Abstract
Increasing evidence shows that puberty onset is largely dependent on body weight rather than chronological age. To investigate the mechanism involved in the energetic control of puberty onset, the present study examined effects of chronic food restriction during the prepubertal period and the resumption of ad libitum feeding for 24 and 48 h on estrous cyclicity, Kiss1 (kisspeptin gene), Tac3 (neurokinin B gene) and Pdyn (dynorphin A gene) expression in the hypothalamus, luteinizing hormone (LH) secretion and follicular development in female rats. When animals weighed 75 g, they were subjected to a restricted feeding to retard growth to 70–80 g by 49 days of age. Then, animals were subjected to ad libitum feeding or remained food-restricted. The growth-retarded rats did not show puberty onset associated with suppression of both Kiss1 and Pdyn expression in the arcuate nucleus (ARC). 24-h ad libitum feeding increased tonic LH secretion and the number of Graafian and non-Graafian tertiary follicles with an increase in the numbers of ARC Kiss1- and Pdyn-expressing cells. 48-h ad libitum feeding induced the vaginal proestrus and a surge-like LH increase with an increase in Kiss1-expressing cells in the anteroventral periventricular nucleus (AVPV). These results suggest that the negative energy balance causes pubertal failure with suppression of ARC Kiss1 and Pdyn expression and then subsequent gonadotropin secretion and ovarian function, while the positive energetic cues trigger puberty onset via an increase in ARC Kiss1 and Pdyn expression and thus gonadotropin secretion and follicular development in female rats.
Keywords: Dynorphin A, Follicular development, Kisspeptin, Luteinizing hormone, Puberty
Attainment of reproductive capacity at puberty has been known to be initiated by an activation of the hypothalamic mechanism controlling tonic hypophyseal gonadotropin secretion in mammals [1,2,3]. It is well accepted that nutritional or energetic cues are important determinants of the initiation of tonic gonadotropin secretion during pubertal development, as evidenced by hyposecretion of gonadotropins when growth is retarded by food restriction in several mammalian species including cattle [4], sheep [5] and rats [6, 7]. Interestingly, such animals exhibited puberty onset when they reached “critical” body weights, at which normally grown animals exhibited puberty onset [5, 6]. The “critical” body weight hypothesis for determining puberty onset was initially proposed by Frisch and Revelle [8]. Their findings showed puberty onset in girls when they reached a body weight of around 47 kg at 17 years old in the 1840s, and at 13 years old in the 1960s along with nutritional improvements [9, 10]. Collectively, several lines of evidence suggested that the timing of puberty onset largely depends on body weight rather than chronological age in mammals [5,6,7,8,9].
Previous studies suggested that hypothalamic kisspeptin neurons, that govern mammalian reproduction, are involved in the mechanism regulating the pubertal increase in hypophyseal gonadotropin secretion [11,12,13,14,15,16,17,18,19,20]. Kisspeptin signaling in the hypothalamus is thought to be fundamental to initiate tonic hypophyseal gonadotropin secretion, because deficiency of kisspeptin or the kisspeptin receptor (also known as GPR54, deorphanized in 2001 [21, 22]) gene resulted in hypogonadotropic hypogonadism associated with pubertal failure in humans [11,12,13] and in animal models [14,15,16,17,18,19,20]. The hypothalamic arcuate nucleus (ARC) kisspeptin neurons, which co-express neurokinin B- and dynorphin A, thus referred to as KNDy neurons, are suggested to play a key role in generating pulsatile secretion of gonadotropin-releasing hormone (GnRH), which controls tonic hypophyseal gonadotropin secretion in mammals [3, 23, 24]. The currently working model suggests that each GnRH pulse is triggered by kisspeptin secreted from KNDy neurons, in which neurokinin B facilitates and dynorphin A inhibits the synchronized activity of KNDy neuronal cluster [23,24,25,26]. Indeed, Keen et al. [27] showed that pulsatile secretion of kisspeptin in the median eminence was well correlated with pulsatile secretion of GnRH detected in the median eminence in pubertal female monkeys. Further, central administration of neurokinin B or a dynorphin A antagonist increased the frequency of luteinizing hormone (LH) pulses and multiple unite activity volleys, which have been considered to reflect the rhythmic oscillations in the activity of KNDy neurons as an intrinsic source of the GnRH pulse generator, in sheep and goats [23,24,25,26]. On the other hand, anterior hypothalamic kisspeptin neuronal population in the anteroventral periventricular nucleus (AVPV) or preoptic area (POA) is now considered as a GnRH surge generator in response to the positive feedback action of estrogen derived from the preovulatory follicle(s) in mammals, because estrogen increases Kiss1 (kisspeptin gene) expression or activates kisspeptin neurons in the AVPV or POA in all mammals examined to date [28,29,30,31,32,33,34]. Since energetic deficiency negatively regulates GnRH/LH pulse [35, 36] and surge [37], both ARC KNDy and AVPV/POA kisspeptin neurons would be major targets of negative energy balance in prepubertal period, during which energy is mainly consumed for body growth rather than reproductive function. Indeed, suppression of hypothalamic Kiss1 and Tac3 expression has been shown in peripubertal female rats subjected to short-time fasting [38, 39]. Suppression of hypothalamic Kiss1 expression by short-time fasting has also been reported in adult rats and mice [40,41,42].
The present study aims to investigate whether energetic cues control puberty onset via regulating Kiss1, Tac3 (neurokinin B gene) and Pdyn (dynorphin A gene) expression in the ARC KNDy neurons and AVPV/POA kisspeptin neurons. To this end, we used prepubertal chronic growth-retarded female rats [6, 43, 44]. This chronic growth-retarded rat model has an advantage to analyze the effect of energetic cues on KNDy gene expression during the pubertal transition, because previous studies demonstrated that the chronic growth-retarded rat model retained hypogonadotropic status during the food restriction and synchronously exhibited puberty onset few days after the resumption of ad libitum feeding [6, 43, 44]. The present study, therefore, examined the effects of chronic food restriction started at prepubertal period and the resumption of ad libitum feeding on pubertal sign, such as vaginal proestrus, Kiss1,Tac3 and Pdyn expression in the ARC, LH secretion, uterine and ovarian weight and follicular development in the chronic growth-retarded rat model. AVPV Kiss1 expression was also determined, because previous studies demonstrated that the growth-retarded rat model showed ovulation a few days after the resumption of ad libitum feeding [6, 43].
Materials and Methods
Animals
Wistar-Imamichi strain rats were purchased from the Institute for Animal Reproduction (Kasumigaura, Japan). The rats were kept in a room with a temperature of 23 ± 3oC with a 14/10 h light/dark cycle (lights on at 0500 h) and, supplied with standard rodent chow (CE-2; CLEA Japan, Tokyo, Japan) and water ad libitum unless otherwise noted. Female rats (7–8 weeks of age) having at least two consecutive regular 4-day estrous cycles, which were determined by vaginal smears, were mated overnight with stud male rats (26–52 weeks of age) on the day of proestrus. Pregnant females, which were determined by the presence of vaginal plugs, were housed individually.
The day that a newborn litter was found at noon was designated postnatal day 0. The litter size was adjusted to eight on day 1 to minimize the growth variation within and between litters. The female pups were weaned on day 20 and housed individually. The body weight, food intake, vaginal opening and vaginal smear, to detect estrous cyclicity, were daily monitored in the morning. Animals were fed ad libitum until the day their body weights reached 75 g (the mean ± SD of age, 28.2 ± 1.4 days (range, 26–31 days); the mean ± SD of food intake at the last day of the ad libitum feeding, 12.8 ± 1.7 g (range, 8.8–15.0 g)), and then fed restricted quantities of chow, which was varied individually dependent on daily weight gain, once daily (0930 h) to keep their body weights at 70–80 g by day 49 (the initial food intake, 6.9 g; the mean ± SD daily food intake during food restriction, 6.1 ± 0.2 g (range, 4.8–6.9 g)). Then, half of the animals were subjected to ad libitum feeding thereafter (the mean ± SD daily food intake, 15.5 ± 1.5 g (range, 12.6–17.7 g)) and the other half remained food-restricted (the mean ± SD daily food intake, 5.7 ± 0.2 g (range, 5.2–6.0 g)). These ad libitum-fed animals and food-restricted controls were subjected to either brain, ovary and uterus sampling (n = 4 for each groups) or blood sampling (n = 4 for each groups) on days 50 or 51 (24 or 48 h after the resumption of ad libitum feeding, respectively), as previous studies showed the resumption of ad libitum feeding resulted in the first ovulation in the growth-retarded rat model at 2 or 3 days later [6, 43].
Care of the animals and all the experimental procedures used in these experiments were approved by the Committee on Animal Experiments of the Graduate School of Bioagricultural Sciences, Nagoya University, Japan.
Brain sampling
Brain samples were obtained from the animals subjected to ad libitum feeding for 24 or 48 h and the age-matched food-restricted controls on 50 and 51 days of age. The descending aorta was clamped and the upper body of rats were perfused with 0.05 M phosphate-buffered saline (PBS) followed by 4% paraformaldehyde in 0.05 M phosphate buffer between 1030 and 1330 h under deep anesthesia with an injection of sodium pentobarbital (40 mg/kg). The brains were immediately removed and post-fixed in the same fixative overnight at 4oC. The brains were then immersed in 30% sucrose in 0.05 M phosphate buffer until the brain sank. Frozen frontal sections containing the AVPV and ARC (50-μm thickness) were made by a cryostat according to a rat brain atlas [45]. Every two AVPV section was used for in situ hybridization to visualize Kiss1 expression as described previously [29]. Every fourth ARC section was used for in situ hybridization to visualize Kiss1, Tac3 and Pdyn expression, respectively.
In situ hybridization for Kiss1, Tac3 and Pdyn
The sections were washed with PBS and treated with 1 μg/ml protease K for 15 min at 37oC. The sections were incubated with 0.25% acetic anhydride in 0.1 M triethanolamine for 10 min and then hybridized overnight at 60oC with a digoxigenin (DIG)-labeled anti-sense cRNA probe for either Kiss1, Tac3 or Pdyn. Each DIG-labeled anti-sense cRNA probe corresponded to nucleotides 33-349 of Kiss1 (GenBank accession no AY196983), 180-304 of Tac3 (GenBank accession no NM_019162) and 315-731 of Pdyn (GenBank accession no NM_019374), respectively. After hybridization, the sections were washed with 2 × SSC containing 50% formamide for 15 min at 60 oC twice. The sections were then treated with 20 μg/ml RNase A for 30 min at 37oC and then immersed sequentially in 2 × SSC, 0.5 × SSC and DIG- 1 buffer [100 mM Tris-HCl (pH7.5), 150 mM NaCl and 0.01% Tween 20] for 15 min, twice each. Following this, the sections were immersed in 1.5% blocking reagent (Boehringer Mannheim, Mannheim, Germany) in DIG-1 buffer for 1 h at 37oC and incubated with an alkaline phosphatase conjugated anti-DIG antibody (1:1,000; Roche Diagnostics, Indianapolis, IN, USA, RRID:AB_514497) for 2 h at 37oC. Then, the sections were washed with DIG-1 buffer and DIG-3 buffer [100 mM Tris-HCl (pH 9.5), 100 mM NaCl and 50 mM MgCl2]. Following this, the sections were treated with a chromogen solution (337 μg/ml 4-nitro blue tetrazolium chloride and 175 μg/ml 5-bromo-4-chloro-3-indolyl-phosphate in DIG-3 buffer, Roche Diagnostics) for 1 h. The reaction was stopped by adding a reaction stop solution [10 mM Tris-HCl (pH 7.6) and 1 mM EDTA (pH 8.0)]. The sections were mounted on gelatin-coated glass slides. The visualized cells throughout the AVPV (from 0.12 mm anterior to 0.60 mm posterior to the bregma) and ARC (from 1.72 to 4.36 mm posterior to the bregma) were unilaterally counted under a light microscope (BX53, Olympus, Tokyo, Japan): two of the authors independently counted the visualized cells, which detected subjectively, in all the sections, and the averages were calculated in each individual. Specificity of antisense cRNA probes for Kiss1, Tac3 and Pdyn were verified by control experiments using sense cRNA probes. No signals were found in the sections incubated with sense cRNA probes for Kiss1, Tac3 or Pdyn.
Ovary and uterus sampling
Ovarian and uteri samples were obtained from the animals subjected to ad libitum feeding for 24 or 48 h and the age-matched food-restricted controls on 50 and 51 days of age. Ovaries and uteri were collected and weighed immediately after the perfusion of the upper body with the fixative solution. The ovaries were then fixed in 10% neutral buffered formalin for 24 h, dehydrated through increasing concentrations of ethanol, and were embedded with paraffin. Serial 8-μm sections of ovaries were made by using a microtome (RM2235, Leica, Wetzlar, Germany) and mounted on gelatin-coated glass slides. Ovarian sections on the slides were deparaffinized and stained with hematoxylin and eosin and then subjected to the quantitative analysis of follicular development.
Quantitative analysis of follicular development
The number of follicles were counted every fifth section under a light microscope (BX53) according to Yamada et al. [46]. To avoid doubly counting the same follicle, only follicles with nucleoli of oocytes were counted. The stages of follicles were classified according to Pedersen and Peters [47]. Briefly, the primordial follicle is defined as a small oocyte surrounded with epithelial cells; the primary follicle is defined as a growing oocyte surrounded with complete single layer of cuboidal epithelial cells; the secondary follicle is defined as a growing oocyte surrounded with multiple layers of granulosa cells without a visible cavity; the tertiary follicle is defined as a large oocyte surrounded with multiple layers of granulosa cells with one or more cavities containing the follicle fluid. The atretic follicles, defined by any one of the following features —loose granulosa layers, dead granulosa cells scattered into antrum, pyknotic granulosa cells, or degenerated oocyte— were not included.
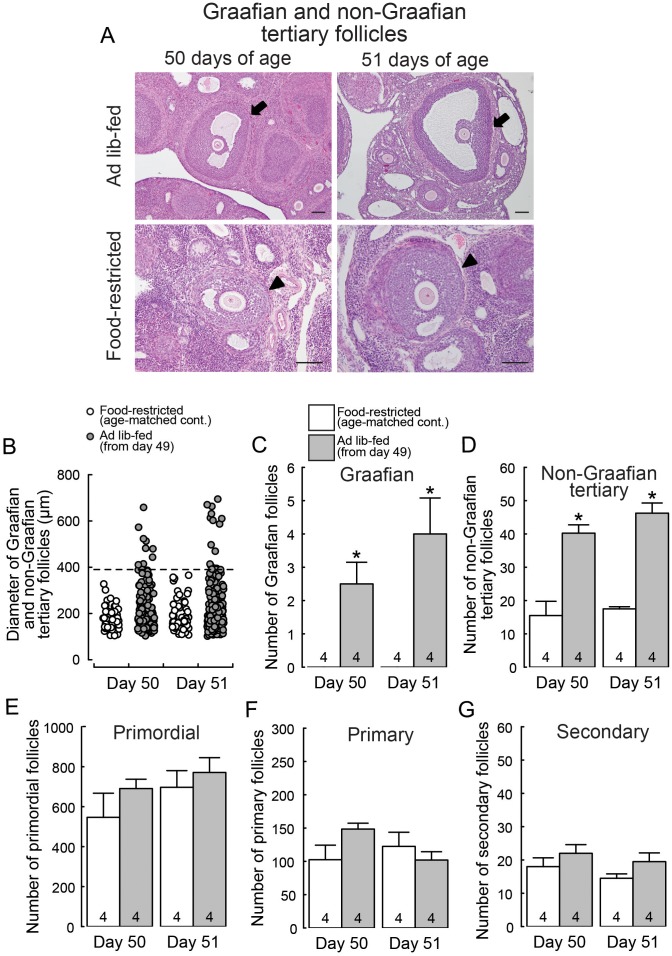
The tertiary follicle was further classified in terms of the mean diameter that was calculated using the maximal diameter and the diameter at a right angle to it by using Image J software (https://imagej.nih.gov/ij/). According to Hirshfield and Midgrey [48], the tertiary follicle with a mean diameter greater than 390 µm was termed as Graafian.
Blood sampling
Blood samples were obtained from the animals subjected to ad libitum feeding for 24 or 48 h and the age-matched food-restricted controls on 50 and 51 days of age. Briefly, blood samples (50 μl) were collected from freely moving conscious rats for 3 h, at 6-min intervals, starting at 1300 h to detect either tonic or surge-mode of LH secretion, through a silicon cannula (0.5 mm inner diameter; 1.0 mm outer diameter; Shin-Etsu polymer, Tokyo, Japan) inserted into the right atrium through the jugular vein on the day before blood sampling under anesthesia with an injection of ketamine (26.7 mg/kg)-xylazine (5.3 mg/kg) mixture and isoflurane inhalation (1–3% in air). An equivalent volume of rat blood cells, taken from donor rats and diluted with heparinized saline, was replaced through the cannula after each blood collection to keep the hematocrit constant. Plasma were separated by centrifugation, collected and stored at –20ºC until assayed for LH.
Radioimmunoassay and LH pulse parameter analysis
Plasma LH concentrations were determined by a double-antibody radioimmunoassay (RIA) using a rat LH RIA kit provided by the National Hormone and Peptide Program (Torrance, CA, USA) and were expressed in terms of the NIDDK rat LH-RP-3. The detectable levels ranged from 3.9 to 2500 pg/tube for 25 μl plasma samples. Intra- and inter-assay coefficients of variation were 5.8% and 7.9% at the level of 1.8 ng/ml, respectively.
LH pulse parameters were determined by the PULSAR computer program [49]. Mean LH concentrations as well as the baseline level, frequency and amplitude of LH pulses were calculated for the 3-h sampling period in each individual and then groups.
Statistical analysis
Statistical differences in the number of Kiss1-, Tac3- or Pdyn-expressing cells, LH pulse parameters, body weights, ovarian weights, uterine weights as well as the number of primordial, primary, secondary and tertiary follicles between the ad libitum-fed rats and age-matched food-restricted controls were determined by Student’s t-test using the SAS University Edition (https://www.sas.com/).
Results
Ad libitum feeding induced catch-up growth and puberty onset in the growth-retarded female rats
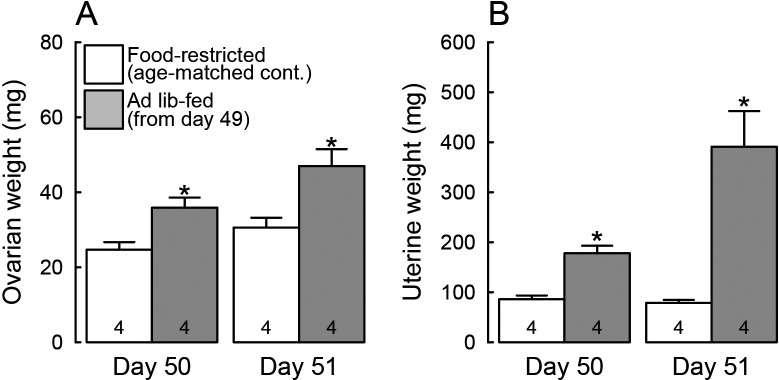
The growth-retarded female rats that received restricted food showed hypogonadotropic status: they showed vaginal opening (the mean ± SD of age, 30 ± 1 days) and then persistent vaginal diestrus during the experimental period (until 51 days of age). On the other hand, all animals subjected to ad libitum feeding for 48 h from 49 days of age showed the first vaginal proestrus on day 51. Body weights were significantly heavier in the ad libitum-fed rats on both 50 and 51 days of age (24 and 48 h after the resumption of ad libitum feeding) compared to the age-matched food-restricted controls (P < 0.05, Fig. 1). Ovarian and uterine weights were also significantly higher in the ad libitum-fed rats on both 50 and 51 days of age compared to the age-matched food-restricted controls (P < 0.05, Fig. 2A, B).
Fig. 1.
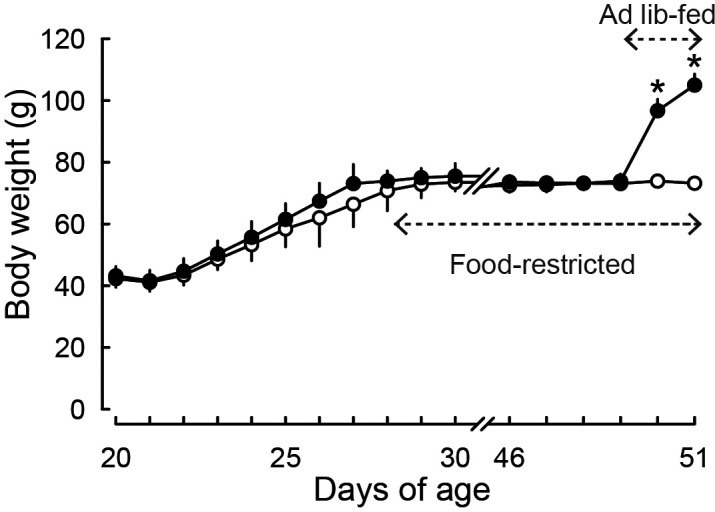
Ad libitum feeding induced catch-up growth in growth-retarded female rats. The rats weaned on day 20 were fed ad libitum until the day when their body weights reached 75 g and then subjected to a restricted feeding to keep their body weights at 70–80 g by day 49. Then, half of the animals were subjected to ad libitum feeding thereafter and the other half were remained food-restricted. Values are expressed as the mean ± SD (n = 8 each). Asterisks indicate significant difference in body weights between the ad libitum-fed rats and the age-matched food-restricted controls (* P < 0.05, Student’s t-test).
Fig. 2.
Ad libitum feeding increased ovarian and uterine weights in the growth-retarded female rats. Ovarian (A) and uterine (B) weights of animals subjected to ad libitum feeding from 49 days of age and the age-matched food-restricted controls on 50 and 51 days of age. Values are expressed as the mean ± SEM. Numbers in each column indicate the numbers of animals used. Asterisks indicate significant difference in ovarian or uterine weight between the ad libitum-fed rats and the age-matched food-restricted controls (* P < 0.05, Student’s t-test).
Ad libitum feeding induced LH secretion in the growth-retarded female rats
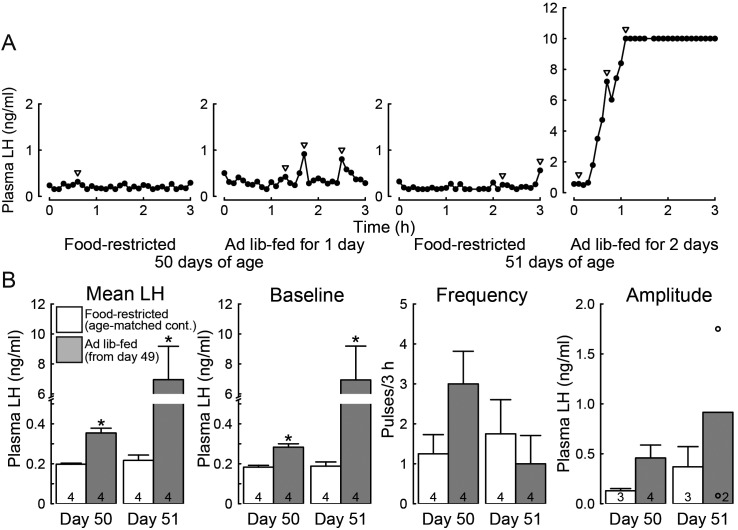
Figure 3A shows representative plasma LH profiles of ad libitum-fed rats and the age-matched food-restricted controls on both 50 and 51 days of age. The female rats subjected to ad libitum feeding for 24 h showed an increase in tonic LH secretion on day 50. 48-h ad libitum feeding induced a surge-like increase in plasma LH concentrations in all females on day 51. On the other hand, LH secretion was strongly suppressed in age-matched food-restricted controls. Specifically, mean LH concentrations and the baseline levels of LH pulses were significantly higher in ad libitum-fed rats than growth-retarded controls on each day (P < 0.05, Fig. 3B). Frequency and amplitude of LH pulses tended to be higher in ad libitum-fed rats than growth-retarded controls in particular on day 50, but no significant difference was detected in these pulse parameters.
Fig. 3.
Ad libitum feeding induced tonic and surge-like increase in luteinizing hormone (LH) secretion in the growth-retarded female rats. (A) Representative plasma LH profiles in the ad libitum-fed rats on 50 and 51 days of age (24 and 48 h after the resumption of ad libitum feeding) and the age-matched food-restricted controls. Arrowheads indicate LH pulses identified with the PULSAR computer program. Blood sampling was started from 1300 h. Note that the ad libitum-fed animals on 51 days of age showed a surge-like increase in plasma LH concentration and the concentrations were often higher than the upper detectable level. (B) Mean LH concentrations, baseline, frequency and amplitude of LH pulses in the ad libitum-fed rats on 50 and 51 days of age (24 and 48 h after the resumption of ad libitum feeding) and age-matched food-restricted controls. Values are expressed as the mean ± SEM. Numbers in each column in the graphs for mean LH concentration, and baseline and frequency of LH pulses, indicate the numbers of animals used. Numbers in each column in the graph for the amplitude of LH pulse indicate the numbers of animals showing detectable LH pulses during the 3-h sampling period. Note that LH pulses were detected in two out of four ad libitum-fed animals that showed a surge-like LH increase. Open circles indicate the mean values of pulse amplitude in those individuals. Asterisks indicate significant difference in mean LH concentration or baseline of LH pulses between the ad libitum-fed rats and the age-matched food-restricted controls (* P < 0.05, Student’s t-test).
Ad libitum feeding increased the number of Kiss1- and Pdyn-expressing cells in the ARC of the growth- retarded female rats
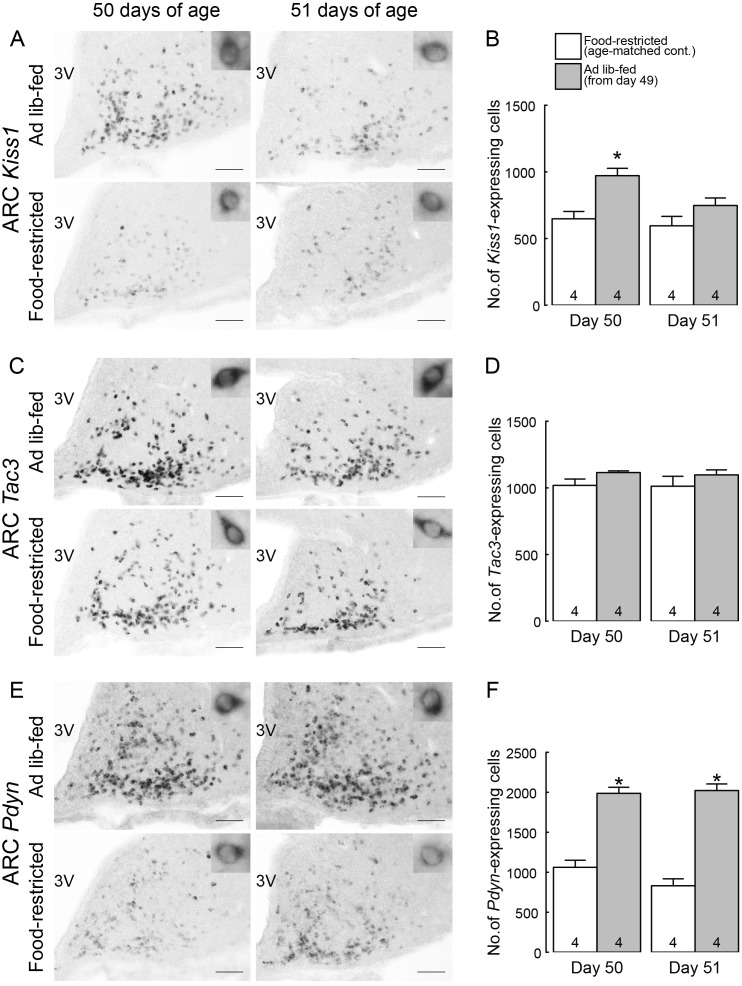
Figure 4A shows Kiss1-expressing cells in the ARC of representative female rats subjected to ad libitum feeding and the age-matched food-restricted controls. In the ad libitum-fed rats, a large number of Kiss1-expressing cells were found throughout the ARC on day 50 (24 h after the resumption of ad libitum feeding) and a moderate number of Kiss1-expressing cells were found scattered in the ARC on day 51 (48 h after the resumption). On the other hand, a small number of Kiss1-expressing cells were found in the ARC of age-matched food-restricted controls on days 50 and 51. The number of ARC Kiss1-expressing cells was significantly higher in ad libitum-fed rats on day 50 compared to the age-matched food-restricted controls (P < 0.05, Fig. 4B). On the other hand, a number of Tac3-expressing cells were found in the ARC of both ad libitum-fed rats and age-matched growth-retarded controls on both 50 and 51 days of age (Fig. 4C). No significant difference in the number of ARC Tac3-expressing cells between groups (Fig. 4D). Figure 4E shows Pdyn-expressing cells in the ARC of female rats subjected to ad libitum feeding and the age-matched food-restricted controls. In the ad libitum-fed rats, a large number of Pdyn-expressing cells were found throughout the ARC on both 50 and 51 days of age (24 and 48 h after the resumption of ad libitum feeding, respectively). On the other hand, a small number of Pdyn-expressing cells were found in the ARC of age-matched food-restricted controls on both 50 and 51 days of age. The number of ARC Pdyn-expressing cells were significantly higher in ad libitum-fed rats on both 50 and 51 days of age (24 and 48 h after the resumption of ad libitum feeding) compared to the age-matched food-restricted controls (P < 0.05, Fig. 4F).
Fig. 4.
Ad libitum feeding increased the number of Kiss1- and Pdyn-expressing cells in the arcuate nucleus (ARC) of the growth-retarded female rats. (A) Kiss1-expressing cells in the ARC of representative female rats subjected to ad libitum feeding on 50 and 51 days of age (24 and 48 h after the resumption of ad libitum feeding) and age-matched food-restricted controls. (B) The number of Kiss1-expressing cells throughout the ARC of ad libitum-fed rats on 50 and 51 days of age and age-matched food-restricted controls. (C) Tac3-expressing cells in the ARC of representative female rats subjected to ad libitum feeding on 50 and 51 days of age and age-matched food-restricted controls. (D) The number of Tac3-expressing cells throughout the ARC of ad libitum-fed rats on 50 and 51 days of age and age-matched food-restricted controls. (E) Pdyn-expressing cells in the ARC of representative female rats subjected to ad libitum feeding on 50 and 51 days of age and age-matched food-restricted controls. (F) The number of Pdyn-expressing cells throughout the ARC of ad libitum-fed rats on 50 and 51 days of age and age-matched food-restricted controls. Insets show representative cells showing Kiss1 (A), Tac3 (C) or Pdyn (E) expression at higher magnification. Scale bars, 100 μm; 3V, third ventricle. Values are expressed as the mean ± SEM. Numbers in each column indicate the numbers of animals used. Asterisks indicate significant difference in the number of ARC Kiss1- or Pdyn-expressing cells between the ad libitum-fed rats and the age-matched food-restricted controls (* P < 0.05, Student’s t-test).
Ad libitum feeding increased the number of Kiss1-expressing cells in the AVPV of the growth-retarded female rats
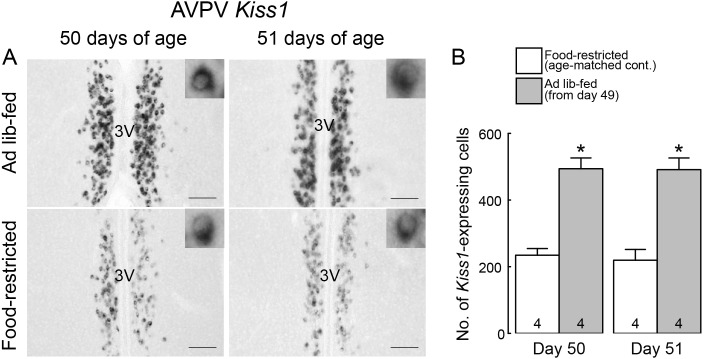
Figure 5A shows Kiss1-expressing cells in the AVPV of representative female rats subjected to ad libitum feeding and the age-matched food-restricted controls. In the ad libitum-fed rats, a large number of Kiss1-expressing cells were found throughout the AVPV on both 50 and 51 days of age (24 and 48 h after the resumption of ad libitum feeding, respectively). On the other hand, a small number of Kiss1-expressing cells were found in the AVPV of age-matched food-restricted controls on both 50 and 51 days of age. The number of AVPV Kiss1-expressing cells was significantly higher in ad libitum-fed rats on both 50 and 51 days of age compared to the age-matched food-restricted controls (P < 0.05; Fig. 5B).
Fig. 5.
Ad libitum feeding increased the number of Kiss1-expressing cells in the anteroventral periventricular nucleus (AVPV) of the growth-retarded female rats. (A) Kiss1-expressing cells in the AVPV of representative female rats subjected to ad libitum feeding on 50 and 51 days of age (24 and 48 h after the resumption of ad libitum feeding) and age-matched food-restricted controls. Insets show Kiss1-expressing cells at higher magnification. Scale bars, 100 μm; 3V, third ventricle. (B) The number of Kiss1-expressing cells throughout the AVPV of ad libitum-fed rats on 50 and 51 days of age and age-matched food-restricted controls. Values are expressed as the mean ± SEM. Numbers in each column indicate the numbers of animals used. Asterisks indicate significant difference in the number of AVPV Kiss1-expressing cells between the ad libitum-fed rats and the age-matched food-restricted controls (* P < 0.05, Student’s t-test).
Ad libitum feeding increased the number of Graafian and non-Graafian tertiary follicles in the growth- retarded female rats
Figure 6A shows Graafian and non-Graafian tertiary follicles in the ovary of representative female rats subjected to ad libitum feeding and the age-matched food-restricted controls. The ad libitum-fed rats showed Graafian follicles with a diameter greater than 390 µm on both 50 and 51 days of age (24 and 48 h after the resumption of ad libitum feeding), while the age-matched food-restricted controls showed only non-Graafian tertiary follicles with a diameter smaller than 390 µm (Fig. 6B). The number of Graafian and non-Graafian tertiary follicles was significantly higher in ad libitum-fed rats on both 50 and 51 days of age compared to the age-matched food-restricted controls (P < 0.05; Fig. 6C and 6D). The number of primordial, primary and secondary follicles were not significantly different between ad libitum-fed rats and the age-matched food-restricted controls (Fig. 6E, 6F and 6G).
Fig. 6.
Ad libitum feeding increased the number of Graafian and non-Graafian tertiary follicles in the growth-retarded female rats. (A) Representative photomicrographs of ovarian sections showing Graafian follicles with a diameter greater than 390 µm (arrows) in the ad libitum-fed rats on 50 and 51 days of age (24 and 48 h after the resumption of ad libitum feeding) and ovarian sections showing only non-Graafian tertiary follicles with a diameter smaller than 390 µm (arrowheads) in the age-matched food-restricted controls. Note that the magnification of the upper two images is different from that of the lower two images. Scale bar, 100 μm. (B) The mean diameter in each Graafian and non-Graafian tertiary follicle. The tertiary follicle with a diameter greater than 390 µm was termed as Graafian. The numbers of Graafian (C) and non-Graafian tertiary (D) follicles in the ad libitum-fed rats on 50 and 51 days of age and age-matched food-restricted controls. Values are expressed as the mean ± SEM. Numbers in each column in the graphs indicate the numbers of animals used. Asterisks indicate significant difference in the numbers of Graafian and non-Graafian tertiary follicles between the ad libitum-fed rats and the age-matched food-restricted controls (* P < 0.05, Student’s t-test). The number of primordial (E), primary (F) and secondary (G) follicles in the ad libitum-fed rats on 50 and 51 days of age and the age-matched food-restricted controls.
Discussion
The present study demonstrated that positive energy balance triggered completion of gene expression in ARC KNDy neurons, which may in turn result in puberty onset, because our chronic food-restricted female rats showed hypogonadotropic status along with suppression of ARC Kiss1 and Pdyn expression. The resumption of ad libitum feeding induced puberty onset along with increases in ARC Kiss1 and Pdyn expression in the chronic growth-retarded female rat model within 24 h. To our knowledge, this is the first report to show the energetic down- and up-regulations of ARC Kiss1 and Pdyn expression associated with pubertal transition in the chronic growth-retarded female rats. On the other hand, it is unlikely that ARC Tac3 expression is affected by chronic undernutrition —albeit ARC Tac3 expression was reportedly suppressed by short-time fasting in the peripubertal period [39]—, because the number of Tac3-expressing cells were comparable between the ad libitum-fed rats and food-restricted controls. Interestingly, ARC Kiss1, Tac3 and Pdyn gene expression were also separately controlled during the lactational anestrus: our recent study [50] showed that Kiss1 expression was solely suppressed in ARC of lactating mother rats. The present results suggest that the chronic negative energy balance impairs KNDy neurons, namely the GnRH pulse generator, via suppression of Kiss1 and Pdyn expression, and the positive energetic cues trigger the completion of the functional GnRH pulse generator via increases in ARC Kiss1 and Pdyn expression. Indeed, the chronic food-restricted rats showed suppression of LH secretion, whereas ad libitum-fed rats showed an increase in tonic LH secretion. Importantly, ARC Pdyn expression and LH secretion was suppressed by chronic food restriction and increased by the resumption of ad libitum feeding, despite of that dynorphin has been consider to play an inhibitory role in tonic LH secretion via inhibition of the synchronized activity of KNDy neuronal cluster [23, 24]. Taken together, the present results suggest that the negative energy balance inhibits pubertal onset of gonadotropin secretion by the suppression of ARC Kiss1 and Pdyn expression, and the positive energetic cues induce pubertal onset of gonadotropin secretion by the completion of gene expression in ARC KNDy neurons in female rats.
Furthermore, it was demonstrated that chronic food restriction inhibited follicular development especially from the secondary to the tertiary stages and the resumption of ad libitum feeding induced follicular development with an increase in the number of Graafian and non-Graafian tertiary follicles within 24 h after feeding. Previously, Lintern-Moore et al. [51, 52] showed recovery of follicular development by the resumption of ad libitum feeding in the chronic growth-retarded rats, at 24 days later. The current study further exhibited that acute follicular development from the secondary follicles to the Graafian follicles occurs within 24 h by the resumption of ad libitum feeding in the chronic growth-retarded rats. Moreover, it showed that the numbers of primordial, primary, and secondary follicles were comparable between ad libitum-fed rats and food-restricted controls. These results are consistent with our understanding of the gonadotropin-dependency for the follicular development in mammals: gonadotropins are indispensable for the promotion of follicular development from the secondary to the tertiary stages, whereas they are dispensable for the promotion of follicular development from the primordial to the secondary stages [53,54,55]. Taken together with the present results of an increase in tonic LH secretion induced by the resumption of ad libitum feeding, an excess amount of energy is required to increase tonic gonadotropin secretion and hence promotes the further follicular development in the ovary.
The present study also indicated an increase in Kiss1 expression in the AVPV of growth-retarded rat model 24 and 48 h after the resumption of ad libitum feeding. The increase in AVPV Kiss1 expression is likely caused by an increase in circulating estrogen that is synthesized by tertiary follicles stimulated by tonic gonadotropin secretion. Indeed, the animals subjected to ad libitum feeding for 24 and 48 h showed an increase in uterine weights and the animals subjected to 48-h ad libitum feeding showed vaginal proestrus, both of which are reliable biomarkers to show an increase in circulating levels of estrogen [56, 57]. It is well known that AVPV Kiss1 expression is positively controlled by estrogen in rodents [28,29,30] and thus kisspeptin neurons are considered to be responsible for GnRH/LH surge generation. Indeed, the current 48-h ad libitum feeding caused a surge-like increase in LH secretion. This result is well consistent with previous studies showing the resumption of ad libitum feeding resulted in the first ovulation in growth-retarded rat model on 2 or 3 days later [6, 43]. It is likely that the circulating estrogen also exert a negative influence on ARC Kiss1 expression 48 h after the resumption of ad libitum feeding. Our previous studies showed that both diestrous and proestrous levels of estrogen increase AVPV Kiss1 expression [29], whereas only proestrous levels of estrogen suppressed ARC Kiss1 expression in female rats [29, 58]. This could be a reason why the number of AVPV Kiss1-expressing cells were comparable between the 24- and 48-h ad libitum-fed rats and why the number of ARC Kiss1-expressing cells tended to decrease in the 48-h ad libitum-fed rats.
Under a positive energy balance, energy storage in body fat has been considered to be a possible determinant for pubertal onset [59]. Body fat is the most labile component of body weight and therefore would reflect environmental changes in food supplies more rapidly than other tissues [59]. Thus, researchers paid much attention to leptin, the first hormone discovered from fat tissue [60]. Leptin is now considered one of the permissive factors that allow pubertal development to proceed [61]. On the other hand, many studies suggest that glucose, fatty acids, and ketone bodies are signals in the energetic control of LH secretion in rats [36, 62, 63]. Further studies are needed to clarify a key signal or a neural pathway, that relays the attainment of energy storage from the peripheral to KNDy neurons at the pubertal onset in mammals.
In conclusion, the present study suggests that energetic cues rather than chronological ages to define the puberty onset via affecting the ARC Kiss1 and Pdyn expression: the negative energy balance causes pubertal failure probably because of the suppression of ARC Kiss1 and Pdyn expression causing deficiency of gonadotropin secretion, while the positive energetic cues induce puberty onset by completion of functional GnRH pulse generator via increases in ARC Kiss1 and Pdyn expression and hence an increase in tonic GnRH/gonadotropin secretion and follicular development, which in turn induces GnRH/LH surge leading to ovulation in female rats.
Acknowledgments
The authors respectfully acknowledge the contributions of the late Prof Kei-ichro Maeda, University of Tokyo, who provided invaluable insights, support and guidance for the present study. We thank Dr Helen I’Anson for her technical support to establish the growth-retarded model with Wistar-Imamichi rats. We also thank Ms Narumi Kawai for her technical support and Dr Nicola Skoulding for editorial assistance. We are grateful to the National Hormone and Peptide Program for the rat LH assay kit and to Dr GR Merriam and Dr KW Wachter for the PULSAR computer program. The radioimmunoassay and analyses of LH pulse parameters were performed at the Nagoya University Radioisotope Center and the Nagoya University Information Technology Center, respectively. This study was supported in part by Grants-in-Aid for Scientific Research JP18H03973, JP18K19267 (to HT) and JP16K07987 (to NI) from the Japanese Society for the Promotion of Science.
References
- 1.Sisk CL, Foster DL. The neural basis of puberty and adolescence. Nat Neurosci 2004; 7: 1040–1047. [DOI] [PubMed] [Google Scholar]
- 2.Uenoyama Y, Inoue N, Ieda N, Pheng V, Maeda K-I, Tsukamura H. Maturation and physiology of hypothalamic regulation of the gonadal axis. In: Kumanov P, Agarwal A (eds.), Puberty: Physiology and Abnormalities. Springer 2016: 1–11. [Google Scholar]
- 3.Uenoyama Y, Pheng V, Tsukamura H, Maeda KI. The roles of kisspeptin revisited: inside and outside the hypothalamus. J Reprod Dev 2016; 62: 537–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Day ML, Imakawa K, Zalesky DD, Kittok RJ, Kinder JE. Effects of restriction of dietary energy intake during the prepubertal period on secretion of luteinizing hormone and responsiveness of the pituitary to luteinizing hormone-releasing hormone in heifers. J Anim Sci 1986; 62: 1641–1648. [DOI] [PubMed] [Google Scholar]
- 5.Foster DL, Olster DH. Effect of restricted nutrition on puberty in the lamb: patterns of tonic luteinizing hormone (LH) secretion and competency of the LH surge system. Endocrinology 1985; 116: 375–381. [DOI] [PubMed] [Google Scholar]
- 6.Bronson FH. Food-restricted, prepubertal, female rats: rapid recovery of luteinizing hormone pulsing with excess food, and full recovery of pubertal development with gonadotropin-releasing hormone. Endocrinology 1986; 118: 2483–2487. [DOI] [PubMed] [Google Scholar]
- 7.Sisk CL, Bronson FH. Effects of food restriction and restoration on gonadotropin and growth hormone secretion in immature male rats. Biol Reprod 1986; 35: 554–561. [DOI] [PubMed] [Google Scholar]
- 8.Frisch RE, Revelle R. Height and weight at menarche and a hypothesis of menarche. Arch Dis Child 1971; 46: 695–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tanner JM. Earlier maturation in man. Sci Am 1968; 218: 21–27. [DOI] [PubMed] [Google Scholar]
- 10.Johnson MH, Everitt BJ. Essential Reproduction. Oxford: Blackwell Science; 2000: 128–130. [Google Scholar]
- 11.Seminara SB, Messager S, Chatzidaki EE, Thresher RR, Acierno JS, Jr, Shagoury JK, Bo-Abbas Y, Kuohung W, Schwinof KM, Hendrick AG, Zahn D, Dixon J, Kaiser UB, Slaugenhaupt SA, Gusella JF, O’Rahilly S, Carlton MB, Crowley WF, Jr, Aparicio SA, Colledge WH. The GPR54 gene as a regulator of puberty. N Engl J Med 2003; 349: 1614–1627. [DOI] [PubMed] [Google Scholar]
- 12.de Roux N, Genin E, Carel JC, Matsuda F, Chaussain JL, Milgrom E. Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor GPR54. Proc Natl Acad Sci USA 2003; 100: 10972–10976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Topaloglu AK, Tello JA, Kotan LD, Ozbek MN, Yilmaz MB, Erdogan S, Gurbuz F, Temiz F, Millar RP, Yuksel B. Inactivating KISS1 mutation and hypogonadotropic hypogonadism. N Engl J Med 2012; 366: 629–635. [DOI] [PubMed] [Google Scholar]
- 14.Messager S, Chatzidaki EE, Ma D, Hendrick AG, Zahn D, Dixon J, Thresher RR, Malinge I, Lomet D, Carlton MB, Colledge WH, Caraty A, Aparicio SA. Kisspeptin directly stimulates gonadotropin-releasing hormone release via G protein-coupled receptor 54. Proc Natl Acad Sci USA 2005; 102: 1761–1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.d’Anglemont de Tassigny X, Fagg LA, Dixon JP, Day K, Leitch HG, Hendrick AG, Zahn D, Franceschini I, Caraty A, Carlton MB, Aparicio SA, Colledge WH. Hypogonadotropic hypogonadism in mice lacking a functional Kiss1 gene. Proc Natl Acad Sci USA 2007; 104: 10714–10719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dungan HM, Gottsch ML, Zeng H, Gragerov A, Bergmann JE, Vassilatis DK, Clifton DK, Steiner RA. The role of kisspeptin-GPR54 signaling in the tonic regulation and surge release of gonadotropin-releasing hormone/luteinizing hormone. J Neurosci 2007; 27: 12088–12095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lapatto R, Pallais JC, Zhang D, Chan YM, Mahan A, Cerrato F, Le WW, Hoffman GE, Seminara SB. Kiss1-/- mice exhibit more variable hypogonadism than Gpr54-/- mice. Endocrinology 2007; 148: 4927–4936. [DOI] [PubMed] [Google Scholar]
- 18.Chan YM, Broder-Fingert S, Wong KM, Seminara SB. Kisspeptin/Gpr54-independent gonadotrophin-releasing hormone activity in Kiss1 and Gpr54 mutant mice. J Neuroendocrinol 2009; 21: 1015–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Uenoyama Y, Nakamura S, Hayakawa Y, Ikegami K, Watanabe Y, Deura C, Minabe S, Tomikawa J, Goto T, Ieda N, Inoue N, Sanbo M, Tamura C, Hirabayashi M, Maeda KI, Tsukamura H. Lack of pulse and surge modes and glutamatergic stimulation of luteinizing hormone release in Kiss1 knockout rats. J Neuroendocrinol 2015; 27: 187–197. [DOI] [PubMed] [Google Scholar]
- 20.Uenoyama Y, Inoue N, Maeda KI, Tsukamura H. The roles of kisspeptin in the mechanism underlying reproductive functions in mammals. J Reprod Dev 2018; 64: 469–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ohtaki T, Shintani Y, Honda S, Matsumoto H, Hori A, Kanehashi K, Terao Y, Kumano S, Takatsu Y, Masuda Y, Ishibashi Y, Watanabe T, Asada M, Yamada T, Suenaga M, Kitada C, Usuki S, Kurokawa T, Onda H, Nishimura O, Fujino M. Metastasis suppressor gene KiSS-1 encodes peptide ligand of a G-protein-coupled receptor. Nature 2001; 411: 613–617. [DOI] [PubMed] [Google Scholar]
- 22.Kotani M, Detheux M, Vandenbogaerde A, Communi D, Vanderwinden JM, Le Poul E, Brézillon S, Tyldesley R, Suarez-Huerta N, Vandeput F, Blanpain C, Schiffmann SN, Vassart G, Parmentier M. The metastasis suppressor gene KiSS-1 encodes kisspeptins, the natural ligands of the orphan G protein-coupled receptor GPR54. J Biol Chem 2001; 276: 34631–34636. [DOI] [PubMed] [Google Scholar]
- 23.Okamura H, Tsukamura H, Ohkura S, Uenoyama Y, Wakabayashi Y, Maeda K. Kisspeptin and GnRH pulse generation. Adv Exp Med Biol 2013; 784: 297–323. [DOI] [PubMed] [Google Scholar]
- 24.Goodman RL, Ohkura S, Okamura H, Coolen LM, Lehman MN. KNDy hypothesis for generation of GnRH pulses: evidence from sheep and goats. In: Herbison AE, Plant TM (eds.), The GnRH Neuron and its Control. Hoboken, NJ: Wiley; 2018: 289–324. [Google Scholar]
- 25.Wakabayashi Y, Nakada T, Murata K, Ohkura S, Mogi K, Navarro VM, Clifton DK, Mori Y, Tsukamura H, Maeda K, Steiner RA, Okamura H. Neurokinin B and dynorphin A in kisspeptin neurons of the arcuate nucleus participate in generation of periodic oscillation of neural activity driving pulsatile gonadotropin-releasing hormone secretion in the goat. J Neurosci 2010; 30: 3124–3132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goodman RL, Coolen LM, Lehman MN. A role for neurokinin B in pulsatile GnRH secretion in the ewe. Neuroendocrinology 2014; 99: 18–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Keen KL, Wegner FH, Bloom SR, Ghatei MA, Terasawa E. An increase in kisspeptin-54 release occurs with the pubertal increase in luteinizing hormone-releasing hormone-1 release in the stalk-median eminence of female rhesus monkeys in vivo. Endocrinology 2008; 149: 4151–4157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smith JT, Cunningham MJ, Rissman EF, Clifton DK, Steiner RA. Regulation of Kiss1 gene expression in the brain of the female mouse. Endocrinology 2005; 146: 3686–3692. [DOI] [PubMed] [Google Scholar]
- 29.Adachi S, Yamada S, Takatsu Y, Matsui H, Kinoshita M, Takase K, Sugiura H, Ohtaki T, Matsumoto H, Uenoyama Y, Tsukamura H, Inoue K, Maeda K. Involvement of anteroventral periventricular metastin/kisspeptin neurons in estrogen positive feedback action on luteinizing hormone release in female rats. J Reprod Dev 2007; 53: 367–378. [DOI] [PubMed] [Google Scholar]
- 30.Homma T, Sakakibara M, Yamada S, Kinoshita M, Iwata K, Tomikawa J, Kanazawa T, Matsui H, Takatsu Y, Ohtaki T, Matsumoto H, Uenoyama Y, Maeda K, Tsukamura H. Significance of neonatal testicular sex steroids to defeminize anteroventral periventricular kisspeptin neurons and the GnRH/LH surge system in male rats. Biol Reprod 2009; 81: 1216–1225. [DOI] [PubMed] [Google Scholar]
- 31.Tomikawa J, Homma T, Tajima S, Shibata T, Inamoto Y, Takase K, Inoue N, Ohkura S, Uenoyama Y, Maeda K, Tsukamura H. Molecular characterization and estrogen regulation of hypothalamic KISS1 gene in the pig. Biol Reprod 2010; 82: 313–319. [DOI] [PubMed] [Google Scholar]
- 32.Inoue N, Sasagawa K, Ikai K, Sasaki Y, Tomikawa J, Oishi S, Fujii N, Uenoyama Y, Ohmori Y, Yamamoto N, Hondo E, Maeda K, Tsukamura H. Kisspeptin neurons mediate reflex ovulation in the musk shrew (Suncus murinus). Proc Natl Acad Sci USA 2011; 108: 17527–17532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Matsuda F, Nakatsukasa K, Suetomi Y, Naniwa Y, Ito D, Inoue N, Wakabayashi Y, Okamura H, Maeda KI, Uenoyama Y, Tsukamura H, Ohkura S. The luteinizing hormone surge-generating system is functional in male goats as in females: involvement of kisspeptin neurones in the medial preoptic area. J Neuroendocrinol 2014; 27: 57–65. [DOI] [PubMed] [Google Scholar]
- 34.Watanabe Y, Uenoyama Y, Suzuki J, Takase K, Suetomi Y, Ohkura S, Inoue N, Maeda KI, Tsukamura H. Oestrogen-induced activation of preoptic kisspeptin neurones may be involved in the luteinising hormone surge in male and female Japanese monkeys. J Neuroendocrinol 2014; 26: 909–917. [DOI] [PubMed] [Google Scholar]
- 35.Maeda K, Tsukamura H. Neuroendocrine mechanism mediating fasting-induced suppression of luteinizing hormone secretion in female rats. Acta Neurobiol Exp (Warsz) 1996; 56: 787–796. [DOI] [PubMed] [Google Scholar]
- 36.Kinoshita M, Moriyama R, Tsukamura H, Maeda KI. A rat model for the energetic regulation of gonadotropin secretion: role of the glucose-sensing mechanism in the brain. Domest Anim Endocrinol 2003; 25: 109–120. [DOI] [PubMed] [Google Scholar]
- 37.Briski KP, Sylvester PW. Role of endogenous opiates in glucoprivic inhibition of the luteinizing hormone surge and fos expression by preoptic gonadotropin-releasing hormone neurones in ovariectomized steroid-primed female rats. J Neuroendocrinol 1998; 10: 769–776. [DOI] [PubMed] [Google Scholar]
- 38.Castellano JM, Navarro VM, Fernández-Fernández R, Nogueiras R, Tovar S, Roa J, Vazquez MJ, Vigo E, Casanueva FF, Aguilar E, Pinilla L, Dieguez C, Tena-Sempere M. Changes in hypothalamic KiSS-1 system and restoration of pubertal activation of the reproductive axis by kisspeptin in undernutrition. Endocrinology 2005; 146: 3917–3925. [DOI] [PubMed] [Google Scholar]
- 39.Navarro VM, Ruiz-Pino F, Sánchez-Garrido MA, García-Galiano D, Hobbs SJ, Manfredi-Lozano M, León S, Sangiao-Alvarellos S, Castellano JM, Clifton DK, Pinilla L, Steiner RA, Tena-Sempere M. Role of neurokinin B in the control of female puberty and its modulation by metabolic status. J Neurosci 2012; 32: 2388–2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kalamatianos T, Grimshaw SE, Poorun R, Hahn JD, Coen CW. Fasting reduces KiSS-1 expression in the anteroventral periventricular nucleus (AVPV): effects of fasting on the expression of KiSS-1 and neuropeptide Y in the AVPV or arcuate nucleus of female rats. J Neuroendocrinol 2008; 20: 1089–1097. [DOI] [PubMed] [Google Scholar]
- 41.Matsuzaki T, Iwasa T, Kinouchi R, Yoshida S, Murakami M, Gereltsetseg G, Yamamoto S, Kuwahara A, Yasui T, Irahara M. Fasting reduces the kiss1 mRNA levels in the caudal hypothalamus of gonadally intact adult female rats. Endocr J 2011; 58: 1003–1012. [DOI] [PubMed] [Google Scholar]
- 42.Luque RM, Kineman RD, Tena-Sempere M. Regulation of hypothalamic expression of KiSS-1 and GPR54 genes by metabolic factors: analyses using mouse models and a cell line. Endocrinology 2007; 148: 4601–4611. [DOI] [PubMed] [Google Scholar]
- 43.Bronson FH. Effect of food manipulation on the GnRH-LH-estradiol axis of young female rats. Am J Physiol 1988; 254: R616–R621. [DOI] [PubMed] [Google Scholar]
- 44.Messer NA, I’Anson H. The nature of the metabolic signal that triggers onset of puberty in female rats. Physiol Behav 2000; 68: 377–382. [DOI] [PubMed] [Google Scholar]
- 45.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates, 6th Edition. San Diego: Academic Press; 2008. [Google Scholar]
- 46.Yamada T, Ichihara G, Wang H, Yu X, Maeda K, Tsukamura H, Kamijima M, Nakajima T, Takeuchi Y. Exposure to 1-bromopropane causes ovarian dysfunction in rats. Toxicol Sci 2003; 71: 96–103. [DOI] [PubMed] [Google Scholar]
- 47.Pedersen T, Peters H. Proposal for a classification of oocytes and follicles in the mouse ovary. J Reprod Fertil 1968; 17: 555–557. [DOI] [PubMed] [Google Scholar]
- 48.Hirshfield AN, Midgley AR., Jr. Morphometric analysis of follicular development in the rat. Biol Reprod 1978; 19: 597–605. [DOI] [PubMed] [Google Scholar]
- 49.Merriam GR, Wachter KW. Algorithms for the study of episodic hormone secretion. Am J Physiol 1982; 243: E310–E318. [DOI] [PubMed] [Google Scholar]
- 50.Sugimoto A, Tsuchida H, Ieda N, Ikegami K, Inoue N, Uenoyama Y, Tsukamura H. Somatostatin-somatostatin receptor 2 signaling mediates LH pulse suppression in lactating rats. Endocrinology 2019; 160: 473–483. [DOI] [PubMed] [Google Scholar]
- 51.Lintern-Moore S, Everitt AV. The effect of restricted food intake on the size and composition of the ovarian follicle population in the Wistar rat. Biol Reprod 1978; 19: 688–691. [DOI] [PubMed] [Google Scholar]
- 52.Lintern-Moore S, Everitt AV, Mariana JC, Mauléon P. The effect of restricted food intake and refeeding on the ovarian follicle population of the pre-puberal Wistar rat. Reprod Nutr Dev 1981; 21 (5A): 611–620. [DOI] [PubMed] [Google Scholar]
- 53.Richards JS. The Ovarian Cycle. Vitam Horm 2018; 107: 1–25. [DOI] [PubMed] [Google Scholar]
- 54.Richards JS, Pangas SA. The ovary: basic biology and clinical implications. J Clin Invest 2010; 120: 963–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.McGee EA, Hsueh AJ. Initial and cyclic recruitment of ovarian follicles. Endocr Rev 2000; 21: 200–214. [DOI] [PubMed] [Google Scholar]
- 56.Dorfman RI, Dorfman AS. Estrogen assays using the rat uterus. Endocrinology 1954; 55: 65–69. [DOI] [PubMed] [Google Scholar]
- 57.Kahnt LC, Doisy EA. The vaginal smear method of assay of the ovarian hormone. Endocrinology 1928; 12: 760–768. [Google Scholar]
- 58.Kinoshita M, Tsukamura H, Adachi S, Matsui H, Uenoyama Y, Iwata K, Yamada S, Inoue K, Ohtaki T, Matsumoto H, Maeda K. Involvement of central metastin in the regulation of preovulatory luteinizing hormone surge and estrous cyclicity in female rats. Endocrinology 2005; 146: 4431–4436. [DOI] [PubMed] [Google Scholar]
- 59.Frisch RE. Body fat, puberty and fertility. Biol Rev Camb Philos Soc 1984; 59: 161–188. [DOI] [PubMed] [Google Scholar]
- 60.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature 1994; 372: 425–432. [DOI] [PubMed] [Google Scholar]
- 61.Urbanski HF. Leptin and puberty. Trends Endocrinol Metab 2001; 12: 428–429. [DOI] [PubMed] [Google Scholar]
- 62.Sajapitak S, Iwata K, Shahab M, Uenoyama Y, Yamada S, Kinoshita M, Bari FY, I’Anson H, Tsukamura H, Maeda K. Central lipoprivation-induced suppression of luteinizing hormone pulses is mediated by paraventricular catecholaminergic inputs in female rats. Endocrinology 2008; 149: 3016–3024. [DOI] [PubMed] [Google Scholar]
- 63.Iwata K, Kinoshita M, Susaki N, Uenoyama Y, Tsukamura H, Maeda K. Central injection of ketone body suppresses luteinizing hormone release via the catecholaminergic pathway in female rats. J Reprod Dev 2011; 57: 379–384. [DOI] [PubMed] [Google Scholar]