Abstract
Purpose:
To evaluate the clinical significance of invasive urothelial carcinoma that is ambiguous for muscularis propria invasion on initial transurethral resection of bladder tumor (TURBT).
Methods:
All consecutive in-house TURBTs with invasive urothelial carcinoma from 1999–2017 that underwent radical cystectomy (RC) were grouped as follows: invasion of the lamina propria (INLP; n=102; 24%), invasion of muscularis propria (INMP; n=296; 69%) and ambiguous for muscularis propria invasion (AMP; n=30; 7%). AMP was defined as extensive invasive carcinoma displaying thin muscle bundles where it is difficult to determine with certainty if those muscle bundles represent muscularis mucosae or muscularis propria (detrusor). Cases with any amount of small cell carcinoma or prior therapy were excluded.
Results:
The average age was 66 years old in INLP, 67 in INMP, and 65 in AMP. RC showed invasive carcinoma stage pT2 or above in 50/102 (49%) of INLP vs. 255/296 (86%) of INMP (P=<.001) vs. 25/30 (83.33%) of AMP (P=0.002). Lymph nodes showed metastatic carcinoma in 18/98 (18.36%) of INLP vs. 96/272 (35.29%) of INMP (P=0.002), and 6/25 (24%) in AMP (P=0.729). The average follow-up was 48 months (range 0–192). Survival of AMP patients was similar to INLP and both were significantly better than INMP (P=0.002 and P=0.016).
Conclusion:
The great majority of patients with AMP on initial TURBT have advanced disease on RC and emphasizes the need for early repeat TURBT or even consideration of early cystectomy to lower the risk of worse pathological findings and to prolong survival.
Keywords: urothelial carcinoma, muscularis propria, cystectomy
INTRODUCTION
Bladder cancer is the fourth most common cancer in men and the ninth most common cancer in women in the Western world [1, 2]. The majority of bladder cancers are primarily diagnosed as non-muscle invasive tumors (pTa-pT1), while around 20% present with muscle invasive disease [1]. Non-muscle invasive bladder cancer (NMIBC) frequently recurs and approximately 20% of initially non-muscle invasive disease will eventually progress to muscle invasive disease [1]. Traditionally, independent variables of disease free survival (DFS) in bladder cancer are grade, pathologic stage, and presence of lymph node involvement [3]. While NMIBC has a five-year survival of approximately 90%, this drops to <50% for patients with detrusor invasion.
Transurethral resection of the bladder tumor (TURBT) is the initial treatment, and provides tissue for diagnosis and staging.[4] Assessment of pT categories is crucial in the management of bladder cancer, as the presence of muscularis propria (MP) invasion (pT2) in TURBT specimens is the main crossroad towards a more aggressive therapy (eg, radical cystectomy [RC], neoadjuvant chemotherapy, or chemoradiation) [5–7]. Presence of MP is considered a surrogate measure for the TURBT adequacy, and repeat-TURBT is indicated if MP is absent in high-grade or pT1 tumors [5]. Thus, reporting the presence of MP and whether it is involved by tumor, is the standard of care [8]. In a small proportion of TURBTs, a definitive assessment of MP invasion cannot be made. Several pathologic features might mimic muscle invasion in urothelial carcinoma of the bladder in TURBT. Infiltration of hypertrophic muscularis mucosa (MM) and extensive desmoplastic stromal reaction can both be misinterpreted as muscle invasion. Furthermore, it is not well-established whether urothelial carcinoma located directly adjacent to but not within the muscularis propria (MP) should be staged as pT2 [1].
In some cases, urothelial carcinoma is present invading in between thin muscle bundles where it is difficult to assess whether these muscle bundles represent MM or MP destroyed by tumor. In these cases, we have been using the term “tumor ambiguous for muscularis propria invasion” (AMP) with recommendation for additional sampling. A study previously described the findings of a repeat TURBT on patients with invasive urothelial carcinoma involving muscle bundles indeterminate between MM and MP [9]. In this study we report the RC findings and survival data on our cohort of cases diagnosed with bladder cancer and AMP on initial TURBT and compared them to cases with invasion into MP or lamina propria (LP) on initial TURBT.
MATERIAL AND METHODS
The study was approved by the Institutional Review Board. All in-house consecutive cases of invasive high-grade urothelial carcinoma on initial TURBT from 1999 to 2017 that underwent RC at our institution were included. All pathology reports were reviewed to gather pathologic information. The slides from cases diagnosed as AMP were reviewed by an expert urologic pathologist (AM) to confirm the findings. We did not use immunohistochemistry previously reported to aid in the differential diagnosis as we do not find them useful and do not use them routinely [10]. Detailed pathological findings in both TURBT and RC including tumor size, depth of invasion, pathologic stage and lymph node status were recorded. Clinical information including age, gender, follow up and treatment were collected from our medical records. Cases with any component of small cell carcinoma or chemotherapy prior to RC were excluded.
The cases were grouped based on the worst depth of invasion in the pre-cystectomy TURBT as follows: (1) Invasion of the LP (INLP), (2) Invasion of MP (INMP), (3) Ambiguous for MP invasion (AMP), even if repeat TURBT was INLP. If the repeat TURBT was INMP, the case was classified as INMP for the purpose of this study.
AMP was defined as tumor with extensive invasion displaying thin muscle bundles where it is difficult to determine with certainty if those muscle bundles represent MM or MP. Differences among dichotomous variables were assessed using Pearson’s chi-squared test with Yates’ continuity correction. Survival estimates were calculated using Kaplan-Meier survival analysis for time from cystectomy to death and Cox regression analysis for split time analysis factoring in time from TURBT to cystectomy. A P value ≤0.05 was considered statistically significant. A P value ≤0.05 was considered statistically significant. All statistical analyses were performed using STATA release 13 (StataCorp LP, College Station, Texas, USA).
RESULTS
The study included 428 patients with paired TURBT and RC, 344 (80%) men and 84 (20%) women. On pre-cystectomy TURBT, 30 (7%) patients were diagnosed with AMP, 102 (24%) patients were diagnosed with INLP, and 296 (69%) patients were diagnosed with INMP. Only patients with initial AMP and who were not reclassified as INMP in repeat TURBT stayed in the AMP group. Six patients were reclassified as INMP after repeat TURBT, and as such were analized within the INMP group. Two patients in the AMP group had partial cystectomies as tumors were located in a diverticulum and 28 patients had RC. All patients initially diagnosed with AMP had cystectomy on follow-up. Time between diagnosis and surgery for those without repeat TURBT was from 37 to 261 days (average 115 days). From TURBT to cystectomy, 12/30 (40%) of patients received intravesical BCG including four patients who received 12 cycles and eight patients who received six cycles. In addition to BCG, one patient also received intravesical mitomycin. The summary of the clinicopathologic findings and the reason for surgery are presented in table 1. Table 2 shows the number of cases with variant differentiation in each group.
Table 1:
Clinicopathological Characteristics of Patients
| INLP (N=102) | AMP (N=30) | INMP (N=296) | P Value | |
|---|---|---|---|---|
| Average age (range) | 66 (42–85) | 65 (50–80) | (67) 40–86 | 0.544 |
| Male:Female | 80:22 | 25:5 | 239:57 | 0.822 |
| Time from diagnosis to cystectomy in days (95%CI) | 309 (237–380) | 207 (124– 289) | 227 (190–265) | 0.353 |
| ≥pT2 at radical cystectomy | 50 (49%)* | 25 (83%)* | 255 (86%) | 0.002 |
| pT4 | 6 (6%)*ϕ | 1 (3.33%)ϕ | 52 (17%)* | *0.007; ϕ0.92 |
| Lymph node involvement | 18/98 (18%) | 6/25 (24%) | 96/272 (35%) | 0.887 |
| Follow up time in months (range) | 54 (2–192) | 58 (14–133) | 43 (0–151) | NA |
| Died of disease | 30 (29%)* | 5 (16.67%)ϕ | 117 (40%)*ϕ | *0.002; ϕ0.016 |
| Reason for surgery | ||||
| Symptoms | 14 (13%) | 7 (23%) | NA | 0.327 |
| Failure intravesical therapy | 67 (66%) | 9 (30%) | NA | 0.001 |
| High-risk high-grade tumor | 11 (11%) | 5 (17%) | NA | 0.583 |
| Unresectable by TUR | 8 (8%) | 6 (20%) | NA | 0.117 |
| Imaging studies convincing of ≥T2 | 2 (2%) | 3 (10%) | NA | 0.138 |
NA: Not applicable.
Values used to calculate the P value.
Values used to calculate the P value.
Table 2:
Variant histology
| INLP (N=102) | AMP (N=30) | INMP (N=296) | P value | |
|---|---|---|---|---|
| Sarcomatoid | 6 | 3 | 9 | 0.4166 |
| Nested | 3 | 1 | 1 | 0.7642 |
| Squamous | 14 | 3 | 0 | 1 |
| Glandular | 19 | 1 | 0 | 0.4237 |
| Plasmacytoid/Rhabdoid/Signet ring cell | 5 | 0 | 4 | 1 |
| Micropapillary | 10 | 2 | 11 | 0.8065 |
| Other (Myxoid, Clear cell change) | 4 | 0 | 0 | 0.8625 |
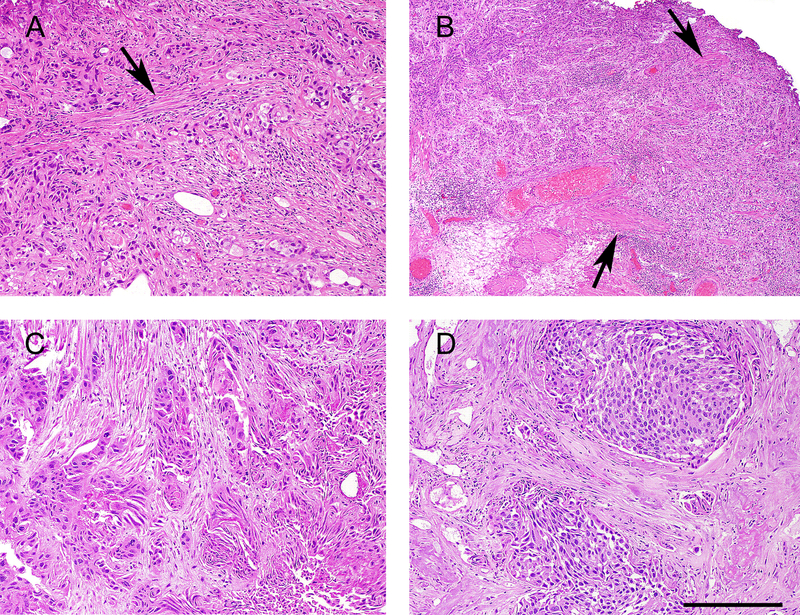
Pathologic review of the slides of the specimens diagnosed with AMP revealed that the reason for a equivocal diagnosis of invasion into the MP included: marked desmoplastic stroma, where it is difficult to assess whether the spindle cells in between nests of invasive carcinoma represent reactive myofibroblasts or smooth muscle fibers being digested; extensive invasive carcinoma with thin muscle bundles difficult to differentiate between hypertrophic MM and MP (Figure 1).
Figure 1:
Examples of Invasive high-grade urothelial carcinoma, ambiguous for muscularis propria invasion. A. Extensive invasive high-grade urothelial carcinoma involving thin muscle bundles (arrow), where it is difficult to determine whether the tumor is within digested muscularis propria versus muscularis mucosae. B. Invasive high grade urothelial around muscle bundles (arrows) where it is difficult to determine whether the smooth muscle is hypertrophic muscularis mucosae versus muscularis propria. C. Nests of invasive urothelial carcinoma surrounded by stroma containing spindle cells with smooth muscle differentiation. It is not possible to differentiate reactive myofibroblasts from muscularis propria being digested by the carcinoma. D. Large nests of invasive carcinoma separated by significant amount of stroma containing spindle cells where it is difficult to differentiate desmoplasia from digested muscularis propria.
On RC 50/102 (49%) of INLP patients were pT2 or higher compared to 25/30 (83.33%) of AMP patients (p=0.002) and 255/296 (86%) of INMP patients (p<.001). The difference between AMP and INMP cases was not statistically significant. Invasion of pelvic organs (pT4) was seen in 6/102 (5.8%) of INLP patients compared to 52/296 (17%) of INMP patients (p=0.007) and 1/30 (3.33%) of AMP patients (P=0.92). The difference in involvement of pelvic organs between AMP and the other two groups was not statistically significant. Lymph nodes were involved by metastatic carcinoma in 18/98 (18.36%) of INLP patients compared to 96/272 (35.29%) of INMP patients (p=0.003) and 6/25 (24%) of AMP patients (P=0.729). The differences in lymph node involvement between AMP and the other two groups were not statistically significant. Imaging findings for AMP cohort revealed six cases with possible direct extravesical extension, one case of suspected bone metastasis, and one case with suspected lymph node involvement.
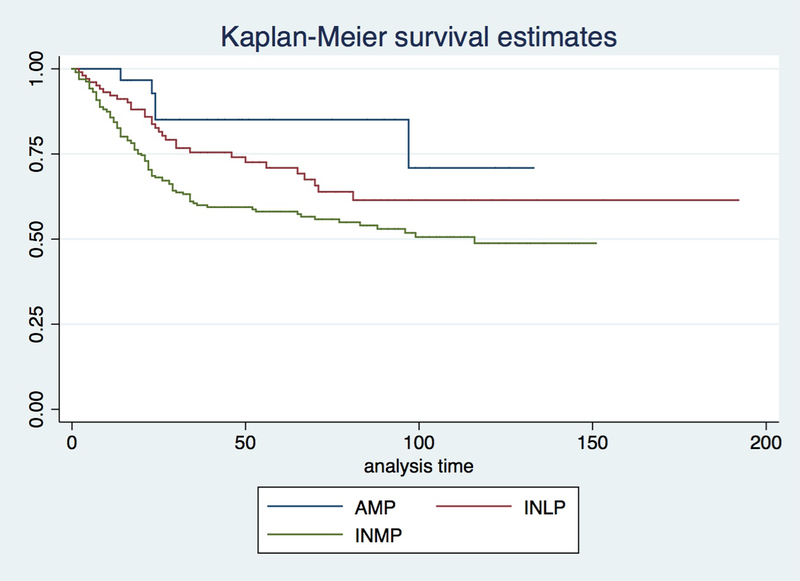
The average follow-up was 54 months for INLP patients, 43 months for INMP patients and 57 months for AMP patients. The disease specific mortality was significantly higher on patients with INMP (117/296, 39.52%) than patients with INLP (30/102, 29.41%), and higher than patients with AMP (5/30, 16.67%; P=0.003). The mortality of INLP and AMP groups were similar (Figure 2). A time-dependent covariate survival analysis using a Cox regression method was performed including the time from diagnosis to surgery and showed similar results. There was no difference between AMP and INLP groups (p=0.478) but survival of INMP was significantly worse than AMP and INLP (p=0.001). In summary, patients diagnosed with AMP in pre-cystectomy TURBT have higher proportion of pT2 or pT3 tumors than INLP and similar to INMP on RC. There was no significant difference in LN involvement or pelvic organ invasion between AMP and INLP or INMP. Mortality of AMP patients is significantly less than INMP patients and similar to INLP patients who undergo RC.
Figure 2:
Survival analysis from cystectomy to death.
The disease specific mortality was significantly higher on patients with invasive urothelial carcinoma involving the muscularis propria (INMP) than patients with invasive carcinoma involving the lamina propria (INLP) and patients with invasive carcinoma ambiguous for invasion of muscularis propria (AMP) (P=0.002; P=0.016). The mortality of INLP and AMP groups were similar.
DISCUSSION
Pathologic stage (pT) is critical in determining the management and prognostication of bladder cancer [6]. Bladder wall possesses a great degree of variation at different regions within the organ, with some differences between the same regions of bladder from different individuals [11]. Other factors that may complicate the interpretation of pT stage category include fragmentation, tangential sectioning, artifacts (eg, cautery, crush), stromal reaction, inflammation and variant tumor histology [5, 8, 11]. Diagnosis of MP invasion (pT2) in TURBT specimens is critical for prognostication and to decide therapy (e.g., RC, neoadjuvant chemotherapy, or chemoradiation) [12]. Infiltrating urothelial carcinoma can partially destroy or splay the MP leading to residual thin muscle bundles such that it is difficult to distinguish whether these thin muscle bundles represent the MM or altered MP [9]. Another problem in assessing muscle invasion is that MM can be hyperplastic and mimic MP, complicating pathologic staging. Although hyperplastic MM frequently have haphazard outline distinguishable from MP, it may have compact parallel muscle fibers and regular outline resembling MP [5, 13, 14].
Some of the clues that might be helpful to identify MM are slender muscle fibers that run parallel to the surface mucosa, location in the LP (superficial to mid vs. deep), nonjuxtaposition to adipose tissue (more common in deep LP), closeness to the surface epithelium, and association with vascular plexus [5]. In contrast, dense bundles of smooth muscle characterize the MP (detrusor muscle). The histologic absence of MM at the bladder neck and trigone, with a gradual diminution in size and organization of the muscle bundles of the MP that extends almost immediately beneath the urothelium, should also be noted to avoid confusing small MP muscle bundles for MM muscles. The presence of adipose tissue should not be used as an indicator of deep tumor invasion, given that fat may be present at any level of the bladder, including the lamina propria and MP [15].
The presence of MP is considered a surrogate measure for the TURBT adequacy and reporting the presence of MP and its status by tumor involvement in TURBT with high-grade lesions is required. Also, the presence or absence of MP independently predicts recurrence rate at first follow-up cystoscopy, which is also influenced by the surgeon’s experience. In cases where the muscular layer is not evident due to tumor infiltration, one should carefully assess the periphery of the tumor, where it may be easier to identify the muscle layer [15]. Absence of MP in TURBT with tumor has been associated with increased risk of residual disease, recurrence and understaging. In fact, a study of non-muscle invasive cancer at TURBT showed a recurrence rate at first follow-up cystoscopy of 44% when MP was absent in initial TURBT compared with 22% when present [16].
Immunohistochemical stains can be a very helpful tool in identification of muscle bundles (desmin) and their relationship with the carcinoma (broad spectrum cytokeratins). However, they are not a particularly useful in distinguishing the level of muscle invasion in bladder cancer. The problem arises in that immunohistochemical stains currently available do not distinguish whether the muscle bundles are of muscularis mucosae versus muscularis propria, which is the most important distinction one attempts to make for accurate staging. Smoothelin has been proposed as a marker smooth muscle fibers of the muscularis mucosae [10]. However, in our practice we do not find it useful, an observation reported by Lindh et el who found that smoothelin detection is strongly dependent on pretreatment conditions [17]. Another study by Miyamoto et al confirmed a differential expression of smoothelin between muscularis mucosae and muscularis propria; however, they found limited utility in cases in which the distinction is difficult [18]. Furthermore, in the International Society of Urologic Pathology (ISUP) recommendations issued in 2014, the experts concluded that there was insufficient data to make the recommendation to use these immunohistochemical stains to determine type of muscle invasion for bladder cancer staging [19].
Some studies have shown that a delay in doing a RC is associated with higher pathological stage at cystectomy [4], with studies reporting that delaying surgery more than 90 days results in a worse pathological stage with locally advanced disease [20], and other studies showing similar results, with decreased survival when surgery was delayed more than 12 weeks [21]. Solsona et al has reported that survival is high in patients with superficial bladder cancer after RC, and that survival is better in patients treated with early RC when compared to those who had surgery after recurrence of tumors [22]. In the current study, the average period between TURBT and RC was 207 days for patients labeled as AMP. Although this overall delay in the surgery for these patients might have contributed to the high percentage of patients with pT2 or higher on their final pathologic staging, this delay in RC did not appear to affect the survival in these patients as their survival was not different from that of the INLP group and both groups had significantly better survival than the IMP group. The question remains whether RC performed earlier would have improved survival of both INLP and AMP patients.
Despite the critical importance of the distinction between muscle invasive and non-muscle invasive bladder cancer on TURBT, this decision is often problematic. In such cases, additional staging TURBT is required, in many cases this distinction cannot be made even on following TURBT and the urologist is left with the dilemma of how to manage these patients. Of the 30 patients diagnosed with AMP on initial TURBT in our study 25 (83.33%) had muscle invasive disease, 11 (44%) had cancer involving perivesical adipose tissue and 1 (3.33%) had disease involving other pelvic organs on their RC. These findings demonstrate that, for the majority of patients, the diagnosis of AMP correlates with advanced disease, and this finding should trigger the need for an early repeat TURBT aimed to definitively stage the tumor. Additionally, consideration should be given to potential early cystectomy to lower the risk of worse pathological findings and to prolong survival.
There is increasing body of evidence that the cases that are initially diagnosed as non-muscle invasive and progressed while despite local treatment tend to behave worse in the long term. A study by Rodney et al reported that patients who progressed to muscle invasive disease on surveillance had similar pathological outcomes and worse survival than those who initially presented with muscle invasive disease , suggesting that a proportion of patients initially labeled as non-muscle invasive are not receiving aggressive treatment early enough [12]. In another study, the investigator reviewed 74 patients who progressed to muscle invasion while on surveillance and intravesical therapy and compared them with 89 patients who presented with muscle invasive disease. Disease specific mortality was twice as high for those who progressed to muscle invasion before RC [23]. In a third study, Guzzo et al analyzed outcome of patients who underwent RC with pre-surgical diagnosis of non-muscle invasive disease and compared outcome to those who had known muscle invasive disease. Researchers found that upstaging to pT2 among patients with high-grade T1 or CIS was associated with worse recurrence free survival (RFS) compared with patients with known muscle invasion before RC [24]. These findings were more significant among patients with a history of bacillus Calmette-Guerin (BCG) treatment [24]. The investigator concluded that understaging or treatment delay may contribute to worse outcomes among this group of patients and should be considered when discussing treatment options [24]. In contrast to the above, in our study we found that patients diagnosed as either AMP or INLP on initial TURBT had significantly better survival than those labeled as INMP. This finding is intriguing because the proportion of patients with tumors stage pT2 or above within the AMP group was similar to the INMP group. However, their survival was similar to INLP group, which suggests that patients with AMP are clinically closer to INLP with extensive invasion of the lamina propria.
It is well accepted that patients with a diagnosis of bladder cancer with AMP should undergo a repeat TURBT to determine the presence of muscle invasion. Despite this being a well-established practice, a previous study showed that 40% of the patients do not get a repeat TURBT within 3 months of an AMP diagnosis. Based on our finding that 83% of patients with AMP diagnosis on initial TURBT have pT2 disease or higher on RC, consideration for early cystectomy for these patients should be given in an attempt to avoid worse pathological findings and prolong survival.
A major limitation of our study is its retrospective design. There was also unavoidable selection bias, since we would expect that patients who have high clinical suspicion for muscle invasion were more inclined to have RC. Strengths of our study include having complete pathological information on both TURBT and RC specimens, along with a well-established program for bladder cancer treatment with clinicians that have ample experience in performing RCs, which creates a perfect clinical setting for excellent clinical care and appropriate gathering of follow-up information.
CONCLUSION
The great majority of patients with AMP on pre-cystectomy TURBT will have muscle invasive disease or a higher pathological stage on RC but disease specific mortality that aligns with patients with INLP who undergo cystectomy. This emphasizes the importance of early restaging TURBT in these patients. If re-staging TURBT still does not prove invasion into the MP, these patients should probably be considered as “high-risk” NMIBC , and in some patients, early cystectomy should be given consideration to lower the risk of worse pathological findings and prolong survival.
Acknowledgements:
This study was supported by the Department of Pathology of Johns Hopkins University.
Footnotes
Conflict of interest: The authors declare no conflict of interest
Ethical approval
All procedures performed in studies involving human participants were in accordance with ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendements or comparable ethical standards.
REFERENCES
- [1].Beukers W, Meijer T, Vissers CJ, et al. (2012) Down-staging (<pT2) of urothelial cancer at cystectomy after the diagnosis of detrusor muscle invasion (pT2) at diagnostic transurethral resection (TUR): is prediction possible? Virchows Arch 461, 149–156. https://www.ncbi.nlm.nih.gov/pubmed/22777576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Siegel RL, Miller KD, Jemal A. (2018) Cancer statistics, 2018. CA Cancer J Clin 68, 7–30. https://www.ncbi.nlm.nih.gov/pubmed/29313949 [DOI] [PubMed] [Google Scholar]
- [3].Salama A, Abdelmaksoud AM, Shawki A, et al. (2016) Outcome of Muscle-Invasive Urothelial Bladder Cancer After Radical Cystectomy. Clin Genitourin Cancer 14, e43–47. https://www.ncbi.nlm.nih.gov/pubmed/26363827 [DOI] [PubMed] [Google Scholar]
- [4].Minardi D, Milanese G, Parri G, et al. (2016) Non-muscle invasive high grade urothelial carcinoma of the bladder. Which factors can influence understaging at the time of radical cystectomy? Arch Ital Urol Androl 88, 13–16. https://www.ncbi.nlm.nih.gov/pubmed/27072170 [DOI] [PubMed] [Google Scholar]
- [5].Paner GP, Montironi R, Amin MB. (2017) Challenges in Pathologic Staging of Bladder Cancer: Proposals for Fresh Approaches of Assessing Pathologic Stage in Light of Recent Studies and Observations Pertaining to Bladder Histoanatomic Variances. Adv Anat Pathol 24, 113–127. https://www.ncbi.nlm.nih.gov/pubmed/28398951 [DOI] [PubMed] [Google Scholar]
- [6].Witjes JA, Comperat E, Cowan NC, et al. (2014) EAU guidelines on muscle-invasive and metastatic bladder cancer: summary of the 2013 guidelines. Eur Urol 65, 778–792. https://www.ncbi.nlm.nih.gov/pubmed/24373477 [DOI] [PubMed] [Google Scholar]
- [7].Spiess PE, Agarwal N, Bangs R, et al. (2017) Bladder Cancer, Version 5.2017, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 15, 1240–1267. https://www.ncbi.nlm.nih.gov/pubmed/28982750 [DOI] [PubMed] [Google Scholar]
- [8].Amin MB, McKenney JK, Paner GP, et al. (2013) ICUD-EAU International Consultation on Bladder Cancer 2012: Pathology. Eur Urol 63, 16–35. https://www.ncbi.nlm.nih.gov/pubmed/23083804 [DOI] [PubMed] [Google Scholar]
- [9].Miyamoto H, Epstein JI. (2010) Transurethral resection specimens of the bladder: outcome of invasive urothelial cancer involving muscle bundles indeterminate between muscularis mucosae and muscularis propria. Urology 76, 600–602. https://www.ncbi.nlm.nih.gov/pubmed/20394971 [DOI] [PubMed] [Google Scholar]
- [10].Paner GP, Shen SS, Lapetino S, et al. (2009) Diagnostic utility of antibody to smoothelin in the distinction of muscularis propria from muscularis mucosae of the urinary bladder: a potential ancillary tool in the pathologic staging of invasive urothelial carcinoma. Am J Surg Pathol 33, 91–98. https://www.ncbi.nlm.nih.gov/pubmed/18936687 [DOI] [PubMed] [Google Scholar]
- [11].Hansel DE, Amin MB, Comperat E, et al. (2013) A contemporary update on pathology standards for bladder cancer: transurethral resection and radical cystectomy specimens. Eur Urol 63, 321–332. https://www.ncbi.nlm.nih.gov/pubmed/23088996 [DOI] [PubMed] [Google Scholar]
- [12].Breau RH, Karnes RJ, Farmer SA, et al. (2014) Progression to detrusor muscle invasion during urothelial carcinoma surveillance is associated with poor prognosis. BJU Int 113, 900–906. https://www.ncbi.nlm.nih.gov/pubmed/24053569 [DOI] [PubMed] [Google Scholar]
- [13].Paner GP, Ro JY, Wojcik EM, et al. (2007) Further characterization of the muscle layers and lamina propria of the urinary bladder by systematic histologic mapping: implications for pathologic staging of invasive urothelial carcinoma. Am J Surg Pathol 31, 1420–1429. https://www.ncbi.nlm.nih.gov/pubmed/17721199 [DOI] [PubMed] [Google Scholar]
- [14].Vakar-Lopez F, Shen SS, Zhang S, et al. (2007) Muscularis mucosae of the urinary bladder revisited with emphasis on its hyperplastic patterns: a study of a large series of cystectomy specimens. Ann Diagn Pathol 11, 395–401. https://www.ncbi.nlm.nih.gov/pubmed/18022122 [DOI] [PubMed] [Google Scholar]
- [15].Kirkali Z, Chan T, Manoharan M, et al. (2005) Bladder cancer: epidemiology, staging and grading, and diagnosis. Urology 66, 4–34. https://www.ncbi.nlm.nih.gov/pubmed/16399414 [DOI] [PubMed] [Google Scholar]
- [16].Mariappan P, Zachou A, Grigor KM, et al. (2010) Detrusor muscle in the first, apparently complete transurethral resection of bladder tumour specimen is a surrogate marker of resection quality, predicts risk of early recurrence, and is dependent on operator experience. Eur Urol 57, 843–849. https://www.ncbi.nlm.nih.gov/pubmed/19524354 [DOI] [PubMed] [Google Scholar]
- [17].Lindh C, Nilsson R, Lindstrom ML, et al. (2011) Detection of smoothelin expression in the urinary bladder is strongly dependent on pretreatment conditions: a critical analysis with possible consequences for cancer staging. Virchows Arch 458, 665–670. https://www.ncbi.nlm.nih.gov/pubmed/21494761 [DOI] [PubMed] [Google Scholar]
- [18].Miyamoto H, Sharma RB, Illei PB, et al. (2010) Pitfalls in the use of smoothelin to identify muscularis propria invasion by urothelial carcinoma. Am J Surg Pathol 34, 418–422. https://www.ncbi.nlm.nih.gov/pubmed/20154589 [DOI] [PubMed] [Google Scholar]
- [19].Amin MB, Trpkov K, Lopez-Beltran A, et al. (2014) Best practices recommendations in the application of immunohistochemistry in the bladder lesions: report from the International Society of Urologic Pathology consensus conference. Am J Surg Pathol 38, e20–34. https://www.ncbi.nlm.nih.gov/pubmed/25029121 [DOI] [PubMed] [Google Scholar]
- [20].Chang SS, Hassan JM, Cookson MS, et al. (2003) Delaying radical cystectomy for muscle invasive bladder cancer results in worse pathological stage. J Urol 170, 1085–1087. https://www.ncbi.nlm.nih.gov/pubmed/14501697 [DOI] [PubMed] [Google Scholar]
- [21].Sanchez-Ortiz RF, Huang WC, Mick R, et al. (2003) An interval longer than 12 weeks between the diagnosis of muscle invasion and cystectomy is associated with worse outcome in bladder carcinoma. J Urol 169, 110–115; discussion 115. https://www.ncbi.nlm.nih.gov/pubmed/12478115 [DOI] [PubMed] [Google Scholar]
- [22].Solsona E, Iborra I, Rubio J, et al. (2004) The optimum timing of radical cystectomy for patients with recurrent high-risk superficial bladder tumour. BJU Int 94, 1258–1262. https://www.ncbi.nlm.nih.gov/pubmed/15610101 [DOI] [PubMed] [Google Scholar]
- [23].Schrier BP, Hollander MP, van Rhijn BW, et al. (2004) Prognosis of muscle-invasive bladder cancer: difference between primary and progressive tumours and implications for therapy. Eur Urol 45, 292–296. https://www.ncbi.nlm.nih.gov/pubmed/15036673 [DOI] [PubMed] [Google Scholar]
- [24].Guzzo TJ, Magheli A, Bivalacqua TJ, et al. (2009) Pathological upstaging during radical cystectomy is associated with worse recurrence-free survival in patients with bacillus Calmette-Guerin-refractory bladder cancer. Urology 74, 1276–1280. https://www.ncbi.nlm.nih.gov/pubmed/19758684 [DOI] [PubMed] [Google Scholar]