Abstract
Objective:
To assess the frequency of gastrostomy tube (GT) placement in extremely low birth weight (ELBW) infants, associated co-morbidities, and long-term outcomes.
Study design:
Analysis of ELBW infants from 25 centers enrolled in the National Institute of Child Health and Human Development Neonatal Research Network’s Generic Database and Follow-up Registry from 2006-2012. Frequency of GT placement before 18-22 months, demographic and medical factors associated with GT placement, and associated long-term outcomes at 18-22 months corrected age were described. Associations between GT placement and neonatal morbidities and long-term outcomes were assessed with logistic regression after adjustment for center and common covariables.
Results:
Of the 4549 ELBW infants included in these analyses, 333 (7.3%) underwent GT placement; 76% had the GT placed post-discharge. Of infants with GTs, 11% had birth weights small for gestational age (SGA), 77% had bronchopulmonary dysplasia (BPD), and 29% severe intraventricular hemorrhage (IVH) or periventricular leukomalacia (PVL). At follow-up, 56% of infants with a GT had weight <10th percentile, 61% had neurodevelopmental impairment (NDI), and 55% had chronic breathing problems. After adjustment, SGA, BPD, IVH/PVL, poor growth and NDI were associated with GT placement. Thirty-two percent of infants with GTs placed were taking full oral feeds at follow-up.
Conclusions:
GT placement is common in ELBW infants, particularly among those with severe neonatal morbidities. GT placement in this population was associated with poor growth, NDI and chronic respiratory and feeding problems at follow-up. The frequency of GT placement post-neonatal discharge indicates the need for close nutritional follow-up of ELBW infants.
Trial registration
Oral feeding difficulties are common among extremely low birth weight (ELBW) infants. Coordination of suck-swallow-breathe patterns are critical to feeding success, but these skills typically evolve and mature starting at 33-34 weeks of gestation.1-4 This process can be delayed in those who require invasive medical interventions (endotracheal intubation and long-term nasogastric (NG) feedings).5,6 ELBW infants with poor oral feeding skills have prolonged hospital stays, incur increased health care costs and may be at increased risk for long-term deficits in feeding skills and neurodevelopment.7,8
ELBW infants with bronchopulmonary dysplasia (BPD) or severe neurologic injury are especially prone to ongoing feeding difficulties.9,10 Infants with severe BPD have been found to have poorer coordination when feeding, poor endurance, and an inability to generate sucking pressures strong enough for successful oral feeding.11,12 At follow-up visits, lower scores on neurodevelopmental testing in the cognitive and language domains have been demonstrated in ELBW infants with dysfunctional feeding behaviors.13
Oral feeding difficulties often necessitate alternative feeding options before or after NICU discharge. There is some evidence in the literature supporting early discharge with home gavage feeding in stable premature infants who have difficulties establishing full oral feeds.14-16 Nonetheless, the overall frequency of GT placement in neonates has increased with one study showing the rate of GTs doubling in very low birthweight infants between 2000 and 2012.17
Although GT feeding in premature infants has been described in the literature, the frequency of use in ELBW infants and its association with other neonatal morbidities is unknown. The purpose of this study was to examine the frequency of GT placement in ELBW infants, to investigate the association with certain neonatal morbidities, and to evaluate longer-term growth and developmental outcomes.
Methods:
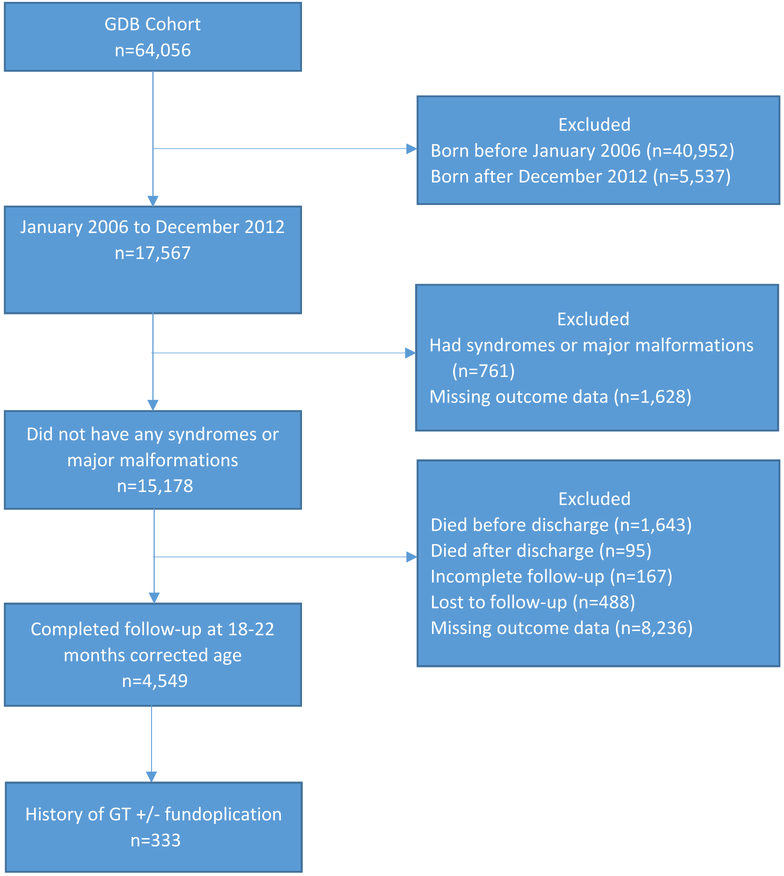
We performed a retrospective cohort analysis of all ELBW infants (birth weight <1,000 g) who were enrolled in the National Institute of Child Health and Human Development (NICHD) Neonatal Research Network’s (NRN) Generic Database (clinicaltrials.gov: ) between January 1, 2006 and December 31, 2012. Prior to discharge, all surgical procedures were noted. If an infant did not have a GT placed before discharge, then GT placement after discharge was determined by a question asked of the caregiver at follow-up. This was a simple yes or no question regarding whether the child had a gastrostomy tube or button placed. The timing of the follow-up evaluation changed during the research study period. Follow-up was performed between 18-22 months corrected age prior to 7/1/2012 and between 22-26 months corrected age afterwards. Infants were excluded from the analysis if they had significant congenital heart disease, an upper airway or GI malformation, or a syndrome or chromosomal abnormality. Infants who developed short bowel syndrome during their NICU stay necessitating a GT were excluded as these infants often require GT management for reasons other than oral feeding difficulties. Infants dying before or after NICU discharge, those not completing the developmental follow-up visit, and those with missing outcome data at discharge and at follow-up also were excluded (Figure; available at www.jpeds.com). Trained research personnel collected clinical data in a standardized manner.
Figure 1. Patient Cohort.
GDB database: January 2006 to December 2012 n=17,567
Excluded
Had syndromes or major malformations (n=761)
Died before discharge (n=1,643)
Died after discharge (n=95)
Incomplete follow-up (n=167)
Lost to follow-up (n=488)
Missing/empty outcome data (n=9,864)
Completed follow-up at 18-22 months corrected age (n=4,549)
Prevalence of GT placement prior to the follow-up visit, demographic and medical factors associated with GT placement, and associated outcomes (including: respiratory, feeding, growth, and neurodevelopmental outcomes) were documented. Demographic factors included race, sex, and maternal education level. Medical factors included in the analyses were a birth weight small for gestational age (SGA), bronchopulmonary dysplasia (BPD), severe intraventricular hemorrhage (IVH), periventricular leukomalacia (PVL), and necrotizing enterocolitis (NEC). SGA was defined as a birth weight <10th percentile for gestational age.18 BPD was defined using the physiologic definition of infants at 36 weeks postmenstrual age.19 IVH included grade III or IV.20 NEC was defined as Stage IIA or greater on modified Bell’s Staging criteria.21,22 Infants diagnosed with surgical NEC leading to short bowel syndrome were excluded and not included in the primary analysis, however, information was gathered regarding the prevalence of GTs in this population. Longer-term outcomes evaluated were poor growth, neurodevelopmental impairment, “chronic feeding problems” (use of thickened feeds, abnormal swallowing, dysphagia, or documented aspiration at follow-up), and “chronic breathing problems” (use of oxygen, diuretics, or bronchodilators at follow-up). Poor growth, measured at follow-up on the National Center for Health Statistics (NCHS) growth curve, was defined as weight, length or head circumference <10th percentile for corrected age.23 Infants were evaluated between 18-26 months adjusted age using the Bayley Scales of Infant and Toddler Development (BSID-III) and a standardized neurosensory examination. Neurodevelopmental impairment (NDI) was defined as any of the following: a cognitive composite score on the BSID III <85, moderate-to-severe cerebral palsy (CP), gross motor function classification system (GMFCS) level ≥2, severe hearing impairment, or bilateral severe visual impairment.13
Descriptive statistics were calculated for baseline characteristics, medical factors, and outcomes at follow-up. Frequencies and percentages were reported for categorical variables with differences in characteristics between groups tested for by chi-square tests or the Fisher exact test. Means, standard deviations, medians, and interquartile ranges were reported for continuous variables with differences tested using the Wilcoxon test. Logistic regression models were used to assess associations between GT placement and neonatal morbidities and outcomes at followup. Models included one characteristic at a time as the primary independent variable with center, gestational age, SGA, BPD, NEC, and severe IVH/PVL as covariables. Odds ratios were estimated with statistical significance determined by Wald chi-square tests. All analyses were conducted in SAS version 9.4.
Data were also gathered for infants discharged on nasogastric or nasojejunal (NG/NJ) feedings. This information, however, was limited and only available from 2008-2011. The characteristics of infants discharged on NG/NJ versus GT tubes were obtained as well as the prevalence of those who were initially discharged with NG/NJ feeds but received a GT prior to follow-up.
Results:
A total of 4549 ELBW infants from 25 centers met the inclusion criteria for the study and were included in the analyses. Of these, 333 (7.3%) of the infants underwent GT placement. Among those with a GT, 77% had BPD (253/333), 29% (96/333) had a grade III or IV IVH or PVL, and 7% (22/333) had NEC (Table 1). Variables that were found to be significantly associated with GT placement were birth weight SGA, BPD, and IVH or PVL, and length of hospital stay after adjustment for center and neonatal morbidities. Demographic factors such as sex, race, and maternal education level were not significantly associated with GT placement. A diagnosis of NEC was not associated with GT placement in our cohort (Table 2). For the 187 infants with surgical NEC, GT placement was significantly more likely in infants with short bowel syndrome (45%, 22/49) than in those without short bowel syndrome (9%, 12/138; p<0.01).
Table 1.
Characteristics of patients with and without gastrostomy tube placement1
| Variables | Total N=4549 |
No GT N=4216 |
GT N=333 |
P-value |
|---|---|---|---|---|
| Baseline characteristics | ||||
| Gestational Age, mean (SD) | 25.73 (1.30) | 25.76 (1.30) | 25.43 (1.30) | <0.01 |
| Small for Gestational Age, n (%) | 315 (7) | 277 (7) | 38 (11) | <0.01 |
| Male, n (%) | 2245 (49) | 2083 (49) | 162 (49) | 0.86 |
| White, n (%) | 2390 (53) | 2203 (53) | 187 (58) | 0.13 |
| Hispanic, n (%) | 751 (17) | 700 (17) | 51 (16) | 0.54 |
| High School degree, n (%) | 2601 (76) | 2417 (76) | 184 (74) | 0.49 |
| Neonatal characteristics | ||||
| Length of hospital stay (days), mean (SD) | 112 (46) | 108 (40) | 169 (72) | <0.01 |
| NEC, n (%) | 250 (5) | 228 (5) | 22 (7) | 0.38 |
| BPD, n (%) | 2288 (51) | 2035 (49) | 253 (77) | <0.01 |
| Severe IVH (grade III or IV) or PVL, n (%) | 745 (16) | 649 (15) | 96 (29) | <0.01 |
| Weight Z-score at 36 weeks, mean (SD) | −1.41 (0.83) | −1.39 (0.83) | −1.62 (0.87) | <0.01 |
| Length Z-score at 36 weeks, mean (SD) | −1.94 (0.92) | −1.91 (0.92) | −2.26 (0.94) | <0.01 |
| Head circumference Z-score at 36 weeks, mean (SD) | −1.34 (1.03) | −1.31 (1.03) | −1.71 (1.01) | <0.01 |
| Outcomes at follow-up | ||||
| Weight Z-score at follow-up, mean (SD) | −1.02 (1.32) | −0.98 (1.30) | −1.59 (1.51) | <0.01 |
| Length Z-score at follow-up, mean (SD) | −0.67 (1.31) | −0.62 (1.31) | −1.32 (1.18) | <0.01 |
| Head circumference Z-score at follow-up, mean (SD) | −0.55 (1.62) | −0.49 (1.60) | −1.32 (1.69) | <0.01 |
| Follow-up weight < 10th %, n (%) | 1852 (41) | 1669 (40) | 183 (56) | <0.01 |
| Follow-up height <10th %, n (%) | 1255 (28) | 1087 (26) | 168 (51) | <0.01 |
| Follow-up Head circumference < 10th %, n (%) | 1216 (27) | 1063 (25) | 153 (47) | <0.01 |
| Weight gain velocity2 (g/mo), mean (SD) | 414.53 (177.09) | 416.24 (182.97) | 394.78 (81.39) | <0.01 |
| Length gain velocity2 (cm/mo), mean (SD) | 1.88 (0.74) | 1.88 (0.76) | 1.82 (0.24) | <0.01 |
| Head circumference gain velocity4 (cm/mo), mean (SD) | 0.77 (0.30) | 0.77 (0.31) | 0.76 (0.15) | 0.31 |
| NDI3, n (%) | 1420 (31) | 1217 (29) | 203 (61) | <0.01 |
| Moderate/severe cerebral palsy, n (%) | 271 (6) | 199 (5) | 72 (22) | <0.01 |
| BSID III Cognitive <70, n (%) | 424 (9) | 315 (8) | 109 (34) | <0.01 |
| BSID III Cognitive <85, n (%) | 1300 (29) | 1117 (27) | 183 (56) | <0.01 |
| BSID III Language <70, n (%) | 787 (18) | 646 (16) | 141 (44) | <0.01 |
| Chronic breathing problems, n (%) | 1318 (32) | 1139 (30) | 179 (55) | <0.01 |
| Chronic feeding problems, n (%) | 1281 (28) | 1085 (26) | 196 (59) | <0.01 |
n (%) and mean (SD) scores were calculated based on non-missing responses.
Velocity is calculated from 36 weeks to follow-up.
NDI was defined as any of the following: a cognitive composite score on the BSID III <85, moderate-to-severe CP, GMFCS level >2, severe hearing impairment, or bilateral severe visual impairment.
Table 2.
Associations between characteristics and GT placement
| Variables | Odds Ratio (95% CI)1 | P-Value |
|---|---|---|
| Gestational Age | 0.91 (0.82,1.00) | 0.06 |
| Small for Gestational Age | 1.92 (1.31,2.84) | <0.01 |
| Male | 0.89 (0.71,1.13) | 0.34 |
| White2 | 0.96 (0.75,1.24) | 0.77 |
| Hispanic | 0.74 (0.51,1.06) | 0.10 |
| Maternal education (High School degree)3 | 0.98 (0.71,1.34) | 0.88 |
| Length of hospital stay | 1.02 (1.02,1.02) | <0.01 |
| NEC | 1.13 (0.70,1.81) | 0.61 |
| BPD | 2.94 (2.20,3.92) | <0.01 |
| Severe IVH (grade III or IV) or PVL | 2.01 (1.54,2.64) | <0.01 |
| Weight Z-score at 36 weeks | 0.65 (0.55,0.76) | <0.01 |
| Length Z-score at 36 weeks | 0.67 (0.58,0.78) | <0.01 |
| Head circumference Z-score at 36 weeks | 0.72 (0.64,0.82) | <0.01 |
| Weight Z-score at follow-up | 0.75 (0.69,0.82) | <0.01 |
| Length Z-score at follow-up | 0.77 (0.70,0.84) | <0.01 |
| Head circumference Z-score at follow-up | 0.77 (0.71,0.83) | <0.01 |
| Follow-up weight < 10th % | 1.65 (1.30,2.10) | <0.01 |
| Follow-up height < 10th % | 2.64 (2.07,3.36) | <0.01 |
| Follow-up head circumference < 10th % | 2.02 (1.58,2.59) | <0.01 |
| Weight gain velocity | 1.00 (1.00,1.00) | 0.25 |
| Length gain velocity | 0.96 (0.87,1.05) | 0.36 |
| Head circumference gain velocity | 0.93 (0.71,1.21) | 0.57 |
| NDI | 3.19 (2.49,4.10) | <0.01 |
| Moderate/severe cerebral palsy | 4.60 (3.25,6.50) | <0.01 |
| BSID III Cognitive <70 | 4.93 (3.69,6.58) | <0.01 |
| BSID III Cognitive <85 | 2.97 (2.31,3.81) | <0.01 |
| BSID Language <70 | 3.53 (2.73,4.56) | <0.01 |
| Chronic breathing problems | 2.42 (1.89,3.10) | <0.01 |
| Chronic feeding problems | 4.06 (3.12,5.28) | <0.01 |
Odds ratios were estimated using logistic regression models. Models included one characteristic at a time as the primary independent variable with center, gestational age, small for gestational age status, physiological bronchopulmonary dysplasia, medically managed necrotizing enterocolitis, and severe intraventricular hemorrhage or periventricular leukomalacia as covariates. Statistical significance was determined by Wald chi-square tests.
Reference all “non-White” other than Hispanic
Reference those without high school degree
Most longer-term adverse outcomes were significantly more likely to occur in infants with GTs than in infants without GTs (Table 1). At follow-up, GT placement was associated with poorer growth, NDI, cerebral palsy, and chronic breathing and feeding problems.
Thirty-two percent (108/333) of the infants with a GT were taking full oral feeds at follow-up. Hispanic ethnicity, as well as all components of NDI, and breathing status at follow-up were independently associated with the ability to attain full oral feedings by 2 years. No specific neonatal morbidities were associated with a resolved need for GT supplementation at follow-up (Table 3).
Table 3.
Demographics of those with or without GT at follow-up.
| Variables | No tube feeding at FU N=108 |
Tube feeding at FU N=225 |
P-value |
|---|---|---|---|
| Small for Gestational Age | 12 (11) | 26 (12) | 0.99 |
| Male | 53 (49) | 109 (49) | 0.99 |
| White | 70 (67) | 117 (53) | 0.02 |
| Hispanic | 29 (27) | 22 (10) | <0.01 |
| High School degree | 57 (72) | 127 (75) | 0.64 |
| Length of hospital stay | 158.20 (62.83) | 174.68 (75.11) | 0.08 |
| NEC | 9 (8) | 13 (6) | 0.48 |
| BPD | 75 (71) | 178 (80) | 0.07 |
| Severe IVH (grade III or IV) or PVL | 31 (29) | 65 (29) | 0.99 |
| Weight Z-score at 36 weeks | −1.53 (0.86) | −1.66 (0.87) | 0.22 |
| Length Z-score at 36 weeks | −2.02 (0.88) | −2.38 (0.94) | 0.14 |
| Head circumference Z-score at 36 weeks | −1.58 (0.94) | −1.77 (1.04) | 0.38 |
| Weight Z-score at follow-up | −1.72 (1.42) | −1.53 (1.55) | 0.17 |
| Length Z-score at follow-up | −1.16 (1.11) | −1.40 (1.21) | 0.48 |
| Head circumference Z-score at follow-up | −1.18 (1.57) | −1.39 (1.74) | 0.03 |
| Follow-up weight <10th % | 60 (57) | 123 (55) | 0.81 |
| Follow-up height <10th % | 51 (48) | 117 (53) | 0.48 |
| Follow-up HC <10th % | 43 (41) | 110 (50) | 0.13 |
| Weight gain velocity (g/month) | 381.73 (70.75) | 401.04 (85.48) | 0.19 |
| Length gain velocity (cm/month) | 1.80 (0.23) | 1.84 (0.25) | 0.74 |
| Head circumference gain velocity (cm/month) | 0.74 (0.14) | 0.76 (0.15) | 0.11 |
| NDI | 47 (44) | 156 (69) | <0.01 |
| Moderate/severe cerebral palsy | 6 (6) | 66 (29) | <0.01 |
| BSID III Cognitive <70 | 16 (15) | 93 (43) | <0.01 |
| BSID III Cognitive <85 | 43 (40) | 140 (65) | <0.01 |
| BSID III Language <70 | 27 (25) | 114 (55) | <0.01 |
| Chronic breathing problems | 39 (37) | 140 (63) | <0.01 |
| Chronic feeding problems | 40 (37) | 156 (69) | <0.01 |
Seventy-six percent (252/333) of those who underwent GT placement did so after discharge from the NICU. Significant differences between patients who had GT placed before and after discharge were slower growth of head circumference and increased chronic breathing and feeding problems among infants who underwent GT placement after discharge. There was no significant difference in weight gain velocity after discharge between these two groups (Table 4).
Table 4.
Demographics of those who had GTs placed pre- and post-discharge
| Variables | GT Pre-discharge N=81 |
GT Post-discharge N=252 |
P-value |
|---|---|---|---|
| Small for Gestational Age | 9 (11) | 29 (12) | 0.99 |
| Male | 37 (46) | 125 (50) | 0.53 |
| White | 47 (59) | 140 (57) | 0.90 |
| Hispanic | 14 (18) | 37 (15) | 0.60 |
| High School degree | 45 (79) | 139 (73) | 0.39 |
| Length of hospital stay (days) | 163 (57) | 171 (76) | 0.99 |
| NEC | 6 (7) | 16 (6) | 0.80 |
| BPD | 58 (73) | 195 (78) | 0.29 |
| Severe IVH (grade III or IV) or PVL | 20 (25) | 76 (30) | 0.40 |
| Weight Z-score at 36 weeks | −1.59 (0.96) | −1.63 (0.84) | 0.39 |
| Length Z-score at 36 weeks | −2.20 (1.05) | −2.28 (0.90) | 0.89 |
| Head circumference Z-score at 36 weeks | −1.72 (1.10) | −1.70 (0.99) | 0.82 |
| Weight Z-score at follow-up | −1.49 (1.39) | −1.62 (1.54) | 0.98 |
| Length Z-score at follow-up | −1.24 (1.16) | −1.35 (1.19) | 0.30 |
| Head circumference Z-score at follow-up | −1.04 (1.59) | −1.42 (1.71) | 0.19 |
| Follow-up weight <10th % | 44 (55) | 139 (56) | 0.90 |
| Follow-up height <10th % | 36 (45) | 132 (53) | 0.25 |
| Follow-up HC <10th % | 33 (41) | 120 (49) | 0.30 |
| Weight gain velocity (g/month) | 401.78 (83.40) | 392.49 (80.77) | 0.38 |
| Length gain velocity (cm/month) | 1.87 (0.26) | 1.81 (0.23) | 0.76 |
| Head circumference gain velocity (cm/month) | 0.79 (0.15) | 0.75 (0.14) | 0.04 |
| NDI | 42 (52) | 161 (64) | 0.07 |
| Moderate/severe cerebral palsy | 12 (15) | 60 (24) | 0.09 |
| BSID III Cognitive <70 | 24 (30) | 85 (35) | 0.50 |
| BSID III Cognitive <85 | 39 (49) | 144 (59) | 0.12 |
| BSID III Language <70 | 32 (40) | 109 (46) | 0.37 |
| Chronic breathing problems | 32 (42) | 147 (59) | 0.01 |
| Chronic feeding problems | 37 (46) | 159 (63) | <0.01 |
Of the 2271 ELBW infants discharged between 2008-2011, 4% (93/2271) were discharged with NG or NJ feeds and 13/93 (14%) of these went on to receive GTs. Infants discharged on NG feeds were more likely to subsequently have a GT placed if they had a severe IVH or poor length and head growth at follow-up and less likely to have a GT placed if male. (Table 5; available at www.jpeds.com).
Table 5.
Characteristics of those discharged on NG with or without GTs placed post-discharge.* (Online only)
| Variables | Discharged on NG, no GT placed post-discharge N=18 |
Discharged on NG, GT placed post-discharge N=13 |
P-value |
|---|---|---|---|
| Small for Gestational Age | 2 (11) | 2 (15) | 0.99 |
| Male | 12 (67) | 3 (23) | 0.03 |
| White | 11 (61) | 8 (62) | 0.99 |
| Hispanic | 1 (6) | 2 (17) | 0.55 |
| High School degree | 14 (93) | 5 (63) | 0.10 |
| Length of hospital stay (days) | 107 (9) | 107 (10) | 0.36 |
| NEC | 2 (11) | 0 (0) | 0.50 |
| BPD | 16 (89) | 8 (62) | 0.10 |
| Severe IVH (grade III or IV) or PVL | 2 (11) | 6 (46) | 0.04 |
| Weight Z-score at 36 weeks | −0.92 (0.41) | −1.27 (0.66) | 0.10 |
| Length Z-score at 36 weeks | −1.53 (0.64) | −1.94 (0.87) | 0.10 |
| Head circumference Z-score at 36 weeks | −0.88 (0.68) | −1.35 (0.80) | 0.18 |
| Weight Z-score at follow-up | −0.47 (1.43) | −1.60 (1.55) | 0.10 |
| Length Z-score at follow-up | −0.17 (0.77) | −1.15 (0.77) | <0.01 |
| Head circumference Z-score at follow-up | −0.07 (1.77) | −0.51 (1.11) | 0.03 |
| Follow-up weight <10th % | 5 (28) | 7 (54) | 0.26 |
| Follow-up height <10th % | 1 (6) | 6 (46) | 0.01 |
| Follow-up head circumference <10th % | 3 (17) | 2 (17) | 0.99 |
| Weight gain velocity (g/month) | 448.44 (108.42) | 389.59 (83.20) | 0.36 |
| Length gain velocity (cm/month) | 1.93 (0.22) | 1.83 (0.16) | 0.10 |
| Head circumference gain velocity (cm/month) | 0.78 (0.08) | 0.78 (0.14) | 0.46 |
| NDI <70 | 4 (22) | 6 (46) | 0.25 |
| NDI <85 | 7 (39) | 6 (46) | 0.73 |
| Moderate/severe cerebral palsy | 2 (11) | 1 (8) | 0.99 |
| BSID III Cognitive <70 | 3 (17) | 2 (15) | 0.99 |
| BSID III Cognitive <85 | 6 (33) | 5 (38) | 0.99 |
| BSID III Language <70 | 5 (28) | 2 (15) | 0.67 |
| Chronic breathing problems | 12 (67) | 5 (38) | 0.16 |
| Chronic feeding problems | 9 (50) | 10 (77) | 0.16 |
Information regarding discharge on NG/NJ feeding was only available from January 2008 through June 2011.
A fundoplication procedure was performed simultaneously with GT placement in 26% of the infants. There was wide center variation for GT placement and fundoplication rates. The rate of GT placement varied from 3-14% by center and fundoplication rates ranged from 0-6.4% among the centers (Table 6; available at www.jpeds.com).
Table 6.
Rate of GT placement and fundoplication by center (Online only)
| Centers | Gastrostomy Tube Placement |
Fundoplication |
|---|---|---|
| A | 0/20 (0%) | 0/20 (0%) |
| B | 0/17 (0%) | 0/17 (0%) |
| C | 5/187 (3%) | 2/187 (1%) |
| D | 3/91 (3%) | 0/91 (0%) |
| E | 6/168 (4%) | 2/168 (1%) |
| F | 9/240 (4%) | 2/240 (1%) |
| G | 3/74 (4%) | 1/74 (1%) |
| H | 1/23 (4%) | 1/23 (4%) |
| I | 20/450 (4%) | 3/450 (1%) |
| J | 12/260 (5%) | 6/260 (2%) |
| K | 15/315 (5%) | 2/315 (1%) |
| L | 4/76 (5%) | 3/76 (4%) |
| M | 1/18 (6%) | 1/18 (6%) |
| N | 29/405 (7%) | 12/405 (3%) |
| O | 17/226 (8%) | 8/226 (4%) |
| P | 32/404 (8%) | 7/404 (2%) |
| Q | 19/232 (8%) | 0/232 (0%) |
| R | 10/119 (8%) | 4/119 (3%) |
| S | 9/96 (9%) | 3/96 (3%) |
| T | 34/342 (10%) | 22/342 (6%) |
| U | 22/201 (11%) | 6/201 (3%) |
| V | 26/231 (11%) | 9/231 (4%) |
| W | 5/37 (14%) | 1/37 (3%) |
| X | 50/312 (16%) | 2/312 (1%) |
| Y | 1/5 (20%) | 0/5 (0%) |
Discussion:
GT placement is common among ELBW infants, especially among those with co-existing morbidities. The NICHD NRN collected information in their Generic Database from 2006-2012 on major surgeries, including GT placement before and after NICU discharge. The database provides a unique opportunity to assess the frequency of use of supplemental GT feeding in extremely preterm infants from multiple academic tertiary centers and to examine associations between this feeding strategy and long-term growth, respiratory, feeding and developmental outcomes in extremely premature infants.
In our cohort, the majority of infants had their GTs placed after discharge (76%) suggesting that a large proportion of ELBW infants were first discharged from the NICU orally feeding but could not maintain these skills. Although there was no significant difference in weight gain velocity between the two groups, ELBW infants were more likely to have a GT placed after discharge if they had chronic respiratory or feeding problems. The high percentage of GT placement post-neonatal discharge indicates a need for close nutritional follow-up of ELBW infants, especially those needing respiratory support such as supplemental oxygen, bronchodilator or diuretic therapy, as well as those with a history of dysphagia or who have risks for aspiration. In addition, oral feeding rehabilitation strategies prior to and following GT placement may be relevant in achieving better overall outcomes, both short-and long-term. Physiological basis for safe oral feeding practices that include volume tolerance and airway safety are both fundamental to successful oral feeding and prevention of need for a GT.1,24 Further work is needed with standardization of oral feeding practices both in the NICU and post-discharge.
GT placement was most strongly associated with BPD followed by severe brain imaging abnormalities (IVH or PVL), then SGA. This is consistent with previous literature documenting the associations of severe BPD and feeding difficulties.9,11,12 The association of significant neonatal brain injury and poor feeding skills is also not unexpected given the oromotor coordination and skills necessary to develop successful oral feeding abilities.
Longer-term outcomes associated with GT placement were assessed at follow-up. At two years of age, GT placement was associated with poor growth, NDI and chronic respiratory and feeding problems. Although the reasons for failure to successfully transition to full oral feeds is poorly described in the literature, ongoing feeding difficulties recently have been described in ELBW infants from a 2006-2008 cohort, with 13% having dysfunctional feeding behaviors at 18-22 months corrected age. These feeding problems were defined as tube feedings, choking or coughing with oral feeds, a history of aspiration or difficulty swallowing.13 Preterm infants are also nearly twice as likely to have oromotor dysfunction and avoidant feeding behaviors at 3 and 12 months corrected age.3 Rommel described 700 infants and young children referred for evaluation and treatment of a severe feeding disorder and reported an overrepresentation of premature infants, especially those born at <34 weeks of gestation or with lower birth weights for gestational age.25
Early feeding problems in premature infants may have significant consequences for growth and development. Overall, children with feeding problems are at risk for nutritional deficiencies and poor growth, regardless GT placement, as well as poorer cognitive, motor, and language outcomes than children without feeding problems. Mizuno and Ueda demonstrated an association between neonatal feeding difficulties and developmental problems at 18-month follow up. In this study, the sensitivity and specificity of early feeding assessments were better predictors of neurodevelopmental outcomes than cranial ultrasound findings.26 Adams-Chapman et al showed an association between feeding difficulties and language delays in ELBW infants at 18-22 month follow-up.13 Behavioral and emotional problems have also been described in children with feeding problems.27,28 Parents often struggle to cope with feeding difficulties in premature infants, and feeding issues may be a primary concern for families after discharge.29,30 Because of this, an increasing number of premature infants are referred for feeding therapy for both infant skill development and parent support, often continuing through school age.31
Given the risk of complications associated with surgically placing a gastrostomy tube in an ELBW infant, as well as possibly increasing length of stay, a better understanding of the duration of “oral feeding failure” in an ELBW infant at NICU discharge will guide providers in making recommendations for discharge feeding plans.32 There is evidence suggesting that supplemental home nasogastric tube feeding may be a safe and effective means for treating oral feeding problems in premature infants, yet many centers do not consider this practice for discharge.14,15 In a Cochrane review comparing early discharge home with gavage feeds and health care support with later discharge home when full oral feeds have been established, it was concluded there were not enough quality trials to make a practice recommendation.16 However, several small studies suggest not only a reduced length of stay, but also a reduction in infection and improved breastfeeding in the home gavage group.15 Although our database did not capture information regarding home NG feeding at NICU discharge in its entirety, the available data showed that 14% of these patients eventually received a GT. This study may provide preliminary evidence to support a clinical trial of home NG vs GT feeding in otherwise stable premature infants with oral feeding problems, which could then identify optimal discharge feeding plans for ELBW infants.
Although our study provided new information about ELBW infants who undergo GT placement, there were several limitations. Primarily, were unable to determine the best method of feeding ELBW patients at discharge: prolonged hospitalization awaiting full oral feeding, NG tube or GT. Optimistically, many babies discharged home with NG supplementation did not require GT placement, but we could not ascertain if complications occurred at home, if these children were readmitted to the hospital or the time interval between discharge and GT placement. We had limited information on the infants discharged with NG feeds who did not progress to GT placement. The study was also limited by the changing criteria for age of followup during the study period (from 18-22 to 22-26 months adjusted age). Lastly, the method of ascertainment for GT placement after discharge depended on caregiver recall, which allows for potential bias, and it was not validated with post-discharge hospital records.
The high percentage of GT placement we observed post-neonatal discharge indicates the need for close nutritional follow-up of ELBW infants. Further studies of nutritional interventions, including standardization of oral infant feeding guidelines, requirements for and timing of GT placement, as well as safety and efficacy of home GT versus NG supplementation are needed to identify optimal discharge feeding plans for ELBW infants.
Acknowledgements
We are indebted to our medical and nursing colleagues and the infants and their parents who agreed to take part in this study. We are thankful for all the participating sites of the NICHD Neonatal Research Network (NRN).
Appendix
Additional members of the Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network
NRN Steering Committee Chair: Michael S. Caplan, MD, University of Chicago, Pritzker School of Medicine. Chicago, Illinois.
Alpert Medical School of Brown University and Women & Infants Hospital of Rhode Island (UG1 HD27904)-Abbott R. Laptook, MD; Martin Keszler, MD; Betty R. Vohr, MD; Angelita M. Hensman, MS RNC-NIC; Barbara Alksninis, PNP; Kristin M. Basso, BSN MaT; Robert Burke, MD; Melinda Caskey, MD; Katharine Johnson, MD; Mary Lenore Keszler, MD; Andrea M. Knoll; Theresa M. Leach, MEd CAES; Emilee Little, RN BSN; Elisabeth C. McGowan, MD; Elisa Vieira, RN BSN; Victoria E. Watson, MS CAS; Suzy Ventura. Providence, Rhode Island.
Case Western Reserve University, Rainbow Babies & Children’s Hospital (UG1 HD21364, M01 RR80) - Michele C. Walsh, MD MS; Anna Maria Hibbs, MD MSCE; Deanne E. Wilson-Costello, MD; Nancy S. Newman, BA RN; Allison H. Payne, MD MS; Bonnie S. Siner, RN; Monika Bhola, MD; Gulgun Yalcinkaya, MD. Cleveland, Ohio.
Children’s Mercy Hospital, University of Missouri Kansas City School of Medicine (UG1 HD68284) - William E. Truog, MD; Eugenia K. Pallotto, MD MSCE; Howard W. Kilbride MD; Cheri Gauldin, RN BS CCRC; Anne Holmes RN MSN MBA-HCM CCRC; Kathy Johnson RN, CCRC; Allison Scott, RNC-NIC BSN CCRC. Kansas City, Missouri.
Cincinnati Children’s Hospital Medical Center, University Hospital, and Good Samaritan Hospital (UG1 HD27853, M01 RR8084) - Kurt Schibler, MD; Edward F. Donovan, MD; Cathy Grisby, BSN CCRC; Kate Bridges, MD; Barbara Alexander, RN; Estelle E. Fischer, MHSA MBA; Holly L. Mincey, RN BSN; Jody Hessling, RN; Teresa L. Gratton, PA; Lenora Jackson, CRC; Kristin Kirker, CRC; Greg Muthig, BS; Jean J. Steichen, MD; Stacey Tepe, BS; Kimberly Yolton, PhD. Cincinnati, Ohio.
Duke University School of Medicine, University Hospital, University of North Carolina, and Duke Regional Hospital (UG1 HD40492, UL1 TR1117, M01 RR30, UL1 TR1111) -C Patricia L. Ashley, MD PhD; Kathy J. Auten, MSHS; Kimberley A. Fisher, PhD FNP-BC IBCLC; Sandra Grimes, RN BSN; Kathryn E. Gustafson, PhD; Melody B. Lohmeyer, RN MSN; Joanne Finkle, RN JD; Matthew M. Laughon, MD MPH; Carl L. Bose, MD; Janice Bernhardt, MS RN; Gennie Bose, RN; Diane Warner, MD MPH; Janice Wereszczak, NNP. Durham, North Carolina.
Emory University, Children’s Healthcare of Atlanta, Grady Memorial Hospital, and Emory University Hospital Midtown (UG1 HD27851, M01 RR39) - David P. Carlton, MD; Barbara J. Stoll, MD; Ellen C. Hale, RN BS CCRC; Yvonne Loggins, RN BSN; Ann Blackwelder, RN MN; Lynn C. Wineski, RN MS; Maureen Mulligan LaRossa, RN; Sheena L. Carter, PhD. Atlanta, Georgia.
Eunice Kennedy Shriver National Institute of Child Health and Human Development - Rosemary D. Higgins, MD; Stephanie Wilson Archer, MA.
Indiana University, University Hospital, Methodist Hospital, Riley Hospital for Children, and Wishard Health Services (UG1 HD27856, M01 RR750) - Gregory M. Sokol, MD; Brenda B. Poindexter, MD MS; Anna M. Dusick, MD (deceased); Lu-Ann Papile, MD; Susan Gunn, NNP CCRC; Faithe Hamer, BS; Heidi M. Harmon, MD; Dianne E. Herron, RN CCRC; Abbey C. Hines, PsyD; Carolyn Lytle, MD MPH; Heike M. Minnich, PsyD HSPP; Lucy Smiley CCRC; Leslie Dawn Wilson, BSN CCRC. Indianapolis, Indiana.
McGovern Medical School at The University of Texas Health Science Center at Houston, Children’s Memorial Hermann Hospital, and Lyndon Baines Johnson General Hospital/Harris County Hospital District (UG1 HD87229, U10 HD21373) - Kathleen A. Kennedy, MD MPH; Jon E. Tyson, MD MPH; Georgia E. McDavid, RN; Nora I. Alaniz, BS; Julie Arldt-McAlister, RN BSN; Katrina Burson, RN BSN; Allison G. Dempsey, PhD; Andrea F. Duncan, MD; Patricia W. Evans, MD; Charles E. Green, PhD; Beverly Foley Harris, RN BSN; Margarita Jiminez, MD MPH; Janice John, CPNP; Patrick M. Jones, MD; Layne M. Lillie, RN BSN; Anna E. Lis, RN BSN; Sara C. Martin, RN BSN; Brenda H. Morris, MD; M. Layne Poundstone, RN BSN; Shawna Rodgers, RN BSN; Saba Siddiki, MD; Maegan C. Simmons, RN; Daniel Sperry, RN; Patti L. Pierce Tate, RCP; Sharon L. Wright, MT(ASCP). Houston, Texas.
Nationwide Children’s Hospital and the Ohio State University Medical Center (UG1 HD68278) - Pablo J. Sanchez, MD; Leif D. Nelin, MD; Patricia Luzader, RN; Christine A. Fortney, PhD RN; Gail E. Besner, MD; Nehal A. Parikh, MD. Columbus, Ohio.
RTI International (U10 HD36790) -Dennis Wallace, PhD; Marie G. Gantz, PhD; W. Kenneth Poole, PhD (deceased); Jamie E. Newman, PhD MPH; Jeanette O’Donnell Auman, BS; Margaret M. Crawford, BS; Jenna Gabrio, MPH; Carolyn M. Petrie Huitema, MS; Kristin M. Zaterka-Baxter, RN BSN. Rockville, Maryland.
Stanford University, Dominican Hospital, El Camino Hospital, and Lucile Packard Children’s Hospital (UG1 HD27880, M01 RR70) - Krisa P. Van Meurs, MD; David K. Stevenson, MD; Susan R. Hintz, MD MS Epi; M. Bethany Ball, BS CCRC; Alexis S. Davis, MD MS Epi; Andrew W. Palmquist, RN; Melinda S. Proud, RCP; Barbara Bentley, PsychD MSEd; Elizabeth Bruno, PhD; Maria Elena DeAnda, PhD; Anne M. DeBattista, RN PNP; Beth Earhart, PhD; Lynne C. Huffman, MD; Jean G. Kohn, MD MPH; Casey Krueger, PhD; Hali E. Weiss, MD. Mountain View, California. Tufts Medical Center, Floating Hospital for Children (U10 HD53119, M01 RR54) - Ivan D. Frantz III, MD; John M. Fiascone, MD; Brenda L. MacKinnon, RNC; Anne Furey, MPH; Ellen Nylen, RN BSN; Elisabeth C. McGowan, MD; Cecelia E. Sibley, PT MHA; Ana K. Brussa, MS OTR/L. Boston, Massachusetts.
University of Alabama at Birmingham Health System and Children’s Hospital of Alabama (UG1 HD34216, M01 RR32) - Waldemar A. Carlo, MD; Namasivayam Ambalavanan, MD; Myriam Peralta-Carcelen, MD MPH; Monica V. Collins, RN BSN MaEd; Shirley S. Cosby, RN BSN; Fred J. Biasini, PhD; Kristen C. Johnston, MSN CRNP; Kathleen G. Nelson, MD; Cryshelle S. Patterson, PhD; Vivien A. Phillips, RN BSN; Sally Whitley, MA OTR-L FAOTA. Birmingham, Alabama.
University of California - Los Angeles, Mattel Children’s Hospital, Santa Monica Hospital, Los Robles Hospital and Medical Center, and Olive View Medical Center (UG1 HD68270) - Uday Devaskar, MD; Meena Garg, MD; Isabell B. Purdy, PhD CPNP; Teresa Chanlaw, MPH; Rachel Geller, RN BSN. Los Angeles, California.
University of California - San Diego Medical Center and Sharp Mary Birch Hospital for Women and Newborns (U10 HD40461) - Neil N. Finer, MD; Yvonne E. Vaucher, MD MPH; David Kaegi, MD; Maynard R. Rasmussen, MD; Kathy Arnell, RNC; Clarence Demetrio, RN; Martha G. Fuller, PhD RN MSN; Wade Rich, BSHS RRT. San Diego, California.
University of Iowa and Mercy Medical Center (UG1 HD53109, M01 RR59) - Tarah T. Colaizy, MD MPH; Michael J. Acarregui, MD; Jane E. Brumbaugh, MD; Dan L. Ellsbury, MD; John A. Widness, MD; Karen J. Johnson, RN BSN; Donia B. Campbell, RNC-NIC; Diane L. Eastman, RN CPNP MA; Jacky R. Walker, RN. Cedar Rapids, Iowa.
University of Miami, Holtz Children’s Hospital (U10 HD21397, M01 RR16587) - Shahnaz Duara, MD; Charles R. Bauer, MD; Ruth Everett-Thomas, RN MSN; Sylvia Fajardo-Hiriart, MD; Arielle Rigaud, MD; Maria Calejo, MS; Silvia M. Frade Eguaras, MA; Michelle Harwood Berkowits, PhD; Andrea Garcia, MS; Helina Pierre, BA; Alexandra Stoerger, BA. Miami, Florida.
University of New Mexico Health Sciences Center (UG1 HD53089, M01 RR997)-Kristi L. Watterberg, MD; Jean R. Lowe, PhD; Janell F. Fuller, MD; Robin K. Ohls, MD; Conra Backstrom Lacy, RN; Andrea F. Duncan, MD MScr; Rebecca Montman, BSN; Sandra Sundquist Beauman, MSN RNC-NIC. Albuquerque, New Mexico.
University of Pennsylvania, Hospital of the University of Pennsylvania, Pennsylvania Hospital, and Children’s Hospital of Philadelphia (UG1 HD68244) – Barbara Schmidt, MD MSc; Haresh Kirpalani, MB MSc; Sara B. DeMauro, MD MSCE; Aasma S. Chaudhary, BS RRT; Soraya Abbasi, MD; Toni Mancini, RN BSN CCRC; Dara M. Cucinotta, RN; Judy C. Bernbaum, MD; Marsha Gerdes, PhD; Hallam Hurt, MD. Philadelphia, Pennsylvania.
University of Rochester Medical Center, Golisano Children’s Hospital, and the University at Buffalo Women’s and Children’s Hospital of Buffalo (UG1 HD68263, U10 HD40521, M01 RR44, UL1 TR42) – Carl T. D’Angio, MD; Dale L. Phelps, MD; Ronnie Guillet, MD PhD; Satyan Lakshminrusimha, MD; Julie Babish Johnson, MSW; Linda J. Reubens, RN CCRC; Cassandra A. Horihan, MS; Diane Hust, MS RN CS; Rosemary L. Jensen; Emily Kushner, MA; Joan Merzbach, LMSW; Gary J. Myers, MD; Holly I.M. Wadkins, MA; Stephanie Guilford, BS; Deanna Maffett, RN; Osman Farooq, MD; Diane Prinzing; Anne Marie Reynolds, MD MPH; Mary Rowan, RN; Michael G. Sacilowski, MAT CCRC; Ashley Williams, MSEd; Karen Wynn, RN; Kelley Yost, PhD; William Zorn, PhD; Lauren Zwetsch, RN MS PNP. Rochester, New York.
University of Texas Southwestern Medical Center at Dallas, Parkland Health & Hospital System, and Children’s Medical Center Dallas (UG1 HD40689, M01 RR633) – Myra H. Wyckoff, MD; Pablo J. Sanchez, MD; Luc P. Brion, MD; Roy J. Heyne, MD; Walid A. Salhab, MD; Charles R. Rosenfeld, MD; Diana M. Vasil, MSN BSN RNC-NIC; Lijun Chen, PhD RN; Alicia Guzman; Gaynelle Hensley, RN; Melissa H. Leps, RN; Nancy A. Miller, RN; Janet S. Morgan, RN; Sally S. Adams, MS RN CPNP; Catherine Twell Boatman, MS CIMI; Elizabeth T. Heyne, MS MA PA-C PsyD; Linda A. Madden, RN CPNP; Lizette E. Lee, RN. Dallas, Texas.
University of Utah Medical Center, Intermountain Medical Center, LDS Hospital, and Primary Children’s Medical Center (UG1 HD87226, U10 HD53124, M01 RR64, UL1 RR25764) – Roger G. Faix, MD; Bradley A. Yoder, MD; Karen A. Osborne, RN BSN CCRC; Cynthia Spencer, RNC BSN; Kimberlee Weaver-Lewis, RN MS; Shawna Baker, RN; Karie Bird, RN BSN; Jill Burnett, RNC BSN; Michael Steffen, MS CPM; Jennifer J. Jensen, RN BSN; Sarah Winter, MD; Karen Zanetti, RN. Salt Lake City, Utah.
Wake Forest University, Baptist Medical Center, Forsyth Medical Center, and Brenner Children’s Hospital (U10 HD40498, M01 RR7122) – T. Michael O’Shea, MD MPH; Robert G. Dillard, MD; Lisa K. Washburn, MD; Barbara G. Jackson, RN, BSN; Nancy Peters, RN; Korinne Chiu, MA; Deborah Evans Allred, MA LPA; Donald J. Goldstein, PhD; Raquel Halfond, MA; Carroll Peterson, MA; Ellen L. Waldrep, MS; Cherrie D. Welch, MD MPH; Melissa Whalen Morris, MA; Gail Wiley Hounshell, PhD. Winston Salem, North Carolina.
Wayne State University, Hutzel Women’s Hospital and Children’s Hospital of Michigan (UG1 HD21385) – Seetha Shankaran, MD; Athina Pappas, MD; John Barks, MD; Rebecca Bara, RN BSN; Laura A. Goldston, MA; Girija Natarajan, MD; Monika Bajaj, MD; Sanjay Chawla, MD; Lilia C. De Jesus, MD; Beena G. Sood, MD; Mary Christensen, RT; Stephanie A. Wiggins, MS; Diane White RT. Detroit, Michigan.
Yale University, Yale-New Haven Children’s Hospital, and Bridgeport Hospital (U10 HD27871, ULTR142) – Richard A. Ehrenkranz, MD; Harris Jacobs, MD; Christine G. Butler, MD; Patricia Cervone, RN; Sheila Greisman, RN; Monica Konstantino, RN BSN; JoAnn Poulsen, RN; Janet Taft, RN BSN; Joanne Williams, RN BSN; Elaine Romano, MSN. New Haven, Connecticut.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The National Institutes of Health, the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), the National Center for Research Resources, and the National Center for Advancing Translational Sciences provided grant support for the Neonatal Research Network’s Generic Database and Follow-up Studies through cooperative agreements. While NICHD staff had input into the study design, conduct, analysis, and manuscript drafting, the comments and views of the authors do not necessarily represent the views of NICHD, the National Institutes of Health, the Department of Health and Human Services, or the U.S. Government. Data collected at participating sites of the NICHD Neonatal Research Network (NRN) were transmitted to RTI International, the data coordinating center (DCC) for the network, which stored, managed and analyzed the data for this study. On behalf of the NRN, A.D. (DCC Principal Investigator) and D.K. (DCC Statistician) had full access to all the data in the study and take responsibility for the integrity of the data and accuracy of the data analysis. The authors declare no conflicts of interest.
List of additional members of the Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network is available at www.jpeds.com (Appendix)
Data Sharing
Data reported in this paper may be requested through a data use agreement. Further details are available at https://neonatal.rti.org/index.cfm?fuseaction=DataRequest.Home.
References:
- 1.).Jadcherla SR, Gupta A, Stoner E, Fernandez S, Shaker R. Pharyngeal swallowing: defining pharyngeal and upper esophageal sphincter relationships in human neonates. The Journal of Pediatrics. 2007. December 1;151:597–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.).Jadcherla SR, Dail J, Malkar MB, McClead R, Kelleher K, Nelin L. Impact of process optimization and quality improvement measures on neonatal feeding outcomes at an all-referral neonatal intensive care unit. Journal of Parenteral and Enteral Nutrition. 2016. July;40:646–55. [DOI] [PubMed] [Google Scholar]
- 3.).Mizuno K, Ueda A. The maturation and coordination of sucking, swallowing, and respiration in preterm infants. The Journal of Pediatrics. 2003. January 1;142:36–40. [DOI] [PubMed] [Google Scholar]
- 4.).DeMauro SB, Patel PR, Medoff-Cooper B, Posencheg M, Abbasi S. Postdischarge feeding patterns in early-and late-preterm infants. Clinical Pediatrics. 2011. October;50:957–62. [DOI] [PubMed] [Google Scholar]
- 5.).Dodrill P, McMahon S, Ward E, Weir K, Donovan T, Riddle B. Long-term oral sensitivity and feeding skills of low-risk pre-term infants. Early human development. 2004. January 1;76:23–37. [DOI] [PubMed] [Google Scholar]
- 6.).Jadcherla SR, Wang M, Vijayapal AS, Leuthner SR. Impact of prematurity and comorbidities on feeding milestones in neonates: a retrospective study. Journal of Perinatology. 2010. March;30:201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.).Lessen BS. Effect of the premature infant oral motor intervention on feeding progression and length of stay in preterm infants. Advances in Neonatal Care. 2011. April 1;11:129–39. [DOI] [PubMed] [Google Scholar]
- 8.).Jadcherla SR, Khot T, Moore R, Malkar M, Gulati IK, Slaughter JL. Feeding methods at discharge predict long-term feeding and neurodevelopmental outcomes in preterm infants referred for gastrostomy evaluation. The Journal of Pediatrics. 2017. February 1;181:125–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.).Jadcherla S Dysphagia in the high-risk infant: potential factors and mechanisms–. The American Journal of Clinical Nutrition. 2016. January 20;103:622S–8S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.).Jadcherla SR, Peng J, Moore R, Saavedra J, Shepherd E, Fernandez S, Erdman SH, DiLorenzo C. Impact of personalized feeding program in 100 NICU infants: pathophysiology-based approach for better outcomes. Journal of Pediatric Gastroenterology and Nutrition. 2012. January;54:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.).Mizuno K, Nishida Y, Taki M, Hibino S, Murase M, Sakurai M, Itabashi K. Infants with bronchopulmonary dysplasia suckle with weak pressures to maintain breathing during feeding. Pediatrics. 2007. October;120:e1035–42. [DOI] [PubMed] [Google Scholar]
- 12.).Gewolb IH, Vice FL. Abnormalities in the coordination of respiration and swallow in preterm infants with bronchopulmonary dysplasia. Developmental Medicine and Child Neurology. 2006. July;48:595–9. [DOI] [PubMed] [Google Scholar]
- 13.).Adams-Chapman I, Bann CM, Vaucher YE, Stoll BJ. Association between Feeding Difficulties and Language Delay in Preterm Infants Using Bayley Scales of Infant Development. The Journal of Pediatrics. 2013;3:680–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.).Rosen D, Schneider R, Bao R, Burke P, Ceballos C, Hoffstadter-Thal K, Benkov K. Home nasogastric feeds: feeding status and growth outcomes in a pediatric population. Journal of Parenteral and Enteral Nutrition. 2016. March;40:350–4. [DOI] [PubMed] [Google Scholar]
- 15.).Meerlo-Habing ZE, Kosters-Boes EA, Klip H, Brand PL. Early discharge with tube feeding at home for preterm infants is associated with longer duration of breast feeding. Archives of Disease in Childhood-Fetal and Neonatal Edition. 2009. July 1;94:F294–7. [DOI] [PubMed] [Google Scholar]
- 16.).Collins CT, Makrides M, McPhee AJ. Early discharge with home support of gavage feeding for stable preterm infants who have not established full oral feeds. Cochrane Database of Systematic Reviews. 2003. [DOI] [PubMed] [Google Scholar]
- 17.).Hatch LD, Scott TA, Walsh WF, Goldin AB, Blakely ML, Patrick SW. National and regional trends in gastrostomy in very low birth weight infants in the USA: 2000–2012. Journal of Perinatology. 2018. September;38:1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.).Olsen IE, Groveman SA, Lawson ML, Clark RH, Zemel BS. New intrauterine growth curves based on United States data. Pediatrics. 2010. February;125:e214–24. [DOI] [PubMed] [Google Scholar]
- 19.).Walsh MC, Yao Q, Gettner P, Hale E, Collins M, Hensman A, Everette R, Peters N, Miller N, Muran G, Auten K. Impact of a physiologic definition on bronchopulmonary dysplasia rates. Pediatrics. 2004. November;114:1305. [DOI] [PubMed] [Google Scholar]
- 20.).Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. The Journal of Pediatrics. 1978. April 1;92:529–34. [DOI] [PubMed] [Google Scholar]
- 21.).Bell MJ, Ternberg JL, Feigin RD, Keating JP, Marshall RI, Barton LE, Brotherton TH. Neonatal necrotizing enterocolitis. Therapeutic decisions based upon clinical staging. Annals of surgery. 1978. January;187:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.).Walsh MC, Kliegman RM. Necrotizing enterocolitis: treatment based on staging criteria. Pediatric Clinics of North America. 1986. February 1;33:179–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.).Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, Flegal KM, Guo SS, Wei R, Mei Z, Curtin LR, Roche AF, Johnson CL. CDC growth charts: United States. Advance data. 2000. June:1–27. [PubMed] [Google Scholar]
- 24.).Kamitsuka MD, Nervik PA, Nielsen SL, Clark RH. Incidence of Nasogastric and Gastrostomy Tube at Discharge Is Reduced after Implementing an Oral Feeding Protocol in Premature (< 30 weeks) Infants. American Journal of Perinatology. 2017. May;34:606. [DOI] [PubMed] [Google Scholar]
- 25.).Rommel N, De AM, Feenstra L, Veereman-Wauters G. The complexity of feeding problems in 700 infants and young children presenting to a tertiary care institution. Journal of Pediatric Gastroenterology and Nutrition. 2003. July;37:75–84. [DOI] [PubMed] [Google Scholar]
- 26.).Mizuno K, Ueda A. Neonatal feeding performance as a predictor of neurodevelopmental outcome at 18 months. Developmental Medicine & Child Neurology. 2005;47:299–304. [DOI] [PubMed] [Google Scholar]
- 27.).Dahl M, Sundelin C. Early feeding problems in an affluent society: I. Categories and clinical signs. Acta Paediatrica. 1986. May;75:370–9. [DOI] [PubMed] [Google Scholar]
- 28.).Esparo G, Canals J, Jane C, Ballespi S, Vinas F, Domenech E. Feeding problems in nursery children: prevalence and psychosocial factors. Acta paediatrica. 2004. May;93:663–8. [PubMed] [Google Scholar]
- 29.).Kavanaugh K, Mead L, Meier P, Mangurten HH. Getting enough: mothers’ concerns about breastfeeding a preterm infant after discharge. Journal of Obstetric, Gynecologic, & Neonatal Nursing. 1995. January;24:23–32. [DOI] [PubMed] [Google Scholar]
- 30.).Pridham K, Saxe R, Limbo R. Feeding issues for mothers of very low-birth-weight, premature infants through the first year. The Journal of Perinatal & Neonatal Nursing. 2004. April 1;18:161–9. [DOI] [PubMed] [Google Scholar]
- 31.).Field D, Garland M, Williams K. Correlates of specific childhood feeding problems. Journal of Paediatrics and Child Health. 2003. May;39:299–304. [DOI] [PubMed] [Google Scholar]
- 32.).Landisch RM, Colwell RC, Densmore JC. Infant gastrostomy outcomes: The cost of complications. Journal of Pediatric Surgery. 2016. December 1;51:1976–82. [DOI] [PubMed] [Google Scholar]