Abstract
Background:
Patterns of inpatient opioid use and their associations with postdischarge opioid use are poorly understood.
Objective:
To measure patterns in timing, duration, and setting of opioid administration in opioid-naive hospitalized patients and to examine associations with postdischarge use.
Design:
Retrospective cohort study using electronic health record data from 2010 to 2014.
Setting:
12 community and academic hospitals in Pennsylvania.
Patients:
148 068 opioid-naive patients (191 249 admissions) with at least 1 outpatient encounter within 12 months before and after admission.
Measurements:
Number of days and patterns of inpatient opioid use; any outpatient use (self-report and/or prescription orders) 90 and 365 days after discharge.
Results:
Opioids were administered in 48% of admissions. Patients were given opioids for a mean of 67.9% (SD, 25.0%) of their stay. Location of administration of first opioid on admission, timing of last opioid before discharge, and receipt of nonopioid analgesics varied substantially. After adjustment for potential confounders, 5.9% of inpatients receiving opioids had outpatient use at 90 days compared with 3.0% of those without inpatient use (difference, 3.0 percentage points [95% CI, 2.8 to 3.2 percentage points]). Opioid use at 90 days was higher in inpatients receiving opioids less than 12 hours before discharge than in those with at least 24 opioid-free hours before discharge (7.5% vs. 3.9%; difference, 3.6 percentage points [CI, 3.3 to 3.9 percentage points]). Differences based on proportion of the stay with opioid use were modest (opioid use at 90 days was 6.4% and 5.4%, respectively, for patients with opioid use for ≥75% vs. ≤25% of their stay; difference, 1.0 percentage point [CI, 0.4 to 1.5 percentage points]). Associations were similar for opioid use 365 days after discharge.
Limitation:
Potential unmeasured confounders related to opioid use.
Conclusion:
This study found high rates of opioid administration to opioid-naive inpatients and associations between specific patterns of inpatient use and risk for long-term use after discharge.
Primary Funding Source:
UPMC Health System and University of Pittsburgh.
The opioid epidemic places a significant burden on families, communities, and health systems across the United States (1, 2). Prescription and illicit opioids are responsible for the highest drug overdose mortality rates ever recorded, accounting for 63 600 deaths in 2016 (3). Health system and policy interventions have largely focused on reducing and monitoring high-risk opioid prescribing (4). Prescription drug monitoring programs seek to mitigate aberrant prescribing and patient misuse (4). The 2016 Centers for Disease Control and Prevention guidelines for opioid prescribing for chronic pain (5) have reduced prescribing volume and some high-risk prescribing (6), and payers have instituted measures to promote better prescribing (7, 8).
These initiatives have largely focused on outpatient prescribing (9). Despite the Joint Commission’s standards for inpatient pain management being implicated as a driver of increased opioid use, opioid prescribing in inpatient settings has received far less empirical evaluation (10). Recent studies reported that up to 13% of opioid-naive patients hospitalized for surgical procedures use opioids as outpatients for extended periods after surgery (11–19). Research on opioid prescribing after nonsurgical hospitalization reported similar rates of persistent use (20). One study of more than 1 million nonsurgical admissions to 286 U.S. hospitals found that opioids were used 51% of the time (21). However, little is known about the timing, duration, and inpatient setting of opioid administration or whether particular inpatient patterns of use correlate with long-term use after discharge.
To fill these knowledge gaps, we linked inpatient and outpatient electronic medical record data from a large health system between 2010 and 2014 to examine inpatient opioid administration among opioid-naive patients. We addressed 3 questions. First, who is most likely to receive opioids during a hospital stay? Second, when, where, and for how long are opioids used during hospital stays? Third, which patterns of opioid prescribing are associated with continued use after discharge?
Methods
Data Source
We obtained data from the UPMC Health System, an integrated delivery and financing system that includes academic and community hospitals and accounts for 41% of hospital admissions in western Pennsylvania. We obtained hospital discharge data from 2010 to 2014 from 12 UPMC-affiliated community and academic hospitals. System-wide implementation of outpatient and inpatient electronic health records was complete before our study period. We linked inpatient electronic health record data (CERNER) to outpatient records (Epic Systems) using the health system’s enterprise master patient index.
We obtained the following data on inpatient encounters: demographic characteristics (age, sex, and race); diagnosis codes; admission type (medical vs. surgical); length of stay; intensive care unit (ICU) stay; source of insurance coverage; in-hospital mortality; and medication administration data, including drug name, route of administration, setting, and day and hour of administration. Information on prior home medications was collected by clinical personnel at the time of admission through patient or caregiver report. When a home medication list was not recorded, medication lists recorded in the discharge summary of a prior hospital stay were used when available.
From the outpatient encounter database, we obtained information on outpatient visits before and after the index hospital stay along with outpatient medication lists. Medication data in the outpatient database represented the reconciled medication information that providers review during each visit and captured both active medications reported by patients and new prescriptions ordered by providers at outpatient visits.
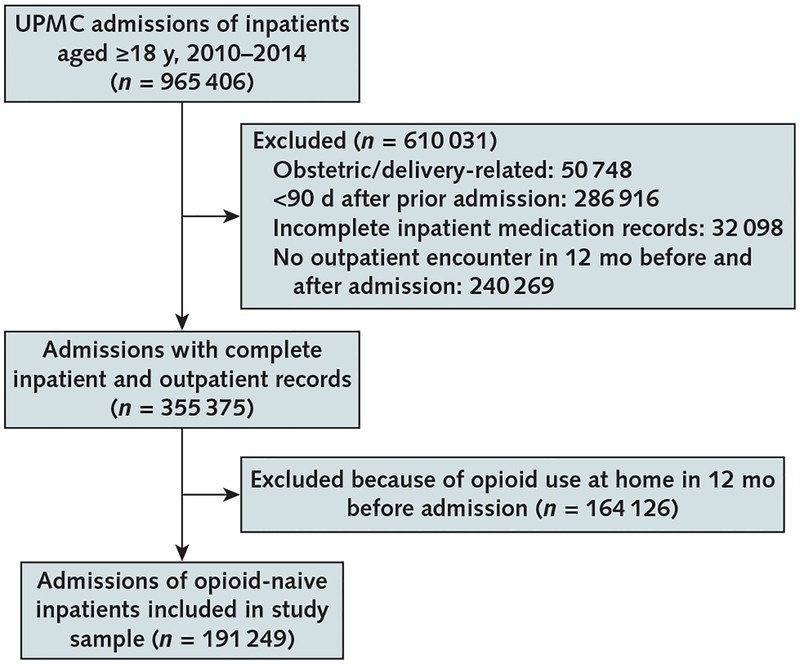
Study Sample
Our sample included adults (aged ≥18 years) admitted to study hospitals from the emergency department, home, or transfers between 2010 and 2014 (Appendix Figure, available at Annals.org). We included admissions for opioid-naive patients, defined as those with no documented opioid use in the inpatient and outpatient encounter databases in the prior 12 months. We excluded admissions that were for deliveries, those occurring less than 90 days after a previous admission (we included only the first admission in such cases to allow for complete measurement of outpatient medication use), those that were missing complete inpatient medication records, and those for patients with no outpatient encounter in the 12 months before and after the admission (to ensure that we could measure outpatient opioid use after discharge). We included admissions for patients with diagnoses that typically prompt opioid treatment (such as burns, major trauma, and advanced cancer) because our objective was not to adjudicate appropriateness of opioid prescription but to analyze associations between inpatient use patterns and long-term use.
Inpatient and Postdischarge Opioid Use
Opioids were identified in inpatient and outpatient encounter databases using the same medication list (Appendix Table 1, available at Annals.org). We excluded partial opioid agonists that are approved for opioid use disorder treatment, such as buprenorphine– naloxone, from our definition of opioid use.
We constructed 3 outcomes related to opioid use. We measured the number of days on which any opioid was administered during the hospital stay, excluding perioperative use (≤24 hours after surgery). Almost all patients admitted for surgery received perioperative opioids, but use varied thereafter. The other 2 measures captured postdischarge use. Using outpatient records, we constructed dichotomous indicators for any opioid use recorded within 90 and 365 days after discharge. Opioid use recorded in the outpatient encounter database could represent self-reported use or prescriptions ordered during the encounter. We could not observe prescription fills in pharmacies by using the outpatient database.
Patterns of Inpatient Opioid Administration
To understand associations between patterns of inpatient analgesia (opioid and nonopioid) and subsequent outpatient use, we created measures reflecting the presence, timing, duration, and location of opioid and nonopioid analgesia administration during each hospital stay. These indicators included the number of calendar days of a stay with any opioid administration, timing of the last opioid administration relative to discharge (in hours), location of first use (for example, emergency department, ICU, or ward), and use of nonopioid analgesics (such as nonsteroidal anti-inflammatory drugs or acetaminophen) (Appendix Table 1).
Covariates
On the basis of prior research and clinical judgment, we selected several patient- and discharge-level characteristics to include as covariates in our multivariable regression models. These included patient sex, age (as an ordinal variable), and race (white, black, or other); calendar year of admission (to account for time trends in opioid prescribing); hospital indicators (to account for differences in case mix and prescribing differences by hospital); payment source (commercial, Medicare, Medicaid, other, or uninsured) as a proxy for socioeconomic status; a 4-category variable that combined type of admission (medical or surgical) with whether the stay involved an ICU stay; Healthcare Cost and Utilization Project Clinical Classifications Software categories based on the International Classification of Diseases, Ninth Revision (22), which we combined into 9 variables to capture reason for admission; Elixhauser Comorbidity Index score (range, 0 to 30); several comorbid conditions previously found to be associated with opioid use, including musculoskeletal pain, depression, other mental disorders, alcohol use disorders, drug dependence, and opioid poisoning (Supplement Table 1, available at Annals.org) (23); and an indicator for whether the patient had used benzodiazepines at home in the 12 months before admission. Length of hospital stay was included in statistical models as either an offset term or a covariate depending on the outcomes of interest. In-hospital mortality was obtained from inpatient records, and postdischarge mortality (treated as a competing event) was measured using monthly data that UPMC obtains from the Social Security Administration.
Statistical Analysis
Admission characteristics were summarized with descriptive statistics (mean and SD or median and interquartile range for continuous variables and frequency and percentage for categorical variables). We then conducted 3 analyses among opioid-naive hospitalized patients. First, we examined factors associated with inpatient opioid use (excluding the perioperative period) using multivariable Poisson models, where the dependent variable was the number of days an opioid was administered in the hospital, length of stay (in days) was log-transformed and treated as an offset term, and all covariates described earlier were included to adjust for case mix. We assessed model fit and found no evidence of overdispersion of the data and thus considered a Poisson model to be appropriate. We included hospital fixed effects to account for hospital-level confounding and robust SEs with clustering by patient to account for those with multiple hospital stays. We report adjusted incidence rate ratios (IRRs), 95% CIs, and [i]P[/i] values for each covariate.
Second, we fit 2 multinomial logistic regression models to examine the association between any inpatient opioid administration (excluding perioperative use for surgical patients) and any outpatient use within 90 or 365 days after discharge. To account for censoring of the outcome due to postdischarge death or readmission, we constructed the outcome variable as 4 mutually exclusive categories: presence of outpatient opioid use, absence of outpatient opioid use (without death or readmission), readmission without outpatient opioid use beforehand, and death without outpatient opioid use beforehand. We adjusted for all covariates measured at the index admission, including length of stay and hospital fixed effects, and we used robust SEs to account for clustering by patient.
Third, we used multinomial logistic regression to examine the association between patterns of inpatient opioid administration (including perioperative use) and any outpatient use at 90 or 365 days after discharge. We limited this analysis to admissions for patients with at least 1 instance of inpatient opioid use during the index admission and used the multinomial outcome described earlier to capture outpatient use in the absence of death or readmission. We included as key independent variables 4 patterns of inpatient opioid use that were determined not to be collinear: the proportion of the hospital stay involving opioid administration (the number of days with opioid administration divided by the total number of days of the hospital stay), specified as 1% to 25%, 26% to 50%, 51% to 75%, or 76% to 100%; length of time between last opioid administration and discharge (0 to 12, 13 to 24, or >24 hours); location of administration of first opioid (stepdown unit or ward, emergency department, ICU, or procedure unit or postanesthesia care unit); and any use of nonopioid analgesics during the stay. We used robust SEs and adjusted for all covariates, including length of stay and hospital fixed effects.
To aid interpretation of both multinomial models, we report marginal effects or predicted probabilities (averaged over the covariates) based on inpatient opioid use patterns and calculated using Stata’s margins command. Fewer than 5% of observations had missing covariates and our sample size is large, so the main analyses were based on complete cases. We assessed the robustness of our results by conducting several sensitivity analyses. We explored the potential for residual confounding by calculating E-values (24). We ran analyses removing hospital stays for conditions for which opioids are commonly indicated and perhaps less discretionary (such as trauma or cancer). We assessed for differences by hospital in covariates and outcomes of interest and reran analyses with removal of the 1 hospital whose patient characteristics diverged from the rest of the sample. Finally, we examined the potential for effect modification by hospital using interaction terms. All statistical analyses were conducted using Stata, version 14 (StataCorp).
Ethics
This study was approved with waiver of informed consent by the Institutional Review Board of the University of Pittsburgh (IRB no. PRO15110454).
Role of the Funding Source
This study was funded via internal departmental support. No funding source had any role in the design, conduct, or analysis of the study or the decision to submit the manuscript for publication.
Results
Descriptive Characteristics of Opioid-Naive Patients
There were 191 249 admissions among 148 068 opioid-naive patients meeting inclusion criteria, of whom 33 696 (22.8%) had 2 or more hospital stays (Appendix Figure). Approximately half the stays were for patients older than 60 years, and 70% were medical admissions (Table 1). At least 1 opioid dose was administered during nearly half of admissions, although un-adjusted rates of administration varied by hospital from 32.7% to 59.1%. There were several differences in characteristics of admissions with and without opioid administration. Generally, inpatients receiving opioids were more likely to be younger and female and to have Medicaid or commercial insurance. They were also nearly twice as likely to have been admitted for a surgical procedure. Comorbid musculoskeletal pain conditions were more common in stays with opioid use.
Table 1.
Descriptive Characteristics of Previously Opioid-Naive Patients*
| Variable | Overall (n = 191 249) | Did Not Receive Opioid in Hospital (n = 98 816 [51.7%]) | Received Opioid in Hospital (n = 92 433 [48.3%]) |
|---|---|---|---|
| Hospital, n (%) | |||
| 1 | 42 456 (22.2) | 17 379 (17.6) | 25 077 (27.1) |
| 2 | 38 950 (20.4) | 21 026 (21.3) | 17 924 (19.4) |
| 3 | 27 064 (14.2) | 14 300 (14.5) | 12 764 (13.8) |
| 4 | 19 859 (10.4) | 10 493 (10.6) | 9366 (10.1) |
| 5 | 16 952 (8.9) | 9346 (9.5) | 7606 (8.2) |
| 6 | 15 277 (8.0) | 8047 (8.1) | 7230 (7.8) |
| 7 | 8663 (4.5) | 4621 (4.7) | 4042 (4.4) |
| 8 | 6042 (3.2) | 4068 (4.1) | 1974 (2.1) |
| 9 | 5829 (3.1) | 3275 (3.3) | 2554 (2.8) |
| 10 | 4680 (2.5) | 2952 (3.0) | 1728 (1.9) |
| 11 | 3903 (2.0) | 2312 (2.3) | 1591 (1.7) |
| 12 | 1574 (0.8) | 997 (1.0) | 577 (0.6) |
| Median age (IQR), y | 58 (35–72) | 61 (40–76) | 54 (33–68) |
| Age, n(%) | |||
| 18–24 y | 14 309 (7.5) | 5987 (6.1) | 8322 (9.0) |
| 25–34 y | 31 591 (16.5) | 13 720 (13.9) | 17 871 (19.3) |
| 35–44 y | 18 383 (9.6) | 8651 (8.8) | 9732 (10.5) |
| 45–54 y | 21 773 (11.4) | 10 662 (10.8) | 11 111 (12.0) |
| 55–64 y | 31 868 (16.7) | 15 658 (15.9) | 16 210 (17.5) |
| 65–74 y | 31 931 (16.7) | 16 994 (17.2) | 14 937 (16.2) |
| ≥75 y | 41 394 (21.6) | 27 144 (27.5) | 14 250 (15.4) |
| Sex, n(%) | |||
| Male | 71 203 (37.2) | 39 860 (40.3) | 31 343 (33.9) |
| Female | 119 980 (62.7) | 58 926 (59.6) | 61 054 (66.1) |
| Race, n(%) | |||
| White | 156 443 (81.8) | 80 994 (82.0) | 75 449 (81.6) |
| Black | 21 776 (11.4) | 11 014 (11.2) | 10 762 (11.6) |
| Other | 5168 (2.7) | 2575 (2.6) | 2593 (2.8) |
| Type of insurance, n(%) | |||
| Commercial | 90 507 (47.3) | 43 085 (43.6) | 47 422 (51.3) |
| Medicare | 66 696 (34.9) | 40 165 (40.7) | 26 531 (28.7) |
| Medicaid | 23 413 (12.2) | 10 036 (10.2) | 13 377 (14.5) |
| Other | 10 633 (5.6) | 5530 (5.6) | 5103 (5.5) |
| Mean Elixhauser Comorbidity Index score (SD) | 0.6 (0.8) | 0.6 (0.9) | 0.5 (0.8) |
| Admission type, n (%) | |||
| Medical without ICU | 120 182 (62.8) | 71 309 (72.2) | 48 873 (52.9) |
| Medical with ICU | 13 913 (7.3) | 7156 (7.2) | 6757 (7.3) |
| Surgical without ICU | 45 235 (23.7) | 18 050 (18.3) | 27 185 (29.4) |
| Surgical with ICU | 11 919 (6.2) | 2301 (2.3) | 9618 (10.4) |
| Median length of hospital stay (IQR), d | 4 (3–6) | 3 (2–5) | 4 (3–6) |
| Deaths, n (%) | |||
| 90 d | 3840 (2.0) | 2144 (2.2) | 1696 (1.8) |
| 365 d | 11 298 (5.9) | 6833 (6.9) | 4465 (4.8) |
| Readmissions to UPMC hospitals, n (%) | |||
| 90 d | 44 005 (23.0) | 23 583 (23.9) | 20 422 (22.1) |
| 365 d | 76 108 (39.8) | 41 566 (42.1) | 34 542 (37.4) |
| HCUP CCS conditions, n (%)† | |||
| Cardiovascular | 39 015 (20.4) | 26 306 (26.6) | 12 709 (13.8) |
| Respiratory | 10 440 (5.5) | 7295 (7.4) | 3145 (3.4) |
| Gastrointestinal | 17 816 (9.3) | 7288 (7.4) | 10 528 (11.4) |
| Oncologic | 19 665 (10.3) | 8486 (8.6) | 11 179 (12.1) |
| Genitourinary/maternal health | 20 019 (10.5) | 10 434 (10.6) | 9585 (10.4) |
| Neurologic and mental health | 11 387 (6.0) | 9324 (9.4) | 2063 (2.2) |
| Musculoskeletal, trauma, and orthopedic | 42 316 (22.1) | 14 441 (14.6) | 27 875 (30.2) |
| Endocrine and hematologic | 11 169 (5.8) | 6252 (6.3) | 4917 (5.3) |
| Other | 19 422 (10.2) | 8990 (9.1) | 10 432 (11.3) |
| Comorbidities, n (%)‡ | |||
| Musculoskeletal pain conditions | 56 285 (29.4) | 26 583 (26.9) | 29 702 (32.1) |
| Depression | 24 698 (12.9) | 12 777 (12.9) | 11 921 (12.9) |
| Other mental disorders | 4982 (2.6) | 3726 (3.8) | 1256 (1.4) |
| Alcohol use disorder | 1953 (1.0) | 1383 (1.4) | 570 (0.6) |
| Drug dependence/opioid poisoning | 1934 (1.0) | 1377 (1.4) | 557 (0.6) |
| History of benzodiazepine use, n (%)§ | 8743 (4.6) | 4479 (4.5) | 4264 (4.6) |
HCUP CCS = Healthcare Cost and Utilization Project Clinical Classifications Software; ICU = intensive care unit; IQR = interquartile range.
All differences are statistically significant (P < 0.01). χ2 test was used for P values for categorical variables, and Mann–Whitney test was used for P values for continuous variables.
Groupings based on International Classification of Diseases, Ninth Revision, codes recorded.
Likely to be associated with higher risk for opioid use (23).
Captured on home medication list collected at time of admission.
Factors Associated With Inpatient Opioid Administration
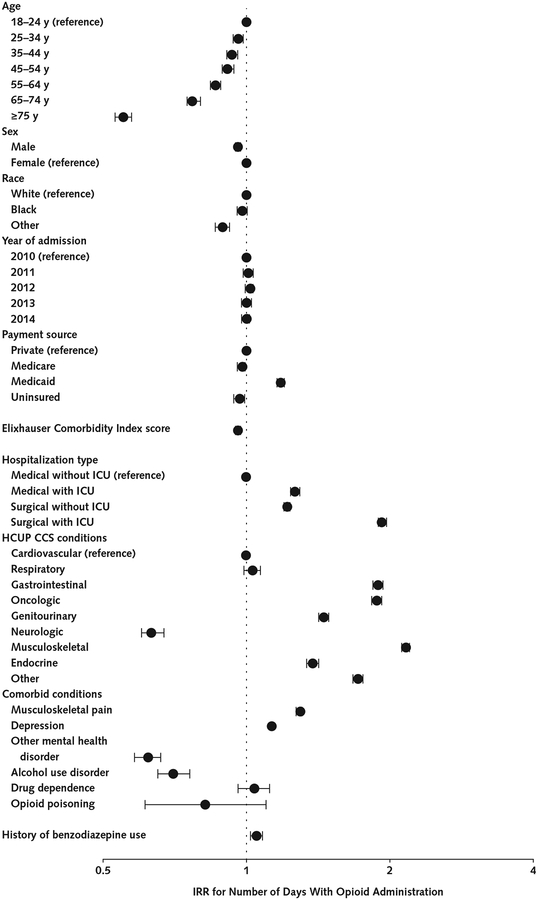
Several characteristics were associated with inpatient opioid receipt (Figure 1). Patients aged 75 years or older were half as likely as those aged 18 to 24 years to receive opioids (IRR, 0.55 [95% CI, 0.53 to 0.57]; P < 0.001). Compared with commercially insured patients, Medicaid enrollees were 18% more likely to receive opioids (IRR, 1.18 [CI, 1.16 to 1.20]; P < 0.001). Surgical admissions with an ICU stay were nearly twice as likely to include opioid administration (during the postoperative period) than medical admissions without an ICU stay (IRR, 1.93 [CI, 1.90 to 1.97]; P < 0.001). Opioid administration was significantly more likely in hospitalizations for gastrointestinal; oncologic; and musculoskeletal, trauma, or orthopedic conditions than in those for cardiovascular conditions. Patients with comorbid musculoskeletal pain (IRR, 1.30 [CI, 1.28 to 1.31]; P < 0.001) and those with depression (IRR, 1.13 [CI, 1.11 to 1.15]; P <0.001) were more likely to receive opioids, whereas other comorbid mental disorders (IRR, 0.62 [CI, 0.58 to 0.66]; P < 0.001) and alcohol use disorder (IRR,0.70 [CI, 0.65 to 0.76]; P < 0.001) were associated with a lower rate of opioid receipt.
Figure 1.
Inpatient opioid use among opioid-naive patients, with adjustment for patient- and stay-level characteristics.
IRRs were estimated using a multivariable Poisson model that included 191 249 hospital stays. The dependent variable was the number of days with opioid administration. Length of hospital stay (log-transformed) was treated as an offset term. Error bars indicate 95% CIs. HCUP CCS = Healthcare Cost and Utilization Project Clinical Classifications Software; ICU = intensive care unit; IRR = incidence rate ratio.
Patterns of Inpatient Opioid Administration
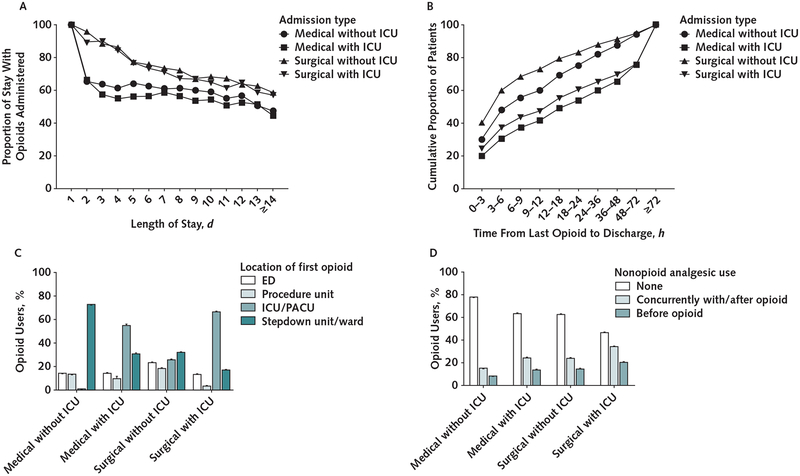
Figure 2 shows patterns of opioid and nonopioid analgesic administration for the 92 433 admissions with opioid use, stratified by hospitalization type. Opioid administration during the 24-hour perioperative period is included to fully characterize patterns for surgical patients. Across all hospital stays, patients who received opioids received them for 67.9% (SD, 25.0%) of days, and the percentage was substantially greater for surgical admissions (Figure 2, A). The proportion of the stay involving opioid use decreased as length of stay increased.
Figure 2.
Duration (A), timing (B), setting of administration of first opioid (C), and nonopioid administration (D) among opioid-naive patients who received opioids in the hospital, by admission type.
Patterns of opioid administration reported for 92 433 stays during which ≥1 opioid was administered and that met all other study inclusion criteria. ED = emergency department; ICU = intensive care unit; PACU = postanesthesia care unit.
Timing of opioid administration varied substantially by admission type. A higher percentage of non-ICU surgical patients received their last opioid less than 12 hours before discharge than non-ICU medical patients (Figure 2, B). Admissions with an ICU stay generally had more nonopioid time before discharge.
Setting of first opioid administration also varied by admission type, although the first opioid was administered in the emergency department in a small proportion of admissions (13.0% to 22.9%) (Figure 2, C). A majority of medical and surgical patients who spent time in the ICU had their first opioid administered in the ICU and the postanesthesia care unit, respectively.
Nonopioid analgesics were rarely used before opioids (7.9% to 22.2%, depending on admission type) and were used at any time during hospital stays in which an opioid was administered in 22.6% to 54.2% of admissions (Figure 2, D). Administration of a nonopioid analgesic after or concurrently with an opioid (rather than before) was more common for surgical than for medical admissions.
Association Between Inpatient Opioid Administration and Outpatient Use
Among opioid-naive inpatients, receipt of opioids was associated with a roughly 2-fold higher relative risk for outpatient use within 90 days than the relative risk for no outpatient use, death, or readmission (relative risk ratio [RRR], 2.07 [CI, 1.97 to 2.18]) (Supplement Table 2, available at Annals.org). Among inpatients who received at least 1 opioid, risk for outpatient use was 5.9% (CI, 5.7% to 6.1%) versus 3.0% (CI, 2.8% to 3.1%) among those with no inpatient use (difference, 3.0 percentage points [CI, 2.8 to 3.2 percentage points]) (Table 2). Results were similar at 365 days, although risk for outpatient use was higher overall.
Table 2.
Association Between Inpatient Opioid Use and Subsequent Outpatient Opioid Use, Death, and Readmission at 90 and 365 Days After Discharge*
| Outcome | No Inpatient Opioid Use, % | Inpatient Opioid Use, % | Difference (95% Cl), percentage points |
|---|---|---|---|
| 90 d | |||
| Outpatient opioid use | 3.0 (2.8 to 3.1) | 5.9 (5.7 to 6.1) | 3.0 (2.8 to 3.2) |
| No outpatient opioid use/death/readmission | 74.7 (74.3 to 75.0) | 72.2 (71.9 to 72.6) | −2.5 (−2.9 to −2.1) |
| Death | 0.2 (0.2 to 0.2) | 0.3 (0.2 to 0.3) | 0.1 (0.0 to 0.1) |
| Readmission | 22.2 (21.9 to 22.5) | 21.6 (21.3 to 21.9) | −0.6 (−0.9 to −0.3) |
| 365 d | |||
| Outpatient opioid use | 4.3 (4.2 to 4.5) | 7.7 (7.5 to 7.9) | 3.4 (3.2 to 3.6) |
| No outpatient opioid use/death/readmission | 54.6 (54.3 to 55.0) | 52.9 (52.5 to 53.3) | −1.7 (−2.1 to −1.3) |
| Death | 0.7 (0.7 to 0.8) | 0.7 (0.6 to 0.8) | −0.0 (−0.1 to 0.0) |
| Readmission | 40.3 (39.9 to 40.7) | 38.7 (38.3 to 39.1) | −1.6 (−2.0 to −1.2) |
HCUP CCS = Healthcare Cost and Utilization Project Clinical Classifications Software; ICU = intensive care unit.
Includes 182 917 cases with complete data from 191 249 inpatient stays for opioid-naive patients. The table shows predicted margins obtained from 2 multinomial logistic regression models (full results shown in Supplement Tables 2 and 3 [available at Annals.org]) that included an indicator of any inpatient opioid use and adjusted for the following covariates: age, sex, race, year of admission, payment source for hospital stay (e.g., Medicare or Medicaid), Elixhauser Comorbidity Index score, admission type (medical with no ICU stay, medical with ICU stay, surgical with no ICU stay, or surgical with ICU stay), length of stay, hospital fixed effects, HCUP CCS comorbid conditions, and history of benzodiazepine use. Outpatient opioid use at 90 and 365 d after discharge was the key outcome of interest, and death and readmission (both measured ≤90 d after discharge) were treated as competing risks and thus as separate levels of the outcome. Robust SEs were used to account for within-patient correlation.
Associations Between Inpatient Opioid Administration Patterns and Outpatient Use
Among the subset of opioid-naive patients receiving opioids during their index admission, several patterns were associated with increased likelihood of outpatient use. Those given opioids for 76% to 100% of their stay were more likely to use opioids within 90 days after discharge than those receiving opioids for 1% to 25% of the stay (RRR, 1.25 [CI, 1.09 to 1.43]) (Supplement Table 4, available at Annals.org). The predicted probability was 6.4% (CI, 6.0% to 6.8%) versus 5.4% (CI, 4.8% to 5.9%) (difference, 1.0 percentage point [CI, 0.4 to 1.5 percentage points]) (Table 3). At 365 days after discharge, the RRR was 1.35 (CI, 1.19 to 1.54), for a difference of 1.8 percentage points (CI, 1.2 to 2.5 percentage points) between those with opioids for 75% to 100% versus 1% to 25% of the stay. Compared with patients who did not receive an opioid during the last 24 hours of their stay, those given an opioid no more than 12 hours before discharge had twice the risk for opioid use 90 days after discharge (RRR, 2.02 [CI, 1.83 to 2.23]) (Supplement Table 4), corresponding to a difference of 3.6 percentage points (CI, 3.3 to 3.9 percentage points). Hospital setting (for example, emergency department or ICU) was not significantly associated with outpatient use at 90 days. Administration of the first opioid in the emergency department was associated with a slightly higher rate of outpatient use at 365 days (RRR, 1.13 [CI, 1.04 to 1.22]).
Table 3.
Association Between Patterns of Opioid Administration and Outpatient Opioid Use at 90 and 365 Days After Discharge Among Inpatients Receiving Opioids*
| Variable | ≤90 Days After Discharge | ≤365 Days After Discharge | ||
|---|---|---|---|---|
| Outpatient Opioid Use (95% CI), % | Difference (95% CI), percentage points | Outpatient Opioid Use (95% CI), % | Difference (95% CI), percentage points | |
| Proportion of stay with opioids administered | ||||
| 1%–25% | 5.4 (4.8 to 5.9) | Reference | 6.6 (6.0 to 7.3) | Reference |
| 26%–50% | 5.6 (5.2 to 5.9) | 0.2 (−0.2 to 0.7) | 7.4 (7.0 to 7.8) | 0.8 (0.2 to 1.5) |
| 51%–75% | 6.5 (6.2 to 6.8) | 1.1 (0.5 to 1.6) | 8.5 (8.1 to 8.8) | 1.9 (1.3 to 2.6) |
| 76%–100% | 6.4 (6.0 to 6.8) | 1.0 (0.4 to 1.5) | 8.4 (7.9 to 8.8) | 1.8 (1.2 to 2.5) |
| Time from last opioid administration to discharge | ||||
| >24 h | 3.9 (3.6 to 4.2) | Reference | 5.4(5.1 to 5.8) | Reference |
| 13–24 h | 5.4 (5.0 to 5.9) | 1.5 (1.1 to 2.0) | 6.9 (6.5 to 7.4) | 1.5 (1.1 to 2.0) |
| 0–12 h | 7.5 (7.2 to 7.8) | 3.6 (3.3 to 3.9) | 9.6 (9.2 to 9.9) | 4.2 (3.8 to 4.6) |
| Setting of first opioid administration | ||||
| Stepdown unit/ward | 6.0 (5.7 to 6.3) | Reference | 7.8 (7.5 to 8.1) | Reference |
| ED | 6.1 (5.6 to 6.5) | 0.1 (−0.4 to 0.5) | 7.9 (7.4 to 8.4) | 0.1 (−0.3 to 0.6) |
| ICU | 6.4 (5.6 to 7.1) | 0.4 (−0.2 to 1.1) | 8.1 (7.2 to 9.0) | 0.3 (−0.6 to 1.2) |
| PACU/procedure unit | 6.0 (5.7 to 6.4) | 0 (−0.3 to 0.4) | 8.0 (7.6 to 8.4) | 0.2 (−0.2 to 0.6) |
| Any inpatient use of nonopioid analgesic | ||||
| None | 6.0 (5.8 to 6.2) | Reference | 7.8 (7.6 to 8.1) | Reference |
| ≥1 time during stay | 6.3 (6.0 to 6.7) | 0.3 (0.1 to 0.7) | 8.2 (7.8 to 8.5) | 0.4 (0.0 to 0.7) |
ED = emergency department; HCUP CCS = Healthcare Cost and Utilization Project Clinical Classifications Software; ICU = intensive care unit; PACU = postanesthesia care unit.
Includes 92 433 stays with inpatient opioid use that met all other inclusion criteria. The table shows predicted margins obtained from 2 multinomial logistic regression models (full results shown in Supplement Tables 4 and 5 [available at Annals.org]) that included indicators of various inpatient opioid use patterns and adjusted for the following covariates: age, sex, race, year of admission, payment source for hospital stay (e.g., Medicare or Medicaid), Elixhauser Comorbidity Index score, admission type (medical with no ICU stay, medical with ICU stay, surgical with no ICU stay, or surgical with ICU stay), length of stay, hospital fixed effects, HCUP CCS comorbid conditions, and history of benzodiazepine use. Outpatient opioid use at 90 and 365 d after discharge was the key outcome of interest, and death and readmission (both measured ≤90 d after discharge) were treated as competing risks and thus as separate levels of the outcome. Robust SEs were used to account for within-patient correlation.
Sensitivity Analyses
To explore the role of potential unmeasured confounders (such as inpatient opioid dosage, history of substance use disorder, or pain severity), we calculated E-values. The observed RRRs for differences in outpatient opioid use at 90 days could be explained by an unmeasured confounder that was associated with the likelihood of any inpatient opioid administration and the risk for outpatient use by a risk ratio of 3.6 or higher for each, beyond measured confounders. The risk ratio of an unmeasured confounder would need to be 3.1 for the 365-day outcome. These E-values are substantially higher than the largest observed confounder for either the 90- or 365-day outcome (risk ratio, 2.20 [CI, 2.00 to2.44]). For analyses of patterns of inpatient opioid use, E-values were 3.5 (time from last opioid administration to discharge) and 1.8 (proportion of stay with opioid use). Our other sensitivity analyses also showed that results were generally robust to inclusion of certain conditions (such as trauma and cancer) and exclusion of 1 hospital that largely served younger women. Examination of effect modification by hospital indicated that the direction of the observed effects was identical to the main analyses. Results of sensitivity analyses are presented in Appendix Tables 2 and 3 and Supplement Tables 6 to 10 (available at Annals.org).
Discussion
Our study has 4 key findings. First, nearly half of opioid-naive patients were given opioids in the hospital. Opioid use was common even for medical admissions without an ICU stay, and opioids were rarely preceded by nonopioid analgesics. Second, any receipt of an opioid in the hospital was associated with roughly twice the probability of outpatient use after discharge. Third, a large percentage of inpatients who received opioids received them for most of their stay, and patients were frequently administered opioids within 12 hours of discharge. Fourth, patterns related to timing and duration of inpatient opioid administration were independently associated with increased probability of outpatient use up to 1 year after discharge.
Studies have shown that a nontrivial minority of surgical patients who fill opioid prescriptions immediately after discharge experience long-term use, misuse, or addiction (11–19). Most of these studies analyzed claims data, which provide detailed information on out-patient use but cannot describe opioid administration during inpatient stays. Our study describes the hitherto unexplored “black box” of inpatient opioid use in a large sample of inpatients from community and academic hospitals. Although we could not assess the appropriateness of opioid administration, we detected several areas that may represent opportunities to modify risk for subsequent use.
For example, most opioid-naive patients who were given opioids in the hospital received them before nonopioid analgesics. Although some patients may have contraindications to nonsteroidal anti-inflammatory drugs, such as a history of peptic ulcer disease or renal impairment, the prevalence of these conditions does not explain the apparent preference for opioids over nonopioid alternatives. The overall duration of opioid use, especially use that continued into the final hours of the hospital stay, was also not explained by case mix yet increased the probability of postdischarge use. Efforts to restrict these inpatient prescribing practices thus seem worthy of evaluation.
Because our observational study could not determine causality, interventional trials are needed to determine whether altering inpatient opioid prescribing will limit opioid-related morbidity and mortality. Multi-pronged strategies that educate hospital-based clinicians together with standardized protocols that guide pain assessment and treatment hold promise (25, 26). Implementation of programs to enhance recovery after surgery, which promote opioid-free and multimodal analgesia, has decreased length of hospital stay and complication rates and may decrease long-term postoperative opioid use (27–29).
Our findings should be viewed in light of several limitations. First, our data were obtained from a single health system, although the system is large and includes both community and academic hospitals. Second, our observational study may be subject to confounding by unobservable factors, such as history of substance use disorder, pain severity, or opioid dosage. We conducted a sensitivity analysis to determine how much unmeasured confounding would be needed to explain the observed RRRs between inpatient and outpatient opioid use. The magnitude of the E-values relative to the confounders we were able to adjust for suggests that our findings on the association between any inpatient opioid use and timing of opioid administration relative to discharge are robust. However, the E-value for the RRR comparing the differences in the proportion of the stay with opioids suggests that this estimate may be more susceptible to unobserved confounding. Third, our use of only a 12-month lookback may have resulted in some patients being misclassified as opioid-naive. Given the high error rates in medication reconciliation systems (30, 31) and the relatively poor agreement we found between inpatient and outpatient medication lists, we required that both medication lists in both databases indicate no opioid use when identifying opioid-naive patients. This conservative approach may limit generalizability to patients with no outpatient records. Fourth, we did not measure the appropriateness of opioid use in our sample, which included some patients who may have received these medications legitimately (for example, those with severe trauma or cancer). Fifth, by relying on medication use recorded in outpatient records, we could have missed patients who exclusively receive opioids illegally or from non-UPMC providers. We also could not determine whether patients filled their prescriptions; thus, our findings may overestimate opioid prescribing.
This 5-year observational study sheds light on patterns of inpatient opioid administration to opioid-naive patients. Receipt of any opioids in the hospital was associated with nearly twice the probability of continued outpatient use. Our findings also highlight specific patterns of inpatient opioid prescribing that are associated with continued outpatient use and are likely amenable to health system interventions.
Supplementary Material
Financial Support:
By the UPMC Health System and the University of Pittsburgh.
Disclosures: Dr. Seymour reports a grant from the National Institutes of Health during the conduct of the study. Authors not named here have disclosed no conflicts of interest. Disclosures can also be viewed at www.acponline.org/authors/icmjeConflictOfInterestForms.do?msNum=M18-2864.
Appendix
Appendix Figure.
Study flow diagram.
Appendix Table 1.
Types
| Opioids |
| Alfentanil |
| Butorphanol |
| Codeine |
| Dihydrocodeine |
| Fentanyl |
| Hydrocodone |
| Hydromorphone |
| Levorphanol |
| Meperidine |
| Methadone |
| Morphine |
| Nalbuphine |
| Oxycodone |
| Oxymorphone |
| Pentazocine |
| Remifentanil |
| Sufentanil |
| Tapentadol |
| Tramadol |
| Nonopioid analgesics |
| Celecoxib |
| Ibuprofen |
| Naproxen |
| Ketorolac |
| Acetaminophen |
Appendix Table 2.
Sensitivity Analysis of Association Between Any Inpatient Opioid Use and Outpatient Opioid Use, Death, and Readmission at 90 and 365 Days After Discharge, With Hospital 1 Excluded
| Outcome | No Inpatient Opioid Use | Inpatient Opioid Use | Difference (95% CI), percentage points |
|---|---|---|---|
| 90 d | |||
| Outpatient Opioid Use | 3.3 (3.1 to 3.4) | 6.3 (6.1 to 6.5) | 3.0 (2.8 to 3.2) |
| No outpatient opioid use/death/readmission | 70.9 (70.6 to 71.3) | 68.8 (68.4 to 69.2) | −2.1 (−2.5 to −1.7) |
| Death | 0.4 (0.4 to 0.5) | 0.5 (0.5 to 0.6) | 0.1 (0.1 to 0.2) |
| Readmission | 25.4 (25.0 to 25.7) | 24.4 (24.1 to 24.8) | −1.0 (−1.4 to −0.6) |
| 365 d | |||
| Outpatient opioid use | 4.7 (4.5 to 4.8) | 8.0 (7.8 to 8.3) | 3.4 (3.1 to 3.6) |
| No outpatient opioid use/death/readmission | 48.8 (48.4 to 49.2) | 48.1 (47.6 to 48.5) | −0.7 (−1.2 to −0.3) |
| Death | 1.3 (1.2 to 1.4) | 1.2 (1.1 to 1.3) | −0.1 (−0.2 to 0.0) |
| Readmission | 45.2 (44.8 to 45.6) | 42.7 (42.2 to 43.1) | −2.5 (−3.0 to −2.1) |
Appendix Table 3.
Sensitivity Analysis of Association Between Patterns of Inpatient Opioid Administration and Outpatient Opioid Use at 90 and 365 Days After Discharge Among Inpatients Receiving Opioids, With Hospital 1 Excluded
| Variable | ≤90 Days After Discharge | ≤365 Days After Discharge | ||
|---|---|---|---|---|
| Outpatient Opioid Use (95% CI), % | Difference (95% CI), percentage points | Outpatient Opioid Use (95% CI), % | Difference (95% CI), percentage points | |
| Proportion of stay with opioids administered | ||||
| 1%–25% | 6.0 (5.3 to 6.7) | Reference | 7.1 (6.4 to 7.8) | Reference |
| 26%–50% | 6.0 (5.6 to 6.4) | 0.0 (−0.7 to 0.7) | 7.9 (7.4 to 8.3) | 0.8 (0.1 to 1.5) |
| 51%–75% | 7.3 (7.0 to 7.7) | 1.3 (0.6 to 2.0) | 9.5 (9.0 to 9.9) | 2.4 (1.7 to 3.1) |
| 76%–100% | 7.2 (6.7 to 7.7) | 1.2 (0.5 to 1.9) | 9.3 (8.7 to 9.8) | 2.2 (1.5 to 2.9) |
| Time from last opioid administration to discharge | ||||
| >24 h | 4.6 (4.2 to 4.9) | Reference | 6.3 (5.9 to 6.7) | Reference |
| 13–24 h | 6.5 (5.9 to 7.0) | 1.9 (1.3 to 2.4) | 8.0 (7.4 to 8.6) | 1.7 (1.1 to 2.3) |
| 0–12 h | 8.5(8.1 to 8.8) | 3.9 (3.5 to 4.2) | 10.5 (10.0 to 10.9) | 4.2 (3.7 to 4.6) |
| Setting of first opioid administration | ||||
| Stepdown unit/ward | 6.7 (6.3 to 7.0) | Reference | 8.5(8.1 to 8.9) | Reference |
| ED | 6.6 (6.1 to 7.1) | −0.1 (−0.6 to 0.4) | 8.4 (7.8 to 8.9) | −0.1 (−0.7 to 0.4) |
| ICU | 7.1 (6.3 to 8.0) | 0.4 (−0.4 to 1.3) | 8.9 (7.9 to 9.8) | 0.4 (−0.6 to 1.3) |
| PACU/procedure unit | 6.7 (6.2 to 7.2) | 0.0 (−0.5 to 0.5) | 8.7 (8.1 to 9.3) | 0.2 (−0.4 to 0.8) |
| Any inpatient use of nonopioid analgesic | ||||
| None | 6.6 (6.4 to 6.9) | Reference | 8.5 (8.2 to 8.8) | Reference |
| ≥1 time during stay | 7.0 (6.6 to 7.4) | 0.4 (0.0 to 0.8) | 8.9 (8.4 to 9.3) | 0.4 (−0.1 to 0.8) |
ED = emergency department; ICU = intensive care unit; PACU = postanesthesia care unit.
Footnotes
Current author addresses and author contributions are available at Annals.org.
Reproducible Research Statement: Study protocol and data set: Not available. Statistical code: Available from Dr. Donohue (jdonohue@pitt.edu).
References
- 1.The White House. Council of Economic Advisers Report: The Underestimated Cost of the Opioid Crisis. 20 November 2017. Accessed at www.whitehouse.gov/briefings-statements/cea-report-underestimated-cost-opioid-crisis on 26 April 2018.
- 2.Krueger AB. Where have all the workers gone? An inquiry into the decline of the U.S. labor force participation rate. Brookings Papers on Economic Activity. 7 September 2017. Accessed at www.brookings.edu/bpea-articles/where-have-all-the-workers-gone-an-inquiry-into-the-decline-of-the-u-s-labor-force-participation-rate on 10 May 2019. [DOI] [PMC free article] [PubMed]
- 3.Hedegaard H, Warner M, Miniño AM. Drug Overdose Deaths in the United States, 1999–2016. NCHS Data Brief no. 294. December 2017. Accessed at www.cdc.gov/nchs/data/databriefs/db294.pdf on 10 May 2019. [PubMed]
- 4.Prescription Drug Abuse Policy System Web site. Accessed at www.pdaps.org on 10 May 2019.
- 5.Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain - United States, 2016. MMWR Recomm Rep. 2016;65:1–49. doi: 10.15585/mmwr.rr6501e1 [DOI] [PubMed] [Google Scholar]
- 6.Bohnert ASB, Guy GP Jr, Losby JL. Opioid prescribing in the United States before and after the Centers for Disease Control and Prevention’s 2016 opioid guideline. Ann Intern Med. 2018;169:367–75. doi: 10.7326/M18-1243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pharmacy Quality Alliance. PQA Opioid Core Measure Set – 2019. Springfield, VA: Pharmacy Quality Alliance; 2016. Accessed at www.pqaalliance.org/index.php?option=com_content&view=article&id=105:opioid-core-measure-set&catid=32:measures on 10 May 2019. [Google Scholar]
- 8.Pharmacy Quality Alliance. PQA Receives NQF Endorsement of Three Performance Measures to Address Opioid Misuse/Abuse [press release]. Alexandria, VA: Pharmacy Quality Alliance; 2017. Accessed at https://myemail.constantcontact.com/Press-Release—PQA-Receives-NQF-Endorsement-of-Three-Performance-Measures-to-Address-Opioid-Misuse-Abuse.html?soid=1108959632030&aid=tfl6y6ucOGo on 13 May 2019. [Google Scholar]
- 9.Schuchat A, Houry D, Guy GP Jr. New data on opioid use and prescribing in the United States. JAMA. 2017;318:425–6. doi: 10.1001/jama.2017.8913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baker DW. History of the Joint Commission’s pain standards: lessons for today’s prescription opioid epidemic. JAMA. 2017;317: 1117–8. doi: 10.1001/jama.2017.0935 [DOI] [PubMed] [Google Scholar]
- 11.Brat GA, Agniel D, Beam A, et al. Postsurgical prescriptions for opioid naive patients and association with overdose and misuse: retrospective cohort study. BMJ. 2018;360:j5790. doi: 10.1136/bmj.j5790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wunsch H, Wijeysundera DN, Passarella MA, et al. Opioids prescribed after low-risk surgical procedures in the United States, 2004–2012. JAMA. 2016;315:1654–7. doi: 10.1001/jama.2016.0130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kulshrestha S, Barrantes F, Samaniego M, et al. Chronic opioid analgesic usage post-kidney transplantation and clinical outcomes. Clin Transplant. 2014;28:1041–6. doi: 10.1111/ctr.12414 [DOI] [PubMed] [Google Scholar]
- 14.Johnson SP, Chung KC, Zhong L, et al. Risk of prolonged opioid use among opioid-naïve patients following common hand surgery procedures. J Hand Surg Am. 2016;41:947–957.e3. doi: 10.1016/j.jhsa.2016.07.113 [DOI] [PubMed] [Google Scholar]
- 15.Raebel MA, Newcomer SR, Bayliss EA, et al. Chronic opioid use emerging after bariatric surgery. Pharmacoepidemiol Drug Saf. 2014;23:1247–57. doi: 10.1002/pds.3625 [DOI] [PubMed] [Google Scholar]
- 16.Lee JS, Hu HM, Edelman AL, et al. New persistent opioid use among patients with cancer after curative-intent surgery. J Clin Oncol. 2017;35:4042–9. doi: 10.1200/JCO.2017.74.1363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goesling J, Moser SE, Zaidi B, et al. Trends and predictors of opioid use after total knee and total hip arthroplasty. Pain. 2016;157: 1259–65. doi: 10.1097/j.pain.0000000000000516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Calcaterra SL, Yamashita TE, Min SJ, et al. Opioid prescribing at hospital discharge contributes to chronic opioid use. J Gen Intern Med. 2016;31:478–85. doi: 10.1007/s11606-015-3539-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brummett CM, Waljee JF, Goesling J, et al. New persistent opioid use after minor and major surgical procedures in US adults. JAMA Surg. 2017;152:e170504. doi: 10.1001/jamasurg.2017.0504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jena AB, Goldman D, Karaca-Mandic P. Hospital prescribing of opioids to Medicare beneficiaries. JAMA Intern Med. 2016;176: 990–7. doi: 10.1001/jamainternmed.2016.2737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Herzig SJ, Rothberg MB, Cheung M, et al. Opioid utilization and opioid-related adverse events in nonsurgical patients in US hospitals. J Hosp Med. 2014;9:73–81. doi: 10.1002/jhm.2102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Agency for Healthcare Research and Quality. Clinical Classifications Software (CCS) for ICD-9-CM. Rockville, MD: Agency for Healthcare Research and Quality; 2017. Accessed at www.hcup-us.ahrq.gov/toolssoftware/ccs/ccs.jsp on 13 May 2019. [Google Scholar]
- 23.Sullivan MD, Edlund MJ, Fan MY, et al. Trends in use of opioids for non-cancer pain conditions 2000–2005 in commercial and Medicaid insurance plans: the TROUP study. Pain. 2008;138:440–9. doi: 10.1016/j.pain.2008.04.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.VanderWeele TJ, Ding P. Sensitivity analysis in observational research: introducing the E-value. Ann Intern Med. 2017;167:268–74. doi: 10.7326/M16-2607 [DOI] [PubMed] [Google Scholar]
- 25.Bachhuber MA, Nash D, Southern WN, et al. Reducing the default dispense quantity for new opioid analgesic prescriptions: study protocol for a cluster randomised controlled trial. BMJ Open. 2018; 8:e019559. doi: 10.1136/bmjopen-2017-019559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Delgado MK, Shofer FS, Patel MS, et al. Association between electronic medical record implementation of default opioid prescription quantities and prescribing behavior in two emergency departments [Letter]. J Gen Intern Med. 2018;33:409–11. doi: 10.1007/s11606-017-4286-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu VX, Rosas E, Hwang J, et al. Enhanced recovery after surgery program implementation in 2 surgical populations in an integrated health care delivery system. JAMA Surg. 2017;152:e171032. doi: 10.1001/jamasurg.2017.1032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ljungqvist O, Scott M, Fearon KC. Enhanced recovery after surgery: a review. JAMA Surg. 2017;152:292–8. doi: 10.1001/jamasurg.2016.4952 [DOI] [PubMed] [Google Scholar]
- 29.Brandal D, Keller MS, Lee C, et al. Impact of enhanced recovery after surgery and opioid-free anesthesia on opioid prescriptions at discharge from the hospital: a historical-prospective study. Anesth Analg. 2017;125:1784–92. doi: 10.1213/ANE.0000000000002510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tam VC, Knowles SR, Cornish PL, et al. Frequency, type and clinical importance of medication history errors at admission to hospital: a systematic review. CMAJ. 2005;173:510–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Phansalkar S, Her QL, Tucker AD, et al. Impact of incorporating pharmacy claims data into electronic medication reconciliation. Am J Health Syst Pharm. 2015;72:212–7. doi: 10.2146/ajhp140082 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.