Abstract
Background
Advanced maternal age, family history of diabetes, pre-gestational obesity, increased level of HbA1c, history of gestational diabetes mellitus (GDM), and poor pregnancy consequences are considered risk factors for antenatal insulin requirement in women with GDM. However, the role of assisted reproductive technology (ART) in increasing the risk of insulin therapy in pregnancies complicated with GDM remained elusive. The current study aimed to determine the role of ART in predicting insulin therapy in GDM women and investigate the clinical and biochemical factors predicting the need for insulin therapy in pregnancies complicated with GDM.
Methods
In this prospective cohort study, 236 Iranian women with GDM were diagnosed by one-step oral glucose tolerance test (OGTT) between October 2014 and June 2017. They were mainly assigned to two groups; the first group (n = 100) was designated as ART which was further subdivided into two subgroups as follows: 60 participants who received medical nutrition therapy (MNT) and 40 participants who received MNT plus insulin therapy (MNT-IT). The second group (n = 136) was labeled as the spontaneous conception (SC), consisting of 102 participants receiving MNT and 34 participants receiving MNT in combination with IT (MNT-IT). The demographic, clinical, and biochemical data were compared between groups. Multivariate logistic regression was performed to estimate prognostic factors for insulin therapy.
Results
A higher rate of insulin therapy was observed in the ART group as compared with the SC group (40% vs. 25%; P < 0.001). Multivariate logistic regression demonstrated that maternal age ≥ 35 years [OR: 2.91, 95% CI: (1.28–6.62)], high serum FBS [1.10: (1.04–1.16)], HbA1c [1.91 (1.09–3.34)], and ART treatment [2.94: (1.24–6.96)] were independent risk factors for insulin therapy in GDM women.
Conclusions
Apart from risk factors mentioned earlier, ART may be a possible prognostic factor for insulin therapy in pregnancies complicated with GDM.
Keywords: Gestational diabetes mellitus, Insulin, Prognostic factors, Assisted reproductive technology, Pregnancy
Introduction
Assisted reproductive technology (ART) is increasingly being practiced worldwide. Besides, gestational diabetes mellitus (GDM) has become more prevalent in obese women conceived via ART treatments [1]. Several lines of evidence demonstrate that both ART and GDM are associated with adverse pregnancy outcomes as compared to those with natural conception [2, 3]. Current reports indicate that a history of infertility can increase the risk of GDM independent of the known risk factors [4, 5]. Furthermore, a higher prevalence of GDM in women who became pregnant by ART was previously reported [5]. Therefore, GDM, as a marked co-morbidity, should be early diagnosed and managed appropriately.
As the pregnancy proceeds, insulin resistance is gradually increased, especially in the third trimester of the gestational period [6]. Obesity and insulin-resistance reduce the functionality of β-cells and induce inflammation thought to play key roles in the development of GDM [7]. In this circumstance, early management of GDM which is of immense importance includes medical nutrition therapy (MNT), self-blood glucose monitoring, physical activity, and regular consumption of medications to control hyperglycemia [8]. Approximately 20–60% of GDM women need pharmacological treatments to control their blood sugar [9]. Insulin is considered a safe and effective medication for women with GDM who failed to respond to medical nutrition therapy [8]. Adequate and accurate control of blood glucose can attenuate adverse maternal and perinatal outcomes [10]. However, factors predicting antenatal insulin requirement in women with GDM have not been fully understood. Some risk factors, such as advanced maternal age, family history of diabetes, pre-gestational obesity, high oral glucose tolerance test (OGTT) values, history of GDM or poor pregnancy consequences, and polycystic ovary syndrome (PCOS) have been previously addressed in the literature [11, 12].
However, the role of ART in increasing the risk of insulin therapy in women with GDM remained opaque. Hence, this study was designed to explore the role of ART in predicting insulin therapy in women with GDM. Moreover, we examined the predictive potential of clinical and biochemical parameters for insulin therapy in the management of women with GDM.
Participants and methods
The present research was a prospective cohort study that included 236 Iranian singleton pregnant women (aged 20–40 years) with GDM who recruited between October 2014 and June 2017. All participants consisted of ART and spontaneous conception (SC) groups enrolled by simple sampling following GDM diagnosis. Medical records of Iranian GDM pregnant women were registered. The ART group included singleton pregnancies conceived following in-vitro fertilization /intra-cytoplasmic sperm injection (IVF/ICSI) or ICSI cycles that referred to the Department of Endocrinology and Female Infertility of the Royan Institute, Tehran, Iran. The protocol of infertility treatment in our institute was in agreement with the standard international guidelines.
The pregnant women with no history of infertility and/or infertility treatments were designated the SC group who referred to the Obstetrics and Gynecology Clinic of Arash Women’s Hospital which affiliated with Tehran University of Medical Science. Written informed consent was taken from all participants before the enrollment.
The diagnosis of GDM was made by a one-step OGTT at 24 and 28 weeks of gestation. The American Diabetes Association/International Association of the Diabetes and Pregnancy Study Groups (ADA/IAPDSG) criteria were considered to diagnose GDM [8]. The exclusion criteria were: (1) any systemic disorders, (2) pregravid diabetes or glucose intolerance, (3) previous insulin treatment, (4) consuming oral glucose-lowering drugs (metformin and glibenclamide), (5) vanishing embryos or selective fetal reduction, (6) history of polycystic ovarian syndrome (PCOS), and (7) twin pregnancies. The Institutional Review Board and Ethics committee of the Iran University of Medical Sciences and Royan institute approved the present study.
Clinical and demographic data were obtained from medical records and face-to-face interviews. In these two centers, the weight (without shoes with the least clothes) of women was measured by Seca scale, and the height was determined by a stadiometer. Pre-pregnancy body mass index (BMI) [pre-pregnancy weight (kg)/height (m) 2] was calculated according to the standard formula. Systolic and diastolic blood pressure was assessed by trained nurses with a mercury sphygmomanometer at 24–28 weeks of gestation. The mean systolic and diastolic blood pressure was recorded in duplicate. Venous blood samples were collected at 24 and 28 weeks of gestation for the determination of fasting blood sugar (FBS), hemoglobin A1c (HbA1c), insulin, a high-sensitivity C-reactive protein (hs-CRP), and interleukin-17 (IL-17), as well as the lipid profile, i.e., cholesterol, triglycerides (TG), high density lipoprotein (HDL), low density lipoprotein (LDL), and very-low-density lipoproteins (VLDL), after 8–12 h’ fast. The Homeostasis Model Assessment of insulin resistance (HOMA-IR) index was also calculated.
All GDM women referred to an endocrinologist and a dietician for blood sugar management, Medical nutrition therapy (MNT), and nutrition plan, and consultation. Medical nutrition therapy (MNT) was defined as the management of GDM with optimal diet (energy content, macronutrient distribution, its quality and amount) to achieve sufficient mother’s weight gain and fetal growth, as well as maintaining near-normoglycemia and avoiding the development of ketone bodies and hypoglycemia. The participants were asked to take three main meals with three snacks per day and perform self-monitoring of blood glucose (SMBG). After 2 weeks of MNT, all participants were visited again by an endocrinologist. Fasting and postprandial sugar (2 h after breakfast, lunch, and dinner) were evaluated. If FBS was lower than 95 mg/dl, the 1-h postprandial blood sugar level was < 140 mg/dl, and 2-h postprandial blood sugar level was < 120 mg/dl, MNT alone continued. Insulin therapy (IT) was initiated by an endocrinologist when medical nutrition therapy failed, and fasting/postprandial blood glucose levels were above the target at any time during pregnancy. Subcutaneous injections of the rapid-acting and/or long-acting insulin were prescribed according to the blood glucose patterns.
The ovarian stimulation protocols and follow-up process after standard IVF/ICSI procedures were described previously in details [13]. ART drugs, the protocol of controlled ovarian stimulation (COS) using standard GnRH agonists or antagonists, as well as the modes of ART (fresh or frozen embryo transfer cycles) were obtained from the medical registry of infertile women. Ovarian hyper-stimulation syndrome (OHSS) is characterized by the increased level of serum estradiol (> 4000 pg/ml) along with a large number (> 20 per ovary) of follicles on the day of human chorionic gonadotropin (hCG) administration. Infertile women who were at higher risk of developing OHSS, frozen embryo transfer was performed by the vitrification method.
Data related to the ART procedures, including menarche age, infertility duration, irregular menstrual cycle, infertility type (secondary vs. primary), history of ovarian hyperstimulation syndrome (OHSS) risk, ovarian stimulation protocol (standard GnRH agonists vs. GnRH antagonists), and the method of ART [fresh embryo transfer (fresh ET) vs. frozen ET)] were obtained from women receiving infertility treatments.
Other variables that were considered in the final analysis were as follows; maternal age, BMI, history of having a first-degree relative with diabetes, prior history of spontaneous abortion, and macrosomic baby, history of GDM, increased OGTT values (GTT-FBS and GTT-2 h), increased levels of HbA1c, mode of conception, and GDM treatment modalities (MNT /MNT- IT).
Statistical analysis
In the current study, continuous variables were presented as the means and standard error of the mean (mean ± SEM) and categorical variables were expressed as the percentage. The chi-square test and independent T-tests were applied to compare variables between the two groups as indicated. The univariate logistic regression analysis was carried out to compare the characteristics of participants receiving either MNT-IT or MNT and select the variables for entering in the multivariate model, as well as determining significant predictive factors for insulin requirement in the study population. All statistical analyses were two-sided, and the p-value of < 0.05 was considered statistically significant. The analysis of the obtained values was performed by the Stata software version 12.
Based on previous studies [14–16], a sample size of 236 GDM women would be necessary to obtain a power of 80% with a significance level α = 5% to detect a relationship between the type of conception and the need for insulin therapy.
Results
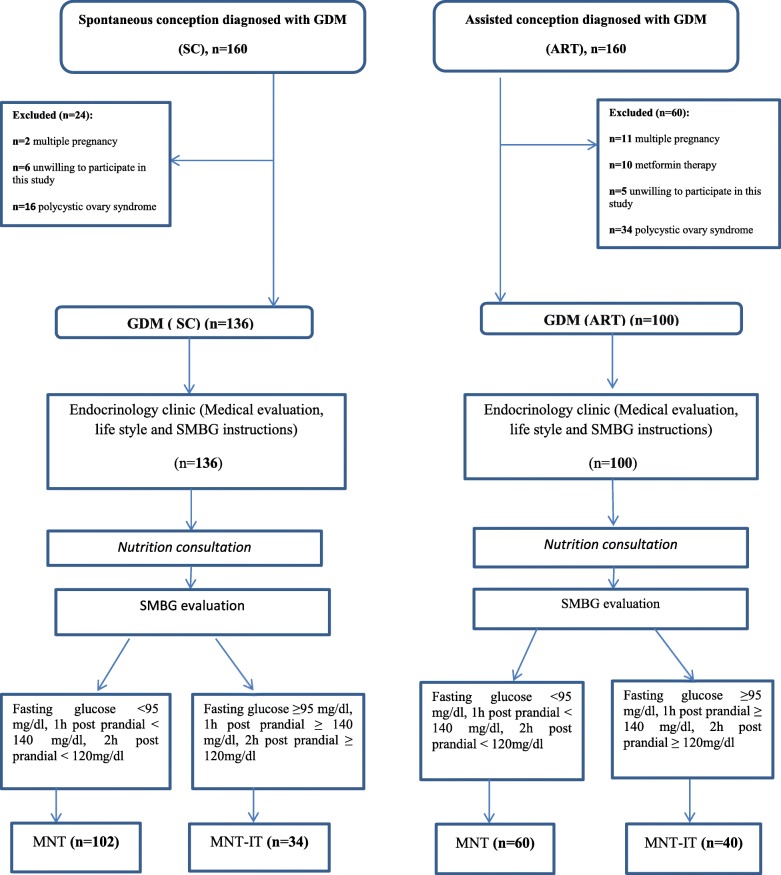
In this study, 100 GDM women conceived via ART and 136 GDM women conceived via spontaneous conception were included. All participants were stratified based on the treatment modalities receiving during pregnancy [i.e., medical nutrition therapy (MNT) or medical nutrition therapy plus insulin therapy (MNT-IT)]. In the ART group, 60 subjects were in the MNT sub-group and 40 subjects in the MNT-IT subgroup. In the SC group, 102 subjects were assigned to the MNT sub-group and 34 subjects in the MNT-IT subgroup. Figure 1 shows a flow diagram of the categorization of participants. The results showed a higher rate of participants in the ART group who required insulin treatment compared with individuals in the SC group [40 (40%) vs. 34 (25%), respectively; P < 0.001].
Fig. 1.
The Flow chart of the study population
The clinical and biochemical characteristics of women in both groups are summarized in Table 1. Based on our results, there was no significant difference between SC and ART groups in terms of the mean maternal age, systolic and diastolic blood pressure, and the number of individuals with a family history of diabetes, prior history of spontaneous abortion, and macrocosmic baby. However, there was a significant difference between the two groups concerning the parity, pre-pregnancy BMI, and history of GDM. Besides, most biochemical characteristics were not significantly different except for serum FBS and hs-CRP levels.
Table 1.
Comparison of clinical and biochemical characteristics between spontaneous conception and ART participants
| Variables | SC (n = 136) | ART (n = 100) | †P-value |
|---|---|---|---|
| Clinical | |||
| Maternal age (Years, Mean ± SE) | 31.57 ± 0.46 | 32.36 ± 0.52 | 0.261 |
| Parity (n = 0), n (%) | 56 (41.2) | 88 (87.1) | 0.001* |
| Family history of DM, (Yes, n %) | 52 (38.2) | 42 (41.6) | 0.602 |
| Systolic blood pressure (Mean ± SE) | 107.07 ± 0.87 | 106.68 ± 0.99 | 0.769 |
| Diastolic blood pressure (Mean ± SE) | 68.59 ± 0.72 | 66.83 ± 0.76 | 0.098 |
| Pre-pregnancy BMI (Mean ± SE) | 25.89 ± 0.42 | 27.32 ± 0.40 | 0.018* |
| Prior history of spontaneous abortion, (Yes), n (%) | 36 (29.1) | 32 (31.7) | 0.670 |
| Prior history of GDM, (Yes), n (%) | 18 (13.2) | 1 (0.9) | 0.001* |
| Prior history of macrosomia, (Yes), n (%) | 6 (4.4) | 1 (0.9) | 0.124 |
| Biochemical | |||
| GTT-FBS (mg/dl)(Mean ± SE) | 93.38 ± 0.87 | 93.13 ± 0.96 | 0.851 |
| GTT-1 h(mg/dl)(Mean ± SE) | 162.93 ± 4.11 | 158.22 ± 4.72 | 0.455 |
| GTT-2 h(mg/dl)(Mean ± SE) | 134.29 ± 3.71 | 133.26 ± 3.67 | 0.847 |
| FBS (mg/dl)(Mean ± SE) | 84.21 ± 1.05 | 88.23 ± 0.95 | 0.006* |
| HbA1c (%) (Mean ± SE) | 4.98 ± 0.10 | 5.07 ± 0.63 | 0.297 |
| TG (mg/dl)(Mean ± SE) | 200.61 ± 6.13 | 193.42 ± 5.62 | 0.254 |
| Cholesterol (mg/dl)(Mean ± SE) | 219.91 ± 3.52 | 210.86 ± 4.43 | 0.107 |
| HDL (mg/dl)(Mean ± SE) | 64.17 ± 1.34 | 63.49 ± 1.26 | 0.715 |
| LDL (mg/dl)(Mean ± SE) | 116.13 ± 3.01 | 110.31 ± 4.01 | 0.238 |
| VLDL (mg/dl)(Mean ± SE) | 40.05 ± 1.24 | 37.19 ± 1.28 | 0.115 |
| Hs-CRP (Mean ± SE) | 4.69 ± 0.40 | 7.21 ± 0.83 | 0.005* |
| IL-17 (Mean ± SE) | 1.19 ± 0.26 | 2.66 ± 0.95 | 0.161 |
| Insulin (Mean ± SD) | 13.57 ± 140 | 13.98 ± 0.96 | 0.821 |
| HOMA-IR | 2.90 ± 0.34 | 3.09 ± 0.23 | 0.672 |
SC spontaneous conception, ART Assisted reproductive technology, IL-17 Interleukin- 17, Hs-CRP High-sensitivity C - reactive protein, The Homeostasis Model Assessment of insulin resistance (HOMA-IR) index = [Glucose] * [Insulin] / 405 (Glucose in mg/dl)
*P < 0.05 was considered statistically significant
† T-test compared the mean difference between the SC group and ART group
The clinical characteristics between the two groups were stratified based on treatment modalities that are displayed in Table 2. Higher rates of maternal age ≥ 35 years, multiparty, pre-pregnancy BMI ≥25 (kg/m2) and prior history of GDM were observed in the MNT-IT subgroup of the SC group; however, such increments were not statistically significant when compared with the MNT subgroup of the ART group. In the ART group, the MNT-IT subgroup had a higher rate of family history of diabetes compared with the MNT subgroup.
Table 2.
Comparison of clinical parameters between parturients with spontaneous and ART conception stratified based on treatment modalities
| Variables | SC (n = 136) | ART (n = 100) | OR3 (CI 5%) c | P-value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| MNT (n = 102) | MNI-IT (n = 34) | OR1 (CI 95%) a | P-value | MNT (n = 60) | MNT-IT (n = 40) | OR2 (CI 95%) b | P-value | |||
| Maternal age (Years, Mean ± SE) | 30.64 ± 0.53 | 34.38 ± 0.73 | 1.17 (1.07–1.28) | 0.001* | 32.05 ± 0.77 | 32.65 ± 0.62 | 1.02 (0.95–1.10) | 0.576 | 1.94 (1.10–3.41) | 0.023* |
| Maternal age ≥ 35 years,n (%) | 26 (25.5) | 16 (47.0) | 2.6 (1.16–5.82) | 0.018* | 19 (31.7) | 15 (37.5) | 1.29 (0.55–2.99) | 0.546 | 1.87 (1.05–3.33) | 0.032* |
| Parity (n = 0), n (%) | 46 (45.1) | 10 (29.4) | 11.97 (0.86–4.54) | 0.108 | 50 (83.3) | 37 (92.5) | 0.41 (0.10–1.58) | 0.182 | 0.84 (0.47–1.47) | 0.535 |
| Family history of DM (Yes, n %) | 35 (34.3) | 17 (50.0) | 1.91 (0.87–4.20) | 0.103 | 20 (33.3) | 22 (55.0) | 2.44 (1.07–5.56) | 0.033* | 2.17(1.24–3.79) | 0.007* |
| Systolic blood pressure (Mean ± SE) | 106.37 ± 1.01 | 109.11 ± 0.72 | 1.03 (0.99–1.07) | 0.176 | 107.25 ± 1.17 | 106.0 ± 1.82 | 0.99 (0.95–1.03) | 0.542 | 1.01 (0.98–1.03) | 0.424 |
| Diastolic blood pressure (Mean ± SE) | 68.22 ± 0.83 | 69.71 ± 1.42 | 1.02 (0.97–1.07) | 0.367 | 66.83 ± 0.98 | 67.0 ± 1.25 | 1.00 (0.96–1.06) | 0.915 | 1.0 (0.97–1.04) | 0.574 |
| Pre-pregnancy BMI (Mean ± SE) | 25.44 ± 0.49 | 27.31 ± 0.81 | 1.08 (1.00–1.17) | 0.059 | 27.22 ± 0.46 | 27.52 ± 0.76 | 1.01 (0.95–1.12) | 0.726 | 1.94 (1.09–3.45) | 0.022* |
| Pre-pregnancy BMI ≥25 (kg/m2), n (%) | 49(49.1) | 23 (71.9) | 2.65 (1.12–6.31) | 0.024* | 43 (71.7) | 29 (72.5) | 1.04 (0.43–2.55) | 0.928 | 1.92 (1.05–3.51) | 0.034* |
| History of spontaneous abortion, n (%) | 26 (26.0) | 13 (38.2) | 1.76 (0.77–4.02) | 0.175 | 16 (26.7) | 16 (40) | 1.83 (0.78–4.30) | 0.161 | 1.90 (1.12–3.23) | 0.018* |
| History of GDM, (Yes, n %) | 8 (7.8) | 10 (29.4) | 4.90 (1.74–13.74) | 0.001* | 1 (1.7) | 0 (0) | – | 1 | 2.66 (1.03–6.84) | 0.043* |
| History of macrosomia, (Yes, n %) | 4 (3.9) | 2 (5.8) | 1.53 (0.27–8.76) | 0.630 | 1 (1.7) | 0 (0) | 0.87 (0.16–4.60) | 0.872 | 0.66 (0.13–3.35) | 0.619 |
CI Confidence Interval, GDM gestational diabetes mellitus, SC Spontaneous Conception, ART Assisted Reproductive Technology, MNT Medical Nutrition Therapy, MNT-IT Medical Nutrition Therapy plus Insulin Therapy
*P < 0.05 was considered statistically significant
a OR1; Fitting GDM treatment modality as the outcome of the univariate regression models. The reference group was the MNT subgroup compared to the MNT-IT in SC group
b OR2; Fitting GDM treatment modality as the outcome of the univariate regression models; The reference group was the MNT subgroup compared to the MNT-IT in ART group
c OR3; Fitting GDM treatment modality as the outcome of the univariate regression models; The reference group was the MNT subgroup compared to the MNT-IT in the total population
The results of the univariate logistic regression analysis are presented in Table 2 and show the risk factors of insulin therapy in the study population. The parameters associated with insulin requirement in total population were as follows; maternal age ≥ 35 years, family history of diabetes mellitus (DM), pre-pregnancy BMI, previous history of spontaneous abortion, and prior history of GDM. However, there were no significant differences between MNT and MNT-IT subgroups of the ART group in terms of ART parameters (P > 0.05) (Table 3). None of the infertility parameters was associated with insulin therapy in this group.
Table 3.
Infertility parameters of ART-conceived parturients stratified based on the treatment modalities
| Variables | MNT (n = 60) |
MNT-IT (n = 40) | P-value |
|---|---|---|---|
| Menarche age, years (Mean ± SE) | 13.1 ± 0.2 | 13.6 ± 0.3 | 0.532 |
| Infertility duration, years, (Mean ± SE) | 6.2 ± 0.5 | 7.5 ± 0.8 | 0.117 |
| Irregular menstrual cycle, n (%) | 7 (11.7) | 3 (7.5) | 0.496 |
| Infertility type (Secondary), n (%) | 25 (41.7) | 17 (42.5) | 0.934 |
| Prior OHSS risk, n (%) | 23 (38.3) | 17 (42.5) | 0.677 |
| COH protocol | |||
| Standard long GnRH agonist, n (%) | 44 (77.2) | 35 (87.5) | 0.199 |
| ART mode | |||
| Fresh ET, n (%) | 28 (51.9) | 23 (57.5) | 0.587 |
MNT Medical Nutrition Therapy, MNT-IT Medical Nutrition Therapy plus Insulin Therapy, Fresh ET Fresh embryo transfer
*P < 0.05 was considered statistically significant
The biochemical values of the SC and ART participants were stratified according to the treatment modalities that are depicted in Table 4. The mean concentrations of FBS and HbA1c were significantly higher in the MNT-IT subgroup of the SC group compared with the MNT subgroup. Similar findings were observed in the MNT-IT subgroup of the ART group when compared with MNT subgroup. The higher levels of one- and two-hour glucose after OGTT were observed in the MNT-IT subgroup of the SC group in comparison with the MNT subgroup. However, such values were not significantly different between the MNT and MNT-IT subgroups of the ART population (P > 0.05). The mean concentrations of TG and VLDL were significantly higher in the MNT-IT subgroup of the ART group as compared with the MNT subgroup. The mean levels of the inflammatory markers were higher in the MNT-IT subgroup compared with the MNT subgroup of the ART group. In the total population, higher levels of GTT-FBS, GTT-1 h, GTT-2 h, FBS, and HbA1c, as well as hs-CRP, were observed in the MNT-IT subgroup compared with the MNT subgroup of the ART group.
Table 4.
Comparison of biochemical parameters between parturients with spontaneous and ART conception stratified based on treatment modalities
| Variables | SC (n = 136) | ART (n = 100) | P-valuec | ||||
|---|---|---|---|---|---|---|---|
| MNT (n = 102) | MNI-IT (n = 34) | P-value a | MNT (n = 60) | MNT-IT (n = 40) | P-valueb | ||
| GTT-FBS (mg/dl)(Mean ± SE) | 91.69 ± 0.95 | 98.18 ± 1.66 | 0.001* | 90.67 ± 1.20 | 96.92 ± 1.42 | 0.001* | 0.002* |
| GTT-1 h (mg/dl)(Mean ± SE) | 156.10 ± 4.46 | 183.90 ± 8.66 | 0.003* | 159.14 ± 5.98 | 156.82 ± 7.98 | 0.813 | 0.014* |
| GTT-2 h (mg/dl)(Mean ± SE) | 129.38 ± 4.19 | 147.40 ± 7.34 | 0.003* | 131.0 ± 4.50 | 136.58 ± 6.39 | 0.464 | 0.046* |
| FBS (mg/dl) (Mean ± SE) | 82.89 ± 1.21 | 88.03 ± 1.77 | 0.032* | 85.98 ± 0.99 | 91.44 ± 1.77 | 0.004* | 0.030* |
| HbA1c (%) (Mean ± SE) | 4.92 ± 0.07 | 5.40 ± 0.12 | 0.005* | 4.85 ± 0.10 | 5.16 ± 0.08 | 0.022* | 0.004* |
| TG (mg/dl) (Mean ± SE) | 201.12 ± 6.83 | 199.11 ± 13.71 | 0.887 | 175.31 ± 8.16 | 204.97 ± 9.69 | 0.026* | 0.065 |
| Cholesterol (mg/dl) (Mean ± SE) | 221.33 ± 4.20 | 215.71 ± 6.36 | 0.491 | 202.09 ± 4.58 | 223.13 ± 7.97 | 0.019* | 0.066 |
| HDL (mg/dl) (Mean ± SE) | 64.61 ± 1.56 | 62.85 ± 2.60 | 0.573 | 65.20 ± 1.74 | 60.81 ± 1.65 | 0.097 | 0.083 |
| LDL (mg/dl) (Mean ± SE) | 117.30 ± 3.89 | 112.52 ± 6.54 | 0.496 | 101.83 ± 4.35 | 121.42 ± 6.93 | 0.014* | 0.105 |
| VLDL (mg/dl) (Mean ± SE) | 40.19 ± 1.36 | 39.59 ± 2.84 | 0.836 | 35.06 ± 1.66 | 40.90 ± 1.92 | 0.029* | 0.089 |
| hs-CRP (mg/l) (Mean ± SE) | 4.60 ± 0.41 | 5.06 ± 1.16 | 0.651 | 6.45 ± 0.75 | 8.69 ± 1.95 | 0.049* | 0.010* |
| IL-17(pg/mL) (Mean ± SE) | 1.21 ± 0.27 | 0.70 ± 0.60 | 0.660 | 0.91 ± 0.19 | 5.52 ± 2.35 | 0.017b | 0.061 |
| Insulin (mU/L) (Mean ± SE) | 12.90 ± 1.64 | 15.87 ± 2.58 | 0.375 | 13.26 ± 1.36 | 14.92 ± 1.29 | 0.411 | 0.191 |
| HOMA-IR | 2.76 ± 0.42 | 3.39 ± 0.49 | 0.443 | 2.86 ± 0.33 | 3.40 ± 0.31 | 0.255 | 0.202 |
GDM Gestational Diabetes Mellitus, SC Spontaneous Conception, ART Assisted Reproductive Technology, MNT Medical Nutrition Therapy, MNT-IT Medical Nutrition Therapy plus Insulin Therapy
The Homeostasis Model Assessment of insulin resistance (HOMA-IR) index = [Glucose] * [Insulin] / 405 (Glucose in mg/dl)
*P < 0.05 was considered significant
a T-test compared the mean difference between MNT and MNT-IT subgroups in the SC group
bT-test compared the mean difference between MNT and MNT-IT subgroups in the ART group
c T-test compared the mean difference between MNT and MNT-IT subgroups in total population
The analysis of multivariate logistic regression was conducted to determine the predictive factors linked with insulin therapy (Table 5). Factors identified as prognostic factors of insulin therapy were the age equal to or above 35 years old [OR: 2.91, 95% CI: (1.28–6.62)], increased levels of GTT- FBS [1.10: (1.04–1.16)], HbA1c [1.91 (1.09–3.34)], as well as ART treatment. The mode of conception (ART treatment) was identified as the independent prognostic factor for insulin requirement in GDM women after the adjustment (or controlling) of other confounding factors or covariates [OR: 2.94, 95% CI: (1.24–6.96)].
Table 5.
The multivariate logistic regression analysis for the risk factors associated with insulin therapy
| Model | OR (CI 95%)a |
|---|---|
| Age ≥ 35 yr. (Yes/No) | 2.91 (1.28–6.62)† |
| Prior history of GDM (Yes/No) | 3.22 (0.83–12.51) |
| GTT-FBS (mg/dl) | 1.10 (1.04–1.16) † |
| GTT-2 h (mg/dl) | 1.01 (0.99–1.02) |
| Family history of DM (Yes/No) | 1.06 (0.45–2.45) |
| HbA1c (%) | 1.91 (1.09–3.34)† |
| BMI ≥ 25 (kg/m2) | 2.12 (0.84–5.37) |
| Mode of conception (ART/SC) | 2.94 (1.24–6.96)† |
Data are presented as odds ratios (95% confidence interval)
MNT-IT was compared to MNT: Reference group was MNT
†P < 0.05 was considered statistically significant
a OR; Fitting GDM treatment modality as the outcome of the multivariate regression models;
Discussion
The present study found that maternal age ≥ 35 years old, elevated fasting glucose, and increased levels of HbA1c were the independent risk factor for insulin therapy. Furthermore, infertility treatment using assisted reproductive technology may be a possible predictive factor for insulin therapy in women with GDM.
The prevalence of GDM in Iran ranges from 1.3 to 18.8% in different geographical regions [16]. A recent systematic review and meta-analysis revealed that GDM treatment reduces the risk of delivering infants with macrosomia (i.e., being large-for-gestational-age birth), shoulder dystocia, and gestational hypertension. Of note, GDM treatment causes no significant increase in the risk of small-for-gestational-age birth [17]. Regarding the global increment in gestational diabetes, determination of high-risk populations requiring insulin therapy is crucial. Lifestyle modification is the first-line therapy for the management of women with GDM. However, some GDM women require insulin therapy as they the change in their lifestyle would not be sufficient alone.
Assisted conception, a standard treatment for infertility, is growing worldwide. Several unknown and concomitant factors in women conceived via ART make them prone to develop complications during their pregnancy, such as gestational diabetes which influences the clinical practice. Recently, Chen et al. [18] observed that peripheral insulin sensitivity is reduced in IVF-conceived women. They also reported the alternation of glucose metabolism (impaired glucose tolerance) in IVF-conceived mice. On the basis of the current data, insulin requirement was significantly higher in the ART group compared with the SC group (40% vs. 25%). Previously, antenatal insulin requirement was reported in 10.8–52.8% of GDM women after spontaneous conception [5, 19].
Several studies investigated clinical and biochemical parameters predicting the need for insulin therapy in GDM women who spontaneously conceived [11, 12, 19–22]. Moreover, some studies suggested similar prognostic factors, including advanced age [11, 23], elevated fasting glucose [14, 15, 20, 23], elevated two-hour glucose [14], prior history of GDM [15], and HbA1c [12, 15, 20, 21] for insulin treatment. Conversely, some reports demonstrated that the elevated pre-pregnancy BMI [12, 14, 15, 23], family history of diabetes [12, 20], and elevated 1-h blood glucose after GTT [14, 15, 20] were potentially independent prognostic factors. More recently, Barens et al. indicated a prediction model for insulin therapy in GDM women. According to this model, seven significant independent prognostic factors have been introduced, namely maternal age > 30 years, pre-gravid obesity (BMI ≥30 kg/m2), prior history of GDM, FBS ≥ 5.3 mmol/l, HbA1c ≥ 5.5% at the initial diagnosis of GDM, early diagnosis of GDM (< 24 weeks of gestation), and family history of diabetes. They concluded that 85.7–93.1% of women had six to seven prognostic factors mentioned above, but 9.3–14% of women had no or one prognostic factor [11].
The present results revealed that age ≥ 35 years, elevated GTT-FBS, and HbA1c were the independent risk factors for insulin therapy. Interestingly, the current study found a new prognostic factor for insulin therapy in pregnant women with GDM. The risk of insulin requirement was 2.94 folds higher in the ART group compared with the SC group. However, little is known about the association between ART and the risk of GDM. Previous evidence showed that infertile women and ART population were susceptible to develop GDM [4, 13]; yet, the mechanism underlying ART-induced insulin resistance and insulin requirement is partially understood. We assessed the impact of some aspects of ART properties on antenatal insulin requirement and observed no significant difference between MNT and MNT-IT subgroups of the ART group with respect to the ART parameters. Several hypotheses may be proposed in this regard.
First, the experimental study showed ART-induced endothelial dysfunction and arterial hypertension, glucose intolerance, and insulin resistance [24]. Second, some ART characteristics may be in charge of insulin resistance and the need for insulin therapy, such as PCOS [25], the number of embryo transfer, and administration of GnRH agonist during the COS cycle in ART. Third, infertile women usually receive exogenous progesterone during the luteal phase and at the first trimester of pregnancy, which may be associated with gestational diabetes and insulin resistance. Similar mechanisms might be proposed for insulin requirement in patients who undergo ART treatment. Nunes et al. found that progesterone- particularly at pharmacological doses- increased the generation of reactive oxygen species (ROS), and it could be toxic to pancreatic β-cells as a result of oxidative stress [26].
Moreover, Wada et al. [27] showed the molecular mechanisms of progesterone involved in the pathogenesis of insulin resistance during pregnancy. They indicated that progesterone could induce insulin resistance by the inhibition of GLUT-4 translocation, a decrease in the expression of the insulin receptor substrate-1 (IRS-1), and the uptake of glucose by adipocytes. The degradation of IRS-1 is one of the primary mechanisms that could cause insulin resistance when exposed to pro-inflammatory cytokines [27]. Notably, our data showed the increased serum concentrations of inflammatory markers in the ART group. Furthermore, higher levels of inflammatory biomarkers were observed in the MNT-IT than those in the MNT group. Previous research also demonstrated the increased levels of hs-CRP in GDM women who underwent IVF-ET cycles [28].
Furthermore, recent evidence showed a correlation of the elevated hs-CRP [29] and pro-inflammatory cytokines [6, 7] with the development of GDM and insulin resistance. More recently, IL-13, as an inflammatory marker, was found to be associated with the conversion of normoglycemia into type 2 diabetes mellitus and the initiation of insulin therapy [30]. Hence, inflammatory biomarkers may have indirectly affected insulin requirement.
The current study demonstrates that assisted conception could be a prognostic factor for insulin requirement in GDM populations. However, there were some limitations in our study. We could not evaluate all aspects of ART characteristics, and only a limited number of inflammatory biomarkers were evaluated. Another limitation was the lower number of women requiring insulin treatment. Moreover, the current research was performed in Iranian GDM women. This may limit the generalizability of the findings to other racial and ethnic groups. It is necessary to study large populations with other racial/ethnic groups in the future. Additionally, it is possible that the underlying infertility, as opposed to ART, could account for the study findings and the present study was not capable of categorizing the effects of assisted conception (ART) and nature of infertility; hence, it is critical to consider this issue in future studies on infertile patients who undergo non-IVF treatment as a separate cohort.
In conclusion, our findings confirm that age ≥ 35 years old, elevated levels of GTT-FBS and HbA1c are regarded as the independent risk factors for insulin therapy in GDM population. Furthermore, assisted conception could be a predictive factor for insulin therapy in pregnancies complicated with GDM. However, this study is the first report in this field, and more studies are warranted to corroborate these results.
Acknowledgments
The authors would like to thank Royan institute and hospitals related to the Ministry of Health and Medical Education, as well as patients for their invaluable contributions to the performance of the current research.
Abbreviations
- ADA/IAPDSG
American diabetes association/ International association of diabetes and pregnancy study groups
- ANOVA
Analysis of variance
- aOR
Adjusted odds ratio
- ART
Assisted reproductive technology
- BSP
Blood sugar profile
- COS
Controlled ovarian stimulation
- GDM
Gestational diabetes mellitus
- HbA1c
Hemoglobin A1c
- HDL
High density lipoprotein
- HOMA-IR
Homeostasis model assessment of insulin resistance
- hs-CRP
High-sensitivity C-reactive protein
- IL-17
Interleukin-17
- IRS-1
Insulin receptor substrate-1
- IT
Insulin therapy
- IVF/ICSI
In-vitro fertilization/ Intra-cytoplasmic sperm injection
- LDL
Low density lipoprotein
- MNT
Medical nutrition therapy
- OGTT
Oral glucose tolerance test
- OHSS
Ovarian hyperstimulation syndrome
- PCOS
Polycystic ovary syndrome
- ROS
Reactive oxygen species
- SC
Spontaneous conception
- SMBG
Self-monitoring of blood glucose
- TG
Triglycerides
- VLDL
Very-low-density lipoproteins
Authors’ contributions
A K, MEK, RP, RH, and HRB: contributed to the research design. AK, AM, RP, RH, HRB, and AA contributed to the selection of patients, data collection, interpretation of the data, and drafting the manuscript. AK wrote the manuscript. AKH assisted in the analysis of the data. All authors read and approved the final version of the manuscript.
Funding
No financial support.
Availability of data and materials
The datasets used and/or analyzed during the current study are available upon the request from the corresponding authors.
Ethics approval and consent to participate
The Institutional Review Boards and the Ethics Committee of the Iran University of Medical Sciences and Royan Institute, Tehran, Iran approved the present study (Date: 2015-09-4, Ethical Code: IR.IUMS.REC.1396.25469 and Date: 2014-09-4, Ethical Code: IR.ACECR.ROYAN.REC.1393.2). All procedures performed in this research, including recruiting participants were in accordance with the ethical guidelines of the Iran University of Medical Science, Royan institute, National Research Committee, and Helsinki Declaration (1964) and its later amendments or comparable ethical standards.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Azam Kouhkan, Phone: +98 21 23562000, Email: akouhkan@royaninstitute.org, Email: azamkouhkan@yahoo.com.
Hamid Reza Baradaran, Email: baradaran.hr@iums.ac.ir.
Roya Hosseini, Email: rohosseini@yahoo.com.
Arezoo Arabipoor, Email: arezoo.arabipoor@gmail.com.
Ashraf Moini, Email: a_moini@royaninstitute.org.
Reihaneh Pirjani, Email: pirjani@razi.tums.ac.ir.
Alireza Khajavi, Email: alireza.khajavi.student@gmail.com.
Mohammad E. Khamseh, Phone: +98 21 86036064, Email: Khamseh.m@iums.ac.ir, Email: khamseh.m@iums.ac.ir
References
- 1.Tobias DK, Chavarro JE, Williams MA, Buck Louis GM, Hu FB, Rich-Edwards J, et al. History of infertility and risk of gestational diabetes mellitus: a prospective analysis of 40,773 pregnancies. Am J Epidemiol. 2013;178(8):1219–1225. doi: 10.1093/aje/kwt110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pinborg A, Wennerholm U-B, Romundstad L, Loft A, Aittomaki K, Söderström-Anttila V, et al. Why do singletons conceived after assisted reproduction technology have adverse perinatal outcome? Systematic review and meta-analysis. Hum Reprod Update. 2012;19(2):87–104. doi: 10.1093/humupd/dms044. [DOI] [PubMed] [Google Scholar]
- 3.Catalano P. M., McIntyre H. D., Cruickshank J. K., McCance D. R., Dyer A. R., Metzger B. E., Lowe L. P., Trimble E. R., Coustan D. R., Hadden D. R., Persson B., Hod M., Oats J. J. N. The Hyperglycemia and Adverse Pregnancy Outcome Study: Associations of GDM and obesity with pregnancy outcomes. Diabetes Care. 2012;35(4):780–786. doi: 10.2337/dc11-1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Holst S, Kjær SK, Jørgensen ME, Damm P, Jensen A. Fertility problems and risk of gestational diabetes mellitus: a nationwide cohort study. Fertil Steril. 2016;106(2):427–434. doi: 10.1016/j.fertnstert.2016.03.040. [DOI] [PubMed] [Google Scholar]
- 5.Wang Y, Nikravan R, Smith H, Sullivan E. Higher prevalence of gestational diabetes mellitus following assisted reproduction technology treatment. Hum Reprod. 2013;28(9):2554–2561. doi: 10.1093/humrep/det270. [DOI] [PubMed] [Google Scholar]
- 6.Saisho Y, Miyakoshi K, Tanaka M, Shimada A, Ikenoue S, Kadohira I, et al. Beta cell dysfunction and its clinical significance in gestational diabetes. Endocr J. 2010;57(11):973–980. doi: 10.1507/endocrj.K10E-231. [DOI] [PubMed] [Google Scholar]
- 7.Abell SK, De Courten B, Boyle JA, Teede HJ. Inflammatory and other biomarkers: role in pathophysiology and prediction of gestational diabetes mellitus. Int J Mol Sci. 2015;16(6):13442–13473. doi: 10.3390/ijms160613442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Association AD 13. Management of diabetes in pregnancy: Standards of Medical Care in Diabetes—2018. Diabetes Care. 2018;41(Supplement 1):S137–SS43. doi: 10.2337/dc18-S013. [DOI] [PubMed] [Google Scholar]
- 9.Simeonova-Krstevska S, Bogoev M, Bogoeva K, Zisovska E, Samardziski I, Velkoska-Nakova V, et al. Maternal and neonatal outcomes in pregnant women with gestational diabetes mellitus treated with diet, metformin or insulin. Open Access Macedonian J Med Sci. 2018;6(5):803. doi: 10.3889/oamjms.2018.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Farrar D, Simmonds M, Bryant M, Sheldon TA, Tuffnell D, Golder S, et al. Treatments for gestational diabetes: a systematic review and meta-analysis. BMJ Open. 2017;7(6):2016–015557. doi: 10.1136/bmjopen-2016-015557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barnes RA, Wong T, Ross GP, Jalaludin BB, Wong VW, Smart CE, et al. A novel validated model for the prediction of insulin therapy initiation and adverse perinatal outcomes in women with gestational diabetes mellitus. Diabetologia. 2016;59(11):2331–2338. doi: 10.1007/s00125-016-4047-8. [DOI] [PubMed] [Google Scholar]
- 12.Sapienza AD, Francisco RPV, Trindade TC, Zugaib M. Factors predicting the need for insulin therapy in patients with gestational diabetes mellitus. Diabetes Res Clin Pract. 2010;88(1):81–86. doi: 10.1016/j.diabres.2009.12.023. [DOI] [PubMed] [Google Scholar]
- 13.Kouhkan Azam, Khamseh Mohammad E., Moini Ashraf, Pirjani Reihaneh, Valojerdi Ameneh Ebrahim, Arabipoor Arezoo, Hosseini Roya, Baradaran Hamid Reza. Predictive factors of gestational diabetes in pregnancies following assisted reproductive technology: a nested case–control study. Archives of Gynecology and Obstetrics. 2018;298(1):199–206. doi: 10.1007/s00404-018-4772-y. [DOI] [PubMed] [Google Scholar]
- 14.Jalaludin B. Gestational diabetes mellitus: who requires insulin therapy? Aust N Z J Obstet Gynaecol. 2011;51(5):432–436. doi: 10.1111/j.1479-828X.2011.01329.x. [DOI] [PubMed] [Google Scholar]
- 15.Mitra S, Nayak PK, Sahoo J, Mathew A, Padma A, Kamalanathan S, et al. Predictors for antenatal insulin requirement in gestational diabetes. Gynecol Endocrinol. 2014;30(8):565–568. doi: 10.3109/09513590.2014.911274. [DOI] [PubMed] [Google Scholar]
- 16.Jafari-Shobeiri M, Ghojazadeh M, Azami-Aghdash S, Naghavi-Behzad M, Piri R, Pourali-Akbar Y, et al. Prevalence and risk factors of gestational diabetes in Iran: a systematic review and meta-analysis. Iran J Public Health. 2015;44(8):1036. [PMC free article] [PubMed] [Google Scholar]
- 17.Poolsup N, Suksomboon N, Amin M. Effect of treatment of gestational diabetes mellitus: a systematic review and meta-analysis. PLoS One. 2014;9(3):e92485. doi: 10.1371/journal.pone.0092485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen M., Wu L., Zhao J., Wu F., Davies M. J., Wittert G. A., Norman R. J., Robker R. L., Heilbronn L. K. Altered Glucose Metabolism in Mouse and Humans Conceived by IVF. Diabetes. 2014;63(10):3189–3198. doi: 10.2337/db14-0103. [DOI] [PubMed] [Google Scholar]
- 19.Watanabe Mayu, Katayama Akihiro, Kagawa Hidetoshi, Ogawa Daisuke, Wada Jun. Risk Factors for the Requirement of Antenatal Insulin Treatment in Gestational Diabetes Mellitus. Journal of Diabetes Research. 2016;2016:1–6. doi: 10.1155/2016/9648798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pertot T, Molyneaux L, Tan K, Ross GP, Yue DK, Wong J. Can common clinical parameters be used to identify patients who will need insulin treatment in gestational diabetes mellitus? Diabetes Care. 2011;34(10):2214–2216. doi: 10.2337/dc11-0499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bakiner O, Bozkirli E, Ozsahin K, Sariturk C, Ertorer E. Risk factors that can predict antenatal insulin need in gestational diabetes. J Clin Med Res. 2013;5(5):381. doi: 10.4021/jocmr1515w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meshel S, Schejter E, Harel T, Maslovitz S, Germez N, Elimelech B, et al. Can we predict the need for pharmacological treatment according to demographic and clinical characteristics in gestational diabetes? J Matern Fetal Neonatal Med. 2016;29(13):2062–2066. doi: 10.3109/14767058.2015.1077225. [DOI] [PubMed] [Google Scholar]
- 23.González-Quintero VH, Istwan NB, Rhea DJ, Tudela CM, Flick AA, De la Torre L, et al. Antenatal factors predicting subsequent need for insulin treatment in women with gestational diabetes. J Women's Health. 2008;17(7):1183–1187. doi: 10.1089/jwh.2007.0667. [DOI] [PubMed] [Google Scholar]
- 24.Cerny D, Sartori C, Rimoldi SF, Meister T, Soria R, Bouillet E, et al. Assisted reproductive technologies predispose to insulin resistance and obesity in male mice challenged with a high-fat diet. Endocrinology. 2017;158(5):1152–1159. doi: 10.1210/en.2016-1475. [DOI] [PubMed] [Google Scholar]
- 25.Szymanska M, Horosz E, Szymusik I, Bomba-Opon D, Wielgos M. Gestational diabetes in IVF and spontaneous pregnancies. Neuro Endocrinol Lett. 2011;32(6):885–888. [PubMed] [Google Scholar]
- 26.Nunes V A, Portioli-Sanches E P, Rosim M P, Araujo M S, Praxedes-Garcia P, Valle M M R, Roma L P, Hahn C, Gurgul-Convey E, Lenzen S, Azevedo-Martins A K. Progesterone induces apoptosis of insulin-secreting cells: insights into the molecular mechanism. Journal of Endocrinology. 2014;221(2):273–284. doi: 10.1530/JOE-13-0202. [DOI] [PubMed] [Google Scholar]
- 27.Wada T, Hori S, Sugiyama M, Fujisawa E, Nakano T, Tsuneki H, et al. Progesterone inhibits glucose uptake by affecting diverse steps of insulin signaling in 3T3-L1 adipocytes. Am J Physiol-Endocrinol Metab. 2010;298(4):E881–E8E8. doi: 10.1152/ajpendo.00649.2009. [DOI] [PubMed] [Google Scholar]
- 28.Yildizfer F, Donma O, Yen M, Ekmekci O, Kul ZAK, Keser Z, et al. In vitro fertilization, levels of pro-inflammatory factors and lipid peroxidation. Int J Fertility Sterility. 2015;9(3):277. doi: 10.22074/ijfs.2015.4541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kumari R, Singh H. The prevalence of elevated high-sensitivity C-reactive protein in normal pregnancy and gestational diabetes mellitus. J Fam Med Primary Care. 2017;6(2):259. doi: 10.4103/2249-4863.219995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brahimaj A, Ligthart S, Ghanbari M, Ikram MA, Hofman A, Franco OH, et al. Novel inflammatory markers for incident pre-diabetes and type 2 diabetes: the Rotterdam study. Eur J Epidemiol. 2017;32(3):217–226. doi: 10.1007/s10654-017-0236-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available upon the request from the corresponding authors.