Abstract
Background
Feeding practices around the time of packed red blood cell transfusion have been implicated in the subsequent development of necrotising enterocolitis (NEC) in preterm infants. Specifically, it has been suggested that withholding feeds around the time of transfusion may reduce the risk of subsequent NEC. It is important to determine if withholding feeds around transfusion reduces the risk of subsequent NEC and associated mortality.
Objectives
• To assess the benefits and risks of stopping compared to continuing feed management before, during, and after blood transfusion in preterm infants
• To assess the effects of stopping versus continuing feeds in the following subgroups of infants: infants of different gestations; infants with symptomatic and asymptomatic anaemia; infants who received different feeding schedules, types of feed, and methods of feed delivery; infants who were transfused with different blood products, at different blood volumes, via different routes of delivery; and those who received blood transfusion with and without co‐interventions such as use of diuretics
• To determine the effectiveness and safety of stopping feeds around the time of a blood transfusion in reducing the risk of subsequent necrotising enterocolitis (NEC) in preterm infants
Search methods
We used the standard search strategy of Cochrane Neonatal to search the Cochrane Central Register of Controlled Trials (CENTRAL; 2018, Issue 11), in the Cochrane Library; MEDLINE (1966 to 14 November 2018); Embase (1980 to 14 November 2018); and the Cumulative Index to Nursing and Allied Health Literature (CINAHL; 1982 to 14 November 2018). We also searched clinical trials databases, conference proceedings, and reference lists of retrieved articles for randomised controlled trials (RCTs), cluster‐RCTs, and quasi‐RCTs.
Selection criteria
Randomised and quasi‐randomised controlled trials that compared stopping feeds versus continuing feeds around the time of blood transfusion in preterm infants.
Data collection and analysis
Two review authors independently selected trials, assessed trial quality, and extracted data from the included studies.
Main results
The search revealed seven studies that assessed effects of stopping feeds during blood transfusion. However, only one RCT involving 22 preterm infants was eligible for inclusion in the review. This RCT had low risk of selection bias but high risk of performance bias, as care personnel were not blinded to the study allocation. The primary objective of this trial was to investigate changes in mesenteric blood flow, and no cases of NEC were reported in any of the infants included in the trial. We were unable to draw any conclusions from this single study. The overall GRADE rating for quality of evidence was very low.
Authors' conclusions
Randomised controlled trial evidence is insufficient to show whether stopping feeds has an effect on the incidence of subsequent NEC or death. Large, adequately powered RCTs are needed to address this issue.
Plain language summary
Stopping feeds for prevention of transfusion‐associated necrotising enterocolitis in preterm infants
Review question
In preterm infants, does stopping feeds around the time of a packed red blood cell transfusion result in decreased risk of developing necrotising enterocolitis (NEC) or death?
Background
NEC is a serious inflammatory gut disease that is associated with high rates of morbidity and mortality in preterm babies. It is well known that certain feeding practices have an impact on the chance of a preterm baby developing NEC, and evidence suggests that packed red cell transfusions, which are often required during a preterm baby’s intensive care admission, may have a role in the development of this disease. The effects of feeding a baby during a red cell transfusion and subsequent development of NEC are currently unclear, and significant practice variation exists.
Study characteristics
Through searches of medical databases up to November 2018, review authors found seven studies that assessed the effects of stopping feeds during blood transfusion. Of these seven, one study was a non‐randomised observational study, four studies are ongoing, and one study was terminated with no results available. Only one study involving 22 preterm infants was eligible for inclusion in the review.
Key results
Randomised controlled trials have provided limited evidence on the effects of feeding practices during blood transfusion and the development of NEC. Only one small trial was included in the analysis, and this trial did not report any cases of transfusion‐associated NEC in the enteral feeding or non‐feeding groups.
Quality of evidence
Data were insufficient to allow any meaningful conclusions based on the very low quality of evidence according to the GRADE rating. Large randomised controlled trials are needed to answer the review question.
Summary of findings
Summary of findings for the main comparison. Stopping feeds compared to continuing feeds during transfusion for prevention of transfusion‐associated necrotising enterocolitis in preterm infants.
| Stopping feeds compared to continuing feeds during transfusion for prevention of transfusion‐associated necrotising enterocolitis in preterm infants | ||||||
| Patient or population: preterm infants receiving transfusion Intervention: stopping feeds during transfusion Comparison: continuing feeds during transfusion | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with stopping feeds during transfusion | Risk with continuing feeds | |||||
| Incidence of NEC within 48 hours after transfusion | Study population | Not estimable | 22 (1 RCT) | ⊕⊝⊝⊝ VERY LOWa,b,c | ||
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| Incidence of NEC any time after first transfusion | Study population | Not estimable | 22 (1 RCT) | ⊕⊝⊝⊝ VERY LOWa,b,c | ||
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| Mortality to 44 weeks' postmenstrual age | Study population | Not estimable | 22 (1 RCT) | ⊕⊝⊝⊝ VERY LOWa,b,c | ||
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| Length of hospital stay (days) | ‐ | See comment | ‐ | (0 studies) | ‐ | No study reported on this outcome |
| Total number of days to full oral feeds | ‐ | See comment | ‐ | (0 studies) | ‐ | No study reported on this outcome |
| Incidence of feed intolerance | Study population | ‐ | (0 studies) | ‐ | No study reported on this outcome | |
| See comment | See comment | |||||
| Growth | ‐ | See comment | ‐ | (0 studies) | ‐ | No study reported on this outcome |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial. | ||||||
| GRADE Working Group grades of evidence. High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aMethodological concerns that lower confidence in the estimate of effect.
bReported by a single study with very few participants; optimal information size would most likely have not been reached with this sample size.
cNo reported events to estimate effect.
Background
Description of the condition
Necrotising enterocolitis (NEC) is a serious inflammatory condition of the intestine that affects up to 10% of very low birth weight (VLBW) infants, leading to increased risk for mortality and significant morbidities (Stoll 2010; Yee 2012). Many factors have been associated with the occurrence of NEC but the pathogenesis has not been clearly elucidated. Transfusion‐associated NEC (TANEC) refers to NEC episodes that are temporally related to the transfusion of packed red blood cells, typically within 48 hours after transfusion (McGrady 1987; Stritzke 2013). In a meta‐analysis of observational studies, exposure to blood transfusion was reported to double the risk of NEC (Mohamed 2012).
TANEC has been estimated to account for up to 20% to 35% of NEC episodes (Gephart 2012). Compared with infants with NEC unrelated to blood transfusion, infants with TANEC were more likely to require surgical intervention and had higher mortality and longer hospitalisation (Josephson 2010; Mohamed 2012; Paul 2011). Several mechanisms have been proposed to contribute to the development of TANEC, including severe anaemia that leads to impaired gut blood flow, exposure to immunological mediators in transfused blood that may trigger an immune reaction in gut mucosa, and ischaemia/reperfusion injury associated with blood transfusion (Blau 2011; Christensen 2010).
Description of the intervention
One intervention that has been suggested to reduce the risk of TANEC is stopping feeds around the time of a blood transfusion (El‐Dib 2011). Types of alterations to feeding during blood transfusions include the following: withholding feeding hours before blood transfusion, during the transfusion, and after transfusion (Keir 2013). However, there are concerns that withholding feeding during this period may result in lower caloric intake, disruption to feeding progress, and metabolic instability of the infant. Studies and protocols have also considered altering types of milk feed and fortifications during the period of blood transfusion (Christensen 2010; Le 2017).
How the intervention might work
Although the pathogenesis of TANEC is not well elucidated, withholding feeding around the time of blood transfusion among preterm infants may decrease the additional effects of any postprandial changes in blood flow and intestine mucosal injury that occur after feeding (El‐Dib 2011). Preterm infants who were fed during blood transfusion were noted to lack the typical postprandial increase in blood flow of the mesenteric arteries as documented by doppler ultrasound (Doty 2016). Feeding surrounding blood transfusion has also been shown to exacerbate mucosal inflammation that may occur as a result of underlying anaemia in the preterm infant (Le 2017).
Why it is important to do this review
The potential impact of this intervention on reducing the risk of developing TANEC needs to be evaluated, as VLBW infants are among the most transfused patients in hospital settings (Ekhaguere 2016; Widness 1996). Evidence‐based guidance regarding the benefits and safety of stopping feeds during blood transfusion for preterm infants is lacking, especially in relation to the risk of NEC.
Objectives
To assess the benefits and risks of stopping compared to continuing feed management before, during, and after blood transfusion in preterm infants
To assess the effects of stopping versus continuing feeds in the following subgroups of infants: infants of different gestations; infants with symptomatic and asymptomatic anaemia; infants who received different feeding schedules, types of feed, and methods of feed delivery; infants who were transfused with different blood products, at different blood volumes, via different routes of delivery; and those who received blood transfusion with and without co‐interventions such as use of diuretics
To determine the effectiveness and safety of stopping feeds around the time of a blood transfusion in reducing the risk of subsequent necrotising enterocolitis (NEC) in preterm infants
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs), cluster‐RCTs, and quasi‐RCTs.
Types of participants
Preterm infants (< 37 weeks' gestation) and very low birth weight infants (VLBW; < 1500 g) who received oral feed (any amount) and transfusion of any blood product (such as whole blood, packed cells, or platelets) for any indication during their stay in the neonatal intensive care unit (NICU).
We planned to exclude infants who received full or partial exchange transfusion; we believe that these infants have different levels of risk for NEC that would best be examined in a separate review, should any RCT report assessment of these infants.
Types of interventions
Interventions
Temporary stopping of feeds before, during, or after transfusion of all blood products. In this review, we considered affected feeds as all feeds that would overlap with administration of blood product should they be given as per feeding schedule. This included any feed that was scheduled to be given before blood transfusion but continued during transfusion, and any feed that was commenced as per schedule during transfusion and was completed during or after transfusion
Control
Continuation of feeding as per routine schedule
We accepted all feeding regimens as implemented by study authors, including various feed intervals (continuous feed, hourly, once every two hours, once every three hours, or other intervals of bolus feed), types of feed (breast milk, formula milk, or mixed), methods of feed delivery (direct oral or oro/nasogastric tube feed, push or gravity feed), and ways of stopping feeds as appropriate to each feeding regimen, as long as enteral feeds were suspended during the process of blood transfusion, as elaborated above.
We also accepted all blood transfusion regimens implemented by the study authors, including the following.
Type of blood product given: packed cell or whole blood or a mixture throughout all transfusion episodes.
Volume of blood transfused: up to 10 mL/kg or higher or a mixture throughout all transfusion episodes.
Route of delivery: umbilical catheter, long line or peripheral catheter, or a mixture throughout all transfusion episodes.
Presence or absence of a co‐intervention such as diuretic administration during blood transfusion or a mixture throughout all transfusion episodes.
Types of outcome measures
Outcomes were measured within 48 hours of transfusion, or when an episode of NEC occurred subsequent to transfusion, or at discharge or at death.
Primary outcomes
Number of infants with necrotising enterocolitis (as defined by modified Bell Stage II or III (Bell 1978), a modified Bell staging system, or investigator‐defined variations of the Bell staging system) within 48 hours after transfusion
Number of infants with necrotising enterocolitis (as defined by modified Bell Stage II or III (Bell 1978), a modified Bell staging system, or investigator‐defined variations of the Bell staging system): any episode(s) after the first blood transfusion. We planned to consider infants with one or more episodes of NEC as an event
Mortality to 44 weeks' postmenstrual age
Secondary outcomes
Length of hospital stay (days)
Total number of days to achieve full oral feed since commencement of oral feeding. Full oral feed was defined as ingestion of all nutrient volumes in a 24‐hour period without gavage (McCain 2001)
Incidence of feed intolerance during NICU stay. Feed intolerance was defined as symptoms that arise from gastrointestinal disturbance, such as vomiting, diarrhoea, and excessive abdominal distension or abnormal gastric aspirates that necessitated ceasing of the oral feed, or both (Young 2012)
Growth (as defined by weight measured at a defined period in the study, e.g. at 44 weeks' postmenstrual age; rate of weight gain (g/kg/d) or time to regain birth weight, or both)
Search methods for identification of studies
We applied the search strategy used by Cochrane Neonatal.
Electronic searches
We searched the following databases.
Cochrane Central Register of Controlled Trials (CENTRAL; current issue), in the Cochrane Library.
MEDLINE (PubMed (National Library of Medicine)) (1950 to 14 November 2018).
Embase (1980 to 14 November 2018).
Cumulative Index to Nursing and Allied Health Literature (CINAHL; 1982 to 14 November 2018).
We have outlined the detailed search strategies for each of the above databases in Appendix 1, Appendix 2, Appendix 3, and Appendix 4, respectively.
We also searched ongoing clinical trials and unpublished studies via the following websites.
clinicaltrials.gov.
controlled‐trials.com.
clinicalstudyresults.org.
We did not apply any language restrictions.
Searching other resources
We searched the references cited in relevant studies, Cochrane Reviews, guidelines, review articles, and conference proceedings, including abstracts from Annual Meetings of the Pediatric Academic Societies (American Pediatric Society/Society for Pediatric Research and European Society for Paediatric Research) and the Perinatal Society of Australia and New Zealand. We also planned to contact experts if necessary to identify further relevant studies.
Data collection and analysis
Selection of studies
We employed standard Cochrane methods, as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a).
Two review authors (NML and KTY) independently searched for relevant studies. Two review authors (JYK and KTY) then independently screened these studies for inclusion in the review by title/abstract using the predefined inclusion and exclusion criteria. They resolved any disagreements with the help of a third review author who acted as an arbiter (NML). We obtained the full texts of any potentially relevant studies and assessed these for inclusion.
We included published and unpublished studies available in full‐text article or abstract form. We planned to contact the authors of unpublished studies and studies available only as abstracts to request additional information not provided in available reports, including details such as methods of sequence generation, allocation and blinding, participant withdrawal and prespecified outcomes, and full outcome data. We listed any studies excluded after full‐text assessment and reasons for their exclusion in an Excluded studies table. We illustrated the study selection process in a PRISMA diagram (Figure 1).
1.
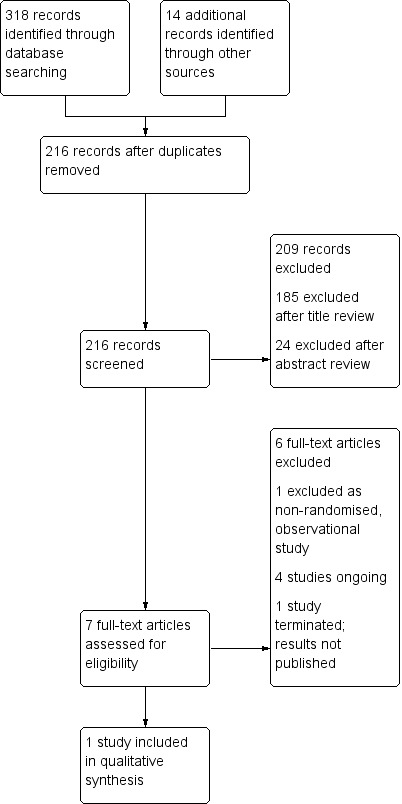
Study flow diagram.
Data extraction and management
Two review authors (TS and KTY) independently extracted and coded all data from each included study using a pro forma designed specifically for this Cochrane Review. We screened for duplicate entry of participants by matching initial numbers of participants recruited against total numbers at each step in the study. If we discovered a discrepancy, we planned to try to identify an explanation in the article (e.g. multiple enrolment of the same participants during different transfusion episodes); if this were the case, we would have excluded the study. We planned to contact study authors for clarification if necessary. We resolved any differences in our data by discussion leading to a consensus.
Assessment of risk of bias in included studies
Two review authors (TS and KTY) independently assessed each included study for risk of bias according to the six criteria stated in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011b).
Sequence generation.
Allocation concealment.
Blinding.
Incomplete outcome data.
Selective outcome reporting.
Other issues (e.g. extreme baseline imbalance).
We accorded a judgement of low, high, or unclear risk of bias, with justifications based on information obtained from the papers. A detailed description on how we judged the study according to each criterion is provided in Appendix 5. We assessed blinding of data for objective and subjective outcomes separately where possible. We completed a 'Risk of bias' table for each eligible study and presented our overall 'Risk of bias' assessment in a 'Risk of bias' summary (Figure 2) ('Risk of bias' graph not required). Any disagreement among the review authors was resolved by discussion to achieve a consensus.
2.
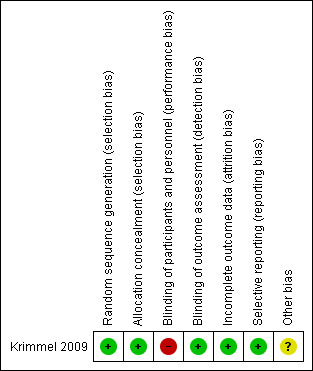
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Measures of treatment effect
We planned to report outcome estimates for categorical data using risk ratios (RRs), risk differences (RDs), the number needed to treat for an additional beneficial outcome (NNTB), and the number needed to treat for an additional harmful outcome (NNTH). For continuous data, we planned to use mean differences (MDs) with their respective 95% confidence intervals (CIs). If pooled analyses were not possible for reasons such as major discrepancies in study characteristics or outcome reporting, as detailed in the Assessment of heterogeneity section, we planned to report study results individually.
Unit of analysis issues
One unit of analysis issue that we expected was how each study handled multiple transfusion episodes in an infant. We anticipated that individual studies may have adopted one of the following two approaches.
Randomise infants to withholding feed or continuing feed at the first blood transfusion, and maintain the same intervention for randomised infants at all subsequent transfusions.
Randomise infants to withholding feed or continuing feed at each blood transfusion episode.
If this approach were used, each infant may have his/her feed withheld during one transfusion episode and continued during another transfusion episode. In this review, we will include only studies that adopt the first approach, namely, each infant receives the allocated intervention before the first transfusion, with the same intervention applied during subsequent transfusion episodes. We planned to exclude studies that adopted the second approach due to the likelihood of contamination secondary to period effect (withholding or continuing feed during blood transfusion may have different effects at different postmenstrual ages and at different stages in the infant feeding regimen) as well as carry‐over effect (the effect of withholding or continuing feed may persist beyond the period of first and subsequent blood transfusion episodes), similar to the issues that may arise in a cross‐over trial.
For cluster‐RCTs (e.g. trials in which assignment to intervention or control groups was made at the NICU level), we planned to assess whether adjustment had been made for the effects of clustering to account for non‐independence among the participants in a cluster via use of an appropriate analysis model such as the Generalised Estimating Equation (GEE) model. If study authors did not state the unit of analysis, we planned to inspect the width of the standard error (SE) or the 95% CI of the estimated treatment effects. If we found an inappropriately small SE or a narrow 95% CI, we would have asked the study authors to provide information on the unit of analysis.
If no adjustment were made for the effects of clustering, we planned to perform adjustment by multiplying the SE of the final effect estimates by the square root of the 'design effect', represented by the formula "1 + (M‐1) x ICC", where M is the average cluster size (number of infants per cluster) and ICC is the intracluster correlation. We planned to determine the average cluster size (M) from each trial by dividing the total number of infants by the total number of clusters. We planned to use a relatively large assumed ICC of 0.10, which is commonly used and is considered a realistic estimate in general (Campbell 2001). We planned to combine the adjusted final effect estimates from each trial with their SEs in a meta‐analysis using generic inverse variance methods, as stated in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a).
If determination of the unit of analysis were not possible, we planned to include the studies concerned in a meta‐analysis using the effect estimates reported by study authors. We would then have performed a sensitivity analysis to assess how the overall results are affected by these studies.
Dealing with missing data
If a study had a 20% or higher rate of missing data, we judged the study as having high risk of bias for incomplete outcome data. If a study had less than 20% missing data, we adopted a 'worse‐case scenario' approach in judging the dropout rate. If we noted an important difference in the effect estimate for the particular outcome after applying the 'worst‐case scenario' (e.g. markedly different effect size, a reverse in the direction of effect), we judged the study as having high risk of bias in incomplete outcome data. If we had considered the missing data to be critical to the final estimates in our meta‐analysis, we planned to contact the study authors for further data.
We planned to perform sensitivity analyses to assess how the overall results are affected by the inclusion of studies with high risk of attrition bias from incomplete outcome data.
Assessment of heterogeneity
We had planned to use the I² statistic to quantify the degree of inconsistency in the results (Higgins 2011a). We intended to use the following cut‐offs for the reporting of heterogeneity, according to the Cochrane Neonatal Group's recommendations: less than 25%, negligible heterogeneity; 25% to 49%, low heterogeneity; 50% to 74%, moderate heterogeneity; and 75% or higher, high heterogeneity. If we found a moderate or high degree of heterogeneity, we planned to evaluate the studies in terms of their clinical and methodological characteristics using the criteria listed as follows to determine whether the degree of heterogeneity may be explained by differences in those characteristics, and whether a meta‐analysis would be appropriate.
Characteristics of study participants (e.g. postmenstrual age, birth weight, indication for blood transfusion, type of blood product received ‐ packed cells or whole blood).
Clinical settings of the studies (e.g. tertiary or secondary NICU).
Co‐interventions.
Risk of bias (as detailed in the Assessment of risk of bias in included studies section).
Assessment of reporting biases
We had planned to use a funnel plot to screen for publication bias if at least 10 studies were included in the analysis of relevant outcomes. If publication bias were suggested by significant asymmetry of the funnel plot, we would have included a statement in our results with a corresponding note of caution in our discussion.
Data synthesis
We planned to perform meta‐analyses using a fixed‐effect model in Review Manager 5 (RevMan 5) (RevMan 2014). Our primary data analyses followed the intention‐to‐treat principle, namely, all infants for whom relevant outcome data were available were analysed in the group originally allocated. We planned to express our results as RRs, RDs, NNTB, NNTH, and MDs with their respective 95% CIs, as detailed in the Measures of treatment effect section. For cluster‐RCTs, our methods of analysis are detailed in the Unit of analysis issues section.
Quality of the evidence
We assessed the quality of evidence for the main comparison at the outcome level using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach (Schünemann 2013). This methodological approach considers evidence from randomised controlled trials as high quality that may be downgraded if there is at least a serious concern for each of these five areas: risk of bias, inconsistency across studies (heterogeneity), indirectness of evidence, imprecision of estimates, and suspicion or presence of publication bias (Schünemann 2013). A serious concern for any of these areas will result in downgrading of the quality of evidence by one level, and a very serious concern will result in downgrading of the quality of evidence by two levels. We will create a 'Summary of findings' table to display findings along with quality of evidence for the major outcomes in this review, as detailed below, using the GRADEpro Guideline Development Tool (GRADEpro GDT 2015).
The GRADE approach results in an assessment of the quality of a body of evidence according to one of four grades (Schünemann 2013).
High: we are very confident that the true effect lies close to that of the estimate of the effect.
Moderate: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different.
Low: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect.
Very low: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect.
Each decision to downgrade the quality of evidence will be accompanied by an explanation, which we have displayed as a footnote in Table 1.
Depending on availability of the data, we had planned to include the following outcomes in our 'Summary of findings' table: NEC (within 48 hours after transfusion), NEC (any episode), and mortality to discharge, as detailed in the Primary outcomes section; and length of hospital stay, days to achieve full feed, incidence of feed intolerance, and growth, as detailed in the Secondary outcomes section.
Subgroup analysis and investigation of heterogeneity
If suitable data were available, we had planned to explore potential sources of clinical heterogeneity through the following subgroup analyses.
-
Population:
gestational age at enrolment (early preterm defined as < 28 completed weeks' gestation, moderate preterm as 28 to 32 completed weeks' gestation, and late preterm as 33 to 36 completed weeks' gestation) (Mangham 2009);
indications for blood transfusion: symptomatic or asymptomatic anaemia or a mixture of both throughout all transfusion episodes;
feeding schedule: continuous feed, one‐hourly, two‐hourly, three‐hourly or at other intervals, applied consistently throughout all transfusion episodes, or a mixture along different transfusion episodes;
type of feed: breast milk, formula, or a mixture throughout all transfusion episodes; and
methods of feed delivery: oral or via nasogastric or orogastric tube or a mixture throughout all transfusion episodes.
-
Intervention:
type of blood product given: packed cell or whole blood or a mixture throughout all transfusion episodes;
volume of blood transfused: up to 10 mL per kg or higher or a mixture throughout all transfusion episodes;
route of delivery: umbilical catheter, long line or peripheral catheter, or a mixture throughout all transfusion episodes; and
presence or absence of a co‐intervention such as diuretic administration during blood transfusion or a mixture throughout all transfusion episodes.
Sensitivity analysis
We had planned to perform sensitivity analyses for the primary outcomes and for any secondary outcomes for which sufficient numbers of studies were available to assess the impact of excluding studies with high risk of the following.
Selection bias (for either criterion or for both criteria of random sequence generation and allocation concealment).
Attrition bias (incomplete outcome data).
Results
Description of studies
Results of the search
The CENTRAL search strategy yielded 59 records, the MEDLINE search strategy 113 records, the EMBASE search strategy 128 records, and the CINAHL search strategy 18 records. Of these, we assessed seven full studies for eligibility, resulting in one included study and one excluded study (see PRISMA diagram in Figure 1).
We assessed one study as awaiting classification (NCT01949896 2013). This study randomised preterm infants to either (1) NPO approximately 4 hours before receiving a blood transfusion and NPO until approximately 24 hours after the blood transfusion, or (2) continued feeding during the transfusion (at the discretion of the medical team). The study was terminated due to an insufficient patient population for enrolment. No results have been reported, and it is unclear if any results are available.
We assessed four studies as ongoing (NCT02733718; ISRCTN62501859; NCT02132819; ACTRN12616000160437).
Of these:
all studies are enrolling preterm infants;
all studies are randomising infants to different feeding regimens during blood transfusions;
two studies are randomising infants to either no enteral feeds or continuing feeds (ISRCTN62501859; NCT02132819); and
two studies are randomising infants to no enteral feeds, restricted feeds, or continuing feeds (NCT02733718; ACTRN12616000160437).
Included studies
We assessed one study that enrolled and randomised preterm infants to different feeding regimens during a blood transfusion (Krimmel 2009).
Types of participants
Infants born at 25 to 32 weeks' gestational age or at ≤ 38 weeks' corrected gestational age, receiving bolus enteral feedings (orally or by a feeding tube) of at least 60 mL/kg/d at the time of packed red blood cell transfusion for anaemia of prematurity.
Types of interventions
All infants received a transfusion of packed red blood cells in two aliquots of 10 mL/kg. Each aliquot was given over two hours with an interval of two hours between aliquots. Infants were randomised to either (1) feeding during the interval between packed red blood cell aliquots, or (2) an intravenous glucose infusion between packed red blood cell aliquots. It is unclear whether any infants enrolled in the study required more than one transfusion, and whether infants were allocated to the same intervention for any subsequent transfusion episodes.
Outcomes
The primary outcome was postprandial change in mesenteric blood flow velocity (MBFV) pre‐transfusion and post transfusion. Other measured outcomes included mean MBFV, peak systolic MBFV, and end‐diastolic MBFV. Clinical outcomes included mortality, serious adverse events, and NEC.
Excluded studies
We excluded one study that enrolled and investigated preterm infants allocated to different feeding regimens during a blood transfusion due to a non‐randomised, observational study design (Marin 2014).
Risk of bias in included studies
Krimmel 2009 was assessed as low risk of bias from selection bias and attrition bias. See 'Risk of bias summary' (Figure 2).
Allocation
Krimmel 2009 was at low risk of selection bias as randomisation was by block design and was concealed via opaque sealed envelopes.
Blinding
Krimmel 2009 was at high risk of performance bias as clinical staff were not blinded to intervention allocation but at low risk of detection bias as investigators were blinded to the feeding assignment.
Incomplete outcome data
Krimmel 2009 was at low risk of attrition bias, reporting complete outcome data for all infants.
Selective reporting
Krimmel 2009 was at low risk of reporting bias, reporting all prespecified outcome measures.
Other potential sources of bias
In Krimmel 2009, baseline data were inadequate to show similarity among groups. It is unclear whether any infants enrolled in the study required more than one transfusion, and whether infants were allocated to the same intervention for any subsequent transfusion episodes. We identified no other potential biases.
Effects of interventions
See: Table 1
Stopping feeds versus continuing feeds during transfusion
Primary outcomes
Incidence of NEC within 48 hours after transfusion (Analysis 1.1): Krimmel 2009 reported no incidence of NEC within 48 hours of transfusion (22 infants). We rated this as low‐quality evidence due to the inclusion of only one study with no reported events and very few participants. Optimal information size would most likely have not been reached with this sample size.
1.1. Analysis.
Comparison 1 Stopping feeds versus continuing feeds during transfusion, Outcome 1 Incidence of NEC within 48 hours after transfusion.
Incidence of NEC any time after first transfusion (Analysis 1.2): Krimmel 2009 reported no incidence of NEC any time after first transfusion (22 infants). We rated this as low‐quality evidence due to the inclusion of only one study with no reported events and very few participants. Optimal information size would most likely have not been reached with this sample size.
1.2. Analysis.
Comparison 1 Stopping feeds versus continuing feeds during transfusion, Outcome 2 Incidence of NEC any time after first transfusion.
Mortality to 44 weeks' postmenstrual age (Analysis 1.3): Krimmel 2009 reported no incidence of mortality (22 infants). We rated this as low‐quality evidence due to the inclusion of only one study with no reported events and very few participants. Optimal information size would most likely have not been reached with this sample size.
1.3. Analysis.
Comparison 1 Stopping feeds versus continuing feeds during transfusion, Outcome 3 Mortality to 44 weeks' postmenstrual age.
Secondary outcomes
Length of hospital stay (days): no study reported length of hospital stay.
Total number of days to full oral feeds: no study reported total number of days to full oral feeds.
Incidence of feed intolerance: no study reported the incidence of feed intolerance.
Growth: no study reported on growth.
Subgroup analyses
Subgroup analyses were not performed as there was only one included study.
Discussion
Summary of main results
Limited evidence is available on the effects of feeding practices during blood transfusion and the development of necrotising enterocolitis (NEC). The one small trial included in this analysis did not report any cases of transfusion‐associated NEC (TANEC) in either enteral feeding or non‐feeding groups (Krimmel 2009). This study had several methodological concerns, which limited the generalisability of results of this review. This study was not primarily focused on NEC and was conducted to determine changes in mesenteric blood flow from pre‐feed to post feed in both anaemic and transfused states.
Overall completeness and applicability of evidence
We included only one eligible study in which a total of 22 preterm infants were randomised from a planned recruitment of 60 (Krimmel 2009). The primary outcome of this study focused on changes in mesenteric blood flow pre‐transfusion and post transfusion in association with feeding. The study reported no incidence of NEC after transfusion in either group (with or without feeding) surrounding blood transfusion. We were unable to draw any conclusions from this one study.
Quality of the evidence
We assessed Krimmel 2009 to have low risk of bias from selection bias and attrition bias. The study was at high risk of performance bias due to lack of blinding of the intervention to the clinical staff, although investigators were blinded to the feeding assignment. We rated the evidence to be of very low quality due to the inclusion of only one study with a low number of participants and no reported events. Optimal information size was unlikely to have been reached with the sample size from this single study. It is also unclear whether any infants enrolled in the study required more than one transfusion, and whether infants were allocated to the same intervention for any subsequent transfusion episodes. Data were inadequate to show similarity among groups at baseline, and the study had lower than planned recruitment rates. No further assessment was possible given the available data.
Potential biases in the review process
We aimed to minimise bias introduced during the review process. Two review authors independently assessed eligibility for inclusion, carried out data extraction, and assessed risk of bias. Our search revealed one randomised controlled trial that reported on our primary outcomes. One study was terminated due to insufficient patient recruitment; no results have been reported nor made available (NCT01949896 2013). Four potentially eligible studies were not included in our analyses as they are still ongoing (NCT02733718; ISRCTN62501859; NCT02132819; ACTRN12616000160437).
Agreements and disagreements with other studies or reviews
Several observational studies aimed to determine the effect of feeding during red blood cell transfusion and the risk of developing TANEC. A prospective, observational study on mesenteric tissue oxygenation response among infants fed and fasted during red blood cell (RBC) transfusion revealed decreased postprandial mesenteric tissue oxygenation patterns compared with infants not fed during RBC transfusion (Marin 2014). A recent systematic review on withholding feeds during red blood cell transfusion for prevention of NEC included a total of seven pre/postfeeding intervention studies (Jasani 2017). Findings of the review suggest that withholding feeds during blood cell transfusion significantly reduced the incidence of NEC (risk ratio (RR) 0.47, 95% confidence interval (CI) 0.28 to 0.80; P = 0.005). The quality of evidence was moderate on GRADE analysis ‐ there were significant differences in the feeding protocol used among different studies with different periods of feeding cessation. The definition of transfusion‐associated NEC differed, with NEC defined as < 48 hours and < 72 hours of red cell transfusion in different studies. Review authors concluded that adequately powered randomised controlled trials are needed.
Authors' conclusions
Implications for practice.
Randomised controlled trial evidence is insufficient to show whether stopping feeds during red blood cell transfusion has an effect on the incidence of subsequent NEC or on mortality.
Implications for research.
Large, adequately powered randomised controlled trials are needed to provide high‐quality evidence and resolve the question whether withholding feeds around red blood cell transfusion substantially reduces the incidence of subsequent NEC or mortality. At least two of the ongoing randomised controlled trials have planned on recruitment of more than 150 participants.
Acknowledgements
We thank Dr Roger Soll, Co‐ordinating Editor, and Colleen Ovelman, Managing Editor of Cochrane Neonatal, for their assistance with the draft protocol.
The methods section of this review is based on a standard template used by Cochrane Neonatal.
Appendices
Appendix 1. CENTRAL search strategy
MeSH descriptor: [Infant, newborn]explode all trees
newborn*: ti,ab,kw
neonat*: ti,ab,tw
infant*: ti,ab,kw
#1 OR #2 OR #3 OR #4
MeSH descriptor: [Transfusion, blood] explode all trees
transfus*: ti,ab,kw
#6 OR #7
feed*: ti,ab,kw
Mesh descriptor: [enteral nutrition] explode all trees
Mesh descriptor: [feeding behaviour] explode all trees
#9 OR #10 OR #11
#5 AND #8 AND #12
Appendix 2. MEDLINE search strategy
Search "Infant, newborn"[Mesh]
Search newborn* [TIAB]
Search neonat* [TIAB]
Search infant* [TIAB]
Search #1 OR #2 OR #3 OR #4
Search blood transfusion [Mesh]
Search transfus* [TIAB]
Search #6 OR #7
Search feed* [TIAB]
Search enteral feeding[MeSH Terms]
Search feeding pattern[MeSH Terms]
Search #9 OR #10 OR #11
Search clinical trial [PT]
Search clinical trials [Mesh]
Search randomised [TIAB]
Search randomly [TIAB]
Search trial [TI]
Search #13 OR #14 OR #15 OR #16 OR #17
Search #5 AND #8 AND #12 AND #18
Appendix 3. Embase search strategy
Explode: "Infant, newborn"/all subheadings
(newborn*) in TI, AB
(neonat*) in TI, AB
(infant*) in TI, AB
Search #1 OR #2 OR #3 OR #4
Explode "transfusion, blood"/all subheadings
(transfus*) in TI, AB
Search #6 OR #7
(feed*) in TI, AB
Explode: "enteral feeding"/all subheadings
Explode: "feeding pattern"/all subheadings
Search #9 OR #10 OR #11
Explode "RANDOMIZED‐CONTROLLED‐TRIAL"/ all subheadings
Explode "RANDOMIZATION"/ all subheadings
Explode "CONTROLLED‐STUDY"/ all subheadings
Explode "MULTICENTER‐STUDY"/ all subheadings
Explode "DOUBLE‐BLIND‐PROCEDURE"/ all subheadings
Explode "SINGLE‐BLIND‐PROCEDURE"/ all subheadings
(RANDOM* or CROSS?OVER* or FACTORIAL* or PLACEBO* or VOLUNTEER*) in TI,AB
(SINGL* or DOUBL* or TREBL* or TRIPL*) AND (BLIND* or MASK*) in TI,AB
Search #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20
Search #5 AND #8 AND #12 AND #21
Appendix 4. CINAHL search strategy
MH "Infant, newborn"
TI newborn* or AB newborn*
TI neonat* or AB neonat*
TI infant* or AB infant*
#1 OR #2 OR #3 OR #4
MH "blood transfusion"
TI transfus* or AB transfus*
#6 OR #7
TI feed* or AB feed*
MH "feeding, enteral"
MH "feeding pattern"
#9 OR #10 OR #11
PT Clinical trial
TI randomised or AB randomised or AB random*
TI trial
MH "Clinical Trials"
#13 OR #14 OR #15 OR #16
#5 AND #8 AND #12 AND #17
Appendix 5. 'Risk of bias' domains and judgement
| 'Risk of bias' judgement | Criteria for this judgement |
| Random sequence generation: selection bias (biased allocation to interventions) due to inadequate generation of a randomised sequence | |
| Low risk of bias | The investigators describe a random component in the sequence generation process such as:
*Minimization may be implemented without a random element, and this is considered to be equivalent to being random |
| High risk of bias | The investigators describe a non‐random component in the sequence generation process. Usually, the description would involve some systematic, non‐random approach, for example:
Other non‐random approaches happen much less frequently than the systematic approaches mentioned above and tend to be obvious. They usually involve judgement or some method of non‐random categorisation of participants, for example:
|
| Unclear risk of bias | Insufficient information about the sequence generation process to permit judgement of ‘low risk’ or ‘high risk’ |
| Allocation concealment: selection bias (biased allocation to interventions) due to inadequate concealment of allocations before assignment | |
| Low risk of bias | Participants and investigators enrolling participants could not foresee assignment because 1 of the following, or an equivalent method, was used to conceal allocation.
|
| High risk of bias | Participants or investigators enrolling participants could possibly foresee assignments and thus introduce selection bias, such as allocation based on:
|
| Unclear risk of bias | Insufficient information to permit judgement of ‘low risk’ or ‘high risk’. This is usually the case if the method of concealment is not described or is not described in sufficient detail to allow a definitive judgement – e.g. if the use of assignment envelopes is described, but it remains unclear whether envelopes were sequentially numbered, opaque, and sealed |
| Blinding of participants and personnel: performance bias due to knowledge of the allocated interventions by participants and personnel during the study | |
| Low risk of bias | Any 1 of the following.
|
| High risk of bias | Any 1 of the following.
|
| Unclear risk of bias | Any 1 of the following.
|
| Blinding of outcome assessment: detection bias due to knowledge of the allocated interventions by outcome assessors | |
| Low risk of bias | Any 1 of the following.
|
| High risk of bias | Any 1 of the following.
|
| Unclear risk of bias | Any 1 of the following.
|
| Incomplete outcome data: attrition bias due to quantity, nature, or handling of incomplete outcome data | |
| Low risk of bias | Any 1 of the following.
|
| High risk of bias | Any 1 of the following.
|
| Unclear risk of bias | Any 1 of the following.
|
| Selective reporting: reporting bias due to selective outcome reporting | |
| Low risk of bias | Any 1 of the following.
|
| High risk of bias | Any 1 of the following.
|
| Unclear risk of bias | Insufficient information to permit judgement of ‘low risk’ or ‘high risk’. It is likely that most studies will fall into this category |
| Other bias: bias due to problems not covered elsewhere in the table | |
| Low risk of bias | Study appears to be free of other sources of bias |
| High risk of bias | There is at least 1 important risk of bias. For example, the study:
|
| Unclear risk of bias | There may be a risk of bias, but there is either:
|
Data and analyses
Comparison 1. Stopping feeds versus continuing feeds during transfusion.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Incidence of NEC within 48 hours after transfusion | 1 | 22 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Incidence of NEC any time after first transfusion | 1 | 22 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Mortality to 44 weeks' postmenstrual age | 1 | 22 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Krimmel 2009.
| Methods | Single‐centre, randomised, controlled trial in USA | |
| Participants |
Inclusion criteria: infants born at 25 to 32 weeks' gestational age; ≤ 38 weeks' corrected gestational age; receiving bolus enteral feedings (orally or by a feeding tube) of at least 60 mL/kg/d at the time of packed red blood cell transfusion for anaemia of prematurity; singleton infants or first infant of twin gestation Exclusion criteria: known congenital anomalies of the heart, brain, kidneys, or intestine; chromosomal abnormality; twinto‐twin transfusion sequence; higher‐order multiples; history of NEC Bell's Stage 2 or greater; concurrent treatment with antibiotics for sepsis; feeding intolerance, defined as gastric aspirate > 30% of feed volume on 3 sequential feeds; concurrent enrolment in another randomised trial; conditions previously shown to alter mesenteric blood flow velocity including intrauterine growth restriction (body weight < 3%) and current patent ductus arteriosus |
|
| Interventions | All infants received a transfusion of packed red blood cells in 2 aliquots of 10 mL/kg. Each aliquot was given over 2 hours with an interval of 2 hours between aliquots Group 1 (n = 11): infants were fed during the interval between packed red blood cell aliquots Group 2 (n = 11): infants received an intravenous glucose infusion between packed red blood cell aliquots |
|
| Outcomes |
Primary outcome: postprandial change in mesenteric blood flow velocity (MBFV) pre‐transfusion and post transfusion Measured outcomes: mean MBFV; peak systolic MBFV; end‐diastolic MBFV Other outcomes: mortality; serious adverse events; NEC |
|
| Notes | Additional information available at clinicaltrials.gov/ct2/show/record/NCT00167388 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomisation was by block design, with block sizes ranging from two to six" |
| Allocation concealment (selection bias) | Low risk | "Randomisation was concealed using opaque sealed envelopes" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Clinical staff not blinded to intervention allocation "Infants will be randomised to feeding or NPO during the PRBC transfusion" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "The investigator performing the Doppler studies will remain masked to the feeding assignment of the infant" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete outcome data provided for all infants |
| Selective reporting (reporting bias) | Low risk | Reported outcome measures, all prespecified |
| Other bias | Unclear risk | Inadequate baseline data to determine similarity of groups; unclear whether any infants enrolled in the study required more than 1 transfusion, and whether infants were allocated to the same intervention for any subsequent transfusion episodes |
MBFV: mesenteric blood flow velocity.
NEC: necrotising enterocolitis.
PRBC: packed red blood cells.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Marin 2014 | Non‐randomised, observational study |
Characteristics of studies awaiting assessment [ordered by study ID]
NCT01949896 2013.
| Methods | Single‐centre, randomised, controlled trial in USA |
| Participants |
Inclusion criteria: infants born at < 31 weeks' gestational age; 3 to 7 days old at time of consent Exclusion criteria: infant with multiple congenital anomalies; infant with suspected/confirmed genetic anomalies; infant with suspected/confirmed congenital immune deficiencies |
| Interventions | Total N = 12 Group 1: NPO approximately 4 hours before receiving a blood transfusion and NPO until approximately 24 hours after the blood transfusion Group 2: continued feeding during the transfusion (at the discretion of the medical team) |
| Outcomes |
Primary outcome: pro‐inflammatory cytokine response Secondary outcomes: none specified |
| Notes | Study terminated due to insufficient patient population for enrolment. No results reported; unclear if available |
Characteristics of ongoing studies [ordered by study ID]
ACTRN12616000160437.
| Trial name or title | The effects of feeding on blood flow to the gut in preterm infants receiving red blood cell transfusion |
| Methods | Single‐centre, randomised, controlled trial in Australia |
| Participants |
Inclusion criteria: preterm infants at < 35 weeks' gestation; receiving red cell transfusion for anaemia; given enteral feeding of at least 120 mL/kg/d Exclusion criteria: < 28 weeks' corrected gestation at time of intervention; growth restriction (BW < third centile); major congenital anomalies (including severe cardiac or cerebral disease, any malformation or disease of the gastrointestinal tract); diagnosis of necrotising enterocolitis, spontaneous intestinal perforation, or history of abdominal surgery; need for vasopressor therapy; cutaneous disease not allowing for placement of near‐infrared spectroscopy (NIRS) sensor |
| Interventions | Total N = 60 Group 1: withholding of enteral feeds during red cell transfusion for 12 hours from the start of the transfusion Group 2: restriction of enteral feed volume to 120 mL/kg/d, maximum calorie concentration 20 kcal/30 mL Group 3: continuing of enteral feeds during red cell transfusion for 24 hours from the start of the transfusion Enteral feeds will be continued according to the feeding regimen before the transfusion, which may be continuous feeds or bolus feeds every 1 to 3 hours |
| Outcomes |
Primary outcomes: mean CSOR, using NIRS; mean mesenteric fractional oxygen extraction, using NIRS (1 hour before transfusion, during transfusion, immediately post transfusion, 12 and 24 hours after transfusion) Secondary outcomes: time to return to full feeds, defined as number of hours after the 24‐hour study period until the infant is receiving the same feed volume as before the transfusion; feed intolerance, defined as gastric aspirates > 30% of feed volume or vomiting; abdominal distension, assessed by review of medical and nursing documentation; adverse events including transfusion reactions, suspected or proven sepsis, necrotising enterocolitis; necrotising enterocolitis; late‐onset sepsis; mortality |
| Starting date | 2016 |
| Contact information | Tim Schindler; email: tim.schindler@health.nsw.gov.au Royal Hospital for Women, Australia |
| Notes |
ISRCTN62501859.
| Trial name or title | Withholding enteral feeds around packed red cell transfusion |
| Methods | Multi‐centre, randomised, controlled trial in UK |
| Participants |
Inclusion criteria: gestational age at birth < 30 weeks (up to and including 29 +6 weeks) Exclusion criteria: packed red cell transfusion with concurrent enteral feeds before enrolment; infants for whom enteral feeding is contraindicated in the first 7 days after birth (e.g. congenital abnormalities) |
| Interventions | Total N = 250 Group 1: nil by mouth from 4 hours before blood transfusion, during blood transfusion (this lasts 3 to 4 hours), and for 4 hours after the blood transfusion is finished (approximately 11 to 12 hours in total) Group 2: milk feeds continued before, during, and after the blood transfusion If the baby requires any further blood transfusions during his or her neonatal unit stay, he or she will remain allocated to the same intervention group |
| Outcomes |
Primary outcome: point‐of‐care trial method feasibility outcomes; clinical primary outcome not specified Secondary outcomes: NEC; spontaneous intestinal perforation; all‐cause mortality; length of neonatal unit stay; duration of any parenteral nutrition; number of days with a central venous line in situ; number of central line‐associated bloodstream infections; growth |
| Starting date | 2018 |
| Contact information | Kayleigh Stanbury; email: wheat@npeu.ox.ac.uk NPEU Clinical Trials Unit, UK |
| Notes |
NCT02132819.
| Trial name or title | The effect of withholding feeds during red blood cell transfusion on development of TRAGI in very low birth weight infants |
| Methods | Single‐centre, randomised, controlled trial in Turkey |
| Participants |
Inclusion criteria: < 32 weeks' gestational age or < 1500 grams; preterm infants > 7 days old; Infants feeding well enterally at the time of transfusion planning Exclusion criteria: infants with signs of severe sepsis; infants with severe hypoxia and asphyxia; infants with a congenital anomaly or a complex cardiac anomaly |
| Interventions | Total N = 150 Group 1: feeding will be continued during the transfusion Group 2: at least 2 feeds before the transfusion, 2 feeds after the transfusion, and feeds during the transfusion are withheld |
| Outcomes |
Primary outcomes: increase in abdominal circumference within 3 days after transfusion; Bell's Stage 1 NEC; Bell's Stage 2 NEC; Bell's Stage 3 NEC Secondary outcomes: increase in volume of gastric residual aspirates within 3 days after transfusion; occult or obvious blood in stool within 1 day after transfusion |
| Starting date | 2014 |
| Contact information | Suzan Sahin; email: suzan_balkan@yahoo.com Zekai Tahir Burak Women's Health Research and Education Hospital, Turkey |
| Notes |
NCT02733718.
| Trial name or title | The impact of different feeding strategies during packed red cell transfusion on intestinal oxygenation |
| Methods | Single‐centre, randomised, controlled trial in Turkey |
| Participants |
Inclusion criteria: prematurity (< 32 completed weeks of gestation at birth); need for PRBC transfusion; feeding at least 30 mL/kg/d at the time of transfusion Exclusion criteria: neonates previously diagnosed with gastrointestinal problems such as NEC, intestinal perforation, or atresia; infants receiving continuous feeds or less than 30 mL/kg/d; major congenital or chromosomal abnormalities or infants unlikely to survive; intraventricular haemorrhage > Grade 3; haemodynamically significant patent ductus arteriosus; infants requiring vasopressor support; skin disruption precluding application of sensors |
| Interventions | Total N = 20 Group 1: no enteral feeding before (2 hours), during (3 hours), and after (2 hours) red blood cell transfusion Group 2: enteral feeding is reduced by 50% before, during, and after the red blood cell transfusion Group 3: the same feeding volume will be continued without decreasing or stopping |
| Outcomes |
Primary outcome: mesenteric oxygenation (incidence of low mesenteric oxygenation after transfusion) Secondary outcomes: feeding intolerance until 12 weeks' post transfusion or discharge; transfusion‐related NEC |
| Starting date | 2015 |
| Contact information | Hülya Selva Bilgen; email: hülya.bilgen@gmail.com Marmara University School of Medicine, Turkey |
| Notes |
BW: birth weight.
CSOR: cerebro‐splanchnic oxygenation ratio.
NEC: necrotising enterocolitis.
NIRS: near‐infrared spectroscopy.
PRBC: packed red blood cells.
TRAGI: transfusion‐associated gut injury.
Differences between protocol and review
It was not possible to assess the quality of evidence as planned due to lack of data. Similarly, planned subgroup and sensitivity analyses were not performed.
There were no differences between the protocol and the review.
Contributions of authors
KTY, JYK, AS, KT, and NML participated in writing the protocol.
TS, KTY, NML, and JYK performed the literature search, independently assessed studies for eligibility, performed critical appraisal of eligible studies and data extraction, and formed a consensus on the conclusions.
TS and KTY wrote the review with input from JYK, AS, KT, and NML.
Sources of support
Internal sources
No sources of support supplied
External sources
-
Vermont Oxford Network, USA.
Cochrane Neonatal Reviews are produced with support from Vermont Oxford Network, a worldwide collaboration of health professionals dedicated to providing evidence‐based care of the highest quality for newborn infants and their families.
Declarations of interest
None.
New
References
References to studies included in this review
Krimmel 2009 {published data only}
- Krimmel GA, Baker R, Yanowitz TD. Blood transfusion alters the superior mesenteric artery blood flow velocity response to feeding in premature infants. American Journal of Perinatology 2009;26(2):99‐106. [DOI: 10.1055/s-0028-1090595; PUBMED: 19021097] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Marin 2014 {published data only}
- Marin T, Josephson CD, Kosmetatos N, Higgins M. Moore JE. Feeding preterm infants during red blood cell transfusion is associated with a decline in postprandial mesenteric oxygenation. Journal of Pediatrics 2014;165(3):464‐71.e1. [DOI: 10.1016/j.jpeds.2014.05.009; PUBMED: 24948351] [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
NCT01949896 2013 {published data only}
- NCT01949896. Impact of feeding on pro‐inflammatory cytokine response in neonates receiving a RBC transfusion. clinicaltrials.gov/show/NCT01949896 (first received 25 September 2013).
References to ongoing studies
ACTRN12616000160437 {published data only}
- ACTRN12616000160437. The effects of feeding on blood flow to the gut in preterm infants receiving red blood cell transfusion. anzctr.org.au/ACTRN12616000160437.aspx (first received 2 May 2016).
ISRCTN62501859 {published data only}
- ISRCTN62501859. Withholding enteral feeds around packed red cell transfusion. isrctn.com/ISRCTN62501859 (first received 27 July 2018).
NCT02132819 {published data only}
- NCT02132819. The effect of withholding feeds during red blood cell transfusion on development of TRAGI in very low birth weight infants. clinicaltrials.gov/show/NCT02132819 (first received 7 May 2014).
NCT02733718 {published data only}
- NCT02733718. The impact of different feeding strategies during packed red cell transfusion on intestinal oxygenation. clinicaltrials.gov/show/NCT02733718 (first received 11 April 2016).
Additional references
Bell 1978
- Bell MJ, Ternberg JL, Feigin RD, Keating JP, Marshall R, Barton L, et al. Neonatal necrotizing enterocolitis. Therapeutic decisions based upon clinical staging. Annals of Surgery 1978;187(1):1‐7. [DOI: 10.1097/00000658-197801000-00001; PUBMED: 413500] [DOI] [PMC free article] [PubMed] [Google Scholar]
Blau 2011
- Blau J, Calo JM, Dozor D, Sutton M, Alpan G, Gamma EF. Transfusion‐related acute gut injury: necrotizing enterocolitis in very low birth weight neonates after packed red blood cell transfusion. Journal of Pediatrics 2011;158(3):403‐9. [DOI: 10.1016/j.jpeds.2010.09.015; PUBMED: 21067771] [DOI] [PubMed] [Google Scholar]
Campbell 2001
- Campbell MK, Mollison J, Grimshaw JM. Cluster trials in implementation research: estimation of intracluster correlation coefficients and sample size. Statistics in Medicine 2001;20(3):391‐9. [PUBMED: 11180309] [DOI] [PubMed] [Google Scholar]
Christensen 2010
- Christensen RD, Lambert DK, Henry E, Wiedmeier SE, Snow GL, Baer VL, et al. Is "transfusion‐associated necrotizing enterocolitis" an authentic pathogenic entity?. Transfusion 2010;50(5):1106‐12. [DOI: 10.1111/j.1537-2995.2009.02542.x; PUBMED: 20051059] [DOI] [PubMed] [Google Scholar]
Doty 2016
- Doty M, Wade C, Farr J, Gomezcoello VC, Martin G, Nasr T. Feeding during blood transfusions and the association with necrotizing enterocolitis. American Journal of Perinatology 2016;33(9):882‐6. [DOI: 10.1055/s-0036-1579651; PUBMED: 27031053] [DOI] [PubMed] [Google Scholar]
Ekhaguere 2016
- Ekhaguere OA, Morriss FH Jr, Bell EF, Prakash N, Widness JA. Predictive factors and practice trends in red blood cell transfusions for very‐low‐birth‐weight infants. Pediatric Research 2016;79(5):736‐41. [DOI: 10.1038/pr.2016.4; PUBMED: 26756783] [DOI] [PMC free article] [PubMed] [Google Scholar]
El‐Dib 2011
- El‐Dib M, Narang S, Lee E, Massaro AN, Aly H. Red blood cell transfusion, feeding and necrotizing enterocolitis in preterm infants. Journal of Perinatology 2011;31(3):183‐7. [DOI: 10.1038/jp.2010.157; PUBMED: 21252964] [DOI] [PubMed] [Google Scholar]
Gephart 2012
- Gephart SM. Transfusion‐associated necrotizing enterocolitis: evidence and uncertainty. Advances in Neonatal Care 2012;12(4):232‐6. [DOI: 10.1097/ANC.0b013e31825e20ee; PUBMED: 22864004] [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADEpro GDT 2015 [Computer program]
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Version accessed 25 July 2017. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015.
Higgins 2011a
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. handbook.cochrane.org.
Higgins 2011b
- Higgins JP, Altman DG, Sterne JAC. Chapter 8. Assessing risk of bias in included studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. handbook.cochrane.org.
Jasani 2017
- Jasani B, Rao S, Patole S. Withholding feeds and transfusion‐associated necrotizing enterocolitis in preterm infants: a systematic review. Advances in Nutrition 2017;8(5):764‐9. [DOI: 10.3945/an.117.015818; PUBMED: 28916576] [DOI] [PMC free article] [PubMed] [Google Scholar]
Josephson 2010
- Josephson CD, Wesolowski A, Bao G, Sola‐Visner MC, Dudell G, Castillejo MI, et al. Do red cell transfusions increase the risk of necrotizing enterocolitis in premature infants?. Journal of Pediatrics 2010;157(6):972‐8.e1‐3. [DOI: 10.1016/j.jpeds.2010.05.054; PUBMED: 20650470] [DOI] [PMC free article] [PubMed] [Google Scholar]
Keir 2013
- Keir AK, Wilkinson D. Question 1 * do feeding practices during transfusion influence the risk of developing necrotising enterocolitis in preterm infants?. Archives of Disease in Childhood 2013;98(5):386‐8. [DOI: 10.1136/archdischild-2013-303791; PUBMED: 23585577] [DOI] [PubMed] [Google Scholar]
Le 2017
- Le VT, Klebanoff MA, Talavera MM, Slaughter JL. Transient effects of transfusion and feeding advances (volumetric and caloric) on necrotizing enterocolitis development: a case‐crossover study. PLoS One 2017;12(6):e0179724. [DOI: 10.1371/journal.pone.0179724; PUBMED: 28632783] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mangham 2009
- Mangham LJ, Petrou S, Doyle LW, Draper ES, Marlow N. The cost of preterm birth throughout childhood in England and Wales. Pediatrics 2009;123(2):e312‐27. [DOI: 10.1542/peds.2008-1827; PUBMED: 19171583] [DOI] [PubMed] [Google Scholar]
McCain 2001
- McCain GC, Gartside PS, Greenberg JM, Lott JW. A feeding protocol for healthy preterm infants that shortens time to oral feeding. Journal of Pediatrics 2001;139(3):374‐9. [4384170] [DOI] [PubMed] [Google Scholar]
McGrady 1987
- McGrady GA, Rettig PJ, Istre GR, Jason JM, Holman RC, Evatt BL. An outbreak of necrotizing enterocolitis. Association with transfusions of packed red blood cells. American Journal of Epidemiology 1987;126(6):1165‐72. [DOI: 10.1093/oxfordjournals.aje.a114754; PUBMED: 3687923] [DOI] [PubMed] [Google Scholar]
Mohamed 2012
- Mohamed A, Shah PS. Transfusion associated necrotizing enterocolitis: a meta‐analysis of observational data. Pediatrics 2012;129(3):529‐40. [DOI: 10.1542/peds.2011-2872; PUBMED: 22351894] [DOI] [PubMed] [Google Scholar]
Paul 2011
- Paul DA, Mackley A, Novitsky A, Zhao Y, Brooks A, Locke RG. Increased odds of necrotizing enterocolitis after transfusion of red blood cells in premature infants. Pediatrics 2011;127(4):635‐41. [DOI: 10.1542/peds.2010-3178; PUBMED: 21402638] [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Schünemann 2013
- Schünemann H, Brożek J, Guyatt G, Oxman A, editor(s). Handbook for Grading the Quality of Evidence and the Strength of Recommendations Using the GRADE Approach (updated October 2013). GRADE Working Group, 2013. gdt.guidelinedevelopment.org/app/handbook/handbook.html.
Stoll 2010
- Stoll BJ, Hansen NI, Bell EF, Shankaran S, Laptook AR, Walsh MC, et al. Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network. Neonatal outcomes of extremely preterm infants from the NICHD Neonatal Research Network. Pediatrics 2010;126(3):443‐56. [DOI: 10.1542/peds.2009-2959; PUBMED: 20732945] [DOI] [PMC free article] [PubMed] [Google Scholar]
Stritzke 2013
- Stritzke AI, Smyth J, Synnes A, Lee SK, Shah PS. Transfusion‐associated necrotising enterocolitis in neonates. Archives of Disease in Childhood. Fetal and Neonatal Edition 2013;98(1):F10‐4. [DOI: 10.1136/fetalneonatal-2011-301282; PUBMED: 22447991] [DOI] [PubMed] [Google Scholar]
Widness 1996
- Widness JA, Seward VJ, Kromer IJ, Burmeister LF, Bell EF, Strauss RG. Changing patterns of red blood cell transfusion in very low birth weight infants. Journal of Pediatrics 1996;129(5):680‐7. [DOI: 10.1016/s0022-3476(96)70150-6; PUBMED: 8917234] [DOI] [PubMed] [Google Scholar]
Yee 2012
- Yee WH, Soraisham AS, Shah VS, Aziz K, Yoon W, Lee SK, et al. Canadian Neonatal Network. Incidence and timing of presentation of necrotizing enterocolitis in preterm infants. Pediatrics 2012;129(2):e298‐304. [DOI: 10.1542/peds.2011-2022; PUBMED: 22271701] [DOI] [PubMed] [Google Scholar]
Young 2012
- Young L, Morgan J, McCormick FM, McGuire W. Nutrient‐enriched formula versus standard term formula for preterm infants following hospital discharge. Cochrane Database of Systematic Reviews 2012, Issue 3. [DOI: 10.1002/14651858.CD004696.pub4] [DOI] [PubMed] [Google Scholar]


