Abstract
The bed nucleus of the stria terminalis (BNST) is a nodal structure in neural circuits controlling anxiety-related defensive behavioral responses. It contains neurons expressing the stress- and anxiety-related neuropeptide corticotropin-releasing hormone (Crh) as well as Crh receptors. Repeated daily subthreshold activation of Crh receptors in the BNST is known to induce a chronic anxiety-like state, but how this affects neurotransmitter-relevant gene expression in target regions of the BNST is still unclear. Since the BNST projects heavily to the dorsal raphe nucleus (DR), the main source of brain serotonin, we here tested the hypothesis that such repeated, anxiety-inducing activation of Crh receptors in the BNST alters the expression of serotonergic genes in the DR, including tph2, the gene encoding the rate-limiting enzyme for brain serotonin synthesis, and slc6a4, the gene encoding the serotonin transporter (SERT). For 5 days, adult male Wistar rats received daily, bilateral, intra-BNST microinjections of vehicle (1% bovine serum albumin in 0.9% saline, n = 11) or behaviorally subthreshold doses of urocortin 1 (Ucn1, n = 11), a potent Crh receptor agonist. Priming with Ucn1 increased tph2 and slc6a4 mRNA expression selectively within the anxiety-related dorsal part of the DR (DRD) and decreased social interaction (SI) time, a measure of anxiety-related defensive behavioral responses in rodents. Decreased social interaction was strongly correlated with increased tph2 mRNA expression in the DRD. Together with previous studies, our data are consistent with the hypothesis that Crh-mediated control of the BNST/DRD-serotonergic system plays a key role in the development of chronic anxiety states, possibly also contributing to stress-induced relapses in drug abuse and addiction behavior.
Keywords: anxiety, chronic anxiety, tryptophan hydroxylase 2, tph2, serotonin transporter
Graphical Abstract
1. Introduction
Emotional disorders, such as social anxiety disorder (SAD), cause significant suffering and are a costly health care burden to society. While both stress-responsive systems and serotonin (5-hydroxytryptamine; 5-HT) systems are often dysregulated in these and other psychiatric disorders (Arango et al., 2002;Gillespie and Nemeroff, 2005), additional research is needed to determine how these systems interact to induce chronic anxiety-like states. Susceptibility to stress-related plasticity changes in the brain are determined by inherited genetic factors, as well as environmental factors, such as adverse events or chronic stressors (Kendler et al., 2004). In rodents, chronically elevated glucocorticoid stress hormones cause anxiety-like behaviors (Donner et al., 2012b) and increase the expression of crh, the gene encoding a key stress-responsive neuropeptide, corticotropin-releasing hormone (Crh), in the bed nucleus of the stria terminalis (BNST) (Makino et al., 1994;Shepard et al., 2006), and tph2, the gene encoding the rate-limiting enzyme for brain 5-HT synthesis in the serotonergic dorsal raphe nucleus (DR) (Donner et al., 2012b). Increased rat tph2 mRNA and Tph2 protein expression after stress exposure (Donner et al., 2018), or chronic, anxiogenic corticosterone treatment is predominantly observed in the dorsal part of the DR (DRD; also referred to as the dorsomedial DR) (Donner et al., 2016;Donner et al., 2012c). As the DRD is activated by a multitude of anxiogenic pharmacologic compounds (Abrams et al., 2005), as well as anxiogenic stimuli, such as exposure to an open-field arena (Hale et al., 2008), we hypothesize that much of corticosterone’s effect on tph2 expression is indirect. One likely input comes from Crh-expressing neurons in limbic forebrain regions, such as the BNST, that project directly to the brainstem DRD (Lee et al., 2008;Sink et al., 2013;Ren et al., 2018;Dabrowska et al., 2016
The BNST is part of the extended amygdala complex, which also includes the basolateral amygdala (BL) and the central nucleus of the amygdala (CE). Specific circuits (neuronal projection systems) between components of this extended amygdala complex and functional subdivisions of the DR are thought to control different types of anxiety. We hypothesize that conflict anxiety (i.e., relevant to generalized anxiety disorder (GAD) and SAD) is controlled by a BNST-DR circuit, while panic-like anxiety is controlled by a BL/CE-DR circuit (Donner et al., 2012a;Donner and Lowry, 2013). Afferents arising from the BNST are known to innervate the DR, including the anxiety-relevant DRD (Peyron et al., 1998;Dabrowska et al., 2016). However, the identity and sensitivity of these afferents to stress-related neuropeptides remains unclear, as does their effect on serotonergic gene expression.
The purpose of this study, therefore, was to investigate mRNA expression of tph2 (tryptophan hydroxylase 2, brain-specific isoform) and slc6a4 (serotonin transporter, SERT) in a circuit-driven animal model of SAD, namely repeated sub-threshold activation of Crh receptors within the BNST. Corticotropin-releasing hormone functions as a neurotransmitter peptide within the extended amygdala (Walker et al., 2009) and also in the DR (Day et al., 2004;Donner et al., 2016; Valentino et al., 2010). It plays a critical role in anxiety-like defensive behavioral responses and can be either anxiolytic or anxiogenic, depending on the brain region, receptor type, and the downstream neuronal projections that it activates (Bale et al., 2002). In the BNST of rodents, both Crh receptor subtypes (Crhr1 and Crhr2) are found (Chalmers et al., 1995), and Crh appears to exert largely anxiogenic actions (Walker et al., 2009;Sink et al., 2013), but see (Henckens et al., 2017). Repeated, sub-threshold activation of Crh receptors in the BNST likely causes long-term plasticity changes and results in a long-lasting anxiety-like state, as measured in the rat social interaction (SI) test (Lee et al., 2008).
Here, we bilaterally infused either vehicle or sub-threshold doses of urocortin 1 (Ucn1), a Crh-related neuropeptide that binds to Crhr1 and Crhr2 receptors (Vaughan et al., 1995), once daily for five days into the BNST of adult male rats. Their anxiety-like state was assessed in the SI test before and after priming with Ucn1, and tph2 and slc6a4 gene expression were measured in nine functionally and neuroanatomically distinct subdivisions of the serotonergic DR.
2. Materials and methods
2.1. Animals
Twenty-four adult male Wistar rats (Harlan Laboratories, Indianapolis, IN, USA; 250-300 g in weight) were single-housed with ad libitum access to food (Cat. No. 8640; Teklad 22/5 Rodent Diet, Harlan Laboratories) and water at 22 °C room temperature and a reversed 12:12 h light/dark cycle (lights on at 0700 h). Rats were used for intra-BNST priming with Ucn1 (n = 12) or vehicle (n = 12), and 12 age-matched conspecifics were used as partner rats in the SI test. All animal procedures were conducted at Indiana University Purdue University at Indianapolis School of Medicine (IUPUI, Indianapolis, IN, USA). All animal experiments complied with the ARRIVE guidelines, were carried out in accordance with IUPUI IACUC committee approval, and the Guide for the Care and Use of Laboratory Animals, Eighth Edition (Institute for Laboratory Animal Research, The National Academies Press, Washington, D.C., 2011). Every effort was made to reduce the number of animals used and their suffering.
2.2. Experimental design
The experimental timeline is illustrated in Figure 1A. After acclimation for one week, rats were deeply anesthetized using isoflurane and underwent stereotaxic surgeries. During stereotaxic surgery, bilateral stainless steel guide cannulae (26 ga, Cat. No. C315GS-4, Plastics One, Roanoke, VA, USA) were implanted, and the guide cannulae were secured with stainless steel screws and dental cement. Bilateral guide cannulae were placed directly above the BNST at the following coordinates with reference to bregma (Paxinos and Watson, 1998), anterior/posterior: −1.0 mm; lateral: +/−2.5 mm; dorsoventral: −8.0 mm, 10° angle of stereotaxic arms, incisor bar at +5.5 mm (Lee et al., 2008). After surgery, guide cannulae were sealed with dummy cannulae (Plastics One). Six days later, experimental rats were habituated by placing them individually into the SI arena for 5 min, and tested in the SI test 24 h later (Day 0) to measure baseline anxiety (File, 1980). For the subsequent five days, rats received daily bilateral intra-BNST microinjections of vehicle (100 nl of 1% bovine serum albumin, Cat. No. A2153, Sigma-Aldrich, St. Louis, MO, USA, in sterile 0.9% saline, n = 12) or Ucn1 treatment (Cat. No. U6631, Sigma-Aldrich; 6 fmoles Ucn1/100 nl vehicle per side, n = 12) at 0900 h via microinjection cannulae that were inserted into the permanent guide cannulae and extended 1 mm from the guide cannula tip into the BNST. This priming paradigm is known to produce long-lasting anxiety-like behavior in the SI test through stimulation of intra-BNST Crh receptors at a dose of Ucn1 that, following acute administration, is not anxiogenic (Lee et al., 2008). Two days after the final injection, rats were tested in the SI test again to confirm development of an anxiety-like state in Ucn1-treated rats. One day later, rats were euthanized between 0900 h and 1000 h via rapid decapitation. The brains were extracted, fresh-frozen, shipped on dry ice to the University of Colorado Boulder (Boulder, CO, USA), and stored at−80 °C until tissue sectioning. Rats were euthanized 24 h after the post-priming SI test, and not earlier, because other socially interactive paradigms, such as social defeat, have been shown to increase rat tph2 mRNA expression 4 h post-exposure (Gardner et al., 2009b). In other words, we conducted the tissue collection 24 h following behavioral testing in order to avoid acute effects of the behavioral testing on tph2 and slc6a4 mRNA expression in the DR, and we assumed that mRNA changes occurred prior to behavioral testing, and otherwise remained static.
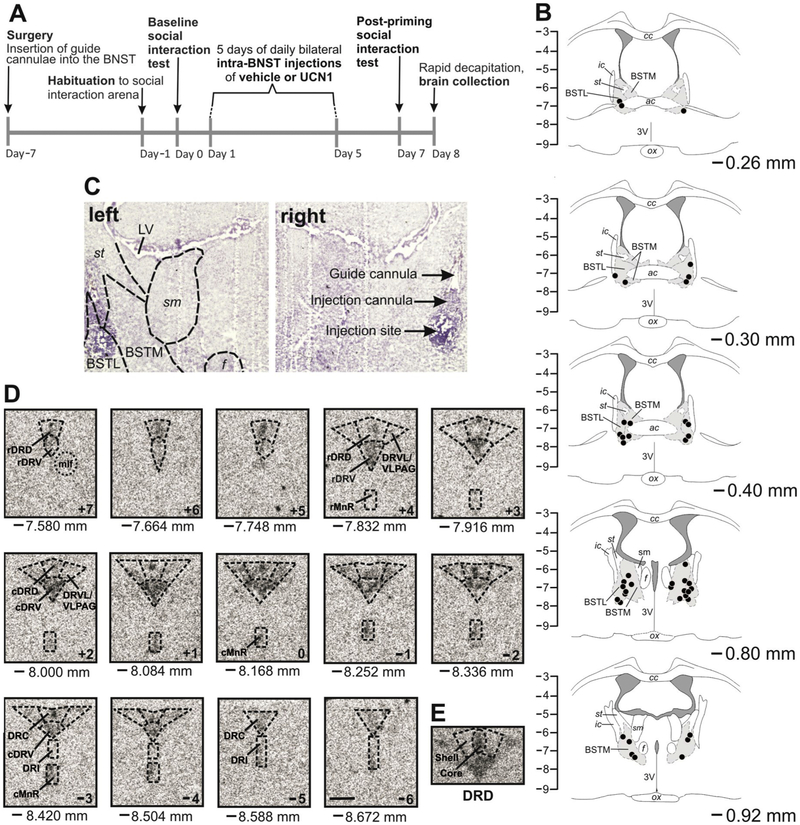
Figure 1.
(A) Experimental timeline. Adult, male rats received five daily bilateral, intra-bed nucleus of the stria terminalis (BNST) microinjections of vehicle (n = 12) or urocortin 1 (UCN1, n = 12), and anxiety-like behavior was assessed in pre- and post-priming social interaction (SI) tests. (B) Verification and mapping of cannula placements. Each black dot represents an injection site from rostral (−0.26 mm bregma) to caudal (−0.92 mm bregma). The orientation of the sections matches the orientation of the viewer (left hemisphere on the left, right hemisphere on the right). Only rats with both injection sites within the BNST were used for analysis (n = 11 per treatment group). The vertical scale indicates dorsoventral coordinates in mm from bregma. (C) Representative photomicrographs of bilateral intra-BNST cannula placements at −0.80 mm bregma. (D) Neuroanatomical analysis atlas of rat serotonergic gene expression within anatomical subdivisions of the dorsal raphe nucleus (DR) and median raphe nucleus (MnR). Shown are 14 representative coronal brain sections of slc6a4 mRNA expression from −7.580 mm to −8.672 mm bregma (designated +7 to −6). Abbreviations are applicable to each following level until indicated differently. Scale bar: 1 mm. (E) Core and shell of the DRD. Abbreviations: 3V, third ventricle; ac, anterior commissure; BSTL, bed nucleus of the stria terminalis, lateral division; BSTM, bed nucleus of the stria terminalis, medial division; cc, corpus callosum; cDRD, caudal aspect of the dorsal raphe nucleus, dorsal part; cDRV, caudal aspect of the dorsal raphe nucleus, ventral part; cMnR, caudal aspect of the median raphe nucleus; DRC, dorsal raphe nucleus, caudal part; DRD, dorsal raphe nucleus, dorsal part; DRI, dorsal raphe nucleus, interfascicular part; DRVL, dorsal raphe nucleus, ventrolateral part; f, fornix; ic, internal capsule; LV, lateral ventricle; mlf, medial longitudinal fasciculus; ox, optic chiasm; rDRD, rostral aspect of the dorsal raphe nucleus, dorsal part; rDRV, rostral aspect of the dorsal raphe nucleus, ventral part; rMnR, rostral aspect of the median raphe nucleus; sm, stria medullaris of the thalamus; st, stria terminalis; VLPAG, ventrolateral periaqueductal gray.
2.3. Social interaction (SI) test
The SI test was performed using a 91.5 cm × 91.5 cm wooden box with 30.5 cm-high walls and an open top under low light conditions (dark room with red light positioned directly above) as previously described (Gehlert et al., 2005). An unfamiliar age- and weight-matched male partner rat was paired with an experimental rat for 5 min between 0900 h and 1300 h. A video camera mounted above recorded the behavior for later analysis using the “The Observer” behavioral analysis software (version 5.0, Noldus Information Technologies, Wageningen, The Netherlands). Social interaction time was defined as any behavior initiated or lully participatory by the experimental rat: face-on contact, sniffing, following, grooming, crawling over or under the partner rat. No experimental rat was exposed to the same partner rat more than once. Decreased SI time is indicative of an anxiogenic treatment effect (File, 1980).
2.4. Brain tissue sectioning and verification of cannula placements
Forebrains were cryosectioned (Leica CM1900, North Central Instruments, Denver, CO, USA) coronally between approximately 0.70 mm and −1.30 mm bregma at 30 μm. Sections were thaw-mounted onto Vista-Vision HistoBond® microscope slides (VWR Scientific, West Chester, PA, USA), stained with cresyl violet (Paxinos and Watson, 1998), cover-slipped with Entellan mounting medium (Cat. No. 14802, EMS, Hatfield, PA, USA), and cannulae placements were identified with reference to a stereotaxic rat brain atlas (Paxinos and Watson, 1998). Only rats with both injection tip sites within the BNST were used for analysis (Figure 1B and 1C). Two animals were excluded from the study based on cannulae placements outside of the BNST (final n = 11 per group). Hindbrains were cryosectioned coronally at 12 μm in series of seven, thaw-mounted onto Vista-Vision HistoBond® microscope slides (VWR Scientific), and stored at −80°C.
2.5. tph2 and slc6a4 in situ hybridization histochemistry
In situ hybridization histochemistry for tph2 mRNA was performed using a synthetic 45-base oligonucleotide probe with the sequence 5’-TCC GTC CAA ATG TTG TCA GGT GGA TTC AGC GTC ACA ATG GTG GTC-3’ (Integrated DNA Technologies, Coralville, IA, USA), complementary to bases 489–533 of rat tph2 mRNA. After labeling with [35S]-deoxyadenosine-5’-trisphosphate (dATP, Cat. No. NEG034H001MC, PerkinElmer, Waltham, MA, USA) on the 3’-end using terminal deoxynucleotidyl transferase (20 U/μl, Cat. No. EP0161, Fermentas, Glen Burnie, MD, USA) for 2 h at 37 °C, the probe was cleaned using a QIAquick® nucleotide removal kit (Cat. No. 28304, Qiagen, Valencia, CA, USA) and used in the assay, as described previously (Lukkes et al., 2013). After overnight hybridization at 42 °C, slides were apposed to an autoradiography film (BioMax MR, Catalogue Number 871 5187, Carestream Health, Rochester, NY, USA) for 14 days. In situ hybridization histochemistry for slc6a4 mRNA was performed at 37 °C using a 3′-tail-[35S]-dATP-labeled synthetic 50-base anti-sense oligonucleotide probe (5′-ACT GCA GAG TAC CCA TTG GAT ATT TGG CTA GGC TCT GCC CTG TCC GCT GT-3′, Integrated DNA Technologies), complementary to bases 207–256 of rat slc6a4 mRNA, as previously described (Gardner et al., 2009a). Slides were apposed to autoradiographic film for 11 days.
2.6. Semi-quantitative analysis of gene expression
Digital autoradiography images obtained for the two genes of interest were analyzed with ImageJ (NIH, Bethesda, MD, USA) while the experimenter was blinded to the treatment groups. Gray value × area was measured using matrices in the shape of the rostral and caudal median raphe nucleus (rMnR and cMnR) and nine functionally different DR subdivisions. Area (mm2) was defined as the pixels falling above a certain gray value threshold that was determined empirically and kept consistent throughout each gene’s analysis. Fourteen rostro-caudal sections, designated levels +7 through −6 (Figure 1D), were analyzed: core and shell of the rostral aspect of the dorsal raphe nucleus, dorsal part (rDRD core and rDRD shell, Figure 1E), −7.580 to −7.916 mm bregma; rostral aspect of the dorsal raphe nucleus, ventral part (rDRV), −7.580 to −7.916 mm bregma; core and shell of the caudal aspect of the dorsal raphe nucleus, dorsal part (cDRD core and cDRD shell), −8.000 to − 8.336 mm bregma; caudal aspect of the dorsal raphe nucleus, ventral part (cDRV), −8.000 to −8.504 mm bregma; dorsal raphe nucleus, ventrolateral part/ventrolateral periaqueductal gray (DRVL/VLPAG), −7.832 to −8.504 mm bregma; dorsal raphe nucleus, caudal part (DRC), −8.420 to −8.672 mm bregma; dorsal raphe nucleus, interfascicular part (DRI), −8.420 to −8.672 mm bregma (Paxinos and Watson, 1998). The lateral wing (DRVL/VLPAG) values were averaged between the left and right hemisphere. In addition, we analyzed the rMnR, −7.832 to −8.084 mm bregma, and the cMnR, −8.168 to −8.504 mm bregma. Background gray value in a region adjacent to the DR was subtracted individually within each image. Gene expression in the entire DR was computed averaging all subdivisions.
2.7. Statistical analysis
Data were analyzed using SPSS (version 22.0, SPSS Inc., Chicago, IL, USA) after removal of extreme outliers (Grubbs, 1969). Social interaction time was analyzed using a linear mixed model (LMM) analysis with treatment (vehicle or Ucn1) as the between-subjects factor, and time (baseline versus post-priming SI test) as the within-subjects factor. Gene expression was assessed using LMM analysis, with treatment as the between-subjects factor and subdivision as the within-subjects factor. Where appropriate, post hoc pairwise comparisons were made using Fisher’s Least Significant Difference tests. Correlation analysis between post-priming SI time and tph2 or slc6a4 mRNA expression in the rDRD and cDRD core and shell were performed using SigmaPlot (Systat Software Inc, San Jose, CA, USA). Graphs were prepared using SigmaPlot, and figures were assembled in CorelDraw (Version 12.0, Corel Inc., Mountain View, CA, USA). Significance was accepted at p < 0.05. Values are shown as the mean + or ± the standard error of the mean (SEM).
3. Results
3.1. tph2 mRNA expression
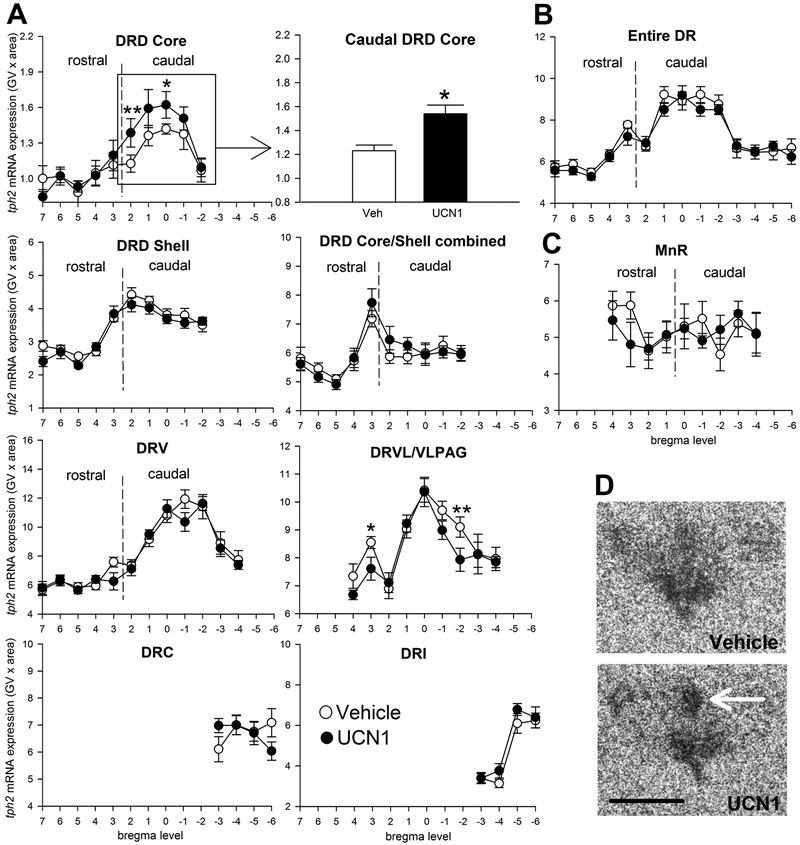
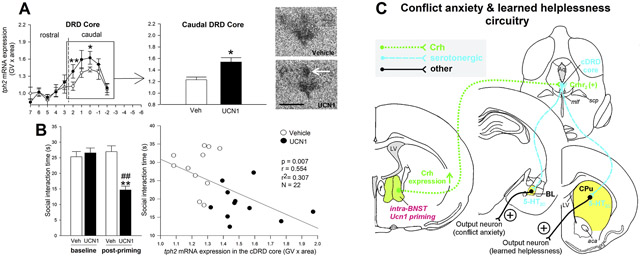
There was a significant interaction between treatment and subdivision in the DR (Figure 2A; subdivisions × treatment, F(8, 4) = 3.22, p < 0.05). Urocortin 1-priming of the BNST elevated tph2 mRNA expression, specifically in the cDRD core, compared to vehicle-primed controls (Figure 2A and 2D; p < 0.05), but not in any other subdivision of the DR or MnR. In contrast, Ucn1 decreased tph2 expression in two rostrocaudal levels of the DRVL/VLPAG compared to controls (Figure 2A, p < 0.05 at level +3; p < 0.01 at level −2). No treatment effect of Ucn1 was detected on overall tph2 mRNA expression in the entire DR or MnR when all subdivisions were averaged (Figure 2B and 2C).
Figure 2.
Effects of five days of daily bilateral intra-bed nucleus of the stria terminalis (BNST) microinjections of vehicle (n = 11, open circles) or urocortin 1 (UCN1, n = 11, closed circles) on tph2 mRNA expression in subdivisions of (A) the dorsal raphe nucleus (DR), (B) in the entire DR (average of all subdivisions), and (C) in the median raphe nucleus (MnR). Tph2 mRNA expression is displayed throughout the entire rostrocaudal extent of each subdivision, with bregma levels (+7 through −6) on the x-axis, and gene expression on the y-axis. Borders between rostral and caudal aspects of each subdivision are indicated with dashed vertical lines. Shown is the mean ± or + the standard error of the mean (SEM). (D) Representative photomicrographs showing tph2 mRNA expression in the DR of vehicle- and Ucn1-primed rats at approximately −8.168 mm from bregma. The white arrow indicates the core of the caudal aspect of the dorsal raphe nucleus, dorsal part(cDRD core). Abbreviations: DRC, dorsal raphe nucleus, caudal part; DRD, dorsal raphe nucleus, dorsal part; DRI, dorsal raphe nucleus, interfascicular part; DRV, dorsal raphe nucleus, ventral part; DRVL/VLPAG, dorsal raphe nucleus, ventrolateral part/ventrolateral periaqueductal gray; Scale bar: 1 mm. p* < 0.05, p** <0.01, compared to vehicle controls.
3.2. slc6a4 mRNA expression
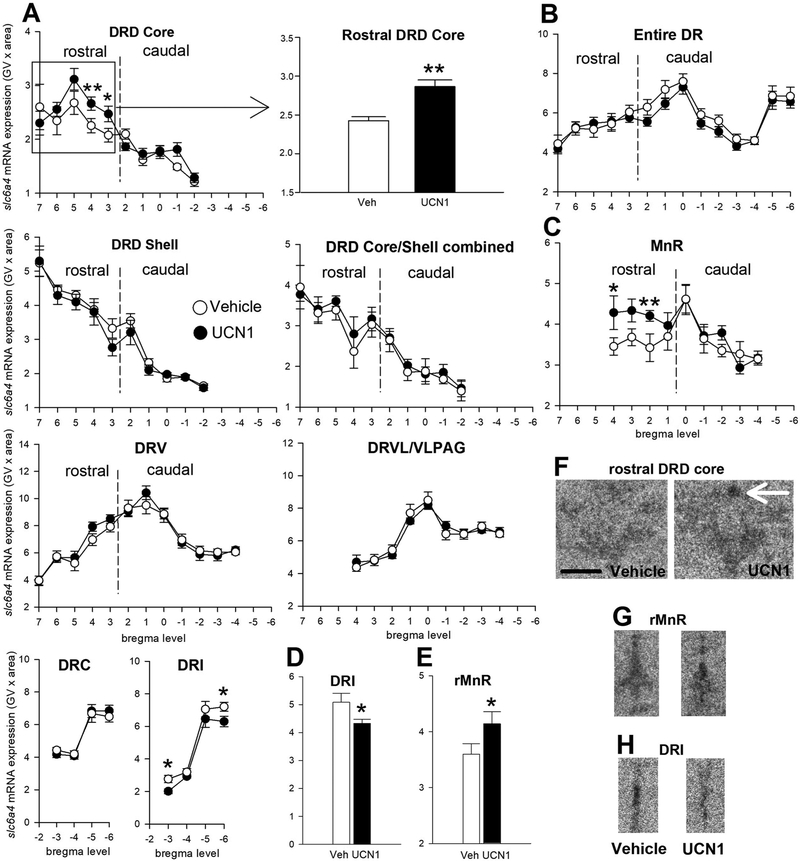
An interaction between intra-BNST Ucn1 treatment and subdivision was found for slc6a4 mRNA expression in the DR (subdivisions × treatment, F(8, 4) = 4.55, p < 0.01). Specifically, in the rDRD core, slc6a4 mRNA expression was significantly higher after Ucn1 treatment compared to controls (Figure 3A and 3F; p < 0.01), while slc6a4 mRNA expression in the DRI was decreased by Ucn1-priming (Figure 3A, 3D and 3H; p < 0.05). In the rMnR, Ucn1-treated rats displayed increased expression of slc6a4 mRNA compared to controls (Figure 3C and 3G; p < 0.01). Expression of slc6a4 mRNA in other subdivisions or the entire DR when all subdivisions were averaged did not differ between treatment groups (Figure 3B).
Figure 3.
Effects of five days of daily bilateral intra-bed nucleus of the stria terminalis (BNST) microinjections of vehicle (n = 11, open circles) or urocortin 1 (UCN1, n = 11, closed circles) on slc6a4 mRNA expression in subdivisions of (A) the dorsal raphe nucleus (DR), (B) in the entire DR (average of all subdivisions), and (C) in the median raphe nucleus (MnR). Slc6a4 mRNA expression is displayed throughout the entire rostrocaudal extent of each subdivision, with bregma levels (+7 through −6) on the x-axis, and gene expression on the y-axis. Borders between rostral and caudal aspects of each subdivision are indicated with dashed vertical lines. (D-E) Bar graphs displaying the overall expression of slc6a4 mRNA expression in the DRI and rostral MnR (rMnR). Shown is the mean ± or + the standard error of the mean (SEM). Representative photomicrographs show slc6a4 mRNA expression in the (F) rostral DRD core at approximately −7.916 mm from bregma, (G) the rMnR at approximately −7.916 mm bregma, and (H) in the dorsal raphe nucleus, interfascicular part (DRI) at approximately −8.588 mm bregma of vehicle-and Ucn1-primed rats. The white arrow indicates the core of the rostral aspect of the dorsal raphe nucleus, dorsal part (rDRD core). Abbreviations: DRC, dorsal raphe nucleus, caudal part; DRD, dorsal raphe nucleus, dorsal part; DRI, dorsal raphe nucleus, interfascicular part; DRV, dorsal raphe nucleus, ventral part; DRVL/VLPAG, dorsal raphe nucleus, ventrolateral part/ventrolateral periaqueductal gray; rMnR, rostral aspect of the median raphe nucleus; Scale bar: 1 mm. p* < 0.05, p** < 0.01, compared to vehicle controls.
3.3. Behavior in the SI test
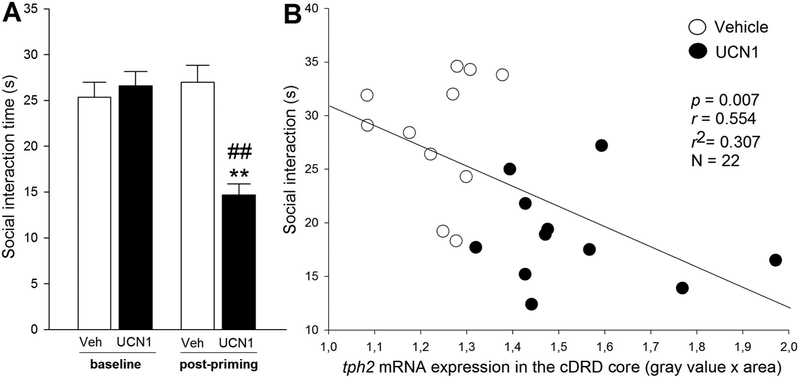
Analysis of defensive behavioral responses in the SI test revealed a time × treatment interaction (F(1, 18) = 33.22, p < 0.001). There was no difference in baseline SI time between the vehicle and the Ucn1 group, but 48 h after the final intra-BNST priming injection, Ucn1-treated rats displayed increased anxiety-like behavior in the SI test (less SI time, p < 0.01), relative to vehicle-primed controls (Figure 4A). Also, Ucn1-primed rats spent significantly less time interacting with the partner rat than they did on Day 0 (p < 0.01), while vehicle-primed control rats displayed no difference in SI time compared to Day 0.
Figure 4.
Behavioral responses to intra-bed nucleus of the stria terminalis (BNST) priming. (A) Baseline (Day 0) and post-priming (Day 7) anxiety-like behavior of adult, male rats that received daily bilateral intra-BNST microinjections of vehicle (Veh, n = 12/11, open bars) or urocortin 1 (Ucn1, n = 12/11, black bars), as measured in the social interaction (SI) test. Shown is the mean SI time (s, seconds) + SEM. p** < 0.01 versus vehicle controls; p## < 0.01 versus baseline SI time on Day 0. (B) Correlation of SI time on Day 7 and tph2 mRNA expression in the caudal core of the dorsal raphe nucleus, dorsal part (cDRD core) of vehicle- (open circles) or Ucn1-(closed circles) primed rats.
3.4. Correlation of behavior and gene expression
Based on the proposed role of the cDRD, but not the rDRD, in anxiety-like behaviors, as well as learned helplessness (Donner et al., 2018;Hale et al., 2012;Rozeske et al., 2011;Hammack et al., 2002), and on reports of increased TPH2 mRNA and protein expression (Bach-Mizrachi et al., 2006;Bach-Mizrachi et al., 2008;Boldrini et al., 2005), as well as increased CRH immunoreactivity (Austin et al., 2003) in the human equivalent of the cDRD in the brains of depressed suicide victims, we performed correlation analyses of SI time and serotonergic gene expression in these areas. Expression of tph2 mRNA, but not slc6a4 mRNA, within the cDRD core was negatively correlated with post-priming SI time (Figure 4B), meaning that increased tph2 mRNA expression in the cDRD core was strongly associated with decreased SI time (r = 0.55, r2 = 0.31, p < 0.01, N = 22). No other correlations of gene expression and behavior were found.
4. Discussion
Intra-BNST priming with Ucn1 induced an anxiety-like state and altered tph2 and slc6a4 mRNA expression. Specifically, Ucn1-priming increased both tph2 mRNA expression within the caudal DRD, a region associated with serotonergic responses to anxiogenic drugs, anxiety-related neuropeptides, anxiogenic behavioral stimuli, and learned helplessness. Altered tph2 mRNA expression was limited to the caudal DRD core and was not observed in the DRD shell. Reduced social interaction time was strongly correlated with tph2 mRNA expression in the DRD core. Furthermore, Ucn1-priming decreased tph2 expression in DRVL/VLPAG serotonergic neurons, a subpopulation of serotonergic neurons that has been implicated in inhibition of panic-like physiological and behavioral responses (Wscieklica et al., 2017;Donner and Lowry, 2013;Hassell Jr et al., 2017). In contrast, Ucn1-priming altered slc6a4 expression in the rostral DRD core, rostral MnR and DRI, regions that are implicated in stress resilience (Paul and Lowry, 2013).
Crh receptors in the BNST
Early studies of the distribution of Crhr1 and Crhr2 receptor mRNA in rat brain reported moderate expression of both Crhr1 and Crhr2 within the BNST (Chalmers et al., 1995). Chalmers and colleagues noted Crhr1 mRNA expression in both the medial and lateral aspects of the posterior BNST, and higher concentrations of Crhr2 mRNA expression in the medial (perifornical) aspects of the posterior BNST. Consistent with these findings, Van Pett and colleagues reported that Crhr1 mRNA expression is evident throughout the BNST, including the rostromedial, rostrolateral, posterodorsal, and posteroventral regions, whereas Crhr2 mRNA expression is restricted to the posterodorsal and posteroventral regions (Van Pett et al., 2000). Thus, both Crhr1 and Crhr2 are present in the BNST, and actions of Ucn1 on Crhr1, Crhr2, or both, could mediate the effects of BNST priming in our study.
Topographical organization of the BNST
Intra-BNST injections spanned a region of approximately 660 μm in the anterior/posterior plane. Thus, injections encompassed diverse subregions of the BNST, including anterior regions of the BNST (i.e., BSTLD, BSTLI, BSTLJ, BSTLV, BSTMA, and BSTMV according to Paxinos and Watson, (1998)), and posterior regions of the BNST (i.e., BSTLP, BSTMPI, BSTMPL, and BSTMPM according to Paxinos and Watson, (1998)); for a detailed discussion of anterior and posterior regions of the BNST, their neurochemical properties, and relevance to anxiety-like defensive behavioral responses, see (Lebow and Chen, 2016). A previous study involving reversible inactivation of the BNST using bilateral infusion of small volumes (100 nl) of the nonselective synapse blocker, CoCl2, with a similar distribution of microinjection sites as used here, found that inactivation of the BNST prevented both anxiety-like defensive behavioral responses in the Vogel conflict test and conditioned freezing responses in a model of contextual fear (Resstel et al., 2008). Furthermore, although many studies have focused on anterior regions of the BNST in control of anxiety-like defensive behavioral responses (Kim et al., 2013;Levita et al., 2004;Hammack et al., 2009), other studies have found that the posterior BNST also plays a role in control of anxiety-like defensive behavioral responses (Henckens et al., 2017). Specifically, activation of neurons in the oval nucleus of the BNST promotes an anxiety-like state, whereas activation of the adjacent anterodorsal BNST (BSTLP) decreases multiple features of anxiety-like states (Kim et al., 2013). Other studies have shown that activation of glutamatergic neurons within the ventral BNST (BSTLV) projecting to the ventral tegmental nucleus increase anxiety-like defensive behavioral responses, whereas activation of GABAergic neurons within the ventral BNST projecting to the ventral tegmental nucleus decrease anxiety-like defensive behavioral responses (Jennings et al., 2013). Meanwhile, selective activation of Crhr2 expressing GABAergic projection neurons in the posterior BNST decreases anxiety and stress-induced anxiety (Henckens et al., 2017). Taken together, the evidence for functional heterogeneity within the anterior and posterior regions of the BNST suggests that BNST priming using Ucn1 may induce a chronic anxiety-like state by sensitizing BNST neurons in the oval subnucleus of the BNST (BSTLD), inhibiting BNST neurons in the anterodorsal/posterior BNST, increasing activity of glutamatergic ventral tegmental-projecting neurons within the ventral BNST, decreasing activity of GABAergic ventral tegmental-projecting neurons within the ventral BNST, or a combination of these effects. Future studies will be required to explore the potential mechanisms involved.
Anxiety-promoting CRF neurons in the oval nucleus of the BNST selectively target the caudal DRD
Recent studies by Ren and colleagues (2018) have demonstrated that BNST projection neurons preferentially innervate DR serotonergic neurons projecting to the central nucleus of the amygdala (DRSERT→CeA neurons) that in turn increase anxiety-like defensive behavioral responses, a finding consistent with previous retrograde tracing from the dorsomedial DR (Peyron et al., 1998). Although not discussed by Ren and colleagues (2018), the BNST neurons projecting to DRSERT→CeA neurons (located in the dorsomedial DR) are almost exclusively located in the oval nucleus of the BNST (BSTLD), the location of a dense population of Crh-expressing neurons in rats and mice (Hu et al., 2019;Nguyen et al., 2016;Dabrowska et al., 2016). Furthermore, using injections of a‘floxed’ anterograde tracer (rAAV5/EF1a-DIO-mCherry) into the oval nucleus of the BNST of CRFp3.0CreGFP transgenic mice and analogous methods in rats, Dabrowska and colleagues showed that CRF neurons in the oval BNST selectively target the DRD, also referred to as the dorsomedial DR (Dabrowska et al., 2016), i.e., the same region where we observed changes in Tph2 mRNA expression in our study. Overexpression of Crh selectively in BNST neurons decreases Crhr2 receptor expression selectively within the dorsomedial DR and increases expression of conditioned emotional memory (Sink et al., 2013). Conversely, serotonergic projections from the DR to BNST Crh-expressing neurons, through activation of 5-HT2C receptors, promote development of an anxiety-like state (Marcinkiewcz et al., 2016). Finally, direct activation of Crh neurons in the oval BNST nucleus induces an anxiety-like state that mimics that observed following chronic stress (Hu et al., 2019). Thus, Crh-expressing neurons in the oval subnucleus of the BNST and serotonergic neurons within the dorsomedial DR appear to have reciprocal connections that play a role in control of emotional behavior, including anxiety-like defensive behavioral responses. Intra-BNST Ucn1 priming may sensitize or dysregulate these reciprocal circuits, leading to development of a chronic anxiety-like state. Nevertheless, as noted above, the BNST is complex in terms of its topographical organization and neurochemical properties (Lebow and Chen, 2016), and further research is needed to determine how intra-BNST priming alters BNST function and downstream signaling.
Intra-BNST priming induces a chronic anxiety-like state
Intra-BNST priming with Ucn1 decreased social interaction time, an anxiogenic effect, measured 48 h following the final Ucn1 injection. Previous studies have shown that Ucn1-induced priming of the BNST increases social anxiety, but not panic-like physiological responses (Lee et al., 2008), suggesting that the BNST plays an important role in anxiety-like defensive behavioral responses that are anticipatory in nature, or involve a conflict between approach and avoidance. In contrast, Ucn1-induced priming of the BL increases both social anxiety and panic-like anxiety (Hale et al., 2012). Ucn1 priming may activate an anxiety-promoting DR-amygdala circuit identified using viral-genetic approaches by Ren and colleagues (2018). DR-amygdala serotonergic neurons are preferentially located in the DRD (Ren et al., 2018). DR-amygdala serotonergic neurons, relative to serotonergic neurons projecting to the orbitofrontal cortex, preferentially receive input from the stress-, anxiety- and fear-related brain regions, including the central nucleus of the amygdala, the paraventricular nucleus of the hypothalamus, the nucleus of the solitary tract, and, importantly, as discussed in detail above, the BNST (Ren et al., 2018). DR-amygdala serotonergic neurons give rise to collateral projections to forebrain systems controlling anxiety- and fear-related defensive behavioral responses and anxiety-like states, including the central nucleus of the amygdala, lateral amygdala, intercalated nucleus of the amygdala, basolateral nucleus of the amygdala, paraventricular nucleus of the hypothalamus, substantia nigra, and the ventral bed nucleus of the stria terminalis (Ren et al., 2018). Activation of a DR-BNST serotonergic circuit, acting on 5-HT2C receptors to stimulate Crh neurons within the BNST, has also been implicated in anxiety-like defensive behavioral responses (Marcinkiewcz et al., 2016). Together with these findings, our data elucidate how serotonergic circuits downstream of the BNST may control chronic conflict anxiety-like states, suggesting that serotonergic neurons within the DRD core play an important role.
Evidence that dorsomedial DR serotonergic neurons promote acute- and chronic anxiety-like states
Ucn1-induced priming of the BNST increased tph2 mRNA expression within serotonergic neurons located in the caudal DRD, also referred to as the dorsomedial DR. Serotonergic neurons within the caudal DRD are activated by anxiogenic drugs, Crh-related neuropeptides, including Ucn2, and anxiogenic stimuli, including acoustic startle, anxiety due to intimate partner violence, the avoidance task on the elevated T-maze, open-field exposure, social defeat, and inescapable shock in a model of learned helplessness (Abrams et al., 2005;Bouwknecht et al., 2007;Rozeske et al., 2011;Staub et al., 2005;Spiacci, Jr. et al., 2012;Cordero et al., 2012) (for review, see Hassell Jr et al., 2017). Activation of caudal DRD serotonergic neurons likely depends on specific neuronal afferents (Hassell Jr et al., 2017;Ren et al., 2018). In support of the assumption that a key source of these afferents is the oval subnucleus of the BNST (Ren et al., 2018;Dabrowska et al., 2016), Sink et al (2013) reported that crh overexpression in the BNST decreases Crhr2 binding selectively in the caudal DRD. Based on the published autoradiograms, this decrease was clearly located in the DRD core, the only subregion of the DR that contains serotonergic neurons that co-express Crh (Commons et al., 2003). The fact that tph2 mRNA expression was elevated in the caudal DRD core in our study reinforces the hypothesis that the BNST selectively innervates and controls serotonergic function in this subregion. Indeed, previous anterograde and retrograde tracing studies have confirmed direct projections from the oval subnucleus of the BNST to the DRD core (Peyron et al., 1998;Dabrowska et al., 2016).
Serotonergic neurons in the DRD core have unique morphological properties, relative to serotonergic neurons in the DRD shell (Steinbusch, 1984). Serotonergic neurons in the DRD core also have unique neurochemical properties; as mentioned above, Commons et al (2003) have described a population of Crh-expressing serotonergic neurons that are restricted to the DRD core. Crh is a potent anxiogenic neuropeptide, and serotonin is a potent anxiogenic neurotransmitter via actions on 5-HT2C receptors located in forebrain target regions of DRD-efferents, including the BL, the BNST, and the dorsal hypothalamic area (Christianson et al., 2010;Strong et al., 2011;Commons et al., 2003;Marcinkiewcz et al., 2016). Activation of this Crh-expressing subset of serotonergic neurons may have particularly potent anxiogenic effects. As mentioned above, a recent study by Marcinkiewcz et al (2016) describes projections from the DR to the BNST that act on 5-HT2C receptors to silence anxiolytic output from the BNST. It is possible that activation of Crh-expressing serotonergic neurons in the DRD core leads to a downstream increase in Crh release within the BL. Acute anxiogenic actions of Crh (Cipriano et al., 2016), as well as development of chronic anxiety-like states following intra-BL Crh priming within the BL (Gehlert et al., 2005;Rainnie et al., 2004), are known to be mediated via Crhr1.
The observation that tph2 expression in the DRD core subregion was strongly correlated with decreased SI time raises the possibility that sensitization of neurons in the DRD core contributes to the persistent anxiety-like state following Ucn1-priming. In separate studies, we have shown that chronic glucocorticoid administration, which also induces a chronic anxiety-like state, increases tph2 mRNA, Tph2 protein expression, and Tph enzyme activity in the DRD, an effect that was prevented by local blockade of Crhr2 receptors (Donner et al., 2016). This hypothesis is consistent with studies of learned helplessness by Maier and colleagues, in which a functional desensitization of 5-HT1A receptors in the DRD accounts for the chronic anxiety-like state associated with learned helplessness (Rozeske et al., 2011). Furthermore, exaggerated fear conditioning and escape deficits measured 24 h following inescapable stress in this model of learned helplessness are prevented by lesions of the BNST, suggesting that the same BNST/caudal DRD circuitry is involved in the behavioral manifestation of learned helplessness (Hammack et al., 2004). Consistent with these observations, learned helplessness can be precipitated in the absence of inescapable stress by microinjections of Crhr2 receptor agonists directly into the region of the caudal DRD, while the exaggerated fear conditioning and escape deficits 24 h following inescapable stress are prevented by blockade of Crhr2 receptors within the region of the DRD (Hammack et al., 2002). Downstream of the DRD, exaggerated anxietylike responses in the juvenile social interaction test 24 h following inescapable stress are prevented by blockade of 5-HT2C receptors in the BL (Christianson et al., 2010), while escape deficits are prevented by blockade of 5-HT2C receptors within the dorsal striatum (Strong et al., 2011). Both the BL (Abrams et al., 2005) and the dorsal striatum (Steinbusch, 1984) are innervated by the DRD core. Figure 5 presents a hypothetical model illustrating how the BNST/DRD-core circuit may contribute to the development of chronic anxiety-like states, including learned helplessness.
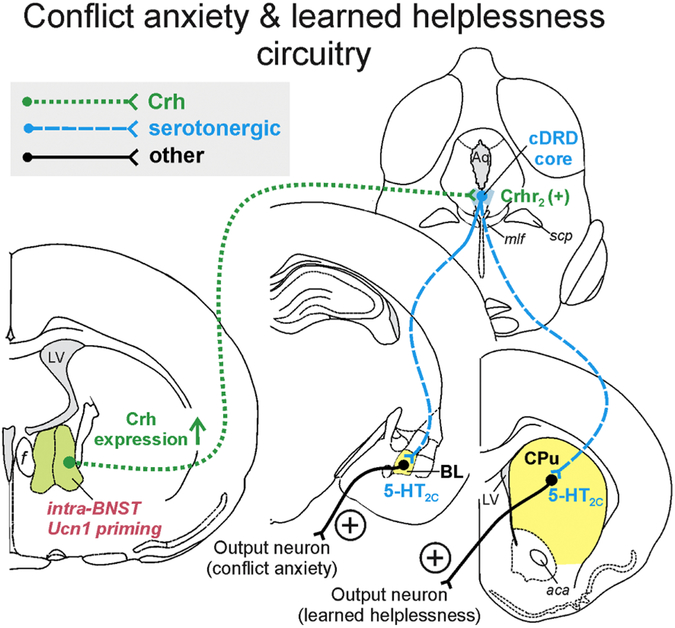
Figure 5.
Hypothetical model of how urocortin 1 (Ucn1) priming within the bed nucleus of the stria terminalis (BNST) may enhance corticotropin-releasing hormone (Crh) expression, Crh release, and Crh actions on Crh receptor type 2 (Crhr2) in serotonergic neurons (Lukkes et al., 2011) of the caudal core of the dorsal raphe nucleus, dorsal part (cDRD core) (Sink et al., 2013 ;Dabrowska et al., 2016), ultimately leading to increased activity of serotonergic projections innervating, for example, the conflict anxiety-related basolateral amygdaloid nucleus (BL) (Hale et al., 2008;Christianson et al., 2010;Ren et al., 2018) and the caudate putamen (CPu), where serotonin acts on 5-hydroxytryptamine receptor type 2C (5-HT2C), contributing to the manifestation of learned helplessness (Christianson et al., 2010;Strong et al., 2011). In addition, increased Crh release within the BL, possibly arising from Crh-expressing serotonergic neurons in the cDRD (Commons et al., 2003), may contribute to enhanced anxiety via Crh receptor type 1 (Crhr1) activation in the BL. Other abbreviations: aca, anterior commissure, anterior part; Aq, mesencephalic aqueduct; f, fornix; LV, lateral ventricle; mlf medial longitudinal fasciculus; scp, superior cerebellar peduncle.
BNST priming decreases tph2 mRNA expression in the lateral wings of the DR
Ucn1-induced priming decreased tph2, but not slc6a4, mRNA expression in DRVL/VLPAG serotonergic neurons. An increase in tph2 expression in the DRD core associated with a decrease in tph2 mRNA expression in the DRVL/VLPAG serotonergic neurons is consistent with a number of studies showing that whenever DRD serotonergic neurons are activated, DRVL/VLPAG serotonergic neurons are not, and vice versa (Hassell Jr et al., 2017). Thus, the chronic anxiety-like state following BNST-priming may involve dysregulation of gene expression in multiple functional subdivisions of the brainstem raphe complex.
BNST priming alters slc6a4 mRNA expression
Slc6a4, but not tph2, expression was altered in the rostral DRD, rostral MnR, and in the DRI following Ucn1-induced priming. The rhombomeric derivation of the MnR and DRI is developmentally distinct from other subdivisions of the brainstem raphe complex (Jensen et al., 2008), and the rostral DRD, MnR, and the DRI are functionally implicated in stress resilience (Paul and Lowry, 2013). For example, direct microinjection of Crh into the DRD region results in a Crhr2-dependent, immediate increase in serotonin release in the central nucleus of the amygdala, which lasts approximately 60 minutes and is associated with freezing behavior. At the termination of freezing behavior, there is a delayed increase in serotonin release in the medial prefrontal cortex, which is dependent on the MnR; if the MnR is inactivated, the serotonin release in the medial prefrontal cortex does not occur (Forster et al., 2008). The medial prefrontal cortex is a key structure for behavioral control and stress resilience (Maier et al., 2006). Thus, changes in slc6a4 expression in these regions may be secondary or temporally delayed relative to the changes observed in the DRD. While the primary anxiogenic effects of BNST priming are likely mediated by direct projections to the DRD core, internal raphe-raphe projections may modulate other subpopulations of serotonergic neurons, e.g. in the MnR and DRI, leading to altered stress resilience.
5. Limitations
One limitation of the current study is that we limited our assessment to analysis of tph2 and slc6a4 mRNA expression, and did not extend this finding to confirm effects of intra-BNST Ucn1 priming on Tph2 and Slc6a4 protein. Although, in previous studies, we have observed parallel effects of treatment, i.e., chronic administration of the glucocorticoid hormone, corticosterone, on tph2 mRNA expression, Tph2 protein expression, and Tph2 activity in the dorsomedial DR (Donner et al., 2012a;Donner et al., 2016), future studies should also evaluate Tph2 protein expression and Tph2 activity within the dorsomedial DR following intra-BNST priming.
6. Conclusions
In summary, our findings are relevant for clinical conditions including anxiety-disorders, stressor- and trauma-related disorders like posttraumatic stress disorder, and affective disorders. Analyses of post mortem tissue from depressed suicide patients have revealed increased CRH immunoreactivity (Austin et al., 2003) and increased TPH2 mRNA and TPH2 protein expression within the caudal DR (Bach-Mizrachi et al., 2006;Bach-Mizrachi et al., 2008). A subregional analysis revealed that TPH2 expression in the DRD of depressed suicides was 136% that of controls (Bach-Mizrachi et al., 2006). Another study of alcohol-addicted depressed suicides indicates that TPH-immunostaining was selectively increased in the DRD, with a striking elevation of TPH-immunostaining in the presumed human equivalent of the DRD core (Bonkale et al., 2006). Our data suggest that increased TPH2 mRNA and TPH2 protein expression in the DRD may be based on overactivity of a BNST/DRD core circuit, resulting in chronic anxiety or a ‘learned helplessness’ state. Based on human post mortem studies, this circuit also may play a role in vulnerability to suicidal self-directed violence
HIGHLIGHTS.
BNST priming induces a chronic anxiety-like state
BNST priming increases tph2 mRNA expression in the dorsomedial dorsal raphe nucleus
Increased tph2 mRNA expression is strongly correlated with anxiety-like behavior
Acknowledgements
We gratefully acknowledge Zachary D. Barger for proof reading the manuscript.
Funding
This study was supported by NIH grant R01MH065702 (AS, CAL) and R01MH086539 (CAL). The sponsor(s) had no role in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the article for publication. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Mental Health or the National Institutes of Health. CAL is supported by the National Institute of Mental Health (grant number 1R21MH116263), Department of the Navy, Office of Naval Research Multidisciplinary University Research Initiative Award (grant N00014-15-1-2809), Department of Veterans Affairs Office of Research and Development RR&D Small Projects in Rehabilitation Research (grant 1-I21-RX002232-01), the Colorado Department of Public Health and Environment (grant DCEED-3510), and the Alfred P. Sloan Foundation (grant G-2016-7077). D.M.K. was supported by a University of Colorado Boulder Undergraduate Research Opportunities Program (UROP) Individual grant (AY 18-19).
Abbreviations
- 3V
third ventricle
- 5-HT
5-hydroxytryptamine; serotonin
- ac
anterior commissure
- BL
basolateral amygdala
- BNST
bed nucleus of the stria terminalis
- BSTL
bed nucleus of the stria terminalis, lateral division
- BSTLD
bed nucleus of the stria terminalis, lateral division, dorsal part
- BSTLI
bed nucleus of the stria terminalis, lateral division, intermediate part
- BSTLJ
bed nucleus of the stria terminalis, lateral division, juxtacapsular part
- BSTLP
bed nucleus of the stria terminalis, lateral division, posterior part
- BSTLV
bed nucleus of the stria terminalis, lateral division, ventral part
- BSTM
bed nucleus of the stria terminalis, medial division
- BSTMA
bed nucleus of the stria terminalis, medial division, anterior part
- BSTMPI
bed nucleus of the stria terminalis, medial division, posterointermediate part
- BSTMPL
bed nucleus of the stria terminalis, medial division, posterolateral part
- BSTMPM
bed nucleus of the stria terminalis, medial division, posteromedial part
- BSTMV
bed nucleus of the stria terminalis, medial division, ventral part
- cc
corpus callosum
- cDRD
caudal aspect of the dorsal raphe nucleus, dorsal part
- cDRD
core, core of the caudal aspect of the dorsal raphe nucleus, dorsal part
- cDRD
shell, shell of the caudal aspect of the dorsal raphe nucleus, dorsal part
- cDRV
caudal aspect of the dorsal raphe nucleus, ventral part
- CE
central nucleus of the amygdala
- cMnR
caudal aspect of the median raphe nucleus
- Crh
corticotropin-releasing hormone
- DR
dorsal raphe nucleus
- DRC
dorsal raphe nucleus, caudal part
- DRD
dorsal raphe nucleus, dorsal part (also referred to as the dorsomedial DR)
- DRI
dorsal raphe nucleus, interfascicular part
- DRVL
dorsal raphe nucleus, ventrolateral part
- DRVL/VLPAG
dorsal raphe nucleus, ventrolateral part/ventrolateral periaqueductal gray, also called lateral wing of the DR
- f
fornix
- ic
internal capsule
- LMM
linear mixed model
- LV
lateral ventricle
- mlf
medial longitudinal fasciculus
- MnR
median raphe nucleus
- ox
optic chiasm
- rDRD
rostral aspect of the dorsal raphe nucleus, dorsal part
- rDRD core
core of the rostral aspect of the dorsal raphe nucleus, dorsal part
- rDRD shell
shell of the rostral aspect of the dorsal raphe nucleus, dorsal part
- rDRV
rostral aspect of the dorsal raphe nucleus, ventral part
- rMnR
rostral aspect of the median raphe nucleus
- SERT
serotonin transporter
- SAD
social anxiety disorder
- SEM
standard error of the mean
- SI
social interaction
- slc6a4
solute carrier family 6 (neurotransmitter transporter, serotonin), member 4, serotonin transporter, SERT
- sm
stria medullaris of the thalamus
- st
stria terminalis
- Tph2
tryptophan hydroxylase 2, brain-specific isoform
- tph2
tph2 gene
- Ucn1
urocortin 1
- VLPAG
ventrolateral periaqueductal gray
Footnotes
Conflict of interest
CAL serves on the Scientific Advisory Board of Immodulon Therapeutics Ltd. All other authors declare no financial conflicts of interest related to this work.
Declarations of interest: CAL serves on the Scientific Advisory Board of Immodulon Therapeutics, Ltd. The other authors report no conflicts of interest related to the submitted manuscript.
Ethical statement
This manuscript is in accordance with the Authorship statement of ethical standards for manuscripts submitted to Progress in Neuro-Psychopharmacology & Biological Psychiatry. All authors have read and approved the submission of the manuscript; the manuscript has not been published and is not being considered for publication elsewhere, in whole or in part, in any language, except as an abstract.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Abrams JK, Johnson PL, Hay-Schmidt A, Mikkelsen JD, Shekhar A, Lowry CA (2005) Serotonergic systems associated with arousal and vigilance behaviors following administration of anxiogenic drugs. Neuroscience 133:983–997. [DOI] [PubMed] [Google Scholar]
- Arango V, Underwood MD, Mann JJ (2002) Serotonin brain circuits involved in major depression and suicide. Prog Brain Res 136:443–453. [DOI] [PubMed] [Google Scholar]
- Austin MC, Janosky JE, Murphy HA (2003) Increased corticotropin-releasing hormone immunoreactivity in monoamine-containing pontine nuclei of depressed suicide men. Mol Psychiatry 8:324–332. [DOI] [PubMed] [Google Scholar]
- Bach-Mizrachi H, Underwood MD, Kassir SA, Bakalian MJ, Sibille E, Tamir H, Mann JJ, Arango V (2006) Neuronal tryptophan hydroxylase mRNA expression in the human dorsal and median raphe nuclei: major depression and suicide. Neuropsychopharmacology 31:814–824. [DOI] [PubMed] [Google Scholar]
- Bach-Mizrachi H, Underwood MD, Tin A, Ellis SP, Mann JJ, Arango V (2008) Elevated expression of tryptophan hydroxylase-2 mRNA at the neuronal level in the dorsal and median raphe nuclei of depressed suicides. Mol Psychiatry 13:507–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bale TL, Lee KF, Vale WW (2002) The role of corticotropin-releasing factor receptors in stress and anxiety. Integr Comp Biol 42:552–555. [DOI] [PubMed] [Google Scholar]
- Boldrini M, Underwood MD, Mann JJ, Arango V (2005) More tryptophan hydroxylase in the brainstem dorsal raphe nucleus in depressed suicides. Brain Res 1041:19–28. [DOI] [PubMed] [Google Scholar]
- Bonkale WL, Turecki G, Austin MC (2006) Increased tryptophan hydroxylase immunoreactivity in the dorsal raphe nucleus of alcohol-dependent, depressed suicide subjects is restricted to the dorsal subnucleus. Synapse 60:81–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouwknecht JA, Spiga F, Staub DR, Hale MW, Shekhar A, Lowry CA (2007) Differential effects of exposure to low-light or high-light open-field on anxiety-related behaviors: Relationship to c-Fos expression in serotonergic and non-serotonergic neurons in the dorsal raphe nucleus. Brain Res Bull 72:32–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalmers DT, Lovenberg TW, De Souza EB (1995) Localization of novel corticotropin-releasing factor receptor (CRF(2)) mRNA expression to specific subcortical nuclei in rat brain: Comparison with CRF(1) receptor mRNA expression. J Neurosci 15:6340–6350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christianson JP, Ragole T, Amat J, Greenwood BN, Strong PV, Paul ED, Fleshner M, Watkins LR, Maier SF (2010) 5-hydroxytryptamine 2C receptors in the basolateral amygdala are involved in the expression of anxiety after uncontrollable traumatic stress. Biol Psychiatry 67:339–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cipriano AC, Gomes KS, Nunes-de-Souza RL (2016) CRF receptor type 1 (but not type 2) located within the amygdala plays a role in the modulation of anxiety in mice exposed to the elevated plus maze. Horm Behav 81:59–67. [DOI] [PubMed] [Google Scholar]
- Commons KG, Connolley KR, Valentino RJ (2003) A neurochemically distinct dorsal raphe-limbic circuit with a potential role in affective disorders. Neuropsychopharmacology 28:206–215. [DOI] [PubMed] [Google Scholar]
- Cordero MI, Poirier GL, Marquez C, Veenit V, Fontana X, Salehi B, Ansermet F, Sandi C (2012) Evidence for biological roots in the transgenerational transmission of intimate partner violence. Transl Psychiatry 2:e106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabrowska J, Martinon D, Moaddab M, Rainnie DG (2016) Targeting Corticotropin-Releasing Factor Projections from the Oval Nucleus of the Bed Nucleus of the Stria Terminalis Using Cell-Type Specific Neuronal Tracing Studies in Mouse and Rat Brain. J Neuroendocrinol 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day HE, Greenwood BN, Hammack SE, Watkins LR, Fleshner M, Maier SF, Campeau S (2004) Differential expression of 5HT-1A, alpha(1b) adrenergic, CRF-R1, and CRF-R2 receptor mRNA in serotonergic, gamma-aminobutyric acidergic, and catecholaminergic cells of the rat dorsal raphe nucleus. J Comp Neurol 474:364–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donner NC, Johnson PL, Fitz SD, Kellen KE, Shekhar A, Lowry CA (2012a) Elevated tph2 mRNA expression in a rat model of chronic anxiety. Depress Anxiety 29:307–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donner NC, Kubala KH, Hassell JE Jr., , Lieb MW, Nguyen KT, Heinze JD, Drugan RC, Maier SF, Lowry CA (2018) Two models of inescapable stress increase tph2 mRNA expression in the anxiety-related dorsomedial part of the dorsal raphe nucleus. Neurobiol Stress 8:68–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donner NC, Lowry CA (2013) Sex differences in anxiety and emotional behavior. Pflugers Arch 465:601–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donner NC, Montoya CD, Lukkes JL, Lowry CA (2012b) Chronic non-invasive corticosterone administration abolishes the diurnal pattern of tph2 expression. Psychoneuroendocrinology 37:645–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donner NC, Montoya CD, Lukkes JL, Lowry CA (2012c) Chronic non-invasive corticosterone administration abolishes the diurnal pattern of tph2 expression. Psychoneuroendocrinology 37:645–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donner NC, Siebler PH, Johnson DT, Villarreal MD, Mani S, Matti AJ, Lowry CA(2016) Serotonergic systems in the balance: CRHR1 and CRHR2 differentially control stress-induced serotonin synthesis. Psychoneuroendocrinology 63:178–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- File SE (1980) The use of social interaction as a method for detecting anxiolytic activity of chlordiazepoxide-like drugs. J Neurosci Methods 2:219–238. [DOI] [PubMed] [Google Scholar]
- Forster GL, Pringle RB, Mouw NJ, Vuong SM, Watt MJ, Burke AR, Lowry CA, Summers CH, Renner KJ (2008) Corticotropin-releasing factor in the dorsal raphe nucleus increases medial prefrontal cortical serotonin via type 2 receptors and median raphe nucleus activity. Eur J Neurosci 28:299–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner KL, Hale MW, Lightman SL, Plotsky PM, Lowry CA (2009a) Adverse early life experience and social stress during adulthood interact to increase serotonin transporter mRNA expression. Brain Res 1305:47–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner KL, Hale MW, Oldfield S, Lightman SL, Plotsky PM, Lowry CA (2009b) Adverse experience during early life and adulthood interact to elevate tph2 mRNA expression in serotonergic neurons within the dorsal raphe nucleus. Neuroscience 163:991–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehlert DR, Shekhar A, Morin SM, Hipskind PA, Zink C, Gackenheimer SL, Shaw J, Fitz SD, Sajdyk TJ (2005) Stress and central Urocortin increase anxiety-like behavior in the social interaction test via the CRF1 receptor. Eur J Pharmacol 509:145–153. [DOI] [PubMed] [Google Scholar]
- Gillespie CF, Nemeroff CB (2005) Hypercortisolemia and depression. Psychosom Med 67 Suppl 1:S26–S28. [DOI] [PubMed] [Google Scholar]
- Grubbs FE (1969) Procedures for Detecting Outlying Observations in Samples. Technometrics 11:1–21. [Google Scholar]
- Hale MW, Hay-Schmidt A, Mikkelsen JD, Poulsen B, Bouwknecht JA, Evans AK, Stamper CE, Shekhar A, Lowry CA (2008) Exposure to an open-field arena increases c-Fos expression in a subpopulation of neurons in the dorsal raphe nucleus, including neurons projecting to the basolateral amygdaloid complex. Neuroscience 157:733–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale MW, Shekhar A, Lowry CA (2012) Stress-related serotonergic systems: implications for symptomatology of anxiety and affective disorders. Cell Mol Neurobiol 32:695–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammack SE, Guo JD, Hazra R, Dabrowska J, Myers KM, Rainnie DG (2009) The response of neurons in the bed nucleus of the stria terminalis to serotonin: implications for anxiety. Prog Neuropsychopharmacol Biol Psychiatry 33:1309–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammack SE, Richey KJ, Schmid MJ, LoPresti ML, Watkins LR, Maier SF (2002) The role of corticotropin-releasing hormone in the dorsal raphe nucleus in mediating the behavioral consequences of uncontrollable stress. J Neurosci 22:1020–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammack SE, Richey KJ, Watkins LR, Maier SF (2004) Chemical lesion of the bed nucleus of the stria terminalis blocks the behavioral consequences of uncontrollable stress. Behav Neurosci 118:443–448. [DOI] [PubMed] [Google Scholar]
- Hassell JE Jr, Yamashita PSM, Johnson PL, Zangrossi H Jr, Shekhar A, Lowry CA (2017) Stress, panic, and central serotonergic inhibition In: Stress: Neuroendocrinology and Neurobiology (Fink G, ed), pp 153–164. Amsterdam: Elsevier. [Google Scholar]
- Henckens MJAG, Printz Y, Shamgar U, Dine J, Lebow M, Drori Y, Kuehne C, Kolarz A, Eder M, Deussing JM, Justice NJ, Yizhar O, Chen A (2017) CRF receptor type 2 neurons in the posterior bed nucleus of the stria terminalis critically contribute to stress recovery. Mol Psychiatry 22:1691–1700. [DOI] [PubMed] [Google Scholar]
- Hu P, Maita I, Kwok C, Gu E, Gergues M, Liu J, Pang Z, Swaab DF, Lucassen PJ, Roepke TA, Samuels BA (2019) Corticotropin-releasing hormone signaling in the oval bed nucleus of the stria terminalis mediates chronic stress-induced negative valence behaviors associated with anxiety. bioRxiv preprint first posted online March 9, 2019; doi: 10.1101/572966:1-42. [DOI] [Google Scholar]
- Jennings JH, Sparta DR, Stamatakis AM, Ung RL, Pleil KE, Kash TL, Stuber GD (2013) Distinct extended amygdala circuits for divergent motivational states. Nature 496:224–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen P, Farago AF, Awatramani RB, Scott MM, Deneris ES, Dymecki SM (2008) Redefining the serotonergic system by genetic lineage. Nat Neurosci 11:417–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Kuhn JW, Prescott CA (2004) Childhood sexual abuse, stressful life events and risk for major depression in women. Psychol Med 34:1475–1482. [DOI] [PubMed] [Google Scholar]
- Kim SY, Adhikari A, Lee SY, Marshel JH, Kim CK, Mallory CS, Lo M, Pak S, Mattis J, Lim BK, Malenka RC, Warden MR, Neve R, Tye KM, Deisseroth K (2013) Diverging neural pathways assemble a behavioural state from separable features in anxiety. Nature 496:219–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebow MA, Chen A (2016) Overshadowed by the amygdala: the bed nucleus of the stria terminalis emerges as key to psychiatric disorders. Mol Psychiatry 21:450–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y, Fitz S, Johnson PL, Shekhar A (2008) Repeated stimulation of CRF receptors in the BNST of rats selectively induces social but not panic-like anxiety. Neuropsychopharmacology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levita L, Hammack SE, Mania I, Li XY, Davis M, Rainnie DG (2004) 5-hydroxytryptamine1A-like receptor activation in the bed nucleus of the stria terminalis: electrophysiological and behavioral studies. Neuroscience 128:583–596. [DOI] [PubMed] [Google Scholar]
- Lukkes JL, Kopelman JM, Donner NC, Hale MW, Lowry CA (2013) Development × environment interactions control tph2 mRNA expression. Neuroscience 237:139–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukkes JL, Staub DR, Dietrich A, Truitt W, Neufeld-Cohen A, Chen A, Johnson PL, Shekhar A, Lowry CA (2011) Topographical distribution of corticotropin-releasing factor type 2 receptor-like immunoreactivity in the rat dorsal raphe nucleus: co-localization with tryptophan hydroxylase. Neuroscience 183:47–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier SF, Amat J, Baratta MV, Paul E, Watkins LR (2006) Behavioral control, the medial prefrontal cortex, and resilience. Dialogues Clin Neurosci 8:397–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makino S, Gold PW, Schulkin J (1994) Effects of corticosterone on CRH mRNA and content in the bed nucleus of the stria terminalis; comparison with the effects in the central nucleus of the amygdala and the paraventricular nucleus of the hypothalamus. Brain Res 657:141–149. [DOI] [PubMed] [Google Scholar]
- Marcinkiewcz CA, Mazzone CM, D'Agostino G, Halladay LR, Hardaway JA, DiBerto JF, Navarro M, Burnham N, Cristiano C, Dorrier CE, Tipton GJ, Ramakrishnan C, Kozicz T, Deisseroth K, Thiele TE, McElligott ZA, Holmes A, Heisler LK, Kash TL (2016) Serotonin engages an anxiety and fear-promoting circuit in the extended amygdala. Nature 537:97–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen AQ, Dela Cruz JA, Sun Y, Holmes TC, Xu X (2016) Genetic cell targeting uncovers specific neuronal types and distinct subregions in the bed nucleus of the stria terminalis. J Comp Neurol 524:2379–2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul ED, Lowry CA (2013) Functional topography of serotonergic systems supports the Deakin/Graeff hypothesis of anxiety and affective disorders. J Psychopharmacol 27:1090–1106. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C (1998) The Rat Brain in Stereotaxic Coordinates, Fourth Edition. San Diego: Academic Press. [Google Scholar]
- Peyron C, Petit J-M, Rampon C, Jouvet M, Luppi P-H (1998) Forebrain afferents to the rat dorsal raphe nucleus demonstrated by retrograde and anterograde tracing methods. Neuroscience 82:443–468. [DOI] [PubMed] [Google Scholar]
- Rainnie DG, Bergeron R, Sajdyk TJ, Patil M, Gehlert DR, Shekhar A (2004) Corticotrophin releasing factor-induced synaptic plasticity in the amygdala translates stress into emotional disorders. J Neurosci 24:3471–3479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren J, Friedmann D, Xiong J, Liu CD, Ferguson BR, Weerakkody T, DeLoach KE, Ran C, Pun A, Sun Y, Weissbourd B, Neve RL, Huguenard J, Horowitz MA, Luo L (2018) Anatomically Defined and Functionally Distinct Dorsal Raphe Serotonin Sub-systems. Cell 175:472–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resstel LB, Souza RF, Guimaraes FS (2008) Anxiolytic-like effects induced by medial prefrontal cortex inhibition in rats submitted to the Vogel conflict test. Physiol Behav 93:200–205. [DOI] [PubMed] [Google Scholar]
- Rozeske RR, Evans AK, Frank MG, Watkins LR, Lowry CA, Maier SF (2011) Uncontrollable, but not controllable, stress desensitizes 5-HT1A receptors in the dorsal raphe nucleus. J Neurosci 31:14107–14115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepard JD, Schulkin J, Myers DA (2006) Chronically elevated corticosterone in the amygdala increases corticotropin releasing factor mRNA in the dorsolateral bed nucleus of stria terminalis following duress. Behav Brain Res 174:193–196. [DOI] [PubMed] [Google Scholar]
- Sink KS, Walker DL, Freeman SM, Flandreau El, Ressler KJ, Davis M (2013) Effects of continuously enhanced corticotropin releasing factor expression within the bed nucleus of the stria terminalis on conditioned and unconditioned anxiety. Mol Psychiatry 18:308–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiacci A Jr., Coimbra NC, Zangrossi H Jr. (2012) Differential involvement of dorsal raphe subnuclei in the regulation of anxiety- and panic-related defensive behaviors. Neuroscience 227:350–360. [DOI] [PubMed] [Google Scholar]
- Staub DR, Spiga F, Lowry CA(2005) Urocortin 2 increases c-Fos expression in topographically organized subpopulations of serotonergic neurons in the rat dorsal raphe nucleus. Brain Res 1044:176–189. [DOI] [PubMed] [Google Scholar]
- Steinbusch HWM (1984) Serotonin-immunoreactive neurons and their projections in the CNS In: Classical Transmitters and Transmitter Receptors in the CNS (Björklund A, Hökfelt T, eds), pp 68–125. Amsterdam: Elsevier Science Publishers B.V. [Google Scholar]
- Strong PV, Christianson JP, Loughridge AB, Amat J, Maier SF, Fleshner M, Greenwood BN (2011) 5-hydroxytryptamine 2C receptors in the dorsal striatum mediate stress-induced interference with negatively reinforced instrumental escape behavior. Neuroscience 197:132–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentino RJ, Lucki I, Van BE (2010) Corticotropin-releasing factor in the dorsal raphe nucleus: Linking stress coping and addiction. Brain Res 1314:29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Pett K, Viau V, Bittencourt JC, Chan RKW, Li H-Y, Arias C, Prins GS, Marilyn P, Vale W, Sawchenko PE (2000) Distribution of mRNAs encoding CRF receptors in brain and pituitary of rat and mouse. J Comp Neurol 428:191–212. [DOI] [PubMed] [Google Scholar]
- Vaughan J, Donaldson C, Bittencourt J, Perrin MH, Lewis K, Sutton S, Chan R, Turnbull AV, Lovejoy D, Rivier C, Rivier J, Sawchenko PE, Vale W (1995) Urocortin, a mammalian neuropeptide related to fish urotensin-I and to corticotropin-releasing factor. Nature 378:287–292. [DOI] [PubMed] [Google Scholar]
- Walker DL, Miles LA, Davis M (2009) Selective participation of the bed nucleus of the stria terminalis and CRF in sustained anxiety-like versus phasic fear-like responses. Prog Neuropsychopharmacol Biol Psychiatry 33:1291–1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wscieklica T, Silva MSCF, Lemes JA, Melo-Thomas L, Cespedes IC, Viana MB (2017) Deep brain stimulation of the dorsal raphe inhibits avoidance and escape reactions and activates forebrain regions related to the modulation of anxiety/panic. Behav Brain Res 321:193–200. [DOI] [PubMed] [Google Scholar]