Abstract
Background
Phenylalanine ammonia-lyase (PAL, EC 4.3.1.24) is the first key enzyme in the phenylpropanoid pathway. The pal gene has been widely studied in plants and participates in plant growth, development and defense systems. However, in Pleurotus ostreatus, the biological functions of pal during organismal development and exposure to abiotic stress have not been reported.
Results
In this study, we cloned and characterized the pal1 (2232 bp) and pal2 (2244 bp) genes from the basidiomycete P. ostreatus CCMSSC 00389. The pal1 and pal2 genes are interrupted by 6 and 10 introns, respectively, and encode proteins of 743 and 747 amino acids, respectively. Furthermore, prokaryotic expression experiments showed that PAL enzymes catalyzed the conversion of L-phenylalanine to trans-cinnamic acid. The function of pal1 and pal2 was determined by constructing overexpression (OE) and RNA interference (RNAi) strains. The results showed that the two pal genes had similar expression patterns during different developmental stages. The expression of pal genes was higher in the reproductive growth stage than in the vegetative growth stage. And the interference of pal1 and pal2 delayed the formation of primordia. The results of heat stress assays showed that the RNAi-pal1 strains had enhanced mycelial tolerance to high temperature, while the RNAi-pal2 strains had enhanced mycelial resistance to H2O2.
Conclusions
These results indicate that two pal genes may play a similar role in the development of P. ostreatus fruiting bodies, but may alleviate stress through different regulatory pathways under heat stress.
Keywords: Pleurotus ostreatus, Phenylalanine ammonia-lyase, Development, Heat stress, Overexpression, RNA interference
Background
Phenylalanine ammonia-lyase (PAL, EC 4.3.1.24) is the first enzyme in the phenylpropanoid pathway and catalyzes the conversion of L-phenylalanine to trans-cinnamic acid by nonoxidative deamination [1–3]. Furthermore, PAL is the first key enzyme in the phenylpropanol pathway, participating in the formation of a series of structural and defensive phenolic compounds, such as lignin, phenolic acid and hydroxybenzoic acid, flavonoids and stilbene in plants [4]. The pal gene has been widely studied in plants and participates in plant growth, development and defense systems [5, 6], including lignin synthesis in cell walls, nutrient transport, and the regulation of seed color [7]. Plants can induce PAL enzymes under abiotic stresses (e.g., ultraviolet-B (UV-B) light, high and low temperature, injury, salt.), leading to the accumulation of phenolic compounds such as flavonoids and phenolic acids [8]. Under salt stress, the antioxidant capacity of plants has been shown to be enhanced by increasing PAL activity [9]. Under UV-B stress, the content of salicylic acid in soybean roots and leaves increased with the increase of PAL activity, showing strong stress resistance [10]. The pal gene has also been studied recently in mushrooms, such as Flammulina velutipes, in which the pal gene was cloned and characterized. The different expression patterns of the F. velutipes pal gene and its activity in different organs of the mushroom indicated that pal is associated with mushroom growth [11]. In Tricholoma matsutake, transcriptome analysis revealed a pattern of pal gene expression that was dependent on the developmental stage, suggesting that pal has many physiological functions in this mushroom [12]. In several basidiomycete fungi, a metabolic pathway for the metabolism of phenylalanine via cinnamic, benzoic, p-hydroxybenzoic, and protocatechuic acids has been reported that is similar to that observed in plants [13]. However, the biological function of pal in P. ostreatus during development and under abiotic stress has not been reported.
Pleurotus ostreatus is one of the most widely cultivated mushroom species globally [14], being an edible mushroom with high nutritional and medicinal value. The mechanism of fruiting body development of edible fungi has been a popular research topic in recent years, with numerous studies investigating fruiting body development and associated signaling pathways. A number of functional genes, such as nicotinamide adenine dinucleotide phosphate oxidase [15], cytochrome P450 [16], superoxide dismutase [17], multicopper oxidases [18] and catalase [19], have been identified and characterized with respect to mushroom development. In addition, the AMP signaling pathway has been reported in mushrooms [20]. The development of mushrooms is a complex process that is regulated by gene products and environmental factors. In China, P. ostreatus is primarily cultivated in horticultural facilities, thus its cultivation is strongly affected by seasonal temperature changes, especially the summer high temperatures. A number of studies have shown that high temperatures can affect mycelial growth and fruiting body development [21], and can even lead to spawn burning [22] and Trichoderma contamination [23]. Previous studies on the heat stress response of P. ostreatus have investigated programmed cell death [24], the role of catalase in fruiting body development and heat stress [19], and the effect of trehalose on mycelial damage mitigation [25]. Recently, Zou et al. studied proteome changes in P. ostreatus mycelia during heat stress and recovery and identified 204 proteins, including PAL, exhibiting altered expression during heat stress or the recovery phase [26]. These finding laid a foundation for studying the biological functions of pal in P. ostreatus under heat stress.
Many studies have used molecular and genetic methods to silence the pal gene to study its biological functions in plant growth, development and environmental stress [7, 27]. In recent years, RNA interference (RNAi) and Overexpression (OE) technologies have been widely used to study gene functions in P. ostreatus. For example, the overexpression of a methionine sulfoxide reductase A gene enhances stress tolerance in P. ostreatus [28], which provides a more effective method for studying the function of genes in P. ostreatus. In this study, we searched and cloned the pal genes from the P. ostreatus genome. On the basis of describing their characteristics, we studied the role of pal genes in fruiting body development and heat stress using RNAi and OE technologies.
Results
Cloning and bioinformatics analysis of pal
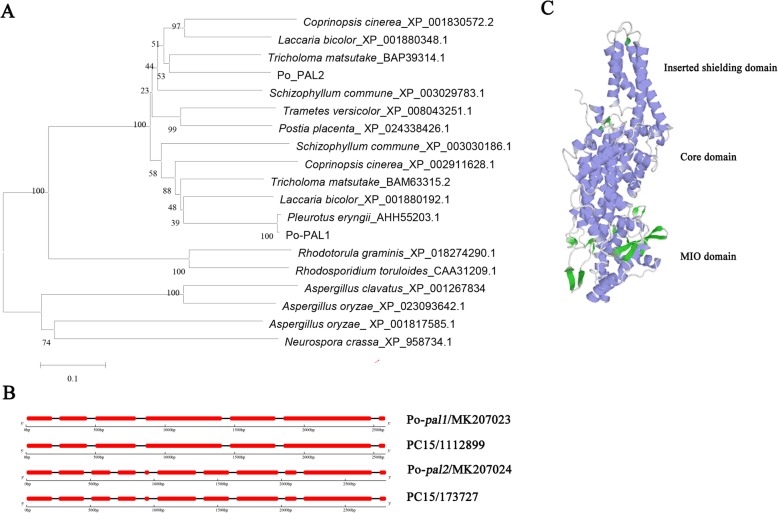
Two pal genes were identified in the P. ostreatus genome and were named pal1 and pal2, respectively. Their full-length cDNA sequences were 2232 and 2244 bp, respectively. DNA sequence analysis showed that 7 exons are interrupted by 6 introns in pal1, while 11 exons are interrupted by 10 introns in pal2 (Fig. 1b). The two sequences were deposited in GenBank with the accession numbers MK207023 and MK207024, respectively. Figure 1b shows that pal1 has an identical gene structure with the genes encoding PC15_1111887 and that pal2 has an identical gene structure with the gene encoding PC15_173,727. The PAL1 and PAL2 protein sequences in both P. ostreatus CCMSSC 00389 and PC15 were highly similar to one another (Additional file 1: Figure S1), and the consistency reached 99.73 and 99.59%, respectively. However, the amino acid sequences and nucleotide sequences of pal1 and pal2 had low similarity in P. ostreatus CCMSSC 00389 (Additional file 1: Figure S1 and Additional file 2: Figure S2).
Fig. 1.
Relationships of fungal PALs, gene structural features and a 3-D structural model. a A neighbor-joining phylogenetic tree of PAL protein sequences from 11 fungal species. b Gene structures of selected pal genes in P. ostreatus CCMSSC00389 and PC15. The exons are represented by red rectangles, and the black lines connecting two exons represent introns. c 3-D structural model of the Po-PAL protein. The structure was divided into three parts: the 4-methylideneimidazole-5-one (MIO) domain, the core domain and the inserted shielding domain. The MIO group is highlighted in green
To understand the phylogenetic relationship between the PAL proteins and other fungal PALs, phylogenetic analysis was performed. Phylogenetic analysis of 19 PAL sequences revealed two distinct branches (Fig. 1a). The phylogenetic tree showed that PAL1 and PAL2 have higher similarities to the protein sequences of other mushrooms or fungi than to each other. The cladogram revealed the variation in the PAL protein sequence among fungi.
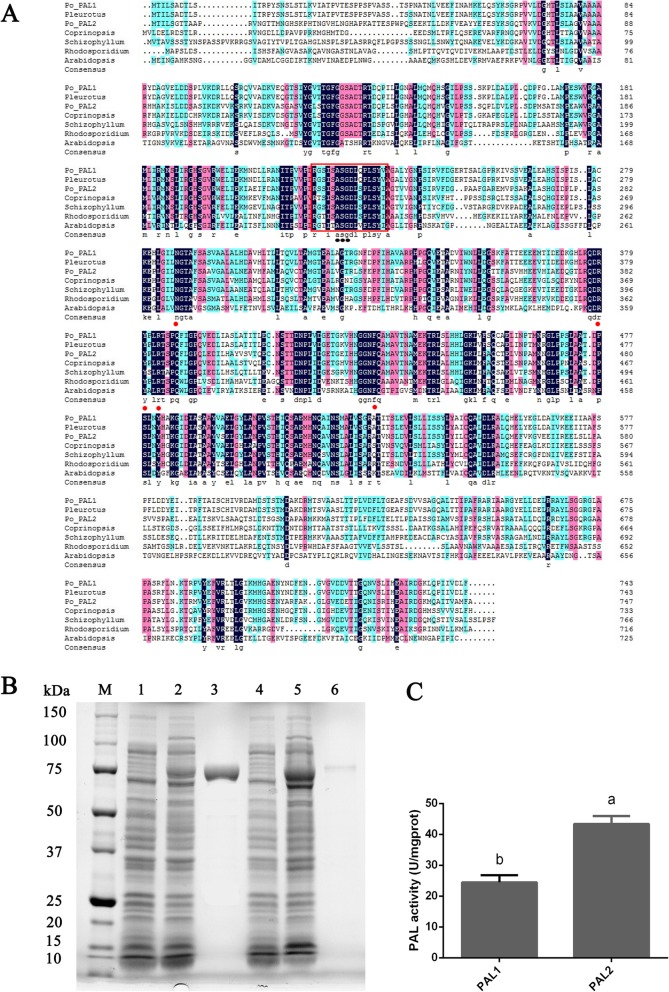
The pal1 and pal2 sequences were bioinformatically analyzed to determine their physicochemical properties and possible structure. The pal1 gene encodes a putative 743-amino-acid polypeptide with an approximate molecular weight and calculated pI of 79.845 kDa and 5.28, respectively. The pal2 gene encodes a putative 747-amino-acid polypeptide of 79.946 kDa with a predicted isoelectric point of 6.11 [29]. An online analysis revealed a Pfam lyase aromatic domain in both pal1 and pal2, whereas only pal2 was observed to have a SCOP d1qj5a_domain. SCOP d1qj5a_domain starts at amino acid position 638 and ends at position 735 and it belongs to the PLP-dependent transferase superfamily. The gene models for pal1 and pal2 from different organisms are shown in Fig. 2a, which primarily describes the amino acid identities and similarities among pal genes in different organisms. The pal motif is labeled with a red box, and the conserved active-site motif (Ala-Ser-Gly) and specific amino acids are also shown in Fig. 2a. The conserved active-site motif is labeled with circles under the specific amino acids, which can be converted into an MIO (4-methylidene-imidazole-5-one) prosthetic group (Fig. 1c). The other active-site residues are labeled with red circles in Fig. 2a. The 3-D structure of PAL (Fig. 1c) showed that it is composed of an MIO domain, a core domain and an inserted shielding domain [30]. Thus, the PAL amino sequences are highly conserved with other characterized PAL proteins in fungi.
Fig. 2.
Partial amino acid sequence alignment and SDS-PAGE results of pal1 and pal2 expressed in E. coli. a Partial amino acid sequence alignment of PAL1 and PAL2 of P. ostreatus CCMSSC00389 and other PALs. Positions with identical amino acid residues are indicated by circles below the sequence. (Pleurotus eryngii_AHH55203.1, Schizophyllum commune_XP_003030186.1, Coprinopsis cinerea_XP_001830572.2, Rhodosporidium toruloides_CAA31209.1, Arabidopsis thaliana_NP_181241.1). b SDS-PAGE analysis of recombinant PAL protein extracted from E. coli BL21 (DE3) cells. M, protein molecular weight standards; 1, crude lysate of pal1 from E. coli BL21 (DE3) grown at 16 °C for 12 h; 2, crude enzyme of pal1 from E. coli BL21 (DE3)/pSMART-V-PAL induced with IPTG (1 mM) at 16 °C for 12 h; 3, pal1 protein purified with a nickel column; 4, crude lysate of pal2 from E. coli BL21 (DE3) grown at 16 °C for 12 h; 5, crude enzyme of pal2 from E. coli BL21(DE3)/pSMART-V-PAL induced with IPTG (1 mM) at 16 °C for 12 h; and 6, pal2 protein purified with a nickel column. c Determination of PAL activity. Three independent biological replicates were performed for all experiments. The values are the means ± SE. Different letters indicate significant differences between the strains (P < 0.05, according to Tukey’s test)
The analysis of prokaryotic expression showed that the purified proteins (PAL1 and PAL2) had molecular weights of approximately 75 kDa (Fig. 2b), which is consistent with predictions. The activity of the purified enzymes was determined by spectrophotometry (Fig. 2c). The results showed that the activity of PAL1 (24.469 ± 2.296 u/mg protein) was significantly lower than that of PAL2 (43.387 ± 2.551 u/mg protein).
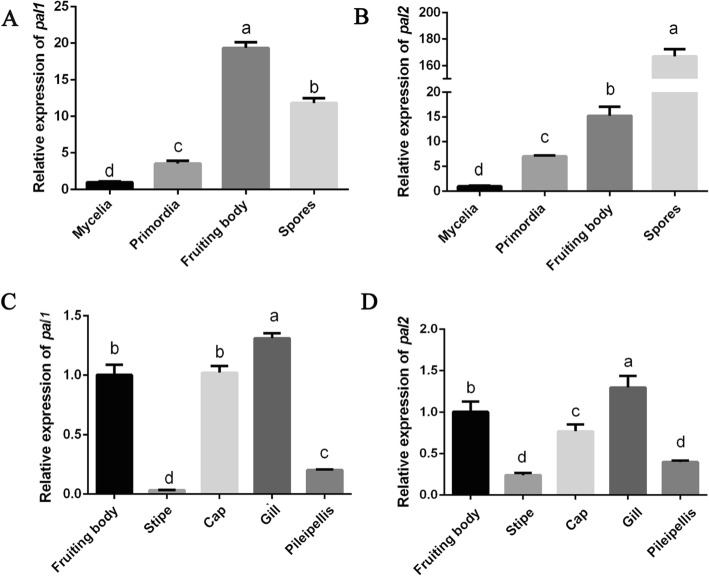
Expression of pal1 and pal2 during different P. ostreatus developmental stages
The four stages composing the life cycle of a mushroom are the mycelium, primordia, fruiting body and spore stages (Additional file 3: Figure S3). To investigate the expression patterns of pal1 and pal2 during P. ostreatus development, the expression of these genes during different developmental stages and different parts of the fruiting bodies of the wild type (WT) strain were assessed (Fig. 3). The results showed that compared with that in mycelia, the expression of pal1 was significantly upregulated in primordia (3.5-fold), fruiting bodies (19.3-fold) and spores (11.8-fold) (Fig. 3a). In addition, the expression of pal2 was upregulated significantly and continuously during P. ostreatus development and was higher than that of mycelia in primordia (7-fold), fruiting bodies (15.2-fold), and spores (68-fold) (Fig. 3b). Figure 3c and d show pal1 and pal2 gene expression in different parts of the P. ostreatus fruiting body. The results showed that the expression of pal1 and pal2 in different parts of fruiting bodies had the same trend, with the highest expression observed in the gills.
Fig. 3.
The expression of pal during different developmental stages and different parts of P. ostreatus fruiting bodies. a qPCR analysis of pal1 expression in WT strain during different cultivation stages. b qPCR analysis of pal2. The relative abundances of the WT transcript levels at different stages were normalized by comparison with that observed in the mycelium stage (relative transcript level = 1). c The expression of pal1 in four parts of the P. ostreatus fruiting body. d qPCR analysis of pal2 expression. The relative abundances of the transcript levels in the WT strain in the different parts of the fruiting body were normalized by comparison with that observed in the fruiting body (relative transcript level = 1). The values are the means ± SE of three independent experiments. Different letters indicate significant differences between the strains (P < 0.05, according to Tukey’s test)
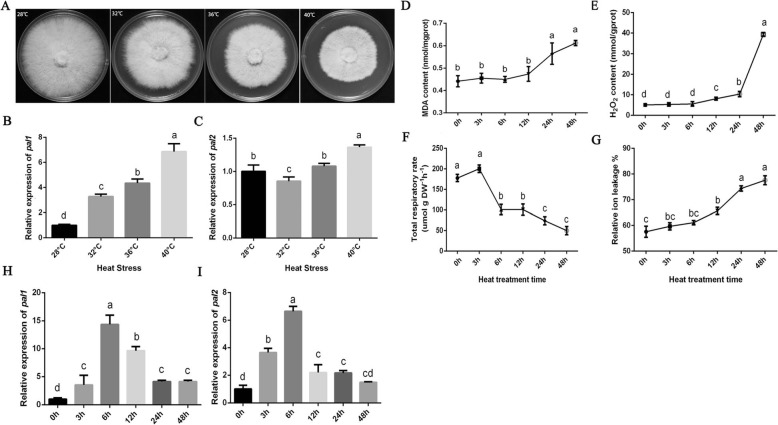
Expression of pal1 and pal2 and damage to mycelia under heat stress
As shown in Fig. 4a, the mycelial growth was slightly affected at 32 °C, severely inhibited at 36 °C and completely abrogated at 40 °C. The expression of pal1 increased significantly with increasing temperature, while pal2 expression was first downregulated and then upregulated through a small series of changes. According to the results, 40 °C was selected as the stress temperature for further study. The H2O2 and MDA content, which are two indicators of oxidative damage, increased with the duration of processing, especially when the stress time exceeded 24 h. The results showed that oxidative damage occurred in mycelia under heat stress (Fig. 4d, e). Figure 4f and g show the changes in the mycelial total respiration rate and relative ion leakage under different temperature stresses. The results showed that with increasing heat stress time, the total respiration rate of mycelia increased temporarily and then decreased rapidly. At the same time, the relative ion leakage increased significantly with increasing stress time, indicating that the degree of mycelial damage increased. Figure 4h and i show the relative expression of pal1 and pal2 in mycelia after different durations of heat stress. pal1 and pal2 expression first increased and then decreased with heat stress time. The expression of the pal1 and pal2 genes peaked at 6 h of heat stress.
Fig. 4.
Effects of heat stress on mycelia. a Effects of different temperature stresses on mycelia. b Relative expression of pal1 in mycelia at different temperatures. c Relative expression of pal2 in mycelia at different temperatures. The relative abundances of transcripts in the mycelia at different temperatures were normalized by comparison with that observed in mycelia at 28 °C (relative transcript level = 1) (d) MDA content. e H2O2 content. f Total respiratory rate. g Relative ion leakage. h Relative expression of pal1. i Relative expression of pal2. The mean values and standard deviations of three biological replicates are shown. The error bars with different letters over the columns denote significant differences (P < 0.05, according to Tukey’s test)
Generation of pal OE and RNAi strains
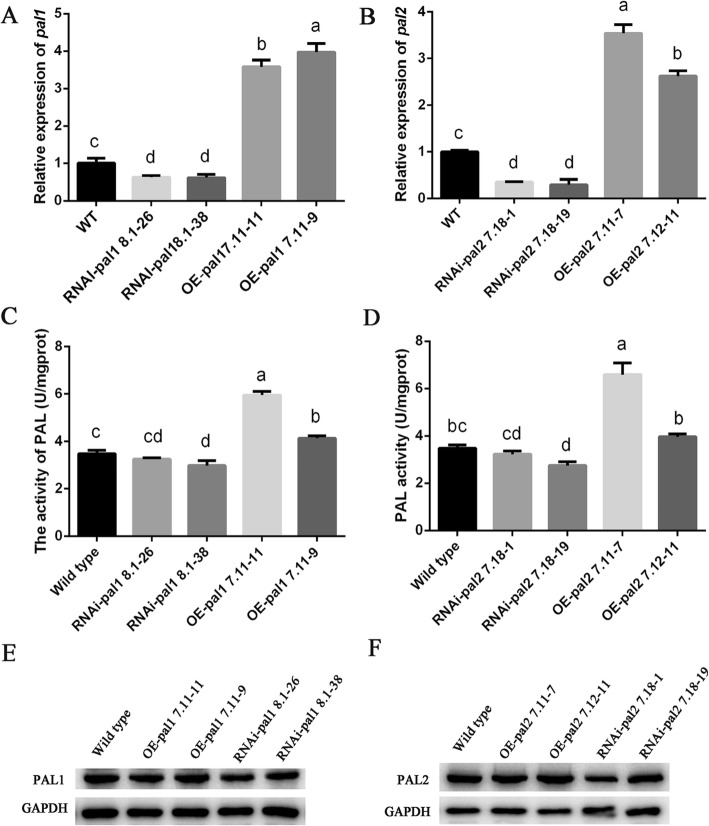
Gene transformation with a gene knockout vector is a useful approach to explore the function of genes in fungi [31]. To study the roles of pal1 and pal2 in P. ostreatus, two RNAi-pal silencing vectors and two OE-pal OE vectors were constructed containing the hyg gene as a selectable marker (Fig. 5). The efficiency of RNAi and OE of the transformants was further confirmed by qPCR analysis. The transcription of pal1 in the OE strains (OE-pal1 7.11–11 and OE-pal1 7.11–9) was approximately 4-fold higher than that of the WT strain, whereas pal1 transcription in the RNAi strains (RNAi-pal1 8.1–26 and RNAi-pal1 8.1–38) decreased by more than 50%. Therefore, these strains were selected for further study (Fig. 6a). The transcription of pal2 in the OE strains (OE-pal2 7.11–7 and OE-pal2 7.12–11) and RNAi strains (RNAi-pal2 7.18–1 and RNAi-pal2 7.18–19) were significantly different from that observed in the WT strain. The pal2 gene expression of the overexpression strains was approximately 3-fold higher than that in the WT strain, while the expression in the RNAi strains decreased to 20% (Fig. 6b). The PAL enzyme activity in the tested strains was also assessed. The results showed that the PAL activity in the OE-pal1 7.11–11 strain was 1.7-fold greater than that in the WT strain and that the PAL activity in the OE-pal2 7.11–7 strain was 1.8-fold greater than that in the WT strain (Fig. 6c, d). In contrast, PAL enzyme activity was slightly decreased in the RNAi strains than that in the WT strain (Fig. 6c, d). Western blotting showed that the expression of PAL protein was slightly increased in the OE-pal strains, and decreased in the RNAi-pal strains relative to the WT strain (Fig. 6e, f).
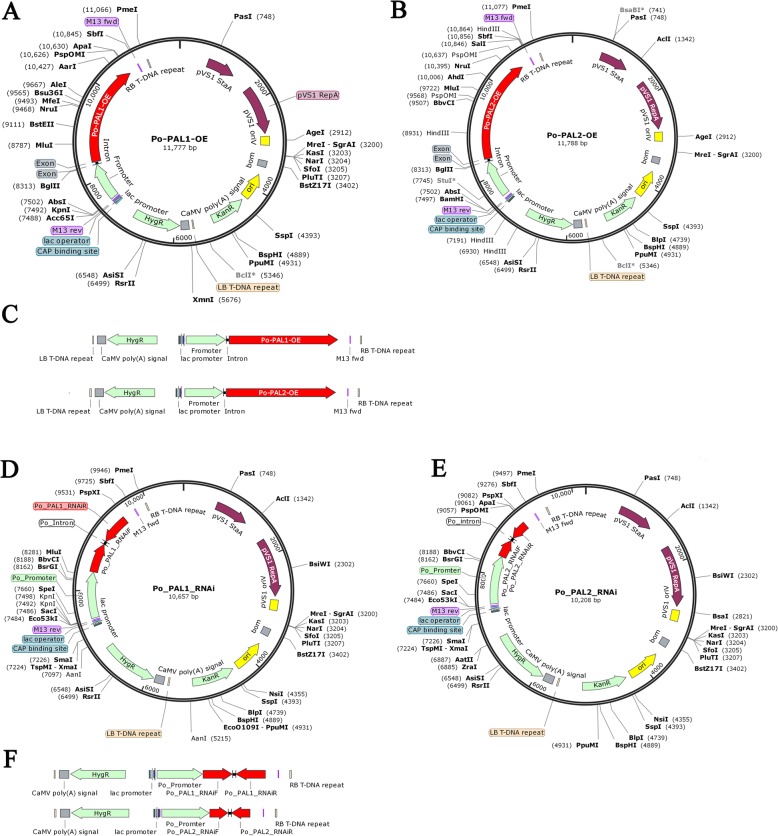
Fig. 5.
Strateg for the OE and RNAi of pal genes in P. ostreatus. a The structure of the pal1 OE vector. b The structure of the pal2 OE vector. c Schematic representation of the OE vector based on the A. tumefaciens right and left borders. On the left side, the hygR cassette is driven by the lac promoter. On the right side, the PAL OE cassette is driven by the P. ostreatus gpd promoter. d The structure of the pal1 gene RNAi vector. e The structure of the pal2 gene RNAi vector. f Schematic representation of the RNAi vector based on the A. tumefaciens right and left borders. On the left side, the HygR cassette is driven by the lac promoter. On the right side, the pal RNAi cassette (pal-RNAiF and pal-RNAiR) is driven by the P. ostreatus gpd promoter
Fig. 6.
Characterization of the pal OE and RNAi strains. a qPCR analysis of the expression of pal1 in the tested strains. b qPCR analysis of the expression of pal2 in the tested strains. c and d Determination of the PAL activity in the tested strains. e and f Protein expression of pal1 and pal2 in the tested strains. Three independent biological replicates were performed for all experiments. The values are the means ± SE. Different letters indicate significant differences between the strains (P < 0.05, according to Tukey’s test)
pal1 and pal2 are involved in primordium formation
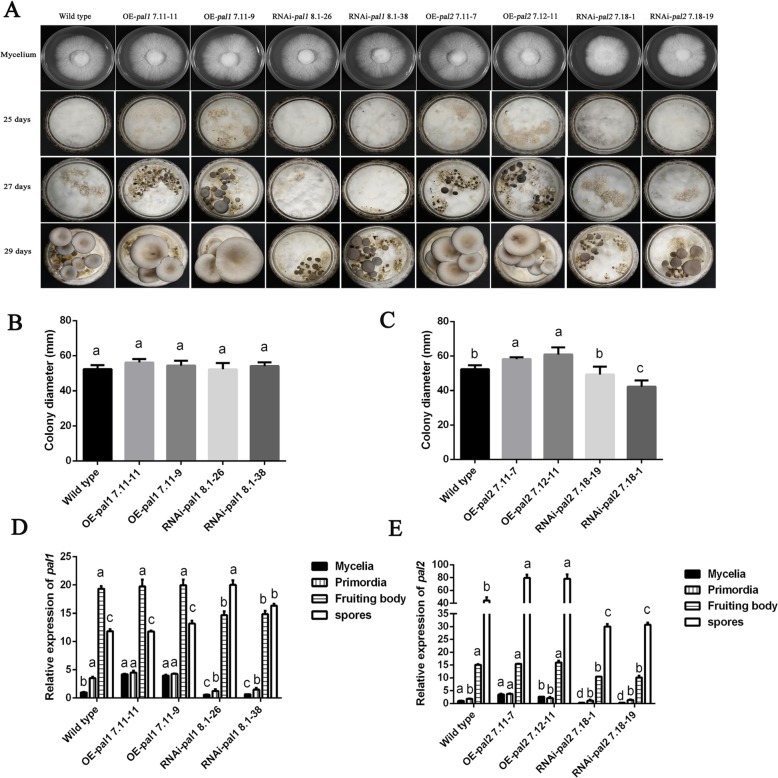
The effects of pal OE and RNAi on mycelial growth are shown in Fig. 7a. The results showed that OE and RNAi of pal1 had no visible phenotypic effects (Fig. 7a, b). However, compared with the WT strain, the OE-pal2 strains exhibited slightly larger colony diameters (Fig. 7a), whereas the RNAi strains exhibited significantly lower growth rates (Fig. 7c). Figure 7c shows that the mycelial growth rate of the OE-pal2 7.11–7 and OE-pal2 7.12–11 strains increased by 11.21 and 16.26% respectively relative to that of the WT strain. Relative to the mycelial growth rate of the WT strain, the rates of the RNAi-pal2 7.18–1 and RNAi-pal2 7.18–19 strains were decreased by 5.82 and 19.43%, respectively. In the mushroom production experiments, we observed that OE-pal strains formed primordia 2 days earlier than the WT strain, whereas the RNAi strains exhibited the opposite phenotype. The results of the mushroom production experiment indicated that the time of primordium formation in the pal interfering strains was 2 days later than that of the WT strain (Fig. 7a). Correspondingly, the period of mushroom cultivation was shortened by pal overexpression and prolonged by RNAi. To further explore the biological role of pal in the development of fruiting bodies, the expression of pal1 and pal2 was assessed in the WT, OE and RNAi strains at different developmental stages by qPCR. Figure 7d and e show that the pal gene expression patterns in the OE-pal and RNAi-pal strains at different developmental stages were similar to those of the WT strain. Pal gene expression during the reproductive growth stage was higher than that during the vegetative reproductive stage except in the spores of the RNAi-pal1 strains. In summary, pal overexpression can promote primordium formation, and pal interference can significantly prolong the time to primordium formation. OE and RNAi of pal have little effect on the expression patterns at different developmental stages.
Fig. 7.
pal1 and pal2 are involved in the development of P. ostreatus. a Developmental stages (mycelia, primordia and fruiting body) in the life cycle of the WT, OE and RNAi strains. b and c The growth rate of the pal mutants. d and e qPCR analysis of pal expression in tested strains at different development stages (mycelia, primordia, fruiting body and spores). The relative abundances of the transcripts at different stages were normalized by comparison with that observed in mycelia in the WT strain (relative transcript level = 1). The values are the means ± SE of three independent experiments. Different letters indicate significant differences between the strains (P < 0.05, according to Tukey’s test)
pal1 and pal2 participate in the regulation of the mycelial response to heat stress
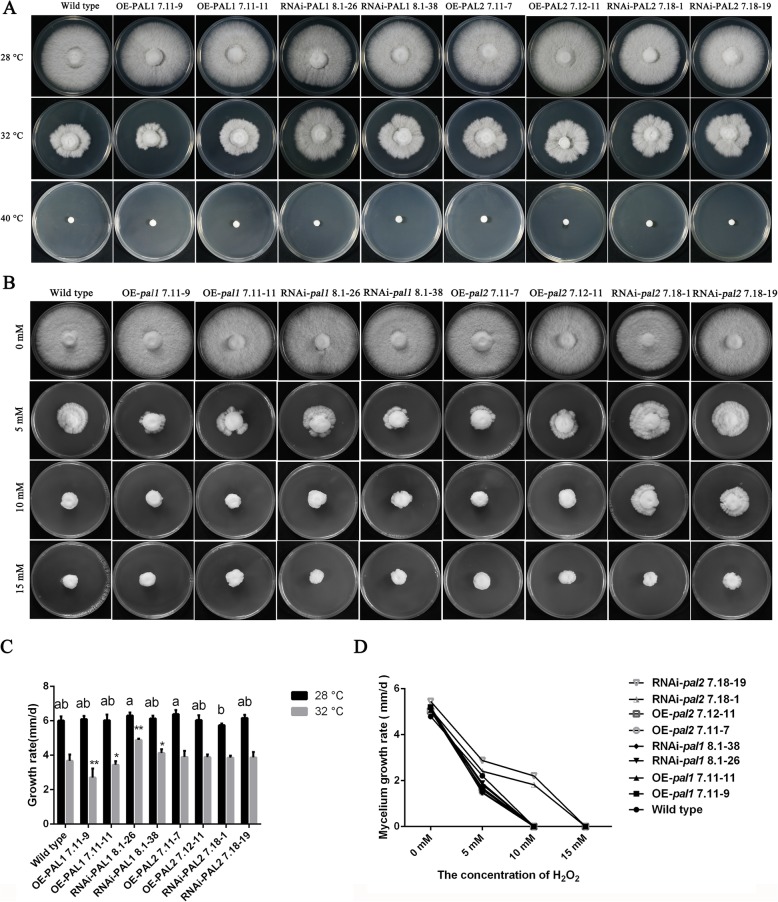
Figure 8a and c show the growth status and rate of the tested strains. The results showed that the growth rate of the WT strain was seriously affected when the mycelial growth temperature was 32 °C, while that of the pal1 interference strains was significantly increased compared with that of the WT strain. Similar to the WT strain, the growth rate of other tested strains was significantly inhibited, and germination actually stopped at 40 °C.
Fig. 8.
pal1 and pal2 participate in the resistance of mycelia to heat stress. a Colony morphology at different temperatures. b The effects of exogenous H2O2 at different concentrations on mycelial growth. c The growth rate of experimental strains at different temperatures. d Effects of different concentrations of exogenous H2O2 on the mycelial growth rate. The values are the means ± SE of three independent experiments. Different letters indicate significant differences between the transformed strains and the WT strain under 28 °C culture conditions. Different asterisks indicate significant differences between the transformed strains and the WT strain under 32 °C culture conditions. (P < 0.05, according to Tukey’s test)
Heat stress can lead to a significant increase in H2O2 in mycelia. To investigate whether pal genes are involved in the reactive oxygen species (ROS) response, the growth rates of the WT, OE and RNAi strains were evaluated on PDA plates with different concentrations of exogenously added H2O2. The resistance of the RNAi-pal2 7.18–1 and RNAi-pal2 7.18–19 strains to H2O2 was higher than that of the WT strain, especially when at a H2O2 concentration of 10 mM (Fig. 8b). In addition, the resistance of other strains to H2O2 did not change significantly under the tested conditions (Fig. 8b and d). In summary, the interference of the pal2 gene reduced the sensitivity of mycelia to H2O2.
Discussion
PAL plays an important role in the acquisition of secondary metabolites. The role of PAL in plants has been extensively studied. However, the biological function of PAL in fungi, which has important research significance, has yet to be fully elucidated. The number and structure of pal genes varies greatly in different organisms, and there are several pal genes in fungi. In Rhodosporidium toruloides, pal is encoded by a single gene. In the genomes of Aspergillus oryzae RIB 40 and Aspergillus nidulan FGSC A4, four and two pal genes are encoded, respectively. In this study, two pal genes were identified within the P. ostreatus genome, which is generally consistent with that observed in other basidiomycetes. For example, two pal genes were identified in Coprinopsis cinerea and Schizophyllum commune. Our phylogenetic tree also supports this result. Po-PAL1 and Po-PAL2 clustered together with PALs from Coprinopsis cinerea and Schizophyllum commune, respectively. In addition, the P. ostreatus pal1 and pal2 genes contained 6 and 10 introns, respectively, differing greatly in genetic structure. Previous studies have shown that the number of introns in Basidiomycota pal genes ranges from 0 to 13 introns, and our results are consistent with these observations [32]. The nucleotide sequence consistency of pal1 and pal2 was 55.41%, and the amino acid consistency was 61.61% (Additional file 1 and 2: Figure S1-S2). In phylogenetic trees, the PAL1 and PAL2 sequences were not phylogenetically closely related to each other, suggesting that PAL1 and PAL2 did not undergo simple gene duplication.
PAL proteins participate in the growth and development of plants, playing different roles in different species. In Arabidopsis, Antje et al. reported that pal1 and pal2 mutants had no unique morphological phenotype but were sterile [7]. Huang et al. showed that three independent pal1 and pal2 double mutants generated yellow seeds due to the lack of condensed tannin pigments in the seed coat [33]. In fungi, many pal genes have been cloned, but little research has been performed to elucidate their biological function. In this study, the results showed that the gene expression patterns of pal1 and pal2 during different developmental stages were essentially the same, with pal1 and pal2 expression increasing during the transformation from the vegetative to the reproductive growth stages. The gene expression of each of pal1 and pal2 was also consistent in different parts of the fruiting body. Overexpression of pal1 and pal2 resulted in earlier formation of primordia, suggesting that these genes may play a similar role in the development of fruiting bodies. In Flammulina velutipes, the expression of pal in the stipe increased significantly, suggesting that pal may be involved in stipe elongation [31]. We observed that pal gene expression in the stipe was significantly lower than that in the fruiting the body, possibly because a long stipe is not a beneficial trait during P. ostreatus development. The transcription pal in the cap was significantly higher than that in the stipe. Considering that phenolic compounds in plants are synthesized through the activity of pal in the phenylpropanoid pathway, pal expression in the P. ostreatus cap may be involved in the production of phenolic compounds, which may improve the antioxidant capacity of mushrooms. In this study, different levels of pal1 and pal2 transcription were observed in spores. Previous studies have shown that among the different parts of the fruiting body of Tricholoma matsutake, the gill exhibits the highest transcription of tmpal2 [34]. Our results also indicate that pal2 transcription is significantly higher than that of pal1 in spores, which suggests that pal2 may play a major role in spore-related progress.
In this study, pal1 gene expression increased significantly after heat stress, and the RNAi-pal1 strains showed a significant growth advantage over the WT strain at 32 °C. The OE-pal2 and RNAi-pal2 strains showed no significant difference in growth at 32 °C but showed marked resistance to exogenous H2O2. In plants, oxidative stress is produced as a secondary stress during the heat stress response, which results in the abundant production of ROS [35]. ROS poses a serious threat to cell function by damaging lipids and proteins [36]. ROS are oxygen-containing compounds, such as superoxide anion (O2−), H2O2, and hydroxyl radicals (·OH) [37]. We speculate that pal2 interference may reduce the sensitivity of mycelia to H2O2, whereas pal1 interference may reduce the sensitivity of mycelia to other types of ROS. Thus, pal1 and pal2 may respond to stress by regulating different pathways. The results showed that pal may have a negative regulatory effect on the response of P. ostreatus to heat stress. Similar observations have been reported in plants. In Arabidopsis thaliana, pal1 and pal2 RNAi strains were more sensitive to UV-B radiation but more resistant to drought stress [33]. In Brachypodium, no significant difference in UV-B radiation or drought resistance was observed between RNAi-pal and WT plants [38].
Conclusions
In summary, in this study, two pal genes were cloned, and the structural characteristics of the encoded proteins were studied. Through qPCR analysis, we observed that the gene expression patterns of pal1 and pal2 were essentially the same during P. ostreatus different developmental stages. In addition, this study confirmed that pal interference can delay the formation of primordia. These results indicate that pal genes are involved in the development of P. ostreatus fruiting bodies. In addition, this study assessed the role of pal in heat stress, providing a basis for exploring the role of the phenylpropanoid pathway in the development and stress response of P. ostreatus.
Methods
Strains, plasmids and media
The dikaryotic P. ostreatus strain CCMSSC00389 from the Center for Mushroom Spawn Standards and Control of China was used in this study as a parent strain for OE and RNAi experiments. The WT, OE and RNAi strains were maintained on potato dextrose agar (PDA) at 4 °C. For the selection and maintenance of transformants, complete media (CM) was supplemented with 90 μg/mL hygromycin (hyg) (Invitrogen, U.S.A.). Agrobacterium tumefaciens (A. tumefaciens) GV3101 (IMCAS, Beijing, China) was grown in Luria-Bertani (LB) medium (Oxoid, England) containing 100 μg/mL kanamycin (kan) (VWR Life Science, U.S.A.) and 50 μg/mL rifampicin (rif) (MP Biomedicals, France) and used to transform P. ostreatus. Escherichia coli (E. coli) DH5α and BL21 (DE3) (Tiangen, Beijing, China) were used for plasmid construction, and grown in LB broth containing kan (50 μg/mL). Restriction endonucleases were purchased from New England Biolabs (NEB), and DNA polymerase, a reverse-transcription kit, and a DNA Gel Extraction kit were purchased from Vazyme (Nanjing, China). Primer synthesis and DNA sequencing were performed by Sangon Biotech (Shanghai, China). The plasmid pCAMIBA 1300 was purchased from YRGen Biotech Company (Changsha, China).
Identification, cloning and sequence analysis of pal genes
The two pal genes were identified by keyword search in the annotated genome database of P. ostreatus strain PC15 [39], via the Joint Genome Institute website (https://genome.jgi.doe.gov/PleosPC15_2/PleosPC15_2.home.html), and two protein sequences with the following protein IDs were obtained: 1112899 and 173,727. Subsequently, the two sequences were used to BLAST against the CCMSSC00389 genome database to identify homologs. The nucleotide sequences were used to design primers (pal1 and pal2, listed in Table 1) to amplify full-length sequences from CCMSSC00389 complementary DNA (cDNA). Total RNA and DNA were extracted using TRIzol (Omega Bio-Tek, U.S.A.) and cetyltrimethylammonium bromide (CTAB), respectively. The first-strand cDNA was synthesized using a PrimeScript™ RT-PCR kit (Vazyme). The amplified products were purified and cloned into the vector pGEM-T (Promega, Madison, WI, U.S.A.) for sequencing. All primers used in the experiment are shown in Table 1.
Table 1.
Primers used in this study
| Primer | Sequence (5′ → 3′) | Note |
|---|---|---|
| Po-gpdF | GGTACCTTTATTGGCGGT | Promoter cloning |
| Po-gpdR | CCAGGTCAGTGAAATTTCC | |
| pal1_gF | ATGACAATCCTATCCGCAGAC | gDNA fragment cloning |
| pal1_gR | TTAGAATAAATCAACGATGATA | |
| pal1_cF | ATGACAATCCTATCCGCAGAC | cDNA fragment cloning |
| pal1_cR | TTAGAATAAATCAACGATGATA | |
| pal2_gF | ATGACTATTCTCTCAGGGA | gDNA fragment cloning |
| pal2_gR | CTACGCGAACATCGCTA | |
| pal2_cF | ATGACTATTCTCTCAGGGA | cDNA fragment cloning |
| pal2_cR | CTACGCGAACATCGCTA | |
| pal1-qF | CTCCTTCACAATCGCATCTA | qPCR |
| pal1-qR | CTTCAGCCGCCTATGTTG | |
| pal2-qF | CAACTGCTGCGTATGTCA | |
| pal2-qR | GATGTAGAGGTATGAGGAGATT | |
| β-actin-F | GCGATGAACAATAGCAGGG | Endogenous control |
| β-actin-R | GCTGGTATCCACGAGACAAC | |
| pal1-OE-F | ttacaggtcaaagttATGACAATCCTATCCGCAGAC | Construction of OE plasmids |
| pal1-OE-R | aattctagagggcccTTAGAATAAATCAACGATGATA | |
| pal2-OE-F | ttacaggtcaaagttATGACTATTCTCTCAGGGA | |
| pal2-OE-R | aattctagagggcccCTACGCGAACATCGCTA | |
| pal1-RNAi-F1 | actgacctggGATTTGCAACCGTTGTCTTACG | Construction of RNAi plasmids |
| pal1-RNAi-R1 | gttggagtgcaactccaCTAAAATCAGATGAGGTTGTAAGCG | |
| pal1-RNAi-F2 | tagTGGAGTTGCACTCCAACGTGA | |
| pal1-RNAi-R2 | catgccaattctagagggcccGATTTGCAACCGTTGTCTTACG | |
| pal2-RNAi-F1 | aatttcactgacctggCCACCGACAATCCTCTCATCG | |
| pal2-RNAi-R1 | gcacaaccaagcagtaaaCTAGAAAATGAGAATAAGACCTTGCTACC | |
| pal2-RNAi-F2 | agTTTACTGCTTGGTTGTGCATTTC | |
| pal2-RNAi-R2 | catgccaattctagagggcccCCACCGACAATCCTCTCATCG | |
| hyg F | CGACAGATCCGGTCGGCATCTACTCTATTTCTT | Detection of transformants |
| hyg R | TCTCGTGCTTTCAGCTTCGATGTAGGAGGG | |
| OE_gpd_pal1F | TGCGTGGTAGAAGAATGG | |
| OE_gpd_pal1R | CGATGAAGAAGGTAGAATGC | |
| OE_gpd_pal2F | CGTTCTCCGAGTCTGTTC | |
| OE_gpd_pal2R | TGATAGCGTCTTGCCATC | |
| pal1-PE-F | TCGCGGATCCGAATTCATGCAATCCTATCCGCA GAC | Construction of prokaryotic expression plasmid |
| pal1-PE-R | GAGTGCGGCCGCTTAGAATAAATCAACGATATAGGC | |
| pal2-PE-F | CGGATCCGAATTCAGACTATTCTCTAGGGACCACCG | |
| pal2-PE-R | TGCTCGAGTGCGGCCGCCTACGCAACATCGCTACCG |
Bioinformatics analysis of the pal genes
DNAMAN software was used for multiple sequence alignment. The molecular weight, distribution of amino acids, isoelectric point, and signal peptide of pal were predicted using the online ProtParam (http://web.expasy.org/protparam/). The structural domains of the PAL proteins were analyzed online (http://smart.embl-heidelberg.de/). A phylogenetic tree was constructed using the neighbor joining method in MEGA 5.0 based on the PAL nucleotide sequences obtained from GenBank and the maximum composite likelihood model. The three-dimensional (3D) structure of the PAL proteins were predicted using Modeller.
Expression and purification of pal1 and pal2 in E. coli
Expression and purification of pal1 and pal2 in E. coli were performed as previously described with slight modifications [40]. The PCR products were digested with two restriction enzymes (EcoRI-HF and NotI-HF) and ligated into the vector pET28a (Novagen, Inc., Madison, WI, U.S.A.) that had been digested with the same enzymes. The recombinant plasmids that were confirmed by DNA sequencing were named pET28a-pal1 and pET28a-pal2 and were subsequently transformed into E. coli BL21 (DE3) cells for protein expression. The transformed strains were inoculated into LB medium containing 50 μg/mL kan and incubated at 37 °C with shaking at 180 rpm until reaching an OD600 nm of 0.6–0.8. Isopropyl-β-D-thiogalactopyranoside (IPTG) was added to reach a final concentration of 1 mM to induce protein expression, and the culture was incubated overnight at 16 °C with shaking at 180 rpm.
The cultured cells were centrifuged at 4 °C and 5000 rpm for 5 min, washed with PBS buffer, and then suspended in lysis buffer. After the cells were lysed by ultrasonication, the enzymes were retained in the supernatant after centrifugation. The supernatant was loaded onto an Ni-NTA column (Qiagen, Duesseldorf, Germany) that had been preequilibrated with binding buffer. Subsequently, the column was eluted with binding buffer, washing I buffer, washing II buffer, elution I buffer and elution II buffer. Finally, the fractions were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) [41]. The PAL activities of the purified protein were determined using a Phenylalanine Ammonia-Lyase Assay kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) according to the manufacturer’s instructions.
OE and RNAi vector construction
The original pCAMBIA1300 vector was modified to harbor the hyg phosphotransferase gene (hyp), which was expressed under the control of the upstream lac promoter [28, 42]. The pal gene OE cassettes were constructed as follows. The P. ostreatus gpd promoter was PCR amplified, after which the pal1 and pal2 cDNA was obtained. The two cassettes were individually cloned into vector to generate the pal gene expression cassette driven by the P. ostreatus gpd promoter (Fig. 5a, b, c). Finally, the vector was introduced into A. tumefaciens GV3101. RNAi-F and RNAi-R fragments were obtained by PCR, after which the two amplicons were individually inserted into the vector to construct the interference vectors (Fig. 5d, e, f). Finally, the interference vectors were transferred into P. ostreatus by A. tumefaciens GV3101. The primers used to construct the vectors are shown in Table 1.
Agrobacterium-mediated transformation
P. ostreatus mycelia were inoculated onto PDA plates and cultured at 28 °C until the colony diameter was 5.5–6 cm. Mycelial pellets were cut from the edge of the colony using a cork borer with a 5-mm diameter. Subsequently, 200 pellets were placed into 100 mL of CM liquid medium at 28 °C for 2 days without shaking. A. tumefaciens GV3101 containing the OE-pal or RNAi-pal plasmid was cultivated at 28 °C with shaking at 180 rpm in LB medium with the selective antibiotics (100 μg/mL kan and 50 μg/mL rif) for at least 16 h. A. tumefaciens cells were collected in sterile tubes (50 mL capacity) by centrifugation at 4500 rpm and 4 °C for 10 min. The bacterial cells were suspended in induction medium (IM,; supplemented with 200 μM acetosyringone) and incubated for 5 h (90 rpm, 28 °C) to preinduce A. tumefaciens GV3101 virulence. Then, the A. tumefaciens GV3101 and the mycelia pellets were cocultured at 28 °C for 5 h without shaking. After incubation, the mycelial pellets were dried with filter paper and placed onto IM solid medium at 28 °C for 3 days and then transplanted onto CM medium with selective antibiotics (90 μg/mL hyg and 50 μg/mL cef). Transformants were obtained after 25 days of culturing and were subsequently selected twice for hyg resistance. PCR analysis of the hyg and pal genes was performed using the primers listed in Table 1 [42].
Mushroom production experiment and sample collection
The strains were cultured on PDA plate for 7 days. Samples mycelia were collected, frozen with liquid nitrogen and stored at − 80 °C. The remaining mycelia were inoculated on cotton seed hull culture medium and cultured at 25 °C in dark. When the mycelia were full, they were transferred to a mushroom room for mushroom production. The primordia, fruiting bodies and spores were collected and stored at − 80 °C after quick freezing with liquid nitrogen.
Western blot analysis
Western blot analysis was performed according to a previous study. Briefly, equal amounts of total protein (20 μg) were loaded into the protein lane and separated in a 12% (w/v) SDS-PAGE. After electrophoresis, proteins were transferred onto a polyvinylidene fluoride membrane. Western blot analysis was performed using antibodies against PAL1 and PAL2, which were synthesized by Shanghai GenScript Company. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH, PC15_1090663 (jgi)) was used as control [19].
Heat stress treatment
WT, pal-overexpressing (OE-pal1 7.11–9, OE-pal1 7.11–11, OE-pal2 7.11–7 and OE-pal2 7.12–11) and RNAi transformant strains (RNAi-pal1 8.1–26, RNAi-pal1 8.1–38, RNAi-pal2 7.18–1 and RNAi-pal2 7.18–19) were used in this study. The WT strain was cultured on PDA plates incubated at 28 °C for 5 days and then subjected to different temperatures to induce heat stress for 2 days. To assess the function of pal in the mycelial response to heat stress, the WT, OE-pal and RNAi-pal strains were cultured on PDA plates at different temperatures (28, 32, and 40 °C) for 6 days [19, 43].
Growth susceptibility assay
To assess the susceptibility of the WT, OE-pal and RNAi-pal strains to oxidative stress, mycelial tip pellets with 5-mm diameter were inoculated onto PDA plates supplemented with 5, 10 or 15 mM H2O2. The control groups were subjected to the nonexogenous addition of H2O2. The diameters of the strains were measured after incubation at 28 °C for 7 days [44].
Quantitative real-time PCR (qPCR)
To analyze the expression of pal at different developmental stages, samples were collected at the mycelia, primordia, fruiting body and spore stages. The levels of gene-specific mRNA expressed by the WT, OE-pal and RNAi-pal strains were analyzed using qPCR according to our previous study [19], with the β-actin gene used as a reference. The qPCR amplification procedure was as follows: 95 °C for 3 min, 40 cycles of 95 °C for 3 s and 60 °C for 32 s, and a final extension at 72 °C for 30 s. The relative gene expression was analyzed according to the 2−△△CT method.
Enzymatic activity assay
The WT, OE-pal and RNAi-pal strains were cultured on PDA medium incubated at 28 °C for 5 days. Subsequently, the mycelia were quickly scraped, mixed, and frozen in liquid nitrogen for further use. The activity of PAL was determined using a Phenylalanine Ammonia-Lyase Assay kit according to the manufacturer’s instructions.
Determination of malondialdehyde (MDA) and H2O2 contents
Intracellular MDA and H2O2 contents were determined using a Malondialdehyde and Hydrogen Peroxide Assay kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) according to the manufacturer’s instructions.
Determination of relative ion leakage and total respiratory rate
Ten pellet pieces (5 mm) were inoculated into 100 mL of potato dextrose broth medium for 5 days at 28 °C with shaking at 180 rpm. Heat stress was then applied for different durations at 40 °C (0, 3, 6, 12, 24, and 48 h). The conductivity of mycelial pellets (C1) was measured by washing off the electrolytes attached to the surface with deionized water and then placing the pellets them into 20 mL of deionized water at 28 °C for 2 h. Then, the sample was autoclaved for 30 min to determine the total conductivity (C2). The relative ion leakage rate (%) = C1/C2*100 [45]. The respiration rate was determined by measuring the production of carbon dioxide with a carbon dioxide meter (MultiRAE IR PGM-54) in sealed containers. The total respiratory rate was measured according to previous studies [22].
Data analysis
GraphPad Prism 6 (GraphPad Software, Inc., San Diego, CA, U.S.A.) was used for statistical analysis. The values are reported as the means ± SE and were analyzed by one-way ANOVA, with a P value of < 0.05 considered significant.
Supplementary information
Additional file 1: Figure S1. Amino acid sequence alignment. (A) PAL1 of CCMSSC00389 and PC15. (B) PAL2 of CCMSSC00389 and PC15. (C) PAL1 and PAL2 of CCMSSC00389.
Additional file 2: Figure S2. Nucleotide sequences alignment. (A) pal1 of CCMSSC00389 and PC15. (B) pal2 of CCMSSC00389 and PC15. (C) pal1 and pal2 of CCMSSC00389.
Additional file 3: Figure S3. Different developmental stages of P. ostreatus CCMSSC00389. (A) Mycelia. (B) Primordia. (C) Fruiting body. (D) Spores (The red arrow points to spores).
Acknowledgements
We thank all the members of the research group for their cooperation in the laboratory.
Abbreviations
- cDNA
Complementary DNA
- CM
Complete media
- CTAB
Cetyltrimethylammonium bromide
- hyg
Hygromycin
- hyp
Hygromycin phosphotransferase gene
- IM
Induction medium
- IPTG
Isopropyl-β-D-thiogalactopyranoside
- kan
Kanamycin
- LB
Luria-Bertani
- MDA
Malondialdehyde
- MIO
4-methylideneimidazole-5-one
- OE
Overexpression
- PAL
Phenylalanine ammonia-lyase
- pal
Phenylalanine ammonia-lyase gene
- PDA
Potato dextrose agar
- pI
Isolectric point
- qPCR
Quantitative real-time PCR
- rif
Rifampicin
- RNAi
RNA interference
- ROS
Reactive oxygen species
- SDS-PAGE
Sodium dodecyl sulfatepolyacrylamide gel electrophoresis
- WT
Wild type
Authors’ contributions
LDH carried out all experiments, data analysis, and manuscript writing. LNW collaborated in bioinformatics analysis and in the manuscript writing. JXZ and CYH participated in the design of the study and in the manuscript writing. WG and XLW collaborated in the manuscript writing. All authors have read and approved the final manuscript.
Funding
This research was supported by the National Basic Research Program of China (No. 2014CB138303) and China Agriculture Research System (CARS20).
Availability of data and materials
The datasets used and analysed during the current study available from the corresponding author on reasonable request.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Ludan Hou, Email: houludan@126.com.
Lining Wang, Email: wanglining90@126.com.
Xiangli Wu, Email: wuxiangli@caas.cn.
Wei Gao, Email: gaowei01@caas.cn.
Jinxia Zhang, Email: zhangjinxia@caas.cn.
Chenyang Huang, Phone: +86-10-82106207, Email: huangchenyang@caas.cn.
Supplementary information
Supplementary information accompanies this paper at 10.1186/s12866-019-1594-4.
References
- 1.Irisarri P, Zhebentyayeva T, Errea P, Pina A. Differential expression of phenylalanine ammonia lyase (PAL) genes implies distinct roles in development of graft incompatibility symptoms in Prunus. Sci Hortic. 2016;204:16–24. doi: 10.1016/j.scienta.2016.03.025. [DOI] [Google Scholar]
- 2.Kamalipourazad Maryam, Sharifi Mohsen, Maivan Hassan Zare, Behmanesh Mehrdad, Chashmi Najmeh Ahmadian. Induction of aromatic amino acids and phenylpropanoid compounds in Scrophularia striata Boiss. cell culture in response to chitosan-induced oxidative stress. Plant Physiology and Biochemistry. 2016;107:374–384. doi: 10.1016/j.plaphy.2016.06.034. [DOI] [PubMed] [Google Scholar]
- 3.Han C, Li J, Jin P, Li X, Wang L, Zheng Y. The effect of temperature on phenolic content in wounded carrots. Food Chem. 2017;215:116–123. doi: 10.1016/j.foodchem.2016.07.172. [DOI] [PubMed] [Google Scholar]
- 4.Vogt T. Phenylpropanoid biosynthesis. Mol Plant. 2010;3(1):2–20. doi: 10.1093/mp/ssp106. [DOI] [PubMed] [Google Scholar]
- 5.Wang XH, Gong M, Tang L, Zheng S, Lou JD, Ou L, Gomeslaranjo J, Zhang C. Cloning, bioinformatics and the enzyme activity analyses of a phenylalanine ammonia-lyase gene involved in dragon's blood biosynthesis in Dracaena cambodiana. Mol Biol Rep. 2013;40(1):97–107. doi: 10.1007/s11033-012-2032-y. [DOI] [PubMed] [Google Scholar]
- 6.Jiang Y, Xia N, Li X, Shen W, Liang L, Wang C, Wang R, Peng F, Xia B. Molecular cloning and characterization of a phenylalanine ammonia-lyase gene (LrPAL) from Lycoris radiata. Mol Biol Rep. 2011;38(3):1935–1940. doi: 10.1007/s11033-010-0314-9. [DOI] [PubMed] [Google Scholar]
- 7.Antje R, Kris M, John R, Geert G, Vanessa H, Riet DR, Sergej K, Jan VD, Jean-Paul J, Marnik V. Molecular phenotyping of the pal1 and pal2 mutants of Arabidopsis thaliana reveals far-reaching consequences on phenylpropanoid, amino acid, and carbohydrate metabolism. Plant Cell. 2004;16(10):2749–2771. doi: 10.1105/tpc.104.023705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tovar MJ, Romero MP, Girona J, Motilva MJ. L-phenylalanine ammonia-yase activity and concentration of phenolics in developing olive (Olea europaea L. cv Arbequina) fruit grown under different irrigation regimes. J Sci Food Agr. 2010;82(8):892–898. doi: 10.1002/jsfa.1122. [DOI] [Google Scholar]
- 9.Şirin S, Aslım B. Determination of antioxidant capacity, phenolic acid composition and antiproliferative effect associated with phenylalanine ammonia lyase (PAL) activity in some plants naturally growing under salt stress. Med Chem Res. 2018;28(3):229–238. doi: 10.1007/s00044-018-2278-6. [DOI] [Google Scholar]
- 10.Bandurska H, Cieślak M. The interactive effect of water deficit and UV-B radiation on salicylic acid accumulation in barley roots and leaves. Environ Exp Bot. 2013;94(6):9–18. doi: 10.1016/j.envexpbot.2012.03.001. [DOI] [Google Scholar]
- 11.Yeo H, Ja S, Seong H, Won S. Cloning and expression analysis of phenylalanine ammonia-lyase gene in the mycelium and fruit body of the edible mushroom Flammulina velutipes. Mycobiology. 2015;43(3):327–332. doi: 10.5941/MYCO.2015.43.3.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yoon H, You YH, Kim YE, Kim YJ, Kong WS, Kim JG. Cloning and mRNA expression analysis of the gene encoding phenylalanine ammonia-lyase of the ectomycorrhizal fungus Tricholoma matsutake. J Microbiol Biotechnol. 2013;23(8):1055–1059. doi: 10.4014/jmb.1303.03064. [DOI] [PubMed] [Google Scholar]
- 13.Moore K, Rao PVS, Towers GHN. Degradation of phenylalanine and tyrosine by basidiomycetes. Life Sci. 1967;6(24):2629–2633. doi: 10.1016/0024-3205(67)90113-0. [DOI] [PubMed] [Google Scholar]
- 14.Jaworska G, Bernaś E, Mickowska B. Effect of production process on the amino acid content of frozen and canned Pleurotus ostreatus mushrooms. Food Chem. 2011;125(3):936–943. doi: 10.1016/j.foodchem.2010.09.084. [DOI] [Google Scholar]
- 15.Mu D, Li C, Zhang X, Li X, Shi L, Ren A, Zhao M. Functions of the nicotinamide adenine dinucleotide phosphate oxidase family in Ganoderma lucidum: an essential role in ganoderic acid biosynthesis regulation, hyphal branching, fruiting body development, and oxidative-stress resistance. Environ Microbiol. 2014;16(6):1709–1728. doi: 10.1111/1462-2920.12326. [DOI] [PubMed] [Google Scholar]
- 16.Hsu KH, Lee YR, Lin YL, Chu FH. Cytochrome P450 genes in medicinal mushroom Antrodia cinnamomea T.T. Chang et W.N. Chou (higher basidiomycetes) are strongly expressed during fruiting body formation. Int J Med Mushrooms. 2011;13(6):513–523. doi: 10.1615/IntJMedMushr.v13.i6.30. [DOI] [PubMed] [Google Scholar]
- 17.Yan JJ, Zhang L, Wang RQ, Xie B, Li X, Chen RL, Guo LX, Xie BG. The sequence characteristics and expression models reveal superoxide dismutase involved in cold response and fruiting body development in Volvariella volvacea. Int J Mol Sci. 2016;17(1):34. doi: 10.3390/ijms17010034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Madhavan S, Krause K, Jung EM, Kothe E. Differential regulation of multi-copper oxidases in Schizophyllum commune during sexual development. Mycol Progress. 2014;13(4):1199–1206. doi: 10.1007/s11557-014-1009-8. [DOI] [Google Scholar]
- 19.Wang L, Wu X, Gao W, Zhao M, Zhang J, Huang C. Differential expression patterns of Pleurotus ostreatus catalase genes during developmental stages and under heat stress. Genes (Basel) 2017;8(11):335. doi: 10.3390/genes8110335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Palmer GE, Horton JS. Mushrooms by magic: making connections between signal transduction and fruiting body development in the basidiomycete fungus Schizophyllum commune. FEMS Microbiol Lett. 2006;262:1):1–1):8. doi: 10.1111/j.1574-6968.2006.00341.x. [DOI] [PubMed] [Google Scholar]
- 21.Chang ST, Miles PG. Mushrooms: cultivation, nutritional value, medicinal effect, and environmental impact. Boca Raton: CRC Press; 2004. pp. 688–692. [Google Scholar]
- 22.Zhang RY, Hu DD, Zhang YY, Goodwin PH, Huang CY, Chen Q, Gao W, Wu XL, Zou YJ, Qu JB. Anoxia and anaerobic respiration are involved in “spawn-burning” syndrome for edible mushroom Pleurotus eryngii grown at high temperatures. Sci Hortic. 2016;199:75–80. doi: 10.1016/j.scienta.2015.12.035. [DOI] [Google Scholar]
- 23.Qiu Z, Wu X, Zhang J, Huang C. High temperature enhances the ability of Trichoderma asperellum to infect Pleurotus ostreatus mycelia. PLoS One. 2017;12(10):e0187055. doi: 10.1371/journal.pone.0187055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Song C, Chen Q, Wu X, Zhang J, Huang C. Heat stress induces apoptotic-like cell death in two Pleurotus species. Curr Microbiol. 2014;69(5):611–616. doi: 10.1007/s00284-014-0634-4. [DOI] [PubMed] [Google Scholar]
- 25.Lei M, Wu X, Zhang J, Wang H, Huang C. Gene cloning, expression, and characterization of trehalose-6-phosphate synthase from Pleurotus ostreatus. J Basic Microbiol. 2017;57(7):580–589. doi: 10.1002/jobm.201700120. [DOI] [PubMed] [Google Scholar]
- 26.Zou Y, Zhang M, Qu J, Zhang J. iTRAQ-based quantitative proteomic analysis reveals proteomic changes in mycelium of Pleurotus ostreatus in response to heat stress and subsequent recovery. Front Microbiol. 2018;9:2368. doi: 10.3389/fmicb.2018.02368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pallas JA, Paiva NL, Lamb C, Dixon RA. Tobacco plants epigenetically suppressed in phenylalanine ammonia-lyase expression do not develop systemic acquired resistance in response to infection by tobacco mosaic virus. Plant J. 1996;10(2):281–293. doi: 10.1046/j.1365-313X.1996.10020281.x. [DOI] [Google Scholar]
- 28.Yin C, Zheng L, Zhu J, Chen L, Ma A. Enhancing stress tolerance by overexpression of a methionine sulfoxide reductase a (MsrA) gene in Pleurotus ostreatus. Appl Microbiol Biotechnol. 2015;99(7):3115–3126. doi: 10.1007/s00253-014-6365-4. [DOI] [PubMed] [Google Scholar]
- 29.Lin W, Liu A, Weng C, Li H, Sun S, Song A, Zhu H. Cloning and characterization of a novel phenylalanine ammonia-lyase gene from Inonotus baumii. Enzyme Microb Technol. 2018;112:52–58. doi: 10.1016/j.enzmictec.2017.10.010. [DOI] [PubMed] [Google Scholar]
- 30.Ritter H, Schulz GE. Structural basis for the entrance into the phenylpropanoid metabolism catalyzed by phenylalanine ammonia-lyase. Plant Cell. 2004;16(12):3426–3436. doi: 10.1105/tpc.104.025288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yun YH, Koo JS, Kim SH, Kong WS. Cloning and expression analysis of phenylalanine ammonia-lyase gene in the mycelium and fruit body of the edible mushroom Flammulina velutipes. Mycobiology. 2015;43(3):327–332. doi: 10.5941/MYCO.2015.43.3.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hyun MW, Yun YH, Kim JY, Kim SH. Fungal and plant phenylalanine ammonia-lyase. Mycobiology. 2011;39(4):257–265. doi: 10.5941/MYCO.2011.39.4.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang J, Gu M, Lai Z, Fan B, Shi K, Zhou Y, Yu J, Chen Z. Functional analysis of the Arabidopsis PAL gene family in plant growth, development, and response to environmental stress. Plant Physiol. 2010;153(4):1526–1538. doi: 10.1104/pp.110.157370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tasaki Y, Miyakawa H. Structure and expression of two phenylalanine ammonia-lyase genes of the basidiomycete mushroom Tricholoma matsutake. Mycoscience. 2015;56(5):503–511. doi: 10.1016/j.myc.2015.03.001. [DOI] [Google Scholar]
- 35.Qu AL, Ding YF, Jiang Q, Zhu C. Molecular mechanisms of the plant heat stress response. Biochem Biophys Res Commun. 2013;432(2):203–207. doi: 10.1016/j.bbrc.2013.01.104. [DOI] [PubMed] [Google Scholar]
- 36.Fahad S, Bajwa AA, Nazir U, Anjum SA, Farooq A, Zohaib A, Sadia S, Nasim W, Adkins S, Saud S. Crop production under drought and heat stress: plant responses and management options. Front Plant Sci. 2017;8:1147. [DOI] [PMC free article] [PubMed]
- 37.Eliseu R, Mariutti LRB, Faria AF, Mercadante AZ. Microcapsules containing antioxidant molecules as scavengers of reactive oxygen and nitrogen species. Food Chem. 2012;134(2):704–711. doi: 10.1016/j.foodchem.2012.02.163. [DOI] [PubMed] [Google Scholar]
- 38.Cass CL, Peraldi A, Dowd PF, Mottiar Y, Santoro N, Karlen SD, Bukhman YV, Foster CE, Thrower N, Bruno LC. Effects of phenylalanine ammonia lyase (pal) knockdown on cell wall composition, biomass digestibility, and biotic and abiotic stress responses in Brachypodium. J Exp Bot. 2015;66(14):4317–4335. doi: 10.1093/jxb/erv269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Robert R, Salamov AA, Brown DW, Nagy LG, Dimitrios F, Held BW, Anthony L, Vincent L, Emmanuelle M, Robert O. Extensive sampling of basidiomycete genomes demonstrates inadequacy of the white-rot/brown-rot paradigm for wood decay fungi. Proc Natl Acad Sci U S A. 2014;111(27):9923–9928. doi: 10.1073/pnas.1400592111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wu X, Hou Z, Huang C, Chen Q, Gao W, Zhang J. Cloning, purification and characterization of trehalose-6-phosphate synthase from Pleurotus tuoliensis. PeerJ. 2018;6(7):e5230. doi: 10.7717/peerj.5230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang J, Yang D, Yan Q, Jiang Z. Characterization of a novel L-phenylalanine oxidase from Coprinopsis cinereus and its application for enzymatic production of phenylpyruvic acid. Process Biochem. 2017;61:102–109. doi: 10.1016/j.procbio.2017.06.021. [DOI] [Google Scholar]
- 42.Ding Y, Liang S, Lei J, Chen L, Kothe E, Ma A. Agrobacterium tumefaciens mediated fused egfp-hph gene expression under the control of gpd promoter in Pleurotus ostreatus. Microbiol Res. 2011;166(4):314–322. doi: 10.1016/j.micres.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 43.Zhang MJ, Liu XM, Zou YJ, Huang CY, Liu B, Zhang JX. Optimization of heat stress for Pleurotus spp. cultivation. Mycosystema. 2015;34(4):662–669. [Google Scholar]
- 44.Zhang G, Sun Z, Ren A, Shi L, Shi D, Li X, Zhao M. The mitogen-activated protein kinase GlSlt2 regulates fungal growth, fruiting body development, cell wall integrity, oxidative stress and ganoderic acid biosynthesis in Ganoderma lucidum. Fungal Genet Biol. 2017;104:6–15. doi: 10.1016/j.fgb.2017.04.004. [DOI] [PubMed] [Google Scholar]
- 45.Tossi V, Amenta M, Lamattina L, Cassia R. Nitric oxide enhances plant ultraviolet-B protection up-regulating gene expression of the phenylpropanoid biosynthetic pathway. Plant Cell Environ. 2011;34(6):909–921. doi: 10.1111/j.1365-3040.2011.02289.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Figure S1. Amino acid sequence alignment. (A) PAL1 of CCMSSC00389 and PC15. (B) PAL2 of CCMSSC00389 and PC15. (C) PAL1 and PAL2 of CCMSSC00389.
Additional file 2: Figure S2. Nucleotide sequences alignment. (A) pal1 of CCMSSC00389 and PC15. (B) pal2 of CCMSSC00389 and PC15. (C) pal1 and pal2 of CCMSSC00389.
Additional file 3: Figure S3. Different developmental stages of P. ostreatus CCMSSC00389. (A) Mycelia. (B) Primordia. (C) Fruiting body. (D) Spores (The red arrow points to spores).
Data Availability Statement
The datasets used and analysed during the current study available from the corresponding author on reasonable request.