Abstract
Pediatric growth charts are used to monitor children’s growth and development, and assist in healthcare delivery and supervision. Researchers have developed syndrome-specific growth charts for several disorders in which affected children grow differently than unaffected children. Typical standardized growth curves have been recently reported in non-growth hormone treated infants with Prader-Willi syndrome (PWS) of both genders from birth to 36 months of age to support monitoring growth and nutritional status during infancy, and to guide growth hormone treatment and follow up. Electronic health record (EHR) systems serving pediatric populations can now incorporate interactive automated growth charts to assist the healthcare providers in clinical settings to monitor growth. The American Academy of Pediatrics has recommended that EHR systems incorporate syndrome-specific growth charts where feasible. This manuscript describes the experiences at two medical centers at different stages of incorporating electronic growth charts into the medical records on infants with rare disorders, using PWS as an illustration. The absence of readily available and accessible syndrome-specific standardized growth charts in other disorders causes challenges for EHR personnel and for healthcare providers who care for these children particularly in the growing electronic medical record environment.
Keywords: medical informatics applications, medical informatics computing, Prader-Willi syndrome, user-computer interface, pediatric growth charts
INTRODUCTION
Pediatric growth charts are specialized tools designed to help healthcare providers screen children for diseases that involve growth, and to monitor management of clinical treatments that may influence growth [2010]. Plotting children’s growth on hard copies of growth charts is a standard of pediatric care around the world for many years [de Onis et al., 2004]. To support this, the Centers for Disease Control and Prevention (CDC) [Ogden et al., 2002] and the World Health Organization (WHO) [Grummer-Strawn et al., 2010] have published growth charts for normally developing children. Additionally, investigators have published specialized growth charts for children with genetic syndromes (e.g., Down syndrome [Cronk et al., 1988; Rosenbloom et al., 2010] and Prader-Willi syndrome (PWS) [Butler and Meaney, 1991; Butler et al., 2011]). As healthcare providers increasingly adopt electronic health record (EHR) systems in practice, there is a growing need to make available growth charts that can be readily integrated. While the authors are not aware of any research evaluating how growth charts have been implemented into the different available EHR systems, reports suggest that most pediatric EHR systems include modest growth charting capabilities for normally developing children [Rosenbloom et al., 2006]. The American Academy of Pediatrics (AAP) Task Force on Medical Informatics has published “Special Requirements for Electronic Medical Record Systems in Pediatrics” [Spooner, 2007] which calls for EHR systems serving children to chart growth based on their diagnoses. However, there are only limited reports describing successes and challenges associated with integrating growth charts into EHR systems for children with genetic syndromes influencing growth [Rosenbloom et al., 2010]. In this manuscript, the authors describe efforts to implement recently developed growth charts for PWS into different EHR systems at two large medical centers at different levels of EHR system deployment.
PWS occurs in about 1 in 10,000–20,000 live births, involving all ethnic groups, and is the most common genetic syndromic cause of life-threatening obesity if not adequately addressed [Butler, 1990, 2009; Butler and Thompson, 2000; Goldstone et al., 2008]. PWS is a complex condition due to errors in genomic imprinting with disturbance of paternally expressed genes in the chromosome 15q11-q13 region, usually from de novo paternal deletions in about 70% of subjects [Butler, 1990, 2011; Bittel and Butler, 2005; Butler et al., 2008; Cassidy and Driscoll, 2009]. Maternal disomy 15 is the second most common form of PWS (about 25% of cases) followed by imprinting center defects due to microdeletions (or epimutations) or other chromosome 15 rearrangements (e.g., translocations) [Bittel and Butler, 2005; Cassidy and Driscoll, 2009; Butler, 2011].
Obesity is the most significant health problem in PWS. Dietary restrictions and increased physical activity are key management issues, but recombinant human growth hormone therapy (GH) has led to increased stature, decreased body weight and fat, increased muscle mass and physical activity and a higher quality of life for PWS individuals [Eiholzer et al., 1998; Ritzen et al., 1999; Myers et al., 2000; Haqq et al., 2003; Mogul et al., 2008; Sode-Carlsen et al., 2010; Sode-Carlsen et al., 2011]. Most children with PWS are born with a normal weight and height, but due to hypotonia and a poor suck, failure-to-thrive develops. By 2–4 years of age, individuals with PWS begin to overeat and develop a rapid weight gain with fat mass increasing more rapidly than muscle mass or stature [Butler et al., 1986, 1988; Butler and Meaney, 1987; Butler, 1990]. Short stature in PWS is more related to shorter lower body segment compared with upper body in both sexes. Small hands and feet are common, but more remarkable in females [Butler et al., 1991]. A narrow forehead and dolichocephaly are also common in this disorder [Butler et al., 1986; Meaney and Butler, 1987; Butler, 1990]. Meaney and Butler [1989] also reported negative correlations between height and age suggesting a relative slowing down of linear growth with age in non-GH treated individuals with PWS.
Because of a paucity of US growth standards in PWS, Butler and Meaney [1991] published standardized curves for 14 anthropometric measurements for individuals with PWS ages 2–22 years for both Caucasian males and females from the USA and not treated with growth hormone. Those growth standards can be used to monitor the growth of individuals with PWS, after infancy particularly before growth hormone treatment. From this study, weight in both male and female PWS subjects was compared and the 50th centile approximated the normal 95th centile. For height in female PWS subjects, the 50th centile closely approximated the normal 5th centile and by 12 years of age the 50th centile fell below the normal 5th centile and continued to drop off relative to the normal curve. Similarly, in male subjects with PWS, the 50th centile fell below the normal 5th centile by 14 years of age, but with a steeper drop-off than in female subjects. For head circumference in both males and females with PWS and healthy individuals, the 50th centile was generally between the normal 50th and 5th centiles, approaching the normal 5th centile by 18 years of age. Standardized growth curves have been published for common anthropometric parameters for individuals with PWS in several countries including the USA, Germany, and Japan useful for comparison purposes and for monitoring growth and development [Butler et al., 2006].
To generate new standardized growth curves for infants and toddlers, Butler et al. [2011] recently reported on weight, length, head circumference, weight/length, and body mass index (BMI) from non-growth hormone treated white infants (males and females) with PWS between 0 and 36 months of age. Five separate standardized growth curves (weight, length, head circumference, weight/length, and BMI) representing seven centile ranges were developed from 186 non-growth hormone treated infants with PWS and the normative 50th centile plotted on each standardized infant growth curve. The data supporting these growth charts are also available as data tables, standardized and configured according to the format used by CDC and WHO for growth chart development [Ogden et al., 2002]. These growth standards can be used to monitor growth when examining infants with PWS and other infants for comparison purposes and assessing nutritional status and responses to growth hormone therapy.
MATERIALS AND METHODS
This report involves two medical centers at two different stages of EHR development and implementation. The Vanderbilt University Medical Center (VUMC) is a primary and tertiary care facility with large local and regional primary referral bases for patients across the Southeast. VUMC includes the 247 bed Monroe Carell Jr. Children’s Hospital at Vanderbilt (VCH) and the Doctors’ Office Tower. In 2009, there were 235,849 pediatric visits to VCH, including 171,000 children seen in outpatient clinics. A total of seven patients diagnosed with PWS were seen at VUMC genetic clinics between 2009 and 2012. The VUMC includes multidisciplinary clinics that address the multiple medical, social, and nutritional needs of children and families with genetic syndromes such as PWS. The VUMC has in place numerous locally developed health information technologies, including a care provider order entry system and a well developed and implemented EHR system with clinical documentation. The EHR system includes pediatric growth charts for normally developing children, for premature infants, and for children with Down syndrome since 2004. Growth charts for normally developing children are based on World Health Organization standards for children 0–24months, and Centers for Disease Control and Prevention (CDC) for ages 24 months through 20 years. As published elsewhere [Rosenbloom et al., 2006, 2010], the VUMC EHR system growth chart software is designed to incorporate additional growth chart standards that are configured according to the format used by the CDC and WHO.
The University of Kansas Medical Center (KUMC) is a primary and tertiary care facility located in Kansas City, and serves as a referral base for the Midwest, primarily Kansas and Missouri. KUMC includes the University of Kansas Hospital (KUH) and clinics. In 2010, KUMC provided care for 113,000 patients, representing all 105 counties in Kansas and 86% of Missouri counties and contributed to 361,652 outpatient visits, 46,209 emergency department visits and 26,108 hospital admissions. KUH averages 4,415 pediatric hospital admissions per year and approximately 37,000 outpatient visits per year based on a 3-year average with services provided by the Department of Pediatrics. During 2011, 40 individuals with PWS (22 infants/children and 18 adults) were seen at KUMC in the outpatient setting, accounting for 85 outpatient visits. The number of individuals seen with PWS is higher than expected at KUMC based on the population served which directly reflects the clinic and research record of Dr. Butler who specializes in treating individuals with PWS and in conducting research related to this disorder. University of Kansas Physicians began using a commercially available EHR system in November 2007. EHR system deployment began in inpatient areas with nursing documentation, medication administration, pharmacy, and clerical order entry. Physician documentation was deployed in 2009, followed by care provider order entry and ambulatory use of the EHR system in 2010 through 2012. At the time of this report, outpatient pediatric clinics are implemented the ambulatory EHR system in April 2012. The EHR system currently has in place standard pediatric growth charts from the CDC database, but no other population-based electronic growth charts for healthcare providers for use in the clinical setting.
RESULTS
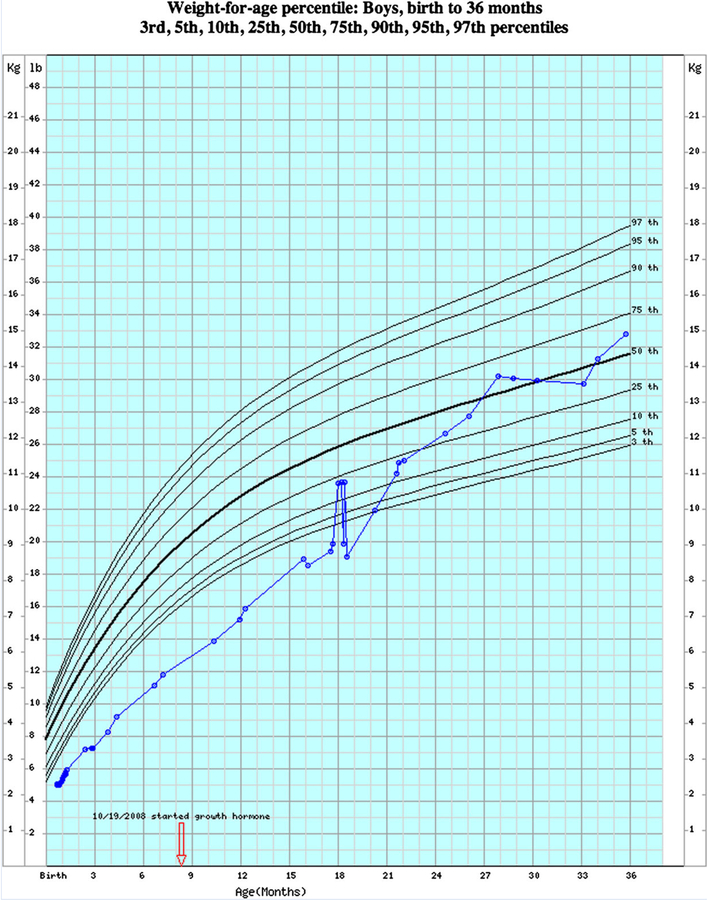
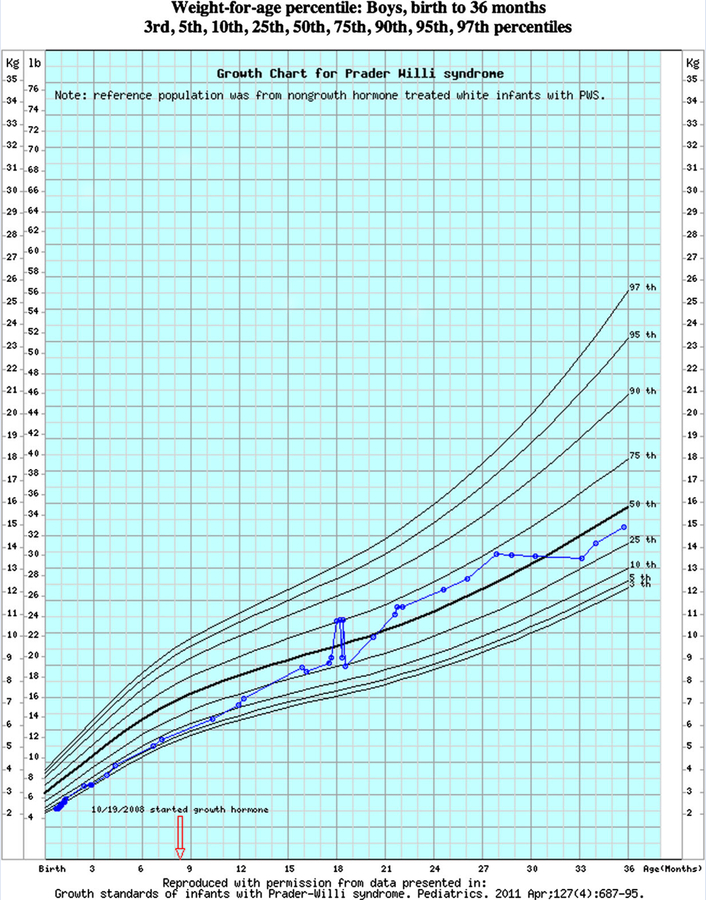
At VUMC, children seen in outpatient clinics typically go through an intake process that includes a measurement of length or height, weight and—for those less than 36 months—head circumference. These values are recorded into an EHR system-based intake form, alongside documentation of the child’s clinical problems, current medications and allergies. For patients receiving care in the hospital or emergency department, measurements of height and weight are also recorded in order entry systems. Once recorded, all values for length or height, weight, and head circumference become automatically available for inclusion into pediatric growth charts. Data tables for PWS growth charts available from Butler et al. [2011] were used to develop and implement PWS-specific electronic growth charts. Developers at VUMC implemented the electronic version of PWS-specific growth charts for children ages 0–36 months in May 2011, 1 month after the charts were initially published. Healthcare providers seeing patients at VUMC can access the PWS charts by launching the standard EHR growth charts and selecting an option, “correct for Prader-Willi syndrome” in a menu of growth chart actions available at the top of the page. Sample growth charts from a male with PWS with several data points plotted electronically at VUMC are shown in Figures 1 and 2.
FIG. 1.
Electronic version of the normative weight-for-age growth chart standardized and configured according to format used by CDC and WHO growth data from boys from birth to 36 months. For illustration, weight measures obtained from birth to 36 months for a boy with Prader-Willi syndrome followed in the clinical setting plotted electronically on the interactive normative non-PWS growth chart.
FIG. 2.
Syndrome-specific electronic growth chart for weight-for-age generated from data on non-growth hormone treated boys with Prader-Willi syndrome from birth to 36 months. For illustration, weight measures obtained from birth to 36 months from a boy with Prader-Willi syndrome followed in the clinical setting plotted electronically on the interactive PWS growth chart.
At KUMC and at the time of this report, the EHR system has been installed throughout the inpatient hospital, and has been implemented in outpatient pediatric settings for 1 month. The EHR system includes standard population-based growth charts for children without PWS. While the EHR system does have in place the necessary capabilities to allow development of special population-based growth chart standards to be configured according to the format used by CDC and WHO, the electronic PWS growth standards have not been implemented as an alternative option at this time. The current practice is to use the standardized growth charts for infants with PWS by placing a paper-based hard copy of the gender specific curves for length, weight, and head circumference into each of the patient’s medical record. Each growth parameter is measured routinely and recorded during the clinic visit on each infant or obtained from an outside source (e.g., weekly measurements by parents or other healthcare providers and sent to us by email or by phone). The data are then hand plotted accordingly on the graphs which are required to monitor weight in relationship to caloric intake and failure to thrive and length in relationship to growth hormone therapy (failure to thrive and growth hormone therapy are common in most infants with PWS). The growth data are then visually analyzed and maintained to compare the rate of growth, both length and weight, with others similarly affected at the same age and sex. If growth hormone is administered, then the onset of therapy and change in dosage is recorded by hand on each paper chart at each date, and similarly if changes occur in nutritional status and feeding information (e.g., G-tube placement) and caloric intake recorded.
DISCUSSION
Anthropometric standards have been developed for several syndromes and used successfully in the medical management of patients. The use of growth standards has become routine in most clinical genetics centers for monitoring growth and development of patients with certain syndromes although there is a paucity of standardized syndrome and age specific growth curves, and even less in the clinical setting, that can be accessed and plotted electronically. Medical care of individuals who have PWS is complex, particularly monitoring abnormal growth parameters (e.g., weight, height, head circumference) before and during growth hormone therapy in infancy. Due to early recognition and genetic diagnosis, growth hormone therapy begins at a young age in PWS which impacts on length, body weight and composition further emphasizing the importance of accessible standards in infants with PWS thereby assisting the clinician and dietitian in monitoring nutrition, growth and development. Growth hormone (GH) deficiency in PWS is lifelong and can cause problems beyond infancy including poor body composition, reduced energy and physical performance, osteoporosis, and disorders of sleep and mood. Although studies in adults with PWS are limited, early studies on GH treatment in adults with PWS showed that 1 year of growth hormone treatment would be beneficial [Davies et al., 1998; Hoybye, 2007; Mogul et al., 2008; Sode-Carlsen et al., 2010]. With the advent of electronic medical records and surveillance, it is crucial that electronic versions of standardized syndrome specific growth curves for all ages become available to healthcare providers involved in a multidisciplinary approach in managing and treating individuals with rare disorders and for natural history studies not only in PWS and Down syndrome.
This manuscript describes the use of growth charts for individuals with PWS at two medical centers. At one center (VUMC), EHR systems were quickly modified to incorporate an electronic version of PWS growth charts once they were published for use in the inpatient or outpatient setting. At the other center (KUMC), growth among children with PWS continues to be monitored using paper-based growth charts despite that electronic growth charts have become fully implemented in both inpatient and outpatient pediatric settings. The authors speculate several reasons contributed to the differential implementation at the two centers. First, by being entirely developed in-house, the EHR system at VUMC allowed local onsite developers to have the flexibility to make rapid changes based on user-perceived needs. By contrast, the EHR system at KUMC is a commercially available system that is used by numerous health centers. Any major changes to that system require prioritization by the vendor itself, and must be balanced by needs that other institutions and customers may have. While the specific EHR system used at KUMC does allow local onsite developers to implement focused changes (e.g., in the outpatient setting), including adding new population-specific growth charts, the fact that the system was developed elsewhere acts as a barrier to modification. Second, at the time of this report, the two centers were at different stages of EHR system rollout and use. At VUMC, growth charts had been implemented into the EHR system across all sites of pediatric care for over 7 years, and included charts for other special populations. At KUMC, growth charts were only implemented in inpatient settings where the EHR system was also deployed, and included no charts for other special populations. The lack of full implementation likely reduced demand for EHR system-based growth charts for children with PWS (or other syndromes). Third, PWS is an uncommon syndrome. Implementing growth charts for children with PWS requires having in place a robust infrastructure and culture around specialized health information technologies that healthcare providers are accustomed to using in the first place if they are going to use new tools. For example, in addition to implementing EHR system-based growth charts for specialized populations, the center would also need to have in place an expectation by clinical users that such charts are likely to be available. In the case that numerous specialized growth charts were available, EHR systems would require methods to make it easy for users to sort through all available growth charts for special populations. Methods might include leveraging simple user interfaces, being driven by diagnoses present in an encoded electronic problem list, or being triggered by the presence in electronic medical records of relevant syndrome-specific genetic testing results (e.g., microarray findings or DNA mutations).
Any new tool proposed for use in the healthcare setting will have certain limitations before development and implementation, including the use of syndrome specific electronic growth charts. One important limitation includes the rarity and recognition of certain disorders affecting growth such as PWS and the awareness of the healthcare provider to seek out and access growth charts for special populations in the general clinic setting. This would be less of a problem in specialty or syndrome-specific clinics in the future serving these patients (e.g., clinics developed by Dr. Butler at KUMC as a regional and national referral base for PWS). As more information is learned about rare disorders affecting growth and advances in genetic testing allowing for early diagnosis and interventions, the need for available EHR system-based growth charts for special populations will be further emphasized, with PWS being a classical example.
REFERENCES
- Bittel DC, Butler MG. 2005. Prader-Willi syndrome: Clinical genetics, cytogenetics and molecular biology. Expert Rev Mol Med 7:1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler MG. 1990. Prader-Willi syndrome: Current understanding of cause and diagnosis. Am J Med Genet 35:319–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler MG. 2009. Genomic imprinting disorders in humans: A mini-review. J Assist Reprod Genet 26:477–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler MG. 2011. Prader-Willi syndrome: Obesity due to genomic imprinting. Curr Genomics 12:204–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler MG, Butler RI, Meaney FJ. 1988. The use of skinfold measurements to judge obesity during the early phase of Prader-Labhart-Willi syndrome. Int J Obes 12:417–422. [PMC free article] [PubMed] [Google Scholar]
- Butler MG, Fischer W, Kibiryeva N, Bittel DC. 2008. Array comparative genomic hybridization (aCGH) analysis in Prader-Willi syndrome. Am J Med Genet A 146:854–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler MG, Haynes JL, Meaney FJ. 1991. Anthropometric study with emphasis on hand and foot measurements in the Prader-Willi syndrome: Sex, age and chromosome effects. Clin Genet 39:39–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler MG, Lee PDK, Whitman BY. 2006. Management of Prader-Willi syndrome. New York, NY: Springer-Verlag; 550 p. [Google Scholar]
- Butler MG, Meaney FJ. 1987. An anthropometric study of 38 individuals with Prader-Labhart-Willi syndrome. Am J Med Genet 26:445–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler MG, Meaney FJ. 1991. Standards for selected anthropometric measurements in Prader-Willi syndrome. Pediatrics 88:853–860. [PMC free article] [PubMed] [Google Scholar]
- Butler MG, Meaney FJ, Palmer CG. 1986. Clinical and cytogenetic survey of 39 individuals with Prader-Labhart-Willi syndrome. Am J Med Genet 23:793–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler MG, Sturich J, Lee J, Myers SE, Whitman BY, Gold JA, Kimonis V, Scheimann A, Terrazas N, Driscoll DJ. 2011. Growth standards of infants with Prader-Willi syndrome. Pediatrics 127:687–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler MG, Thompson T. 2000. Prader-Willi syndrome: Clinical and genetic findings. Endocrinologist 10:3S–16S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassidy SB, Driscoll DJ. 2009. Prader-Willi syndrome. Eur J Hum Genet 17:3–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronk C, Crocker AC, Pueschel SM, Shea AM, Zackai E, Pickens G, Reed RB. 1988. Growth charts for children with Down syndrome: 1 month to 18 years of age. Pediatrics 81:102–110. [PubMed] [Google Scholar]
- Davies PS, Evans S, Broomhead S, Clough H, Day JM, Laidlaw A, Barnes ND. 1998. Effect of growth hormone on height, weight, and body composition in Prader-Willi syndrome. Arch Dis Child 78:474–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Onis M, Wijnhoven TM, Onyango AW. 2004. Worldwide practices in child growth monitoring. J Pediatr 144:461–465. [DOI] [PubMed] [Google Scholar]
- Eiholzer U, Gisin R, Weinmann C, Kriemler S, Steinert H, Torresani T, Zachmann M, Prader A. 1998. Treatment with human growth hormone in patients with Prader-Labhart-Willi syndrome reduces body fat and increases muscle mass and physical performance. Eur J Pediatr 157: 368–377. [DOI] [PubMed] [Google Scholar]
- Goldstone AP, Holland AJ, Hauffa BP, Hokken-Koelega AC, Tauber M. 2008. Recommendations for the diagnosis and management of Prader-Willi syndrome. J Clin Endocrinol Metab 93:4183–4197. [DOI] [PubMed] [Google Scholar]
- Grummer-Strawn L, Reinold C, Krebs NF. 2010. Use of World Health Organization and CDC growth charts for children aged 0–59 months in the United States. Morb Mortal Wkly Rep 59:1–15. [PubMed] [Google Scholar]
- Haqq AM, Stadler DD, Jackson RH, Rosenfeld RG, Purnell JQ, LaFranchi SH. 2003. Effects of growth hormone on pulmonary function, sleep quality, behavior, cognition, growth velocity, body composition, and resting energy expenditure in Prader-Willi syndrome. J Clin Endocrinol Metab 88:2206–2212. [DOI] [PubMed] [Google Scholar]
- Hoybye C. 2007. Five-years growth hormone (GH) treatment in adults with Prader-Willi syndrome. Acta Paediatr 96:410–413. [DOI] [PubMed] [Google Scholar]
- Meaney FJ, Butler MG. 1987. Craniofacial variation and growth in the Prader-Labhart-Willi syndrome. Am J Phys Anthropol 74:459–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meaney FJ, Butler MG. 1989. The developing role of anthropologists in medical genetics: Anthropometric assessment of the Prader-Labhart-Willi syndrome as an illustration. Med Anthropol 10:247–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogul HR, Lee PD, Whitman BY, Zipf WB, Frey M, Myers S, Cahan M, Pinyerd B, Southren AL. 2008. Growth hormone treatment of adults with Prader-Willi syndrome and growth hormone deficiency improves lean body mass, fractional body fat, and serum triiodothyronine without glucose impairment: Results from the United States multicenter trial. J Clin Endocrinol Metab 93:1238–1245. [DOI] [PubMed] [Google Scholar]
- Myers SE, Carrel AL, Whitman BY, Allen DB. 2000. Sustained benefit after 2 years of growth hormone on body composition, fat utilization, physical strength and agility, and growth in Prader-Willi syndrome. J Pediatr 137:42–49. [DOI] [PubMed] [Google Scholar]
- Ogden CL, Kuczmarski RJ, Flegal KM, Mei Z, Guo S, Wei R, Grummer-Strawn LM, Curtin LR, Roche AF, Johnson CL. 2002. Centers for Disease Control and Prevention 2000 Growth Charts for the United States: Improvements to the 1977 National Center for Health Statistics Version. Pediatrics 109:45–60. [DOI] [PubMed] [Google Scholar]
- Ritzen EM, Lindgren AC, Hagenas L, Marcus C, Muller J, Blichfeldt S. 1999. Growth hormone treatment of patients with Prader-Willi syndrome. Swedish Growth Hormone Advisory Group. J Pediatr Endocrinol Metab 12:345–349. [PubMed] [Google Scholar]
- Rosenbloom ST, McGregor TL, Chen Q, An AQ, Hsu S, Dupont WD. 2010. Specialized pediatric growth charts for electronic health record systems: The example of Down syndrome. AMIA Annu Symp Proc 2010:687–691. [PMC free article] [PubMed] [Google Scholar]
- Rosenbloom ST, Qi X, Riddle WR, Russell WE, DonLevy SC, Giuse D, Sedman AB, Spooner SA. 2006. Implementingpediatric growth charts into an electronic health record system. J Am Med Inform Assoc 13:302–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sode-Carlsen R, Farholt S, Rabben KF, Bollerslev J, Schreiner T, Jurik AG, Christiansen JS, Hoybye C. 2010. One year of growth hormone treatment in adults with Prader-Willi syndrome improves body composition: Results from a randomized, placebo-controlled study. J Clin Endocrinol Metab 95:4943–4950. [DOI] [PubMed] [Google Scholar]
- Sode-Carlsen R, Farholt S, Rabben KF, Bollerslev J, Schreiner T, Jurik AG, Frystyk J, Christiansen JS, Hoybye C. 2011. Growth hormone treatment for two years is safe and effective in adults with Prader-Willi syndrome. Growth Horm IGF Res 21:185–190. [DOI] [PubMed] [Google Scholar]
- Spooner SA. 2007. Special requirements of electronic health record systems in pediatrics. Pediatrics 119:631–637. [DOI] [PubMed] [Google Scholar]