Abstract
Background:
Living kidney donors (LKDs) with obesity have increased perioperative risks and risk of end-stage renal disease post-donation. Consequently, obesity serves as a barrier to donation, as many transplant centers encourage or require weight loss prior to donation for obese LKD candidates. Therefore, this study sought to assess patients’ perspectives on weight management strategies prior to donation among obese LKD candidates. We hypothesized that willingness to participate in a weight loss program may be associated with donor-recipient relationship.
Materials and Methods:
Obese (BMI ≥30 kg/m2) LKD candidates evaluated at a single institution from 9/2017-8/2018 were recruited. A survey was administered to assess LKD candidates’ baseline exercise and dietary habits and their interest in weight management strategies for the purpose of donation approval. Participants were grouped by relationship to the recipient (close relatives: first-degree relatives or spouses (n=29), compared with all other relationships (n=21)). Descriptive statistics were employed to summarize the data.
Results:
50 of 51 obese LKD candidates who were approached completed the survey. 90% of participants expressed willingness to lose weight if necessary to become eligible for donor nephrectomy. Compared with all other LKD candidates, close relatives were more likely to be interested in combined diet and exercise programs at our institution (p=0.01).
Conclusion:
Among obese LKD candidates, there was an interest in weight loss for the purposes of living kidney donation approval, particularly among close relatives of potential recipients. Future programs designed to promote weight management efforts for obese LKD candidates should be considered.
Keywords: Kidney Transplantation, Living Kidney Donor, Obesity, Weight Loss, Qualitative, Survey
INTRODUCTION
Over the past several decades, obesity, defined as body mass index (BMI) of 30 kg/m2 or greater, has become an increasing health concern in the United States.1,2 With the rise of the obesity epidemic, the general population is at increased risk for other medical comorbidities, such as metabolic syndrome, that are associated with the development of cardiovascular3,4 and chronic kidney diseases.5-8 Not surprisingly, the potential donor pool for living kidney donor transplantation has also become less healthy over time, and reflects the trends observed in the general population.9,10 In recent eras, living kidney donors (LKDs) have been found to have higher BMIs, and the percentage of LKDs with BMIs in the normal range has decreased over time.10 Medically complex donors, including LKDs with hypertension or obesity, have also been reported to account for nearly one-fourth of all LKDs in the United States.9 Additionally, the increased utilization of medically complex donors reflects the transplant community’s response to the organ shortage through efforts to increase access to transplantation by optimizing the use of living kidney donors.11,12
However, many individuals willing to serve as LKDs for their family members or loved ones are excluded from organ donation, as LKD candidates who exceed transplant centers’ BMI thresholds are required to lose weight prior to donation approval. Accordingly, several studies have demonstrated that obesity serves as a significant medical barrier to living kidney donation.13-15 Although there is no general consensus on BMI threshold for organ donation, many centers in the United States use a BMI cutoff of 35 kg/m2.16 These practices are in place in part due to an established association of increased risk for cardiovascular disease and end stage renal disease (ESRD) with increasing BMI in the general patient population17, in addition to the increasing prevalence of obesity-related kidney diseases.18 Moreover, the risks associated with living kidney donation may be compounded by the presence of obesity, evidenced by an observed increased risk of post-donation end-stage renal disease among LKDs with obesity, compared with their non-obese counterparts.19,20 Likewise, other studies have demonstrated increased perioperative risks for obese LKDs, compared with non-obese donors.21-23
In determining candidacy for donation and assessing long-term post-donation risks, certain risk factors, such as obesity, are modifiable and may be correctable prior to donation. For example, among obese living donor candidates, weight management may be pursued prior to donation in order to increase the safety of living donor nephrectomy in both the perioperative and long-term periods. Nevertheless, there is sparse information in the literature regarding preoperative weight management strategies for obese living donor candidates. Importantly, no prior study, to our knowledge, has assessed donor candidates’ perspectives on weight loss for the purposes of living kidney donation approval.
Consequently, through administration of a survey among obese LKD candidates at our institution, we sought to evaluate the lifestyle habits of obese LKD candidates as well as their perceptions of weight management for the purposes of living kidney donation. We theorized that many prospective obese donors may be interested in weight loss but may lack the knowledge and support for lifestyle modifications necessary to achieve and sustain their goals. We therefore sought to assess interest in weight management programs among obese LKD candidates. Additionally, as previous literature has demonstrated improved psychosocial outcomes post-donation among spouses and first-degree relatives who serve as LKDs24,25, we further hypothesized that an association may exist between donor-recipient relationship and willingness to participate in weight loss programs for the purposes of living kidney donation approval.
MATERIALS AND METHODS
Study Population
Utilizing a brief medical questionnaire completed either on-line or via telephone by the potential donor candidate, the living donor team screened 1338 donor referrals during the study period (9/1/17 – 8/31/18). Among the 1338 screened, only 247 donor candidates met initial screening criteria and were formally scheduled for evaluation. Respondents for our survey were identified in our LKD clinic at the time of formal evaluation. Donor candidates were screened on the basis of their BMI measured in clinic at the time of donor evaluation, with a minimum BMI of 30kg/m2 chosen as our cutoff for participant selection. 59 obese living donor candidates were identified. After clinical evaluation and donor education, the donor candidates were then provided the opportunity to join the study and if interested, informed consent was obtained. We successfully approached 51 obese donor candidates (84.7%; among the 8 not approached, 2 were sent home prior to formal evaluation and 6 were evaluated when no member of the study team was available to consent), and 50 agreed to participate for a 98% survey response rate. Inclusion criteria included obese LKD candidates undergoing in-person evaluation in clinic, English-speaking, age ≥18 years, and willingness to discuss their health, weight and lifestyle habits.
Survey Tool
A 27 question survey was developed by two physicians, with questions detailing patient demographics, baseline exercise and diet habits (Supplemental material). The survey also included questions regarding participants’ interest in weight loss for the purposes of serving as LKDs. Additionally, the survey evaluated the donor candidates’ understanding of the association between obesity and end-stage renal disease in the context of kidney donation. The survey was created specifically for our patient population in order to gauge their interest in creation of a weight management program at our institution. The tool was therefore not externally or internally validated. However, the questionnaire was tested in a cognitive interview setting among a lay audience prior to study implementation.
Participants were asked to report their age, gender and race/ethnicity as well as their relationship to the potential transplant recipient. They were also asked to list their height, weight, and BMI in addition to disclosing the weight loss goal that was required by the transplant center prior to donation. Respondents were also asked to report how many days per week they exercised, which forms of exercise best described their typical activities and which, if any, weight management strategies they had previously used in attempts to lose weight. They were also asked about typical dietary habits, including the number of fast food meals per week, sugary beverages consumed per day (examples including sweet tea, Coca-Cola products, or Kool aid) and microwavable or ready-made meals.
Using a five-point Likert scale ranging from “1= strongly interested” to “5=strongly disinterested” the participants were queried regarding their interests in losing weight for the purposes of kidney donation and to decrease their risk of end-stage renal disease post-donation. Participants were also asked whether or not they agreed that being overweight increases the risk for kidney disease (from 1=”strongly agree” to 5=”strongly disagree.”) With the Likert scale, we next assessed their interest in exercising, dieting or a combined diet and exercise program to lose weight in order to become a donor. We also assessed their interest in joining one of those programs at our institution (diet, exercise, and combined diet/exercise programs). Finally, participants were queried regarding their interest in bariatric surgery for the purposes of donation approval as well.
Data Analysis
Data from the survey were collected and analyzed using basic descriptive statistics. Based on the distribution of the data and the sample size, for questions assessed via the Likert scale, scores of 1 (“strongly interested”) and 2 (“interested”) were collapsed to indicate an interest, while scores of 3-5 (“neutral”, “disinterested”, “strongly disinterested”) were collapsed to indicate no interest.26 LKD candidate participants were then categorized according to their relationship with the potential recipient. Donor candidates who were first-degree relatives (parents, siblings, or children) and spouses were included as “close relatives,” while all other donor-recipient relationships, such as second-degree relatives, friends or altruistic donors, were included as “all other relationships.” When comparing the two donor candidate groups, categorical variables were assessed using chi-squared tests or Fisher’s exact tests, and continuous variables were assessed with student’s t-tests or non-parametric tests, depending on the distribution. All analyses were performed in SAS 9.4 (Cary, NC). The institutional review board at the University of Alabama at Birmingham approved this study.
RESULTS
Donor Candidate Demographics
Among 59 obese LKD candidates evaluated in our clinic from September 2017 through August 2018 who met inclusion criteria, 51 were approached for the study due to logistical reasons. Of those, 50 donor candidates agreed to participate in a weight management survey. The mean age of the participants was 39 years (standard deviation of 13 years), and the majority of the obese donor candidates were female (60.0%) (Table 1). When describing their race/ethnicity, 62.0% of the obese LKD candidates described themselves as white/Caucasian, while 26.0% of the LKD candidates were black/African American, and the remainder of the candidates were classified as “other” races/ethnicities, including American Indian, Asian, Hispanic/Latino or multiracial candidates. Among all participants, 29 (58.0%) donor candidates were close relatives (either first-degree relatives or spouses of the potential donors), while 21 (42.0%) donor candidates had other donor-recipient relationships, including second-degree relatives, in-laws, non-related friends or non-directed donors. The median BMI measured in clinic was 32.8 kg/m2 (interquartile range (IQR) 31.1-34.8), while the mean self-reported BMI prior to clinic evaluation was 31.5 kg/m2 (IQR 30.0-33.0).
Table 1.
Survey participants demographics (n=50)
| Characteristic | Participants (n=50) |
|---|---|
| Age (Mean ± standard deviation) |
39 ± 13 |
| Male Gender % (n) |
40.0 (20) |
| Race % (n) |
|
| Black | 26.0 (13) |
| White | 62.0 (31) |
| Other | 12.0 (6) |
| Donor-Recipient Relationship % (n) |
|
| First-degree relative or spouse | 58.0 (29) |
| All other relationship | 42.0 (21) |
| BMI Clinic Median (Interquartile Range) |
32.8 (31.1-34.8) |
| BMI Self-reported Median (Interquartile Range) |
31.5 (30.0-33.0) |
| Weight loss required for donation approval (self-reported) % (n) |
|
| None | 63.0 (29) |
| 1-15 lbs | 10.9 (5) |
| 15-30 lbs | 17.4 (8) |
| 30-60 lbs | 8.7 (4) |
When asked whether or not they were required to lose weight prior to donation, 46 of the 50 participants responded to the survey question. Of those, 63.0% (n=29) of the LKD candidates claimed that they were not required to lose weight, while 10.9% (n=5) were required to lose 15 pounds or less, 17.4% (n=8) were required to lose 15-30 pounds, and 8.7% (n=4) were asked to lose 30-60 pounds (Table 1). Interestingly, 23.1% (n=6) of donor candidates who had indicated on the survey that they were required to lose weight had in fact not been required to lose weight by the transplant program. Conversely, 45.0% (n=9) of donor candidates who reported that they were not required to lose weight had been given a weight loss requirement prior to donation approval. Ultimately, 24 (48.0%) of the donor candidates were approved for donation, and 17 (34.0%) of evaluated donor candidates went on to donate.
Donor Candidate Baseline Health Behaviors by Relationship to Recipient
Participant baseline dietary and exercise habits were then explored overall and grouped according to the relationship between the donor candidate and potential kidney transplant recipient. Obese donor candidates were asked how many days per week they exercised, and approximately 38% of all obese LKD candidates reported that they exercised at least 3 days per week (37.9% of close relative donor candidates and 38.1% of all other donor-recipient relationships). When reporting previous weight management strategies attempted, 82.8% of close relative LKD candidates reported that they attempted individual exercise, compared with 71.4% of all other LKD candidates (p=0.34). Donor candidates who were not close relatives more commonly reported attending exercise classes (47.6%, compared with 20.7% of close relative donor candidates, p=0.04). 10.3% of donor candidates defined as close relatives had tried nutritional classes in the past, such as Weight Watchers, compared with 23.8% of all other donor candidates (p=0.10). No LKD candidates reported prior bariatric surgery. 13.8% of close relative donor candidates and 19.1% of all other donor candidates had tried “other” weight loss strategies in the past. While all close relative donor candidates reported trying at least one weight loss strategy, nearly 24% of all other donor candidates reported that they had no previous weight loss strategy attempts (Table 2). When describing their typical exercise activity, 78.6% of close relative LKD candidates reported walking for exercise, compared with 60.0% of other LKD candidates (p=0.16). Nearly 40% of close relative donor candidates reported that they ran for exercise, compared with 20.0% of all other donor candidates (p=0.15). Fewer candidates reported biking, swimming, fitness classes or sports participation (Table 2). Among close relative donor candidates, 32.1% participated in weightlifting, while only 25.0% of all other donor candidates lifted weights (p=0.59).
Table 2.
Participant baseline health behaviors, by relationship to potential recipient (n=50)
| Characteristic | Close relative donor candidates (n=29) |
All other relationships (n=21) |
P value |
|---|---|---|---|
| Exercise ≥3 days/week | 37.9 (11) | 38.1 (8) | 0.99 |
| Previous weight loss strategies attempted | |||
| Individual Exercise | 82.8 (24) | 71.4 (15) | 0.34 |
| Exercise Classes | 20.7 (6) | 47.6 (10) | 0.04 |
| Nutrition classes (ie Weight Watchers) | 10.3 (3) | 28.6 (6) | 0.10 |
| Weight loss supplements | 34.5 (10) | 23.8 (5) | 0.42 |
| Bariatric Surgery | 0 (0) | 0 (0) | |
| Other | 13.8 (4) | 19.1 (4) | 0.62 |
| None | 0 (0) | 23.8 (5) | 0.01 |
| Typical Exercise Activity | |||
| Walking | 78.6 (22) | 60.0 (12) | 0.16 |
| Running/Jogging | 39.3 (11) | 20.0 (4) | 0.15 |
| Biking | 10.7 (3) | 10.0 (2) | 0.94 |
| Swimming | 3.6 (1) | 10.0 (2) | 0.36 |
| Fitness Classes | 10.7 (3) | 5.0 (1) | 0.48 |
| Sports | 10.7 (3) | 0 (0) | 0.13 |
| Weightlifting | 32.1 (9) | 25.0 (5) | 0.59 |
| Other | 3.6 (1) | 15.0 (3) | 0.16 |
| None | 3.6 (1) | 10.0 (2) | 0.36 |
| Fast food meals per week | 2.5 ± 1.7 | 3.0 ± 3.0 | 0.51 |
| Sugar beverages per day | 1.0 ± 1.4 | 2.0 ± 4.3 | 0.30 |
| Microwave meals per day | 0.2 ± 0.4 | 0.1 ± 0.2 | 0.09 |
Among all obese LKD candidate participants, there were no statistically significant differences between the two groups (close relatives and all other donor-recipient relationships) based on reported dietary consumption. When describing their dietary habits, close relative LKD candidates reported a mean 2.5 fast food meals per week, compared with a mean of 3.0 for all other LKD candidates (p=0.51). Close relative donor candidates had 1 sugary beverage per day, compared with a mean of 2 sugary beverages per day among other donor candidates (p=0.30). Donor candidates reported very few microwavable meals consumed per day, independent of donor-recipient relationship (Table 2).
Donor Candidate Knowledge and Perspectives on Weight Management
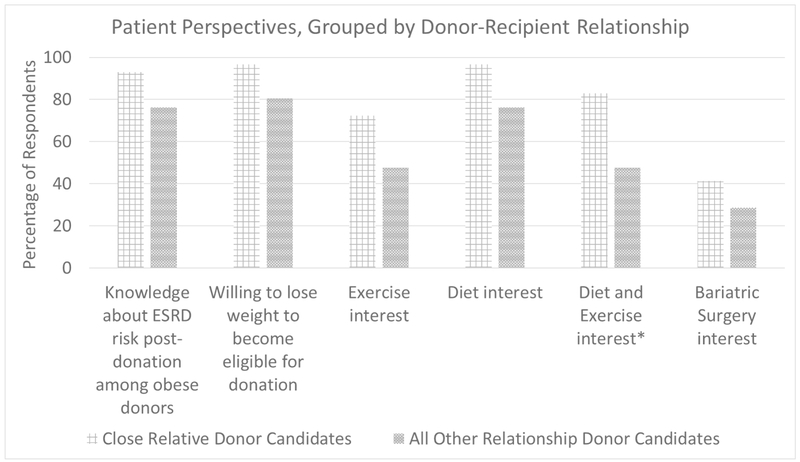
Many of the obese LKD candidates expressed an understanding about the relationship between obesity and kidney disease. 93.1% of close relative donor candidates endorsed this understanding, compared with 76.2% of all other donor candidates (p=0.12) (Figure 1). Despite this, 96.6% of close relative donor candidates stated that if necessary they would be willing to lose weight in order to become approved LKDs, compared with 80.6% of all other donor candidates (p=0.15). All of the close relative donor candidates stated that they would be interested in weight-loss pre-donation to decrease their post-donation risk of end-stage renal disease, compared with 95.2% of donor candidates with other donor-recipient relationships (p=0.42).
Figure 1.
Patient Perspectives by donor-recipient relationship. Exercise interest and combined diet and exercise interest refer to interest in creation of a program at our institution. *p<0.05
Donor Candidate Interest in Development of Diet and Exercise Programs
We next assessed LKD candidates’ interest in specific dietary and exercise programs. Greater than 95% of donor candidates claimed that they would be interested in exercising to lose weight prior to kidney donation (96.4% of close relative donor candidates and 95.2% of all other donor relationships, p=0.83). When queried about an interest in joining an exercise program at our institution, 72.4% of close relative donor candidates expressed an interest in joining such a program, compared with 47.6% of all other donor candidates (p=0.07)(Figure 1). Many of the close relative donor candidates expressed an interest in joining a diet program prior to kidney donation approval (96.6%) compared with other donor candidates (76.2%) p=0.07), but there was again less interest in joining a diet program specifically at our institution (82.8% for close relatives and 71.4% for all other relationships, p=0.49). Many of the LKD candidates also expressed an interest in a combined diet and exercise program (93.1% of close relative donor candidates and 76.2% of other donor candidates, p=0.12). However, there was a greater interest in a dietary and exercise program at our institution among close relative donor candidates (82.8%) compared with other donor candidates (47.6%, p=0.01). Finally, 41.4% of close relative donor candidates stated that they would be willing to pursue bariatric surgery if necessary prior to kidney donation, compared with 28.6% of all other donor candidates (p=0.35).
DISCUSSION
The results of this study demonstrated an interest in weight loss for the purposes of donation approval among obese LKD candidates. Based on the responses from the survey, obese donor candidates confirmed baseline knowledge of the risks associated with obesity in the setting of living kidney donation. Moreover, acknowledging these risks, they expressed a willingness to lose weight prior to donation approval and to improve their long-term health post-donation. Although fewer than half of the donor candidates reported regular exercise of at least three days per week, greater than 95% of respondents expressed an interest in pursuing an exercise program for the purposes of donation approval. Likewise, many candidates were interested in diet or combined diet and exercise programs. When assessing the role of donor-recipient relationship, close relative donor candidates were more commonly interested in a combined diet and exercise program at our institution, compared with all other donor candidates.
To our knowledge, there are not many formal weight loss or weight management programs that have been developed specifically for obese LKD candidates. However, the Center for Medicare Services requires that transplant centers include a registered dietician as part of the transplant team. Some centers have specifically utilized dieticians to disseminate nutritional information for donor candidates, particularly medically complex donors.27 Moreover, the literature suggests that formalized diet and exercise programs exist successfully in other fields. The Look Action for Health in Diabetes (Look AHEAD) trial was a randomized trial that assessed cardiovascular risk reduction with intensive lifestyle interventions, compared with a control (diabetes support and education alone) among overweight and obese diabetic patients.28 In their study, the authors found that while there were no statistically significant differences in cardiovascular mortality following intensive lifestyle interventions compared with diabetes education, there was significant improvement in physical activity, fitness, weight loss and body composition.28 Furthermore, the importance of both diet and exercise in weight loss efforts has been previously emphasized in the literature, with combined diet and exercise programs associated with improvements in both metabolic syndrome prevalence and weight loss.29
The findings of our study also revealed that LKD candidates who were close relatives were particularly engaged and interested in weight loss programs for the purpose of kidney donation. This may be secondary to the fact that among close relative donor candidates, achieving transplantation for the intended recipient also translates to an improved quality of life for the donor as well. Terasaki and colleagues have previously established that spousal donors derive personal benefits following donation, such as improved marital relationships.25 The authors also noted that in their survey among spousal donors, greater than 99% of respondents reported that they would recommend spousal kidney donation to others. Likewise, based on data from a questionnaire sent to prior LKDs, Johnson et. al found that LKDs with relationships other than first-degree relatives had greater than 3-fold odds of reporting they would not serve as LKDs again, if it was feasible.24 Additionally, Reese et. al previously demonstrated that donor-recipient spousal relationship was associated with medical complexity of the donor, suggesting that the donor-recipient relationship is considered in determining candidacy for donation nephrectomy, even in the setting of medically complex donors.9
This study has several strengths, in that it is the first of its kind to assess obese LKD candidates’ willingness to lose weight to become approved for kidney donation. Likewise, the single center study allowed for the capture of granular information in addition to the patient survey responses. However, the study has several limitations as well. This preliminary formative research sheds light on patient perspectives on weight management for the purposes of donation, but the small sample size from a single institution may limit the generalizability of the results. Many of the LKD candidates were advised to lose weight at the time of their donor evaluation, which may have biased the participants towards reporting willingness to participate in a program. In addition, when assessing the donor candidates’ willingness to participate in diet and/or exercise programs at our transplant center, many of the donor candidates mentioned that travel distance would be a potential barrier to future participation. As such, many participants expressed that they would not be interested in pursuing programs at our institution for that reason, and therefore the level of interest in potential programs may be attenuated due to logistical travel concerns. Also, the survey was created for our patient population and did not undergo validation, which may also limit the generalizability of our findings.
CONCLUSIONS
These data demonstrated an interest in weight loss among obese LKD candidates for the purposes of living kidney donation approval. Interest in a combination of dietary and exercise program at our institution was higher among close relatives of the potential transplant recipient, compared with other donor-recipient relationships. Future goals of this study include the creation of a weight management program at our institution. The aim of such a program would be to help obese donor candidates achieve healthier lifestyles so they may then be able to pursue their goals of providing the gift of life. This survey marks the beginning of our program development through the engagement of stakeholders –obese LKD candidates—in order to develop a patient-centered lifestyle modification program.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the National Institutes of Health (NIH)- National Research Service Award, through Grant Award Number T32 DK007545 (PI Mustian, mentored), and the National Institute of Diabetes and Digestive and Kidney Diseases – R01 DK113980 (PI Locke) and K23 DK103918 (PI Locke, mentored). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
DISCLOSURE
The authors have no conflicts of interest to disclose related to this project.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Ortiz SE, Kawachi I, Boyce AM. The medicalization of obesity, bariatric surgery, and population health. Health (London) 2017;21:498–518. [DOI] [PubMed] [Google Scholar]
- 2.Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999–2004. Jama 2006;295:1549–55. [DOI] [PubMed] [Google Scholar]
- 3.Mottillo S, Filion KB, Genest J, Joseph L, Pilote L, Poirier P, et al. The metabolic syndrome and cardiovascular risk a systematic review and meta-analysis. J Am Coll Cardiol 2010;56:1113–32. [DOI] [PubMed] [Google Scholar]
- 4.Dekker JM, Girman C, Rhodes T, Nijpels G, Stehouwer CD, Bouter LM, et al. Metabolic syndrome and 10-year cardiovascular disease risk in the Hoorn Study. Circulation 2005;112:666–73. [DOI] [PubMed] [Google Scholar]
- 5.Chen J, Muntner P, Hamm LL, Jones DW, Batuman V, Fonseca V, et al. The metabolic syndrome and chronic kidney disease in U.S. adults. Ann Intern Med 2004;140:167–74. [DOI] [PubMed] [Google Scholar]
- 6.Zammit AR, Katz MJ, Derby C, Bitzer M, Lipton RB . Metabolic Syndrome and Smoking Are Associated with Future Development of Advanced Chronic Kidney Disease in Older Adults. Cardiorenal medicine 2016;6:108–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bomback AS, Kshirsagar AV, Whaley-Connell AT, Chen SC, Li S, Klemmer PJ, et al. Racial differences in kidney function among individuals with obesity and metabolic syndrome: results from the Kidney Early Evaluation Program (KEEP). Am J Kidney Dis 2010;55:S4–s14. [DOI] [PubMed] [Google Scholar]
- 8.Mendy VL, Azevedo MJ, Sarpong DF, Rosas SE, Ekundayo OT, Sung JH, et al. The association between individual and combined components of metabolic syndrome and chronic kidney disease among African Americans: the Jackson Heart Study. PLoS One 2014;9:e101610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reese PP, Feldman HI, McBride MA, Anderson K, Asch DA, Bloom RD. Substantial variation in the acceptance of medically complex live kidney donors across US renal transplant centers. Am J Transplant 2008;8:2062–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sachdeva M, Rosen LM, Varghese J, Fishbane S, Molmenti EP. Weight trends in United States living kidney donors: Analysis of the UNOS database. World J Transplant 2015;5:137–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davis CL. Evaluation of the living kidney donor: current perspectives. Am J Kidney Dis 2004;43:508–30. [DOI] [PubMed] [Google Scholar]
- 12.Reese PP, Caplan AL, Kesselheim AS, Bloom RD. Creating a Medical, Ethical, and Legal Framework for Complex Living Kidney Donors. 2006;1:1148–53. [DOI] [PubMed] [Google Scholar]
- 13.Norman SP, Song PX, Hu Y, Ojo AO. Transition from donor candidates to live kidney donors: the impact of race and undiagnosed medical disease states. Clin Transplant 2011;25:136–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lapasia JB, Kong SY, Busque S, Scandling JD, Chertow GM, Tan JC. Living donor evaluation and exclusion: the Stanford experience. Clin Transplant 2011;25:697–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sachdeva M, Sunday S, Israel E, Varghese J, Rosen L, Bhaskaran M, et al. Obesity as a barrier to living kidney donation: a center-based analysis. Clin Transplant 2013;27:882–7. [DOI] [PubMed] [Google Scholar]
- 16.Mandelbrot DA, Pavlakis M, Danovitch GM, Johnson SR, Karp SJ, Khwaja K, et al. The medical evaluation of living kidney donors: a survey of US transplant centers. Am J Transplant 2007;7:2333–43. [DOI] [PubMed] [Google Scholar]
- 17.Hsu CY, McCulloch CE, Iribarren C, Darbinian J, Go AS. Body mass index and risk for end-stage renal disease. Ann Intern Med 2006;144:21–8. [DOI] [PubMed] [Google Scholar]
- 18.Kambham N, Markowitz GS, Valeri AM, Lin J, D'Agati VD. Obesity-related glomerulopathy: an emerging epidemic. Kidney Int 2001;59:1498–509. [DOI] [PubMed] [Google Scholar]
- 19.Locke JE, Reed RD, Massie A, MacLennan PA, Sawinski D, Kumar V, et al. Obesity increases the risk of end-stage renal disease among living kidney donors. Kidney Int 2017;91:699–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grams ME, Sang Y, Levey AS, Matsushita K, Ballew S, Chang AR, et al. Kidney-Failure Risk Projection for the Living Kidney-Donor Candidate. N Engl J Med 2016;374:411–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heimbach JK, Taler SJ, Prieto M, Cosio FG, Textor SC, Kudva YC, et al. Obesity in living kidney donors: clinical characteristics and outcomes in the era of laparoscopic donor nephrectomy. Am J Transplant 2005;5:1057–64. [DOI] [PubMed] [Google Scholar]
- 22.Chow GK, Prieto M, Bohorquez HE, Stegall MD. Hand-assisted laparoscopic donor nephrectomy for morbidly obese patients. Transplantation Proceedings 2002;34:728. [DOI] [PubMed] [Google Scholar]
- 23.Jacobs SC, Cho E, Dunkin BJ, Bartlett ST, Flowers JL, Jarrell B, et al. Laparoscopic nephrectomy in the markedly obese living renal donor. Urology 2000;56:926–9. [DOI] [PubMed] [Google Scholar]
- 24.Johnson EM, Anderson JK, Jacobs C, Suh G, Humar A, Suhr BD, et al. Long-term follow-up of living kidney donors: quality of life after donation. Transplantation 1999;67:717–21. [DOI] [PubMed] [Google Scholar]
- 25.Terasaki PI, Cecka JM, Gjertson DW, Cho YW. Spousal and other living renal donor transplants. Clin Transpl 1997:269–84. [PubMed] [Google Scholar]
- 26.de Vaus D Surveys in Social Research Sixth ed: Allen & Unwin; 2014. [Google Scholar]
- 27.Bergen CR, Reese PP, Collins D. Nutrition assessment and counseling of the medically complex live kidney donor. Nutr Clin Pract 2014;29:207–14. [DOI] [PubMed] [Google Scholar]
- 28.Dutton GR, Lewis CE. The Look AHEAD Trial: Implications for Lifestyle Intervention in Type 2 Diabetes Mellitus. Prog Cardiovasc Dis 2015;58:69–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baetge C, Earnest CP, Lockard B, Coletta AM, Galvan E, Rasmussen C, et al. Efficacy of a randomized trial examining commercial weight loss programs and exercise on metabolic syndrome in overweight and obese women. Appl Physiol Nutr Metab 2017;42:216–27. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.