The accurate diagnosis of the composition of intestinal strictures is critical in the management of Crohn’s disease (CD) [1], as inflammatory strictures may respond to anti-inflammatory therapy, but late-stage fibrotic strictures require surgical resection. In addition, the intestinal expression of extracellular matrix genes, i.e. fibrosis, is an important predictor of future complicated and obstructive CD [2]. Mucosal biopsies are limited by sampling depth, rarely reaching the muscular layers where fibrosis occurs. Conventional imaging modalities can identify the presence of intestinal strictures by structural changes [3]. However, none of the modalities can assess the pathological markers of fibrosis in contrast to inflammation [3]. Previous studies by us and other groups have examined detecting collagen deposition in intestinal fibrosis using magnetization transfer MRI [4] and photoacoustic (PA) imaging [5-7]. Our pilot study using ultrasound (US) elastography has demonstrated that the increased stiffness is a mechanical biomarker of intestinal fibrosis [8]. A challenge in quantitative elastography of the intestinal stricture is that the stress components are difficult to measure in deep abdomen. This study introduces an alternative approach of quantifying the stiffness of intestinal strictures without the necessity of stress measurement using PA-US parallel imaging.
Description of technology
We have successfully identified increased hemoglobin content in inflammatory intestinal strictures and collagen deposition in fibrotic intestinal strictures using spectroscopic PA imaging in animals in vivo [5-7]. In the meantime, we found that parallel PA-US imaging is capable of capturing the blood content reduction in intestinal stricture during compression of abdominal walls of animals. We define such observation strain-PA phenomenon, which reflects the stiffness of the observed tissue.
Video Description
In the video, we first briefly reviewed the physics of spectroscopic PA imaging and our previous studies on PA-US parallel imaging of intestinal strictures. Cineloops demonstrating the strain-PA phenomenon were shown. We spelled out our derivation of the mathematical expression mapping the strain-PA ratio to the strain-stress ratio.
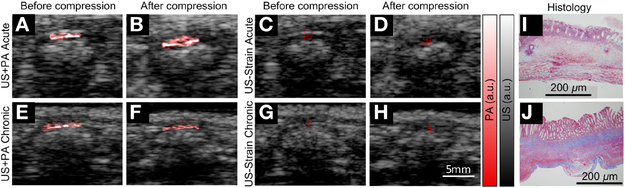
The mathematical expression was examined using Trinitrobenzenesulfonic (TNBS) acid model in rats [8] in vivo. The animal model mimics the acute disease condition where the intestinal stricture only contains inflammation and the chronic condition where both intestinal fibrosis and inflammation present. The experiment was performed using a light-emitting-diode (LED) based PA imaging system (AcousticX, Prexion, Japan). The sensor array of the system possesses a 10 MHz central frequency and 80.9% bandwidth. The LED light sources flashed at 4 kHz and 200 uJ per pulse. PA signals generated by 384 LED flashes were averaged for improved signal-to-noise ratio, producing an imaging frame rate of approximately 10 per second. A vertical translation stage slowly pushed the sensor array against the abdominal wall of the animals at approximately 0.4 mm/s for 25 seconds. Fig. 1 shows the representative images captured in an acute and a chronic animal, and corresponding histology. In Fig. 2(a), the averaged PA signal magnitudes within the top quadrant of the bowel loop were plotted against the bowel wall strain measured by speckle tracking (Echoinsight, Epsilon Imaging Inc., Ann Arbor, MI) in the parallel US images. The descending trend of the strain-PA curves were quantified by fitting to linear models. The slopes of the linear models derived from the chronic (i.e. fibrotic) animals are approximately two times of those derived from the acute (i.e. inflammatory) ones (n=14, p=8×10−7), as shown in Fig. 2(b). The Young’s moduli of the samples were solved from the strain-PA ratios using our mathematical expression. An approximately two times stiffness increase was observed in the chronic animals compared to the acute ones (n=14, p=4×10−5) in Fig. 2(c), which agrees with the conclusion in our previous study [8]. The video concludes with our vision for clinical translation of the presented technology and a preliminary feasibility study in human subjects.
Fig. 1.
Representative PA-US images and histology of the intestinal strictures in acute and chronic TNBS rats. (a)(e) and (b)(f) are the first and last frames of the coregistered PA-US videos, respectively. (c)(g) and (d)(h) are the first and last frames of the US videos for strain measurements, respectively. (i)(j) are the histology of the two animals, respectively.
Fig. 2.
Quantitative analysis of the strain-PA phenomenon. (a) shows the normalized mean PA magnitude within the top quadrant of the intestinal stricture loops as a function of the strain of the stricture walls. As illustrated by the purple dashed line, the curves were fit to linear models. The slope of the linear model, i.e. tanθ, is defined as strain-PA ratio. (b) Strain-PA ratios derived from (a). (c) The Young’s moduli, i.e. the stiffness, of the intestinal strictures derived from the strain-PA ratios in (b).
Supplementary Material
Take home message.
This video demonstrates the capability of PA-US parallel imaging in quantifying the mechanical properties of intestinal strictures, in addition to their molecular components. Such non-invasive and nonionizing radiation imaging procedure is readily translatable to clinics for quick disease assessment and therapeutic planning.
Acknowledgements
This project is partly supported by American Gastroenterological Association Boston Scientific Career Development Technology and Innovation Award, National Institute of Allergy and Infectious Diseases under grant number: R21AI12209801A1, Crohn’s and Colitis Foundation Senior Research Award under grant number 581584, National Cancer Institute under grant number: 1R37CA22282901A1, and National Institute of Arthritis and Musculoskeletal and Skin Diseases under grant number: 5R01AR060350.
We thank Dr. Thomas Wang at University of Michigan for his advice on revising the manuscript. We thank Dr. Ting Feng at Nanjing University of Technology for assisting in preparing the animations illustrating the experiment setup. We also thank Drs. Sato Naoto and Toshitaka Agano with PreXion and Cyberdyne for providing the light emitting diode based photoacoustic ultrasound dual modality imaging system.
Footnotes
Conflict of Interest
This is no conflict of interest for any author.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rieder F, et al. , "Predictors of fibrostenotic Crohn's disease", Inflammatory Bowel Diseases 17 (9), 2000–2007 (2011). [DOI] [PubMed] [Google Scholar]
- 2.Kugathasan S, et al. , "Prediction of complicated disease course for children newly diagnosed with Crohn's disease: a multicentre inception cohort study", The Lancet 389 (10080), 1710–1718 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stidham RW, et al. , "Imaging of intestinal fibrosis: current challenges and future methods", United European Gastroenterology Journal 4 (4), 515–522 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wolff SD, et al. , "Magnetization transfer contrast: method for improving contrast in gradient-recalled-echo images", Radiology 179 (1), 133–137 (1991). [DOI] [PubMed] [Google Scholar]
- 5.Lei H, et al. , "Characterizing intestinal inflammation and fibrosis in Crohn's disease by photoacoustic imaging: feasibility study", Biomedical Optics Express 7 (7), 2837–2848 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhu Y, et al. , "Identifying intestinal fibrosis and inflammation by spectroscopic photoacoustic imaging: an animal study in vivo", Biomedical Optics Express 9 (4), 1590–1600 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lei H, et al. , "Characterizing intestinal strictures of Crohn's disease in vivo by endoscopic photoacoustic imaging", Biomedical Optics Express 10 (5), 2542–2555 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stidham RW, et al. , "Ultrasound Elasticity Imaging for Detecting Intestinal Fibrosis and Inflammation in Rats and Humans With Crohn's Disease", Gastroenterology 141 (3), 819–826.e811 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.