Abstract
Human body is subject to many and variegated mechanical stimuli, actuated in different ranges of force, frequency, and duration. The process through which cells “feel” forces and convert them into biochemical cascades is called mechanotransduction. In this review, the effects of fluid shear stress on bone cells will be presented. After an introduction to present the major players in bone system, we describe the mechanoreceptors in bone tissue that can feel and process fluid flow. In the second part of the review, we present an overview of the biological processes and biochemical cascades initiated by fluid shear stress in bone cells.
Keywords: Mechanotransduction, Fluid shear stress, Bone cells
Introduction
The term “mechanotransduction” indicates the set of mechanisms that enables the cell to convert a mechanical stimulus into biochemical activity. Human body is subject to many and various mechanical stimuli, including flow shear stress, compression, and traction, all of them actuated in different ranges of force magnitude, frequency, and duration (Thompson et al. 2012). One of the main tissues able to feel mechanical strengths is bone, which is characterized by a porous but compact structure (Mirzaali et al. 2016; Yavropoulou and Yovos 2016). For example, it is well known that bone remodeling, the physiological lifelong process responsible for old bone resorption and substitution with new bone (Florencio-Silva et al. 2015; Wittkowske et al. 2016), is guided by forces felt by involved skeletal cells (Stoltz et al. 2018; Wang et al. 2018). In bone tissue, gravitational force and microscopic and macroscopic manifestations of muscle contractions induce mechanical stimuli, leading to bone matrix strain and interstitial fluid flow filling bone porosities (Case et al. 2011; Liu et al. 2010; Piekarski and Munro 1977; Wittkowske et al. 2016).
Many studies have shed light on the effects of fluid flow on bone cells and on what happens at molecular levels when muscles stress bone tissue. Most of them are in vitro experiments performed on bone cells progenitors of mesenchymal origin, called mesenchymal stem cells (MSC), on bone forming cells, called osteoblasts, and on cells included in mature bone tissue, called osteocytes. Most studies expose these cells to controlled fluid flows and measure parameters including cell proliferation rates, maturation or differentiation mostly through the assessment of bone morphogenetic proteins (BMPs) (Delaine-Smith and Reilly 2012), osteopontin (OPN) (Yourek et al. 2010), or osteocalcin (OC) (Nagaraja and Jo 2014) levels, or variations in calcium mobilization (Godin et al. 2007). Just a limited number of studies evaluated an extended list of targeted molecules, trying to highlight biomolecular interactions involved in cellular response to mechanical stimuli. Nevertheless, a comprehensive idea about molecular players activated by stressing bone cells through fluid shear stress is still missing.
In this review paper, a rational summary of the current scientific knowledge regarding the effects of fluid shear stress on bone tissue cells is provided, with particular interest for how bone cells feel the applied forces and for which mechanically induced biochemical cascades are activated. Mechanoreceptors present in bone cells and able to feel and process fluid flow are introduced, followed by an overview of the biochemical pathways initiated by this stress in bone environment.
Bone microstructure and interstitial fluid
Bone is a poroelastic material physiologically subject to a range of stresses in due to daily activities. It is composed of two different tissue types: cortical bone, also called compact, and cancellous bone, also called trabecular or spongy. Both cortical and cancellous bones are porous structures. Pores influence mechanical behavior of the tissue, providing robustness and elasticity where necessary. Three levels of porosities have been identified in bone tissue, presenting different sizes (Cardoso et al. 2013; Cowin and Cardoso 2015): (1.) the vascular porosities within Volkmann and Haversian canals, which are microscopic structures measuring 20 μm in radius and transmit blood vessels in cortical bones from the periosteum into the bone to provide energy and nourishments for osteons; (2.) the lacunar-canalicular system (LCS), a complex network formed by lacunar pores and 0.1 μm radius canalicular channels in the mineralized tissue matrix; (3.) the collagen-hydroxyapatite porosity, which has the smallest pore size.
LCS is composed of lacunar pores occupied by osteocytes, the most abundant cell type in bone, and canaliculi, which are few hundred nanometers in diameter canals running through the bone solid matrix that contain the cell processes of contiguous osteocytes, thus permitting communication between neighboring bone cells. LCS is saturated by interstitial fluids, composed of water, which represents an ideal medium for diffusion-driven ion transport, and other molecules such as sugars, salts, fatty acids, amino acids, coenzymes, and hormones (Wehrli and Fernández-Seara 2005). Fluids can be found in both cortical and cancellous bone, filling the porosities of the tissue. The movement of fluid through the extracellular matrix of tissues, often between blood and lymphatic vessels, is called interstitial fluid flow. Other than transporting these substances to the cells within the bone and while removing metabolic wastes from the cells (Burger and Klein-Nulend 1999; Fritton and Weinbaum 2009), movement of the interstitial fluid provides a specific mechanical environment, represented by fluid shear stress, that is important for the physiological activities of interstitial cells (Knothe Tate 2003; Wittkowske et al. 2016).
Piekarski and Munro (Piekarski and Munro 1977) proposed that interstitial fluid flow in the lacunar-canalicular system is enhanced by daily mechanical loading of bone, increasing the transport of nutrients and metabolic waste between the vascular porosity and the lacunar-canalicular porosity. The interstitial fluid travels inside the canaliculi and lacunae, passing through the space between the osteocyte dendritic process and the canalicular wall, dragging the osteocyte’s cell process and deflecting the tethering elements that attach the cell process to the canalicular wall. Bone tissue would not survive without fluid flow. In vitro experiments were carried out exploring different types of fluid flow in an attempt to mimic physiological interstitial fluid flowing on bone cells (Bonewald and Johnson 2008). These include (i) steady, or laminar flow (Reich et al. 1990; Williams et al. 1994); (ii) pulsatile flow (Hillsley and Frangos 1997), which presumes changes in flow frequency; and (iii) oscillatory flow (Jacobs et al. 1998), where there are changes in flow direction. Directionality of fluid flow appeared to be important (Chen and Jacobs 2013).
Mechanoreceptors of fluid flow
All osteogenic cells, from MSC to osteoblasts and osteocytes, are mechanosensitive and therefore can sense and respond to applied force. Even osteocytes, embedded in bone and therefore for long viewed as inactive, quiescent cells, are highly dynamic. Their cell bodies undergo deformation and they can extend and retract their dendritic processes, redirecting signaling between cells, thus performing as crucial mechanosensors in bone tissue (Zhang et al. 2006). Osteocytes are believed to be the primary sensor of mechanical stimuli in bone, which orchestrate osteoblasts and osteoclasts to adapt bone structure and composition to meet physiological loading demands (Verbruggen et al. 2014). In particular, all bone cells are able to respond to fluid flow (Huang et al. 2015; Riehl et al. 2015; Vezeridis et al. 2006).
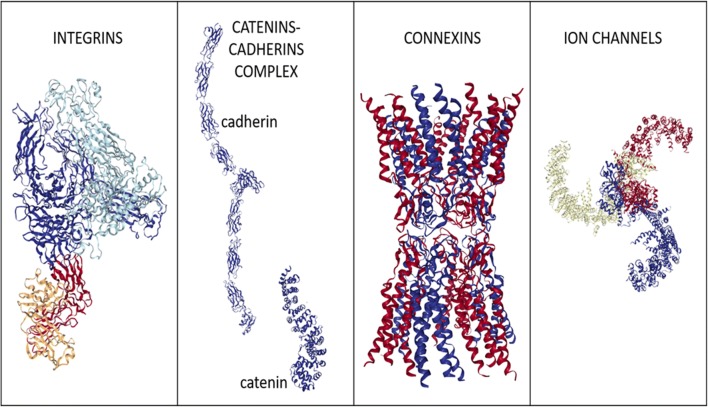
In bone cells, mechanoreceptors can detect alterations in forces that are converted into biomolecular signals. The principal mechanoreceptors in bone cells include the cytoskeleton, transmembrane integrins, cadherins, connexins, lipid rafts, ion channels, and primary cilia. An overview of these players is provided in Fig. 1, collecting cell mechanoreceptors constituted by a single molecule or a protein complex, and in Fig. 2, showing mechanoreceptors represented by organized cell structures. Note that many of them tightly interact within the cell. For example, the cortical actin cytoskeleton is undoubtedly one of the most important factors influencing membrane organization (Johnson 2017) and the cytoskeleton is linked to the nucleoskeleton (Jahed et al. 2014). Table 1 lists mechanoreceptors associated to biomolecular pathways they are able to trigger.
Fig. 1.
Cartoon showing examples of mechanoreceptors constituted by single molecules or protein complexes. Protein structures were obtained from Protein Data Bank (rcsb.org; (Berman et al. 2000)) and were generated by NGL Viewer (Rose et al. 2018). Integrin cartoon represents human AlphaVBeta3 integrin structure (PDB reference: 4O02; (Mahalingam et al. 2014)). Catenin cartoon represents human beta-catenin structure (PDB reference: 4DJS; (Grossmann et al. 2012)). Cadherin cartoon represents mouse N-cadherin ectodomain crystal structure (PDB reference: 3Q2W; (Harrison et al. 2011)). Connexin cartoon represents human Connexin-26 structure (PDB reference: 5ERA; (Bennett et al. 2016)). Ion channel cartoon represents mouse mechanosensitive Piezo1 channel structure (PDB reference: 5Z10; (Zhao et al. 2018))
Fig. 2.
Figure showing cartoons of mechanoreceptors constituted by organized cellular structures
Table 1.
Association between mechanoreceptors recognized in bone cells to feel fluid flow (“Mechanoreceptor for FSS”) and pathways they are known to activate in response to fluid flow (“Downstream pathway induced by FSS”). “Reference pathway” indicates the name of the paragraph of the manuscript where a description is provided. FSS, fluid shear stress
| Mechanoreceptor for FSS | Downstream pathway induced by FSS | Reference pathway |
|---|---|---|
| Integrins and focal adhesions | COX-2 and PGE2 signaling | G Protein–mediated signaling |
| Integrins and focal adhesions | Release of prostaglandin | G Protein–mediated signaling |
| Integrins and focal adhesions | FAK pathways | FAK signaling |
| Integrins and focal adhesions | ERK1/2 pathways | MAPK signaling |
| Cadherins and catenin complexes | Wnt/β-catenin signaling pathway | Wnt/β-catenin signaling |
| Connexins | Release of prostaglandin | G Protein–mediated signaling |
| Connexins | Intracellular Ca2+ oscillations | Calcium signaling |
| Connexins | ERK1/2 MAP kinase | MAPK signaling |
| Cytoskeleton | RhoA/ROCK pathway | G Protein–mediated signaling |
| Mechanosensitive ion channels | Ca2+ signal pathways | Calcium signaling |
| Glycocalyx | PGE2 pathway | G Protein–mediated signaling |
| Lipid rafts and caveolae | ERK1/2 and c-fos expression | MAPK signaling |
| Primary cilia | increase of COX2 | FAK signaling |
| Primary cilia | PGE2 signaling | G Protein–mediated signaling |
Integrins and focal adhesions
Integrins are transmembrane cell surface receptors, comprising heterodimers of α and β subunits. They are considered direct mechanosensors that physically connect the extracellular matrix (ECM) to the cytoskeleton (Hynes 2002), thus acting as mechanic signal receptors. Cell integrins bind the ECM externally, while are associated with the actin cytoskeleton through an elaborate network of proteins that is collectively known as the “integrin adhesome” (Winograd-Katz et al. 2014), which theoretically counts more than 200 components (Horton et al. 2016). The term “adhesome” mainly includes (1) focal complexes, small dot-like adhesions of around 1 μm in width, mainly found in lamellipodia, and are characterized by transient adhesion sites formed during cell migration; (2) focal adhesions, stable integrin-mediated, cell–substrate adhesion structures that anchor the termini of actin filaments and mediate strong attachments to substrates; (3) fibrillar adhesions, a form of adhesion typically associated with fibronectin fibers and less mechanosensitive than focal adhesions (Winograd-Katz et al. 2014). Focal adhesions are the core of the integrin signaling platform, enabling cells to sense the chemical and mechanical properties of the external environment and to respond by activating signaling networks that regulate cell structure, dynamics, behavior, and fate. They contain high levels of vinculin, talin, paxillin, zyxin, α-actinin, VASP, FAK, phosphotyrosine proteins, integrin αVβ3 (Zaidel-Bar et al. 2004), actopaxin (Nikolopoulos and Turner 2000), and p130Cas (Machiyama et al. 2014).
The integrin adhesome is known to be involved in MSC, osteoblasts, and osteocytes mechanoreception of fluid shear stress. Fluid flow stimuli on osteoblasts up-regulates β1 integrin, and its association with Shc, an adaptor protein containing C-terminal Src homology domain-2 (SH2) domain, leads to the activation of ERK ((Kapur et al. 2003); Lee et al. 2008). Moreover, fluid flow was proven to activate αVβ3 integrin (Weyts et al. 2002) in osteoblasts. It is known since long time that integrins play as mechanosensors for fluid flow shear stress even in osteocytes (Salter et al. 1997). In fact, the integrin α5 subunit may act as a tethering protein that, when perturbed by shear stress, opens hemichannels in osteocytes, allowing the release of prostaglandin (Siller-Jackson et al. 2006). In addition, Litzenberger et al. (Litzenberger et al. 2010) elucidated the role of integrins as the molecular driver in osteocytes for COX-2 and PGE2 signaling in response to dynamic fluid flow, independent of intracellular calcium release. Upon application of fluid shear stress to MSC, activation of FAK and extracellular signal–regulated kinase 1/2 (ERK1/2) was observed, which resulted to be inhibited when blocking β1 integrins (Liu et al. 2012). This highlights not only the importance of β1 integrin in mediating the response of human bone marrow-derived MSCs to mechanical stimulation, but also its relation with FAK and ERK1/2 pathways activation.
Cadherins and catenin complexes
Cadherins are a family of calcium-dependent glycoproteins that mediate cell–cell adhesion in all solid tissues. They have a major role in morphogenesis and tissue formation. They link to the cytoskeleton by forming junctional complexes that contain β-catenin and γ-catenin, which bind actin filaments to intermediate filaments, respectively.
Concerning bone cells, it is known that in osteoblasts, β-catenin associates with cadherins on the inner leaflet of the plasma membrane, and that cadherins control osteoblast differentiation by mediating cell–cell adhesion and signals that promote phenotypic osteoblast gene expression (Marie and Haÿ 2013). When investigating the effect of fluid flow on osteoblasts, Norvell et al. (Norvell et al. 2004) found that stress decreases the amount of β-catenin bound to N-cadherin thus increasing the cytoplasmic pool of β-catenin. Analogously, Arnsdorf et al. (Arnsdorf et al. 2009b) reported that, in MSC, the osteogenic effect of fluid flow triggers nuclear beta-catenin activity coupled to liberation of β-catenin from its association with N-cadherin. In addition, fluid flow in MSC was demonstrated (Kelly and Jacobs 2010) to lead to a significant decrease in catenin–adherin association, which could produce increases in cytoplasmic β-catenin levels by liberating it from adherens junctions or cell–cell contacts. The increase in unbound β-catenin coupled with activation of GSK3β and Akt that occurs after fluid shear stress has been proposed as a potential upstream regulator of β-catenin nuclear translocation. Nuclear translocation of β-catenin combined with mechanical stimulation leads to expression of Wnt targets such as Wisp-1 and COX-2 (Case et al. 2008). All these reports suggest that activation of Wnt/β-catenin signaling pathway modulated by these mechanoreceptors. β-catenin appears to be one of the most important molecules in the response to fluid flow even in osteocyte-like MLO-Y4 cell line (Kamel et al. 2010), which showed nuclear translocation of β-catenin at very low levels of shear stress.
Connexins
Connexins are membrane-spanning protein hexamer complexes forming pores within the cell membrane. They are found in gap junctions mediating cell–cell signaling: alignment of connexins with their counterparts on an adjacent cell creates functional connections between cells, which allow for intracellular communication isolated from the extracellular environment, thus allowing the passage of small molecules such as calcium, PGE2, inositol phosphates, ATP, and cAMP (Thompson et al. 2012).
Gap junctions are present in bone cells (Stains and Civitelli 2016) where they are able to feel forces. This is suggested by the increased expression of connexins in vitro and in vivo in response to mechanical stimulation of osteocytes, presumably generating enhanced connections with neighboring cells to enforce proper transmission of mechanical information within the skeletal network (Turner et al. 2002). Alford et al. (Alford et al. 2003) investigated the effects of oscillating fluid flow, for 1 h at a shear stress of 1 Pa and a frequency of 1 Hz, in osteocyte-like MLOY-4 cells. They concentrated on gap junction intercellular communication, particularly the gap junction protein connexin 43 (Cx43), the most abundant gap junction channel on bone cells found both on cell bodies and on their dendritic processes. They verified that mechanically stimulated cells established more gap junctions than the control cells, and underwent a 2-fold phosphorylation, and that the two events were significantly correlated. They also shed light on the fact that oscillating fluid flow regulates gap junctional intercellular communication in osteocytes via the ERK1/2 MAP kinase. When a bone cell is stimulated by fluid flow, the activation of Cx43 induces ATP efflux from the cytosol to the pericellular environment, thus eliciting a significant [Ca2+] response (Hung et al. 1997). Analogously, in more recent studies, gap junction-dependent propagation of Ca2 + oscillations was recorded in response to fluid shear stress (Jing et al. 2013). In addition, Yavropoulou and Yovos (Yavropoulou and Yovos 2016) reported that, independent of cell–cell gap junctions, connexins serve as portals through which prostaglandins are released from the osteocyte in response to fluid shear stress.
Mechanosensitive ion channels
Mechanosensitive ion channels, activated by mechanical stress alone, can drive them over their full dynamic range. Essentially, there are two classes of mechanosensitive ion channels. The non-selective-depolarizing stretch-activated ion channels (SACs) include the DEG/ENAC family of cation channels, the transient receptor potential (TRP) channels, and the Piezo1 and Piezo2 channels, which are grouped as mechanically activated cation channels (Chesler and Szczot 2018; Coste et al. 2010; Cox et al. 2016; Lewis and Grandl 2015; Poole et al. 2014; Soattin et al. 2016), and the selective-hyperpolarizing stretch-activated K channels (SAKs), which include TREK-1, TREK-2, and TRAAK channel types. In addition, other than direct activation of intracellular signaling cascades, the influx of a charged species such as Ca2+ can also alter membrane potential, thus activating voltage-sensitive channels that are not directly mechanosensitive. Thus, multimeric voltage sensitive calcium channels (VSCC) and the voltage-gated calcium channels (VGCCs) play essential roles in responding to mechanical cues (Haut Donahue et al. 2004; Srinivasan et al. 2015).
Different mechanosensitive ion channels are expressed in bone cells. For at least three decades, scientists have studied the direct effects of mechanical stimulation on ion channel activity in bone. They highlight the presence of mechanosensitive channels distinguishable on the basis of their conductance, ionic selectivity, and sensitivity to mechanical stimulus (Davidson et al. 1990). In general, fluid shear stress can induce deformation of bone cell membrane and alteration of membrane proteins, causing mechano-activated ion channels to open and allow the influx of cations, such as Ca2+, Na+, and K+, into the cell (McMahon et al. 2008). An example of mechanosensitive ion channels in bone cells are TRP channels (Abed et al. 2009), although the expression of TRPV6 is modest in murine osteoblasts (Little et al. 2011). Even the multimeric voltage sensitive calcium channels (VSCC) (Li et al. 2002; Shao et al. 2005), and L-type and T-type VSCCs have been implicated in mechanically induced bone formation. Osteoblasts predominantly express the L-type (long lasting) VSCC variant (Li et al. 2002), while in osteocytes, T-type (transient) VSCCs are expressed (Shao et al. 2005; Thompson et al. 2011). Annexin V VGCC, which binds extracellular collagen and cytoskeletal actin, seemed to be present in osteoblasts and are involved in their intracellular calcium response to flow (Haut Donahue et al. 2004). Concerning MSCs, only 15% express functional L-type Ca2+ channel (Heubach et al. 2004), while a major source of Ca2+ oscillations in progenitor cells seems to be the intracellular calcium stores and Ca2+ entry through the plasma membrane store-operated Ca2+ channels (SOC), with little contribution of voltage-operated Ca2+ currents (VOCC) (Kawano et al. 2002). It has been verified that intracellular Ca2+ concentration increases in cultured MSC after fluid flow stimulation (Riddle et al. 2006; Stiehler et al. 2009). Piezo type mechanosensitive ion channel component 1 (Piezo1) was been recently found to be crucial for bone formation (Tsimbouri et al. 2017), and it is present in osteoblasts, osteocytes (Sun et al. 2019) and MSCs (Sugimoto et al. 2017). Piezo1 is activated by various forms of mechanical stimulation, including shear stress. This is highlighted by the fact that when subjected to fluid shear stress treatment, mouse primary osteoblasts significantly increased their expression of Piezo1 (Sun et al. 2019). This channel mediates osteogenesis by Ca2+ signaling pathways.
Cytoskeleton
The ability of a eukaryotic cell to move, deform, and transport intracellular components depends on the cytoskeleton, an interconnected network of filamentous polymers and regulatory proteins. It can be represented as a hard-wired structure that connects the ECM to the cell nucleus. It is composed by three main types of cytoskeletal filaments: actin microfilaments, microtubules and a group of polymers known collectively as intermediate filaments (Fletcher and Mullins 2010). Microtubules are the stiffest of the three polymers and have the most complex assembly and disassembly dynamics, while actin filaments are much less rigid than microtubules. Nevertheless, the presence of high concentrations of crosslinkers that bind to actin filaments promote the assembly of highly organized, stiff structures, including isotropic networks, bundled networks and branched networks (Dos Remedios et al. 2003). Intermediate filaments are the least stiff of the three types of cytoskeletal polymer. Due to the fact that cytoskeleton provide the cell with mechanical integrity, or, better, tensegrity, as suggested more than a decade ago (Ingber 2003a; Ingber 2003b; Volokh et al. 2002), the cytoskeleton is a strong candidate for being a cell mechanoreceptor, since extracellular forces can directly translate into forces on cytoskeletal proteins.
Concerning bone cells, there are significant differences in cytoskeleton reorganization between osteoblasts and MSCs. MSCs display many thick actin bundles or stress fibers, extending throughout the cytoplasm and terminating at focal contacts on the cell membrane. In contrast, osteoblasts have fewer stress fibers and show, predominantly, a thin dense meshwork structure of actin. The main cytoskeleton that plays a critical role in the mechanotransduction during fluid shear stress–induced osteogenic differentiation of MSC is the actin cytoskeleton (Arnsdorf et al. 2009a). Osteoblasts stimulated by fluid shear stress showed a reorganization of actin filaments (Pavalko et al. 1998) and, accordingly, when actin cytoskeleton is disrupted, the response of osteoblasts to fluid flow appears modified (Malone et al. 2007). Analogous response seems to be obtained when microtubule networks are broken (Myers et al. 2007). Disruption of microfilament assembly/disassembly prevents fluid flow-induced osteogenic differentiation even in MSC (Chen and Jacobs 2013). Electron microscopy and cytoskeleton staining on osteocyte-like MLO-Y4 cells exposed to 1 Pa fluid shear stress for 2 h revealed well-organized cytoskeleton and increased filopodia processes (Yan et al. 2018). This, and other evidence, testifies to the involvement of the cytoskeleton in responding to fluid flow. Cytoskeleton stimulation via fluid shear stress seems to be related to activation of RhoA/ROCK pathway, which inactivates myosin phosphatase thereby on inducing the stabilization of actin filaments and stress fiber formation (Arnsdorf et al. 2009a).
Glycocalyx
The glycocalyx is a cell surface proteoglycan layer that covalently bond the ECM to cellular membrane proteins and lipids (Reilly et al. 2003). Glycocalyx expression affects the response to shear stress in vascular endothelial cells (Yao et al. 2007) and the reorganization of actin cytoskeleton.
Concerning bone cells, Lanctot et al. (Lanctot et al. 2007) reported that glycocalyx structures are present in MSCs and play a significant role in their differentiation. Moreover, bone cells possess a hyaluronic-acid-rich glycocalyx which contributes to bone cell mechanotransduction via oscillating fluid flow. Reilly et al. (Reilly et al. 2003) found glycocalyx even in an osteoblasts (MC3T3-E1) cell line, and in an osteocyte (MLO-Y4) cell line, verifying that in osteocytes the PGE2 pathway is widely dependent on mechanical signals transmitted through the glycocalyx.
Cell membrane structures: lipid rafts and caveolae
Lipid raft domains are small, highly dynamic, and transient plasma membrane entities enriched in saturated phosho-, sphingo-, and glycol-lipids, cholesterol, lipidated proteins, and glycosylphosphatidylinositol (GPI)-anchored proteins. Although their characterization is not completely elucidated, the principle of lateral membrane compartmentalization by lipids is intuitive. There are clear differences in the interaction affinities between various lipids needed to produce a heterogeneous lipid distribution (Sezgin et al. 2017), where plasma membrane has the potential to change its fluidity and to segregate itself into smaller subdomains, called rafts, and to have barriers, called fences (Jacobs et al. 2010). The most apparent function of raft domains is the recruitment of regulatory molecules in one location to create the appropriate concentration of signaling effectors, whereby crosstalk and directionality of signals can occur efficiently. The enrichment of lipid rafts in caveolin proteins produces the characteristic 50–100 nm invaginations of the plasma membrane, originating lipid rafts subdomains called caveolae.
Lipid rafts (Gillette and Nielsen-Preiss 2004) and caveolae (Solomon et al. 2000) are structures present in osteogenic cells. Caveolins-1 and -2 have been described in human fetal osteoblasts and in murine MC3T3-E1 cells along with multiple caveolar flasks in their membrane (Solomon et al. 2000a). Similar findings are corroborated in osteoblastic outgrowths from normal adult bone (Lofthouse et al. 2001). Lipid rafts were found involved in shear stress reception, since membrane fluidity increases with fluid shear stress exposure (Haidekker et al. 2000). They appeared to be crucial in osteoblasts for fluid shear stress–induced activation of ERK1/2 and c-fos expression (Ferraro et al. 2004).
Primary cilia
Primary cilia are nonmotile, microtubule-based organelles that project from the membrane surface of the cell. They are active mechanoreceptors in kidney through their PKD1 and PKD2 components.
Primary cilia are even present in bone cells (Federman and Nichols Jr 1974) where they are important for feeling various biophysical signals. In particular, primary cilia appear to be sensitive to deflection, and tiny motions could be sufficient to produce a response (Malone et al. 2007a). PKD1 and PKD2 were found even in bone, on primary bone cells, osteoblast, and osteocyte cell lines (Xiao et al. 2006). Nevertheless, their mechanism of mechanosensation has not yet been clarified in bone (Klein-Nulend et al. 2013). In particular, Jacobs’ group (Malone et al. 2007b) showed that, unlike kidney cells, primary cilia in bone cells do not mediate calcium flux in response to fluid flow. Primary cilia are required for cellular responses to dynamic fluid flow involved in PGE2 signaling—independent of intracellular Ca2+ influx—in osteoblast-like and osteocyte-like cells, respectively, modeled by MC3T3-E1 and MLO-Y4 cell lines (Malone et al. 2007a). The presence of primary cilia has been demonstrated even in MSCs, where they mediate mechanotransduction (Hoey et al. 2012). Exposure of MSCs to fluid flow increased cyclooxygenase2 and BMP2 gene expression, which indicate an early osteogenic response. However, when treated with small interfering RNA to inhibit intraflagellar transport 88, a protein important for primary cilia formation, MSCs did not respond by increasing osteogenic gene expression (Chen and Jacobs 2013). Primary cilia might also play a role even in paracrine signaling in bone cells mediated by fluid flow. The C3H10T1/2 mouse MSC cell line evolved towards osteogenic lineage, testified by osteopontin upregulation, when they were exposed to conditioned media from fluid shear stressed MLO-Y4 osteocyte-like cells. This did not happen when exposed to conditioned media from fluid shear stressed MLO-Y4 cells with inhibited primary cilia formation (Hoey et al. 2011).
Other mechanoreceptors
Other cell structures are present in bone cells and can process forces, translating them into intracellular biochemical signals. Nevertheless, there is no evidence associating them to a direct action of fluid shear stress on bone cells. The most relevant of them are ephrins, G Protein–Coupled Receptors, and the LINK complex.
Erythropoietin-producing hepatocellular (Eph) receptors are a subfamily of receptor tyrosine kinases, which bind ephrins, in particular, EphA receptors that interact with ephrinA and EphB receptors with ephrinB. Bone cells seem to express ephrin ligands and Eph receptors. Several reports have shown that Eph/ephrin molecules are involved in MSC-mediated cell attachment, migration, and differentiation (Arthur et al. 2011; Compagni et al. 2003; Matsuo and Otaki 2012). They seem to be necessary in osteoblasts for their differentiation and communication with osteoclasts for bone remodeling (Martin et al. 2010). Nevertheless, their role in transducing fluid flow remains to be determined.
G Protein–Coupled Receptors (GPCR) are the largest family of membrane proteins, characterized by the presence of seven membrane-spanning α-helical segments separated by alternating intracellular and extracellular loop regions. The binding of an external signal molecule, a ligand, to a GPCR stimulates signaling pathways inside the cell that are involved in a large variety of processes. GPCRs have been postulated to mediate mechanotransduction. Interestingly, members of a subgroup of GPCR, known as adhesion GPCRs, also contain an extracellular domain that is thought to interact with components of the extracellular matrix, a scaffold-like structure that surrounds cells to provide structural support (Langenhan et al. 2016). Recent studies suggest that some adhesion GPCRs may detect mechanosensory information through the extracellular domains when tethered to the extracellular matrix (Petersen et al. 2015; Scholz et al. 2015). GPCRs are known to intervene in feeling fluid flow stimulation of endothelial cells (Chachisvilis et al. 2006), although Dela Paz et al. (Dela Paz et al. 2017) recently obtained contrasting results. Critical roles of GPCRs have been established in bone development, remodeling, and disease (Luo et al. 2019). Nevertheless, their role in bone transduction of fluid flow stimuli has not been completely assessed.
The Linker of Nucleoskeleton and Cytoskeleton (LINK) complex, composed of nesprins and Sad1p/UNC(uncoordinated)-84 (SUN) proteins, may connect cytoplasmic cytoskeleton, nuclear envelope, and nucleoskeleton (Jahed et al. 2014). The cellular model of “hard-wired” tensegrity proposed by Ingber (Ingber 1993; Ingber 2003a; Ingber 2003b) considers that individual cytoskeletal filaments in the cell bear significant mechanical cues in living cells, and their structural integrity is maintained for longer than the turnover time of individual protein monomers. In this scenario, mechanical forces applied to the cell surface act by promoting mechanochemical transduction not only into cytoplasm but also in the nucleus (Wang et al. 2009), although the pathway has not yet been well clarified. This vision is in line with previous studies, which demonstrated the mechanical coupling of the nucleus and cytoskeleton by exerting forces on the cell surface and observing nuclear deformations (Maniotis et al. 1997). In this perspective, the LINK complex enables the physical coupling of the cytoskeleton to the nucleoskeleton and act as a nuclear mechanoreceptor. Mechanical forces applied to the cell surface involve membrane mechanoreceptors that, through coupling with cytoskeletal filaments, are linked by LINC connectivity to the nuclear envelope, altering shape and location of nuclear lamin. Thus, there is a structural relationship between lamin A/C and nuclear heterochromatin that may affect the accessibility and action of transcriptional regulators (Jahed et al. 2014; Martins et al. 2012). The LINK complex is emerging as an integrated dynamic signaling platform to regulate MSC mechanobiology (Lombardi et al. 2011). Indeed, nucleoskeletal structure and its connection with the cytoskeleton have been shown to be critical in controlling and may arise in response to dynamic mechanical cues, that are important for MSC fate selection. This appears to be a LINC-mediated process (Driscoll et al. 2015). Nevertheless, there is currently no evidence that the LINK complex acts as a transducer of fluid shear stress in bone.
Mechanosignaling pathways activated by fluid flow
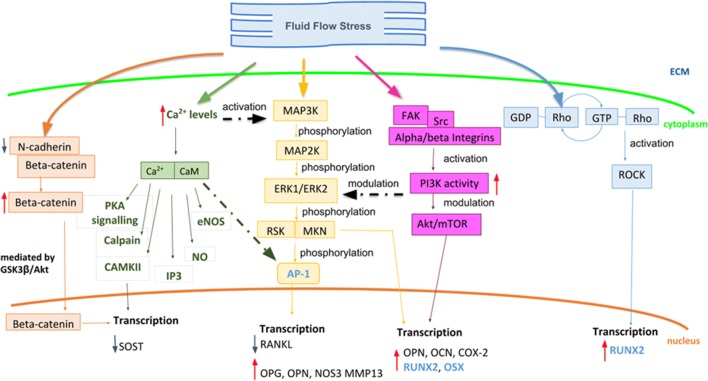
Stimuli that can be felt by cells (such as biochemical, chemical, thermic, electrical and mechanical) can be codified and transformed in biochemical signaling pathways within the cell, thus enabling the cellular response. As summarized by Wittkowske et al. (Wittkowske et al. 2016), the response of in vitro cultured osteoblasts to fluid shear stress, both in high-stress condition, considered between 0.5 and 2 Pa, and in low stress condition, considered below 0.5 Pa, triggers a rapid increase of cellular components such as ITP, intracellular calcium, Ca2+-dependent nitric oxide, prostaglandin E2, and ATP. These regulate the expression of osteopontin, cyclooxygenase-2, c-FOS, and, as late response—days to weeks—collagen1 matrix for bone mineralization. Similarly, it is known from literature that in vitro cultured osteocyte response to FSS activating Ca2+, ATP, NO, and PGE2, and regulating the release of RANKL and OPG and the expression of E11, a dendritic protein that is one of the earliest osteocyte markers of the dendritic elongating processes, which is absent on primary osteoblasts but increases with time following differentiation into an osteocyte-like cell type. Nevertheless, the chain leading from the stimulus experienced by cell to the biochemical response requires delicate and precise steps involving many different proteins and compounds. The first players in the mechanotransduction process are the mechanoreceptors in charge of activating first messengers. This phase has been named mechanocoupling (Duncan and Turner 1995). First messengers enable the conversion of the mechanical force into a biochemical outcome, triggering secondary downstream biomolecular cascades. This step has been called biochemical coupling (Turner and Pavalko 1998). Through downstream cascades, this reaches the nucleus: force signal acts on the nucleus to promote changes in the shape, folding, or kinetics of specific hub molecules, such as transcription factors, or might modify chromatin organization, thereby modulating gene transcription. Here an extensive description of the major signaling pathways activated by fluid shear stress in bone cells is reported (Fig. 3).
Fig. 3.
Schematic representation of the principal signaling events occurring during fluid shear stress in bone cells. The signalling cascades through which the mechanical stress impacts at molecular level is reported. The MAPK/FAK signaling (orange and pink respectively), RhoA-ROCK pathway (blue), calcium and beta-catenin signaling (green and red respectively) activated after fluid shear stress have the main effects at transcriptional level into the nucleus. As reported in the figure, these pathways up- (red arrows) or down- (blue arrows) regulate the expression of genes having a crucial role in bone physiology, and genes encoding for transcription factors (reported in light blue)
MAPK signaling
The mitogen-activated protein kinases (MAPKs) are a family of conserved serine threonine kinases that respond to many different extracellular stimuli to activate intracellular processes including gene expression, metabolism, proliferation, differentiation, and apoptosis. The classic MAPK cascade includes the following pathways: ERK1/2, the p38 MAPK, the JNK, and the ERK5. These are characterized by three levels of sequential activation of kinases (Cargnello and Roux 2012). At the top of the cascade is the MAPK kinase kinase kinase (MAPKKK), which is phosphorylated on serine and threonine residues in response to external stimuli. This phosphorylation often occurs in the context of an interaction between the MAPKKK protein and a member of the RAS/RHO family of small GTP-binding proteins. Activated MAPKKK proteins phosphorylate the dual-specificity MAPK kinase protein (MAPKK) that ultimately phosphorylate the MAPK proteins in a conserved Thr-X-Tyr motif. Both cytosolic and nuclear targets of MAPK proteins have been identified, and upon stimulation, a proportion of the phosphorylated MAPKs relocate from the cytoplasm to the nucleus.
The MAP2Ks 1 and 2 phosphorylate the extracellular signal–regulated kinases (ERKs) 1 and 2, also known as MAPK3 and MAPK1, in response to a wide range of extracellular stimuli to promote differentiation, proliferation, cell motility, cell survival, metabolism, and transcription (reviewed in McKay and Morrison 2007; Roskoski Jr 2012; Raman et al. 2007). In the classical pathway, MAPK1/3 activation is triggered by the GEF-mediated activation of RAS at the plasma membrane, leading to the activation of the RAF MAP3Ks (McKay and Morrison 2007). However, many physiological and pathological stimuli have been found to activate MAPK1/3 independently of RAF and RAS, acting instead through MAP3Ks such as MOS, TPL2, and AMPK (Dawson et al. 2008; Wang et al. 2009; Kuriakose et al. 2014; Awane et al. 1999). Activated MAPK1/3 phosphorylate numerous targets in both the nucleus and cytoplasm (Roskoski Jr 2012). In fact, phosphorylated ERK1/2 can up-regulate and activate transcription factors, such as AP-1 (Haasper et al. 2008).
In bone cells, various types of mechanical stimulation, among which fluid shear stress, activate ERK1/2 (Liu et al. 2008; Thompson et al. 2011). In particular, ERK1/2 activation causes RANKL down-regulation, OPG, OPN, NOS3, and MMP13 upregulation, which lead to decreased osteoclastic activity and an increase in bone formation. ERK1/2 activation is associated with ATP-dependent mechanical regulation of voltage-sensitive Ca2+ channels (VSCCs). In fact, fluid shear stress activation of ERK1/2 in osteoblastic cells requires Ca2+ influx via the VSCCs and it is ATP dependent (Liu et al. 2008). Additionally, a recent study demonstrated that activation of ERK1/2 is necessary for TGFβ-induced osteogenic differentiation of mesenchymal stem cells (Arita et al. 2011).
FAK signaling
Focal adhesion kinase (FAK), a non-receptor cytoplasmic protein tyrosine kinase (non-RTK), is concentrated near focal adhesions and plays an important role in signaling events involving stress signals (Schlaepfer et al. 1999). The non-RTKs lack a transmembrane domain but are recruited into signal transduction cascades through interaction with other plasma-bound receptors, which may or may not themselves have intrinsic catalytic activity. In this way, non-RTKs essentially function as an enzymatic subunit of the signaling complex and contribute to many of the same downstream signaling pathways. FAK forms a functional network of integrin-stimulated signaling pathways by associating with various signaling proteins including Src family PTKs (Orr and Murphy-Ullrich 2004), phosphatidylinositol 3-kinases (PI3K), and paxillin (Cukierman et al. 2001; Hehlgans et al. 2007). This network results in activation of downstream targets including the MAPK pathways. Upon activation, FAK is autophosphorylated, generating interactions with Src-family proteins and other molecules containing Src homology 2 (SH2) domains. Phosphorylation of FAK participates in MAPK activation by interacting with c-Src, Grb2, and Ras (Schlaepfer et al. 1999).
In osteoblasts the fluid flow shear stress induces a sustained association of Src and FAK with alpha and beta integrin, which results in an enhanced PI3K activity and a modulation of downstream ERK and Akt/mTOR/p70S6K pathways increasing osteoblast proliferation. In particular, FAK activation by fluid shear stress increases the OPN, OCN, COX-2, Runx2, and Osx expression levels (Young et al. 2009). It is noteworthy that Runx2 and Osx are two transcription factors upregulated by FAK. Runx2 is essential for osteoblast differentiation and skeletal morphogenesis, while Osx plays a major role, along with Runx2, in driving the differentiation of mesenchymal precursor cells into osteoblasts and osteocytes. Taken together, these demonstrate the importance of FAK in regulating osteoblast differentiation and osteogenesis (Wang et al. 2011).
Calcium signaling
Calcium ions are important for cellular signaling. Once they enter the cytosol, they have allosteric regulatory effects on many enzymes and proteins. Calcium can act in signal transduction resulting from activation of ion channels or as a second messenger caused by indirect signal transduction pathways such as GPRs.
It has been clearly demonstrated that intracellular Ca2+ levels increase in response to mechanical stimulation of bone cells. Moreover, changes in intracellular Ca2+ levels alter proliferation, differentiation, and mobility through the activation of signaling events, such as MAPK signaling, protein kinase A (PKA), c-Fos, IP3, ATP, nitric oxide, and eNOS (Yavropoulou et al. 2016) and CaMKII (Coste et al. 2010). A novel mechanism regulating calcium intracellular levels, microtubules, and ROS signals was recently demonstrated by Lyons et al. in an osteocyte cell model of bone remodeling under mechanical stress (Lyons et al. 2017). In particular, it has been demonstrated that fluid shear stress stimulus acted through the microtubules network to activate NOX2 to generate reactive oxygen species (ROS). NADPH oxidases (NOX) are membrane-associated enzymatic complexes that use NADPH as an electron donor to reduce oxygen and produce superoxide that functions as a secondary messenger (Brown and Griendling 2009). NOX2 complex consists of CYBB (NOX2), CYBA, NCF1, NCF2, and NCF4. RAC2:GTP can activate the NOX2 complex by binding to CYBB and NCF2. NOX2-dependent ROS signals targeted TRPV4 channels to elicit Ca2+ influx, activate Ca/calmodulin-dependent kinase II (CaMKII), and decrease SOST abundance in the osteocyte enhancing bone formation.
G protein mediated signaling
G proteins are a family of proteins that bind the guanine nucleotides guanosine triphosphate (GTP) and guanosine diphosphate (GDP) and possess intrinsic GTPase activity. G proteins play a central role in signal transduction as well as in a myriad of cellular processes, including membrane vesicle transport, cytoskeletal assembly, cell growth, and protein synthesis. The small GTPases are a family of enzymes that function as molecular switches and are involved in regulating many cellular processes. All small GTPases transduce information through signaling pathways by alternating between an active GTP-bound and an inactive GDP-bound state. To speed up this process, the small GTPases are themselves regulated by guanine nucleotide exchange factors (GEFs), which assist in GDP dissociation, and also by GTPase activating proteins (GAPs) that stimulate GTP hydrolysis. Along with GEFs and GAPs, guanine dissociation inhibitors (GDIs) can bind to small GTPases and regulate their location to the membrane or cytoplasm (Yavropoulou et al. 2016; Thompson et al. 2012).
Osteocytes exposed to fluid shear stress demonstrated a transient decrease in cAMP production, which is dependent on adenylate cyclase 6 (AC6), an enzyme activated by GTPases. AC6 activation in osteocytic cells regulated COX-2 gene expression (Kwon et al. 2010) and PGE2 signaling. COX is a crucial enzyme in the production of prostaglandins (PGE) and its isoform 2 (COX-2) is more expressed in trauma, inflammation, and/or proliferative disorders. PGE2 is involved in widespread regulation of various processes of inflammation and bone metabolism and it acts through four GPR subtypes present in bone cells. The expression of COX-2 after fluid shear stress can be enhanced also through the phospholipase C (PLC) activation which is linked to the activation of IP3 signaling via G protein-dependent mechanism. Mechanical signals such as fluid shear stress can activate small GTPases homologous to Ras (RhoA GTPases). RhoA has a crucial role in the organization of actin cytoskeleton through the regulation of stress fiber formation in response to mechanical stimulation. The guanine nucleotide exchange factors (GEFs) mediate the activation of the Rho/Rho-associated kinases (ROCK). The upstream signals regulating Rho GTPase activity, such as integrin and TGF-β, may induce the relocalization of GEFs to membrane structures containing the GTPase targets. GEF binds to inactivated Rho-GDP to form a Rho-GEF dimer that destabilizes the GDP binding. GTP replaces GDP to activate Rho and GEFs are released from Rho-GTP. Active Rho interacts with ROCK and, in turn, to phosphorylate myosin phosphatase. Activated myosin phosphatase activates myosin light chain to increase the contraction of actin cytoskeleton. Moreover, the activated Rho signaling after shear stress regulates the expression of Runx2 (Liu et al. 2010). As mentioned before, Runx2 is a transcription factor with a crucial role in bone development.
Wnt/β-catenin signaling
WNT signaling pathways control a wide range of developmental and adult processes including cell proliferation, cell fate decisions, cell polarity, and stem cell maintenance (Saito-Diaz et al. 2013). The pathway is named after the WNT ligands, a large family of secreted cysteine-rich glycoproteins. At least 19 WNT members have been identified in humans and mice with distinct expression patterns during development (Willert and Nusse 2012). These ligands can activate different downstream signaling cascades depending on which receptors they engage. In the so-called “canonical” WNT signaling pathway, WNT ligands bind one of the 10 human Frizzled (FZD) receptors in conjunction with the LRP5/6 co-receptors to activate a transcriptional cascade that controls processes such as cell fate, proliferation, and self-renewal of stem cells. When WNT ligand recruits FZD-LRP receptor, the stabilization and translocation of cytosolic beta-catenin to the nucleus occurs. Nuclear beta-catenin is a co-activator for lymphoid enhancer-binding factor (LEF)- and T cell factor (TCF)-dependent transcription. In the absence of WNT ligand, cytosolic beta-catenin is phosphorylated by a degradation complex consisting of glycogen synthase kinase 3 (GSK3), casein kinase 1 (CK1), Axin and Adenomatous Polyposis Coli (APC), and subsequently ubiquitinated and degraded by the 26S proteasome (Saito-Diaz et al. 2013).
It has been demonstrated that Wnt-beta-catenin signaling pathway has an important role in bone biology (Case and Rubin 2010). Mutations in the co-receptor LRP5 were found to be “activating,” i.e., inducing bone density increase, and “inactivating,” i.e., causing bone mass reduction (Boyden et al. 2002). Β-catenin, a downstream effector of the pathway, is the main regulator of the bone remodeling both in osteoblasts and osteocytes. β-catenin can be a direct target of mechanotransduction of the classical activation pathway through LRP5 receptor (Case et al. 2011), involving the inhibition of the activity of GSK3β through the mTORPs2 activation by mechanical shear stress. In osteoblasts, β-catenin associates with cadherins on the inner leaflet of the plasma membrane. Fluid shear stress increases the cytoplasmic pool of β-catenin by decreasing the amount of β-catenin bound to N-cadherin (Norvell et al. 2004). β-catenin nuclear translocation occurs after fluid shear stress and it is mediated by the activation of GSK3β and Akt. Thus, cadherins may serve as launching platforms for β-catenin in response to mechanical stimulation (Bidwell and Pavalko 2010). SOST, a gene constitutively expressed in osteocytes, inactivates the Wnt receptor, thus it is a negative regulator of bone formation. After mechanical stress, SOST is down-regulated and osteoblast activity increases (Robling et al. 2008).
The down-regulation of SOST seems to be also mediated by estrogen receptor beta (Galea et al. 2013). A recent study in osteocyte-like cells ((Yan et al. 2018)) reveals a direct regulation on osteocytic morphology and apoptotic characteristics after fluid shear stress and demonstrates that osteocyte-secreted bone metabolism-modulating molecule expression was regulated by fluid flow in a time-dependent manner. Yan and colleagues demonstrated that fluid shear stress stimulation promoted Wnt and β-catenin expression in 0, 3, and 6 h after stimulation. The stimulated cells after 3 h exhibited more significant effects on the promotion of OCN and Cx43 and inhibition of DKK1 and SOST expression than the 0 and 6 h groups. The 3 h group with fluid flow stimulation also showed the most prominent effects on suppressing RANKL and RANKL/OPG expression. Wnt/β-catenin signaling pathway was activated in the process of the 2-h fluid shear stress stimulation, and β-catenin can enter into the nucleus to regulate gene transcription. Moreover, SOST and DKK1, two antagonists of the Wnt signaling pathway (Gifre et al. 2013; Ueland et al. 2015), were significantly inhibited in the mRNA expression by fluid flow 3 h post stimulation, revealing that flow stimulation can attenuate the inhibitory effects of SOST and DKK1 on the Wnt/β-catenin signaling pathway in osteocytes. This revealed prominent time-dependent expression of SOST and DKK1 of osteocytes in response to external fluid stimulation.
Conclusions
Although mechanotransduction pathways have been under analysis for almost two decades, a comprehensive idea of the molecular interactions following a mechanical stimulus is still missing. The reason is the high complexity of the pathways involved. These are different, multiple, and potentially still not totally identified, mechanical sensors able to sense the mechanical stimulus. These are physiologically activated in parallel when the mechanical stimulus reaches the cell. First messengers are responsible for the conversion of the mechanical stimulus into a biochemical outcome in the form of secondary biomolecular cascades. These involve transcription factors that carry the messages to the nucleus, where they are activated and chromatin is remodeled to modulate gene expression in response to stimulus. This comprehensive overview of the mechanotransduction pathways of fluid flow stimulated bone cells provides an up-to-date knowledge in the field, and an organized view on the underlying mechanisms. Eight modes are present in bone cells, but the list is destined to grow due as other mechanoreceptors are discovered. When exposed to fluid flow, bone cells mechanoreceptors trigger downstream cascades that have been identified and grouped into five major groups: MAPK, FAK, calcium, G protein-mediated, and Wnt/β-catenin signaling pathways. These processes convert the information carried by the mechanical stimulus into molecular signals, passing through the cell into to the nucleus, thus regulating gene expression and transcription factors activity. Interestingly, it was possible to identify contact points among separate signaling cascades (Fig. 1), in terms of either convergent points within processes or common gene expression regulation. The FAK pathway converges on the MAPK pathway since both proceed into the ERK1/2 signaling pathway. In the calcium signaling pathway, AP-1 is activated, and in a way that resembles the MAPK cascade. G protein mediated signaling pathway activates RUNX2 such as MAPK pathways; Wnt/β-catenin signaling pathway inactivates SOST analogously to the end of one possible way of calcium signaling pathway. These considerations lead to observe a certain level of redundancy among the signaling paths, highlighting the fact that, although coming from diverse biochemical ways, the messages carried from the extracellular environment to the nucleus often involve, inactivate, or activate, and regulate the same transcription factors, RANKL, RUNX2, OSX, SOST, and AP-1, thus producing an overlapping and enforced response of the bone cells to the fluid flow stimulus.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abed E, Labelle D, Martineau C, Loghin A, Moreau R. Expression of transient receptor potential (TRP) channels in human and murine osteoblast-like cells. Mol Membr Biol. 2009;26:146–158. doi: 10.1080/09687680802612721. [DOI] [PubMed] [Google Scholar]
- Alford AI, Jacobs CR, Donahue HJ. Oscillating fluid flow regulates gap junction communication in osteocytic MLO-Y4 cells by an ERK1/2 MAP kinase-dependent mechanism small star, filled. Bone. 2003;33:64–70. doi: 10.1016/s8756-3282(03)00167-4. [DOI] [PubMed] [Google Scholar]
- Arita NA, Pelaez D, Cheung HS. Activation of the extracellular signal-regulated kinases 1 and 2 (ERK1/2) is needed for the TGFbeta-induced chondrogenic and osteogenic differentiation of mesenchymal stem cells. Biochem Bioph Res Co. 2011;405:564–569. doi: 10.1016/j.bbrc.2011.01.068. [DOI] [PubMed] [Google Scholar]
- Arnsdorf EJ, Tummala P, Jacobs CR. Non-canonical Wnt signaling and N-cadherin related beta-catenin signaling play a role in mechanically induced osteogenic cell fate. PLoS One. 2009;4(4):e5388. doi: 10.1371/journal.pone.0005388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsdorf EJ, Tummala P, Kwon RY, Jacobs CR. Mechanically induced osteogenic differentiation - the role of RhoA, ROCKII and cytoskeletal dynamics. J Cell Sci. 2009;122:546–553. doi: 10.1242/jcs.036293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arthur A, Zannettino A, Panagopoulos R, Kobla S, Sims NA, Stylianou C, Matsuo K, Gronthos S. EphB/ephrin-B interactions mediate human MSC attachment, migration and osteochondral differentiation. Bone. 2011;48:533–542. doi: 10.1016/j.bone.2010.10.180. [DOI] [PubMed] [Google Scholar]
- Awane M, Andres PG, Li DJ, Reinecker HC. NF-kappa B-inducing kinase is a common mediator of IL-17-, TNF-alpha-, and IL-1 betainduced chemokine promoter activation in intestinal epithelial cells. J Immunol. 1999;162(9):5337–5344. [PubMed] [Google Scholar]
- Bennett BC, Purdy MD, Baker KA, Acharya C, McIntire WE, Stevens RC, Zhang Q, Harris AL, Abagyan R, Yeager M. An electrostatic mechanism for Ca(2+)-mediated regulation of gap junction channels. Nat Commun. 2016;7:8770–8770. doi: 10.1038/ncomms9770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman HM, Westbrook J, Feng Z, Gilliland G, Bhat TN, Weissig H, Shindyalov IN, Bourne PE. The Protein Data Bank. NAR. 2000;28:235–242. doi: 10.1093/nar/28.1.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bidwell JP, Pavalko FM. The load-bearing mechanosome revisited. Clin Rev Bone Miner Metab. 2010;8:213–223. doi: 10.1007/s12018-010-9075-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonewald LF, Johnson ML. Osteocytes, mechanosensing and Wnt signaling. Bone. 2008;42(4):606–615. doi: 10.1016/j.bone.2007.12.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyden LM, Mao J, Belsky J, Mitzner L, Farhi A, Mitnick MA, Wu D, Insogna K, Lifton RP. High bone density due to a mutation in LDL-receptor-related protein 5. N Engl J Med. 2002;346:1513–1521. doi: 10.1056/NEJMoa013444. [DOI] [PubMed] [Google Scholar]
- Brown DI, Griendling KK. Nox proteins in signal transduction. Free Radic Biol Med. 2009;47(9):1239–1253. doi: 10.1016/j.freeradbiomed.2009.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger EH, Klein-Nulend J. Mechanotransduction in bone--role of the lacuno-canalicular network. FASEB J. 1999;13(Suppl):S101–S112. [PubMed] [Google Scholar]
- Cardoso L, Fritton SP, Gailani G, Benalla M, Cowin SC. Advances in assessment of bone porosity, permeability and interstitial fluid flow. J Biomech. 2013;46(2):253–265. doi: 10.1016/j.jbiomech.2012.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cargnello M, Roux PP. Activation and function of the MAPKs and their substrates, the MAPK-activated protein kinases. Microbiol Mol Biol Rev. 2012;76(2):496. doi: 10.1128/MMBR.00031-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Case N, Ma M, Sen B, Xie Z, Gross TS, Rubin J. Beta-catenin levels influence rapid mechanical responses in osteoblasts. J Biol Chem. 2008;283:29196–29205. doi: 10.1074/jbc.M801907200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Case N, Rubin J. Beta-catenin--a supporting role in the skeleton. J Cell Biochem. 2010;110:545–553. doi: 10.1002/jcb.22574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Case N, Sen B, Thomas JA, Styner M, Xie Z, Jacobs CR, Rubin J. Steady and oscillatory fluid flows produce a similar osteogenic phenotype. Calcif Tissue Int. 2011;88:189–197. doi: 10.1007/s00223-010-9448-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chachisvilis M, Zhang YL, Frangos JA. G protein-coupled receptors sense fluid shear stress in endothelial cells. Proc Natl Acad Sci U S A. 2006;103(42):15463–15468. doi: 10.1073/pnas.0607224103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JC, Jacobs CR. Mechanically induced osteogenic lineage commitment of stem cells. Stem Cell Res Ther. 2013;4(5):107. doi: 10.1186/scrt318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesler AT, Szczot M. Portraits of a pressure sensor. eLife. 2018;7:e34396. doi: 10.7554/eLife.34396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compagni A, Logan M, Klein R, Adams RH. Control of skeletal patterning by ephrinB1-EphB interactions. Dev Cell. 2003;5:217–230. doi: 10.1016/s1534-5807(03)00198-9. [DOI] [PubMed] [Google Scholar]
- Coste B, Mathur J, Schmidt M, Earley TJ, Ranade S, Petrus MJ, Dubin AE, Patapoutian A. Piezo1 and Piezo2 are essential components of distinct mechanically activated cation channels. Science. 2010;330(6000):55–60. doi: 10.1126/science.1193270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowin SC, Cardoso L. Blood and interstitial flow in the hierarchical pore space architecture of bone tissue. J Biomech. 2015;48(5):842–854. doi: 10.1016/j.jbiomech.2014.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox CD, Bae C, Ziegler L, Hartley S, Nikolova-Krstevski V, Rohde PR, Ng CA, Sachs F, Gottlieb PA, Martinac B. Removal of the mechanoprotective influence of the cytoskeleton reveals PIEZO1 is gated by bilayer tension. Nat Commun. 2016;7:10366. doi: 10.1038/ncomms10366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cukierman E, Pankov R, Stevens DR, Yamada KM. Taking cell-matrix adhesions to the third dimension. Science. 2001;294(5547):1708–1712. doi: 10.1126/science.1064829. [DOI] [PubMed] [Google Scholar]
- Davidson RM, Tatakis DW, Auerbach AL. Multiple forms of mechanosensitive ion channels in osteoblast-like cells. Pflugers Arch. 1990;416(6):646–651. doi: 10.1007/BF00370609. [DOI] [PubMed] [Google Scholar]
- Dawson CW, Laverick L, Morris MA, Tramoutanis G, Young LS. Epstein-Barr virus-encoded LMP1 regulates epithelial cell motility and invasion via the ERK-MAPK pathway. J Virol. 2008;82(7):3654–3664. doi: 10.1128/JVI.01888-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dela Paz NG, Melchior B, Frangos JA. Shear stress induces Gαq/11 activation independently of G protein-coupled receptor activation in endothelial cells. Am J Phys Cell Phys. 2017;312(4):C428–C437. doi: 10.1152/ajpcell.00148.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaine-Smith RM, Reilly GC. Mesenchymal stem cell responses to mechanical stimuli. MLTJ. 2012;2(3):169–180. [PMC free article] [PubMed] [Google Scholar]
- Driscoll TP, Cosgrove BD, Heo SJ, Shurden ZE, Mauck RL. Cytoskeletal to nuclear strain transfer regulates YAP signaling in mesenchymal stem cells. Biophys J. 2015;108(12):2783–2793. doi: 10.1016/j.bpj.2015.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dos Remedios CG, Chhabra D, Kekic M, Dedova IV, Tsubakihara M, Berry DA, Nosworthy NJ. Actin and actin binding proteins: regulation of cytoskeletal microfilaments. Physiol Rev. 2003;83:433–473. doi: 10.1152/physrev.00026.2002. [DOI] [PubMed] [Google Scholar]
- Duncan RL, Turner CH. Mechanotransduction and the functional response of bone to mechanical strain. Calcif Tissue Int. 1995;57(5):344–358. doi: 10.1007/BF00302070. [DOI] [PubMed] [Google Scholar]
- Federman M, Nichols G., Jr Bone cell cilia: vestigial or functional organelles? Calcif Tissue Res. 1974;17:81–85. doi: 10.1007/BF02547216. [DOI] [PubMed] [Google Scholar]
- Ferraro JT, Daneshmand M, Bizios R, Rizzo V. Depletion of plasma membrane cholesterol dampens hydrostatic pressure and shear stress-induced mechanotransduction pathways in osteoblast cultures. Am J Phys Cell Phys. 2004;286(4):C831–C839. doi: 10.1152/ajpcell.00224.2003. [DOI] [PubMed] [Google Scholar]
- Fletcher DA, Mullins RD. Cell mechanics and the cytoskeleton. Nature. 2010;463(7280):485–492. doi: 10.1038/nature08908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florencio-Silva Rinaldo, Sasso Gisela Rodrigues da Silva, Sasso-Cerri Estela, Simões Manuel Jesus, Cerri Paulo Sérgio. Biology of Bone Tissue: Structure, Function, and Factors That Influence Bone Cells. BioMed Research International. 2015;2015:1–17. doi: 10.1155/2015/421746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritton SP, Weinbaum S. Fluid and solute transport in bone: flow-induced mechanotransduction. Annu Rev Fluid Mech. 2009;41:347–374. doi: 10.1146/annurev.fluid.010908.165136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galea GL, Meakin LB, Sugiyama T, Zebda N, Sunters A, Taipaleenmaki H, Stein GS, van Wijnen AJ, Lanyon LE, Price JS. Estrogen receptor alpha mediates proliferation of osteoblastic cells stimulated by estrogen and mechanical strain, but their acute down-regulation of the Wnt antagonist Sost is mediated by estrogen receptor beta. J Biol Chem. 2013;288:9035–9048. doi: 10.1074/jbc.M112.405456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gifre L, Ruiz-Gaspa S, Monegal A, Nomdedeu B, Filella X, Guanabens N, Peris P. Effect of glucocorticoid treatment on Wnt signalling antagonists (sclerostin and Dkk-1) and their relationship with bone turnover. Bone. 2013;57:272–276. doi: 10.1016/j.bone.2013.08.016. [DOI] [PubMed] [Google Scholar]
- Gillette JM, Nielsen-Preiss SM. The role of annexin 2 in osteoblastic mineralization. J Cell Sci. 2004;117(Pt3):441–449. doi: 10.1242/jcs.00909. [DOI] [PubMed] [Google Scholar]
- Godin LM, Suzuki S, Jacobs CR, Donahue HJ, Donahue SW. Mechanically induced intracellular calcium waves in osteoblasts demonstrate calcium fingerprints in bone cell mechanotransduction. Biomech Model Mechanobiol. 2007;6(6):391–398. doi: 10.1007/s10237-006-0059-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossmann TN, Yeh JT, Bowman BR, Chu Q, Moellering RE, Verdine GL. Inhibition of oncogenic Wnt signaling through direct targeting of beta-catenin. Proc Natl Acad Sci U S A. 2012;109:17942–17947. doi: 10.1073/pnas.1208396109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haasper C, Jagodzinski M, Drescher M, Meller R, Wehmeier M, Krettek C, Hesse E. Cyclic strain induces FosB and initiates osteogenic differentiation of mesenchymal cells. Exp Toxicol Pathol. 2008;59(6):355–363. doi: 10.1016/j.etp.2007.11.008. [DOI] [PubMed] [Google Scholar]
- Haidekker MA, L’Heureux N, Frangos JA. Fluid shear stress increases membrane fluidity in endothelial cells: a study with DCVJ fluorescence. Am J Physiol Heart Circ Physiol. 2000;278:H1401–H1406. doi: 10.1152/ajpheart.2000.278.4.H1401. [DOI] [PubMed] [Google Scholar]
- Harrison OJ, Jin X, Hong S, Bahna F, Ahlsen G, Brasch J, Wu Y, Vendome J, Felsovalyi K, Hampton CM, Troyanovsky RB, Ben-Shaul A, Frank J, Troyanovsky SM, Shapiro L, Honig B. The extracellular architecture of adherens junctions revealed by crystal structures of type I cadherins. Structure. 2011;19:244–256. doi: 10.1016/j.str.2010.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haut Donahue TL, Genetos DC, Jacobs CR, Donahue HJ, Yellowley CE. Annexin V disruption impairs mechanically induced calcium signaling in osteoblastic cells. Bone. 2004;35:656–663. doi: 10.1016/j.bone.2004.04.025. [DOI] [PubMed] [Google Scholar]
- Hehlgans S, Haase M, Cordes N. Signalling via integrins: implications for cell survival and anticancer strategies. Biochim Biophys Acta. 2007;1775(1):163–180. doi: 10.1016/j.bbcan.2006.09.001. [DOI] [PubMed] [Google Scholar]
- Heubach JF, Graf EM, Leutheuser J, Bock M, Balana B, Zahanich I, Christ T, Boxberger S, Wettwer E, Ravens U. Electrophysiological properties of human mesenchymal stem cells. J Physiol. 2004;554:659–672. doi: 10.1113/jphysiol.2003.055806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillsley MV, Frangos JA. Alkaline phosphatase in osteoblasts is down-regulated by pulsatile fluid flow. Calcif Tissue Int. 1997;60:48–53. doi: 10.1007/s002239900185. [DOI] [PubMed] [Google Scholar]
- Hoey DA, Kelly DJ, Jacobs CR. A role for the primary cilium in paracrine signaling between mechanically stimulated osteocytes and mesenchymal stem cells. Biochem Biophys Res Commun. 2011;412(1):182–187. doi: 10.1016/j.bbrc.2011.07.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoey DA, Tormey S, Ramcharan S, O’Brien FJ, Jacobs CR. Primary cilia-mediated mechanotransduction in human mesenchymal stem cells. Stem Cells. 2012;30:2561–2570. doi: 10.1002/stem.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton ER, Humphries JD, James J, Jones MC, Askari JA, Humphries MJ. The integrin adhesome network at a glance. J Cell Sci. 2016;129:4159–4163. doi: 10.1242/jcs.192054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang LW, Ren L, Yang PF, Shang P. Response of osteoblasts to the stimulus of fluid flow. Crit Rev Eukaryot Gene Expr. 2015;25(2):153–162. doi: 10.1615/critreveukaryotgeneexpr.2015013564. [DOI] [PubMed] [Google Scholar]
- Hung CT, Allen FD, Mansfield KD, Shapiro IM. Extracellular ATP modulates [Ca2+]i in retinoic acid-treated embryonic chondrocytes. Am J Phys. 1997;272:C1611–C1617. doi: 10.1152/ajpcell.1997.272.5.C1611. [DOI] [PubMed] [Google Scholar]
- Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110(6):673–687. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- Ingber DE. Cellular tensegrity: defining new rules of biological design that govern the cytoskeleton. J Cell Sci. 1993;104:613–627. doi: 10.1242/jcs.104.3.613. [DOI] [PubMed] [Google Scholar]
- Ingber DE. Mechanobiology and diseases of mechanotransduction. Ann Med. 2003;35:564–577. doi: 10.1080/07853890310016333. [DOI] [PubMed] [Google Scholar]
- Ingber DE, Tensegrity I. Cell structure and hierarchical systems biology. J Cell Sci. 2003;116(Pt7):1157–1173. doi: 10.1242/jcs.00359. [DOI] [PubMed] [Google Scholar]
- Jacobs CR, Temiyasathit S, Castillo AB. Osteocyte mechanobiology and pericellular mechanics. Annu Rev Biomed Eng. 2010;12:369–400. doi: 10.1146/annurev-bioeng-070909-105302. [DOI] [PubMed] [Google Scholar]
- Jacobs CR, Yellowley CE, Davis BR, Zhou Z, Cimbala JM, Donahue HJ. Differential effect of steady versus oscillating flow on bone cells. J Biomech. 1998;31:969–976. doi: 10.1016/s0021-9290(98)00114-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahed Z, Shams H, Mehrbod M, Mofrad MR. Mechanotransduction pathways linking the extracellular matrix to the nucleus. Int Rev Cell Mol Biol. 2014;310:171–220. doi: 10.1016/B978-0-12-800180-6.00005-0. [DOI] [PubMed] [Google Scholar]
- Jing D, Lu XL, Luo E, Sajda P, Leong PL, Guo XE. Spatiotemporal properties of intracellular calcium signaling in osteocytic and osteoblastic cell networks under fluid flow. Bone. 2013;53:531–540. doi: 10.1016/j.bone.2013.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson WA. Two views of the same stimulus. Elife. 2017;6:e30191. doi: 10.7554/eLife.30191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamel MA, Picconi JL, Lara-Castillo N, Johnson ML. Activation of β-catenin signaling in MLO-Y4 osteocytic cells versus 2T3 osteoblastic cells by fluid flow shear stress and PGE2: implications for the study of mechanosensation in bone. Bone. 2010;47(5):872–881. doi: 10.1016/j.bone.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapur S, Baylink DJ, Lau KH. Fluid flow shear stress stimulates human osteoblast proliferation and differentiation through multiple interacting and competing signal transduction pathways. Bone. 2003;32(3):241–251. doi: 10.1016/s8756-3282(02)00979-1. [DOI] [PubMed] [Google Scholar]
- Kawano S, Shoji S, Ichinose S, Yamagata K, Tagami M, Hiraoka M. Characterization of Ca2+ signaling pathways in human mesenchymal stem cells. Cell Calcium. 2002;32:165–174. doi: 10.1016/s0143416002001240. [DOI] [PubMed] [Google Scholar]
- Kelly DJ, Jacobs CR. The role of mechanical signals in regulating chondrogenesis and osteogenesis of mesenchymal stem cells. Birth Defects Res C Embryo Today. 2010;90(1):75–85. doi: 10.1002/bdrc.20173. [DOI] [PubMed] [Google Scholar]
- Klein-Nulend J, Bakker AD, Bacabac RG, Vatsa A, Weinbaum S. Mechanosensation and transduction in osteocytes. Bone. 2013;54(2):182–190. doi: 10.1016/j.bone.2012.10.013. [DOI] [PubMed] [Google Scholar]
- Knothe Tate ML, Niederer P, Knothe U. In vivo tracer transport through the lacunocanalicular system of rat bone in an environment devoid of mechanical loading. Bone. 1998;22:107–117. doi: 10.1016/s8756-3282(97)00234-2. [DOI] [PubMed] [Google Scholar]
- Knothe Tate ML. Mixing mechanisms and net solute transport in bone. Ann Biomed Eng. 2001;29:810–816. doi: 10.1114/1.1397788. [DOI] [PubMed] [Google Scholar]
- Knothe Tate ML. “Whither flows the fluid in bone?” an osteocyte’s perspective. J Biomech. 2003;36(10):1409–1424. doi: 10.1016/s0021-9290(03)00123-4. [DOI] [PubMed] [Google Scholar]
- Kuriakose T, Rada B, Watford WT. Tumor progression locus 2-dependent oxidative burst drives phosphorylation of extracellular signalregulated kinase during TLR3 and 9 signaling. J Biol Chem. 2014;289(52):36089–36100. doi: 10.1074/jbc.M114.587121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon RY, Temiyasathit S, Tummala P, Quah CC, Jacobs CR. Primary cilium-dependent mechanosensing is mediated by adenylyl cyclase 6 and cyclic AMP in bone cells. FASEB J. 2010;24:2859–2868. doi: 10.1096/fj.09-148007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanctot PM, Gage FH, Varki AP. The glycans of stem cells. Curr Opin Chem Biol. 2007;11:373–380. doi: 10.1016/j.cbpa.2007.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langenhan T, Piao X, Monk KR. Adhesion G protein-coupled receptors in nervous system development and disease. Nat Rev Neurosci. 2016;17:550–561. doi: 10.1038/nrn.2016.86. [DOI] [PubMed] [Google Scholar]
- Lewis AH, Grandl J. Mechanical sensitivity of Piezo1 ion channels can be tuned by cellular membrane tension. eLife. 2015;4:e12088. doi: 10.7554/eLife.12088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Duncan RL, Burr DB, Turner CH. L-type calcium channels mediate mechanically induced bone formation in vivo. J Bone Miner Res. 2002;17(10):1795–1800. doi: 10.1359/jbmr.2002.17.10.1795. [DOI] [PubMed] [Google Scholar]
- Little R, Muimo R, Robson L, Harris K, Grabowski PS. The transient receptor potential ion channel TRPV6 is expressed at low levels in osteoblasts and has little role in osteoblast calcium uptake. PLoS One. 2011;6(11):e28166. doi: 10.1371/journal.pone.0028166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litzenberger JB, Kim JB, Tummala P, Jacobs CR. Beta1 integrins mediate mechanosensitive signaling pathways in osteocytes. Calcif Tissue Int. 2010;86(4):325–332. doi: 10.1007/s00223-010-9343-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D, Genetos DC, Shao Y, Geist DJ, Li J, Ke HZ, Turner CH, Duncan RL. Activation of extracellular-signal regulated kinase (ERK1/2) by fluid shear is Ca(2+)- and ATP-dependent in MC3T3-E1 osteoblasts. Bone. 2008;42:644–652. doi: 10.1016/j.bone.2007.09.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Yuan W, Wang J. Mechanisms for osteogenic differentiation of human mesenchymal stem cells induced by fluid shear stress. Biomech Model Mechanobiol. 2010;9(6):659–670. doi: 10.1007/s10237-010-0206-x. [DOI] [PubMed] [Google Scholar]
- Lofthouse RA, Davis JR, Frondoza CG, Jinnah RH, Hungerford DS, Hare JM. Identification of caveolae and detection of caveolin in normal human osteoblasts. J Bone Joint Surg (Br) 2001;83:124–129. doi: 10.1302/0301-620x.83b1.10604. [DOI] [PubMed] [Google Scholar]
- Lombardi ML, Jaalouk DE, Shanahan CM, Burke B, Roux KJ, Lammerding J. The interaction between nesprins and sun proteins at the nuclear envelope is critical for force transmission between the nucleus and cytoskeleton. J Biol Chem. 2011;286(30):26743–26753. doi: 10.1074/jbc.M111.233700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo J, Sun P, Siwko S, Liu M, Xiao J. The role of GPCRs in bone diseases and dysfunctions. Bone Res. 2019;7:19. doi: 10.1038/s41413-019-0059-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons JS, Joca HC, Law RA, Williams KM, Kerr JP, Shi G, Khairallah RJ, Martin SS, Konstantopoulos K, Ward CW, Stains JP. Microtubules tune mechanotransduction through NOX2 and TRPV4 to decrease sclerostin abundance in osteocytes. Sci Signal. 2017;10:506. doi: 10.1126/scisignal.aan5748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machiyama H, Hirata H, Loh XK, Kanchi MM, Fujita H, Tan SH, Kawauchi K, Sawada Y. Displacement of p130Cas from focal adhesions links actomyosin contraction to cell migration. J Cell Sci. 2014;127(Pt16):3440–3450. doi: 10.1242/jcs.143438. [DOI] [PubMed] [Google Scholar]
- Mahalingam B, Van Agthoven JF, Xiong JP, Alonso JL, Adair BD, Rui X, Anand S, Mehrbod M, Mofrad MR, Burger C, Goodman SL, Arnaout MA. Atomic basis for the species-specific inhibition of alpha V integrins by monoclonal antibody 17E6 is revealed by the crystal structure of alpha V beta 3 ectodomain-17E6 Fab complex. J.Biol.Chem. 2014;289:13801–13809. doi: 10.1074/jbc.M113.546929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malone AM, Anderson CT, Tummala P, Kwon RY, Johnston TR, Stearns T, Jacobs CR. Primary cilia mediate mechanosensing in bone cells by a calcium-independent mechanism. Proc Natl Acad Sci U S A. 2007;104(33):13325–13330. doi: 10.1073/pnas.0700636104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malone AM, Batra NN, Shivaram G, Kwon RY, You L, Kim CH, Rodriguez J, Jair K, Jacobs CR. The role of actin cytoskeleton in oscillatory fluid flow-induced signaling in MC3T3-E1 osteoblasts. Am J Phys Cell Phys. 2007;292(5):C1830–C1836. doi: 10.1152/ajpcell.00352.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniotis AJ, Chen CS, Ingber DE. Demonstration of mechanical connections between integrins, cytoskeletal filaments, and nucleoplasm that stabilize nuclear structure. Proc Natl Acad Sci U S A. 1997;94(3):849–854. doi: 10.1073/pnas.94.3.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marie PJ, Haÿ E. Cadherins and Wnt signalling: a functional link controlling bone formation. Bonekey Rep. 2013;2:330. doi: 10.1038/bonekey.2013.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin TJ, Allan EH, Ho PW, Gooi JH, Quinn JM, Gillespie MT, Krasnoperov V, Sims NA. Communication between ephrinB2 and EphB4 within the osteoblast lineage. Adv Exp Med Biol. 2010;658:51–60. doi: 10.1007/978-1-4419-1050-9_6. [DOI] [PubMed] [Google Scholar]
- Martins RP, Finan JD, Guilak F, Lee DA. Mechanical regulation of nuclear structure and function. Annu Rev Biomed Eng. 2012;14:431–455. doi: 10.1146/annurev-bioeng-071910-124638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuo K, Otaki N. Bone cell interactions through Eph/ephrin: bone modeling, remodeling and associated diseases. Cell Adhes Migr. 2012;6:148–156. doi: 10.4161/cam.20888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay MM, Morrison DK. Integrating signals from RTKs to ERK/MAPK. Oncogene. 2007;26(22):3113–3121. doi: 10.1038/sj.onc.1210394. [DOI] [PubMed] [Google Scholar]
- McMahon LA, Campbell VA, Prendergast PJ. Involvement of stretch-activated ion channels in strain-regulated glycosaminoglycan synthesis in mesenchymal stem cell-seeded 3D scaffolds. J Biomech. 2008;41:2055–2059. doi: 10.1016/j.jbiomech.2008.03.027. [DOI] [PubMed] [Google Scholar]
- Mirzaali MJ, Schwiedrzik JJ, Thaiwichai S, Best JP, Michler J, Zysset PK, Wolfram U. Mechanical properties of cortical bone and their relationships with age, gender, composition and microindentation properties in the elderly. Bone. 2016;93:196–211. doi: 10.1016/j.bone.2015.11.018. [DOI] [PubMed] [Google Scholar]
- Myers KA, Rattner JB, Shrive NG, Hart DA. Osteoblast-like cells and fluid flow: cytoskeleton-dependent shear sensitivity. Biochem Biophys Res Commun. 2007;364(2):214–219. doi: 10.1016/j.bbrc.2007.09.109. [DOI] [PubMed] [Google Scholar]
- Nagaraja MP, Jo H. The role of mechanical stimulation in recovery of bone loss-high versus low magnitude and frequency of force. Life (Basel) 2014;4(2):117–130. doi: 10.3390/life4020117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolopoulos SN, Turner CE. Actopaxin, a new focal adhesion protein that binds paxillin LD motifs and actin and regulates cell adhesion. J Cell Biol. 2000;151(7):1435–1448. doi: 10.1083/jcb.151.7.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norvell SM, Alvarez M, Bidwell JP, Pavalko FM. Fluid shear stress induces beta-catenin signaling in osteoblasts. Calcif Tissue Int. 2004;75:396–404. doi: 10.1007/s00223-004-0213-y. [DOI] [PubMed] [Google Scholar]
- Orr AW, Murphy-Ullrich JE. Regulation of endothelial cell function BY FAK and PYK2. Front Biosci. 2004;9:1254–1266. doi: 10.2741/1239. [DOI] [PubMed] [Google Scholar]
- Pavalko FM, Chen NX, Turner CH, Burr DB, Atkinson S, Hsieh YF, Qiu J, Duncan RL. Fluid shear-induced mechanical signaling in MC3T3-E1 osteoblasts requires cytoskeleton-integrin interactions. Am J Phys. 1998;275(6Pt1):C1591–C1601. [PubMed] [Google Scholar]
- Petersen SC, Luo R, Liebscher I, Giera S, Jeong SJ, Mogha A, Ghidinelli M, Feltri ML, Schöneberg T, Piao X, Monk KR. The adhesion GPCR GPR126 has distinct, domain-dependent functions in Schwann cell development mediated by interaction with laminin-211. Neuron. 2015;85:755–769. doi: 10.1016/j.neuron.2014.12.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piekarski K, Munro M. Transport mechanism operating between blood supply and osteocytes in long bones. Nature. 1977;269(5623):80–82. doi: 10.1038/269080a0. [DOI] [PubMed] [Google Scholar]
- Poole K, Herget R, Lapatsina L, Ngo HD, Lewin GR. Tuning piezo ion channels to detect molecular-scale movements relevant for fine touch. Nat Commun. 2014;5:3520. doi: 10.1038/ncomms4520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raman M, Chen W, Cobb MH. Differential regulation and properties of MAPKs. Oncogene. 2007;26:3100–3112. doi: 10.1038/sj.onc.1210392. [DOI] [PubMed] [Google Scholar]
- Reich KM, Gay CV, Frangos JA. Fluid shear stress as a mediator of osteoblast cyclic adenosine monophosphate production. J Cell Physiol. 1990;143:100–104. doi: 10.1002/jcp.1041430113. [DOI] [PubMed] [Google Scholar]
- Reilly GC, Haut TR, Yellowley CE, Donahue HJ, Jacobs CR. Fluid flow induced PGE2 release by bone cells is reduced by glycocalyx degradation whereas calcium signals are not. Biorheology. 2003;40(6):591–603. [PubMed] [Google Scholar]
- Riddle RC, Taylor AF, Genetos DC, Donahue HJ. MAP kinase and calcium signaling mediate fluid flow-induced human mesenchymal stem cell proliferation. Am J Phys Cell Phys. 2006;290:C776–C784. doi: 10.1152/ajpcell.00082.2005. [DOI] [PubMed] [Google Scholar]
- Riehl BD, Lee JS, Ha L, Lim JY. Fluid-flow-induced mesenchymal stem cell migration: role of focal adhesion kinase and RhoA kinase sensors. J R Soc Interface. 2015;12(104):20141351. doi: 10.1098/rsif.2014.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robling AG, Niziolek PJ, Baldridge LA, Condon KW, Allen MR, Alam I, Mantila SM, Gluhak-Heinrich J, Bellido TM, Harris SE, Turner CH. Mechanical stimulation of bone in vivo reduces osteocyte expression of Sost/sclerostin. J Biol Chem. 2008;283:5866–5875. doi: 10.1074/jbc.M705092200. [DOI] [PubMed] [Google Scholar]
- Rose AS, Bradley AR, Valasatava Y, Duarte JD, Prlić A, Rose PW. NGL viewer: web-based molecular graphics for large complexes. Bioinformatics. 2018;34(21):3755–3758. doi: 10.1093/bioinformatics/bty419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roskoski R., Jr ERK1/2 MAP kinases: structure, function, and regulation. Pharmacol Res. 2012;66(2):105–143. doi: 10.1016/j.phrs.2012.04.005. [DOI] [PubMed] [Google Scholar]
- Saito-Diaz K, Chen TW, Wang X, Thorne CA, Wallace HA, Page-McCaw A, Lee E. The way Wnt works: components and mechanism. Growth Factors. 2013;31(1):1–31. doi: 10.3109/08977194.2012.752737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salter DM, Robb JE, Wright MO. Electrophysiological responses of human bone cells to mechanical stimulation: evidence for specific integrin function in mechanotransduction. J Bone Miner Res. 1997;12(7):1133–1141. doi: 10.1359/jbmr.1997.12.7.1133. [DOI] [PubMed] [Google Scholar]
- Schlaepfer DD, Hauck CR, Sieg DJ. Signaling through focal adhesion kinase. Prog Biophys Mol Biol. 1999;71(3-4):435–478. doi: 10.1016/s0079-6107(98)00052-2. [DOI] [PubMed] [Google Scholar]
- Sezgin E, Levental I, Mayor S, Eggeling C. The mystery of membrane organization: composition, regulation and roles of lipid rafts. Nat Rev Mol Cell Biol. 2017;18(6):361–374. doi: 10.1038/nrm.2017.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao Y, Alicknavitch M, Farach-Carson MC. Expression of voltage sensitive calcium channel (VSCC) L-type Cav1.2 (alpha1C) and T-type Cav3.2 (alpha1H) subunits during mouse bone development. Dev Dyn : an official publication of the American Association of Anatomists. 2005;234:54–62. doi: 10.1002/dvdy.20517. [DOI] [PubMed] [Google Scholar]
- Scholz N, Gehring J, Guan C, Ljaschenko D, Fischer R, Lakshmanan V, Kittel RJ, Langenhan T. The adhesion GPCR latrophilin/CIRL shapes mechanosensation. Cell Rep. 2015;11:866–874. doi: 10.1016/j.celrep.2015.04.008. [DOI] [PubMed] [Google Scholar]
- Siller-Jackson AJ, Burra S, Gu S, Harris SE, Bonewald LF, Sprague E, Jiang JX. The role of α5 integrin as a mechanosensor in the regulation of connexin 43 hemichannel release of prostaglandin in response to mechanical stress. J Bone Miner Res. 2006;21(Suppl 1):S72. [Google Scholar]
- Soattin L, Fiore M, Gavazzo P, Viti F, Facci P, Raiteri R, Difato F, Pusch M, Vassalli M. The biophysics of piezo1 and piezo2 mechanosensitive channels. Biophys Chem. 2016;208:26–33. doi: 10.1016/j.bpc.2015.06.013. [DOI] [PubMed] [Google Scholar]
- Solomon KR, Adolphson LD, Wank DA, McHugh KP, Hauschka PV. Caveolae in human and murine osteoblasts. J Bone Miner Res. 2000;15:2391–2401. doi: 10.1359/jbmr.2000.15.12.2391. [DOI] [PubMed] [Google Scholar]
- Solomon KR, Danciu TE, Adolphson LD, Hecht LE, Hauschka PV. Caveolin-enriched membrane signaling complexes in human and murine osteoblasts. J Bone Miner Res. 2000;15:2380–2390. doi: 10.1359/jbmr.2000.15.12.2380. [DOI] [PubMed] [Google Scholar]
- Srinivasan PP, Parajuli A, Price C, Wang L, Duncan RL, Kirn-Safran CB. Inhibition of T-type voltage sensitive calcium channel reduces load-induced OA in mice and suppresses the catabolic effect of bone mechanical stress on chondrocytes. PLoS One. 2015;10(5):e0127290. doi: 10.1371/journal.pone.0127290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stains JP, Civitelli R. Connexins in the skeleton. Semin Cell Dev Biol. 2016;50:31–39. doi: 10.1016/j.semcdb.2015.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiehler M, Bunger C, Baatrup A, Lind M, Kassem M, Mygind T. Effect of dynamic 3-D culture on proliferation, distribution, and osteogenic differentiation of human mesenchymal stem cells. J Biomed Mater Res A. 2009;89:96–107. doi: 10.1002/jbm.a.31967. [DOI] [PubMed] [Google Scholar]
- Stoltz JF, Magdalou J, George D, Chen Y, Li Y, De Isla N, He X, Remond Y. Influence of mechanical forces on bone: introduction to mechanobiology and mechanical adaptation concept. J Cell Immunother. 2018;4(1):10–12. [Google Scholar]
- Sugimoto A, Miyazaki A, Kawarabayashi K, Shono M, Akazawa Y, Hasegawa T, Ueda-Yamaguchi K, Kitamura T, Yoshizaki K, Fukumoto S, Iwamoto T. Piezo type mechanosensitive ion channel component 1 functions as a regulator of the cell fate determination of mesenchymal stem cells. Sci Rep. 2017;7(1):17696. doi: 10.1038/s41598-017-18089-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun W, Chi S, Li Y, Ling S, Tan Y, Xu Y, Jiang F, Li J, Liu C, Zhong G, Cao D, Jin X, Zhao D, Gao X, Liu Z, Xiao B, Li Y. The mechanosensitive Piezo1 channel is required for bone formation. Elife. 2019;8:e47454. doi: 10.7554/eLife.47454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson WR, Majid AS, Czymmek KJ, Ruff AL, Garcia J, Duncan RL, Farach-Carson MC. Association of the alpha(2)delta(1) subunit with Ca(v)3.2 enhances membrane expression and regulates mechanically induced ATP release in MLO-Y4 osteocytes. J Bone Miner Res. 2011;26:2125–2139. doi: 10.1002/jbmr.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson WR, Rubin CT, Rubin J. Mechanical regulation of signaling pathways in bone. Gene. 2012;503(2):179–193. doi: 10.1016/j.gene.2012.04.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsimbouri PM, Childs PG, Pemberton GD, Yang J, Jayawarna V, Orapiriyakul W, Burgess K, González-García C, Blackburn G, Thomas D, Vallejo-Giraldo C, Biggs MJP, Curtis ASG, Salmerón-Sánchez M, Reid S, Dalby MJ. Stimulation of 3D osteogenesis by mesenchymal stem cells using a nanovibrational bioreactor. Nat Biomed Eng. 2017;1:758–770. doi: 10.1038/s41551-017-0127-4. [DOI] [PubMed] [Google Scholar]
- Turner CH, Pavalko FM. Mechanotransduction and functional response of the skeleton to physical stress: the mechanisms and mechanics of bone adaptation. J Orthop Sci. 1998;3(6):346–355. doi: 10.1007/s007760050064. [DOI] [PubMed] [Google Scholar]
- Turner CH, Robling AG, Duncan RL, Burr DB. Do bone cells behave like a neuronal network? Calcif Tissue Int. 2002;70(6):435–442. doi: 10.1007/s00223-001-1024-z. [DOI] [PubMed] [Google Scholar]
- Ueland T, Olarescu NC, Jorgensen AP, Otterdal K, Aukrust P, Godang K, Lekva T, Bollerslev J. Increased serum and bone matrix levels of the secreted Wnt antagonist DKK-1 in patients with growth hormone deficiency in response to growth hormone treatment. J Clin Endocrinol Metab. 2015;100:736–743. doi: 10.1210/jc.2014-2912. [DOI] [PubMed] [Google Scholar]
- Verbruggen SW, Vaughan TJ, McNamara LM. Fluid flow in the osteocyte mechanical environment: a fluid-structure interaction approach. Biomech Model Mechanobiol. 2014;13(1):85–97. doi: 10.1007/s10237-013-0487-y. [DOI] [PubMed] [Google Scholar]
- Vezeridis PS, Semeins CM, Chen Q, Klein-Nulend J. Osteocytes subjected to pulsating fluid flow regulate osteoblast proliferation and differentiation. Biochem Biophys Res Commun. 2006;348(3):1082–1088. doi: 10.1016/j.bbrc.2006.07.146. [DOI] [PubMed] [Google Scholar]
- Volokh KY, Vilnay O, Belsky M. Cell cytoskeleton and tensegrity. Biorheology. 2002;39(1–2):63–67. [PubMed] [Google Scholar]
- Wang L, Cowin SC, Weinbaum S, Fritton SP. Modeling tracer transport in an osteon under cyclic loading. Ann Biomed Eng. 2000;28:1200–1209. doi: 10.1114/1.1317531. [DOI] [PubMed] [Google Scholar]
- Wang N, Tytell JD, Ingber DE. Mechanotransduction at a distance: mechanically coupling the extracellular matrix with the nucleus. Nat Rev Mol Cell Biol. 2009;10:75–82. doi: 10.1038/nrm2594. [DOI] [PubMed] [Google Scholar]
- Wang W, Liu Y, Liao K. Tyrosine phosphorylation of cortactin by the FAK-Src complex at focal adhesions regulates cell motility. BMC Cell Biol. 2011;12:49. doi: 10.1186/1471-2121-12-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Jia L, Zheng Y, Li W. Bone remodeling induced by mechanical forces is regulated by miRNAs. Biosci Rep. 2018;38(4):BSR20180448. doi: 10.1042/BSR20180448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehrli FW, Fernández-Seara MA. Nuclear magnetic resonance studies of bone water. Ann Biomed Eng. 2005;33(1):79–86. doi: 10.1007/s10439-005-8965-8. [DOI] [PubMed] [Google Scholar]
- Weyts FA, Li YS, van Leeuwen J, Weinans H, Chien S. ERK activation and alpha v beta 3 integrin signaling through Shc recruitment in response to mechanical stimulation in human osteoblasts. J Cell Biochem. 2002;87(1):85–92. doi: 10.1002/jcb.10278. [DOI] [PubMed] [Google Scholar]
- Willert K, Nusse R. Wnt proteins. Cold Spring Harb Perspect Biol. 2012;4(9):a007864. doi: 10.1101/cshperspect.a007864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JL, Iannotti JP, Ham A, Bleuit J, Chen JH. Effects of fluid shear stress on bone cells. Biorheology. 1994;31:163–170. doi: 10.3233/bir-1994-31204. [DOI] [PubMed] [Google Scholar]
- Winograd-Katz SE, Fässler R, Geiger B, Legate KR. The integrin adhesome: from genes and proteins to human disease. Nat Rev Mol Cell Biol. 2014;15(4):273–288. doi: 10.1038/nrm3769. [DOI] [PubMed] [Google Scholar]
- Wittkowske C, Reilly GC, Lacroix D, Perrault CM. In vitro bone cell models: impact of fluid shear stress on bone formation. Front Bioeng Biotechnol. 2016;4:87. doi: 10.3389/fbioe.2016.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Z, Zhang S, Mahlios J, Zhou G, Magenheimer BS, Guo D, Dallas SL, Maser R, Calvet JP, Bonewald L, Quarles LD. Cilia-like structures and polycystin-1 in osteoblasts/osteocytes and associated abnormalities in skeletogenesis and Runx2 expression. J Biol Chem. 2006;281(41):30884–30895. doi: 10.1074/jbc.M604772200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Z, Wang P, Wu J, Feng X, Cai J, Zhai M, Li J, Liu X, Jiang M, Luo E, Jing D. Fluid shear stress improves morphology, cytoskeleton architecture, viability, and regulates cytokine expression in a time-dependent manner in MLO-Y4 cells. Cell Biol Int. 2018;42(10):1410–1422. doi: 10.1002/cbin.11032. [DOI] [PubMed] [Google Scholar]
- Yao Y, Rabodzey A, Dewey CF., Jr Glycocalyx modulates the motility and proliferative response of vascular endothelium to fluid shear stress. Am J Physiol Heart Circ Physiol. 2007;293:H1023–H1030. doi: 10.1152/ajpheart.00162.2007. [DOI] [PubMed] [Google Scholar]
- Yavropoulou MP, Yovos JG. The molecular basis of bone mechanotransduction. J Musculoskelet Neuronal Interact. 2016;16(3):221–236. [PMC free article] [PubMed] [Google Scholar]
- Young SR, Gerard-O'Riley R, Kim JB, Pavalko FM. Focal adhesion kinase is important for fluid shear stress-induced mechanotransduction in osteoblasts. J Bone Miner Res. 2009;24:411–424. doi: 10.1359/JBMR.081102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yourek G, McCormick SM, Mao JJ, Reilly GC. Shear stress induces osteogenic differentiation of human mesenchymal stem cells. Regen Med. 2010;5(5):713–724. doi: 10.2217/rme.10.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaidel-Bar R, Cohen M, Addadi L, Geiger B. Hierarchical assembly of cell-matrix adhesion complexes. Biochem Soc Trans. 2004;32(Pt3):416–420. doi: 10.1042/BST0320416. [DOI] [PubMed] [Google Scholar]
- Zhang K, Barragan-Adjemian C, Ye L, Kotha S, Dallas M, Lu Y, Zhao S, Harris M, Harris SE, Feng JQ, Bonewald LF. E11/gp38 selective expression in osteocytes: regulation by mechanical strain and role in dendrite elongation. Mol Cell Biol. 2006;26:4539–4552. doi: 10.1128/MCB.02120-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Q, Zhou H, Chi S, Wang Y, Wang J, Geng J, Wu K, Liu W, Zhang T, Dong MQ, Wang J, Li X, Xiao B. Structure and mechanogating mechanism of the Piezo1 channel. Nature. 2018;554:487–492. doi: 10.1038/nature25743. [DOI] [PubMed] [Google Scholar]