Abstract
Background & Aims:
Patients with drug-induced liver injury (DILI) frequently have comorbid conditions, but the effects of non-liver comorbidities on outcome is not well understood. We investigated the association between co-morbidity burden and outcomes of patients with DILI, and developed and validated a model to calculate risk of death within 6 months.
Methods:
A multiple logistic regression model identified variables independently associated with death within 6 months of presenting with suspected DILI (6-month mortality) for 306 patients enrolled in the DILIN prospective study at Indiana University (discovery cohort). The model was validated using data from 247 patients with suspected DILI enrolled in the same study at the University of North Carolina (validation cohort). Medical comorbidity burden was calculated using the Charlson comorbidity index—patients with scores higher than 2 were considered to have significant comorbidities.
Results:
Six-month mortality was 8.5% in the discovery cohort and 4.5% in the validation cohort. In the discovery cohort, significant comorbidities (odds ratio [OR], 5.4; 95% CI 2.1 – 13.8), model for end-stage liver disease score (OR, 1.11; 95% CI, 1.04–1.17), and serum level of albumin at presentation (OR, 0.39; 95% CI, 0.2–0.76) were independently associated with 6-month mortality. A model based on these 3 variables identified patients who died within 6 months with c-statistic values of 0.89 (95% CI, 0.86–0.94) in the discovery cohort and 0.91 (95% CI, 0.83–0.99) in the validation cohort. We developed a web-based calculator to determine risk of death within 6 months for patients with suspected DILI for use in the clinic.
Conclusions:
We developed and validated a model based on comorbidity burden, model for end-stage liver disease score, and serum level of albumin that predicts 6-month mortality in patients with suspected DILI.
Keywords: MELD, CCI, DILIN Prospective Study
Introduction
Drug-induced liver injury (DILI) is an important cause of liver-related morbidity and mortality that is likely under-recognized. Its diagnosis depends on high index of suspicion, compatible temporal relationship, and thorough exclusion of competing etiologies1. DILI by an implicated drug, commonly occurs in patients with one or several comorbid conditions such as hypertension, diabetes mellitus, cardiovascular disease, renal disease and malignancy. The Drug-Induced Liver Injury Network (DILIN) Prospective Study enrolls individuals with suspected DILI at several clinical centers across the United States (1). A recent paper from the DILIN Prospective Study reported suspected agents and selected clinical features and outcomes of patients with DILI (2). Approximately 82% of patients enrolled in DILIN with suspected DILI and completing the causality assessment were determined to have probable, very likely or definite DILI2. Importantly, 10% of over 1,000 patients with DILI enrolled in DILIN suffered death or required liver transplantation within 2 years of DILI onset, with DILI playing a primary or contributory role in 80% of those deaths or transplants3. In approximately three quarters of these cases, there was evidence of acute liver failure, although in other cases there was more prolonged injury and chronicity preceding death3. A new Hy’s law was proposed in patients with a hepatocellular injury pattern and serum total bilirubin greater than 2.5 mg/dL being predictive of DILI-related mortality or liver transplantation, although 40% of cases of death or liver transplantation presented with a mixed or cholestatic injury pattern3. The most common non-DILI causes of death were related to co-morbid conditions, including malignancy, cardiovascular or cerebrovascular diseases, acute or chronic respiratory diseases, neurologic diseases, diabetes mellitus, and renal disease3.
A recent study suggested that medical comorbidity contributes to mortality risk in patients with hepatitis C infection independent of cirrhosis4. Additionally, medical comorbidity also predicts short-term mortality in acutely ill patients receiving intensive care5. In one study, a model combining medical comorbidity scores with a liver specific model was more discriminating in predicting hospital mortality in cirrhosis6. Anecdotally, we observed that some individuals with significant comorbidities succumbed to relatively minor DILI events whereas others without significant comorbidities survived severe DILI events. However, the impact of comorbidity burden on mortality in patients with suspected DILI has not been investigated previously. In this study we show that comorbidity burden is independently associated with mortality in individuals presenting with suspected DILI. Further, we developed and validated a robust model consisting of comorbidity burden, MELD and serum albumin to predict 6 month mortality among individuals presenting with suspected DILI.
Methods
Our discovery cohort consisted of consecutive patients with suspected DILI enrolled in the DILIN Prospective Study at Indiana University between 2003 and 2017. The DILIN Prospective study () is a multicenter observational study of patients aged 2 years or older with suspected liver injury meeting predefined biochemical criteria for liver test abnormalities7. Enrolled patients were clinically characterized in a standardized fashion and the likelihood of DILI was adjudicated by consensus7. We analyzed demographic and clinical data, including implicated agents, and outcomes of DILI. The pattern of liver injury at presentation was defined using R value (the ratio of alanine aminotransferase (divided by its upper limit of normal) to alkaline phosphatase (divided by the upper limit of normal)), as cholestatic (R<2), mixed (R=2 to 5) or hepatocellular (R>5). We recorded the comorbidities and long term mortality data from medical records and from the DILIN case report forms. The primary outcome of interest was mortality within 6 months of onset of liver injury, and we also described overall mortality up to last follow-up. Participants in this report have been part of a number of papers published by the DILIN3, 8–12. This study has been approved the Institutional Review Boards at Indiana University and the University of North Carolina, and all participants signed an informed consent.
The medical comorbidity burden at presentation was quantified using the Charlson Comorbidity Index (CCI)13. For this study purposes, we grouped patients into those with no or mild medical comorbidity (CCI range from 0 to 75th percentile value) and significant comorbidity (CCI >75th percentile). Descriptive analyses included comparison of clinical characteristics according to CCI groups. The first available INR, serum total bilirubin and creatinine, at or after onset of liver injury, were used to calculate MELD. We analyzed the association of significant comorbidity, with 6-month mortality in all patients. Furthermore, we assessed the interaction of significant comorbidity and severe liver injury, defined as an initial MELD>19, on 6-month mortality risk, as a previously described threshold for increased mortality in the DILIN experience3.
Comparison of categorical variables between study groups, including 6-month mortality, was performed using the chi-square test. Comparison of continuous data between groups was performed using nonparametric tests, including Wilcoxon or Kruskal Wallis tests. Overall mortality was analyzed using the Kaplan Meier method with log-rank for differences between groups. The independent predictors of 6-month mortality were examined using simple and multiple logistic regression analyses. The resulting model was validated on a separate cohort of prospectively enrolled subjects in the DILIN Prospective Study during the same period at the University of North Carolina. A calculator based on the regression coefficients was developed to estimate 6-month mortality in patients with suspected acute DILI. A p-value <0.05 was considered statistically significant for all analyses.
Results
Patient and liver injury characteristics
The discovery cohort consisted of 306 patients, mean (SD) age 49 ± 19 years and 48% male. The most frequent conditions contributing to CCI were diabetes and pulmonary disease (15% each), and malignancy, liver disease, peripheral vascular disease, renal disease, congestive heart failure and stroke (4.8 to 6% each). The median CCI was 0 (interquartile range 0, 2), and 253 patients (83%) with CCI ≤ 2 were classified to have “none to mild comorbidity,” whereas 53 patients (17%) with CCI > 2 were classified to have “significant comorbidity”. We compared select participant and liver injury characteristics of individuals with suspected DILI according to this categorization of comorbidity (Table 1). Compared with none to mild comorbidity group, patients with significant comorbidity were older, and more likely to be male and black. The mean MELD in the entire cohort was 15 ± 7, and it was higher in those with significant comorbidity, which was driven primarily by worse renal function in that subgroup. The pattern of liver injury in patients with significant comorbidity was more frequently cholestatic and less frequently hepatocellular, compared to none/mild comorbidity. The most common class of implicated agent was antimicrobials with no significant differences between groups. However, herbal and dietary supplements were predominantly implicated in patients with none to mild comorbidity, while cardiovascular agents were predominantly implicated in patients with significant comorbidity. Patients with significant comorbidity had a trend towards a higher number of implicated agents and significantly more concomitant medications compared to patients with none to mild comorbidity (Table 1).
Table 1.
Selected characteristics of patients with suspected drug-induced liver injury (DILI) according to Charlson Comorbidity Index (CCI) category (Indiana Cohort)*
| Entire Cohort (N=306) |
No/Mild comorbidity (CCI = 0 to 2) (n=253) |
Significant comorbidity (CCI >2) (n=53) |
P-value** | |
|---|---|---|---|---|
| Age (years) | 49 ± 19 | 47 ± 19 | 57 ± 15 | <0.001 |
| Male (%) | 48 | 43 | 70 | <0.001 |
| Body mass index (kg/m2) | 28 ± 7 | 28 ± 7 | 28.3 ± 7.6 | 0.9 |
|
Race/ethnicity (%) White Black Asian |
81.3 9.5 3.3 |
84.1 6.7 3.6 |
67.9 22.6 1.9 |
0.006 |
| Latino ethnicity (%) | 5.9 | 5.9 | 6 | >0.999 |
| Latency (days) | 161± 506 | 139±481 | 267±610 | 0.7 |
| Serum ALT (IU/L) | 745 ± 996 | 806±1062 | 439±452 | 0.001 |
| Serum Alk Phos (IU/L) | 297 ± 306 | 274 ± 226 | 408±539 | 0.17 |
| Serum bilirubin (mg/dL) | 6.3 ± 5.6 | 6.5 ± 5.6 | 5.4 ± 5.6 | 0.08 |
| INR (at onset) INR (at peak MELD) |
1.4 ± 1 1.9 ± 1.9 |
1.4 ± 1.09 1.8 ± 1.9 |
1.5 ± 0.5 2.3 ± 1.6 |
0.001 <0.001 |
| Serum creatinine (mg/dL) | 1.2 ± 0.6 | 1.2 ± 0.5 | 1.6 ± 1 | <0.001 |
| MELD | 15.7 ± 6.7 | 15.3 ± 6.3 | 17.9 ± 8.2 | 0.037 |
| Albumin (g/dL) | 3.4 ± 0.7 | 3.5 ± 0.7 | 3± 0.7 | <0.001 |
| R-value | 11.6 ± 17.3 | 12.8±18.3 | 5.9±9.5 | <0.001 |
|
Injury pattern (%) Hepatocellular Mixed Cholestatic |
49.7 21.2 29.1 |
53.4 22.5 24.1 |
32.1 15.1 52.8 |
<0.001 |
|
Class of agents (%) Antimicrobials Antineoplastics Cardiovascular agents Herbal and dietary supplements Neurologic agents |
48.7 4.6 10.1 15 7.5 |
47.4 4 8.3 17.8 7.9 |
54.7 7.5 18.9 1.9 5.7 |
0.3 0.3 0.02 0.003 0.8 |
| Number of implicated agents | 1.4 ± 0.7 | 1.4 ± 0.7 | 1.6 ± 0.8 | 0.08 |
| Number of concomitant drugs | 8.7 ± 8.7 | 7.6 ± 8 | 13.8 ± 9.7 | <0.001 |
| Number of comorbidities included in CCI | 0.84 ± 1.25 | 0.42 ± 0.61 | 2.85 ± 1.56 | <0.001 |
| Comorbidities included in CCI (%) Diabetes uncomplicated/complicated Chronic Pulmonary Disease Malignancy non-metastatic/metastatic Liver disease mild / moderate to severe Peripheral vascular disease Renal disease (moderate to severe) Congestive heart failure Stroke or transient ischemic attack Connective tissue disease Peptic ulcer disease Myocardial infarction Acquired Immune Deficiency Syndrome Leukemia Lymphoma Dementia Hemiplegia or Paraplegia |
11.1 / 4.9 15 4.6 / 1.3 2.3 / 2.9 6.2 5.1 5.2 5.2 4.6 3.9 3.6 2.9 2.3 1.3 1.3 None |
9.1 / 1.2 10.3 2.4 / none 1.6 / none 2.4 2 1.6 1.6 3.6 1.6 1.6 None 2 0.4 0.8 None |
20.8 / 22.6 37.7 15.1 / 7.5 5.7 / 17% 24.5 20.8 22.6 22.6 9.4 15.1 13.2 17 3.8 5.7 3.8 None |
<0.001 <0.001 <0.001 <0.001 <0.001 <0.001 <0.001 <0.001 0.06 <0.001 <0.001 <0.001 0.4 0.002 0.08 NA |
Values shown as mean ± standard deviation unless specified otherwise;
P-values compare No/mild comorbidity and significant comorbidity groups.
Abbreviations: ALT: Alanine aminotransferase; Alk P: Alkaline Phosphatase; CCI, Charlson Comorbidity Index; DILI, Drug-induced liver injury; INR, International Normalized Ratio; MELD, Model for End-stage Liver Disease; NA, not applicable.
Comorbidity and clinical outcomes
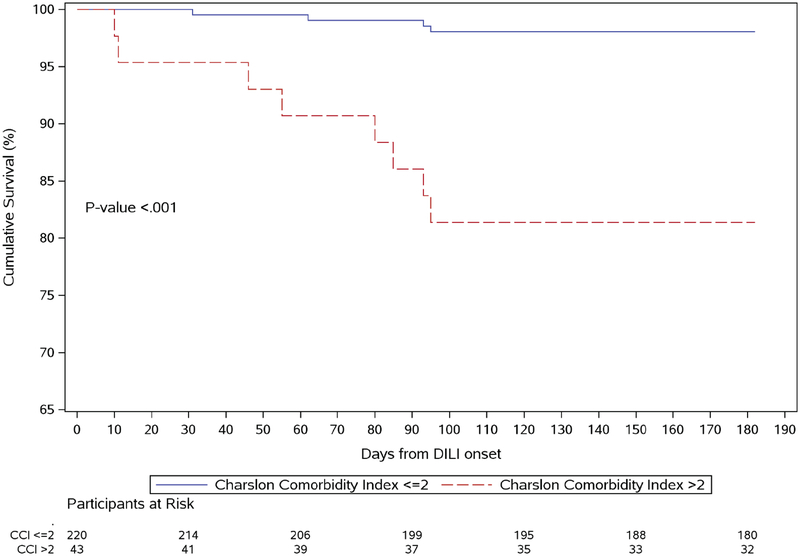
The mean follow-up in the study cohort was 49 ± 44 months (median 38 months, interquartile range 8, 84). Of the 306 patients included, 4 (1.3%) underwent subsequent liver transplantation, and all 4 had no comorbidity. The implicated agents in these 4 cases included: mercaptopurine, diphenoxylate with atropine sulfate and 2 herbal and dietary supplements labelled as ephedrine and vitamins with minerals not otherwise specified. Overall, 49 patients (16%) died at any time after the onset of liver injury. Higher mortality was observed in patients with significant comorbidity, compared to those with none to mild comorbidity (Figure 1). However, death was assessed as liver-related in only 36% of patients with significant comorbidity who died, compared with 64% with no/mild comorbidity (p=0.1) using previously reported methodology to adjudicate the role of DILI in death or liver transplantation3. Mortality within 6 months of suspected DILI presentation was observed in 26/253 (10.3 %) of patients with none to mild, but in 15/53 (28.3%) in patients with significant comorbidity (p<0.001). The drugs implicated in these 26 cases included antimicrobial (13), antineoplastic (6), cardiovascular (6) and a unknown (1) agent (Supplemental Table 1). The causes of death in patients with and without significant comorbidity are described in Supplemental Table 2, with 4 cases of death due to acute on chronic liver failure.
Figure 1.
Survival in patients with significant comorbidity (Charslon Comorbidity Index > 2) (dashed line) vs. mild comorbidity (Charlson Comorbidity Index ≤ 2) (solid line) using the Kaplan-Meier method in patients with suspected drug-induced liver injury (DILI) in the Indiana University Cohort (Figure 1-A) and University of North Carolina cohort (Figure 1-B).
Predictors of mortality
Multiple logistic regression analysis identified significant comorbidity (OR 5.2, 95% CI 2.1 – 13.3), MELD (OR 1.09, 95% CI 1.03–1.15), and serum albumin (OR 3.7, 95% CI 0.2 −0.7) as the independent predictors of 6-month mortality in individuals with suspected DILI (Table 2). The multiple logistic regression model performed well in predicting 6-month mortality, with a c-statistic of 0.89 (95% confidence interval: 0.86 – 0.94) with good fit (Hosmer and Lemeshow Goodness-of-Fit Test, Chi-square = 9.2, p = 0.3).
Table 2.
Factors at presentation associated with 6-month overall mortality in patients with suspected drug-induced liver injury (N=306)
| Variable | Simple logistic regression¶ OR (95%CI) P-value |
Multiple logistic regression OR (95%CI) P-value |
|---|---|---|
|
Comorbidity category Reference category none/mild comorbidity (CCI≤2) Significant comorbidity (CCI > 2) |
8.7 (3.7, 20.3) p< 0.0001 |
5.6 (2.2, 14.1) p<0.001 |
| MELD | 1.14 (1.08, 1.20) p<0.0001 |
1.10 (1.04, 1.17) p<0.001 |
| Albumin (g/dL) | 0.26 (0.15, 0.46) p<0.0001 |
0.40 (0.20, 0.78) p=0.007 |
| Age (years) | 1.03 (1.005, 1.054) p=0.02 |
Factors not associated with 6-month mortality on simple logistic regression included gender, race, ethnicity, body mass index and pattern of liver injury at onset.
Abbreviations: CCI, Charlson Comorbidity Index; CI, Confidence Interval; DILI, Drug-Induced Liver Injury; MELD, Model for End-stage Liver Disease; OR, Odds Ratio.
Significant comorbidity was significantly associated with six-month mortality when the analysis was restricted to those who were adjudicated to have definite, highly likely, or probable DILI (OR: 7.4, 95% CI: 1.9 – 28.6, p=0.004). A previous study [3] has shown that MELD is a strong predictor of mortality in patients with suspected DILI3 For descriptive purposes, we examined the relationship between CCI and 6 month mortality after stratifying our cohort into MELD ≤ 19 and MELD > 19 subgroups, since that MELD threshold was sensitive and specific for predicting mortality in DILI3. Compared to none to mild comorbidity, significant comorbidity was associated with higher 6-month mortality among individuals with MELD ≤ 19 (20% vs 2.4%, p=0.001) as well as MELD > 19 (39.1% vs 13.3%, p=0.01) (Table 3). There was no interaction between CCI and MELD >19 (p value=0.31). This highlights the increased risk of short-term mortality associated with significant comorbidity in patients with suspected DILI, regardless of MELD related risk. In a sensitivity analysis, when we limited our analysis to 231 patients with high confidence DILI cases (causality scores: definite, highly likely, and probable), CCI>2 was a significant predictor of 6 month mortality, even after controlling for albumin and MELD, with the model achieving a c-statistic of 0.92 (95% confidence interval: 0.87 – 0.98) with good fit (Hosmer and Lemeshow Goodness-of-Fit Test, Chi-square = 4.3, p = 0.8).
Table 3.
Relationship between comorbidity burden and 6-month and overall mortality in patients with DILI, stratified according to MELD ≤ 19 vs MELD>19¶
| MELD ≤19 (low risk) | P value | ||
|---|---|---|---|
| None/Mild comorbidity (CCI 0–2) (n=207) |
Significant comorbidity (CCI>2) (n=30) |
||
| 6-month mortality (%) | 2.4 | 20 | <0.001 |
| Overall mortality (%) | 4.8 | 26.7 | <0.001 |
| MELD >19 (high risk) | |||
| None/Mild comorbidity (CCI 0–2) (n=45) |
Significant comorbidity (CCI>2) (n=23) |
||
| 6-month mortality (%) | 13.3 | 39.1 | 0.015 |
| Overall mortality (%) | 13.3 | 39.1 | 0.015 |
Abbreviations: CCI, Charlson Comorbidity Index; MELD, Model for End-stage Liver Disease.
MELD ≤ 19 and >19 were categorized as low-risk and high-risk categorized based on Ref 3.
The validation cohort comprised 247 patients enrolled in DILIN at the University of North Carolina. Significant comorbidity was noted in 40 subjects (16%) and they were older with more males but similar racial distribution, more frequent cholestatic liver injury and less frequent hepatocellular injury compared with those with mild comorbidity, (Supplemental Table 3). Their 6-month mortality was 4.5%. Our multiple logistic regression model based on CCI, MELD, and serum albumin achieved a c-statistic of 0.91 (95% confidence interval: 0.83 – 0.99) in the validation cohort, with good fit (Hosmer and Lemeshow Goodness-of-Fit Test, Chi-square = 5.4, p = 0.7) for predicting 6-month mortality in this validation cohort. We calculated the Brier score, which measures the accuracy of probabilistic predictions, in both derivation and validation cohorts. The lower the Brier score for a set of predictions, the better the prediction are calibrated. The Brier score for our model in the Indiana University derivation cohort was 0.038, and in the University of North Carolina validation cohort was 0.0386.
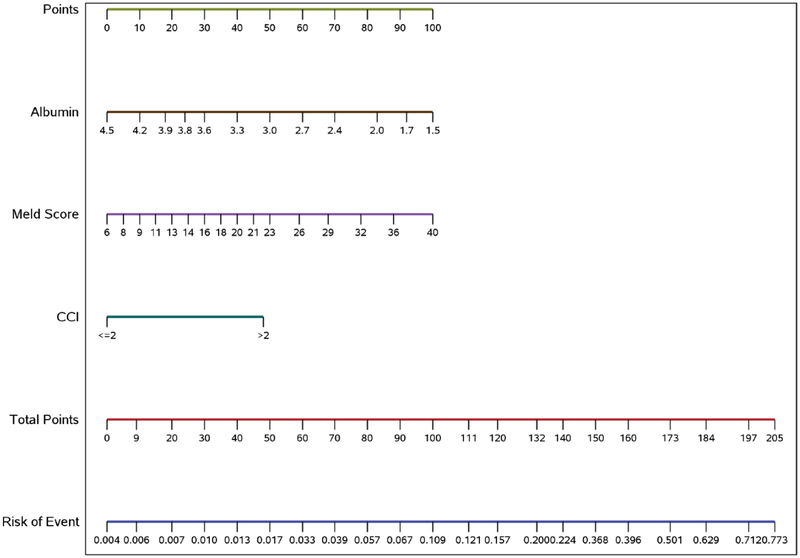
A DILI mortality calculator representing the model incorporating CCI category, albumin and MELD to estimate the risk of 6-month mortality in patients with suspected acute DILI was developed and calibrated using the model derived from the Indiana University cohort (Figure 2). The regression equations for calculating the risk score and the six-month mortality are described in Supplemental Table 4.
Figure 2.
A calculator that incorporates Charlson Comorbidity Index, Model for End-stage Liver Disease (MELD) and serum albumin in predicting 6-month mortality in patients with suspected acute drug-induced liver injury. Points are assigned for Charlson Comorbidity Index, MELD and serum albumin scales using the linear points scale at the top of the figure. The risk of 6-month mortality correlating with the total points is on the two linear scales at the bottom of the figure.
Two cases are provided as examples to demonstrate the use of the calculator. A patient presenting with CCI 0 (0 points), serum albumin 3 g/dL (41 points) and MELD 10 (12 points), will have a total of 53 points and corresponding 25% probability of 6-month mortality. A patient presenting with CCI 5 (50 points), serum albumin 2.9 g/dL (44 points) and MELD 23 (50 points) has a total of 143 points with 36% probability of 6-month mortality.
Discussion
In this prospective study of well-characterized patients with suspected DILI, approximately half of the patients suffered from medical comorbidity, as quantified using the Charlson Comorbidity Index. The main finding of this study was that medical comorbidity, and significant comorbidity in particular (CCI>2), were associated with increased risk of 6-month and overall mortality. Patients with significant comorbidity did not undergo liver transplantation, but mortality was more frequently non liver-related, and likely related to the underlying comorbidity burden.
While not surprising, this finding is novel and highlights a previously unrecognized mortality risk for patients with DILI. Significant comorbidity independently predicted 6-month mortality in addition to MELD and albumin, which have been described as important risk factors for mortality in patients with DILI3. A model comprising severity of comorbidity, MELD and albumin performed well (c-statistic 0.89) in the Indiana University derivation cohort. This model was validated in a prospectively studied cohort at the University of North Carolina and performed equally well in that cohort (c-statistic 0.91). A DILI mortality calculator was developed based on the model’s regression coefficients to estimate 6-month mortality based on model parameters. This dynamic web-based calculator was developed to facilitate application of the model in clinical practice, and can be accessed at http://gihep.com/calculators/hepatology/dili-cam/. Significant comorbidity burden remained an independent predictor of 6-month mortality when excluding patients with liver injury attributed to a non-DILI etiology. Since DILI is not a unique cause of liver injury, it is conceivable that models incorporating comorbidity burden and severity of liver injury could prove useful in improving the prediction of mortality in a variety of liver injuries and diseases, and as such warrants further studies.
A CCI score >2 would not be considered a high score in some studies exploring the impact of CCI that use a threshold of >3 based on highest quartile of CCI14. The CCI threshold of >2 in our study was derived mathematically using the 75th percentile for CCI in the derivation cohort for descriptive purposes. Subsequently, its significance with respect to risk of mortality was demonstrated in both the derivation and validation cohorts, lending credibility to the descriptor of “significant comorbidity” using this definition. This threshold (CCI > 2) has also been associated with mortality risk in general population studies and in patients present with acute heart failure15,16.
While MELD was higher in patients with significant comorbidity, the difference was predominantly related to chronic kidney disease and higher baseline serum creatinine in that subgroup. Significant comorbidity was associated with advanced age and male gender which may explain the association with predominantly cholestatic liver injury17.
An interesting observation in our cohort was that herbal and dietary supplements were almost exclusively implicated in patients with no or mild comorbidity as scored by the CCI, with no deaths amongst those patients. Based on this finding, DILI related to herbal and dietary supplements might then be expected to result in lower mortality. However, this was not observed in an analysis of the whole prospective DILIN cohort, wherein 6-month mortality was similar in cases of herbal and dietary supplement related DILI or other DILI11. This could be explained by younger age of patients using herbal and dietary supplements with mild or no comorbidities.
We observed lower alanine transaminase levels at presentation in patients with significant comorbidity. This may be explained by similar findings in patients with acute liver failure and underlying non-cirrhotic liver disease18. The higher rate of cholestatic pattern liver injury in patients with significant comorbidity who are older and with potential lower capacity for liver regeneration19, 20. While the pattern of liver injury was not associated with 6-month mortality per se, we observed cholestatic injury in 5 of the 10 patients with liver-related death, and in 5 of 16 with non-liver related death. Whereas, cholestatic liver injury was observed in 1 of 6 patients with DILI-related death, in contrast with 9 of 20 patients with non-DILI related death. The latter finding supports the postulation of the late Hy Zimmerman that most patients with cholestatic DILI die from something other than DILI21.
The strengths of the study include the prospective design of the DILIN study and standardized phenotyping of patient factors and both liver and non-liver related outcomes in both the study and validation cohorts. This analysis demonstrates that, while the majority of patients with suspected DILI have no or mild comorbidity, a sizable subset (93/593 (15.7%)) has significant comorbidity. The latter patients have higher short-term mortality with or without DILI as the etiology of liver injury. Significant medical comorbidity was an independent risk factor for 6-month mortality. The resulting model, incorporating severity of comorbidity, MELD and albumin was externally validated and calibrated. A practical calculator was developed to facilitate stratification of mortality risk in patients with suspected DILI in clinical practice. There are several limitations to note. The model was based on single center data and the medical comorbid conditions comprising the CCI were not all systematically documented per protocol and therefore may be incomplete in some instances. In addition, the subgroup of patients with significant comorbidities in both the study and validation cohorts were relatively small. Further examination of comorbidity burden and outcomes of DILI in larger and geographically diverse cohorts would be helpful.
In summary, we observed that medical comorbidity burden is significantly associated with 6 month and overall mortality in individuals with suspected DILI. A model consisting of Charlson Comorbidity Index, MELD score, and serum albumin strongly predicts 6 month mortality in patients with suspected DILI. It identifies patients in need of closer comprehensive monitoring in the wake of acute liver injury.
Supplementary Material
What you need to know:
BACKGROUND AND CONTEXT: We identified a strong relationship between significant comorbidity burden and outcomes of patients with suspected drug-induced liver injury (DILI), and developed and validated a model to calculate risk of death within 6 months.
NEW FINDINGS: Our model calculates risk of death within 6 months for patients with suspected DILI based on comorbidity burden, model for end-stage liver disease score, and serum level of albumin.
LIMITATIONS: This was a post-hoc analysis of data from 2 centers enrolling patients into a prospective study.
IMPACT: Our model can accurately determine risk of death within 6 months for patients with suspected DILI using easily available bedside variables.
Lay Summary: We developed a system to determine risk of death in patients suspected of having drug-induced liver injury.
Acknowledgments
Authors sincerely thank Dr Herbert Bonkovsky for this critical review of this manuscript and important input. Authors also thank Savannah Musselman for her secretarial assistance with this manuscript.
Source of Funding
This work was supported in part by DK065176 (Duke), DK065201 (UNC) and DK065211 (Indiana University). The DILIN Network is structured as a U01 cooperative agreement between the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), the participating clinical centers and the data coordinating center.
Disclosures
Dr. Chalasani has ongoing consulting activities (or had in preceding 12 months) with NuSirt, Abbvie, Eli Lilly, Afimmune (DS Biopharma), Allergan (Tobira), Madrigal, Shire, Axovant, Coherus, Siemens, Centurion, and Genentech. These consulting activities are generally in the areas of nonalcoholic fatty liver disease and drug hepatotoxicity. Dr. Chalasani received research grant support from Intercept, Lilly, Galectin Therapeutics, Exact Sciences and Cumberland where his institution receives the funding. None represents a conflict of interest for this paper. Marwan Ghabril, Lindsey Yoder, Laura Corbito, Paul H. Hayashi and Jiezhun Gu report no potential conflicts of interest. Dr. Vuppalanchi has consulting agreements with Covance and Enanta, and research support from Gilead, Intercept, and Zydus (Cadilla) where his institution receives the funding.
Abbreviations
- CCI
Charlson Comorbidity Index
- DILI
drug-induced liver injury
- DILIN
Drug-Induced Liver Injury Network
- MELD
model for end-stage liver disease
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chalasani NP, Hayashi PH, Bonkovsky HL, et al. ACG Clinical Guideline: the diagnosis and management of idiosyncratic drug-induced liver injury. Am J Gastroenterol 2014;109:950–66; quiz 967. [DOI] [PubMed] [Google Scholar]
- 2.Chalasani N, Bonkovsky HL, Fontana R, et al. Features and Outcomes of 899 Patients With Drug-Induced Liver Injury: The DILIN Prospective Study. Gastroenterology 2015;148:1340–52.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hayashi PH, Rockey DC, Fontana RJ, et al. Death and liver transplantation within 2 years of onset of drug-induced liver injury. Hepatology 2017;66:1275–1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Natarajan Y, White DL, El-Serag HB, et al. Role of Non-hepatic Medical Comorbidity and Functional Limitations in Predicting Mortality in Patients with HCV. Dig Dis Sci 2017;62:76–83. [DOI] [PubMed] [Google Scholar]
- 5.Ladha KS, Zhao K, Quraishi SA, et al. The Deyo-Charlson and Elixhauser-van Walraven Comorbidity Indices as predictors of mortality in critically ill patients. BMJ Open 2015;5:e008990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xu Y, Li N, Lu M, et al. Comparison of risk adjustment methods in patients with liver disease using electronic medical record data. BMC Gastroenterol 2017;17:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fontana RJ, Watkins PB, Bonkovsky HL, et al. Drug-Induced Liver Injury Network (DILIN) prospective study: rationale, design and conduct. Drug Saf 2009;32:55–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dakhoul L, Ghabril M, Gu J, et al. Heavy Consumption of Alcohol is Not Associated With Worse Outcomes in Patients With Idiosyncratic Drug-induced Liver Injury Compared to Non-Drinkers. Clin Gastroenterol Hepatol 2018;16:722–729 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chalasani N, Reddy KRK, Fontana RJ, et al. Idiosyncratic Drug Induced Liver Injury in African-Americans Is Associated With Greater Morbidity and Mortality Compared to Caucasians. Am J Gastroenterol 2017;112:1382–1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fontana RJ, Hayashi PH, Barnhart H, et al. Persistent liver biochemistry abnormalities are more common in older patients and those with cholestatic drug induced liver injury. Am J Gastroenterol 2015;110:1450–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fontana RJ, Hayashi PH, Gu J, et al. Idiosyncratic drug-induced liver injury is associated with substantial morbidity and mortality within 6 months from onset. Gastroenterology 2014;147:96–108.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Navarro VJ, Barnhart H, Bonkovsky HL, et al. Liver injury from herbals and dietary supplements in the U.S. Drug-Induced Liver Injury Network. Hepatology 2014;60:1399–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373–83. [DOI] [PubMed] [Google Scholar]
- 14.Rattanasompattikul M, Feroze U, Molnar MZ, et al. Charlson comorbidity score is a strong predictor of mortality in hemodialysis patients. Int Urol Nephrol 2012;44:1813–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Formiga F, Moreno-Gonzalez R, Chivite D, et al. High comorbidity, measured by the Charlson Comorbidity Index, associates with higher 1-year mortality risks in elderly patients experiencing a first acute heart failure hospitalization. Aging Clin Exp Res 2018;30:927–933. [DOI] [PubMed] [Google Scholar]
- 16.Fraccaro P, Kontopantelis E, Sperrin M, et al. Predicting mortality from change-over-time in the Charlson Comorbidity Index: A retrospective cohort study in a data-intensive UK health system. Medicine (Baltimore) 2016;95:e4973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chalasani N, Fontana RJ, Bonkovsky HL, et al. Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology 2008;135:1924–34, 1934.e1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gottlieb A, Kottmann M, Manka P, et al. How to Define Acute Liver Failure Patients with Pre-Existing Liver Disease without Signs of Cirrhosis. Dig Dis 2019;37:147–154. [DOI] [PubMed] [Google Scholar]
- 19.Canbay A, Chen SY, Gieseler RK, et al. Overweight patients are more susceptible for acute liver failure. Hepatogastroenterology 2005;52:1516–20. [PubMed] [Google Scholar]
- 20.Canbay A, Jochum C, Bechmann LP, et al. Acute liver failure in a metropolitan area in Germany: a retrospective study (2002 – 2008). Z Gastroenterol 2009;47:807–13. [DOI] [PubMed] [Google Scholar]
- 21.Zimmerman HJ. Hepatotoxicity : the adverse effects of drugs and other chemicals on the liver. Philadelphia: Lippincott Williams & Wilkins, 1999. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.