Abstract
The Fontan procedure, although an imperfect solution for children born with a single functional ventricle, is the only reconstruction at present short of transplantation. The hemodynamics associated with the total cavopulmonary connection, the modern approach to Fontan, are severely altered from the normal biventricular circulation and may contribute to the long-term complications that are frequently noted. Through recent technological advances, spear-headed by advances in medical imaging, it is now possible to virtually model these surgical procedures and evaluate the patient-specific hemodynamics as part of the pre-operative planning process. This is a novel paradigm with the potential to revolutionize the approach to Fontan surgery, help to optimize the hemodynamic results, and improve patient outcomes. This review provides a brief overview of these methods, presents preliminary results of their clinical usage, and offers insights into its potential future directions.
Introduction
The Fontan palliation for single ventricle congenital heart defects1 is associated with decreased life expectancy2 and numerous long-term complications that reduce quality of life3. There is evidence to suggest that the surgical design and hemodynamics of the total cavopulmonary connection, the modern approach to the Fontan procedure in which the caval veins are connected to the pulmonary arteries, may play a role in the cause or exacerbation of some of these complications. For example, pulmonary arteriovenous malformations may develop from a lack of hepatic factors in the pulmonary blood supply4, such as when there is preferential streaming of inferior venous flow through the Fontan baffle to a single lung5, 6. Thus, achieving a balanced distribution of blood flow through the Fontan baffle to the pulmonary arteries is an important consideration in the surgical design. The hemodynamic power loss through the total cavopulmonary connection is also important given that elevated power loss (that is, inefficient flow through the connection) has been associated with decreased exercise capacity7, ventricular preload and cardiac output8. These losses have also been shown to be sensitive to the surgical design of the Fontan connection, such as vessel size9, connection angle10, or choice of baffle design11, 12.
Despite the importance of optimizing Fontan hemodynamics, the anatomic heterogeneity associated with single ventricle heart defects precludes a “one-size-fits-all” approach to Fontan surgery. Instead, the connection design and patient-specific hemodynamics can vary considerably. Furthermore, the surgical design that is hemodynamically optimal may not be intuitively obvious, particularly in complex cases. Hence, there is a need for an advanced toolset that allows for visualization of patient-specific connection options and can concurrently predict the hemodynamics associated with each option to facilitate selection of the optimal surgical approach. As a potential solution, a set of engineering tools in which cardiac magnetic resonance is used to inform computer-aided design and fluid dynamics simulations has been developed to assist in patient-specific surgical planning for the Fontan procedure. This review will provide a brief overview of these techniques, and highlight preliminary patient experiences.
Materials and Methods
As shown in Figure 1, the surgical planning paradigm for Fontan surgery consists of four major steps: 1) medical image acquisition, 2) image processing to reconstruct the patient-specific anatomy, 3) virtual surgery, and 4) computational fluid dynamics simulations. The following sections will discuss each of these points in more detail, followed by a patient case study demonstrating their clinical usage.
Figure 1.
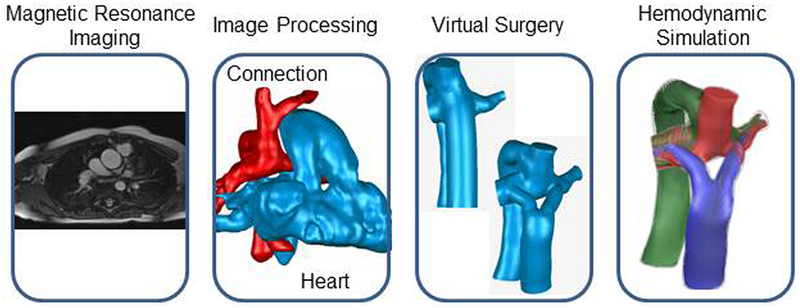
Visual overview of the surgical planning workflow (from right to left): magnetic resonance imaging provides patient anatomy and flow information, which is extracted from post-processing. Virtual surgery enables the design of multiple connection options, which can then be evaluated with assistance from computational fluid dynamics simulations.
Imaging and Image Processing (steps 1 and 2):
Cardiac magnetic resonance is the modality of choice for this application given its ability to image the vascular anatomy and blood flow velocity, both without use of a contrast agent. For anatomic data, a contiguous stack of axial “bright blood” images are acquired through the thorax (far left of Figure 1), and are used to reconstruct a 3-dimensional model of the patient vessels (most often, the inferior and superior caval veins and the pulmonary arteries to their primary branches; second panel of Figure 1). This model serves as the input to both the virtual surgery software and the computational solver. The velocity data are acquired with phase contrast imaging to measure the through-plane velocity. These data inform the computational flow solver as to the relevant blood flow rates into and out of the connection that are to be simulated; that is they provide boundary conditions. Importantly, both the anatomic and velocity techniques described are already routine components of most cardiac magnetic resonance studies. Thus the integration of image-guided surgical planning into clinical studies would not require additional scan time or tradeoffs of other clinical data.
Finally, measurement of a 4-dimensional flow field13 can also provide significant insights. While not directly used in the surgical planning workflow, this in vivo flow information can be beneficial in cases with an existing ‘failed’ Fontan connection to easily ascertain the adverse flow patterns that the new surgical connection must address. The study by Sundareswaran and de Zélicourt provide an excellent example of such a scenario6.
Virtual surgery and computational fluid dynamics simulations (steps 3 and 4):
The virtual surgery tool provides a unique interface that takes in the reconstructed patient anatomy from medical imaging and uses free-form haptic devices to allow the user to position the surgical baffle in 3-dimensional space14. The clinician can thus use this system to create and study the array of potential connection options under consideration for a given patient such as the comparison of an extracardiac conduit and a bifurcated Y-graft (third panel of Figure 1). Models of the surrounding anatomical structures, such as the heart, aorta, and pulmonary veins are also included in the interface to provide relevant surgical landmarks. Comparison of the resulting Fontan models to post-operative image reconstructions has shown that this tool can mimic these surgical connections with high fidelity15.
The final step is to simulate the local flow fields through each of the virtual connection models, quantify the relevant metrics of interest, and select the optimal surgical approach. Any validated fluid dynamics software package can be used for this analysis; our group primarily uses the custom solver developed by de Zélicourt et al.16. Depending on the complexity of the connection, the size of the computational mesh, and the number of computational nodes used to run the solver, a single simulation can be completed on the order of hours to days. The result of the simulation is a high resolution velocity field from which power loss, local flow distributions or any other relevant metric of interest can be derived.
Case Study
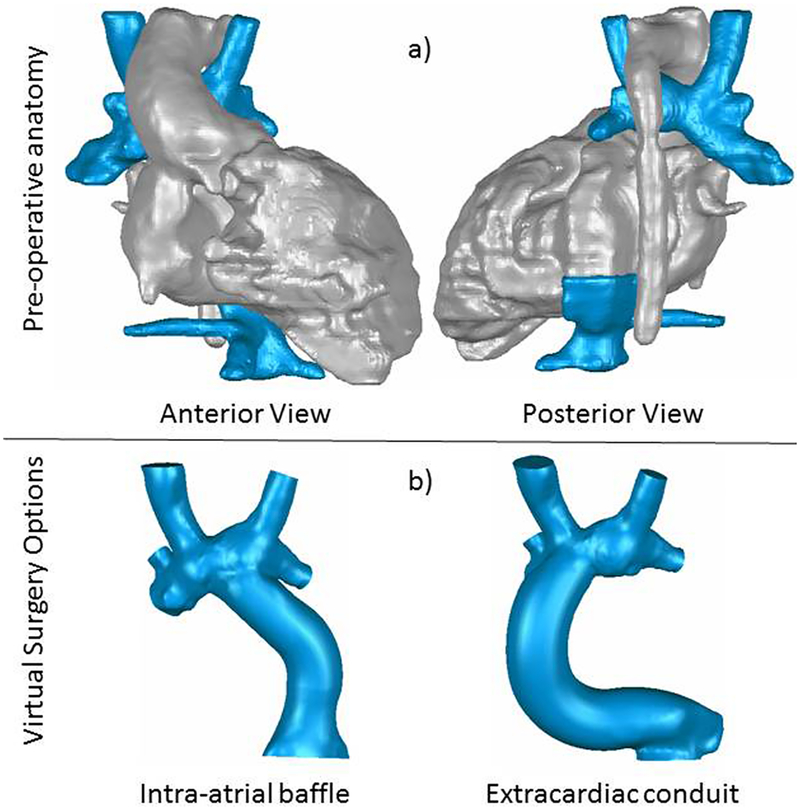
The following case example of a 2 year-old male with heterotaxy syndrome of asplenia type who is after bilateral bidirectional Glenn illustrates the procedure and issues faced. Pulmonary arteriovenous malformations were suspected in the right lung. A primary challenge in the surgical approach to this patient, as shown in the anatomic reconstruction in Figure 2a, was the left-sided positioning of the inferior caval vein. Hence, the primary modeling objective was to identify the optimal approach to baffling the inferior caval flow to the pulmonary arteries that a) ensured sufficient flow from the inferior caval vein to the right lung, and b) minimized power losses.
Figure 2.
a) Anatomic reconstructions from patient case study. The heart and surrounding organs, which are included to provide surgical landmarks and points of reference, are colored in gray, while the vessels that make up the Fontan connection are colored blue. b) Representative virtual surgery models considered for the patient: an intra-atrial baffle on the left, and an extracardiac connection on the right.
Six different surgical options and three flow conditions were simulated; only the major findings are highlighted here. As shown in Figure 2b, the surgical approach was ultimately a decision between a left-sided intra-atrial baffle (16 mm) and an extracardiac conduit (18 mm) that traversed the inferior aspect of the right atrium before turning superiorly toward the bidirectional Glenn connection.
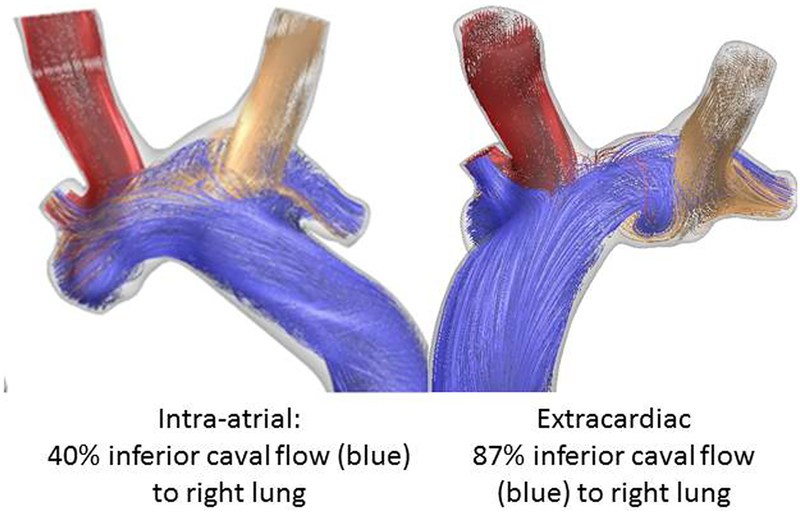
Figure 3 shows representative velocity streamlines for the two surgical models, simulated under the pre-operatively measured flow conditions. The streamlines are colored by vessel of origin to facilitate visualization of flow streaming; flow through the baffle is colored blue. In both cases, inferior caval vein flow was seen to divide between the two lungs, however, the extracardiac model with the baffle positioned further to the right favored inferior caval vein flow to the right lung (the side with the malformations) to a greater extent (87% versus 40%, respectively). Furthermore, with a larger conduit and more streamlined flow through the connection, the right-sided extracardiac connection also had the lowest connection resistance (0.13 Wood Units versus 0.20 Wood Units). Hence, the extracardiac connection was ultimately selected for surgical implementation.
Figure 3.
Flow streaming results from computational fluid dynamics simulations for the considered surgical options. Flow from the inferior veins is colored blue.
To summarize, this patient presented with both a clinically important single ventricle complication (pulmonary arteriovenous malformation), and a particularly complex anatomical configuration of the great vessels which both make for a challenging approach to create the optimal Fontan procedure. In this setting, the image-guided surgical planning simulations produced valuable clinical insights by 1) providing a means to virtually explore the options available and 2) evaluating those options to identify the best approach.
Results Verification
Clearly, these novel methods can only provide relevant clinical insights if the results generated are accurate representations of the post-operative hemodynamics. While capturing the requisite post-operative data to make these important comparisons can be a challenge, preliminary findings have been promising. We recently reported an in depth analysis of four such patient cases with pre- and post-operative data, inclusive of anatomic, vessel flow rate, power loss and flow distribution comparisons15. The conclusions of that study were that model representations of the surgical connections and the flow distribution results, which are critical in cases of pulmonary arteriovenous malformations, were accurate within the uncertainty introduced by acute changes in flow split to the left and right lungs. Other groups have reported similar challenges in accurately accounting for post-operative pulmonary flow splits17. Our model predictions of power losses, although close, require continued development given significant sensitivities to minimum vessel dimensions and systemic venous flow rates and distributions. Continued method development and data collection are ongoing to implement these findings and improve predictive accuracy.
Future Directions
Toward the stated goal of optimizing the Fontan connection using image-guided surgery planning, there is still much more work to be done but the experience thus far has demonstrated that it is an attainable goal. To this end, the following is a brief overview of where the future of this technology is heading.
Lumped parameter modeling:
Lower order computational models of the entire cardiovascular system, in addition to the full fluid dynamics model, can provide a more complete analysis of patient physiology17. Lumped parameter models provide a means to evaluate cardiac function, prescribe dynamic and responsive boundary conditions for the fluid simulation, or evaluate the pressures in other critical organs such as the liver. Hence, the continued development of such systems, and using them in tandem with more complex models, is an active area of research for many groups.
Planning for growth:
The great challenge of Fontan physiology is not improving the acute surgical outcomes but achieving good long term results and avoiding chronic failure. Thus, to truly optimize the connection, the ability to predict how it will grow with the patient and adapt to changing physiologic demands will be an important but daunting task. Building imaging libraries of current patients, inclusive of serial follow-up, is likely the best way to begin to approach this objective in order to understand the modeling challenges which must be incorporated.
Expanding clinical indications for image-guided surgical planning:
Patients with pulmonary arteriovenous malformations represent the majority of surgical planning experience to date as there is a solid understanding of the coupling between a measurable hemodynamic end point (equal hepatic flow to both lungs) and the cure for the disease (increased systemic oxygen saturation). As research identifies similar relationships for other complications, it is likely that this surgical planning paradigm can be applied to more such cases. With that said, preliminary experiences, such as the work described by Mirabella et al.18, have begun to show what else is possible, such as investigating the effect of reopening a fenestration in a patient with elevated central venous pressure and gastrointestinal complications.
Test bed of innovation:
Until such time as patients born with a single ventricle can be expected to have a normal quality and longevity of life, surgical innovation and refinement will continue. Image-guided surgery planning can play a very important role in that process by providing an early outlet to establish proof of concept for these new ideas. Recent experiences with the Fontan Y-Graft12, 19 and flow bifurcation20 serve as excellent examples of this capability. The clinical use of the Y-Graft in particular grew from several surgical planning cases in which such a design was predicted to produce a favorable outcome; it is now being evaluated for potential hemodynamic improvements on a broader set of patients.
Conclusions
Image-guided virtual surgery for the Fontan procedure is a new and exciting paradigm that has the potential to revolutionize cardiovascular surgery for patients born with a single ventricle. By leveraging routine clinical imaging data, this technique enables the clinician to virtually visualize and evaluate patient-specific treatment options before even entering the operating room. By combining these models with state-of-the-art computational tools, the hemodynamic implications of each surgical design can be evaluated. Given the complexity and importance of hemodynamics in the long-term health and viability of the single ventricle circulation, these data represent important clinical insights. Much work is needed to continue to develop and perfect these techniques but one day they may help achieve the elusive goal of patient-specific hemodynamic optimization.
Acknowledgements
The authors wish to acknowledge Jarek Rossignac for development of the virtual surgery interface, Diane de Zélicourt for development and implementation of the computational fluid dynamics solver, and Thomas Spray, J. William Gaynor, Kirk Kanter, and Pedro del Nido for lending their expertise and surgical insights.
Financial Support
This work was supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health (R01HL67622 and R01HL098252), and the American Heart Association (10PRE3720002).
Footnotes
Conflicts of Interest
Dr. Fogel has a grant from Edwards Life Sciences, Siemens Medical Solutions and is medical monitor for an imaging agent from Kereos. Drs. Haggerty and Yoganathan have no disclosures.
References
- 1.Fontan F, Baudet E. Surgical repair of tricuspid atresia. Thorax. 1971;26:240–248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khairy P, Fernandes SM, John E. Mayer J, et al. Long-term survival, modes of death, and predictors of martality in patients with fontan surgery. Circulation. 2008;117:85–92 [DOI] [PubMed] [Google Scholar]
- 3.Anderson PAW, Sleeper LA, Mahony L, et al. Contemporary outcomes after the fontan procedure. Journal of the American College of Cardiology. 2008;52:85–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Duncan BW, Desai S. Pulmonary arteriovenous malformations after cavopulmonary anastomosis. Annals of Thoracic Surgery. 2003;76:1759–1766 [DOI] [PubMed] [Google Scholar]
- 5.De Zelicourt D, Haggerty CM, Sundareswaran KS, et al. Individualized computer-based surgical planning to address pulmonary ateriovenous malformations in patients with a single ventricle with an interrupted inferior vena cava and azygous continuation. Journal of Thoracic and Cardiovascular Surgery. 2011;141:1170–1177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sundareswaran K, de Zélicourt D, Sharma S, et al. Correction of pulmonary arteriovenous malformation using image based surgical planning. JACC Imaging. 2009;2:1024–1030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Whitehead KK, Pekkan K, Kitajima HD, et al. Nonlinear power loss during exercise in single-ventricle patients after the fontan: Insights from computational fluid dynamics. Circulation. 2007;116:I-165–I-171 [DOI] [PubMed] [Google Scholar]
- 8.Sundareswaran KS, Pekkan K, Dasi LP, et al. The total cavopulmonary connection resistance: A significant impact on single ventricle hemodynamics at rest and exercise. American Journal of Physiology 2008;295:H2427–H2435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dasi LP, Krishnankutty R, Kitajima H, et al. Fontan hemodynamics: Importance of pulmonary artery diameter. Journal of Thoracic and Cardiovascular Surgery. 2009;137:560–564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ensley A, Lynch P, Chatzimavroudis GP, et al. Toward designing the optimal total cavopulmonary connection: An in vitro study. Annals of Thoracic Surgery. 1999;68:1384–1390 [DOI] [PubMed] [Google Scholar]
- 11.Marsden AL, Bernstein AJ, Reddy VM, et al. Evaluation of a novel y-shaped extracardiac fontan baffle using computational fluid dynamics. Journal of Thoracic and Cardiovascular Surgery. 2009;137:394–403 [DOI] [PubMed] [Google Scholar]
- 12.Haggerty CM, Kanter KR, Restrepo M, et al. Simulating hemodynamics of the fontan y-graft based on patient-specific in vivo connections. Journal of Thoracic and Cardiovascular Surgery. 2013;145:663–670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sundareswaran KS, Haggerty CM, de Zélicourt D, et al. Visualization of flow structures in fontan patients using 3-dimensional phase contrast magnetic resonance imaging. The Journal of Thoracic and Cardiovascular Surgery. 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pekkan K, Whited B, Kanter K, et al. Patient-specific surgical planning and hemodynamic computational fluid dynamics optimization through free-form haptic anatomy editing tool (surgem). Medical & biological engineering & computing. 2008;46:1139–1152 [DOI] [PubMed] [Google Scholar]
- 15.Haggerty CM, de Zelicourt D, Restrepo M, et al. Comparing pre- and post-operative fontan hemodynamic simulations: Implications for the reliability of surgical planning. Annals of Biomedical Engineering. 2012;40:2639–2651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Zélicourt D, Ge L, Wang C, et al. Flow simulations in arbitrarily complex cardiovascular anatomies–an unstructured cartesian grid approach. Computers & Fluids. 2009;38:1749–1762 [Google Scholar]
- 17.Pennati G, Corsini C, Cosentino D, et al. Boundary conditions of patient-specific fluid dynamics modelling of cavopulmonary connections: Possible adaptation of pulmonary resistances results is a critical issue for virtual surgical planning. Interface Focus. 2011;1:297–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mirabella L, Haggerty CM, Passerini T, et al. Treatment planning for a tcpc test case: A numerical investigation under rigid and moving wall assumptions. International Journal for Numerical Methods in Biomedical Engineering. 2012:n/a–n/a [DOI] [PubMed] [Google Scholar]
- 19.Kanter KR, Haggerty CM, Restrepo M, et al. Preliminary clinical experience with a bifurcated y-graft fontan procedure- a feasibility study. Journal of Thoracic and Cardiovascular Surgery. 2012;(accepted) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Desai K, Haggerty CM, Kanter KR, et al. Haemodynamic comparison of a novel flow-divider optiflo geometry and a traditional total cavopulmonary connection. Interactive cardiovascular and thoracic surgery. 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]