Abstract
Objective:
To identify number of children who received live vaccines outside recommended intervals between doses and calculate corrective revaccination costs.
Methods:
We analyzed >1.6 million vaccination records for children aged 12 months through 6 years from six immunization information system (IIS) Sentinel Sites from 2014–15 when live attenuated influenza vaccine (LAIV, FluMist® Quadrivalent) was recommended for use, and from 2016–17, when not recommended for use. Depending on the vaccine, insufficient intervals between live vaccine doses are less than 24 or 28 days from a preceding live vaccine dose. Private and public purchase costs of vaccines were used to determine revaccination costs of live vaccine doses administered during the live vaccine conflict interval. Measles, mumps, rubella (MMR), varicella, combined MMRV, and LAIV were live vaccines evaluated in this study.
Results:
Among 946,659 children who received at least one live vaccine dose from 2014–15, 4,873 (0.5%) received at least one dose too soon after a prior live vaccine (revaccination cost, $786,413) with a median conflict interval of 16 days. Among 704,591 children who received at least one live vaccine dose from 2016–17, 1,001 (0.1%) received at least one dose too soon after a prior live vaccine (revaccination cost, $181,565) with a median conflict interval of 14 days. The live vaccine most frequently administered outside of the recommended intervals was LAIV from 2014–15, and varicella from 2016–17.
Conclusions:
Live vaccine interval errors were rare (0.5%), indicating an adherence to recommendations. If all invalid doses were corrected by revaccination over the two time periods, the cost within the IIS Sentinel Sites would be nearly one million dollars. Provider awareness about live vaccine conflicts, especially with LAIV, could prevent errors, and utilization of clinical decision support functionality within IISs and Electronic Health Record Systems can facilitate better vaccination practices.
Keywords: Live vaccines, Vaccination errors, Children, Immunization information systems
Introduction
The Advisory Committee on Immunization Practices (ACIP) provides recommendations for vaccinations in the United States (U.S.) [1, 2], and recommends administration of multiple live vaccines on the same day or separated by at least 24–30 days depending on the vaccines [2]. This recommendation affects all live vaccines to include measles, mumps, rubella (MMR), and varicella, which are live vaccines routinely administered to children ages 12 months through 6 years [1]. Regardless of a vaccine containing a live antigen or an inactive antigen, ACIP has recommendations in common for all vaccines to include age at administration and minimal intervals [2]; however, live vaccines must also be administered outside of conflict intervals specific to live vaccines [2]. A recent study found frequencies of vaccinations outside of the ACIP recommended ages were generally low [3], but little is known about errors that may occur for other ACIP recommendations to include number of days to wait between administrations of live vaccines. In contrast to live vaccines, vaccines containing inactivated antigens are generally not affected by circulating antibody and can be administered before, after or same day as the antibody [4]. Vaccines containing live antigens must replicate in order to cause an immune response and antibody against live antigen will interfere with replication [4]. Thus, if a live vaccine is administered sooner than the minimal interval after a prior live vaccine, the result is a less than optimal immune response causing the subsequent dose to be categorized as invalid, and requiring revaccination [2, 4, 5]. A study among children ages 12 months through 6 years showed that when varicella vaccination followed vaccination of measles, mumps, rubella (MMR) by fewer than 28 days, breakthrough infections occurred 3 times more frequently than after greater separation of the vaccinations [6].
Reports of vaccination errors have increased from 2000–2013 [7] with an increased number of vaccines and changes to the U.S. immunization schedule. Most vaccination errors were due to inappropriate schedule to include vaccines administered at the incorrect age or incorrect timing between vaccines [7]. A study using a sample of children from the 2000 National Immunization Survey found at least one invalid dose was administered before the minimal age or minimal interval in 10.5% of 19–35 month olds, and among the invalid doses 15% were varicella and 12% were measles-containing vaccines [8]. Previous studies have quantified vaccination errors related to inappropriate schedule [3, 8]: however, little has been done to measure live-vaccine related errors.
The ACIP recommends a 2-dose schedule for live vaccines that prevent MMR and varicella, with the first dose administered at age 12–15 months and the second dose at age 4–6 years [1, 9–11]. The intra-nasally administered quadrivalent live attenuated influenza vaccine (LAIV) has been licensed for use in the U.S. since 2012. However, ACIP did not recommend LAIV for use during the 2016–17 and 2017–18 flu seasons [12, 13] because of concerns regarding effectiveness against influenza viruses circulating during those seasons. LAIV was recommended again for use in the 2018–19 flu season [14]. Understanding live vaccine-related errors for MMR, varicella, combined MMR and varicella (MMRV), and LAIV are needed to identify non-compliance and find opportunities to prevent live vaccine-related errors among children.
Using provider-submitted, population-based immunization information systems (IIS) data from six IIS Sentinel Sites, our objectives were: 1) determine frequency of invalid MMR, varicella, MMRV, and LAIV vaccine doses administered sooner than the minimal interval of a prior live vaccine among children aged 12 months through 6 years from 2014–15 and 2016–17, and 2) estimate cost of revaccination to correct invalid vaccine doses of MMR, varicella, MMRV, and LAIV associated with live vaccine violations among these children from 2014–15 and 2016–17.
Methods
IIS are confidential, computerized systems that consolidate immunization data submitted by vaccine providers [15]. An IIS does not contain all data contained in Electronic Medical Records, only core data elements about vaccination [16]. Furthermore, IIS Sentinel Site data only contains essential data elements such as vaccine type and dates. IIS Sentinel Sites are located in North Dakota, Michigan, Minnesota, New York City, Wisconsin, and six counties in Oregon; these sites contain 10% of the US pediatric population [17]. IIS Sentinel Sites submitted de-identified record-level IIS data to CDC to enable evaluation of vaccination coverage. Selection of the six IIS Sentinel Sites was based on high-quality IIS data to include an enrollment of ≥ 85% of child and adolescent provider sites participating in the IIS, ≥ 85% of children aged <19 years participating in the IIS, and at least 70% of doses administered are submitted to IIS within 30 days of vaccine administration [17].
An ad hoc study design was used to assess retrospective cohort data from the IIS. We analyzed > 1.6 million records for children aged 12 months through 6 years from July 1, 2014 through December 31, 2015 when LAIV was recommended for use, and from July 1, 2016 through December 31, 2017 when LAIV was not recommended for use. Children aging into or out of an age group were excluded to allow all children in the study sample to have equal opportunity to obtain routinely recommended vaccine doses of MMR, varicella, MMRV, or LAIV, which are the focus of our analysis and collectively referred to as ‘live vaccines of interest’.
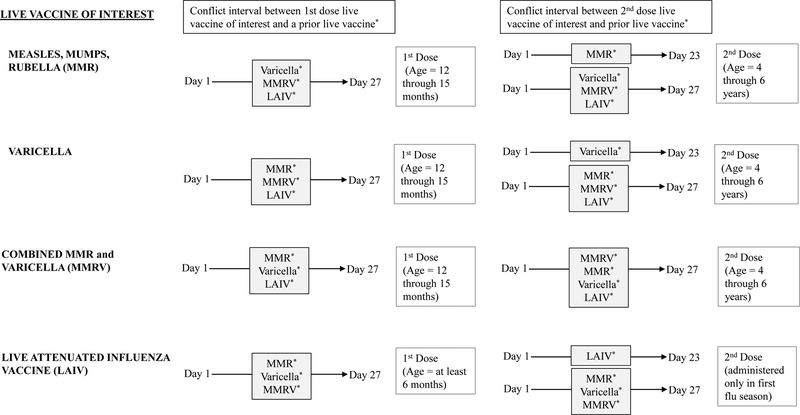
IISs and Electronic Health Record systems have the capability to evaluate administered doses and forecast needed vaccinations according to ACIP recommendations. CDC provides technical resources in order to assist in the creation and maintenance of this functionality through the Clinical Decision Support for Immunizations (CDSi) project. The number of days required to wait between live vaccine doses, also referred to as the ‘conflict interval’ (Figure 1), is described in CDSi supporting data tables [18]. Minimum intervals between live vaccine doses are generally 4 weeks. A grace period of 4 days [2, 4] was included in our analyses. Although the second dose of varicella is recommended 12 weeks after the first dose, ACIP allows 24 days as the minimum interval for retrospective evaluation of dose validity. Therefore, we adopted this in our analysis. Live vaccine doses are deemed “invalid” when a recommended dose was administered during the conflict interval of a prior live vaccine (Figure 1). The number of children who received at least one invalid dose were counted for each live vaccine of interest. Doses of prior live vaccines that may cause a live vaccine of interest to be invalid include MMR, varicella, MMRV, and LAIV. We considered inclusion of travel vaccines containing live antigens, such as yellow fever, but found few vaccination errors related to the yellow fever vaccine as these are only administered in case of travel and had minimal effect on overall vaccination error. Because more than one live vaccine of interest may be administered during the conflict interval, we identified single doses and multiple doses of live vaccines that caused a subsequent live vaccine dose to be invalid. The percentage of children who received invalid doses within the conflict interval were calculated among those who received at least one dose of the live vaccine of interest, stratified by the live vaccine of interest. Median number of days between the preceding live vaccine and the following invalid live vaccine dose were calculated to represent median conflict intervals among children.
Figure 1.
Age of recommendation with dose number and conflict intervals between measles, mumps, rubella, varicella, and live attenuated influenza vaccines. Live vaccines of interest administered during conflict interval of a prior live vaccine will cause the live vaccine of interest dose to be invalid and require revaccination.
Revaccination costs were calculated for total number of invalid doses administered to children for each live vaccine of interest. Following Rodgers et al (2018), revaccination costs (c) were calculated for total number of invalid doses as c = n (p + a + w + t), where n is number of invalid doses, p is price per vaccine dose [19, 20], a is administrative cost per vaccine (set to $8.56 for publicly-funded doses and $30.54 for privately-funded doses during 2014–15, and $9.11 for publicly-funded doses and $32.50 for privately-funded doses during 2016–17), w is cost for patient or caregiver time (set to $18.51 per hour for 2014–15 and $19.14 per hour for 2016–17) for 2 hours of time taken off from work, and t is transit cost to provider’s office (set to $23.86 for 2014–15 and $24.68 for 2016–17) [3]. We used the price for LAIV for estimating cost of revaccination of invalid LAIV doses, and did not use prices for other influenza vaccines that could be used to correct an invalid LAIV dose from 2014–15 [19]. To estimate the vaccine and administration costs associated with revaccination, we assumed half of the doses needed for revaccination were publicly purchased based on a previous study that used the six IIS Sentinel Sites and found approximately half of all doses were publicly purchased vaccines [3]. Costs were based on previous reports, and adjusted to 2015 dollars for 2014–15, and to 2017 dollars for 2016–17 [3, 21]. We performed analyses using SAS version 9.4 (SAS Institute, Inc.).
Results
Among the six IIS Sentinel Sites, the number of children aged 12 months through 6 years who received a live vaccine of interest was 946,659 in 2014–15 and 704,591 in 2016–17 (Tables 1 and 2). Across sites, the number of children ranged from 24,234 in North Dakota to 262,108 in Michigan during 2014–15, and from 16,895 in North Dakota to 200,490 in Michigan during 2016–17. The number of children in the sample size decreased from 2014–15 to 2016–17 as a result of LAIV not being recommended by ACIP in the later time period.
Table 1.
Percentage of children aged 12 months through 6 years who received an invalid dose of MMR, varicella, MMRV, or LAIV due to live vaccine conflict following a prior live vaccine at six IIS Sentinel Sites* from July 1, 2014 – December 31, 2015.
| Live vaccine of interest† | Prior live vaccine causing live vaccine of interest to be invalid | Live vaccine conflict interval⁋, days | Number of children who received ≥ 1 dose of live vaccine of interest, n | Number of children who received ≥ 1 invalid dose of live vaccine of interest, n (%) | Median number of days between prior dose and invalid dose (IQR††) | Revaccination cost§ for invalid doses∞ of live vaccine of interest, $ |
|---|---|---|---|---|---|---|
| Varicella | 347,251 | 1,424 (0.4) | 250,522 | |||
| MMR | 1–27 | 754 (52.9) | 10 (16) | |||
| Varicella | 1–23 | 16 (1.1) | 15 (8) | |||
| MMRV | 1–27 | 16 (1.1) | 10 (22) | |||
| LAIV | 1–27 | 597 (41.9) | 18 (12) | |||
| §§Multiple | 35 (2.5) | NA | ||||
| MMR | 346,098 | 1,001 (0.3) | 120,816 | |||
| MMR | 1–23 | 1 (0.1) | 11 (0) | |||
| Varicella | 1–27 | 447 (44.7) | 14 (15) | |||
| MMRV | 1–27 | 1 (0.1) | 22 (0) | |||
| LAIV | 1–27 | 445 (44.5) | 18 (12) | |||
| §§Multiple | 101 (10.0) | NA | ||||
| MMRV | 350,023 | 990 (0.3) | 217,636 | |||
| MMR | 1–27 | 10 (1.0) | 12.5 (13) | |||
| Varicella | 1–27 | 11 (1.1) | 12 (12) | |||
| MMRV | 1–27 | 30 (3.0) | 22 (10) | |||
| LAIV | 1–27 | 919 (92.8) | 17.5 (12.5) | |||
| §§Multiple | 13 (1.3) | NA | ||||
| LAIV | 380,666 | 1,927 (0.5) | 197,439 | |||
| MMR | 1–27 | 298 (15.5) | 15 (14) | |||
| Varicella | 1–27 | 352 (18.3) | 15 (13) | |||
| MMRV | 1–27 | 37 (1.9) | 16 (12) | |||
| LAIV | 1–23 | 641 (33.3) | 14 (10) | |||
| §§Multiple | 599 (31.1) | NA | ||||
| TOTAL | 946,659 | 4,873 (0.5) | 16 (14) | 786,413 |
Michigan, Minnesota, North Dakota, Wisconsin, New York City, and 6 contiguous Oregon counties
Trade name for live vaccines: Varicella (Varivax [Merck and Co., Inc., Whitehouse Station, NJ]);MMR (M-M-R-II [Merck and Co., Inc., Whitehouse Station, NJ]); MMRV (Proquad); LAIV (Flumist Quadrivalent [MedImmune, LLC, Gaithersburg, MD]).
Number of days referred to as the ‘live vaccine conflict interval’ which begins the day after a dose of the prior live vaccine identified in CDSi version 3.7. A live vaccine administered during the conflict period specific to the prior live vaccine indicates an invalid dose.
Interquartile range of median
Revaccination cost calculated as c = n (p + a + w + t) (Rodgers, et al. 2018), where n is the number of invalid doses, p is the price per vaccine dose,a is administrative cost per vaccine (set to $8.56 public clinic and $30.54 private practice 2014–15), w is cost for patient or caregiver time (set $18.51 per hour for 2014–15) for 2 hours for time take off work, and t is transit cost to provider’s office (set to $23.86 for 2014–15).
Number of total invalid doses: varicella = 1,451, MMR = 1,004, MMRV = 993, LAIV = 1,941.
More than one live vaccine of interest was administered during the conflict interval.
NA Median not available due to more than one conflict interval for multiple live vaccines
Table 2.
Percentage of children aged 12 months through 6 years who received an invalid dose of MMR, varicella, or MMRV due to live vaccine conflict following a prior live vaccine at six IIS Sentinel Sites* from July 1, 2016 – December 31, 2017†.
| Live vaccine of interest⁋ | Prior live vaccine causing live vaccine of interest to be invalid | Live vaccine conflict interval§, days | Number of children who received ≥ 1 dose of live vaccine of interest, n | Number of children who received ≥ 1 invalid dose of live vaccine of interest, n (%) | Median number of days between prior live vaccine and invalid dose (IQR††) | Revaccination cost∞ for invalid doses** of live vaccine of interest, $ |
|---|---|---|---|---|---|---|
| Varicella | 280,351 | 639 (0.2) | 120,131 | |||
| MMR | 1–27 | 544 (85.1) | 12 (14) | |||
| Varicella | 1–23 | 34 (5.3) | 14 (9) | |||
| MMRV | 1–27 | 11 (1.7) | 13 (15) | |||
| §§Multiple | 50 (7.8) | NA | ||||
| MMR | 289,128 | 391 (0.1) | 49,499 | |||
| MMR | 1–23 | 8 (2.0) | 14 (9) | |||
| Varicella | 1–27 | 294 (75.2) | 13 (15) | |||
| MMRV | 1–27 | 11 (2.8) | 14 (16) | |||
| §§Multiple | 77 (19.7) | NA | ||||
| MMRV | 382,380 | 48 (0.01) | 11,934 | |||
| MMR | 1–27 | 15 (31.3) | 19 (17) | |||
| Varicella | 1–27 | 6 (12.5) | 19 (16) | |||
| MMRV | 1–27 | 12 (25.0) | 19 (9.5) | |||
| §§Multiple | 14 (29.2) | NA | ||||
| TOTAL | 704,591 | 1,001 (0.1) | 14 (13) | 181,565 |
Michigan, Minnesota, North Dakota, Wisconsin, New York City, and 6 contiguous Oregon counties
LAIV was not recommended for use from 2016–17
Number of days referred to as the ‘live vaccine conflict interval’ which begins the day after a dose of the prior live vaccine identified in CDSi version 3.7. A live vaccine administered during the conflict period specific to the prior live vaccine indicates an invalid dose.
Interquartile range of median
Trade name for live vaccines: Varicella (Varivax [Merck and Co., Inc., Whitehouse Station, NJ]); MMR (M-M-R-II [Merck and Co., Inc., Whitehouse Station, NJ]); MMRV (Proquad).
Revaccination cost calculated as c = n (p + a + w + t) (Rodgers, et al. 2018), where n is the number of invalid doses, p is the price per vaccine dose,a is administrative cost per vaccine (set to $9.11 public clinic and $32.50 private practice for 2016–17), w is cost for patient or caregiver time (set to $19.14 per hour for 2016–17) for 2 hours for time take off work, and t is transit cost to provider’s office (set to $24.68 for 2016–17).
Number of total invalid doses: varicella = 640, MMR = 398, MMRV = 50.
More than one live vaccine of interest was administered during the conflict interval.
NA Median not available due to more than one conflict interval for multiple live vaccines
Among children receiving a live vaccine of interest in 2014–15, 4,873 (0.5%) received at least one invalid dose during the conflict interval of a prior live vaccine (Table 1). Overall, invalid live vaccine doses were administered during the median conflict interval of 16 days following another live vaccine (Table 1). The live vaccine most frequently administered during the conflict interval was LAIV, for which 1,927 children received an invalid dose of LAIV following a prior live vaccine, and accounted for 0.5% of the 380,666 children who received at least one dose of LAIV (Table 1). The prior live vaccine most frequently administered to cause the subsequent LAIV dose to be invalid was LAIV (33.3%) followed by varicella (18.3%) and MMR (15.5%) (Table 1). Among children who received invalid doses of LAIV, 31.1% were given more than one live vaccine during the conflict interval of LAIV (Table 1). Invalid doses of LAIV were administered between median conflict intervals of 14–16 days from a previous live vaccine dose (Table 1). Among the 347,251 children who received at least one dose of varicella, 1,424 (0.4%) children received invalid doses of varicella. Over half (52.9%) of the children who received invalid doses of varicella were caused by a prior dose of MMR (Table 1) with a median conflict interval of only 10 days between the two doses. Similarly, among the 1,001 children who received invalid doses of MMR, 44.7% were caused by a prior dose of varicella followed by 44.5% of the children who received a prior dose of LAIV (Table 1). Among the 990 children who received invalid doses of MMRV, 92.8% were caused by a prior dose of LAIV (Table 1).
Among children receiving a live vaccine of interest in 2016–17, 1,001 (0.1%) received at least one invalid dose during the conflict interval of a prior live vaccine (Table 2). Overall, invalid live vaccine doses were administered during the median conflict interval of 14 days following another live vaccine (Table 2). The live vaccine most frequently administered during the conflict interval was varicella, for which 639 children received an invalid dose of varicella following a prior live vaccine, and accounted for 0.2% of the 280,351 children who received at least one dose of varicella (Table 2). MMR was most frequently administered as the prior dose, causing the subsequent invalid dose of varicella in 544 children, and accounted for 85.1% of the 639 children who received an invalid dose of varicella (Table 2) with a median conflict interval of 12 days between the two doses. Likewise, among the 391 children who received an invalid dose of MMR, 75.2% were caused by a prior dose of varicella (Table 2). Though LAIV was not recommended for use from 2016–17, 564 children were reported to have received at least one dose of LAIV, 4 of which received an invalid dose due to administration during conflict interval of a prior live vaccine (data not shown).
For both time periods, we found less than 0.01% of children received more than one invalid dose of a live vaccine. For the children who received invalid live vaccine doses requiring revaccination from 2014–15 and 2016–17, the highest revaccination cost was for varicella, with an estimated cost of repeating doses as $250,522 from 2014–15 and $120,131 from 2016–17, including direct and indirect costs (Tables 1–2). The revaccination cost was highest for varicella due to both number of invalid doses and the higher price of varicella vaccination as compared to MMR and LAIV. Across the six IIS Sentinel Sites, we estimated the total revaccination cost of the live vaccines administered during the conflict interval as $787,413 for 2014–15, and $181,565 for 2016–17.
Discussion
This study provides an in-depth analysis of administration of MMR, varicella, MMRV, and LAIV vaccines during conflict intervals of live vaccines among children aged 12 months through 6 years in six IIS Sentinel Sites. Our analyses showed that live vaccine-related errors were rare among children, indicating a compliance to administration of live vaccines on the same day or outside of the conflict interval. Similar findings showing adherence to age-related recommendations were previously reported in the same IIS Sentinel Sites [3]. This study identified differences for adhering to the recommendations among children who received live vaccines in 2014–15 compared to 2016–17. When LAIV was recommended for use in 2014–15, approximately 1 in 200 children were administered an invalid dose of a live vaccine of interest compared to 2016–17 when LAIV was not recommended for use, approximately 1 in 1,000 children were administered an invalid dose of a live vaccine of interest. Improved messaging of live vaccine intervals may be needed as the lower number of children with invalid doses in 2016–17 compared to 2014–15 is likely due to the annual flu vaccine recommended during the 2014–15 season, which does not follow other schedules based on age of recommendation.
From 2014–15, LAIV was most frequently administered during the conflict interval of a prior live vaccine, and consistently caused invalid doses of the other live vaccines of interest. While the routine immunization schedule recommends children receive MMR and varicella vaccines at ages 12–15 months and 4–6 years, influenza vaccine is recommended annually for children ≥ 6 months of age. Although children typically receive MMR and varicella vaccines at a healthcare facility, it is not unusual for children to receive influenza vaccine in other settings (e.g., school). In addition to varying rates of immunization errors among different provider types [22], if vaccination history is not available to the provider or if the provider doesn’t inquire about vaccination history then there may be opportunities for error; therefore, immunization history should be reviewed at every healthcare visit [4]. Children requiring two doses of LAIV may be at risk for this type of vaccination error as we found a prior dose of LAIV was most frequently administered during the conflict interval of a subsequent invalid dose of LAIV.
When LAIV was not recommended for use, the proportion of children who received invalid doses of MMRV notably decreased from 0.3% (2014–15) to 0.01% (2016–17). Likewise, a decrease was identified for children who received invalid doses of MMR and varicella though not as noteworthy. In both time periods, varicella was consistently administered during the shortest median conflict interval of 10–12 days from a prior dose of MMR. Though caregivers may be uncomfortable with multiple vaccinations in a single visit [23, 24], same day administration of MMR and varicella, is preferred to ensure full vaccination [2], and reduce errors between these two live vaccines. The probability of live vaccine-related errors may also increase when there is a deviation from recommended schedules that can be caused by local outbreaks [25], travel plans [26, 27], health conditions [28], and an over-burdened healthcare system where time allotted for immunization completes with non-immunization needs [29]. However, information describing these circumstances was not available in our dataset.
This study estimated direct and indirect revaccination costs that would have been required to correct the invalid doses of live vaccines of interest in the six IIS Sentinel Sites as $786,413 for 2014–15 and $181,565 for 2016–17. As noted in a previous study [3], these revaccination costs likely underestimate national costs since the six IIS Sentinel Sites represent only 10% of the pediatric population nationwide. Because we included the cost of privately funded vaccines, the revaccination costs found for live vaccine-related errors among children were slightly higher when compared to age-related errors among children and adolescents, which was estimated from $111,964 to $179,179, in the six IIS Sentinel Sites [3]. Other types of errors such as non-compliance of minimal intervals among other childhood vaccines, improper storage, incorrect dosage, wrong vaccine and route of administration [30] may incur additional revaccination costs. In 2000, a national sample of childhood immunizations was evaluated, and the estimated cost of correcting an invalid dose for all children aged 19–35 months ranged from $10 million to $18 million dollars [8].
We did not analyze whether children received or did not receive doses to correct invalid live vaccine doses. Though revaccination costs are associated with children who receive corrective doses, there is also a cost for children who do not receive corrective doses of live vaccines. For those children who do not receive corrective doses, the live vaccine dose administered during the live vaccine conflict interval would be considered ineffective thus potentially leading to increased morbidity [6, 31, 32]. This study showed that a low number of children received doses of live vaccines during the conflict interval; however, an increased awareness of the conflict between LAIV and other live vaccines will help to further reduce live vaccine-related errors.
Computerized tracking of patient records may help to reduce vaccination errors [27] and consequently lower costs associated with correcting errors. An IIS is a confidential, computerized system of immunization records that are consolidated from multiple vaccine providers, and is an important tool to ensure children receive recommended immunizations [33]. A fully operational IIS tracks administered vaccines, reduces over-vaccination of new patients, and forecasts recommended immunizations [16, 33, 34]. In the case of live vaccines, providers can obtain vaccination histories from IIS to avoid live vaccine conflicts. Because of the seasonality associated with LAIV, providers are encouraged to submit flu administration data to the IIS since the flu vaccine may be administered outside provider offices, and to check children’s live vaccination history to determine the minimum interval needed from a prior live vaccine. Clinical decision support functionality with an IIS can also help prevent, detect, and correct live vaccine errors.
Study findings are subject to at least three limitations. First, data from the six IIS Sentinel Sites may not represent the U.S. population. The six sites are located in geographic regions that may not represent regionally diverse populations. As a result, live vaccine-related errors may be different regionally and for the U.S. Second, we were unable to determine if the invalid live vaccine administrations were the result of misreporting or misadministration. Third, we were not able to distinguish between public and private doses when calculating revaccination cost and assumed even distribution of purchase costs. Therefore, revaccination costs may be under- or over-estimated if the number of invalid doses were not evenly distributed.
Data from the Sentinel Sites revealed that live vaccine-related errors were rare, indicating an adherence to live vaccine recommendations among children aged 12 months through 6 years. Though live vaccine-related errors were few among children, the total revaccination cost for the 6 sites included in the current analysis is close to one million dollars. The slightly higher proportion associated with LAIV administration error may reflect schedule complexity with routinely recommended live vaccines and with seasonal LAIV. Increased awareness among providers about proper intervals for live vaccines not administered on the same day and improving access to full vaccination history at the time of vaccination administration are needed. A fully functional IIS that provides clinical decision support can assist providers by evaluating and forecasting live vaccinations to detect, prevent, and correct administration errors associated with live vaccines.
Acknowledgements
The IIS sentinel site project is funded by Centers for Disease Control and Prevention. We thank the following representatives from the IIS Sentinel Sites for reviewing the study proposal, manuscript, and providing vaccination records: Rachel Potter, DVM, MS1; Cristi Bramer, MPH1; Miriam Muscoplat, MPH2; Sydney Kuramoto, MPH2; Vikki Papadouka, PhD, MPH3; Alexandra Ternier, MPH3; Dominick Fitzsimmons4; Mary Woinarowicz, MA4; Andrew Osborn, MBA5; Aaron Dunn, MPH5; Danielle Sill, MSPH6, and Stephanie Schauer, PhD6.
1Michigan Department of Health and Human Services
2Minnesota Department of Health
3North Dakota Department of Health
4New York City Department of Health and Mental Hygiene
5Oregon Health Authority
6Wisconsin Department of Health Services
Footnotes
Conflict of Interest Statement:
All authors of this manuscript have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
References
- 1.Centers for Disease Control and Prevention. Recommended immunization schedule for children and adolescents aged 18 years or younger, United States, 2018. Available at: https://www.cdc.gov/vaccines/schedules/downloads/child/0-18yrs-combined-schedule-bw.pdf. Accessed December 21, 2018.
- 2.Kroger AT, Duchin J, Vázquez M. General best practices for immunization. Best practices guidance of the advisory committee on immunization practices (ACIP). Available at: https://www.cdc.gov/vaccines/hcp/acip-recs/general-recs. Accessed December 21, 2018.
- 3.Rodgers L, Shaw L, Strikas R, Hibbs B, Wolicki J, Cardemil C, Weinbaum C. Frequency and cost of vaccinations administered outside recommended minimal and maximum ages – 2014 data from six Sentinel Sites of immunization information systems. J Pediatr 2018;193:164–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. Epidemiology and Prevention of Vaccine-Preventable Diseases. Hamborsky J, Kroger A, Wolfe S, eds. 13th ed Washington D.C. Public Health Foundation, 2015. [Google Scholar]
- 5.Englund JA, Suarez CS, Kelly J, Tate DY, Balfour HH. Placebo-controlled trial of varicella vaccine given with or after measles-mumps-rubella vaccine. J Pediatr 1989;114:37–44. [DOI] [PubMed] [Google Scholar]
- 6.Verstraeten T, Jumaan A, Mullooly JP, Seward JF, Izurieta HS, DeStefano F, Black SB, Chen RT, Vaccine Safety Datalink Research Group. A retrospective cohort study of the association of varicella vaccine failure with asthma, steroid use, age at vaccination and measles-mumps-rubella vaccination. Pediatrics. 2003:112:e98–e103. [DOI] [PubMed] [Google Scholar]
- 7.Hibbs BF, Moro PL, Lewis P, Miller ER, Shimabukuro TT. Vaccination errors reported to the Vaccine Adverse Event Reporting System (VAERS) United States, 2000–2013. Vaccine. 2015;33:317–3178. [DOI] [PubMed] [Google Scholar]
- 8.Stokley S, Maurice E, Smith PJ, Klevens RM. Evaluation of invalid vaccine doses. Am J Prev Med 2004;26:34–40. [DOI] [PubMed] [Google Scholar]
- 9.McLean HQ, Fiebelkorn AP, Temte JL, Wallace GS. Prevention of measles, rubella, congenital rubella syndrome, and mumps, 2013: summary recommendations of the Advisory Committee on Immunizations Practices (ACIP). MMWR Recomm Rep 2013;62:1–34. [PubMed] [Google Scholar]
- 10.Marin M, Güris D, Chaves SS, Schmid S, Seward JF. Prevention of varicella: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2007;56:1–40. [PubMed] [Google Scholar]
- 11.Marin M, Broder KR, Temte JL, Snider DE, Seward JF. Use of combination measles, mumps, rubella, and varicella vaccine: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2010. May 7;59(RR-3):1–12. [PubMed] [Google Scholar]
- 12.Grohskopf LA, Sokolow LZ, Broder KR, Olsen SJ, Karron RA, Jernigan DB, Bresee JS. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices — United States, 2016–17 influenza season. MMWR Recomm Rep.2016;65:1–54. [DOI] [PubMed] [Google Scholar]
- 13.Grohskopf LA, Sokolow LZ, Broder KR, Walter EB, Bresee JS, Fry AM, Jernigan DB. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices — United States, 2017–18 influenza season. MMWR Recomm Rep.2017;66:1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grohskopf LA, Sokolow LZ, Fry AM, Walter EB, Jernigan DB. Update: ACIP recommendations for the use of quadrivalent live attenuated influenza vaccine (LAIV4) – United States, 2018–19 influenza season. MMWR Morb Mortal Wkly Rep 2018;67:643–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Centers for Disease Control and Prevention. Progress in immunization information systems - United States, 2012. MMWR Morb Mortal Wkly Rep 2013;62(49):1005–8. [PMC free article] [PubMed] [Google Scholar]
- 16.Centers for Disease Control and Prevention. Core data elements for IIS functional standards v4.0. Available at: https://www.cdc.gov/vaccines/programs/iis/core-data-elements/iis-func-stds.html. Accessed August 11, 2019.
- 17.Centers for Disease Control and Prevention. Immunization information systems (IIS), Q&A about IIS Sentinel Sites. Available at: https://www.cdc.gov/vaccines/programs/iis/activities/sentinel-sites.html. Accessed December 21, 2018.
- 18.Centers for Disease Control and Prevention. Immunization information systems (IIS), clinical decision support for immunization (CDSi). Available at: https://www.cdc.gov/vaccines/programs/iis/cdsi.html. Accessed December 21, 2018.
- 19.Centers for Disease Control and Prevention. Archived CDC vaccine price list as of April 1, 2015. Available at: https://www.cdc.gov/vaccines/programs/vfc/awardees/vaccine-management/price-list/2015/2015-04-01.html. Accessed December 21, 2018.
- 20.Centers for Disease Control and Prevention. Archived CDC vaccine price list as of April 3, 2017. Available at: https://www.cdc.gov/vaccines/programs/vfc/awardees/vaccine-management/price-list/2017/2017-04-03.html. Accessed December 21, 2018.
- 21.Zhou F, Shefer A, Wenger J, Messonnier M, Wang LY, Lopez A, Moore M, Murphy TV, Cortese M, Rodewald L. Economic evaluation of the routine childhood immunization program in the United States, 2009. Pediatrics, 2014;133:577–85. [DOI] [PubMed] [Google Scholar]
- 22.ISMP national vaccine errors reporting program 2017 analysis (part 1) vaccine errors continue with little change. Available at: https://www.ismp.org/resources/ismp-national-vaccine-errors-reporting-program-2017-analysis-part-i-vaccine-errors. Accessed August 3, 2019.
- 23.Meyerhoff A, Jacobs RJ, Greenberg DP, Yagoda B, Castles CG. Clinician satisfaction with vaccination visits and the role of multiple injections, results from the COVISE Study (Combination Vaccines Impact on Satisfaction and Epidemiology). Clin Pediatr (Phila). 2004;43(1):87–93. [DOI] [PubMed] [Google Scholar]
- 24.Hulsey E, Bland T. Immune overload: parental attitudes toward combination and single antigen vaccines. Vaccine, 2015;33(22):2546–50. [DOI] [PubMed] [Google Scholar]
- 25.High P, Handschur EF, Eze OS, Montana B, Robertson C, Tan C, et al. Update: Mumps Outbreak - New York and New Jersey, June 2009--January 2010 MMWR Morb Mortal Wkly Rep 2010;59:125–9. [PubMed] [Google Scholar]
- 26.Poland GA, Schaffner W, Hopkins RH Jr, U.S. Department of Health and Human Services Immunization guidelines in the United States: new vaccines and new recommendations for children, adolescents, and adults. Vaccine. 2013;31(42):4689–4693. [DOI] [PubMed] [Google Scholar]
- 27.Ventola CL: Immunization in the United States: Recommendations, Barriers, and Measures to Improve Compliance: Part 1: Childhood Vaccinations. P T. 2016;41(7):426–36. [PMC free article] [PubMed] [Google Scholar]
- 28.Centers for Disease Control and Prevention. Vaccine for when your child is sick. Available at: https://www.cdc.gov/vaccines/hcp/patient-ed/conversations/downloads/fs-child-sick.pdf. Accessed December 21, 2018.
- 29.Institute of Medicine. 2003. Setting the Course: A Strategic Vision for Immunization: Part 4: Summary of the Washington, D.C., Workshop. Washington, DC: The National Academies Press. [PubMed] [Google Scholar]
- 30.Bundy DG, Shore AD, Morlock LL, Miller MR. Pediatric vaccination errors: application of the “5 rights” framework to a national error reporting database. Vaccine. 2009;27(29):3890–6. [DOI] [PubMed] [Google Scholar]
- 31.Petralli JK, Merigan TC, Wilbur JR. Circulating interferon after measles vaccination. N Eng J Med. 1965;273:198–201. [DOI] [PubMed] [Google Scholar]
- 32.Kroger AT, Strikas RA. General recommendations for vaccination and immunoprophylaxis. Chapter 2 - The pretravel consultation. Available at: https://wwwnc.cdc.gov/travel/yellowbook/2018/the-pre-travel-consultation/general-recommendations-for-vaccination-immunoprophylaxis. Accessed December 21, 2018. [Google Scholar]
- 33.Community Preventive Services Task Force. Recommendations for use of immunization information systems to increase vaccination rate. J. Public Health Manag Prac 2015;21;249–52. [DOI] [PubMed] [Google Scholar]
- 34.Groom H, Hopkins DP, Pabst LJ, Murphy Morgan J, Patel M, Calonge N, et al. Immunization information systems to increase vaccination rates: a community guide systematic review. J. Public Health Manag Prac 2015;21;227–48. [DOI] [PubMed] [Google Scholar]