping your mind on what you were doing?”. Memory problem was determined by asking the participant “Has a doctor ever told you that you have a memory problem?”.
Definition of motoric cognitive risk syndrome
MCR was defined by the association of subjective cognitive complaint and slow walking speed. Subjective cognitive complaint was defined using the variable trouble concentrating or the self-reported memory problem variable. Slow walking speed was defined as a walking speed one standard deviation (SD) below the average of the cohort. The cohort was defined using sex and age groups. The mean walking speed cutoff was found to be 0.85 m/s (for 45–54-year-old males), 0.84 m/s (for 45–54-year-old females), 0.81 m/s (for 55–64-year-old males), 0.79 m/s (for 55–64-year-old females), 0.77 m/s (for 65–74-year-old males), 0.73 m/s (for 65–74-year-old females), 0.68 m/s (for 75+ males), and 0.64 m/s (for 75+ females).
Definition of anxio-depressive disorders and depression
Expressions of ADD and depression were distinguished based on the items recorded. First, ADD was defined to have one of the following components: anxiety, mood, or depressive disorders. Anxiety disorder was determined by asking the participant “Has a doctor ever told you that you have an anxiety disorder such as a phobia, obsessive-compulsive disorder or a panic disorder?”. Mood disorder was determined by asking the participant “Has a doctor ever told you that you have a mood disorder such as depression (including manic depression), bipolar disorder, mania, or dysthymia?”. Depressive disorder was determined by asking the participant “Has a doctor ever told you that you suffer from clinical depression?”. Moreover, ADD severity was estimated using the accumulation of ADD components expressed per individual and separated into three levels: at least 1, 2, or all 3 components. In addition, depression was also considered and defined by the Center for Epidemiological Studies Depression Scale (CES-D) score ≥ 10 (Andresen et al. 1994).
Ethics
The study was conducted in accordance with the ethical standards set forth in the Helsinki Declaration (1983). Participants in the CLSA were included after obtaining their written and informed consent for the CLSA. The Jewish General Hospital Ethics Committee approved the study protocol. This research has been conducted using the CLSA (led by Drs. Parminder Raina, Christina Wolfson, and Susan Kirkland) baseline comprehensive dataset version 3.0 under Application No. 180902, and data access was approved by the CLSA Data and Sample Access Committee.
Statistics
Participants’ characteristics were summarized using means and standard deviations (SD) or frequencies and percentages, as deemed appropriate. Participants were categorized by age groups (45–54, 55–65, 65–74, 75+), and in each age group, they were separated in participants with and without MCR. Between-group comparisons were completed using unpaired t test or Chi-square test, as deemed appropriate. Due to multiple comparisons (n = 84), significant P values was fixed ≤ 0.0006. Multiple logistic regressions were performed to examine the association of ADD and depression (used as independent variable separately) and MCR (used as a dependent variable) for the entire population and the different age groups (i.e., 45–54; 55–64; 65–74; ≥ 75). This analysis was adjusted for age (only in the total population category), sex, Aboriginal identity, country of birth Canada, independent place of living, living alone, low household income, high education level, number of medications taken daily, and BMI. P values less than 0.05 were considered statistically significant for logistic regressions. All statistics were performed using SPSS (version 23.0; SPSS, Inc., Chicago, IL).
Results
Table 1 provides a comparison of participants’ characteristics according to age groups (i.e., 45–54, 55–64, 65–74, ≥ 75) in participants with and without MCR. The overall prevalence of MCR was 7.0 % and differed significantly when comparing age groups (P ≤ 0.001). The prevalence of MCR in 45–54 (8.9 %) was higher as compared to 55–64 (6.8 %, P ≤ 0.001), 65–74 (5.0 %, P ≤ 0.001), and ≥ 75 (7.4 %, P = 0.004). This prevalence of MCR in 55–64 was also significantly higher compared to 65–74 (P ≤ 0.001) but not different for ≥ 75 (P = 0.198), whereas it was lower in 65–74 compared to ≥ 75 (P ≤ 0.001).
Table 1.
Comparisons of participants’ characteristics according to age groups (i.e., 45–64, 55–64, 65–74, ≥ 75) and motoric cognitive risk syndrome in participants of the Canadian longitudinal study on aging (n = 29,569)
| Age 45–54 | P value* | Age 55–64 | P value* | Age 65–74 | P value* | Age ≥ 75 | P value* | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No MCR (n = 6863) | MCR (n = 666) | No MCR (n = 9036) | MCR (n = 663) | No MCR (n = 6850) | MCR (n = 359) | No-MCR (n = 4752) | MCR (n = 380) | |||||
| Age, mean value ± SD | 50.3 ± 2.7 | 50.4 ± 2.6 | 0.721 | 59.7 ± 2.8 | 59.9 ± 2.8 | 0.118 | 68.9 ± 2.8 | 69.5 ± 2.9 | ≤ 0.001 | 78.8 ± 2.9 | 79.8 ± 3.1 | ≤ 0.001 |
| Female, n (%) | 3492 (50.9) | 403 (60.5) | ≤ 0.001 | 4818 (53.3) | 190 (28.7) | ≤ 0.001 | 3368 (49.2) | 252 (70.2) | ≤ 0.001 | 2389 (50.3) | 149 (39.2) | ≤ 0.001 |
| Aboriginal, n (%) | 371 (5.4) | 58 (8.7) | ≤ 0.001 | 334 (3.7) | 32 (4.8) | 0.028 | 195 (2.8) | 16 (4.5) | 0.182 | 84 (1.8) | 6 (1.6) | 0.001 |
| Country of birth Canada, n (%) | 5802 (84.5) | 545 (81.8) | 0.064 | 7739 (85.6) | 556 (83.9) | 0.207 | 5339 (77.9) | 287 (79.9) | 0.377 | 3650 (76.8) | 303 (79.7) | 0.194 |
| Independent place of living†, n (%) | 6859 (99.9) | 666 (100) | 0.824 | 9018 (99.8) | 660 (99.5) | 0.176 | 6822 (99.6) | 348 (96.9) | ≤ 0.001 | 4663 (98.1) | 362 (95.3) | 0.001 |
| Living alone‡, n (%) | 1614 (23.5) | 203 (30.5) | ≤ 0.001 | 2520 (27.9) | 234 (35.3) | ≤ 0.001 | 2172 (31.7) | 163 (45.4) | ≤ 0.001 | 2094 (44.1) | 182 (47.9) | 0.340 |
| Low household income|| | 832 (12.1) | 171 (25.7) | ≤ 0.001 | 1890 (20.9) | 225 (33.9) | ≤ 0.001 | 2264 (33.1) | 202 (56.3) | ≤ 0.001 | 1914 (40.3) | 190 (50.0) | 0.002 |
| High education level¶, n (%) | 6840 (99.7) | 661 (99.2) | 0.147 | 8984 (99.4) | 654 (98.6) | 0.023 | 6701 (97.8) | 340 (94.7) | ≤ 0.001 | 4546 (95.7) | 356 (93.7) | 0.197 |
| Number of medications daily taken | 2.1 ± 1.8 | 2.8 ± 2.2 | ≤ 0.001 | 2.5 ± 2.0 | 3.3 ± 2.3 | ≤ 0.001 | 2.7 ± 2.0 | 3.7 ± 2.3 | ≤ 0.001 | 3.05 ± 2.0 | 3.6 ± 2.4 | ≤ 0.001 |
| Body mass index (kg/m2) | ||||||||||||
| Mean value ± SD | 27.7 ± 5.5 | 29.5 ± 7.1 | ≤ 0.001 | 28.3 ± 5.6 | 30.6 ± 7.0 | ≤ 0.001 | 28.2 ± 5.1 | 30.8 ± 7.4 | ≤ 0.001 | 27.3 ± 4.5 | 27.9 ± 5.3 | 0.008 |
| Overweight/obese§, n (%) | 4453 (64.9) | 490 (73.6) | ≤ 0.001 | 6341 (70.2) | 535 (80.7) | 0.001 | 4993 (72.9) | 281 (78.3) | 0.021 | 3216 (67.7) | 264 (69.5) | 0.408 |
| Anxio-depressive disorders | ||||||||||||
| Type of disorders | ||||||||||||
| Anxiety disorder‡‡ ††† | 234 (3.4) | 28 (4.2) | 0.285 | 289 (3.2) | 29 (4.4) | 0.101 | 223 (3.3) | 13 (3.6) | 0.704 | 120 (2.5) | 7 (1.8) | 0.409 |
| Mood disorder|||| ††† | 188 (2.7) | 22 (3.3) | 0.399 | 250 (2.8) | 30 (4.5) | 0.009 | 190 (2.8) | 19 (5.3) | 0.006 | 108 (2.3) | 13 (3.4) | 0.156 |
| Depressive disorder†† ††† | 148 (2.2) | 12 (1.8) | 0.545 | 251 (2.8) | 13 (2.0) | 0.212 | 160 (2.3) | 17 (4.7) | 0.004 | 75 (1.6) | 12 (3.2) | 0.022 |
| Number of disorders, n (%) | ||||||||||||
| ≥ 1 | 1609 (23.4) | 265 (39.8) | ≤ 0.001 | 2317 (25.6) | 265 (40) | ≤ 0.001 | 1462 (21.3) | 144 (40.1) | ≤ 0.001 | 665 (14.0) | 83 (21.8) | ≤ 0.001 |
| ≥ 2 | 1039 (15.1) | 203 (30.5) | ≤ 0.001 | 1527 (16.9) | 193 (29.1) | ≤ 0.001 | 889 (13.0) | 95 (26.5) | ≤ 0.001 | 362 (7.6) | 51 (13.4) | ≤ 0.001 |
| 3 | 309 (4.5) | 87 (13.1) | ≤ 0.001 | 426 (4.7) | 72 (10.9) | ≤ 0.001 | 222 (3.2) | 36 (10.0) | ≤ 0.001 | 80 (1.7) | 13 (3.4) | 0.015 |
| Depression*** | 970 (14.1) | 278 (41.7) | ≤ 0.001 | 1250 (13.8) | 288 (43.4) | ≤ 0.001 | 840 (12.3) | 143 (39.8) | ≤ 0.001 | 643 (13.3) | 135 (25.5) | ≤ 0.001 |
MCR, motoric cognitive risk
*Comparison based on unpaired t test or Chi-square test, as appropriate
†Defined as not residing in an assisted living dwelling/institution
‡Defined as single, separated, divorced or widowed
||Defined as CAD $ < 50,000
¶High education level is defined as grade 9 or higher
§Overweight defined as body mass index ≥ 25 kg/m2
#Trouble concentrating: how often did you have trouble keeping your mind on what you were doing?
**Memory problem: has a doctor ever told you that you have a memory problem?
††Defined as “Has a doctor ever told you that you suffer from clinical depression?”
‡‡Defined as “Has a doctor ever told you that you have an anxiety disorder such as a phobia, obsessive-compulsive disorder or a panic disorder?”
||||Defined as “Has a doctor ever told you that you have a mood disorder such as depression (including manic depression), bipolar disorder, mania, or dysthymia?”
†††Exclusive (i.e., no overlap with another depressive symptom)
***Center for Epidemiological Studies Depression Scale (CES-D) score (/X) ≥ 10
P value significant if ≤ 0.0006 because of multiple comparisons (n = 84) indicated in italics
In each age group, the following participants’ characteristics were found to be significant (P ≤ 0.001): sex (higher prevalence of females in participants with MCR compared to those without MCR in 45–54 and 65–74, with opposite results in other age group); number of medications taken (higher prevalence in participants with MCR compared to those without MCR, in all age groups); at least 1 and 2 ADD component (higher prevalence in participants with MCR compared to those without MCR, in all age groups); 3 ADD components cumulated (higher prevalence in participants with MCR compared to those without MCR, in all age groups except in the oldest); and depression (higher prevalence in participants with MCR compared to those without MCR, in all age groups).
Significant (P ≤ 0.001) difference between MCR and non-MCR participants was found for specific age groups for the following characteristics: mean age (older participants with MCR compared to those without MCR, in 65–74, ≥ 75, other age groups were not significant); aboriginal identity (higher prevalence in participants with MCR compared to those without MCR in 45–54, other age groups were not significant); living alone (higher prevalence in participants with MCR compared to those without MCR, in 45–54, 55–64, 65–74, the other age group was not significant); low household income (higher prevalence in participants with MCR compared to those without MCR in 45–54, 55–64, 65–74, older age group was not significant); high education level (lower prevalence in participants with MCR compared to those without MCR in 65–74, other age groups were not significant); mean value of BMI (higher prevalence in participants with MCR compared to those without MCR in 45–54, 55–64, 65–74, older age group was not significant); overweight/obese (higher prevalence in participants with MCR compared to those without MCR, in the ≥ 75, other age groups were not significant); and 3 ADD components (higher prevalence in participants with MCR compared to those without MCR in 45–54, 55–64, 65–74, the oldest age group was not significant).
Table 2 shows the results of multiple logistic regressions examining the association of ADD and depression (used as an independent variable separately) and MCR (used as a dependent variable) for all population and the different age groups (i.e., 45–54; 55–64; 65–74; ≥ 75) adjusted on participant’s clinical characteristics. Mood disorder was positively associated with MCR in the total population (OR = 1.49 with P = 0.001) and in 55–64 (OR = 1.66 with P = 0.013). For the age groups 45–54, 55–64, and 65–74, to have one, two, or all ADD components, as well as depression, was positively associated with MCR (OR ≥ 1.55 with P ≤ 0.002). For the oldest age group (i.e., ≥ 75) only to have at least one ADD components, as well as depression, was positively associated with MCR (OR = 1.52 with P = 0.003 and OR = 3.74 with P ≤ 0.001).
Table 2.
Multiple logistic regressions presenting the association of motoric cognitive risk syndrome (dependent variable) with depressive symptoms (independent variable, separated models for each symptom) in all population and the different age groups (i.e., 45–54; 55–64; 65–74; ≥ 75) adjusted on participant’s clinical characteristics (n = 29,569)
| OR (95 % CI), P value | |||||
|---|---|---|---|---|---|
| Total population* (n = 29,569) |
Age groups* | ||||
| 45–54 (n = 7529) |
55–64 (n = 9699) |
65–74 (n = 7209) |
≥ 75 (n = 5132) |
||
| Anxio-depressive disorders | |||||
| Type of disorders | |||||
| Clinical depression‡# |
0.85 (0.64; 1.14) 0.273 |
0.57 (0.31; 1.06) 0.074 |
0.59 (0.33; 1.05) 0.072 |
1.40 (0.82; 2.39) 0.214 |
1.49 (0.79; 2.83) 0.221 |
| Anxiety disorder||# |
1.23 (0.96; 1.56) 0.097 |
1.34 (0.90; 2.01) 0.153 |
1.42 (0.95; 2.11) 0.091 |
1.10 (0.62; 1.97) 0.736 |
0.77 (0.36; 1.69) 0.523 |
| Mood disorder¶# |
1.49 (1.18; 1.88) 0.001 |
1.16 (0.73; 1.82) 0.531 |
1.66 (1.11; 2.47) 0.013 |
1.63 (0.99; 2.70) 0.055 |
1.67 (0.92; 3.03) 0.090 |
| Number of disorders | |||||
| ≥ 1 |
1.64 (1.48; 1.82) ≤ 0.001 |
1.64 (1.37; 1.97) ≤ 0.001 |
1.78 (1.48; 2.13) ≤ 0.001 |
1.69 (1.33; 2.15) ≤ 0.001 |
1.52 (1.15; 2.00) 0.003 |
| ≥ 2 |
1.68 (1.50; 1.88) ≤ 0.001 |
1.79 (1.47; 2.18) ≤ 0.001 |
1.81 (1.48; 2.21) ≤ 0.001 |
1.55 (1.18; 2.02) 0.002 |
1.53 (1.10; 2.14) 0.074 |
| 3 |
2.10 (1.78; 2.47) ≤ 0.001 |
2.28 (1.74; 2.99) ≤ 0.001 |
2.08 (1.56; 2.77) ≤ 0.001 |
2.15 (1.44; 3.21) ≤ 0.001 |
1.72 (0.93; 3.18) 0.085 |
| Depression§# |
4.03 (3.65; 4.45) ≤ 0.001 |
3.75 (3.15; 4.47) ≤ 0.001 |
4.85 (4.06; 5.81) ≤ 0.001 |
3.65 (2.88; 4.63) ≤ 0.001 |
3.74 (2.95; 4.75) ≤ 0.001 |
OR, odds ratio; CI, confidence interval
*All models adjusted for age (only in the “All Age Groups” category) sex, aboriginal, country of birth Canada, independent place of living, living alone, low household income, high education level, number of medications daily taken and body mass index
‡Defined as “Has a doctor ever told you that you suffer from clinical depression?”
||Defined as “Has a doctor ever told you that you have an anxiety disorder such as a phobia, obsessive-compulsive disorder or a panic disorder?”
¶Defined as “Has a doctor ever told you that you have a mood disorder such as depression (including manic depression), bipolar disorder, mania, or dysthymia?”
§Center for Epidemiological Studies Depression Scale (CES-D) score (/X) ≥ 10
#Exclusive (i.e., no overlap with another depressive symptom)
P value significant (i.e., ≤ 0.05) in italics
Discussion
Our findings show that firstly the highest prevalence of MCR was observed in the youngest age group. Second, there was a higher prevalence of ADD and depression in individuals with MCR compared to those without MCR, for all age groups. Third, this prevalence of ADD and depression was lower in the oldest individuals with MCR compared to the other MCR age groups. Fourth, ADD and depression were positively associated with MCR, irrespective of age group, but this association was weaker for ADD in oldest age groups compared to the other age groups.
The study showed a higher prevalence of MCR in younger individuals as compared to older individuals. This result is not consistent with previous studies that found an opposite association (Verghese et al. 2012, 2014a, b). There are potential reasons for this discrepancy. Firstly, this is the first study that includes the prevalence of MCR in a young age group like 45–54. The majority of previous studies examined the prevalence of MCR in individuals age 65 and above (Callisaya et al. 2016). The youngest age group examined in the literature was individuals age 60 and over with a mean of 74.9 (Callisaya et al. 2016). It has been found that the prevalence of MCR increased with age, particularly after 75 (Verghese et al. 2014a). Our study showed a similar trend as the highest prevalence of MCR was found in the oldest age group when using the 55–64 age group as the reference group (excluding the 45–54 group). Second, previous studies have mainly recruited individuals from geriatric or memory clinics, thus resulting in a bias of selection of older individuals who had a high prevalence of cognitive complaint. In contrast, the CLSA is a population-based study including community-dwelling individuals, who have fewer concerns compared to those consulting in geriatric or memory clinics. Furthermore, as age is associated with slow gait speed, there is a higher probability to have more individuals with MCR in those consulting in geriatric or memory clinics compared to those who are not seen in these clinics who are younger. This last point may explain the fact that we found a lower prevalence of MCR in older age groups compared to the usual prevalence reported in the literature, respectively 7 % versus 10 %.
The second main result of our study is that ADD and depression were more prevalent in individuals with MCR compared to those without MCR, regardless of the age groups. There are two complementary explanations for this result. First, MCR, ADD, and depression present similar symptoms associating slow gait and cognitive complaint, which may result in the overlap. Moreover, it has also been previously reported that depression may be an early manifestation of neurocognitive disorders dues to neurodegenerative or vascular brain diseases, thus explaining the prevalence of ADD and depression in individuals with MCR which is a condition at risk for mild and major neurocognitive disorders (Panza et al. 2010; Baquero and Martín 2015). Second, MCR and ADD or depression are highly prevalent in the Canadian population and thus, there is a high probability to have an individual with both syndromes (Canada S. Table 13-10-0096-18 n.d.; Knoll and MacLennan 2017). Third, it is important to consider that MCR diagnosis in previous studies has typically used criteria extracted from depression scales. This association is important to take into consideration as depression may negatively impact the effect of another morbidity. For instance, it has been reported that comorbidity associated with depression worsens greater health condition compared to depression alone or any morbidity alone (Moussavi et al. 2007).
Another main result of our study is that ADD and depression were more prevalent in younger adults compared to older adults, regardless of the MCR status. The prevalence reported in the sample of younger as well as older MCR participants was similar to that reported in the Canadian population. The prevalence of major depressive disorders is reported to be almost double in younger Canadians (25–64), as compared to elderly Canadians (≥ 65), 12.5 % and 7.2 %, respectively (Canada S. Table 13-10-0465-01 n.d.). To explain this trend of higher prevalence in younger individuals, it can be suggested that they are very active and involved in various roles and activities, increasing their levels of stress. Moreover, older adults are less likely to express their ADD (possible underreporting and diagnosis in this age cohort) as compared to younger adults, possibly due to a shift in recent times of increasing awareness and lessening stigma surrounding mental health (Lyness et al. 1995; Knäuper and Wittchen 1994). Similar to the Canadian population, the age trend was observed more strongly in participants with MCR, as compared to participants without MCR, possibly due to the fact that those with MCR have reported a cognitive complaint and thus, are more likely to express and be open to discussing ADD symptomology/complaint. Lastly, it must also be considered that the scale used to detect depression in the CLSA, the CES-D is not a scale specific to the geriatric population, rather a general scale (Radloff 1977).
Finally, our study underscores that MCR is positively associated with ADD and depression in all age groups. However, this association was weaker in older individuals as compared to younger individuals, suggesting that the nature of the disease explaining the symptomatology influence this association. In younger individuals, MCR may be a clinical manifestation of ADD and depression, whereas in older adults it may be related both to depression or pre-dementia stage, thus explaining the higher prevalence of MCR in the younger population (Verghese et al. 2012, 2014a, b). Over the past 5 years, two clinical characteristics have been reported as a predictor of dementia. First, individuals with perceived changes in memory and/or cognition in the absence of objective evidence are commonly given a “diagnosis” of subjective cognitive impairment (SCI) (Jessen et al. 2014). SCI has been defined as a pre-mild cognitive impairment stage and thus, is considered the earliest clinical stage of AD. Second, low gait performance, such as slow walking speed, has been also associated with the occurrence of dementia (Scherder et al. 2007; Beauchet et al. 2016b). MCR is a combination of these two symptoms and has been recognized as a pre-dementia stage like MCI (Verghese et al. 2012, 2014a, b; Sekhon et al. 2017).
Our study has various strengths. The CLSA Comprehensive is a multicenter population-based cohort study with over 30,000 participants and a wide age range of 45-85-years of age at the time of recruitment. Moreover, a comprehensive clinical assessment of the participants has been completed. However, there are limitations that must be considered. Firstly, the cross-sectional design of our study does not afford causal inferences between depression and depressive symptomatology and MCR. Secondly, this study is a secondary analysis of the exsisting data, and the CLSA was not specially designed for our research question, explaining that characterization of ADD and depression were not supported by a psychiatric diagnosis but as answers to questions or questionnaires like the CES-D. Thirdly, it must be noted that the definition of cognitive complaint of MCR came from one item of the CES-D. Moreover, it must be considered that the question regarding cognitive complaint may not only refer to the subjective cognitive complaint but to the objective cognitive complaint as well. Finally, even if we took into consideration various variables were when analyzing the possible association of MCR with ADD and depression, it is possible that there are other confounders not considered.
In conclusion, the findings of this study underscore that ADD and depression are associated with MCR; aging leading to a weaker strength of association for depression and mixed results for ADD. These findings suggest that MCR may be a clinical manifestation of ADD or depression in younger individuals, whereas it may be related to both depression and pre-dementia stage in older adults.
Acknowledgments
This research was made possible using the data collected by the Canadian Longitudinal Study on Aging (CLSA). Funding for the Canadian Longitudinal Study on Aging (CLSA) is provided by the Government of Canada through the Canadian Institutes of Health Research (CIHR) under grant reference: LSA 9447 and the Canada Foundation for Innovation. This research has been conducted using the CLSA dataset [Baseline Comprehensive dataset version 3.0], under Application No. 180902. The CLSA is led by Drs. Parminder Raina, Christina Wolfson and Susan Kirkland.
Authors’ contributions
Study design HS and OB. Study conduct: HS and OB. Data collection: CLSA team. Data interpretation: HS and OB. Drafting manuscript: HS and OB. Revising manuscript content: GA. Approving final version of manuscript HS, OB, and GA. HS takes responsibility for the integrity of the data analysis.
Compliance with ethical standards
The study was conducted in accordance with the ethical standards set forth in the Helsinki Declaration (1983). Participants in the study were included after obtaining their written and informed consent for the study. The Jewish General Hospital Ethics Committee approved the study protocol.
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Allali G, Ayers EI, Verghese J. Motoric cognitive risk syndrome subtypes and cognitive profiles. J Geront Series A: Biomed Sci Med Sci. 2015;71(3):378–384. doi: 10.1093/gerona/glv092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andresen EM, Malmgren JA, Carter WB, Patrick DL. Screening for depression in well older adults: evaluation of a short form of the CES-D. Am J Prev Med. 1994;10(2):77–84. doi: 10.1016/S0749-3797(18)30622-6. [DOI] [PubMed] [Google Scholar]
- Baquero M, Martín N. Depressive symptoms in neurodegenerative diseases. World J Clin Cases. 2015;3(8):682. doi: 10.12998/wjcc.v3.i8.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchet O, Allali G, Annweiler C, Verghese J. Association of motoric cognitive risk syndrome with brain volumes: results from the GAIT study. J Gerontol Series A: Biomed Sci Med Sci. 2016;71(8):1081–1088. doi: 10.1093/gerona/glw012. [DOI] [PubMed] [Google Scholar]
- Beauchet O, Annweiler C, Callisaya ML, De Cock A-M, Helbostad JL, Kressig RW, et al. Poor gait performance and prediction of dementia: results from a meta-analysis. J Am Med Dir Assoc. 2016;17(6):482–490. doi: 10.1016/j.jamda.2015.12.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callisaya ML, Ayers E, Barzilai N, Ferrucci L, Guralnik JM, Lipton RB, Otahal P, Srikanth VK, Verghese J. Motoric cognitive risk syndrome and falls risk: a multi-center study. J Alzheimers Dis. 2016;53(3):1043–1052. doi: 10.3233/JAD-160230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canada S. Table 13-10-0096-18 Mood disorders, by age group
- Canada S. Table 13-10-0465-01 Mental health indicators
- Canadian longitudinal study on aging – Etude longitudinale canadienne sur le vieillissement www.clsa-elcv.ca: Canadian longitudinal study on aging;
- Doi T, Verghese J, Shimada H, Makizako H, Tsutsumimoto K, Hotta R, Nakakubo S, Suzuki T. Motoric cognitive risk syndrome: prevalence and risk factors in Japanese seniors. J Am Med Dir Assoc. 2015;16(12):1103. e21–1103. e25. doi: 10.1016/j.jamda.2015.09.003. [DOI] [PubMed] [Google Scholar]
- Jessen F, Amariglio RE, Van Boxtel M, Breteler M, Ceccaldi M, Chételat G, et al. A conceptual framework for research on subjective cognitive decline in preclinical Alzheimer’s disease. Alzheimers Dement. 2014;10(6):844–852. doi: 10.1016/j.jalz.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knäuper B, Wittchen H-U. Diagnosing major depression in the elderly: evidence for response bias in standardized diagnostic interviews? J Psychiatr Res. 1994;28(2):147–164. doi: 10.1016/0022-3956(94)90026-4. [DOI] [PubMed] [Google Scholar]
- Knoll AD, MacLennan RN. Prevalence and correlates of depression in Canada: findings from the Canadian Community Health Survey. Canadian Psychology/psychologie canadienne. 2017;58(2):116. doi: 10.1037/cap0000103. [DOI] [Google Scholar]
- Lyness JM, Cox C, Curry J, Conwell Y, King DA, Caine ED. Older age and the underreporting of depressive symptoms. J Am Geriatr Soc. 1995;43(3):216–221. doi: 10.1111/j.1532-5415.1995.tb07325.x. [DOI] [PubMed] [Google Scholar]
- Maguire FJ, Killane I, Creagh AP, Donoghue O, Kenny RA, Reilly RB. Baseline association of motoric cognitive risk syndrome with sustained attention, memory, and global cognition. J Am Med Dir Assoc. 2018;19(1):53–58. doi: 10.1016/j.jamda.2017.07.016. [DOI] [PubMed] [Google Scholar]
- Moussavi S, Chatterji S, Verdes E, Tandon A, Patel V, Ustun B. Depression, chronic diseases, and decrements in health: results from the World Health Surveys. Lancet. 2007;370(9590):851–858. doi: 10.1016/S0140-6736(07)61415-9. [DOI] [PubMed] [Google Scholar]
- Panza F, Frisardi V, Capurso C, D'introno A, Colacicco AM, Imbimbo BP, et al. Late-life depression, mild cognitive impairment, and dementia: possible continuum? Am J Geriatr Psychiatry. 2010;18(2):98–116. doi: 10.1097/JGP.0b013e3181b0fa13. [DOI] [PubMed] [Google Scholar]
- Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1(3):385–401. doi: 10.1177/014662167700100306. [DOI] [Google Scholar]
- Raina PS, Wolfson C, Kirkland SA, Griffith LE, Oremus M, Patterson C, Tuokko H, Penning M, Balion CM, Hogan D, Wister A, Payette H, Shannon H, Brazil K. The Canadian longitudinal study on aging (CLSA) Canadian Journal on Aging/La Revue canadienne du vieillissement. 2009;28(3):221–229. doi: 10.1017/S0714980809990055. [DOI] [PubMed] [Google Scholar]
- Scherder E, Eggermont L, Swaab D, van Heuvelen M, Kamsma Y, de Greef M, van Wijck R, Mulder T. Gait in ageing and associated dementias; its relationship with cognition. Neurosci Biobehav Rev. 2007;31(4):485–497. doi: 10.1016/j.neubiorev.2006.11.007. [DOI] [PubMed] [Google Scholar]
- Sekhon H, Allali G, Launay C, Chabot J, Beauchet O. The spectrum of pre-dementia stages: cognitive profile of motoric cognitive risk syndrome and relationship with mild cognitive impairment. Eur J Neurol. 2017;24(8):1047–1054. doi: 10.1111/ene.13331. [DOI] [PubMed] [Google Scholar]
- Verghese J, Wang C, Lipton RB, Holtzer R. Motoric cognitive risk syndrome and the risk of dementia. J Gerontol Series A: Biomed Sci Med Sci. 2012;68(4):412–418. doi: 10.1093/gerona/gls191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verghese J, Annweiler C, Ayers E, Barzilai N, Beauchet O, Bennett DA, Bridenbaugh SA, Buchman AS, Callisaya ML, Camicioli R, Capistrant B, Chatterji S, de Cock AM, Ferrucci L, Giladi N, Guralnik JM, Hausdorff JM, Holtzer R, Kim KW, Kowal P, Kressig RW, Lim JY, Lord S, Meguro K, Montero-Odasso M, Muir-Hunter SW, Noone ML, Rochester L, Srikanth V, Wang C. Motoric cognitive risk syndrome: multicountry prevalence and dementia risk. Neurology. 2014;83(8):718–726. doi: 10.1212/WNL.0000000000000717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verghese J., Ayers E., Barzilai N., Bennett D. A., Buchman A. S., Holtzer R., Katz M. J., Lipton R. B., Wang C. Motoric cognitive risk syndrome: Multicenter incidence study. Neurology. 2014;83(24):2278–2284. doi: 10.1212/WNL.0000000000001084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang N, Allali G, Kesavadas C, Noone ML, Pradeep VG, Blumen HM, Verghese J. Cerebral small vessel disease and motoric cognitive risk syndrome: results from the Kerala-Einstein study. J Alzheimers Dis. 2016;50(3):699–707. doi: 10.3233/JAD-150523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter Y, Korchounov A, Zhukova TV, Bertschi NE. Depression in elderly patients with Alzheimer dementia or vascular dementia and its influence on their quality of life. Journal of Neurosciences in Rural Practice. 2011;2(1):27–32. doi: 10.4103/0976-3147.80087. [DOI] [PMC free article] [PubMed] [Google Scholar]