Abstract
Although the current smoking prevalence among Hispanics/Latinxs (10%) is lower than in non-Hispanic whites (15%), higher prevalence is observed among certain subgroups (e.g., Puerto Rican males, 19%). Hispanic/Latinx smokers face unique challenges such as lower awareness and acceptability of nicotine replacement aids, lower prevalence of using counseling or medication, and receiving less advice to quit by their health care providers. Despite these barriers to smoking cessation, few interventions specifically targeted to Hispanic/Latinx smokers have been developed and evaluated. This paper summarizes the design, methods, analysis plan, and sample baseline characteristics of an ongoing randomized controlled trial to assess the efficacy of a Spanish-language self-help smoking cessation intervention among Hispanics/Latinxs. Current smokers who prefer health education materials in Spanish were randomized to one of two conditions. The usual care group received a standard smoking cessation booklet developed by the National Cancer Institute. The intervention group received 10 booklets, 9 pamphlets and a booklet for family and friends mailed monthly over 18 months. All participants complete self-report surveys every 6 months over 2 years. Smoking abstinence is biochemically verified at 12-and 24-month follow-up. A total of 2,387 smokers were screened, 2,056 were eligible and 1417 were enrolled in the study. The primary outcome is self-reported 7-day point prevalence abstinence. If the intervention is deemed efficacious, it has potential to have a large public health impact with respect to reducing smoking rates and smoking related morbidity and mortality among a large underserved minority population.
Keywords: Hispanic, Latino, smoking cessation intervention, randomized controlled trial, self-help intervention, tobacco cigarette
1. Introduction
Hispanics/Latinxs (those of Latin American or Spanish origin) are the largest ethnic minority group in the US, currently comprising 17.8% of the population, and projected to constitute 28.6% by 2060 [1]. The current prevalence of cigarette smoking among US Hispanics/Latinxs (10%) is lower than in non-Hispanic whites (16%). However, rates vary by subgroups, with highest prevalence among Puerto Ricans (19% men; 16% women) and Mexicans (15% men, 7% women) and lowest among Dominicans (6% overall) [2].
Despite smoking fewer cigarettes and being less likely to be daily smokers, Hispanics/Latinxs experience a high burden of tobacco-related morbidity and mortality [3]. Four of the five leading causes of death among Hispanics/Latinxs (cancer, heart disease, stroke, and diabetes) are smoking-related [4]. Lung cancer is the leading cause of cancer death among Hispanic/Latinos and the second among Hispanic/Latinas [2]. Thus, reducing cigarette smoking is a significant public health issue for this population.
Hispanic/Latinx smokers have lower rates of quitting when compared to non-Hispanic whites, despite being more likely to have made a quit attempt [5–7]. They less frequently report receiving advice to quit smoking from health care providers, and have a lower prevalence of using counseling, medication, and/or nicotine replacement therapy, than non-Hispanic whites [5, 6, 8–10]. Other factors associated with poor cessation outcomes include limited access to health care, financial strains, and language barriers [11–19].
Hispanic/Latinx smokers’ utilization of smoking cessation materials has been shown to be higher when delivered in the preferred language [20]. Yet, language-specific services and interventions are lacking [16, 19, 21]. In addition, research evaluating smoking cessation materials for Hispanics/Latinxs is scarce; few randomized trials have been conducted to date [22–24]. Therefore, evidence-based smoking cessation interventions that are culturally sensitive and responsive to the distinct sociocultural needs of Hispanic/Latinx smokers are needed [25, 26].
To address this gap, an existing validated English-language self-help smoking cessation intervention developed by Brandon and colleagues, Forever Free®: Stop Smoking for Good (SSFG) was adapted for Spanish-speaking smokers [27, 28]. SSFG is based on cognitive behavioral theory and empirical evidence on tobacco dependence, cessation and relapse. The series of 10 booklets and 9 pamphlets was found to be cost-effective and highly efficacious among self-quitting smokers. Compared to usual care, the SSFG intervention produced significantly greater smoking abstinence rates through the 30-month follow-up time point (33% vs. 23%) [29].
The process of “transcreating” the evidence-based SSFG into a Spanish-language extended self-help intervention involved translation and cultural adaptation. This systematic approach included incorporating distinctive cultural values such as familism, and relevant images and context into the materials. The resulting intervention presents a potentially cost-effective and highly accessible opportunity to reach the largest and fastest growing population of smokers [28].
This paper describes the design, methods, and baseline sample characteristics of an ongoing randomized controlled trial (RCT). The primary aim is to test the efficacy of an extended “transcreated” evidence-based self-help intervention for Hispanics/Latinxs in producing 7-day point-prevalence abstinence compared to a Spanish-language usual care. We hypothesized that new intervention would produce higher rates of smoking abstinence than usual care. The secondary aims are (1) to evaluate prospective moderator variables (e.g., gender, sub-ethnicity) that may aid in refining and targeting the intervention to maximize impact and (2) to calculate the cost-effectiveness in order to facilitate comparisons with other cessation interventions.
2. Methods
2.1. Study I: Intervention Development
The initial part of the study (Study I) was the transcreation process of the Forever Free®: Stop Smoking for Good (SSFG) intervention. The first of the 10 SSFG booklets provides a general summary of the process of quitting smoking; preparing to quit; using pharmacotherapies (e.g., nicotine replacement therapy, varenicline, etc.); and potential challenges. Each of the remaining 9 booklets includes more extensive information about maintaining abstinence. The content of each booklet is listed in Table 1. The booklets ranged from 13 to 33 pages in length, with a mean of 21 pages. The 9 single-sheet tri-fold color pamphlets, designed to induce a sense of social support, reinforce key messages about quitting smoking (e.g., dealing with stress, keeping weight gain in perspective, finding other forms of positive reinforcement, the effects of second hand smoke). The stories in the pamphlets are communicated via a first-person narrative from a former smoker in order to further enhance the perceived social support, which has been found to benefit smoking cessation [27, 30, 31].
Table 1.
Overview of Study Timeline: Interventions and Assessments
| Month | UC | LDC |
|---|---|---|
| 0 | Baseline Assessment | |
| Usual Care Booklet (NCI Booklet) | Booklet 1: Introduction Booklet for Family and Friends |
|
| One-Time Support Phone Call | ||
| 1 | Booklet 2: Smoking Urges | |
| 2 | Booklet 3: Smoking and Weight | |
| 3 | Booklet 4: What if You Have a Cigarette? | |
| 4 | Pamphlet 1: Angelica’ Story (Quitting smoking) | |
| 5 | Booklet 5: Your Health | |
| 6 | Pamphlet 2 Mario’ Story (Pharmacotherapy) | |
| 6-Month Follow-up Assessment | ||
| 7 | Booklet 6: Smoking, Stress, and Mood | |
| 8 | Pamphlet 3: Rafael’ Story (Smoking urges) | |
| 9 | Booklet 7: Lifestyle Balance | |
| 10 | Pamphlet 4: Jorge Luis’ Story (Managing a slip) | |
| 11 | Pamphlet 5: Carmen’ Story (A healthier lifestyle) | |
| 12 | Booklet 8: Life Without Cigarettes | |
| 12-Month Follow-up Assessment | ||
| CO Collection | ||
| 13 | Pamphlet 6: Maria Estela’ Story (Weight gain concerns) | |
| 14 | Pamphlet 7: Jose Luis’ Story (Social support) | |
| 15 | Booklet 9: The Benefits of Quitting Smoking | |
| 16 | Pamphlet 8: Carolina’ Story (Remaining smoking free around other smokers) | |
| 17 | Pamphlet 9: Ramiro’ Story (Positive activities/pastimes) | |
| 18 | Booklet 10: The Road Ahead | |
| 18-Month Follow-up Assessment | ||
| 24 | 24-Month Follow-up Assessment | |
| CO and Saliva Collection | ||
The steps of the translation and adaptation process, as well as the results of the focus groups and learner verification interviews were described in greater detail by Piñeiro et al (2018) [28]. Briefly, we utilized a series of systematic and iterative procedures to translate and culturally adapt the SSFG booklets and pamphlets. The first step (Phase I) was to conduct formative research to identify novel smoking cessation themes relevant to the target Hispanic/Latinx population. Focus groups were conducted with bilingual smokers to gather data on topics such as cultural-specific characteristics of Hispanic/Latinx smokers and barriers and facilitators to achieving smoking abstinence. Focus group participants were also asked to provide feedback on the visual elements of the booklets and pamphlets of the existing SSFG intervention, as well as the name for the new series in Spanish. Key findings from the focus groups (e.g., negative perception of nicotine replacement therapy, stressors related to immigration experience, important role of family, etc.) were then used to modify the existing booklets and create additional content relevant to Hispanic/Latinx smokers.
Subsequently, the 10 booklets and 9 pamphlets of the English-language SSFG intervention were re-created in Spanish. During Phase II, two rounds of learner verification interviews were conducted, each with a sample of 10 participants interviewed individually. Learner verification assesses attraction, comprehension, self-efficacy, cultural acceptability, and persuasion to verify the suitability of the educational materials with the intended audience [32, 33]. Feedback from the first round of learning verification interviews were used to refine the intervention. Revisions made after the first round of interviews were presented to participants in the second round for additional feedback. As a result of the learner verification interviews, vignettes and text were added to address culturally salient daily stressors related to the participants’ home countries, as well as ways in which family and friends can assist smokers in the quitting process. In addition, photos portraying families, places, and activities depicting the diverse countries of origin of Hispanics/Latinxs were added to the booklets. Lastly, in Phase III the findings from the focus groups and learner verification interviews were used to create the final version of the booklets and pamphlets in Spanish. The result of Study I was the creation of the Spanish-language version of the SSFG intervention, titled Libre del cigarrillo, por mi familia y por mí: Guía para dejar de fumar (LDC). In response to direct feedback from focus group participants, an additional booklet, Para mis familiares y amigos, was written for friends and family members to provide support in quitting smoking [28]. An emergent theme among participants in Study I was the desire for some form of personal contact along with the booklets. Thus, a single, brief telephone call was added to the LDC intervention (described further in Methods section).
2.2. Study II: The Randomized Controlled Trial (RCT)
The RCT consists of two arms, Usual Care (UC) and the newly transcreated Libre del Cigarrillo (LDC) intervention. Participants in the UC condition receive a single Spanish-language smoking-cessation booklet developed by the National Cancer Institute (NCI) [34]. Those in the LDC condition receive the newly translated and adapted self-help intervention developed in Study I, a series comprising 10 booklets, 9 pamphlets, and the newly developed booklet for family and friends. Table 1 shows a timeline of the distribution of intervention materials and follow-up assessments.
The primary outcome is self-reported 7-day point-prevalence abstinence rates at 6-month intervals up to 24 months. We hypothesized that participants in the LDC condition will have higher rates of smoking abstinence than participants in the UC condition. This trial received both state and federal funding, with the former supporting data collection within the state of Florida, and the latter supporting data collection elsewhere in the United States.
2.2.1. Participants
To maximize generalizability, participants were enrolled in the RCT if they met the following inclusion criteria: 1) age ≥ 18; 2) smoking ≥ 5 tobacco cigarettes per week over the past year; 3) not currently enrolled in a face-to-face smoking cessation program; and 4) monolingual Spanish-speaking, or bilingual Spanish-English, who prefer receiving educational health materials in Spanish. The smoking inclusion criterion is based on past research demonstrating higher rates of non-daily and light smoking among Hispanics/Latinxs [11, 35]. To minimize treatment contamination between the intervention and control groups, only one participant per house or apartment building was enrolled in the study. In addition, potential participants who were unable to provide a mailing address in the US were excluded from the study. Individuals who did not meet inclusion criteria were offered the same booklet provided to participants in the UC condition. The sample included 1,417 current cigarette smokers living within the contiguous US and Puerto Rico at the end of accrual (555 from Florida and 852 elsewhere).
2.2.2. Procedures
Recruitment for the RCT was conducted between October, 2016 and June, 2018 via multimedia advertising including: website banners, TV (advertisements and news stories), public transportation signage, social media, radio and newsletters. In addition, recruitment was informed by a Cultural Advisory Board (CAB) comprised of researchers from key focused areas (i.e., Florida, Texas, New Mexico, Puerto Rico, Arizona, and California). CAB members used the established rapport with their local communities to assist in recruiting participants, primarily through distribution of study recruitment flyers.
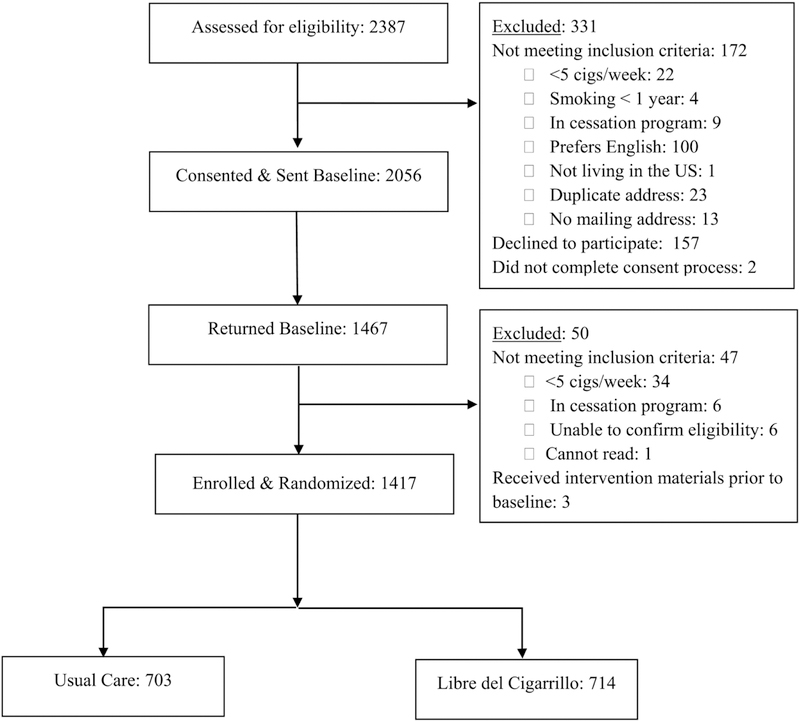
Smokers who responded to the recruitment efforts advertising written materials were screened over the phone. During initial contact, Spanish-speaking research staff collected basic demographic information and a brief smoking history, and assessed eligibility for inclusion in the study. Eligible participants who provided verbal consent were sent the baseline assessment by postal mail or through a link via email (as per their preference) and were told that they would receive $20 for completing the baseline assessment. Participants who completed and returned a baseline assessment and still met the inclusion criteria were enrolled in the RCT. Randomization was conducted using a balanced-permuted block strategy, stratified by sex, smoking status (daily vs. non-daily smoking), and income (household annual income < $10,000 vs. ≥ $10,000). A total of 2,387 smokers were screened, 2,056 met inclusion criteria and consented to participate, 1,467 completed and returned the baseline assessment, and 1,417 remained eligible and were randomized and enrolled in the study. See Figure 1 for a summary of study recruitment and enrollment.
Figure 1.
Study Recruitment and Accrual
Follow-up assessments occur at 6-month intervals after the date of enrollment through 24 months. Participants were provided the option of completing the assessments online or via postal mail, and receive a $20 gift card for completing each assessment. To encourage prompt completion, participants who returned the assessment within a week received a gift valued at less than $2.00 (e.g., coin pouch, eyeglass cleaning cloth, etc.). Participants who complete all four follow-up assessments receive a $50 bonus gift card after the 24 month assessment. Participants are told that compensation is for their research contributions, regardless of their smoking status. In addition, participants living within 75 miles from the cancer center who report abstinence at 12 months are asked to provide a Carbon Monoxide (CO) breath sample. Similarly, at 24 months those living within 75 miles from the cancer center who report abstinence are asked to provide a CO breath sample and a saliva sample for cotinine testing. Participants who complete the biochemical testing are compensated $20, and an additional $20 if they agree to travel to the laboratory to complete the biochemical assessment. This study was reviewed and approved by the Chesapeake Institutional Review Board and is registered on clinicaltrials.gov ().
2.2.3. Study Conditions
2.2.3.1. Usual Care (UC)
Participants in this condition receive NCI’s standard smoking-cessation booklet, Guia: Viva de forma más saludable para usted y su familia, deje de fumar hoy mismo (Live Healthier for You and Your Family Quit Smoking Today). This comprehensive 40-page Spanish-language booklet with high quality content and visual presentation covers topics such as why it is difficult to quit smoking; health risks associated with smoking and the benefits of quitting; practical advice to quit smoking, including how to deal with cravings and relapse; and available options to aid in the quit attempt (e.g., pharmacotherapy, counseling, and quitlines).
2.2.3.2. LDC (Libre del Cigarrillo)
Participants in this condition receive the newly transcreated series of 10 booklets, 9 social support pamphlets (sent during the months that a booklet is not sent), the booklet created for family and friends, and a one-time support phone call to introduce participants to the intervention. The initial booklet and the booklet for family and friends are mailed to participants immediately after randomization, and the rest are mailed sequentially once a month over a period of 18 months. (See Table 1). All participants receive hard copies of the intervention materials delivered by postal mail. However, participants were also given the option to receive the intervention materials electronically (through a link sent via email). Support Phone Call. A 10 minute phone call, intended to build rapport and to provide a personal connection to garner trust and credibility in the intervention, was placed a week after a participant was randomized to the LDC condition. During the phone call, which was conducted in Spanish, a staff member briefly presented the rationale for the intervention, offered suggestions for the intended use of the booklets and pamphlets, and inquired about the reasons for wanting to quit smoking. Of the 714 participants randomized to the intervention arm, 91% completed the support call. If found to be effective, this intervention could blend seamlessly into existing telephone tobacco cessation services such as state quitlines.
2.2.4. Measures
2.2.4.1. Baseline Assessment
All assessments were carried out in Spanish. At baseline, we assessed self-reported socio-demographic characteristics (race/ethnicity, country of origin for each parent, years living in the U.S. if not native born, self-identified Hispanic subethnicity, marital status, etc.), smoking history (including use of electronic cigarettes and smoking cessation aids), and nicotine dependence (using the Spanish-validated Fagerström Test for Nicotine Dependence (FTND) [36]). Socioeconomic status (SES) was measured by education, household income, number of members in household, and debt level [37]. In addition, an 11-point measure of readiness to quit, the Contemplation Ladder [38], and the Stages of Change Algorithm (SOC) [39, 40] were administered to assess motivation to quit smoking. We also administered three motivation-related measures: 1) a brief measure of abstinence-related motivational engagement (ARME) [41], 2) a 9-item short form of the Smoking Situations Confidence Scale (SSC) [42], and 3) the validated Smoking Consequences Questionnaire-Spanish (SCQ-S) [43, 44]. To reduce the response burden on participants, we administered 25 items of the 40-item SCQ-Spanish. We selected the three highest loading items for seven of the eight factors (negative affect reduction, stimulation/state enhancement, health risks, taste/sensory motor, social facilitation, weight control, and craving/addiction). For the eighth factor, boredom reduction, we included all four items. The Short Acculturation Scale for Hispanics (SASH) [45] was administered to assess level of acculturation. The SASH has demonstrated high reliability across Hispanic/Latinx subgroups [11]. In addition, we assessed level of familism using the Attitudinal Familism Scale [46]. Finally, we administered the trait version of the Spanish Positive and Negative Affect Schedule (SPANAS) [47, 48], which has been validated in younger and older populations [49, 50]. These measures of demographics, motivation, mood, acculturation, and familism will be tested as potential moderator variables.
2.2.4.2. Follow-up Assessments
Participants receive follow-up assessments (by mail or e-mail link, as per their preference) at 6-month intervals through 24 months (Table 1). This follow-up duration provides for an assessment of smoking outcomes six months beyond the final mailing of the LDC intervention materials. The assessments include a questionnaire assessing combustible cigarette use since the previous contact, as well as any use of other tobacco products, electronic cigarettes, and pharmacotherapy or other smoking cessation assistance. Participants’ use and evaluation of the self-help material is assessed using the 8-item Client Satisfaction Questionnaire developed in Spanish [51], plus additional items that distinguish the benefits of the content and the repeated contact. To assess the impact of the smoking cessation intervention on health outcomes, the Spanish version of the SF-12 health survey [52, 53] is included in the 18- and 24-month follow-up assessments. Finally, the trait version of the SPANAS is administered at 18 and 24 month follow-up. We attempt to collect a breath CO sample after receipt of the 12- and 24-month follow-up assessments from participants who report smoking abstinence and reside within 75 miles of the research base. Additionally, we attempt to collect a saliva sample at the 24-month follow-up assessment. A portable CO monitor (Vitalograph® by Vitalograph Inc.), is employed to collect the breath sample. The saliva sample is collected in a 2mL tube for cotinine analysis using the NicAlert™ dipstick (Nymox Pharmaceutical Corporation, Hasbrouck Heights, NJ). Abstinence is determined by using a cut-off of 4 ppm for CO and 10ng/mL for cotinine [54, 55]. The disconfirmation rates of this subsample will be used to estimate adjusted smoking rates for the entire sample.
2.2.5. Data Analyses Plan
2.2.5.1. Analysis Overview
Demographics and smoking characteristics at baseline will be compared across intervention conditions using one-way analysis of variance and chi-square tests.
The primary outcome is 7-day point-prevalence abstinence at the four follow-ups. All analyses of treatment effectiveness and prospective moderators will be performed on complete data sets following multiple imputation (see below). To evaluate treatment effectiveness, generalized estimating equations (GEE) will be used to fit population-averaged models of the longitudinally measured 7-day point prevalence abstinence rates, with the main covariates of intervention condition (UC vs. LDC), time (months from baseline, continuous), and the interaction of condition and time. The GEE analysis will allow for assessment of linear changes in abstinence rates over time and group differences in changes. Logistic regression will be used to assess condition differences at the 24-month (final) follow-up, which is six months after the final mailing of the LDC intervention materials. In both the GEE models and logistic regression, potential confounding variables (e.g., group differences on demographic variable) that appear despite randomization will be included.
The secondary aim is to assess prospective moderators of the expected intervention effect (e.g., gender, SES, quitting motivation, nicotine dependence, country of origin, acculturation, trait affect, and self-efficacy). Each moderator, assessed at baseline, will be assessed individually within a logistic regression for the 24-month assessment and within a GEE model by adding the moderator and the interaction term for the moderator and condition. The GEE models will also include interaction terms for the moderator with time and with the time x condition interaction. Significant interaction terms in a GEE model will be further explored using time-specific analyses. Significant interaction terms for the logistic regression at 24 months will be explored by assessing condition differences for each level of categorical moderators.
To manage missing data, multiple imputation under the Missing at Random assumption will be applied using a Markov Chain Monte Carlo method [56] via PROC MI in SAS, given the expected large number of non-monotonic missing data patterns and the expected large number of auxiliary variables (e.g., baseline measures that predict smoking status at follow-up) to be determined by preliminary analyses. A post hoc [57] approach will address the influence of Missing Not at Random (MNAR) data on smoking status (i.e., missing is due to smoking). Sensitivity analyses will be performed by comparing results from different multiple imputation data sets that were generated using different levels of MNAR influence.
2.2.5.2. Sample Size Estimation
The primary statistical analyses will assess the efficacy of LDC in producing 7-day abstinence compared to UC in two ways: (1) across all four follow-up points using generalized estimating equations (GEE), and (2) at the 24-month follow-up using logistic regression. The estimated 7-day point prevalence abstinence rates at each 6-month assessment were based on previous research [27], with an adjustment of 0.625 standard error units to decrease group differences. Abstinence rates were estimated to increase from approximately 12% to 19% for the UC condition and from approximately 16% to 29% for the LDC condition. As previously stated, Study 2 (the RCT) received both state and federal funding; thus, target sample sizes were calculated separately for participants residing in Florida (Florida sample) and those residing elsewhere within the US (non-Florida sample). Both the Florida and the non-Florida sample sizes were estimated with alpha=.05 and a two-sided test. Power was required to be at least .80 for each sample. The larger sample for non-Florida was driven by an expected greater diversity in that population, which may decrease the chances of detecting an effective LDC intervention. Sample size estimates for GEE (GEESIZE version 3.1 [58, 59]) and logistic regression (PASS 16 [60]) resulted in target sample sizes of 250/condition for Florida and 370/condition for non-Florida, for a total sample of 1,240 participants. However, because there was a lower-than-expected survey return rate at the first follow-up we increased the target sample size to 1,400 participants, 550 from Florida and 850 elsewhere.
2.2.5.3. Cost-effectiveness Analysis
Assessment of an intervention such as the Spanish-language LDC smoking cessation intervention must include evaluation of the resources needed to successfully implement the intervention. Our resource utilization methodology is based on collecting information on all resources used in the intervention and affixing a standardized “price” to those resources. This approach, recommended as the most appropriate means of calculating true resource costs [61–63], gives us information on resource utilization and costs which can be compared across organizations. In our evaluation, we will calculate overall resource use/cost of the program, and use the overall cost figures to estimate several measures of the cost-effectiveness of the intervention (e.g., cost per individual enrolled in each intervention arm; cost per person quit).
3. Baseline Results
Descriptive statistics for demographic and smoking variables for the 1,417 participants are presented in Table 2. Of the total sample, 48.3% were female, the mean age was 49.8 (SD = 11.7), 46.8% were married or cohabiting, 29.9% had an education below high school, 58.1% were employed, and 41.1% had a household annual income below $10,000. The sample was 46.4% White, but included diverse Hispanic/Latino subgroups with the largest group being of Mexican descent. Regarding smoking-related variables, participants had been smoking for a mean of 28.1 years (SD = 12.9), the vast majority were daily smokers (93.5%), and they smoked a mean of 14.8 (SD = 8.4) cigarettes per day. They had a low to moderate nicotine dependence according to the FTND (M = 4.9, SD = 2.4). Comparisons between study conditions at baseline indicated that there were more American Indians in the UC condition (4.3%) than in the LDC condition (1.7%) (χ2 = 9.0; p = 0.003). No other significant differences were found between conditions for the remaining variables presented in Table 2.
Table 2.
Baseline Sample Characteristics by Treatment Condition
| Characteristics | UC n = 703 |
LDC n = 714 |
Total N = 1417 |
|---|---|---|---|
| M (SD) or n (%) | M (SD) or n (%) | M (SD) or n (%) | |
| Age | 49.2 (11.5) | 50.3 (11.9) | 49.8 (11.7) |
| Sex (Women) | 339 (48.2%) | 346 (48.5%) | 685 (48.3%) |
| Marital Status (Married or cohabiting) | 316 (45.3%) | 343 (48.4%) | 659 (46.8%) |
| Education (<High school) | 201 (29.4%) | 211 (30.5%) | 412 (29.9%) |
| Employed | 398 (57.8%) | 405 (58.4%) | 803 (58.1%) |
| Income (< $10,000) | 274 (41.4%) | 273 (40.9%) | 547 (41.1%) |
| Race | |||
| White/Caucasian | 313 (44.5%) | 347 (48.6%) | 660 (46.6%) |
| Black or African American | 18 (2.6%) | 23 (3.2%) | 41 (2.9%) |
| American Indian or Alaska Native* | 30 (4.3%) | 12 (1.7%) | 42 (3.0 %) |
| Asian | 0 (0.0%) | 2 (0.3%) | 2 (0.1%) |
| Native Hawaiian or Other Pacific Islander | 13 (1.9%) | 17 (2.4%) | 30 (2.1%) |
| Multiple Races | 28 (4.0%) | 20 (2.8%) | 48 (3.4%) |
| Not reported | 301 (42.8%) | 293 (41.0%) | 594 (41.9%) |
| Born outside the US | 540 (77.5%) | 543 (76.8%) | 1083 (77.1%) |
| Years in the US** | 15.8 (11.4) | 15.3 (12.7) | 15.6 (12.0) |
| Sub-ethnicity | |||
| Puerto Rican | 118 (16.9%) | 117 (16.4%) | 235 (16.7%) |
| Central American | 39 (5.6%) | 48 (6.7%) | 87 (6.2%) |
| Mexican/Mexican American | 233 (33.4%) | 244 (34.2%) | 477 (33.8%) |
| South American | 61 (8.8%) | 64 (9.0%) | 125 (8.9%) |
| Cuban | 154 (22.1%) | 162 (22.7%) | 316 (22.4%) |
| Dominican | 21 (3.0%) | 16 (2.2%) | 37 (2.6%) |
| Other | 13 (1.9%) | 7 (1.0%) | 20 (1.4%) |
| More than one sub-ethnicity | 58 (8.3%) | 55 (7.7%) | 113 (8.0%) |
| Smoking-related variables | |||
| Years smoking | 27.6 (12.8) | 28.5 (13.0) | 28.1 (12.9) |
| Smoke daily | 660 (93.9%) | 665 (93.1%) | 1325 (93.5%) |
| Cigarettes per day | 14.7 (8.8) | 14.9 (8.0) | 14.8 (8.4) |
| FTND1 | 4.9 (2.4) | 5.0 (2.4) | 4.9 (2.4) |
| Contemplation ladder | 6.9 (2.8) | 6.9 (2.8) | 6.9 (2.8) |
Notes:
p < 0.05,
only includes those who were not born in the US or Puerto Rico
LDC = Libre del Cigarrillo; UC= Usual Care
Fagerström Test of Nicotine Dependence
4. Discussion
There is great need for efficacious Spanish-language smoking cessation intervention materials for the Hispanic/Latinx population [20, 64]. Although a prior study found that culturally specific materials and preferred language delivery were associated with both intervention intended use and abstinence at two weeks post intervention [20], there has been little research on the long term efficacy of culturally specific materials. This study addresses this gap through the development, testing, and long-term evaluation of a Spanish-language intervention, Libre del Cigarrillo. The study also represents the first nationwide RCT to test the efficacy of a culturally relevant Spanish-language adaption of a validated self-help smoking cessation intervention. The intervention, which provides self-help materials, delivered via mail over an 18 month period, is based on the efficacious SSFG intervention, which draws from empirical and theoretical research in smoking cessation and relapse prevention [27].
This study has several strengths. First, we used a broad recruitment strategy that included a myriad of local, state and national efforts, augmented by a CAB of national members. Our recruitment strategies yielded a large and diverse sample of Hispanic/Latinx smokers from 39 states across the US, including Puerto Rico. Furthermore, participants in the current sample self-identified with one or more Hispanic/Latinx subgroups, representing 19 Latin American countries, the US, and Spain. This allows us to examine differences in smoking characteristics by Hispanic/Latinx subgroups. Second, this study is the first RCT to provide longitudinal, prospective data on a national sample of Hispanic/Latinx smokers. Thus, we will be able to study demographic and psychological moderators of the expected effect of the intervention over time. Third, self-reported smoking abstinence at 12 and 24 month follow-up is confirmed using biochemical verification among a subset of participants. Few studies testing the efficacy of smoking cessation interventions among Hispanics/Latinxs have biochemically verified self-reported abstinence [21, 65]. This is important because prior studies have demonstrated that Hispanic/Latinx smokers underreport cigarette consumption [66]. Fourth, the distribution of materials is offered electronically, in addition to hard copy. Data collected regarding use and satisfaction with the internet-based materials will aid in assessing this as a potential delivery modality that could reduce the cost and expand the reach of the intervention. Lastly, materials evaluated via this RCT were translated and adapted following a systematic and iterative process, which was informed by the unique cultural context and information needs of Hispanic/Latinx smokers, thus increasing the acceptability and efficacy of the intervention.
The limitations of the present study should be noted. First, there is no true no-treatment control group; participants in the UC condition received NCI’s comprehensive smoking-cessation Spanish-language booklet. We chose to use credible usual care as a comparison condition rather than a no-treatment control for two reasons: 1) when using a reactive recruitment strategy that publicizes an offer of cessation assistance, we believe that there is an ethical obligation to provide at least high quality usual care to all participants; and 2) for public health significance to be high it is more meaningful to evaluate a novel self-help intervention (our LDC booklets and pamphlets) against an existing credible intervention. Second, the generalizability of the results may be affected by self-selection. Compared to the US Hispanic/Latinx population, the study sample has a higher proportion of participants of Puerto Rican and Cuban descent, and a lower proportion of participants of Mexicans descent, despite implementing recruitment strategies targeting regions with varying concentrations of Hispanic/Latinx subgroups. For example, we ran TV ads for a longer period of time in Texas, which has a high population of Hispanics/Latinxs of Mexican descent [1]. However, Puerto Ricans and Cubans have the highest prevalence of cigarette smoking among Hispanic/Latinx subgroups [11, 67]; thus, our sample may be more representative of Hispanic/Latinx smokers per se. Finally, we did not include smokers who prefer to receive their health information in English. Hence, we will be unable to generalize the findings towards Hispanics/Latinxs who are monolingual English-speaking, or bilingual but prefer English. These groups may include Hispanics/Latinxs who are younger and/or have higher levels of acculturation [68, 69].
In summary, this ongoing study is the first national RCT to test the efficacy of a Spanish-language extended self-help smoking cessation intervention among Hispanics/Latinxs. Through a comprehensive recruitment strategy, we were able to enroll a large and diverse sample of smokers that prefer smoking cessation information in Spanish. This study has the potential to offer multiple insights into the feasibility of recruitment, follow-up, and retention of Hispanic/Latinx smokers. If demonstrated to be effective, it would present a low-cost intervention with high dissemination potential and the opportunity to reduce health disparities associated with tobacco smoking and contribute to improve health equity.
Acknowledgments
Funding: This work was supported by the National Institutes of Health [grant number R01CA199143]; and James and the Esther King Biomedical Research Program of the Florida Department of Health [grant number 5JK03].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].U.S. Census Bureau. Facts for features: Hispanic Heritage Month (Release number: CB17-FF.17) 2017. https://www.census.gov/newsroom/facts-for-features/2017/hispanic-heritage.html (accessed 11 February 2019).
- [2].Society AC. Cancer Facts & Figures for Hispanics/Latinos 2018–2020 2018. https://doi:10.3322/caac.21494.
- [3].Trinidad DR, Pérez-Stable EJ, Emery SL, White MM, Grana RA, Messer KS. Intermittent and light daily smoking across racial/ethnic groups in the United States. Nicotine Tob Res 2009;11:203–10. https://doi:10.1093/ntr/ntn018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Statistics NCfH. Leading Causes of Death and Number of Deaths, by Sex, Race, and Hispanic Origin: United States, 1980 and 2016 2017. https://www.cdc.gov/nchs/data/hus/2015/019.pdf (accessed 11 February 2019).
- [5].Babb S Quitting smoking among adults—United States, 2000–2015. MMWR Morbidity and mortality weekly report 2017;65 10.15585/mmwr.mm6552a1.d [DOI] [PubMed]
- [6].Trinidad DR, Perez-Stable EJ, White MM, Emery SL, Messer KS. A nationwide analysis of US racial/ethnic disparities in smoking behaviors, smoking cessation, and cessation-related factors. Am J Public Health 2011;101:699–706. 10.2105/AJPH.2010.1916688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Kahende JW, Malarcher AM, Teplinskaya A, Asman KJQacasbre. Quit attempt correlates among smokers by race/ethnicity. International journal of environmental research and public health. Int J Environ Res Public Health 2011;8:3871–88. https://doi:10.3390/ijerph8103871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Levinson A, Perez-Stable EJ, Espinoza PA, Tanchiva Flores E, Byers TE. Latinos report less use of pharmaceutical aids when trying to quit smoking. Am J Prev Med 2004;26:105–11. [DOI] [PubMed] [Google Scholar]
- [9].Lopez-Quintero C, Crum RM, Neumark YD. Racial/ethnic disparities in report of physician-provided smoking cessation advice: analysis of the 2000 National Health Interview Survey. Am J Public Health 2006;96:2235–9. 10.2105/AJPH.2005.071035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Sonnenfeld N, Schappert SM, Lin SX. Racial and ethnic differences in delivery of tobacco-cessation services. Am J Prev Med 2009;36:21–8. 10.1016/j.amepre.2008.09.028.12. [DOI] [PubMed] [Google Scholar]
- [11].Kaplan RC, Bangdiwala SI, Barnhart JM, Castaneda SF, Gellman MD, Lee DJ, et al. Smoking among U.S. Hispanic/Latino adults: the Hispanic community health study/study of Latinos. Am J Prev Med 2014;46:496–506. 10.1016/j.amepre.2014.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Businelle MS, Kendzor DE, Costello TJ, Cofta-Woerpel L, Li Y, Vidrine JI, et al. Mechanisms linking socioeconomic status to smoking cessation: a structural equation modeling approach. Health Psychol 2010;29:262–73. https://10.1037/a0019285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Kendzor DE, Businelle MS, Costello TJ, Castro Y, Reitzel LR, Cofta-Woerpel LM, et al. Financial strain and smoking cessation among racially/ethnically diverse smokers. Am J Public Health 2010;100:702–6. 10.2105/AJPH.2009.172676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Barbeau EM, Krieger N, Soobader MJ. Working class matters: socioeconomic disadvantage, race/ethnicity, gender, and smoking in NHIS 2000. Am J Public Health 2004;94:269–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Sheffer CE, Stitzer M, Landes R, Brackman SL, Munn T, Moore P. Socioeconomic disparities in community-based treatment of tobacco dependence. Am J Public Health 2012;102:e8–16. 10.2105/AJPH.2011.300519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Baezconde-Garbanati L, Beebe LA, Perez-Stable EJ. Building capacity to address tobacco-related disparities among American Indian and Hispanic/Latino communities: conceptual and systemic considerations. Addiction 2007;102 Suppl 2:112–22. 10.1111/j.1360-0443.2007.01962.x. [DOI] [PubMed] [Google Scholar]
- [17].Vargas Bustamante A, Chen J, Rodriguez HP, Rizzo JA, Ortega AN. Use of preventive care services among Latino subgroups. Am J Prev Med 2010;38:610–9. https://doi:10.1016/j.amepre.2010.01.029. [DOI] [PubMed] [Google Scholar]
- [18].Vinci C, Guo L, Spears CA, Correa-Fernandez V, Etcheverry PE, Lam CY, et al. Socioeconomic indicators as predictors of smoking cessation among Spanish-Speaking Mexican Americans. Ethn Health 2017;1 https://10.1080/13557858.2017.1373074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Wetter D, Mazas C, Daza P, Nguyen L, Fouladi R, Li Y, et al. Reaching and treating Spanish-speaking smokers through the National Cancer Institute’s Cancer Information Service: A randomized controlled trial. Cancer 2007;109 (Suppl 2): 406–413. 10.1002/cncr.22360. [DOI] [PubMed] [Google Scholar]
- [20].Rodriguez Esquivel D, Webb Hooper M, Baker EA, McNutt MD. Culturally specific versus standard smoking cessation messages targeting Hispanics: An experiment. Psychol Addict Behav 2015;29:283–9. 10.1037/adb0000044. [DOI] [PubMed] [Google Scholar]
- [21].Webb MS, Rodriguez-Esquivel D, Baker EA. Smoking cessation interventions among Hispanics in the United States: A systematic review and mini meta-analysis. Am J Health Promot 2010;25:109–18. 10.4278/ajhp.090123-LIT-25. [DOI] [PubMed] [Google Scholar]
- [22].de Dios MA, Cano MÁ, Vaughan EL, Childress SD, McNeel MM, Harvey LM, et al. A pilot randomized trial examining the feasibility and acceptability of a culturally tailored and adherence-enhancing intervention for Latino smokers in the U.S. PLoS One 2019;14:e0210323-e. 10.1371/journal.pone.0210323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Pollak KI, Lyna P, Bilheimer AK, Gordon KC, Peterson BL, Gao X, et al. Efficacy of a couple-based randomized controlled trial to help Latino fathers quit smoking during pregnancy and postpartum: the Parejas trial. Cancer Epidemiol Biomarkers Prev 2015;24:379–85. https://doi:10.1158/1055-9965.EPI-14-0841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Stanton CA, Papandonatos GD, Shuter J, Bicki A, Lloyd-Richardson EE, de Dios MA, et al. Outcomes of a Tailored Intervention for Cigarette Smoking Cessation Among Latinos Living With HIV/AIDS. Nicotine Tob Res 2015;17:975–82. https://doi:10.1093/ntr/ntv014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Zsembik BA, Fennell D. Ethnic variation in health and the determinants of health among Latinos. Soc Sci Med 2005;61:53–63. 10.1016/j.socscimed.2004.11.040. [DOI] [PubMed] [Google Scholar]
- [26].Santisteban D, Vega RR, Suarez-Morales L. Utilizing dissemination findings to help understand and bridge the research and practice gap in the treatment of substance abuse disorders in Hispanic populations. Drug Alcohol Depend 2006;84 (Suppl 1):S94–101. 10.1016/j.drugalcdep.2006.05.011. [DOI] [PubMed] [Google Scholar]
- [27].Brandon TH, Simmons VN, Sutton SK, Unrod M, Harrell PT, Meade CD, et al. Extended Self-Help for Smoking Cessation: A Randomized Controlled Trial. Am J Prev Med 2016;51:54–62. 10.1016/j.amepre.2015.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Piñeiro B, Díaz DR, Monsalve LM, Martínez Ú, Meade CD, Meltzer LR, et al. Systematic Transcreation of Self-Help Smoking Cessation Materials for Hispanic/Latino Smokers: Improving Cultural Relevance and Acceptability. J Health Commun 2018;23:350–9. 10.1080/10810730.2018.1448487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Simmons VN, Sutton SK, Meltzer LR, Unrod M, Meade CD, Brandon TH. Long-term outcomes from a self-help smoking cessation randomized controlled trial. Psychol Addict Behav 2018;32:710–4. https://doi:10.1037/adb0000401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Cresswell KG, Cheng Y, Levine MD. A test of the stress-buffering model of social support in smoking cessation: is the relationship between social support and time to relapse mediated by reduced withdrawal symptoms? Nicotine Tob Res 2015;17:566–71. https://doi:10.1093/ntr/ntu192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Scholz U, Stadler G, Ochsner S, Rackow P, Hornung R, Knoll N. Examining the relationship between daily changes in support and smoking around a self-set quit date. Health Psychol 2016;35:514–7. https://doi:10.1037/hea0000286. [DOI] [PubMed] [Google Scholar]
- [32].Doak LG, Doak CC, Meade CD. Strategies to improve cancer education materials. Oncol Nurs Forum 1996;23:1305–12. [PubMed] [Google Scholar]
- [33].Doak C, Doak L, Root J. Teaching patients with low literacy skills. 1996 Philadelphia, PA: JB Lippencott Co Google Scholar; 1996. [Google Scholar]
- [34].National Cancer Institute. Guía: Viva de forma más saludable para usted y su familia, deje de fumar hoy mismo (Guide: Live Healthier for You and Your Family Quit Smoking Today) Publication Number: 11–7780. 2011. https://smokefree.gov/sites/default/files/pdf/guia-para-dejar-de-fuma-2011.pdf (accessed 11 February 2019).
- [35].Okuyemi KS, Harris KJ, Scheibmeir M, Choi WS, Powell J, Ahluwalia JS. Light smokers: issues and recommendations. Nicotine Tob Res 2002;4:S103–S12. 10.1080/1462220021000032726. [DOI] [PubMed] [Google Scholar]
- [36].Becoña E, Vazquez FL. The Fagerstrom Test for Nicotine Dependence in a Spanish sample. Psychol Rep 1998;83:1455–8. 10.2466/pr0.1998.83.3f.1455. [DOI] [PubMed] [Google Scholar]
- [37].Chiang K, Borrelli B. Income predictors of smoking cessation among Hispanics. J Health Psychol 2014;19:869–76. 10.1177/1359105313481076. [DOI] [PubMed] [Google Scholar]
- [38].Biener L, Abrams DB. The Contemplation Ladder: validation of a measure of readiness to consider smoking cessation. Health Psychol 1991;10:360–5. 10.1037/0278-6133.10.5.360. [DOI] [PubMed] [Google Scholar]
- [39].Becoña E, Becoña Iglesias E, Pontevedra L. Evaluación de la conducta de fumar. Adicciones 2004;16:201–36. [Google Scholar]
- [40].Nath V, Herzog T, Brandon T. A comparison of the contemplation ladder and the stages of change in a college student sample. Poster presented at the annual convention of the Society for Research on Nicotine and Tobacco, Savannah, GA 2002. [Google Scholar]
- [41].Simmons VN, Heckman BW, Ditre JW, Brandon TH. A measure of smoking abstinence-related motivational engagement: development and initial validation. Nicotine Tob Res 2010;12:432–7. 10.1093/ntr/ntq020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Velicer WF, Diclemente CC, Rossi JS, Prochaska JO. Relapse situations and self-efficacy: an integrative model. Addict Behav 1990;15:271–83. 10.1016/0306-4603(90)90070-E. [DOI] [PubMed] [Google Scholar]
- [43].Vidrine JI, Vidrine DJ, Costello TJ, Mazas C, Cofta-Woerpel L, Mejia LM, et al. The Smoking Consequences Questionnaire: Factor structure and predictive validity among Spanish-speaking Latino smokers in the United States. Nicotine Tob Res 2009;11:1280–8. 10.1093/ntr/ntp128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Reig-Ferrer A, Cepeda-Benito A. Smoking expectancies in smokers and never smokers: an examination of the smoking Consequences Questionnaire-Spanish. Addict Behav 2007;32:1405–15. 10.1016/j.addbeh.2006.10.004. [DOI] [PubMed] [Google Scholar]
- [45].Marin G, Sabogal F, Marin BV, Otero-Sabogal R, Perez-Stable EJ. Development of a short acculturation scale for Hispanics. Hisp J Behav Sci 1987;9:183–205. 10.1177/07399863870092005. [DOI] [Google Scholar]
- [46].Steidel AGL, Contreras JM. A new familism scale for use with Latino populations. Hisp J Behav Sci 2003;25:312–30. 10.1177/0739986303256912. [DOI] [Google Scholar]
- [47].Joiner TE Jr., Sandin B, Chorot P, Lostao L, Marquina G. Development and factor analytic validation of the SPANAS among women in Spain: (more) cross-cultural convergence in the structure of mood. J Pers Assess 1997;68:600–15. 10.1207/s15327752jpa6803_8. [DOI] [PubMed] [Google Scholar]
- [48].Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. J Pers Soc Psychol 1988;54:1063–70. 10.1037//0022-3514.54.6.1063.46. [DOI] [PubMed] [Google Scholar]
- [49].Buz J, Perez-Arechaederra D, Fernandez-Pulido R, Urchaga D. Factorial Structure and Measurement Invariance of the PANAS in Spanish Older Adults. Span J Psychol 2015;18:E3 10.1017/sjp.2015.6. [DOI] [PubMed] [Google Scholar]
- [50].Ortuno-Sierra J, Santaren-Rosell M, Albeniz AP, Fonseca-Pedrero E. Dimensional structure of the Spanish version of the Positive and Negative Affect Schedule (PANAS) in adolescents and young adults. Psychol Assess 2015;27:e1–9. 10.1037/pas0000107. [DOI] [PubMed] [Google Scholar]
- [51].Roberts RE, Atrkisson CC, Mendias RM. Assessing the client satisfaction questionnaire in English and Spanish. Hisp J Behav Sci 1984;6:385–96. 10.1177/07399863840064004. [DOI] [Google Scholar]
- [52].Vilagut G, Valderas JM, Ferrer M, Garin O, Lopez-Garcia E, Alonso J. [Interpretation of SF-36 and SF-12 questionnaires in Spain: physical and mental components]. Med Clin (Barc) 2008;130:726–35. [DOI] [PubMed] [Google Scholar]
- [53].Ware J Jr., Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care 1996;34:220–33. 10.2307/3766749. [DOI] [PubMed] [Google Scholar]
- [54].Cropsey KL, Trent LR, Clark CB, Stevens EN, Lahti AC, Hendricks PS. How low should you go? Determining the optimal cutoff for exhaled carbon monoxide to confirm smoking abstinence when using cotinine as reference. Nicotine Tob Res 2014;16:1348–55. 10.1093/ntr/ntu085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].SRNT Subcommittee on Biochemical Verification. Biochemical verification of tobacco use and cessation. Nicotine Tob Res 2002;4:149–59. 10.1080/14622200210123581. [DOI] [PubMed] [Google Scholar]
- [56].Schafer JL. Analysis of incomplete multivariate data: Chapman and Hall/CRC; 1997. [Google Scholar]
- [57].Rubio DB. Multiple imputation for nonresponse in surveys 1987.
- [58].Rochon J Application of GEE procedures for sample size calculations in repeated measures experiments. Stat Med 1998;17:1643–58. . [DOI] [PubMed] [Google Scholar]
- [59].Dahmen G, Rochon J, Konig IR, Ziegler A. Sample size calculations for controlled clinical trials using generalized estimating equations (GEE). Methods Inf Med 2004;43:451–6. 10.1055/s-0038-1633896. [DOI] [PubMed] [Google Scholar]
- [60].PASS 16 Power Analysis and Sample Size Software Kaysville, Utah, USA: NCSS, LLC; 2018. [Google Scholar]
- [61].Meltzer D Valuing health care: Costs, benefits, and effectiveness of pharmaceuticals and other medical technologies New York: Cambridge University Press; 1996. 10.1002/pam.4050150323. [DOI] [Google Scholar]
- [62].Gold MR. Panel on cost-effectiveness in health and medicine. Med Care 1996;34:DS197–9. [PubMed] [Google Scholar]
- [63].Weinstein MC, Siegel JE, Gold MR, Kamlet MS, Russell LB. Recommendations of the Panel on Cost-Effectiveness in Health and Medicine. JAMA 1996;276:1253–8. [PubMed] [Google Scholar]
- [64].Blumenthal DS. Barriers to the provision of smoking cessation services reported by clinicians in underserved communities. J Am Board Fam Med 2007;20:272–9. 10.3122/jabfm.2007.03.060115. [DOI] [PubMed] [Google Scholar]
- [65].Borrelli B, McQuaid EL, Novak SP, Hammond SK, Becker B. Motivating Latino caregivers of children with asthma to quit smoking: a randomized trial. J Consult Clin Psychol 2010;78:34–43. 10.1037/a0016932. [DOI] [PubMed] [Google Scholar]
- [66].Perez-Stable EJ, Marin BV, Marin G, Brody DJ, Benowitz NL. Apparent underreporting of cigarette consumption among Mexican American smokers. Am J Public Health 1990;80:1057–61. 10.2105/AJPH.80.9.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Martell BN, Garrett BE, Caraballo RS. Disparities in Adult Cigarette Smoking - United States, 2002–2005 and 2010–2013. MMWR Morb Mortal Wkly Rep 2016;65:753–8. 10.15585/mmwr.mm6530a1. [DOI] [PubMed] [Google Scholar]
- [68].Pew Research Center. A majority of English-speaking Hispanics in the U.S. are bilingual 2015. http://www.pewresearch.org/fact-tank/2015/03/24/a-majority-of-english-speaking-hispanics-in-the-u-s-are-bilingual/ (accessed 11 February 2019).
- [69].Lara M, Gamboa C, Kahramanian MI, Morales LS, Bautista DE. Acculturation and Latino health in the United States: a review of the literature and its sociopolitical context. Annu Rev Public Health 2005;26:367–97. 10.1146/annurev.publhealth.26.021304.144615. [DOI] [PMC free article] [PubMed] [Google Scholar]