Abstract
Introduction
The pathophysiology behind the subacute but persistent hypercoagulable state following traumatic brain injury (TBI) is poorly understood but contributes to morbidity induced by venous thromboembolism (VTE). Because platelets and their microvesicles have been hypothesized to play a role in posttraumatic hypercoagulability, administration of commonly utilized agents may ameliorate this coagulability. We hypothesized that utilization of aspirin, ketorolac, amitriptyline, unfractionated heparin, or enoxaparin would modulate the platelet aggregation response following TBI.
Methods
Concussive TBI was induced by weight drop. Mice were then randomized to receive aspirin, ketorolac, amitriptyline, heparin, enoxaparin, or saline control at 2 and 8 hours post-TBI. Mice were sacrificed at 6 or 24 hours after injury to determine coagulability by thromboelastometry (ROTEM), platelet function testing with impedance aggregometry, and microvesicle enumeration. Platelet sphingolipid metabolites were analyzed by mass spectrometry.
Results
ROTEM demonstrated increased platelet contribution to maximum clot firmness (%MCF-Platelet) at 6 hours following TBI in mice that received aspirin or amitriptyline, but this did not persist at 24 hours. By contrast, ADP- and arachidonic acid-induced platelet aggregation at 6 hours was significantly lower in mice receiving ketorolac, aspirin, and amitriptyline compared to mice receiving saline at 6 hours post-injury and only arachidonic acid initiated platelet aggregation was decreased by aspirin at 24 hours. There were no differences in microvesicle production at either timepoint. Platelet sphingosine-1-phosphate (S1P) levels were decreased at 6 hours in the group receiving amitriptyline and increased at 24 hours along with platelet ceramide levels at 24 hours in the amitriptyline group.
Conclusion
Following TBI, amitriptyline decreased platelet aggregability and increased contribution to clot in a manner similar to aspirin. The amitriptyline effects on platelet function and sphingolipid metabolites may represent a possible role of acid sphingomyelinase in the hypercoagulability observed following injury. Additionally, inhibition of platelet reactivity may be an underappreciated benefit of low molecular weight heparins, such as enoxaparin.
Keywords: trauma, traumatic brain injury, venous thromboembolism, chemoprophylaxis, sphingolipids
Introduction
Traumatic or unintentional injury is a common cause of morbidity and mortality in the United States. The leading causes of death in trauma patients, who survive the first day of hospitalization, include central nervous system injury, multi-system organ failure and venous thromboembolism (VTE)(1). In 2010, traumatic brain injury (TBI) was associated with 17 deaths per 100,000 people in the United States (2). VTE has been shown to occur in up to 50% of trauma patients (3–5). Isolated TBI is associated with up to a 3.5 fold increase in risk of venous thromboembolism and this hypercoagulable state can persist for days after injury (6, 7).
VTE prophylaxis with unfractionated heparin (UFH) and enoxaparin has been shown to be safe and effective in patients with TBI (8–10). Further studies have demonstrated enoxaparin as superior to UFH in prevention of deep venous thrombosis (DVT) in this population, but the time to initiate chemoprophylaxis after TBI remains controversial (11). However, enoxaparin is similar in rates of pulmonary embolism (PE) and mortality(3, 12). There is a paucity of data evaluating other chemoprophylaxis agents in the prevention of VTE, as other agents such as those modulating sphingolipid metabolism have been shown to play a role in platelet aggregability (13).
The aim of this study was to evaluate the effect of commonly utilized agents for inflammation, VTE chemoprophylaxis, and sphingolipid modulation on this subacute and persistent hypercoagulable state. With sphingolipid metabolism potentially playing a role in platelet function, this study was also designed to investigate the effects of these drugs on intraplatelet ceramide and other sphingolipid metabolites. We hypothesized that utilization of aspirin, ketorolac, amitriptyline, unfractionated heparin, and enoxaparin would modulate the platelet aggregation and whole blood coagulation response following traumatic brain injury.
METHODS
Animal Model
All murine experiments were approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Cincinnati. Male, 8–10 week old C57BL6/J mice were obtained from Jackson Laboratory (Bar Harbor, Maine). They were allowed food and water ad lib and acclimated in a controlled housing environment for at least one week prior to experimentation.
Traumatic Brain Injury Model
A previously described closed weight drop model was used to induce a global, concussive TBI of moderate severity (14–16). Briefly, mice were anesthetized with 2% inhaled isoflurane in 100% oxygen at 1 L/min for 2 minutes, then placed prone on the model platform with the head centered beneath the weight. A 400-gram weight was dropped from 2.5 cm above the surface of the table to induce head injury. The mice were observed to confirm appropriate recovery following injury.
Drug Treatment Groups
Following injury, mice were randomized into drug treatment groups to receive aspirin (100 mg/kg)(17), ketorolac (5 mg/kg) (18), amitriptyline (10 mg/kg) (19), heparin (75 IU/kg) (20), enoxaparin (3 mg/kg) (21), or saline control (100μL) by intraperitoneal injection at 2 and 8 hours post-TBI. Mice were then sacrificed at 6 or 24 hours after injury and blood was drawn via cardiac puncture.
Following initial studies that demonstrated unanticipated platelet response to treatment, increasing doses of enoxaparin (1, 3, and 8 mg/kg) and amitriptyline (0.1, 1, and 1 mg/kg) were administered to evaluate dose response. These drugs were also given in twice daily dosing for a total of three doses to evaluate their effect at steady state.
Rotational Thromboelastometry: EXTEM, FIBTEM
The ROTEM delta whole blood analyzer (Instrumentation Laboratory, Munich, Germany) was utilized to perform viscoelastic testing per manufacturer’s instructions. Three hundred microliters of whole blood were aliquoted and the test was initiated with 20 μL of each respective test reagent. EXTEM and FIBTEM testing were initiated with 20 μL of thromboplastin. FIBTEM testing was performed with the addition of cytochalasin D for platelet inhibition. The temperature was maintained at 37°C. Samples were allowed to run until 60 minutes after maximal clot firmness (MCF) was reached. This allowed for assessment of clotting time (CT), clot formation time (CFT), alpha angle and clot lysis (LI30). The platelet contribution to clot strength (%MCFPlatelet = [MCFEXTEM - MCFFIBTEM]/MCFEXTEM) was calculated as previously described by Midura et al. (22).
Platelet Aggregation
A multiplate impedance aggregometer (Roche Diagnostics, Risch-Rotkreuz, Switzerland) was utilized to measure platelet aggregation. Adenosine diphosphate (ADP) and arachidonic acid (ASPi) were used as agonists. The tests were initiated by incubating 300 μL of whole blood with 300 μL of 0.9% sodium chloride for 3 minutes. After incubation, 20 μL of 6.5 μmol/L ADP or 0.5 mmol/L ASPi were added. Total platelet aggregation (AU), platelet aggregation velocity and area under the curve (AUC) were measured.
Microvesicle Isolation and Quantification
Microvesicles were isolated and quantified as previously described (22, 23). Briefly, whole blood collected via cardiac puncture was anticoagulated with heparin and then centrifuged at 450g for 10 min. The supernatant was collected and centrifuged at 10,000g for an additional 10 minutes. This platelet-free supernatant was then diluted with Roswell Park Memorial Institute media and stained with 10 μL per sample CD41 antibody (BD Diagnostic Systems, Sparks, MD). Nanoparticle Tracking Analysis (NanoSight; Malvern Instruments Ltd, Worcestershire, United Kingdom) was then used to quantify total and CD41+ MP concentrations.
Acid Sphingomyelinase (Asm) Activity
Platelets were isolated by adding whole blood to 10% citrate and spinning at 260g for 5 minutes. The platelet rich plasma was collected and subsequently spun again at 640g for 5 minutes to create the platelet pellet which was then stored at −80°C. Platelet pellets were then lysed in 250 mM sodium acetate (pH 5.0) and 1% NP40 for 5 min. Platelets were then homogenized by two rounds of sonication for 10 sec each with a tip sonicator. Aliquots of the lysates were diluted to 250 mM sodium acetate (pH 5.0) and 0.1% and incubated with 50 nCi per sample [14C]sphingomyelin for 60 min at 37°C. The reaction was stopped by the addition of 800 μL chloroform/methanol (2:1, v/v), phases were separated and radioactivity was determined by using liquid scintillation in an aliquot of the aqueous phase to determine the release of [14C]phosphorylcholine from [14C]sphingomyelin as a measure of Asm activity.
Sphingolipid quantification
Platelets were isolated as noted above and subjected to lipid extraction using 1.5 mL methanol/chloroform (2:1, v:v) as previously described (24). The extraction solvent contained d7sphingosine (d7-Sph), d7--sphingosine-1-phosphate (d7-S1P), C17-lysosphingomyelin (C17-LysoSM), C17-ceramide (C17-Cer) and C16-d31-sphingomyelin (C16-d31-SM) (all Avanti Polar Lipids, Alabaster, USA) as internal standards. Sample analysis was carried out by liquid chromatography tandem-mass spectrometry using either a TQ 6490 mass spectrometer for sphingosine (Sph), S1P, and lysosphingomyelin (LysoSM), or a QTOF 6530 mass spectrometer for ceramide and sphingomyelin species (both Agilent Technologies, Waldbronn, Germany) operating in the positive electrospray ionization mode. The following selected reaction monitoring transitions were used for quantification: m/z 300.3 → 282.3 for Sph, m/z 380.3 → 264.3 for S1P, m/z 465.4 → 184.1 for LysoSM, m/z 307.3 → 289.3 for d7-Sph, m/z 387.3 → 271.3 for d7-S1P and m/z 451.3 → 184.1 for C17-LysoSM. The precursor ions of ceramide or sphingomyelin species (differing in their fatty acid chain lengths) were cleaved into the fragment ions m/z 264.270 or m/z 184.074, respectively (25). Quantification was performed with Mass Hunter Software (Agilent Technologies).
Statistical Analysis
All data are presented as the mean ± standard deviation or median (interquartile range). Student’s t-test was used to compare continuous variables between two groups. The Kruskal-Wallis test was used to compare continuous nonparametric data between groups of three or more. A p value of less than 0.05 was considered significant. Prism 6 (GraphPad Software, La Jolla, California) was used for all statistical analyses.
RESULTS
Increased platelet contribution to clot with aspirin and amitriptyline
Thromboelastometry results demonstrated that the platelet contribution to maximum clot firmness (%MCFPlatelet) at 6 hours was significantly higher in mice that received aspirin (69%, p<0.002) or amitriptyline (68%, p<0.007) compared to mice that received saline (57%). (Figure 1) By 24 hours after TBI, the difference in %MCFPlatelet between aspirin or amitriptyline and saline did not persist. The %MCFPlatelet was similar in ketorolac, heparin and enoxaparin compared to saline at 6 and 24 hours.
Figure 1:
At 6 hours following TBI, mice that received aspirin and amitriptyline had an increased platelet contribution to clot compared to saline utilizing EXTEM and FIBTEM testing. N=6 per group, * = p < 0.05
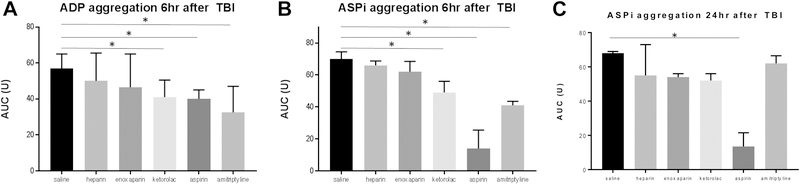
Aspirin, ketorolac and amitriptyline decrease platelet aggregability following TBI
The overall ADP- and arachidonic acid-induced platelet aggregation was significantly lower in mice receiving ketorolac, aspirin, and amitriptyline compared to mice receiving saline at 6 hours post-injury. By 24 hours after injury, only arachidonic acid-induced platelet aggregation was lower in mice receiving aspirin. (Figure 2a–c)
Figure 2a-c:
Platelet aggregation comparison between groups. a-b) At 6 hours following TBI, mice that received ketorolac, aspirin and amitriptyline had decreased platelet aggregation compared to saline when stimulated with both adenosine diphosphate (ADP) and arachidonic acid (ASPi). c) At 24 hours following TBI, only the mice that received aspirin continued to have decreased platelet aggregation when stimulated with ASPi. N=6 per group, * = p < 0.05
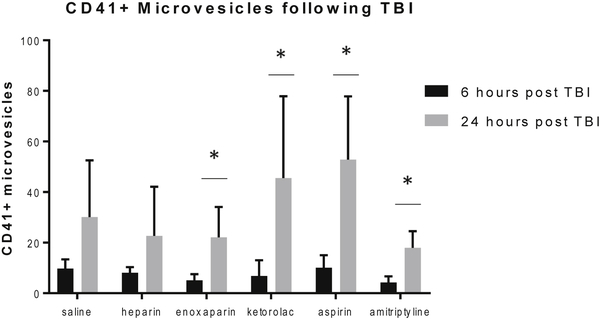
CD41+ microvesicle changes
There were no differences in the total or percentage of platelet-derived (CD41+) microvesicles between any treatment group at both 6 and 24 hours compared to the saline control group. However, CD41+ microvesicles significantly increased over time in the enoxaparin, ketorolac, aspirin, and amitriptyline groups. This change in microvesicle production was not seen in the saline and heparin groups. (Figure 3)
Figure 3:
Platelet derived (CD41+) microvesicles were increased at from 6 to 24 hours following TBI in the groups treated with enoxaparin, ketorolac, aspirin and amitriptyline. No difference seen between groups at either timepoint. N=6 per group, * = p < 0.05
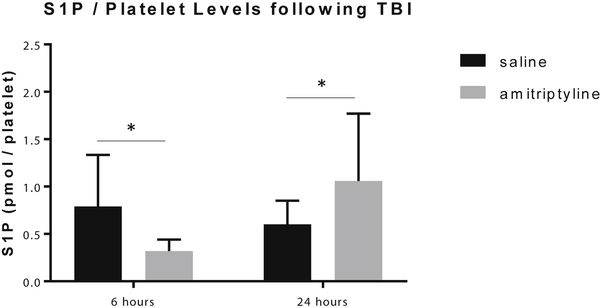
Platelet sphingosine-1-phosphate and ceramide levels increased at 24 hours
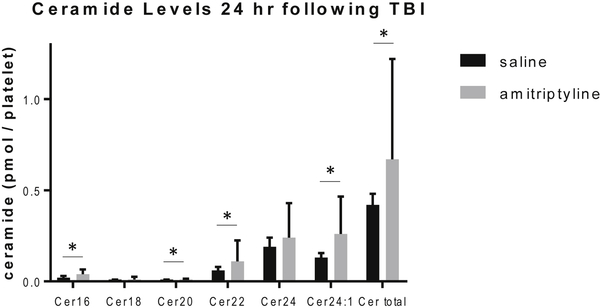
Ceramide and S1P have been shown to affect platelet activation and function (26). Therefore, the levels of acid sphingomyelinase activity and S1P and ceramide levels per platelet were evaluated to assess the functional changes observed in platelet aggregation. There were no differences amongst groups at either timepoint for platelet acid sphingomyelinase activity. At 6 hours following TBI, platelet S1P levels were lower in the amitriptyline group (0.34±0.1 pmol/platelet, p=0.004) compared to saline (0.97±0.5 pmol/platelet). At 24 hours, the S1P levels were higher in the amitriptyline group compared to the saline group (1.3±0.5 vs. 0.68±0.3, amitriptyline vs. saline, p=0.02). (Figure 4) Ceramide levels were also increased in the amitriptyline group compared to saline at 24 hours, including C16, C20, C22, C24:1 and total ceramide per platelet. C18 and C24 ceramide were no different in the amitriptyline treated animals. (Figure 5) By contrast, aspirin, ketorolac, heparin, and enoxaparin did not induce any changes in platelet S1P or ceramide levels despite some changes noted in platelet aggregation.
Figure 4:
In mice treated with amitriptyline, sphingosine-1-phosphate (S1P) per platelet levels were decreased at 6 hours and increased at 24 hours following TBI compared to saline by mass spectrometry. N=6 per group, * = p < 0.05
Figure 5:
At 24 hours following TBI, the mice treated with amitriptyline had increased ceramide levels compared to saline by mass spectrometry. N=6 per group, * = p < 0.05
Enoxaparin and Amitriptyline Show Dose Response Effect
Due to the effect of enoxaparin on platelet aggregation in previous literature and our previous findings of subacute platelet hyperaggregability after TBI, we further investigated the enoxaparin effect by evaluating the dose response with and without TBI (27, 28). Enoxaparin demonstrated decreased platelet aggregation after sham TBI with both ADP and ASPi as the agonist. Interestingly, in the TBI group, the AUC was similar when initiated with ADP, but showed an increase in platelet aggregation when ASPi was used as the agonist. (Figure 6a–d) These findings suggest that enoxaparin may mitigate the post-TBI ADP but not arachidonic acid-induced platelet hyperaggregability.
Figure 6a-d:
In mice treated with enoxaparin, ADP-induced platelet aggregation is decreased with high doses in the setting of sham TBI. However, this is not seen in the mice who underwent TBI and a paradoxical increase in platelet aggregation is seen when initiated with arachidonic acid. N=6 per group, * = p < 0.05
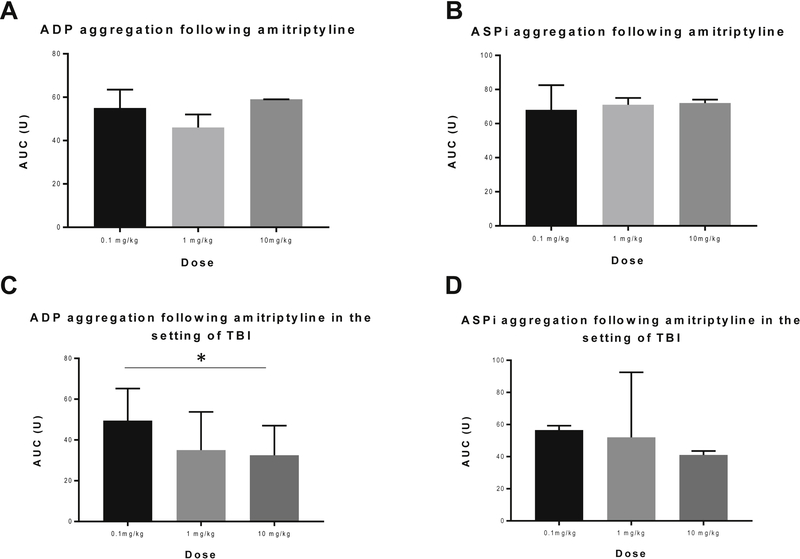
Acid sphingomyelinase and S1P have been previously proposed to have an effect on platelet aggregation (26, 29). This previous literature and our current findings demonstrating decreased platelet aggregation following administration of amitriptyline prompted further investigation into the dose response of amitriptyline. In the sham TBI group, amitriptyline did not show an effect on the platelet aggregation when initiated with ADP or ASPi. However, in the TBI group, amitriptyline reduced platelet aggregation with increased dosing when initiated with ADP. (Figure 7a–d)
Figure 7a-d:
In mice treated with amitriptyline, platelet aggregation is similar between all doses in the setting of sham TBI. However, a decrease in platelet aggregation, when initiated with </P/> ADP, is demonstrated in mice who underwent TBI. N=6 per group, * = p < 0.05
DISCUSSION
In this study, we demonstrated decreased platelet aggregation induced by ADP or ASPi at 6 hours following TBI in mice treated with ketorolac, aspirin and amitriptyline. This correlated with decreased platelet S1P levels at 6 hours following TBI in the amitriptyline group. However, the platelet S1P level was increased in the group receiving amitriptyline compared to saline at 24 hours in which platelet aggregation was restored. Platelet derived microvesicles and acid sphingomyelinase activity were similar amongst all groups. Contrary to the aggregation findings, the platelet contribution to clot formation was significantly increased in the amitriptyline group compared to saline group at both 6 and 24 hours. Overall, these findings suggest that S1P levels may contribute to platelet activity and amitriptyline is a potential, novel medication to modulate platelet function following TBI.
This study demonstrates decreased platelet aggregation and platelet S1P levels at 6 hours in mice treated with amitriptyline following TBI. By 24 hours, the amitriptyline-induced reduction in platelet aggregation had resolved and platelet S1P levels were then elevated. A recent review discussed the possible role of S1P in thrombin-associated inflammatory reactions and cell signaling(30). The regulation of sphingosine kinase and S1P levels by thrombin and other coagulation factors may provide a possible mechanism of thrombotic disease. Another study concluded that S1P may have a physiologic role in thrombosis, hemostasis, and wound healing. These authors demonstrated a significant effect of S1P on platelet shape and aggregation, with ADP and S1P demonstrating a synergistic effect on platelet aggregation (29). Onuma et al. suggested that S1P might inhibit collagen-induced platelet activation by demonstrating a significant decrease in platelet derived growth factor and soluble CD40 ligand induced by S1P. However, their study utilized human platelets and did not stimulate the platelets with either agonist used in the present study (31). Münzer et al. demonstrated an increase in platelet aggregation utilizing sphingosine kinase 1 knockout mice. However, in contrast to our study, S1P levels were not measured and platelet aggregation was measured using a flow chamber (32). Taken together, our data and the previous literature continue to support the role of S1P in platelet aggregation, but its exact mechanism and role will need further investigation, especially in the posttraumatic setting.
Enoxaparin has been found to be more effective in preventing VTE in the trauma population and is the preferred form of chemoprophylaxis according to the Eastern Association for the Surgery of Trauma practice management guidelines (33, 34). Given the findings in this study, we hypothesize that the improved performance of enoxaparin over unfractionated heparin in VTE chemoprophylaxis may be, at least in part, due to the decreased platelet reactivity as demonstrated by treatment of mice with enoxaparin following TBI. Our data demonstrate an enoxaparin dose-dependent response in ADP-induced platelet aggregation. Although this effect is not present following TBI, we have previously demonstrated subacute increased platelet function at 6 hours post-TBI in a murine model that may be mitigated by the enoxaparin effect (16). Previous studies have shown similar results, but ours is the first in a murine injury model. The majority of studies have been from the interventional cardiology literature. Aggarwal et al. demonstrated increased P-selectin expression, which is a surrogate marker of platelet activation, in blood treated with UFH compared to enoxaparin in response to ADP (28). A second study evaluated the blood of patients undergoing hemodialysis that was anticoagulated with either enoxaparin or UFH. The blood anticoagulated with enoxaparin had significantly less platelet reactivity compared to UFH as defined by surface expression of P-selectin in response to ADP (27). In contrast to our findings, a randomized controlled trial evaluating the efficacy and platelet effect of enoxaparin vs. UFH in patients with unstable angina and found greater inhibition of platelet aggregation in the patients who received UFH. However, the dosing and monitoring may have not allowed for adequate antiplatelet effect to be seen (35). These results suggest that the improved outcomes in VTE in the trauma population may be due to the greater impact on platelet aggregation observed with enoxaparin compared to UFH.
Aspirin is a common anti-platelet agent that works via the arachidonic acid pathway and is used in many disease processes to reducing venous and arterial thromboses. This study demonstrated reduced platelet aggregation initiated by both ADP and arachidonic acid following TBI. The effect was seen in both pathways at 6 hours and the ASPi only pathway at 24 hours. At 6 hours following TBI, the platelet contribution to clot formation was significantly increased compared to the group who received saline. These findings suggest that aspirin may have some effect of inhibiting platelet aggregation rather than solely by the arachidonic acid pathway. This effect may also explain the variability in clinical response observed between patients. Dunne et al. evaluated platelet aggregation of patients on dual antiplatelet therapy (P2Y12 inhibitor and aspirin), aspirin alone or healthy volunteers. Their results showed several patients who took aspirin only to have platelet aggregation reduced below the level that was considered to have a therapeutic response to P2Y12 inhibition (36). Another study evaluated the platelet aggregation of “aspirin resistant” patients and found that these patients had an increase in platelet aggregation in response to ADP (37). These studies demonstrate the variability in aspirin effect of platelet aggregation in response to ADP. The variability in aspirin effect may demonstrate an additional mechanistic effect on platelet function and is a future direction of investigation in our laboratory.
There are several limitations of our study that should be addressed. First, our TBI model lacks the angular and rotational forces that are observed in humans involved in motor vehicle accidents or other traumatic accidents. Second, we only used ADP and ASPi as agonists for platelet aggregation. Other factors such as thrombin and collagen involved in platelet aggregation may be missed. Third, our data only represent platelet aggregation in the first 24 hours following traumatic injury and is limited to intraperitoneal route of medication, as additional changes may occur over time following injury. A future direction for this work is to compare different routes of administration, such as utilizing amitriptyline in the drinking water and evaluating its effect over longer time periods. Fourth, the increased downstream sphingolipids in the setting of normal acid sphingomyelinase activity may be due to our time points and enzymatic activity returns to normal prior to our collection times. Further investigation is need into the exact mechanism of increased ceramide and sphingolipid levels. The subsequent use of unique transgenic animals with acid sphingomyelinase deletion and overexpression will allow us to further elucidate this mechanism. Finally, the data for early aspirin and amitriptyline treatment show a reduction in platelet activation by impedance aggregometry with a concomitant increase in platelet contribution to clot by ROTEM. These findings suggest that conclusions about platelet function may be discrepant depending on the in vitro test selected and should be further correlated with clinical findings, including bleeding time or increased intracranial hemorrhage in the setting of TBI.
Conclusions
Following traumatic brain injury, amitriptyline decreased subacute platelet aggregability and increased contribution to clot in a manner similar to aspirin. The effect of amitriptyline on platelet function and changes in S1P levels reflect a possible role of acid sphingomyelinase in the hypercoagulability observed following injury and suggest sphingolipid metabolism as a novel target for multimodal VTE chemoprophylaxis. Additionally, inhibition of platelet reactivity may be an underappreciated benefit of low molecular weight heparins, such as enoxaparin, compared to unfractionated heparin use. Appropriate selection and timing of agents may optimize VTE chemoprophylaxis with multimodal treatment following TBI.
Acknowledgments
The authors wish to thank Lou Ann Friend and Rosalie Veile for their expertise in veterinary and laboratory techniques.
Grants and funding: This work was supported by the National Institutes of Health R01 GM124156-01A1 (MDG)
Footnotes
Conflicts of interest: All authors have no conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Acosta JA, Yang JC, Winchell RJ, Simons RK, Fortlage DA, Hollingsworth-Fridlund P, et al. Lethal injuries and time to death in a level I trauma center. J Am Coll Surg. 1998;186(5):528–33. Epub 1998/05/16. [DOI] [PubMed] [Google Scholar]
- 2.Taylor CA, Bell JM, Breiding MJ, Xu L. Traumatic Brain Injury-Related Emergency Department Visits, Hospitalizations, and Deaths - United States, 2007 and 2013. MMWR Surveill Summ. 2017;66(9):1–16. Epub 2017/03/17. doi: 10.15585/mmwr.ss6609a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Geerts WH, Code KI, Jay RM, Chen E, Szalai JP. A prospective study of venous thromboembolism after major trauma. N Engl J Med. 1994;331(24):1601–6. Epub 1994/12/15. doi: 10.1056/NEJM199412153312401. [DOI] [PubMed] [Google Scholar]
- 4.Knudson MM, Ikossi DG, Khaw L, Morabito D, Speetzen LS. Thromboembolism after trauma: an analysis of 1602 episodes from the American College of Surgeons National Trauma Data Bank. Ann Surg. 2004;240(3):490–6; discussion 6–8. Epub 2004/08/21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hamada SR, Espina C, Guedj T, Buaron R, Harrois A, Figueiredo S, et al. High level of venous thromboembolism in critically ill trauma patients despite early and well-driven thromboprophylaxis protocol. Ann Intensive Care. 2017;7(1):97 Epub 2017/09/14. doi: 10.1186/s13613-017-0315-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mohseni S, Talving P, Lam L, Chan LS, Ives C, Demetriades D. Venous thromboembolic events in isolated severe traumatic brain injury. J Emerg Trauma Shock. 2012;5(1):11–5. Epub 2012/03/15. doi: 10.4103/0974-2700.93102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Massaro AM, Doerfler S, Nawalinski K, Michel B, Driscoll N, Ju C, et al. Thromboelastography defines late hypercoagulability after TBI: a pilot study. Neurocrit Care. 2015;22(1):45–51. Epub 2014/08/17. doi: 10.1007/s12028-014-0051-3. [DOI] [PubMed] [Google Scholar]
- 8.Depew AJ, Hu CK, Nguyen AC, Driessen N. Thromboembolic prophylaxis in blunt traumatic intracranial hemorrhage: a retrospective review. Am Surg. 2008;74(10):906–11. Epub 2008/10/24. [PubMed] [Google Scholar]
- 9.Frisoli FA, Shinseki M, Nwabuobi L, Zeng XL, Adrados M, Kanter C, et al. Early Venous Thromboembolism Chemoprophylaxis After Traumatic Intracranial Hemorrhage. Neurosurgery. 2017;81(6):1016–20. Epub 2017/10/04. doi: 10.1093/neuros/nyx164. [DOI] [PubMed] [Google Scholar]
- 10.Mesa Galan LA, Egea-Guerrero JJ, Quintana Diaz M, Vilches-Arenas A. The effectiveness and safety of pharmacological prophylaxis against venous thromboembolism in patients with moderate to severe traumatic brain injury: A systematic review and meta-analysis. J Trauma Acute Care Surg. 2016;81(3):567–74. Epub 2016/06/01. doi: 10.1097/TA.0000000000001134. [DOI] [PubMed] [Google Scholar]
- 11.Strollo BP, Bennett GJ, Chopko MS, Guo WA. Timing of venous thromboembolism chemoprophylaxis after traumatic brain injury. J Crit Care. 2018;43:75–80. Epub 2017/08/30. doi: 10.1016/j.jcrc.2017.08.012. [DOI] [PubMed] [Google Scholar]
- 12.Barrera LM, Perel P, Ker K, Cirocchi R, Farinella E, Morales Uribe CH. Thromboprophylaxis for trauma patients. Cochrane Database Syst Rev. 2013(3):CD008303.. Epub 2013/04/02. doi: 10.1002/14651858.CD008303.pub2. [DOI] [PubMed] [Google Scholar]
- 13.Munzer P, Borst O, Walker B, Schmid E, Feijge MA, Cosemans JM, et al. Acid sphingomyelinase regulates platelet cell membrane scrambling, secretion, and thrombus formation. Arterioscler Thromb Vasc Biol. 2014;34(1):61–71. Epub 2013/11/16. doi: 10.1161/ATVBAHA.112.300210. [DOI] [PubMed] [Google Scholar]
- 14.Flierl MA, Stahel PF, Beauchamp KM, Morgan SJ, Smith WR, Shohami E. Mouse closed head injury model induced by a weight-drop device. Nat Protoc. 2009;4(9):1328–37. Epub 2009/08/29. doi: 10.1038/nprot.2009.148. [DOI] [PubMed] [Google Scholar]
- 15.Yang SH, Gustafson J, Gangidine M, Stepien D, Schuster R, Pritts TA, et al. A murine model of mild traumatic brain injury exhibiting cognitive and motor deficits. J Surg Res. 2013;184(2):981–8. Epub 2013/04/30. doi: 10.1016/j.jss.2013.03.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martin GE, Xia B, Kim Y, Johnson MD, Veile R, Friend LA, et al. Platelet Function Changes in a Time-Dependent Manner Following Traumatic Brain Injury in a Murine Model. Shock. 2018;50(5):551–6. Epub 2017/11/16. doi: 10.1097/SHK.0000000000001056. [DOI] [PubMed] [Google Scholar]
- 17.Tuinman PR, Muller MC, Jongsma G, Hegeman MA, Juffermans NP. High-dose acetylsalicylic acid is superior to low-dose as well as to clopidogrel in preventing lipopolysaccharide-induced lung injury in mice. Shock. 2013;40(4):334–8. Epub 2013/07/17. doi: 10.1097/SHK.0b013e3182a384f0. [DOI] [PubMed] [Google Scholar]
- 18.Zapata-Morales JR, Aragon-Martinez OH, Adriana Soto-Castro T, Alonso-Castro AJ, Castaneda-Santana DI, Isiordia-Espinoza MA. Isobolographic Analysis of the Interaction Between Tapentadol and Ketorolac in a Mouse Model of Visceral Pain. Drug Dev Res. 2016;77(4):187–91. Epub 2016/05/14. doi: 10.1002/ddr.21310. [DOI] [PubMed] [Google Scholar]
- 19.Johnson BL 3rd, Rice TC, Xia BT, Boone KI, Green EA, Gulbins E, et al. Amitriptyline Usage Exacerbates the Immune Suppression Following Burn Injury. Shock. 2016;46(5):541–8. Epub 2016/10/19. doi: 10.1097/SHK.0000000000000648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nagata K, Kumasaka K, Browne KD, Li S, St-Pierre J, Cognetti J, et al. Unfractionated heparin after TBI reduces in vivo cerebrovascular inflammation, brain edema and accelerates cognitive recovery. J Trauma Acute Care Surg. 2016;81(6):1088–94. Epub 2016/08/18. doi: 10.1097/TA.0000000000001215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kerr NA, de Rivero Vaccari JP, Abbassi S, Kaur H, Zambrano R, Wu S, et al. Traumatic Brain Injury-Induced Acute Lung Injury: Evidence for Activation and Inhibition of a Neural-Respiratory-Inflammasome Axis . J Neurotrauma. 2018;35(17):2067–76. Epub 2018/04/13. doi: 10.1089/neu.2017.5430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Midura EF, Jernigan PL, Kuethe JW, Friend LA, Veile R, Makley AT, et al. Microparticles impact coagulation after traumatic brain injury. J Surg Res. 2015;197(1):25–31. Epub 2015/04/08. doi: 10.1016/j.jss.2015.02.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Prakash PS, Caldwell CC, Lentsch AB, Pritts TA, Robinson BR. Human microparticles generated during sepsis in patients with critical illness are neutrophil-derived and modulate the immune response. J Trauma Acute Care Surg. 2012;73(2):401–6; discussion 6–7. Epub 2012/08/01. doi: 10.1097/TA.0b013e31825a776d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gulbins A, Schumacher F, Becker KA, Wilker B, Soddemann M, Boldrin F, et al. Antidepressants act by inducing autophagy controlled by sphingomyelin-ceramide. Mol Psychiatry. 2018;23(12):2324–46. Epub 2018/07/25. doi: 10.1038/s41380-018-0090-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kachler K, Bailer M, Heim L, Schumacher F, Reichel M, Holzinger CD, et al. Enhanced Acid Sphingomyelinase Activity Drives Immune Evasion and Tumor Growth in Non-Small Cell Lung Carcinoma. Cancer Res. 2017;77(21):5963–76. Epub 2017/09/09. doi: 10.1158/0008-5472.CAN-16-3313. [DOI] [PubMed] [Google Scholar]
- 26.Simon CG Jr., Gear AR. Sphingolipid metabolism during human platelet activation. Thromb Res. 1999;94(1):13–23. Epub 1999/04/23. [DOI] [PubMed] [Google Scholar]
- 27.Aggarwal A, Whitaker DA, Rimmer JM, Solomon RJ, Gennari FJ, Sobel BE, et al. Attenuation of platelet reactivity by enoxaparin compared with unfractionated heparin in patients undergoing haemodialysis. Nephrol Dial Transplant. 2004;19(6):1559–63. Epub 2004/03/23. doi: 10.1093/ndt/gfh209. [DOI] [PubMed] [Google Scholar]
- 28.Aggarwal A, Sobel BE, Schneider DJ. Decreased platelet reactivity in blood anticoagulated with bivalirudin or enoxaparin compared with unfractionated heparin: implications for coronary intervention. J Thromb Thrombolysis. 2002;13(3):161–5. Epub 2002/10/02. [DOI] [PubMed] [Google Scholar]
- 29.Yatomi Y, Ruan F, Hakomori S, Igarashi Y. Sphingosine-1-phosphate: a platelet-activating sphingolipid released from agonist-stimulated human platelets. Blood. 1995;86(1):193–202. Epub 1995/07/01. [PubMed] [Google Scholar]
- 30.Rauch BH. Sphingosine 1-phosphate as a link between blood coagulation and inflammation. Cell Physiol Biochem. 2014;34(1):185–96. Epub 2014/07/01. doi: 10.1159/000362994. [DOI] [PubMed] [Google Scholar]
- 31.Onuma T, Tanabe K, Kito Y, Tsujimoto M, Uematsu K, Enomoto Y, et al. Sphingosine 1-phosphate (S1P) suppresses the collagen-induced activation of human platelets via S1P4 receptor. Thromb Res. 2017;156:91–100. Epub 2017/06/14. doi: 10.1016/j.thromres.2017.06.001. [DOI] [PubMed] [Google Scholar]
- 32.Munzer P, Schmid E, Walker B, Fotinos A, Chatterjee M, Rath D, et al. Sphingosine kinase 1 (Sphk1) negatively regulates platelet activation and thrombus formation. Am J Physiol Cell Physiol. 2014;307(10):C920–7. Epub 2014/09/19. doi: 10.1152/ajpcell.00029.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Geerts WH, Jay RM, Code KI, Chen E, Szalai JP, Saibil EA, et al. A comparison of low-dose heparin with low-molecular-weight heparin as prophylaxis against venous thromboembolism after major trauma. N Engl J Med. 1996;335(10):701–7. Epub 1996/09/05. doi: 10.1056/NEJM199609053351003. [DOI] [PubMed] [Google Scholar]
- 34.Rogers FB, Cipolle MD, Velmahos G, Rozycki G, Luchette FA. Practice management guidelines for the prevention of venous thromboembolism in trauma patients: the EAST practice management guidelines work group. J Trauma. 2002;53(1):142–64. Epub 2002/07/20. [DOI] [PubMed] [Google Scholar]
- 35.Malhotra S, Bhargava VK, Grover A, Pandhi P, Sharma YP. A randomized trial to compare the efficacy, safety, cost and platelet aggregation effects of enoxaparin and unfractionated heparin (the ESCAPEU trial). Int J Clin Pharmacol Ther. 2001;39(3):110–5. Epub 2001/06/09. [DOI] [PubMed] [Google Scholar]
- 36.Dunne E, Egan K, McFadden S, Foley D, Kenny D. Platelet aggregation in response to ADP is highly variable in normal donors and patients on anti-platelet medication. Clin Chem Lab Med. 2016;54(7):1269–73. Epub 2015/11/13. doi: 10.1515/cclm-2015-0802. [DOI] [PubMed] [Google Scholar]
- 37.Macchi L, Christiaens L, Brabant S, Sorel N, Allal J, Mauco G, et al. Resistance to aspirin in vitro is associated with increased platelet sensitivity to adenosine diphosphate. Thromb Res. 2002;107(1–2):45–9. Epub 2002/11/05. [DOI] [PubMed] [Google Scholar]