Abstract
Protrusile jaws are a highly useful innovation that has been linked to extensive diversification in fish feeding ecology. Jaw protrusion can enhance the performance of multiple functions, such as suction production and capturing elusive prey. Identifying the developmental factors that alter protrusion ability will improve our understanding of fish diversification. In the zebrafish protrusion arises postmetamorphosis. Fish metamorphosis typically includes significant changes in trophic morphology, accompanies a shift in feeding niche and coincides with increased thyroid hormone production. We tested whether thyroid hormone affects the development of zebrafish feeding mechanics. We found that it affected all developmental stages examined, but that effects were most pronounced after metamorphosis. Thyroid hormone levels affected the development of jaw morphology, feeding mechanics, shape variation, and cranial ossification. Adult zebrafish utilize protrusile jaws, but an absence of thyroid hormone impaired development of the premaxillary bone, which is critical to jaw protrusion. Premaxillae from early juvenile zebrafish and hypothyroid adult zebrafish resemble those from adults in the genera Danionella, Devario, and Microdevario that show little to no jaw protrusion. Our findings suggest that evolutionary changes in how the developing skulls of danionin minnows respond to thyroid hormone may have promoted diversification into different feeding niches.
Keywords: functional morphology of feeding, metamorphosis, skull
1 |. INTRODUCTION
Many if not most fishes undergo a metamorphosis during which their bodies are extensively remodeled (McMenamin & Parichy, 2013). It is during metamorphosis and postmetamorphic development that most fishes acquire the behavioral and morphological characters that allow them to occupy their adult niches. Premetamorphic (larval) and postmetamorphic (juvenile and adult) fishes of the same species frequently live in different habitats and occupy disparate feeding niches (Leis & McCormick, 2006; McCormick & Makey, 1997; McCormick, Makey, & Dufour, 2002; McMenamin & Parichy, 2013). The ecological diversification of adults in many fish lineages is therefore closely linked to evolutionary changes in the processes that shape their postlarval development. Most molecular developmental studies have focused on early development, particularly embryonic stages (Albertson & Yelick, 2004; Cooper, Wirgau, Sweet, & Albertson, 2013; McMenamin & Parichy, 2013; Parsons, Andreeva, Cooper, Yelick, & Albertson, 2010), but if we are to understand the developmental changes that have permitted the adaptive diversification of adult fish feeding we need a better understanding of the controls of morphogenesis in late development.
Protrusile jaws are an important evolutionary innovation in fish feeding. Highly moveable skull linkages that allow the jaws to protrude forward from the face have evolved independently in at least six lineages of bony fishes and protrusion has been lost, gained, reduced, and enhanced many times in these clades (Ferry-Graham, Gibb, & Hernandez, 2008; Staab, Holzman, Hernandez, & Wainwright, 2012; Wainwright, McGee, Longo, & Hernandez, 2015). Two of these, Cypriniformes (~3,200 species, including the zebrafish) and Acanthomorpha (~17,000 species), have been particularly successful and together comprise more than one-third of living vertebrates (Near et al., 2013; Staab et al., 2012; Wainwright et al., 2015; Yang et al., 2010). Maximum jaw protrusion distance has been closely linked with diet (Cooper, Carter, Conith, Rice, & Westneat, 2017) and an ability to rapidly transition between morphs capable of different degrees of protrusion appears to support diversification into different feeding niches (Cooper & Westneat, 2009; Cooper et al., 2010; Ferry-Graham et al., 2008; Staab et al., 2012; Wainwright et al., 2015).
Although protrusile jaws can confer multiple functional abilities in adults, particularly the enhancement of suction production via rapid expansion of the mouth cavity (i.e., buccal cavity; Ferry-Graham et al., 2008; Holzman, Collar, Mehta, & Wainwright, 2012; Konow & Bellwood, 2005; Staab et al., 2012), the relatively high water viscosities experienced by small aquatic organisms severely limits the utility of protrusile jaws in fish larvae (Hernandez, 2000; Hernández, Barresi, & Devoto, 2002; Yaniv, Elad, & Holzman, 2014). Small fishes live in a low Reynolds number environment in which viscous forces are greater than inertial forces (Hernandez, 1995; Hernandez, 2000). These animals experience water as a “sticky” fluid that can reduce the effectiveness of feeding techniques that are viable at larger body sizes (Hernandez, 1995; Hernandez, 2000). Protrusile jaws would most likely interfere with suction feeding in fish larvae and jaw protrusion does not arise until after metamorphosis in the zebrafish (Hernández et al., 2002; McMenamin, Carter, & Cooper, 2017).
The reshaping of multiple skull bones must be coordinated during the larva-to-juvenile transition for a fish to have a properly integrated mature skull. This need for integrated development should be particularly strong for fishes with complex, highly kinetic adult skulls in which motion is transferred through multiple linkages. Hormones reach all body organs essentially simultaneously via circulating blood, and therefore have the potential to act as agents of developmental coordination that stimulate multiple organs, including different bones, to transform at the same time. Along with additional endocrine factors, thyroid hormone (TH) signaling plays a major role in directing late-developmental skeletal remodeling in vertebrates, and TH stimulates metamorphosis or metamorphosis-like processes in many species (Das et al., 2006; Hu et al., 2019; Laudet, 2011; McMenamin & Parichy, 2013; Paris et al., 2010; Wojcicka, Bassett, & Williams, 2013). Thyroid hormone is known to play an important role in skull morphogenesis and multiple cranial malformations are associated with aberrant TH signaling (Desjardin et al., 2014; Hanken & Hall, 1988; Hanken & Summers, 1988; Hirano, Akita, & Fujii, 1995).
Thyroid hormone exerts extensive effects on the growth and remodeling of both endochondral and intramembranous bone by regulating gene expression (Bassett & Williams, 2003; Gogakos, Bassett, & Williams, 2010; Harvey et al., 2002; Waung, Bassett, & Williams, 2012; Wojcicka et al., 2013). The membranes of most skeletal cells possess TH transporters that facilitate hormone uptake (Gogakos et al., 2010). Active (T3) and inactive (T4) forms of TH are secreted by thyroid follicles and both are transported into bone cells where T4 is activated by cytoplasmic enzymes via the removal of an iodine molecule (Waung et al., 2012). Gene transcription is activated by T3 when it enters the nucleus and forms complexes with TH receptors bound to TH response elements in a number of target genes (Gogakos et al., 2010; Waung et al., 2012). The transcription products of these genes mediate the initiation and duration of bone matrix formation by regulating the differentiation and activity of bone-building cells (osteoblasts) and bone-resorbing cells (osteoclasts; Bassett & Williams, 2003; Harvey et al., 2002; Wojcicka et al., 2013).
In addition to the known roles of TH in both vertebrate metamorphosis and bone morphogenesis, changes to TH signaling may have been an important component of the diversification of the functional morphology of cypriniform feeding (McMenamin et al., 2017; Shkil & Smirnov, 2015; Shkil et al., 2015; Shkil, Kapitanova, Borisov, Abdissa, & Smirnov, 2012). To better understand the controls of fish metamorphosis and the developmental determinants of cypriniform jaw protrusion ability we measured the effects of different TH levels on the development of the functional morphology of feeding in the zebrafish. We also sought to determine whether modulating TH levels would alter zebrafish skull development in ways that could affect evolutionary potential. Variation in heritable traits facilitates evolutionary change and higher levels of variation tend to increase evolutionary potential (i.e., “evolvability”; Klingenberg, Duttke, Whelan, & Kim, 2012; Lavergne & Molofsky, 2007). Covariation among traits will facilitate their coordinated evolution, but will tend to constrain independent evolutionary changes among subsets of covarying traits (Cooper, Wernle, Mann, & Albertson, 2011; Klingenberg, 2008; Sanger, Mahler, Abzhanov, & Losos, 2012; Young & Hallgrimsson, 2005). To gain insight into whether changes in TH levels could affect the evolutionary potential of fish skulls we compared both cranial shape variation and patterns of shape covariation between different skull regions among zebrafish that experienced a range of TH levels during development.
We collected morphological and functional data from a developmental range of zebrafish in which TH production was elevated (TH+), normal, or eliminated (TH−). We also collected morphological data from the premaxillary bones of nine additional species of danionin minnows (Danionini; Danioninae; Cyprindae) that exhibit extensive diversity in adult jaw protrusion ability. Most cypriniform fishes possess a jaw protrusion mechanism that differs from those typically found in acanthomorphs in that they have a medial, mobile kinethmoid bone in the anterodorsal region of the skull that contributes motion to jaw protrusion (Gidmark, Staab, Brainerd, & Hernandez, 2012; Hernandez, Bird, & Staab, 2007; Motta, 1984). Although variation in jaw protrusion mechanisms have evolved among the ~20,000 cypriniform and acanthomorph fishes, in both clades the ascending arm of the premaxilla (sometimes referred to as the ascending process) typically slides anteriorly over the rostrum (the anterior portion of the neurocranium) during jaw protrusion and the length of the ascending arm is an important determinant of maximum protrusion distance (Cooper, Carter, et al., 2017; Motta, 1984).
We tested the following predictions: (a) normal TH levels are required for the development of functional abilities important to adult zebrafish feeding; (b) TH levels lead to alterations in the level of developmental variation in zebrafish head shape; (c) normal TH levels are required for the development of the wild-type pattern of covariation between different regions of the zebrafish skull; and (d) the functional morphology of jaw protrusion in adult TH− zebrafish closely resembles that in related minnows with limited protrusion abilities.
2 |. MATERIALS AND METHODS
2.1 |. Study system
We utilized three zebrafish lines to study the effects of TH on the development of their feeding biomechanics: (a) the transgenic line Tg(tg:nVenus-2a-nfnB)wp.rt8 in which the thyroid follicles can be chemically ablated; (b) the mutant line opallusb1071, hereafter TH+, which has a missense mutation in thyroid stimulating hormone receptor (tshr) that causes constitutive hyperthyroidism (i.e., elevated TH levels; McMenamin et al., 2014); and (c) the AB wild-type line (euthyroid, i.e., normal TH). Both the transgenic and the mutant lines originated in the AB line (McMenamin et al., 2014). All fish were maintained under standard conditions at 28°C and a 14-hr light/10-hr dark cycle under approved WSU IACUC Protocol 04285.
Hypothyroid specimens (TH−) were produced via nitroreductase-mediated cell ablation of thyroid follicles in Tg(tg:nVenus-2a-nfnB)wprt8 larvae at 4 days postfertilization (dpf) following McMenamin et al. (2014). Ablation was performed immediately after formation of the thyroid follicles so that they were rendered permanently incapable of hormone production. In brief, TH-fish were produced by placing specimens in a solution of 1% dimethyl sulfoxide (DMSO) and 10 mM metronidazole for 4 hr. Control Tg (tg:nVenus-2a-nfnB)wp.rt8 fish treated with 1% DMSO only for 4 hr will be referred to as “DMSO” hereafter.
All specimens were fed live paramecia exclusively from 5 dpf until they were large enough to begin feeding on live Artemia. Artemia were introduced slowly into the diet from ~14dpf until all specimens in an aquarium were capable of consuming brine shrimp (as indicated by exhibiting pink, distended bellies after feeding), at which point paramecia were no longer used. To provide more nutritionally complete Artemia, newly hatched brine shrimp were collected after 24 hr and fed for an additional 24 hr with an infusion of Spirulina sp. algae (RGcomplete, Reed Mariculture, Inc., Campbell, CA). To prevent exposure to exogenous TH present in most prepared fish foods, all fish were fed exclusively with enriched Artemia after they were large enough to be fully weaned off of paramecia. All specimens were maintained in 9 L aquaria from 5 dpf onward on a recirculating system with carbon filters. Specimens from each treatment were sampled for kinematic and shape analyses at 8, 15, 30, 60, and 100 dpf.
2.2 |. Kinematic analyses
To test our first prediction that normal TH levels are required for the development of functional abilities important to adult zebrafish feeding we used high speed video to measure multiple aspects of feeding performance in zebrafish from all TH treatment groups. Fish were filmed in lateral view while feeding on either paramecia (8 and 15 dpf) or Artemia (30, 65, and 100 dpf). We analyzed kinematic data from five feeding strikes for each individual and examined five specimens of each age class from each treatment group. Feeding strikes were recorded at 500 frames/second using an Edgertronic monochrome high-speed video camera (Sanstreak Corp., San Jose, CA). Kinematic analyses of feeding strikes were performed using the ImageJ software program (Schneider et al., 2012). We measured the following variables in each video frame of all feeding strikes (see Figure 1a for reference): gape angle (the angle created between landmarks A, B, and C with B as the vertex), jaw protrusion (linear distance between landmarks A and F minus the minimum distance recorded between A and F for that strike), hyoid depression (linear distance between landmarks D and E minus the minimum distance recorded between D and E for that strike), and cranial elevation angle (the angle created by the intersection of a line running along the dorsal edge of the head with a line running along the dorsal edge of the body, with landmark F denoting the point of head rotation). All measurements were made by the same researcher to minimize introduction of operator error. Jaw protrusion and hyoid depression were standardized by fish standard length (SL). Maximum values for every variable were recorded for each feeding strike of every specimen. These maxima were used to calculate a mean value for each specimen. For each age class we used analysis of variance (ANOVA) to test for differences between treatment groups for the maximum value of each variable. When a significant difference was detected we used a Tukey’s honestly significant difference (HSD) test to determine which treatment groups were significantly different from each other.
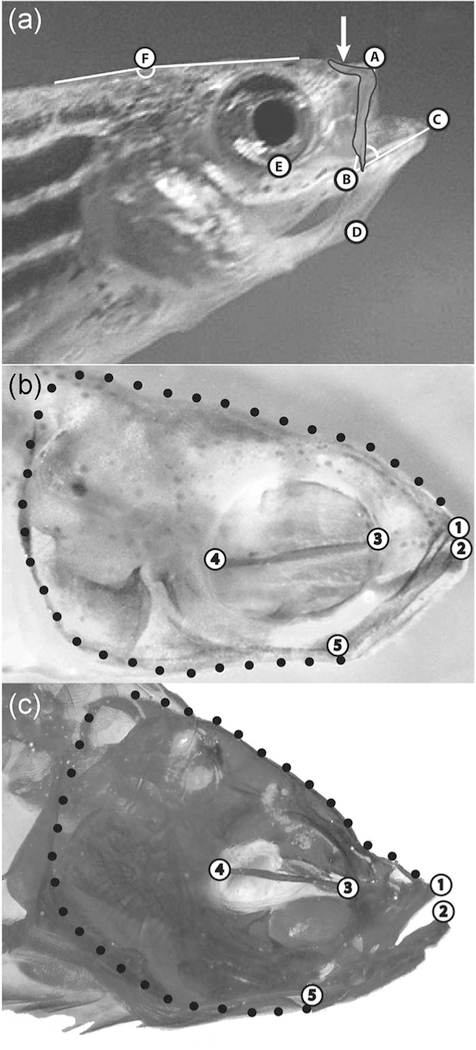
FIGURE 1.
Landmarks and semilandmarks used in analyses of movement and shape. (a) Landmarks used in kinematic analyses with an image of the premaxillary bone of the upper jawsuperimposed its correct anatomical position in a fully protruded upper jaw. Arrow indicates the ascending arm of the premaxilla. Landmarks: (A) Anterior tip of the upper jaw; (B) corner of the mouth; (C) anterior tip of the lower jaw; (D) anterior tip of the hyoid; (E) ventral‐most point of the orbit; and (F) vertex of the angle used to measure head rotation during cranial elevation (the dorsal surface of the head anterior to this point rotated upward during cranial elevation when feeding, while the dorsal surface of the trunk posterior to this point did not). (b) Anatomical landmarks and semilandmarks used in shape analyses of all specimens at all ages sampled shown on a larval zebrafish. Landmarks: (1) anterior tip of the premaxilla in the upper jaw; (2) anterior tip of the dentary bone in the lower jaw; (3) junction of the parasphenoid with the anterior wall of the orbit; (4) junction of the parasphenoid with the posterior wall of the orbit; and (5) lower jaw joint (articularquadrate joint). Black circles indicate semi‐landmarks evenly spaced between LM 1 and 2 to capture overall head shape. (c) Anatomical landmarks and semi‐landmarks used in shape analyses of all specimens at all ages sampled shown on an adult zebrafish (LM are the same as those in panel b). LM, landmark
2.3 |. Morphological analyses
The specimens used in feeding trials were also used for morphological analyses. Euthanized specimens were fixed in 4% formalin for 24 hr and then stepped over into 70% ethanol. They were then cleared and stained for bone and cartilage. The smaller specimens (8–30 dpf) were processed according to Walker and Kimmel’s (2007) acid-free staining protocol. Larger specimens (65 and 100 dpf) were cleared and stained according to Potthoff (1984). Specimens were then stepped into 80% glycerol and photographed in lateral view using an Olympus DP25 digital camera interfaced with an Olympus SZ61 dissecting microscope.
The program tpsDIG2 (http://life.bio.sunysb.edu/morph/ ) was used to place landmarks (LM) and semi-landmarks (semi-LM) on digital images of fish heads (Figure 1b,c). We chose skeletal LM that are present at all of the developmental stages examined. Semi-LM were used to capture the shape of curved surfaces LM (Figure 1b,c). The programs tpsUtil and tpsrelW (http://life.bio.sunysb.edu/morph/) were then used to superimpose semi-LM using a chord-distance (Procrustes distance) based “sliders” method and to remove size and orientation differences from LM and semi-LM position data via Procrustes transformations. Pooled shape data from all specimens of all ages in each hormonal treatment group (N = 25 for each treatment) were used to: (a) test for differences in head shape variation over the course of development; and (b) test for differences in the patterns of covariation between LM and semi-LM locations over the course of development.
To test our second prediction that changes in TH levels will affect developmental variation we measured head shape variation in each of our TH treatment groups. We then used a resampling approach to test for differences in shape variation. Head shape variation was quantified by calculating the Foote disparity value for each treatment group (Foote, 1993). A permutation procedure (2,000 iterations) was used to test for differences in disparity between pairs of data sets. If actual differences in shape disparity values were greater than the upper bound of a 95% confidence interval calculated via permutation, then the disparity values were considered to be significantly different. We used DisparityBox, which is an analytical tool available within the PCAGen8 program, to perform these calculations.
We used a principal components based resampling method to test our third prediction that normal TH levels are required for the development of the wild-type pattern of covariation between different regions of the zebrafish skull. We performed principal components analysis (PCA) of LM and semi-LM positions using the program PCAGen8. These PCAs utilized covariation matrices that capture patterns of positional covariation. To determine if patterns of covariation were significantly different between the hormonal treatments we conducted pairwise comparisons of PC axis orientations using a bootstrapping procedure (4,900 sets).
Although the different PC axes derived from the same data are orthogonal to each other, they are not independent, since only those aspects of covariation that were not associated with PC1 can be used to define subsequent axes. It therefore impossible to compare the orientations of PC axes subsequent to PC1 individually. All analyses that involved multiple axes determined whether the alignments of planes (2 axes) or multidimensional hyperplanes (≥3 axes) were significantly different.
The shape of the premaxillary bone in the upper jaw is a major determinant of maximum jaw protrusion distance in fishes (Cooper, Carter, et al., 2017; Motta, 1984). We used a principal components based resampling method to test our fourth prediction that the functional morphology of jaw protrusion in adult TH− zebrafish closely resembles that in related minnows with limited protrusion abilities. We compared developmental variation in the premaxillary shape of AB and TH− zebrafish to the variation in adult premaxillary shape that has evolved among other members of the cyprinid tribe Danionini (sensu Tang et al., 2010). We obtained specimens of the following fishes through the pet trade: Danio albolineatus, Danio erythromicron, Danio feegradei, Danio kyathit, Danio nigrofasciatus, Danionella translucida, Devario aequipinnatus, Devario maetaengensis, and Mi-crodevario kubotai. Adult specimens (2–4 per species) were cleared and stained following Potthoff (1984). AB zebrafish at 35, 65, and 100 dpf were also cleared and stained (five specimens per age class). We used 35 dpf specimens instead of 30 dpf fish because some 30 dpf specimens did not have well-ossified premaxillae. Premaxillae were removed from all specimens after clearing and staining and then photographed as descried above. Anatomical LM and semi-LM (Figure 4b,c) were used to quantify premaxillary shape. PC scores were used to calculate a developmental trajectory for premaxillary shape change in AB zebrafish.
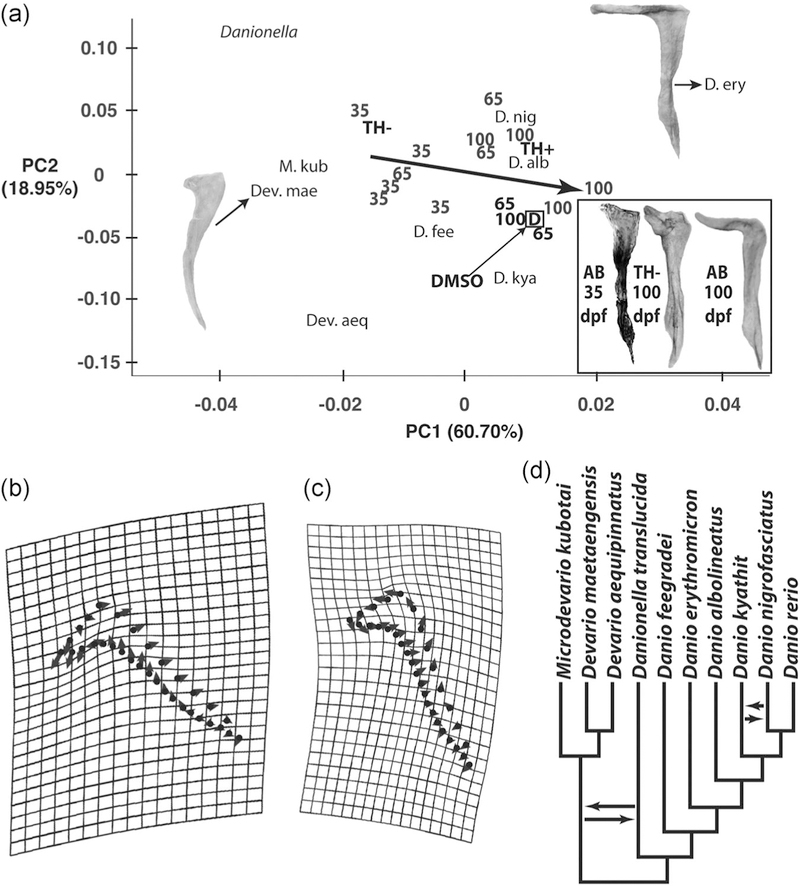
FIGURE 4.
Comparisons of premaxillary morphology. (a) Principal component score plot derived from coordinate-based analyses of premaxilla shape in the following specimens: (1) 35, 65, and 100 dpf AB zebrafish; (2) 100 dpf DMSO, TH−, and TH+ zebrafish; and (3) adults of nine additional species of danionin minnows. The difference in premaxillary shape between Devario maetaengensis and Danio erythromicron exemplify the shape diversity explained by PCI. PC1 is strongly associated with the length of the ascending arm relative to the length of the dentigerous arm. Numbers indicate the distributions of 5 specimens of AB zebrafish from each of the three ages sampled (numbers correspond to dpf). The location of the Procrustes mean premaxillary shapes of TH+ and TH− specimens are indicated by their respective symbols. The location of the Procrustes mean premaxillary shape of DMSO specimens is indicated by “D”. The location of Procrustes mean shapes of the nine nonzebrafish premaxillae are indicated by an abbreviation of the scientific name of each species (see panel (d) for full species names), except for Danionella translucida, where the complete genus name is used. Images of premaxilla shapes of particular interest are inserted. The largest arrow denotes the developmental shape trajectory for AB premaxillae. (b) Landmarks used in shape analyses (black circles), deformation grid and vector plot that shows the shape variation associated with PC1. (c) Landmarks used in shape analyses (black circles), deformation grid and vector plot that shows the shape variation associated with PC2. (d) Phylogenetic relationships of the 10 species whose premaxillae were compared. The phylogeny depicted is taken from Tang et al. (2010). Arrows indicate branch positions that are swapped in the relationships reported by McCluskey and Postlethwait (2015), who did not examine Devario maetaengensis. DMSO, dimethyl sulfoxide; PC, principal component; TH, thyroid hormone
For all shape analyses we used the program Coord-Gen8 to transform the LM and semi-LM coordinate data from the format used by the tpsDIG2 program into the format utilized by Mac OS (Apple Inc.) versions of the IMP-8 series of programs. The IMP-8 programs Coord-Gen8, PCAGen8, TwoGroup8, and PCAGen8 were developed by David Sheets and are available for download at: http://www3.canisius.edu/~sheets/IMP8.htm.
3 |. RESULTS
3.1 |. Ossification patterns
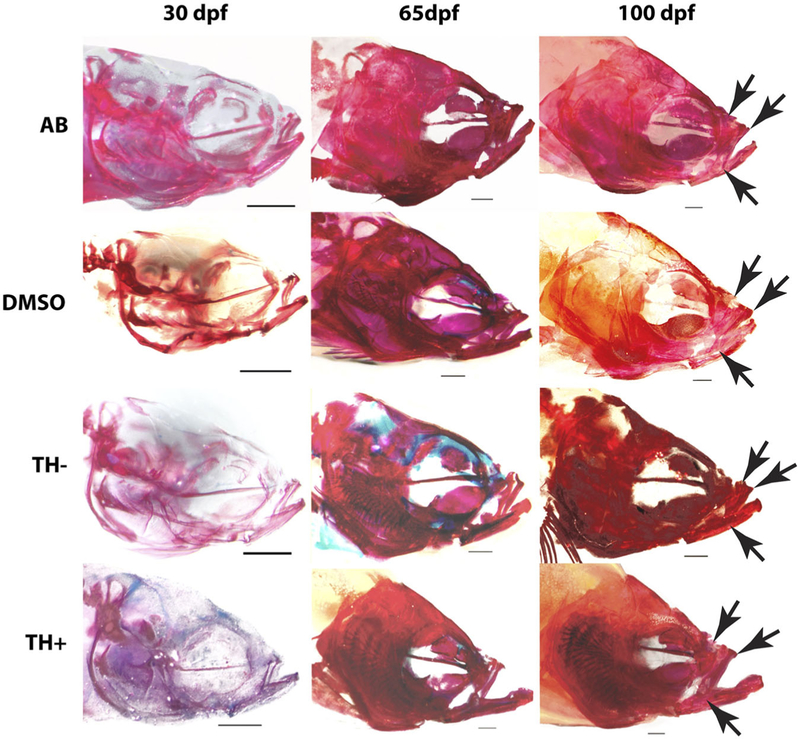
All specimens possessed largely cartilaginous crania at 8 and 15 dpf. Skulls were mostly, but not completely, ossified by 30 dpf in specimens from all treatment groups except TH− (Figure 2). We will refer to 8 and 15 dpf specimens as “premetamorphic,” 30 dpf specimens as “mid-metamorphic” and 65 and 100 dpf specimens as “postmetamorphic.” Euthyroid and TH+ fish had fully ossified skulls by 65 dpf, while TH− specimens retained cartilaginous regions in the calvarium (skull vault) at this stage and in some TH− fish this region failed to ossify by 100 dpf (Figure 2).
FIGURE 2.
Representative cleared and stained specimens of postmetamorphic specimens from all treatment groups. Blue coloration indicates cartilage stained by alcian blue. Red indicates bone stained by alizarin red. Arrows represent the anterio-dorsal and posterio-dorsal edges of the ascending arm of the premaxillary bone. Arrowheads indicate the posterio-ventral tip of the dentigerous arm of the premaxilla (scale bars = 1 mm). TH− specimens exhibited delayed cranial ossification. In many of our TH− specimens the calvarium (skull vault) was not fully ossified by 100 dpf. Both the overall size of the premaxilla and the length of its ascending arm relative to the length of its dentigerous arm were reduced in TH− fish. TH+ fish exhibited hypertrophied lower jaws. TH, thyroid hormone
3.2 |. Standard length
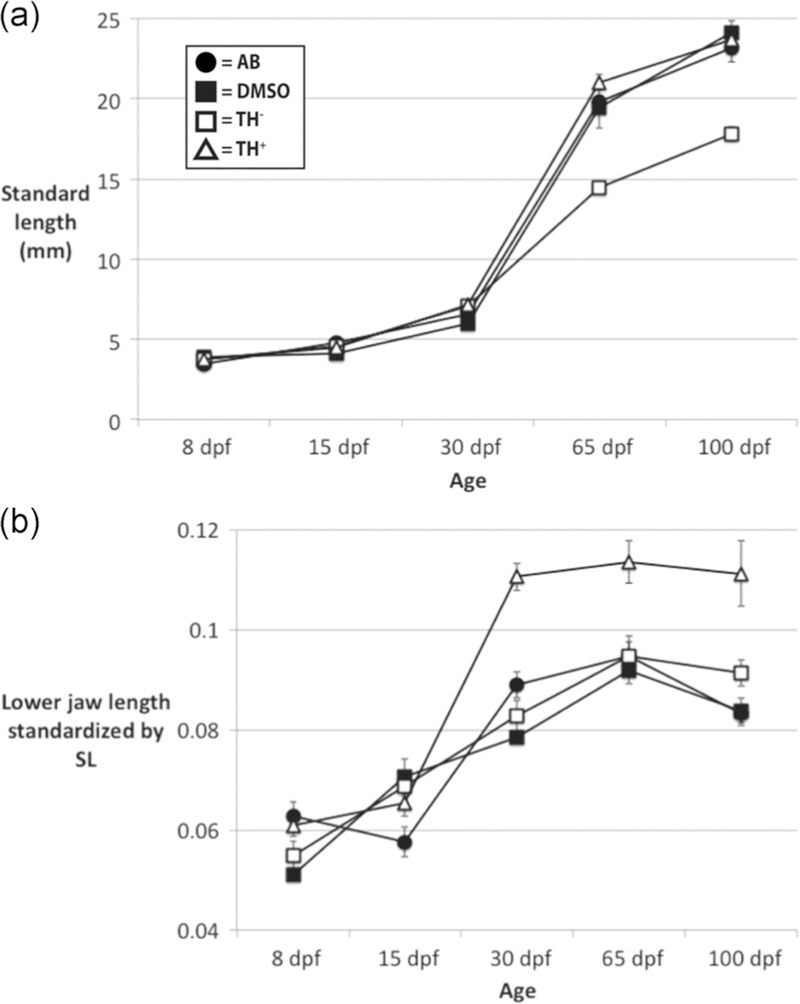
Fish SLs did not differ significantly between the AB, DMSO, and TH+ treatments at any developmental stage (Figure 3). Note that since age alone is a limited proxy for development (Parichy, 2009; McMenamin, Chandless, & Parichy, 2016), individuals within these age categories likely represented a range of developmental stages. Nonetheless, SL did not differ significantly different for any treatment before 65 dpf. At 65 and 100 dpf, TH− fish were shorter than those in other treatments, suggesting TH is required for normal growth rates (Figure 3).
FIGURE 3.
Comparisons of anatomical growth among treatment groups. Mean sizes with standard error bars are given for all treatments at each age sampled. Key to symbols in panel (a). (a) All treatment groups exhibited similar increases in body length until 30 dpf, after which TH− fish exhibited lower growth rates. (b) All treatment groups exhibited similar increases in the relative length of the lower jaw until 15 dpf. After 15 dpf the lower jaws of TH+ fish grew much more quickly than those of the other treatment groups and then stabilized at a larger relative size. TH, thyroid hormone
3.3 |. Jaw morphology
The lower jaws of TH+ specimens began to show abnormal enlargement by 30 dpf (Figures 2 and 3). Lower jaw morphology was normal in TH− specimens (Figures 2 and 3). The maxillary and premaxillary bones of the upper jaw were smaller in 65 and 100 dpf TH− zebrafish relative to both euthyroid and TH+ specimens (Figure 2). Upper jaw morphology in TH+ specimens appeared comparable to that in euthyroid specimens (Figure 2).
3.4 |. Kinematic analyses
Three kinematic variables could be measured at all of the developmental stages examined (cranial elevation, gape distance and hyoid depression). At each developmental stage these three variables were compared among all treatment groups (six comparisons per variable). In regard to these three variables there were therefore 36 comparisons of kinematic performance both before (8 and 15 dpf) and after (65 and 100 dpf) metamorphosis. Of the 36 premetamorphic comparisons, 11 of them exhibited significant differences (30.6%), while there were significant differences between 13 of the 36 postmeta-morphic comparisons (36.1%; Table 1). Treatment groups were most similar in regard to cranial elevation (five significant differences total) and most different in regard to gape angle (13 significant differences total; Table 1). Fish from the euthyroid treatments (AB and DMSO) showed the most similarity in cranial movement, with only three significant kinematic differences between these two treatments (all developmental stages combined; Table 1). Fish from the TH− treatment exhibited the most limited range of cranial element motion during feeding (Table 1 and Figures 5 and S2). There were also 22 significant kinematic differences (all developmental stages combined) between TH− specimens and all other treatment groups, which was the highest number for any of the treatment groups (Table 1).
TABLE 1.
ANOVA and Tukey’s HSD results for comparisons of kinematic variables among treatment groups for each developmental stage sampled
| Premetamorphic |
Midmetamorphic |
Postmetamorphic |
|||
|---|---|---|---|---|---|
| 8 dpf | 15 dpf | 30 dpf | 65 dpf | 100 dpf | |
| Gape angle | AB > DMSO | AB > TH− | TH+ > DMSO | AB > TH− | AB > DMSO |
| AB > TH− | AB > TH+ | TH+ > TH− | DMSO > TH− | AB > TH− | |
| AB > TH+ | DMSO > TH−
TH+ > AB |
||||
| Hyoid depression | AB > TH− | AB > TH− | AB > DMSO | DMSO > AB | |
| AB > TH+ | AB > TH− | ANOVA | DMSO > TH+ | ||
| DMSO > TH− | TH+ > DMSO | not sig. | TH− > TH+ | ||
| DMSO > TH+ | TH+ > TH− | ||||
| Cranial elevation | DMSO > TH+ | TH+ > TH− | AB > DMSO | ||
| ANOVA | AB > TH− | ANOVA | |||
| not sig. | TH+ > DMSO | not sig. | |||
| TH+ > TH− | |||||
| Jaw protrusion | AB > TH− | AB > TH− | |||
| NA | NA | NA | DMSO > TH− | DMSO > TH− | |
| TH+ > TH− | TH+ > TH− | ||||
Note: Significant Tukey’s HSD post-hoc comparisons are listed if the ANOVA results were significant (α = .05). Bold/shaded comparisons had p < .001. The treatment with the higher maximum value is indicated for each comparison.
Abbreviations: ANOVA, analysis of variance; DMSO, dimethyl sulfoxide; dpf, days postfertilization; HSD, honestly significant difference; TH, thyroid hormone.
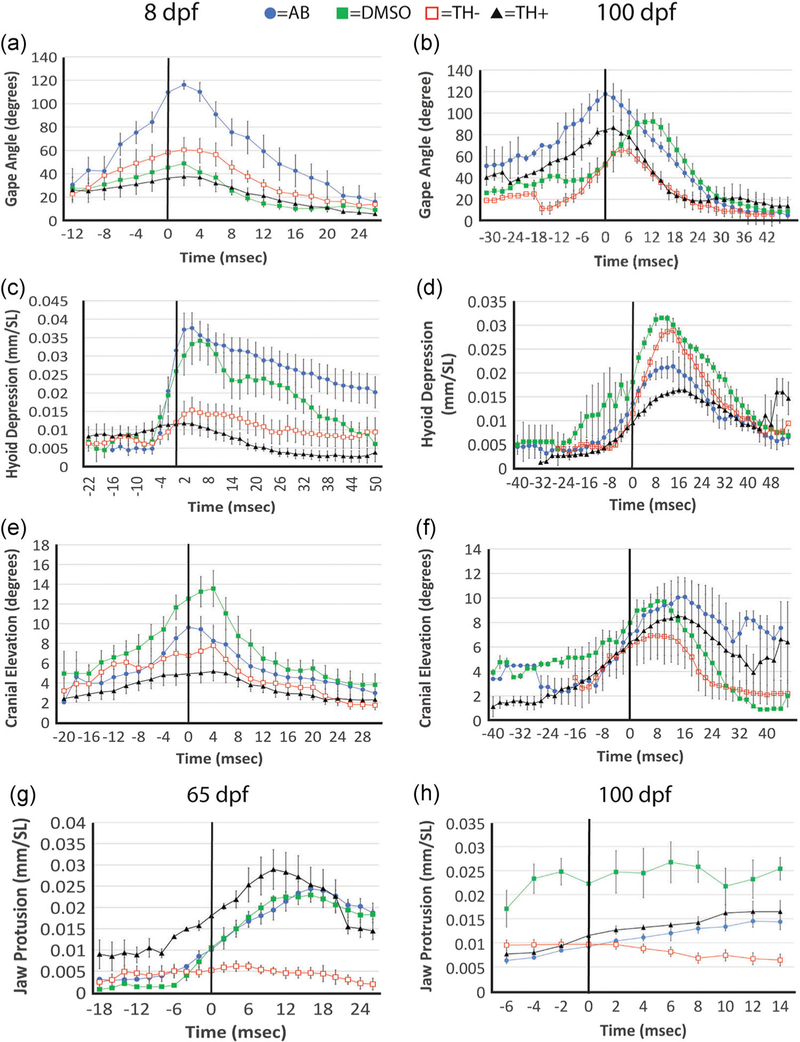
FIGURE 5.
Comparisons of cranial movements during feeding among the four TH treatment groups. In all cases time zero represents the time point when live prey (paramecia at 8 dpf and brine shrimp nauplii at 65 and 100 dpf) passed the tips of the upper and lower jaws as they were being engulfed. Units and standardizations are given in parentheses in each case. (a-h) Plots for gape angle (a,b), hyoid depression (c,d) and cranial elevation (e,f) for specimen of ages 8 and 100 dpf, respectively (kinematic plots for these four variables at 15, 30, and 65 dpf are presented in Figure S2). (g) Jaw protrusion at 65 dpf. (h) Jaw protrusion at 100 dpf. SL, standard length; TH, thyroid hormone
Maximum gape angle occurred at or near time 0 for all treatment groups throughout development (Figure 5a,b and S2). AB fish exhibited high gape angles throughout development (Figures 5a,b and S2), while TH− fish exhibited low gape angles throughout development (Figures 5a,b and S2). Maximum hyoid depression occurred after time 0 for all treatment groups throughout development (Figure 5c,d and S2). Hypothyroid fish exhibited low levels of hyoid depression throughout development until 100 dpf, when they displayed a high degree of hyoid depression (Figures 5c,d and S2). Maximum cranial elevation occurred at or immediately after time 0 throughout development (Figures 5e,f and S2). Hypothyroid fish exhibited low levels of cranial elevation throughout development (Figures 5e,f and S2). Measureable upper jaw protrusion was not observed before 30 dpf in any treatment group, but a small number of euthyroid and TH+ specimens exhibited marginal upper jaw movement at this stage. Specimens from all treatment groups except TH− exhibited protrusile upper jaws by 65 dpf. In contrast, hyperthyroid fish demonstrated low levels of jaw protrusion at both 65 and 100 dpf and were significantly different from all other treatment groups at both stages (Table 1 and Figure 5g,h).
3.5 |. Developmental variation in head shape
Hypothryoid fish showed significantly less developmental variation in head shape than any other treatment group (Table 2). Hyperthyroid fish showed significantly greater developmental variation in head shape than both TH− and DMSO specimens.
TABLE 2.
Shape disparity comparisons for head morphology
| AB | DMSO | TH− | |
|---|---|---|---|
| ABFD = 0.00622046 | NA | ||
| DMSOFD = 0.00593240 | DD = 0.000288 | NA | |
| UBCI = 0.00132652 | |||
| TH−FD = 0.00410838 | DD = 0.002112 (AB) | DD = 0.001824 (DMSO) | |
| UBCI = 0.00139538 | UBCI = 0.00134556 | NA | |
| TH+FD = 0.00780772 | DD = 0.001587 | DD = 0.001875 (TH+) | DD = 0.003699 (TH+) |
| UBCI = 0.00182215 | UBCI = 0.00174844 | UBCI = 0.00188894 |
Abbreviations: DD, difference in the disparities of the treatments compared; FD, Foote disparity; UBCI, upper bound of 95% CI. Disparities were significant when DD > UBCI. Treatments with higher disparities are indicated when significant (bold/shaded).
3.6 |. Shape covariation
Patterns of shape covariation were highly similar in the skulls of all treatments with one exception: TH− versus TH+. These two treatments produced zebrafish skulls that exhibited similar patterns of shape covariation in that the orientations of their PC1 axes were not found to be significantly different from parallel to one another (Table 3). However, their PC2 axes were not parallel, which indicates a moderate difference in anatomical trait covariation (Table 3). Less pronounced differences in TH levels did not produce detectable differences in patterns of skull shape covariation. The first four PC axes described similar covariation patterns when the skulls of euthyroid fish (both AB and DMSO) were compared to those of either TH− or TH+ specimens (Table 3). The first four PC axes also described similar covariation patterns in skulls from the two euthyroid treatments (Table 3).
TABLE 3.
Shape space orientation comparisons for head and premaxilla morphology
| Angle (degrees) |
95% CI | |
|---|---|---|
| PC1 | ||
| AB vs. DMSO | 21.78 | 16.35–31.81 |
| AB vs. TH− | 34.73 | 29.24–44.89 |
| AB vs. TH+ | 23.71 | 19.65–31.17 |
| DMSO vs. TH− | 18.26 | 15.50–30.77 |
| DMSO vs. TH+ | 17.55 | 15.99–24.94 |
| TH− vs. TH+ | 19.13 | 15.92–27.90 |
| AB pramaxilla development vs. Danionini premaxillae diversity | 33.34 | 25.76–64.45 |
| PC1-PC2 | ||
| AB vs. DMSO | 61.51 | 50.06–85.52 |
| AB vs. TH− | 88.02 | 46.32–94.51 |
| AB vs. TH+ | 86.73 | 47.20–91.12 |
| DMSO vs. TH− | 65.94 | 40.42–90.94 |
| DMSO vs. TH+ | 74.15 | 42.80–91.19 |
| TH− vs. TH+ | 29.86 | 34.57–89.13 |
| AB pramaxilla development vs. Danionini premaxillae diversity | 52.15 | 38.73–84.78 |
| PC1-PC3 | ||
| AB vs. DMSO | 80.03 | 73.32–96.61 |
| AB vs. TH− | 65.80 | 55.39–102.35 |
| AB vs. TH+ | 59.58 | 53.65–86.61 |
| DMSO vs. TH− | 84.21 | 67.73–102.18 |
| DMSO vs. TH+ | 77.30 | 65.46–100.39 |
| AB pramaxilla development vs. Danionini premaxillae diversity | 52.83 | 50.17–96.84 |
| PC1-PC4 | ||
| AB vs. DMSO | 101.83 | 84.63–116.01 |
| AB vs. TH− | 93.18 | 71.75–109.74 |
| AB vs. TH+ | 77.48 | 66.79–106.49 |
| DMSO vs. TH− | 86.38 | 74.52–111.07 |
| DMSO vs. TH+ | 97.36 | 77.98–117.97 |
| AB pramaxilla development vs. Danionini premaxillae diversity | 79.00 | 74.07–109.85 |
Note: The observed angle between the shape spaces is given for each comparison. 95% confidence intervals (CIs) for this angle were calculated by bootstrapping the data from both groups (700 bootstraps). Significant differences in bold/shaded. The TH− and TH+ shape spaces were not significantly different for the first PC axis only. All other comparisons were not significantly different for PC1-PC4.
3.7 |. Head and premaxilla shape
Head shape differences between TH treatments were present at all ages examined, but those from fish in different treatments were most similar at the youngest ages (8 and 15 dpf; Figure S1). By 100 dpf TH+ and TH− head shapes were highly distinct from each other (Figure S1).
The ascending arm of the premaxilla (Figure 1), is extremely short at 35 dpf in AB fish, but elongates significantly by 100 dpf (Figure 4). Premaxilla shape in 100 dpf TH− specimens was similar to that of newly ossified premaxillae in 35 dpf AB fish, in that they had very short ascending arms (Figure 4). Both the maxillae and premaxillae in the upper jaws of TH− fish exhibited limited growth in size that was unaccompanied by the shape changes seen in those of postmetamorphic euthyroid and TH+ fish (Figures 2 and 4).
Variation in ascending arm length was strongly associated with both postmetamorphic AB zebrafish development and danionin evolution (Figure 4). Developmental variation in AB premaxilla shape was not significantly different from the variation in premaxilla shape that has evolved among nine additional danionin species (i.e., the first 4 PC axes derived from these two data sets were parallel; Table 3). Among the species that we examined, those from the genus Danio have longer premaxillary ascending arms than those from other danionin genera, with Danio erythromicron having the longest arms (Figure 4). The premaxillae of Danionella, Devario, and Microdevario were most similar to those from 35 dpf AB and 100 dpf TH− zebrafish. Manipulation of freshly euthanized specimens from these three genera, as well as observations of their feeding using high-speed video (personal observation; S. McMenamin et al., 2017), indicate that jaw protrusion is extremely limited to nonexistent in these species.
4 |. DISCUSSION
We found strong evidence that TH levels affect the development of zebrafish feeding biomechanics (prediction 1), that TH increases developmental variation in zebrafish head shape (prediction 2), and that the functional morphology of jaw protrusion in adult TH− zebrafish duplicates that of closely related minnows with limited protrusion abilities (prediction 4). Normal TH levels do not appear to be required for the development of the wild-type pattern of covariation between different regions of the zebrafish skull (prediction 3), but specimens with highly divergent TH levels (TH− and TH+) did exhibit limited differences in cranial covariation patterns. We also found evidence that the developmental trajectory of premaxillary shape change in AB zebrafish is parallel to an important evolutionary axis of danionin diversification (premaxilla shape and jaw protrusion ability).
We found that differences in feeding biomechanics between our TH treatment groups were present at all of the developmental stages we examined, but that these differences increased with age and were more pronounced after metamorphosis (Table 1 and Figure S1). The presence of TH appears to be particularly important for the transition from larval to adult feeding mechanics in the zebrafish. An absence of TH delayed cranial ossification and arrested premaxillae formation so that upper jaw protrusion was absent or severely limited in postmetamorphic TH− zebrafish development to permit upper jaw protrusion (Table 1 and Figures 2, 4, and 5; S. McMenamin et al., 2017).
Hypothyroidism appeared to truncate aspects of zebrafish cranial development. In addition to arresting premaxilla morphogenesis immediately after ossification (Figure 4), lack of TH caused a significant reduction in the skull shape variation that arose between 8 and 100 dpf (Table 2). However, TH− fish grow more slowly than the other groups (Figure 3) and some of this decrease in variation may be attributable to TH− specimens’ slow growth or narrow range of developmental stages. Conversely, TH+ fish showed an increase in developmental variation in skull shape relative to both DMSO and TH−, though they were not significantly different from AB specimens in this regard (Table 2).
The only significant differences in cranial shape covariation that we observed were between the TH− and TH+ treatments, but these differences did not include PC1, which is the largest axis of shape variation (Table 3). Changes to patterns of anatomical trait covariation can have debilitating effects, especially in biomechanical systems where there is a high level of functional integration between different elements (Armbruster, Pelabon, Bolstad, & Hansen, 2014; Kimmel et al., 2015; Walker, 2007) and both TH− and TH+ fish retained a sufficient level of integration between the various bones of the skull to feed successfully.
Premaxillary ascending arm length is an important determinant of maximum upper jaw protrusion distance (Figure 1; Cooper, Carter, et al., 2017; Hulsey, Hollings-worth, & Holzman, 2010; Motta, 1984). Eliminating TH produces zebrafish with highly reduced ascending arms and feeding mechanisms that resemble those of close relatives with nonprotrusile jaws (Table 1 and Figures 4 and 5; S. McMenamin et al., 2017). Many of these have been described as being paedomorphic (Britz, Conway, & Rueber, 2014; Kullander & Britz, 2015), a condition that is strongly associated with changes in TH signaling in vertebrates (De Groef, Grommen, & Darras, 2018; Ivanovic, Cvijanovic, Denoel, Slijepcevic, & Kalezic, 2014; Laudet, 2011). Differences in jaw protrusion ability are strongly associated with differences in diet in marine damselfishes (Cooper, McGraw, Khazanchi, & Ieee., 2017), and although diet data for wild danionins is limited or nonexistent, we suspect that the same may be true for the species examined here. Although TH− zebrafish did not develop adult jaw protrusion abilities, they retained sufficient functional integration to be able to feed successfully in the lab environment. It has been suggested previously that evolutionary modulation of TH signaling may have contributed to the diversification of cypriniform fishes (McMenamin et al., 2017; Shkil & Smirnov, 2015; Shkil et al., 2012, 2015). We conclude that evolutionary changes in TH signaling may be capable of inducing some adaptive changes in the development of fish feeding mechanics without causing severe disruptions to cranial morphogenesis that produce nonfunctional skulls.
4.1 |. The importance of late developmental periods to fish evo-devo
The field of evo-devo is focused on understanding the connections between developmental processes and evolutionary change (Carroll, 2008). Phylogenetic analyses of comparative data have expanded tremendously in recent years and the evolutionary patterns traced by many lineages have been described in great detail (Freckleton, Harvey, & Pagel, 2002; Garland, Bennett, & Rezende, 2005; Mouquet et al., 2012). A particularly rich source of comparative information exists for the field of fish feeding biomechanics and we now know a great deal about the ecological, morphological, and functional evolution of many fish clades. Due to the use of multiple fish species as model organisms for developmental study (e.g., zebrafish, medaka, Mexican tetra, fugu, stickleback, multiple African rift-lake cichlids, etc.) it is possible to experimentally explore aspects of fish development that have played important roles in shaping evolutionary diversification. However, since most evolutionary studies of fish feeding have focused on adult specimens and since most fishes do not develop adult feeding biomechanics until late in their development, often after a pronounced metamorphosis, merging these two areas of investigation will require an increased focus on the later stages of skull morphogenesis.
Evo-devo studies of jaw protrusion provide an illustration of this point. Protrusile jaws constitute one of the most significant biomechanical innovations to arise in fish skulls (Motta, 1984; Wainwright & Longo, 2017; Wainwright et al., 2015), but we know of no species in which protrusion arises before the larva-to-juvenile transition. The fact that small fish larvae will experience much high water viscosities than larger adult forms (Hernandez, 1995; Hernandez, 2000) may predispose many species to undergo developmental changes in feeding mechanics. We suggest that this may be particularly true for fishes that utilize jaw protrusion during adult feeding. Thyroid hormone signaling has pervasive effects on skeletal morphogenesis during late development (Okada, Tanaka, & Tagawa, 2003; Pascual & Aranda, 2013; Shkil et al., 2012), spikes in TH are associated with metamorphosis of larval fishes into juveniles (McMenamin & Parichy, 2013; Okada et al., 2003), and adult jaw protrusion abilities do not arise in TH− zebrafish (Table 1, Figure 4, and 5; S. McMenamin et al., 2017). The range of effects that TH has on bone remodeling (Bassett & Williams, 2003; Gogakos et al., 2010; Harvey et al., 2002; Waung et al., 2012; Wojcicka et al., 2013) suggests that there are likely to be a number of mechanisms by which changes to TH signaling could alter the development of fish skulls and jaws. Our data support the assertion that a better understanding of how TH affects cranial remodeling has important relevance to the evo-devo of adaptive diversification in fish feeding.
Evolutionary changes to TH signaling may have contributed to the diversification of cyprinid fishes. Miniaturization has arisen multiple times in danionine minnows (e.g., the genera Danionella, Fangfangia, Microdevario, Paedocypris, and Sundadanio) and it has been speculated that developmental truncation (i.e., paedomorphosis) has contributed to the divergence of these species from their ancestral forms (Britz, Conway, & Ruber, 2009; Britz et al., 2014; Britz, Kottelat, & Tan, 2011; Ruber, Kottelat, Tan, Ng, & Britz, 2007). We observed that loss of TH production retarded zebrafish growth (Figure 3) and arrested premaxillary shape development at an early stage so that adult TH− specimens did not develop adult jaw protrusion abilities (Table 1, Figures 4 and 5). McMenamin et al. (2017) demonstrated that adult TH− zebrafish retained feeding mechanics similar to those of both larval euthyroid zebrafish and adult specimens from the highly paedomorphic danionine genus Danionella (Britz et al., 2009). Shkil et al. have provided evidence consistent with the hypothesis that changes in TH production and/or tissue responsiveness to TH has contributed to heterochronic evolution during the adaptive divergence of cyprinid fishes outside of the danionine lineage (Shkil & Smirnov, 2015; Shkil et al., 2012, 2015). Taken together, these findings suggest that evolutionary changes in TH signaling may have shaped aspects of cypriniform diversification, especially during the repeated evolution of miniature species that exhibit heterochronic development in which they retain aspects of larval feeding mechanics as adults.
5 |. CONCLUSIONS
TH signaling induced development of an adult premaxillary shape and adult feeding kinematics in metamorphosing zebrafish. Hypothyroidism inhibited the development of jaw protrusion by severely reducing the development of premaxillary ascending arm length. Normal ontogenetic changes in zebrafish premaxillary shape represent developmental variation that could underlie evolutionary changes in jaw protrusion ability. The pronounced effects of thyroid hormone on the development of the functional morphology of the skull during the juvenile to adult transition suggest that changes in TH or pathways affected by TH may have contributed to adaptive diversification of fish feeding biomechanics.
Supplementary Material
ACKNOWLEDGMENTS
We thank Nicolai Konow and two anonymous reviewers for their comments, which greatly improved this manuscript. The work was supported by NSF 1755433 (to W. J. C.) and NIH R00GM105874 and R03HD091634 (to S. K. M.).
Funding information
National Science Foundation, Grant/Award Number: 1755433; NIH, Grant/Award Numbers: R00GM105874, R03HD091634
Footnotes
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
REFERENCES
- Albertson RC, & Yelick PC (2004). Morphogenesis of the jaw: Development beyond the embryo, Zebrafish: 2nd Edition cellular and developmental biology (Vol. 76, pp. 437–454). San Diego, CA: Elsevier Academic Press Inc. [DOI] [PubMed] [Google Scholar]
- Armbruster WS, Pelabon C, Bolstad GH, & Hansen TF (2014). Integrated phenotypes: Understanding trait covariation in plants and animals. Philosophical Transactions of the Royal Society B-Biological Sciences, 369(1649), 10.1098/rstb.2013.0245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassett J, & Williams G. (2003). The molecular actions of thyroid hormone in bone (Vol. 14). [DOI] [PubMed] [Google Scholar]
- Britz R, Conway KW, & Ruber L. (2009). Spectacular morphological novelty in a miniature cyprinid fish, Danionella dracula n. sp. Proceedings of the Royal Society B: Biological Sciences, 276(1665), 2179–2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britz R, Conway KW, & Rueber L. (2014). Miniatures, morphology and molecules: Paedocypris and its phylogenetic position (Teleostei, Cypriniformes). Zoological Journal of the Linnean Society, 172(3), 556–615. 10.1111/zoj.12184 [DOI] [Google Scholar]
- Britz R, Kottelat M, & Tan H. (2011). Fangfangia spiniclei-thralis, a new genus and species of miniature cyprinid fish from the peat swamp forests of Borneo (Teleostei: Cyprinidae) (Vol. 22). [Google Scholar]
- Carroll SB (2008). Evo-devo and an expanding evolutionary synthesis: A genetic theory of morphological evolution. Cell, 134, 134–136. 10.1016/j.cell.2008.06.030 [DOI] [PubMed] [Google Scholar]
- Cooper K, McGraw A, Khazanchi D, & Ieee; (2017). Bioinformatics for middle school aged children: activities for exposure to an interdisciplinary field. In Proceedings of the 2017 7th IEEE Integrated Stem Education Conference (pp. 1–9). [Google Scholar]
- Cooper WJ, Carter CB, Conith AJ, Rice AN, & Westneat MW (2017). The evolution of jaw protrusion mechanics is tightly coupled to bentho-pelagic divergence in damselfishes (Pomacentridae). The Journal of Experimental Biology, 220(4), 652–666. 10.1242/jeb.143115 [DOI] [PubMed] [Google Scholar]
- Cooper WJ, Parsons K, McIntyre A, Kern B, McGee-Moore A, & Albertson RC (2010). Bentho-pelagic divergence of cichlid feeding architecture was prodigious and consistent during multiple adaptive radiations within African rift-lakes. PLOS One, 5(3), A38–A50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper WJ, Wernle J, Mann KA, & Albertson RC (2011). Functional and genetic integration in the skulls of lake Malawi cichlids. Evolutionary Biology, 38(3), 316–334. 10.1007/s11692-011-9124-9 [DOI] [Google Scholar]
- Cooper WJ, & Westneat MW (2009). Form and function of damselfish skulls: Rapid and repeated evolution into a limited number of trophic niches. BMC Evolutionary Biology, 9, 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper WJ, Wirgau RM, Sweet EM, & Albertson RC (2013). Deficiency of zebrafish fgf20a results in aberrant skull remodeling that mimics both human cranial disease and evolutionarily important fish skull morphologies. Evolution & Development, 15(6), 426–441. 10.1111/ede.12052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das B, Cai LQ, Carter MG, Piao YL, Sharov AA, Ko MSH, & Brown DD (2006). Gene expression changes at metamorphosis induced by thyroid hormone in Xenopus laevis tadpoles. Developmental Biology, 291(2), 342–355. 10.1016/j.ydbio.2005.12.032 [DOI] [PubMed] [Google Scholar]
- Desjardin C, Charles C, Benoist-Lasselin C, Riviere J, Gilles M, Chassande O, & Schibler L. (2014). Chondrocytes play a major role in the stimulation of bone growth by thyroid hormone. Endocrinology, 155(8), 3123–3135. 10.1210/en.2014-1109 [DOI] [PubMed] [Google Scholar]
- Ferry-Graham LA, Gibb AC, & Hernandez LP (2008). Premaxillary movements in cyprinodontiform fishes: An unusual protrusion mechanism facilitates “picking” prey capture. Zoology, 111(6), 455–466. 10.1016/j.zool.2007.11.003 [DOI] [PubMed] [Google Scholar]
- Foote M. (1993). Contributions of individual taxa to overall morphological disparity. Paleobiology, 19(4), 403–419. [Google Scholar]
- Freckleton RP, Harvey PH, & Pagel M. (2002). Phylogenetic analysis and comparative data: A test and review of evidence. The American Naturalist, 160(6), 712–726. 10.1086/343873 [DOI] [PubMed] [Google Scholar]
- Garland T, Bennett AF, & Rezende EL (2005). Phylogenetic approaches in comparative physiology. Journal of Experimental Biology, 208(16), 3015–3035. 10.1242/jeb.01745 [DOI] [PubMed] [Google Scholar]
- Gidmark NJ, Staab KL, Brainerd EL, & Hernandez LP (2012). Flexibility in starting posture drives flexibility in kinematic behavior of the kinethmoid-mediated premaxillary protrusion mechanism in a cyprinid fish, Cyprinus carpio. The Journal of Experimental Biology, 215(13), 2262–2272. 10.1242/jeb.070516 [DOI] [PubMed] [Google Scholar]
- Gogakos AI, Bassett JHD, & Williams GR (2010). Thyroid and bone. Archives of Biochemistry and Biophysics, 503(1), 129–136. 10.1016/j.abb.2010.06.021 [DOI] [PubMed] [Google Scholar]
- De Groef B, Grommen SVH, & Darras VM (2018). Forever young: Endocrinology of paedomorphosis in the Mexican axolotl (Ambystoma mexicanum). General and Comparative Endocrinology, 266, 194–201. 10.1016/j.ygcen.2018.05.016 [DOI] [PubMed] [Google Scholar]
- Hanken J, & Hall BK (1988). Skull development during anuran metamorphosis. 2. Role of thyroid-hormone in osteogenesis. Anatomy and Embryology, 178(3), 219–227. 10.1007/bf00318225 [DOI] [PubMed] [Google Scholar]
- Hanken J, & Summers CH (1988). Skull development during anuran metamorphosis. 3. Role of thyroid-hormone in chondrogenesis. Journal of Experimental Zoology, 246(2), 156–170. 10.1002/jez.1402460208 [DOI] [PubMed] [Google Scholar]
- Harvey CB, O’Shea PJ, Scott AJ, Robson H, Siebler T, Shalet SM, & Williams GR (2002). Molecular mechanisms of thyroid hormone effects on bone growth and function. Molecular Genetics and Metabolism, 75(1), 17–30. 10.1006/mgme.2001.3268 [DOI] [PubMed] [Google Scholar]
- Hernandez LP (1995). The functional morphology of feeding in three ontogenetic stages of the zebrafish, Danio rerio. American Zoologist, 35(5), 104A. [Google Scholar]
- Hernandez LP (2000). Intraspecific scaling of feeding mechanics in an ontogenetic series of zebrafish, Danio rerio. Journal of Experimental Biology, 203(19), 3033–3043. [DOI] [PubMed] [Google Scholar]
- Hernandez LP, Bird NC, & Staab KL (2007). Using zebrafish to investigate cypriniform evolutionary novelties: Functional development and evolutionary diversification of the kinethmoid. Journal of Experimental Zoology Part B-Molecular and Developmental Evolution, 308B(5), 625–641. 10.1002/jez.b.21166 [DOI] [PubMed] [Google Scholar]
- Hernández LP, Barresi MJF, & Devoto SH (2002). Functional morphology and developmental biology of zebrafish: Reciprocal illumination from an unlikely couple. Integrative and comparative biology, 42(2), 222–231. [DOI] [PubMed] [Google Scholar]
- Hirano A, Akita S, & Fujii T. (1995). Craniofacial deformities associated with juvenile hyperthyroidism. Cleft Palate-Craniofacial Journal, 32(4), 328–333. [DOI] [PubMed] [Google Scholar]
- Holzman R, Collar DC, Mehta RS, & Wainwright PC (2012). An integrative modeling approach to elucidate suction-feeding performance. Journal of Experimental Biology, 215(1), 1–13. 10.1242/jeb.057851 [DOI] [PubMed] [Google Scholar]
- Hu Y, Mauri A, Donahue J, Singh R, Acosta B, & McMenamin SK (2019). Thyroid hormone coordinates developmental trajectories but does not underlie developmental truncation in Danionins. BioRxiv, 562074. doi: 10.1101/562074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulsey CD, Hollingsworth PR Jr., & Holzman R. (2010). Co-evolution of the premaxilla and jaw protrusion in cichlid fishes (Heroine: Cichlidae). Biological Journal of the Linnean Society, 100(3), 619–629. [Google Scholar]
- Ivanovic A, Cvijanovic M, Denoel M, Slijepcevic M, & Kalezic ML (2014). Facultative paedomorphosis and the pattern of intra- and interspecific variation in cranial skeleton: Lessons from European newts (Ichthyosaura alpestris and Lissotriton vulgaris). Zoomorphology, 133(1), 99–109. 10.1007/s00435-013-0202-7 [DOI] [Google Scholar]
- Kimmel CB, Watson S, Couture RB, McKibben NS, Nichols JT, Richardson SE, & Noakes DLG (2015). Patterns of variation and covariation in the shapes of mandibular bones of juvenile salmonids in the genus Oncor-hynchus. Evolution & Development, 17(5), 302–314. 10.1111/ede.12135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klingenberg CP (2008). Morphological integration and developmental modularity. Annual Review of Ecology Evolution and Systematics, 39, 115–132. 10.1146/annurev.ecolsys.37.091305.110054 [DOI] [Google Scholar]
- Klingenberg CP, Duttke S, Whelan S, & Kim M. (2012). Developmental plasticity, morphological variation and evolvability: A multilevel analysis of morphometric integration in the shape of compound leaves. Journal of Evolutionary Biology, 25(1), 115–129. 10.1111/j.1420-9101.2011.02410.x [DOI] [PubMed] [Google Scholar]
- Konow N, & Bellwood DR (2005). Prey-capture in Pomacanthus semicirculatus (Teleostei, Pomacanthidae): Functional implications of intramandibular joints in marine angelfishes. Journal of Experimental Biology, 208(8), 1421–1433. [DOI] [PubMed] [Google Scholar]
- Kullander SO, & Britz R. (2015). Description of Danio absconditus, new species, and redescription of Danio feegradei (Teleostei: Cyprinidae), from the Rakhine Yoma hotspot in south-western Myanmar. Zootaxa, 3948(2), 233–247. [DOI] [PubMed] [Google Scholar]
- Laudet V. (2011). The origins and evolution of vertebrate metamorphosis. Current Biology, 21(18), R726–R737. 10.1016/j.cub.2011.07.030 [DOI] [PubMed] [Google Scholar]
- Lavergne S, & Molofsky J. (2007). Increased genetic variation and evolutionary potential drive the success of an invasive grass. Proceedings of the National Academy of Sciences of the United of States of America, 104(10), 3883–3888. 10.1073/pnas.0607324104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leis JM, & McCormick MI (2006). The biology, behavior and ecology of the pelagic, larval stage of coral reef fishes In Sale PF (Ed.), Coral reef fishes: Dynamics and diversity in a complex ecosystem (pp. 171–200). Burlington, MA: Elsevier, Inc. [Google Scholar]
- McCluskey BM, & Postlethwait JH (2015). Phylogeny of Zebrafish, a “Model Species,” within Danio, a “Model Genus”.Molecular Biology and Evolution, 32(3), 635–652. 10.1093/molbev/msu325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick MI, Makey L, & Dufour V. (2002). Comparative study of metamorphosis in tropical reef fishes. Marine Biology, 141(5), 841–853. [Google Scholar]
- McCormick MI, & Makey LJ (1997). Post-settlement transition in coral reef fishes: Overlooked complexity in niche shifts. Marine Ecology Progress Series, 153, 247–257. 10.3354/meps153247 [DOI] [Google Scholar]
- McMenamin SK, Bain EJ, McCann AE, Patterson LB, Eom DS, Waller ZP, & Parichy DM (2014). Thyroid hormone-dependent adult pigment cell lineage and pattern in zebrafish. Science, 345(6202), 1358–1361. 10.1126/science.1256251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMenamin S, Carter C, & Cooper WJ (2017). Thyroid hormone stimulates the onset of adult feeding kinematics in zebrafish. Zebrafish, 14(6), 517–525. 10.1089/zeb.2017.1453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMenamin SK, Chandless MN, & Parichy DM (2016). Working with zebrafish at postembryonic stages In Detrich HW, Westerfield M, & Zon LI (Eds.), Zebrafish: Cellular and Developmental Biology, Part B: Developmental Biology (pp. 587–607). San Diego, CA: Elsevier Academic Press Inc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMenamin SK, & Parichy DM (2013). Metamorphosis in teleosts In Shi YB (Ed.), Animal metamorphosis (Vol. 103, pp. 127–165). San Diego, CA: Elsevier Academic Press Inc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motta PJ (1984). Mechanics and functions of jaw protrusion in teleost fishes—A review. Copeia, 1984(1), 1–18. [Google Scholar]
- Mouquet N, Devictor V, Meynard CN, Munoz F, Bersier LF, Chave J, & Thuiller W. (2012). Ecophylogenetics: Advances and perspectives. Biological Reviews, 87(4), 769–785. 10.1111/j.1469-185X.2012.00224.x [DOI] [PubMed] [Google Scholar]
- Near TJ, Dornburg A, Eytan RI, Keck BP, Smith WL, Kuhn KL, & Wainwright PC (2013). Phylogeny and tempo of diversification in the superradiation of spiny-rayed fishes. Proceedings of the National Academy of Sciences of the United States of America, 110, 12738–12743. 10.1073/pnas.1304661110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada N, Tanaka M, & Tagawa M. (2003). Bone development during metamorphosis of the Japanese flounder (Paralichthys olivaceus): differential responses to thyroid hormone. Bergen: Inst Marine Research. [Google Scholar]
- Parichy DM, Elizondo MR, Mills MG, Gordon TN, & Engeszer RE (2009). Staging by externally visible anatomy of the living Fish. Developmental Dynamics, 238, 2975–3015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paris M, Escriva H, Schubert M, Brunet F, Brtko J, Ciesielski F, & Laudet V. (2010). Amphioxus thyroid hormone signaling pathway and the evolution of metamorphosis in chordates. Integrative and comparative biology, 50, E133. [Google Scholar]
- Parsons K, Andreeva V, Cooper WJ, Yelick PC, & Albertson RC (2010). Morphogenesis of the zebrafish jaw: Development beyond the embryo In Westerfield M, Detrich HW, & Zon LI (Eds.), Methods in cell biology: The Zebrafish (Vol. 1, 3rd ed). San Diego, CA: Elsevier Academic Press Inc. [Google Scholar]
- Pascual A, & Aranda A. (2013). Thyroid hormone receptors, cell growth and differentiation. Biochimica Et Biophysica Acta-General Subjects, 1830(7), 3908–3916. 10.1016/j.bbagen.2012.03.012 [DOI] [PubMed] [Google Scholar]
- Potthoff T. (1984). Clearing and staining techniques In Moser HG, Richards WJ, Cohen DM, Fahay MP, Kendall AW Jr, & Richardson SL (Eds.), Ontogeny and systematics of fishes (pp. 35–57). Lawrence, KS: Allen Press. [Google Scholar]
- Ruber L, Kottelat M, Tan HH, Ng PKL, & Britz R. (2007). Evolution of miniaturization and the phylogenetic position of Paedocypris, comprising the world’s smallest vertebrate. BMC Evolutionary Biology, 7,10 10.1186/1471-2148-7-38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger TJ, Mahler DL, Abzhanov A, & Losos JB (2012). Roles for modularity and constraint in the evolution of cranial diversity among anolis lizards. Evolution, 66(5), 1525–1542. 10.1111/j.1558-5646.2011.01519.x [DOI] [PubMed] [Google Scholar]
- Shkil FN, Kapitanova DV, Borisov VB, Abdissa B, & Smirnov SV (2012). Thyroid hormone in skeletal development of cyprinids: Effects and morphological consequences. Journal of Applied Ichthyology, 28(3), 398–405. 10.1111/j.1439-0426.2012.01992.x [DOI] [Google Scholar]
- Shkil FN, Lazebnyi OE, Kapitanova DV, Abdissa B, Borisov VB, & Smirnov SV (2015). Ontogenetic mechanisms of explosive morphological divergence in the Lake Tana (Ethiopia) species flock of large African barbs (Labeobarbus; Cyprinidae; Teleostei). Russian Journal of Developmental Biology, 46(5), 294–306. 10.1134/s1062360415050069 [DOI] [PubMed] [Google Scholar]
- Shkil FN, & Smirnov SV (2015). Experimental approach to the hypotheses of heterochronic evolution in lower vertebrates. Paleontological Journal, 49(14), 1624–1634. 10.1134/s0031030115140178 [DOI] [Google Scholar]
- Staab KL, Holzman R, Hernandez LP, & Wainwright PC (2012). Independently evolved upper jaw protrusion mechanisms show convergent hydrodynamic function in teleost fishes. Journal of Experimental Biology, 215(9), 1456–1463. 10.1242/jeb.066308 [DOI] [PubMed] [Google Scholar]
- Tang KL, Agnew MK, Hirt MV, Sado T, Schneider LM, Freyhof J, & Mayden RL (2010). Systematics of the subfamily Danioninae (Teleostei: Cypriniformes: Cyprinidae). Molecular Phylogenetics and Evolution, 57(1), 189–214. 10.1016/j.ympev.2010.05.021 [DOI] [PubMed] [Google Scholar]
- Wainwright PC, & Longo SJ (2017). Functional innovations and the conquest of the oceans by acanthomorph fishes. Current Biology, 27(11), R550–R557. 10.1016/j.cub.2017.03.044 [DOI] [PubMed] [Google Scholar]
- Wainwright PC, McGee MD, Longo SJ, & Hernandez LP (2015). Origins, innovations, and diversification of suction feeding in vertebrates. Integrative and Comparative Biology, 55(1), 134–145. 10.1093/icb/icv026 [DOI] [PubMed] [Google Scholar]
- Walker JA (2007). A general model of functional constraints on phenotypic evolution. American Naturalist, 170(5), 681–689. 10.1086/521957 [DOI] [PubMed] [Google Scholar]
- Walker MB, & Kimmel CB (2007). A two-color acid-free cartilage and bone stain for zebrafish larvae. Biotechnic and Histochemistry, 82, 23–28. [DOI] [PubMed] [Google Scholar]
- Waung JA, Bassett JHD, & Williams GR (2012). Thyroid hormone metabolism in skeletal development and adult bone maintenance. Trends in Endocrinology and Metabolism, 23(4), 155–162. 10.1016/j.tem.2011.11.002 [DOI] [PubMed] [Google Scholar]
- Wojcicka A, Bassett JHD, & Williams GR (2013). Mechanisms of action of thyroid hormones in the skeleton. Biochimica Et Biophysica Acta-General Subjects, 1830(7), 3979–3986. https://doi.Org/10.1016/j.bbagen.2012.05.005 [DOI] [PubMed] [Google Scholar]
- Yang L, Mayden RL, Sado T, He SP, Saitoh K, & Miya M. (2010). Molecular phylogeny of the fishes traditionally referred to Cyprinini sensu stricto (Teleostei: Cypriniformes). Zoologica Scripta, 39(6), 527–550. https://doi.org/10.1111Zj.1463-6409.2010.00443.x [Google Scholar]
- Yaniv S, Elad D, & Holzman R. (2014). Suction feeding across fish life stages: Flow dynamics from larvae to adults and implications for prey capture. Journal of Experimental Biology, 217(20), 3748–3757. 10.1242/jeb.104331 [DOI] [PubMed] [Google Scholar]
- Young NM, & Hallgrimsson B. (2005). Serial homology and the evolution of mammalian limb covariation structure. Evolution, 59(12), 2691–2704. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.