Abstract
This study aimed at investigating the efficacy of two protocols of estrous synchronization on follicular changes and hemodynamics. Pluriparous Egyptian buffaloes (n = 36) were synchronized either with controlled internal drug release (CIDR)-PGF2α (7-days CIDR insert with PGF2α injected on the 6th day; n = 18) or Ovsynch-CIDR (Ovsynch protocol concurrent with 7-days CIDR insert; n = 18). Blood sampling and ovarian ultrasound examinations (Grayscale, color and power Doppler modes) were conducted on the Day of CIDR removal, estrus, and luteal phase. Mean follicle diameter (MFD), first (1st-LF) and second (2nd-LF) largest follicle diameters, and E2 levels significantly increased in the CIDR-PGF2α group at CIDR withdrawal. Ovsynch-CIDR markedly fortified higher follicle population, MFD, and 1st-LF diameter at estrus and corpus luteum (CL) volume at the luteal phase in concomitant with increases (P < 0.05) in E2 (at estrus) and P4 (at luteal phase). At CIDR removal, the blue pixels in the dominant follicle (DF) were higher (1.5 times; P = 0.054) in the Ovsynch-CIDR than in the CIDR-PGF2α. At estrus, total blood flow (TBF) and power Doppler pixels (PDP) to DF(s) were noticeably higher (seven and 1.6 times; respectively) in the Ovsynch-CIDR than in CIDR-PGF2α (5906 ± 237 vs. 830 ± 60 pixels, P < 0.01 and 5479 ± 322 vs. 3377 ± 19 pixels, P < 0.05; respectively). At the luteal phase, TBF and PDP to the CL increased in the Ovsynch-CIDR group than in the CIDR-PGF2α group (11060 ± 965 vs. 7963 ± 480 pixels, 1.4 times, P = 0.05 and 18900 ± 1350 vs. 13220 ± 568 pixels, 1.1 times, P = 0.005; respectively). In conclusion, based on the improvement in synchronized follicular activity and hemodynamics, the Ovsynch-CIDR regimen is persuaded in Egyptian buffaloes.
Keywords: Buffalo, CIDR-PGF2α, Doppler ultrasonography, Ovary, Ovsynch-CIDR
Buffalo (Bubalus bubalis), the main dairy animal in Egypt (3.69 million heads), produces about 10.90% and 1.97% of the world buffalo’s milk and meat, respectively [1]. Compared to cattle, buffaloes show greater adaptability to the tropical climate and resistance to various diseases [2].
However, Egyptian buffaloes are characterized by lowering the productive and reproductive potential in terms of less pronounced estrus expression and high incidence of silent heat, differences in ovulation and estrus duration, long inter-calving interval and seasonality of breeding activities [3]. Therefore, various estrus synchronization protocols have been admitted in buffaloes the past years to improve their reproductive performance and for the successful application of artificial insemination (AI) [4, 5]. For this purpose, many hormones have been tried for estrus synchronization in farm animals, but at variable success rates [4, 6].
Previous studies found the use of combined progesterone regimens seems better for estrous synchronization in buffaloes because of poor estrus expression and seasonality of breeding activities [3,4,5]. The controlled internal drug release (CIDR)-PGF2α is the classical estrus synchronization regimen, in which PGF2α administrated the day before CIDR removal. The Ovsynch protocol has been used efficiently to synchronize ovulation in buffaloes to achieve more precise timing of insemination without references to observing estrus signs with reasonable conception rates varied between 33 and 60% [4, 7]. To improve the efficiency of the Ovsynch protocol, some authors tried to incorporate the progesterone-releasing devices into this regimen to improve the conception rate [8]. Studies in cattle have emphasized the effect of CIDR-PGF2α and Ovsynch-CIDR estrus synchronization regimens on pregnancy outcome [9] and calving interval [10]. However, to the best of our knowledge, scarce studies have assessed the influence of CIDR-PGF2α and Ovsynch-CIDR regimens on ovarian activity and hemodynamic in buffaloes. Therefore, this study aimed at evaluating the effect of CIDR-PGF2α and Ovsynch-CIDR protocols on follicles population and ovarian hemodynamic ascertained by color Doppler ultrasonography, hormonal evaluation, and the subsequent pregnancy rate.
Material and Methods
Animals
Pluriparous Egyptian buffaloes (n = 36) were used in the current study from February to May 2016. Animals belonging to the educational farm, Faculty of Agriculture, Al-Azhar University, Kaliobeya governorate, Egypt, and weighed 460 ± 25 kg, aged 5.2 ± 1.6 years and their body condition score was 3.25 ± 0.59. Buffaloes were housed under natural daylight conditions away from bulls during the study. They were fed a commercially prepared pelleted ration (16% crude protein/dry matter) with Egyptian sweet clover (Trifolium alexandrinum), and wheat straw as bulk material, and were allowed for clean water and mineralized salt licks (Asulick-H, Assuit, Assuit, Egypt) ad libitum. The animals were regularly prophylactic vaccinated against important infectious endemic diseases such as foot and mouth disease, Rift Valley Fever, and enterotoxemia. Other preventive treatments such as deworming were performed using appropriate broad-spectrum anthelmintic medications, and in none of the buffaloes was there any evidence of disease upon physical examination prior to beginning this study. All buffaloes were monitored behaviorally (twice a day for estrus onset) and ultrasonographically (twice a week to ensure cyclicity i.e. presence of corpus luteum: CL), for at least two consecutive estrous cycles before the beginning of the protocols.
This study was performed in accordance with the Use and Animal Care Guidelines of the Cairo and Benha Universities.
Estrous synchronization and breeding
Animals were randomly assigned into two groups based on the protocol of estrus synchronization [11]. The first group (CIDR-PGF2α group, n = 18) received CIDR device impregnated with 1.38 mg progesterone (EAZIBREED, inter Age, Hamilton, New Zealand) inserted intra-vaginally for 7 days and an intramuscular injection of a synthetic prostaglandin F2α analogue (PGF2α; 500 µg cloprostenol sodium, Estrumate, Intervet, Merck Animal Health, USA) one day before the CIDR withdrawal. The second group (Ovsynch-CIDR group, n = 18) received treatment according to the Ovsynch protocol, accompanied by an intra-vaginal insertion of CIDR for 7 days. Briefly, two doses of 20 μg of gonadotropin-releasing hormone analog (GnRH; 5.0 ml Receptal, MSD Animal Health, Intervet International GmBH, Germany) were given intramuscularly seven days before and two days after a single dose of PGF2α. After the end of the treatment, all buffaloes were checked for behavioral signs of estrus by a vasectomized bull twice a day (at 12 h interval) to stimulate estrus behavior. All buffaloes were subjected to timed AI 12 h after synchronized estrus onset using frozen-thawed semen from a known fertile bull.
Ultrasonographic examinations
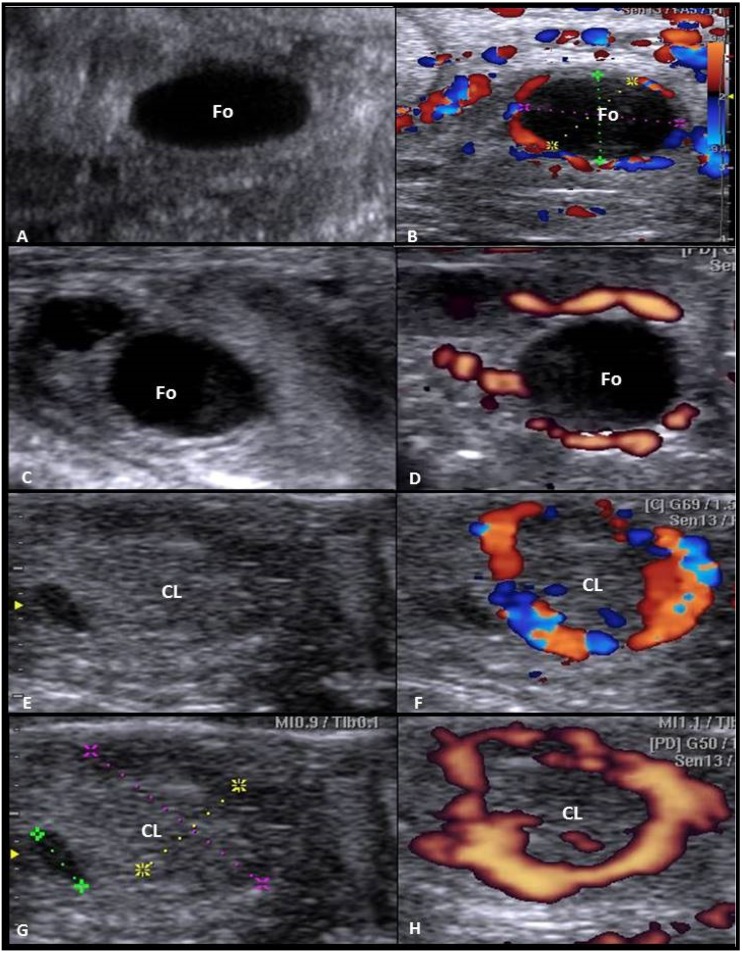
All ultrasonographic examinations were performed by the same operator using B-mode/color Doppler diagnostic ultrasound (SonoAce R3, Samsung, Medison, South Korea) equipped with a 12-MHz endorectal transducer. Ovarian structures (follicles and corpus luteum) as well as follicular (FBF) and luteal blood flow (LBF) were examined daily in both groups from CIDR removal until ovulation. The occurrence of ovulation was anticipated by the disappearance of the large antral follicle(s) observed in the previous examination(s) and confirmed by the detection of corpora lutea on Day 7 later [7]. For pregnancy diagnosis, another transrectal B-mode ultrasonography of the uterus was performed on Days 28–35 post-insemination. The ovary was visualized rectally by B-mode ultrasonography in at least three times per examination in each animal, and follicles and CL number and diameters were assessed using the electronic calipers. After that, color then power Doppler modes were activated in slow continuous motion, constant color gain, velocity, and color-flow filter settings to display signals for blood flow [12] in the observed dominant follicles and CL (Fig. 1). Color and power Doppler images were analyzed for the blood flow perfusions area (s) in pixels using an image analysis program (Adobe Photoshop CC software, Adobe Systems, USA) as described in buffaloes [13]. Blood flow which is colored either blue (away from the transducer) or red (toward the transducer), and total color, and power areas were counted.
Fig. 1.
Ultrasonographic imaging demonstrating the preovulatory follicle (Fo) and the corpus luteum (CL) in Egyptian buffaloes. The left panel is by B-mode ultrasonography (A, C, E, G), while the right panel is representing the color (B, F) and power Doppler modes (D, H).
Blood samplings and hormonal measurements
Blood samples (10 ml) were collected on Day 7 post-insertion, Day of estrus, and Day 7 post-ovulation from the jugular vein of each animal, and the harvested sera were stored at −20°C until the hormonal assay. Concentrations of estradiol (E2) and progesterone (P4) in the serum were measured using ELISA commercial kits (Equipar S.r.I Saronno, VA, Italy) [14]. The sensitivity for E2-EIA (Cat. No. 74070) was 1 pg/ml, and the intra-and inter-assay coefficients of variation were 7.2% and 8.5%, respectively. The sensitivity for P4-EIA (Cat. No. 74020) was 2 pg/ml, and the intra-and inter-assay coefficients of variation were 6.1% and 11.4%, respectively.
Statistical analysis
The data are presented as means ± standard error of the mean (SEM). Statistical analysis of the data was performed. Values in both groups at each time point were compared statistically using the Mann-Whitney U test using GraphPad Prism Ver. 5.01. A probability value of less than 0.05 was considered significant.
Results
Influence of estrus synchronization protocol on ovarian activity in buffaloes
At CIDR removal, there was tendency (P = 0.06) for the total follicular area to be greater in CIDR-PGF2α (254.70 ± 38.41 mm2) than Ovsynch-CIDR (158.80 ± 10.83 mm2) and this was associated with a significant increase in mean follicle diameter (6.53 ± 0.83 vs. 4.63 ± 0.12 mm, P < 0.05), 1st largest follicle (13.08 ± 1.21 vs. 8.29 ± 0.22 mm, P < 0.001) and 2nd large follicle (7.46 ± 0.75 vs. 4.73 ± 0.41 mm, P < 0.05) diameters (Table 1).
Table 1. Comparative efficacy of controlled internal drug release (CIDR)-PGF2α and Ovsynch-CIDR protocols on follicular, hemodynamics and hormonal changes in Egyptian buffaloes at CIDR removal.
| Item | CIDR-PGF2α | Ovsynch-CIDR | P-value (t-test) | |
|---|---|---|---|---|
| Follicular dynamics | ||||
| Follicle population | 8.20 ± 1.20 | 7.60 ± 0.60 | 0.82 | |
| Total follicular surface area | 254.70 ± 38.41 | 158.80 ± 10.83 | 0.06 | |
| Mean follicle diameter (mm) | 6.53 ± 0.83 * | 4.63 ± 0.12 | 0.05 | |
| 1st LF diameter (mm) | 13.08 ± 1.21 *** | 8.29 ± 0.22 | 0.001 | |
| 2nd LF diameter (mm) | 7.46 ± 0.75 * | 4.73 ± 0.41 | 0.05 | |
| Follicle deviation (mm) | 5.62 ± 0.75 | 4.11 ± 0.43 | 0.19 | |
| CL volume (cm3) | 1.22 ± 0.22 | 0.80 ± 0.17 | 0.34 | |
| Hemodynamics | ||||
| Dominant follicle | ||||
| Flow towards transducer (Red pixels) | 1618 ± 376 | 1874 ± 237 | 0.69 | |
| Flow away transducer (Blue pixels) | 1611 ± 356 | 2454 ± 283 * | 0.054 | |
| Total blood flow (Red and blue pixels) | 3362 ± 377 | 4329 ± 480 | 0.37 | |
| Power Doppler pixels | 2521 ± 165 | 3470 ± 509 | 0.73 | |
| Hormonal changes | ||||
| Estradiol (pg/ml) | 6.65 ± 0.86 * | 4.52 ± 0.36 | 0.05 | |
| Progesterone (ng/ml) | 2.25 ± 0.37 | 2.92 ± 0.28 | 0.21 | |
LF: large follicle, CL: corpus luteum. Data presented as mean (± SEM). * and *** referred to significant differences at P < 0.05 and P < 0.001, respectively.
At estrus, Ovsynch-CIDR group showed higher follicle population (P < 0.05), total follicular surface area, (287.30 ± 38.32 vs. 161.71 ± 25.91 mm2, P < 0.05), mean follicle diameter (8.43 ± 0.35 vs. 6.29 ± 0.31 mm, P < 0.01) and 1st largest follicle diameter (13.56 ± 0.81 vs. 10.41 ± 0.95, P < 0.05). At that time, the follicle deviations between the 1st and 2nd largest follicles were higher in Ovsynch-CIDR than CIDR-PGF2α group (9.10 ± 0.54 and 5.65 ± 0.74 mm, respectively, P < 0.01) (Table 2).
Table 2. Comparative efficacy of controlled internal drug release (CIDR)-PGF2α and Ovsynch-CIDR protocols on follicular, hemodynamics and hormonal changes in Egyptian buffaloes at estrus onset.
| Item | CIDR-PGF2α | Ovsynch-CIDR | P-value (t-test) | |
|---|---|---|---|---|
| Follicular dynamics | ||||
| Follicle population | 5.00 ± 0.45 | 6.80 ± 0.37 * | 0.05 | |
| Total follicular surface area | 161.71 ± 25.91 | 287.30 ± 38.32 * | 0.05 | |
| Mean follicle diameter (mm) | 6.29 ± 0.31 | 8.43 ± 0.35 ** | 0.01 | |
| 1st LF diameter (mm) | 10.41 ± 0.95 | 13.56 ± 0.81 * | 0.05 | |
| 2nd LF diameter (mm) | 5.76 ± 0.43 | 4.46 ± 0.40 | 0.09 | |
| Follicle deviation (mm) | 5.65 ± 0.74 | 9.10 ± 0.54 ** | 0.01 | |
| Hemodynamics | ||||
| Dominant follicle | ||||
| Flow towards transducer (Red pixels) | 812 ± 56 | 2461 ± 195 *** | 0.001 | |
| Flow away transducer (Blue pixels) | 718 ± 49 | 3239 ± 150 ** | 0.01 | |
| Total blood flow (Red and blue pixels) | 830 ± 60 | 5906 ± 237 ** | 0.01 | |
| Power Doppler pixels | 3377 ± 19 | 5479 ± 322 * | 0.05 | |
| Hormonal changes | ||||
| Estradiol (pg/ml) | 24.31 ± 4.02 | 35.55 ± 2.95 * | 0.05 | |
| Progesterone (ng/ml) | 0.76 ± 0.09 | 0.98 ± 0.22 | 0.17 | |
LF: large follicle, CL: corpus luteum. Data presented as mean (± SEM). *, ** and *** referred to significant differences at P < 0.05, P < 0.01 and P < 0.001, respectively.
At the luteal phase, the total follicular surface area and follicle deviation tended to be greater in Ovsynch-CIDR than CIDR-PGF2α group (P = 0.07 and P = 0.09, respectively). Also, the volume of synchronized CL was bigger in Ovsynch-CIDR than CIDR-PGF2α (3.06 ± 0.83 and 2.22 ± 0.46 cm3, respectively, P < 0.05) (Table 3).
Table 3. Comparative efficacy of controlled internal drug release (CIDR)-PGF2α and Ovsynch-CIDR protocols on follicular, hemodynamics and hormonal changes in Egyptian buffaloes during the luteal phase.
| Item | CIDR-PGF2α | Ovsynch-CIDR | P-value (t-test) | |
|---|---|---|---|---|
| Follicular dynamics | ||||
| Follicle population | 3.40 ± 0.24 | 5.00 ± 1.10 | 0.19 | |
| Total follicular surface area | 118.97 ± 13.44 | 138.43 ± 10.87 | 0.07 | |
| Mean follicle diameter (mm) | 6.24 ± 0.66 | 7.73 ± 0.81 | 0.64 | |
| 1st LF diameter (mm) | 9.90 ± 1.14 | 11.96 ± 0.83 | 0.18 | |
| 2nd LF diameter (mm) | 4.68 ± 0.58 | 4.74 ± 0.48 | 0.94 | |
| Follicle deviation (mm) | 5.22 ± 0.80 | 7.22 ± 0.66 | 0.09 | |
| CL volume (cm3) | 2.22 ± 0.46 | 3.06 ± 0.83 * | 0.05 | |
| Hemodynamics | ||||
| Dominant follicle | ||||
| Flow towards transducer (Red pixels) | 1306 ± 64 | 1156 ± 67 | 0.18 | |
| Flow away transducer (Blue pixels) | 1387 ± 51 | 2361 ± 154 * | 0.05 | |
| Total blood flow (Red and blue pixels) | 2509 ± 98 | 3475 ± 163 | 0.05 | |
| Power Doppler pixels | 1552 ± 71 | 2362 ± 100 ** | 0.01 | |
| Corpus luteum | ||||
| Flow towards transducer (Red pixels) | 4284 ± 337 | 6032 ± 592 * | 0.05 | |
| Flow away transducer (Blue pixels) | 3679 ± 249 | 5093 ± 416 * | 0.05 | |
| Total blood flow (Red and blue pixels) | 7963 ± 480 | 11060 ± 965 * | 0.05 | |
| Power Doppler pixels | 13220 ± 568 | 18900 ± 1350 ** | 0.005 | |
| Hormonal changes | ||||
| Estradiol (pg/ml) | 4.74 ± 0.64 | 5.42 ± 0.63 | 0.23 | |
| Progesterone (ng/ml) | 1.81 ± 0.49 | 3.94 ± 0.62 | 0.05 | |
LF: large follicle, CL: corpus luteum. Data presented as mean (± SEM). *, ** and *** referred to significant differences at P < 0.05, P < 0.01 and P < 0.001, respectively.
Influence of estrus synchronization protocol on ovarian blood perfusions in buffaloes
Ovarian hemodynamic changes at CIDR removal in terms of TBF, Red and blue pixels; outflow and inflow, respectively were comparable in DF in the CIDR-PGF2α group. But, in the Ovsynch-CIDR group, the blue pixels in DF were plentiful compared to red pixels, and these values were higher (1.5 times; P = 0.054) than its contemporaries’ values in the CIDR-PGF2α group (2454 ± 283 and 1611 ± 356 pixels, respectively, P = 0.054) (Table 1).
On Day of estrus, TBF and PDP to the DF(s) were noticeably higher in the Ovsynch-CIDR group than in CIDR-PGF2α (5906 ± 237 vs. 830 ± 60 pixels, seven times, P < 0.01 and 5479 ± 322 vs. 3377 ± 19 pixels, 1.6 times P < 0.05, respectively). Also, DF showed an upsurge in the red pixels (Three times; P < 0.001), and blue pixels (4.5 times; P < 0.01) in Ovsynch-CIDR than CIDR-PGF2α (Table 2). In this vein, the blood inflow was higher than outflow in the Ovsynch-CIDR group (3239 ± 150 and 2461 ± 195 pixels, respectively), but it nearly similar in the CIDR-PGF2α group (812 ± 56 and 718 ± 49 pixels, respectively).
At the luteal phase, the blue pixels, TBF, and PDP to the DF(s) were noticeably higher (P < 0.05, P < 0.05, and P < 0.01; respectively) in the Ovsynch-CIDR (2361 ± 154, 3475 ± 163 and 2362 ± 100 pixels, respectively) than in the CIDR-PGF2α group (1387 ± 51, 2509 ± 98 and 1552 ± 71 pixels, respectively). Also, TBF and PDP to the CL were significantly higher in the Ovsynch-CIDR group than in CIDR-PGF2α (11060 ± 965 vs. 7963 ± 480 pixels, 1.4 times; P = 0.05 and 18900 ± 1350 vs. 13220 ± 68 pixels, 1.1 times; P = 0.005; respectively). Furthermore, CL red pixels (1.4 times, P = 0.05), blue pixels (1.3 times; P = 0.05), increased in Ovsynch-CIDR than CIDR-PGF2α. In this regard, the luteal blood inflow (blue pixels) was higher than outflow (red pixels) in the Ovsynch-CIDR group and closely equivalent in the CIDR-PGF2α group (Table 3).
Influence of estrus synchronization protocol on circulating steroid hormonal levels in buffaloes
On Day of CIDR removal, a significant (P < 0.05) increase in E2 levels was found in the CIDR-PGF2α group (6.65 ± 0.86 pg/ml) than in the Ovsynch-CIDR group (4.52 ± 0.36 pg/ml). Whereas, E2 at estrus and P4 at luteal phase were substantially (P < 0.05) elevated in the Ovsynch-CIDR group (35.55 ± 2.95 pg/ml and 3.94 ± 0.62 ng/ml) compared to the other group (24.31 ± 4.02 pg/ml and 1.81 ± 0.49 ng/ml).
Influence of estrus synchronization protocol on fertility indices in buffaloes
The rate of estrus synchronization and pregnancy rates (on Day 28–35 post-AI) were higher in Ovsynch-CIDR than CIDR-PGF2α, but unfortunately, these values did not reach to a significant level (P = 0.46 and P = 0.31; respectively) (Table 4).
Table 4. Comparative efficacy of controlled internal drug release (CIDR)-PGF2α and Ovsynch-CIDR protocols on the fertility of Egyptian buffaloes.
| Item | CIDR-PGF2α | Ovsynch-CIDR | P-value (Chi-test) |
|---|---|---|---|
| Estrus response rate a | 12/18 (67%) | 14/18 (78%) | 0.46 |
| Pregnancy rate b | 9/18 (56%) | 12/18 (67%) | 0.31 |
a Estrus response rate was defined as the percentage of animals that expressed heat after the final dose of each regimen. b Pregnancy rate was calculated as the number of pregnant buffaloes divided by the total number of synchronized buffaloes × 100.
Discussion
Estrus synchronization and AI are effective management tools to help animal producers to incorporate superior genetics and to maximize the reproductive performance of their herd [15]. Yet, small buffalo breeders in many areas of Egypt are reluctant to implement this knowledge, primarily due to costs, time and labor constraints as well as the low success rate of the synchronization regimens in terms of conception and/or pregnancy rates. The data presented in the current study will help in the decision of selection between two synchronization regimens in buffaloes. Here, the incorporation of CIDR in the Ovsynch protocol was advantageous in improving the synchronization response judged by enhancing the follicular activity, CL status and blood perfusion to ovarian structures. In addition, it will open new insight to apply further experiments to solve different reproductive disorders in Egyptian buffaloes such as anoestrum, silent heat, and poor conception rate [3, 4].
In the current study, follicular activity was amended at CIDR removal, with an obvious improvement was in the CIDR-PGF2α group in terms of follicular area and dominant follicles diameters (1st and 2nd large follicles) accompanied by a marginal increase in E2 levels. But these changes seem to be temporal because the Ovsynch-CIDR group expressed an enhanced ovarian activity at the time of estrus and later during the luteal phase. These changes were parallel with increasing of blood perfusion to the DF(s) (at estrus) and CL (at luteal phase). In addition, it was concomitant with an increase in E2 (at estrus) and P4 (at luteal phase). The enhanced ovarian activity and the increase in E2 in the CIDR-PGF2α group on the Day of CIDR removal might be attributed to the impact of PGF2α. In this group, PGF2α was administered one day before the CIDR withdrawal that might induce regression of active corpora lutea that might still exist prior to CIDR withdrawal [16]. This action, in turn, decreased endogenous P4 that has an inhibitory effect on follicular growth [16, 17]. Besides, the increase in follicle diameter that had larger granulosa and theca cells mass, in turn, improved the follicular steroidogenic capacity [18].
In the meantime, the results of B-mode, as well as color Doppler ultrasonography in the Ovsynch-CIDR group, support the benefits of GnRH in enhancing the response to CIDR synchronization protocol. These findings agree with former studies in non-cyclic buffalo that indicated that the supplementation of progesterone between the first GnRH and PGF2α administration was associated with an increased conception rate [19].
The increases in follicular blood flow observed in the Ovsynch-CIDR group might be due to the increased pre-ovulatory follicle angiogenesis (indicated by vascular endothelial growth factor expression) under gonadotrophic treatment [20]. The theca layer of the pre-ovulatory follicle develops a thin capillary tissue over the granulosa layer, and the increase in blood flow into the theca layer facilitates follicular development [21]. This might responsible for the upsurge of circulating E2 levels in the Ovsynch-CIDR group. E2 has been supposed to be one of the essential factors in the regulation of blood perfusion in the reproductive tract due to its vasodilatory role [22]. An increase in follicular blood flow and E2 levels were noticed after GnRH administration, and these increases cause metabolic function changes in follicle cells [23]. High levels of E2 at the time of estrus and ovulation activate endothelial nitric oxide synthase production, which induces rapid vasodilation and increases the blood flow to the specified reproductive organs [22].
The increase in luteal blood flow in Ovsynch-CIDR compared to CIDR-PGF2α was verified with color and power Doppler modes. This might be attributed to the effect of GnRH [24] which is assumed to have direct, gonadotropin-independent effects on the ovarian hemodynamic [25]. Explaining the mechanism by which GnRH affect luteal blood flow was beyond the current study aims. Yet, GnRH-dependent luteal blood flow mechanism might be mediated by some factors such as vascular endothelial growth factor, angiopoietin and vascular permeability [26].
Although follicular activity and blood perfusions at the estrus and luteal phases varied markedly between the two groups, the differences in estrus response and pregnancy rates were not reached to the statistical level. Perhaps due to the small animal’s population used in the current study. Nevertheless, numerically, the Ovsynch-CIDR regimen used under the present study conditions was more effective for improving fertility in the cyclic buffaloes. An evaluation of CL hemodynamic during the early luteal phase was used to predict the ongoing pregnancy in Egyptian buffaloes [13]. Early studies showed a positive relationship between greater blood flow of the pre-ovulatory follicle and the cows that became pregnant [27].
The striking feature of this paper is the evaluation of the efficacy of the two protocols on ovarian hemodynamics. Characterization of ovarian response to each regimen based on this parameter provided fundamental information and understanding of its influence on follicular and CL development, and their possible association with animal fertility after AI. However, authors think the importance to provide all various parameters including the pregnancy rate as an index of the fertility response for comparison between the two regimens, even it showed only numerical improvement in animal response that may be attributable to a small sample size of animals used. A further correlation study on a larger animals’ sample may be considered important to validate the applicability of this protocol in improving Egyptian buffalo fertility.
Based on the presented findings, Ovsynch-CIDR protocol substantially increases ovarian activity, follicular (at estrus) and luteal (post-ovulation) blood flow in association with an improvement of the fertility indices as compared with the CIDR-PGF2α regimen, though the differences in pregnancy rate were not reached to a significant level.
Conflict of interest
No conflict of interest.
Acknowledgments
We are grateful to Mr Paul Nyametease, (College of Basic and Applied Science, University of Ghana) for revising the manuscript in correct scientific English. Authors are grateful also to the staff of the dairy farm for allowing use of animals. This research did not receive any specific grant from funding agencies in the public or commercial sectors.
References
- 1.Food, Agriculture Organization of the United N. FAOSTAT statistics database. FAO [Roma]; 2016.
- 2.Abdel-Salam SA, Sayed AI, Elsayed M, Abou-Bakr S. Genetic gain in open nucleus breeding scheme to improve milk production in Egyptian Buffalo. Livest Sci 2010; 131: 162–167. [Google Scholar]
- 3.El-Wishy AB. The postpartum buffalo. II. Acyclicity and anestrus. Anim Reprod Sci 2007; 97: 216–236. [DOI] [PubMed] [Google Scholar]
- 4.Kandiel MM, Gad BA, Sosa GA, El-Azab AI. Follicular dynamics and uterine status after synchronization of ovulation in early post-parturient Egyptian buffaloes. Buffalo Bull 2013; 32: 165–181. [Google Scholar]
- 5.Hoque MN, Talukder AK, Akter M, Shamsuddin M. Evaluation of ovsynch protocols for timed artificial insemination in water buffaloes in Bangladesh. Turk J Vet Anim Sci 2014; 38: 418–424. [Google Scholar]
- 6.Barile VL, Galasso A, Marchiori E, Pacelli C, Montemurro N, Borghese A. Effect of PRID treatment on conception rate in mediterranean buffalo heifers. Livest Prod Sci 2001; 68: 283–287. [Google Scholar]
- 7.Hussein HA, Mohamed RH, Hossam M, Wehrend A. Ovarian response and conception rate following oestrus synchronization using three protocols in Egyptian buffalo heifers. Tierarztl Prax Ausg G Grosstiere Nutztiere 2016; 44: 287–294. [DOI] [PubMed] [Google Scholar]
- 8.Chebel RC, Al-Hassan MJ, Fricke PM, Santos JE, Lima JR, Martel CA, Stevenson JS, Garcia R, Ax RL. Supplementation of progesterone via controlled internal drug release inserts during ovulation synchronization protocols in lactating dairy cows. J Dairy Sci 2010; 93: 922–931. [DOI] [PubMed] [Google Scholar]
- 9.Kasimanickam R, Hall JB, Currin JF, Whittier WD. Sire effect on the pregnancy outcome in beef cows synchronized with progesterone based Ovsynch and CO-Synch protocols. Anim Reprod Sci 2008; 104: 1–8. [DOI] [PubMed] [Google Scholar]
- 10.Azevedo C, Maia I, Canada N, Simões J. Comparison of fertility, regular returns-to-estrus, and calving interval between Ovsynch and CO-synch + CIDR protocols in dairy cows. Theriogenology 2014; 82: 910–914. [DOI] [PubMed] [Google Scholar]
- 11.Kelley DE, Ibarbia L, Daetz R, Bittar JH, Risco CA, Santos JE, Ribeiro ES, Galvão KN. Combined use of progesterone inserts, ultrasongraphy, and GnRH to identify and resynchronize nonpregnant cows and heifers 21 days after timed artificial insemination. Theriogenology 2016; 85: 230–237. [DOI] [PubMed] [Google Scholar]
- 12.Herzog K, Bollwein H. Application of Doppler ultrasonography in cattle reproduction. Reprod Domest Anim 2007; 42 (Suppl 2): 51–58. [DOI] [PubMed] [Google Scholar]
- 13.Lasheen ME, Badr HM, Kandiel MMM, Abo El-Maaty AM, Samir H, Farouk M, Eldawy MH. Predicting early pregnancy in Egyptian buffalo cows via measuring uterine and luteal blood flows, and serum and saliva progesterone. Trop Anim Health Prod 2018; 50: 137–142. [DOI] [PubMed] [Google Scholar]
- 14.Check JH, Ubelacker L, Lauer CC. Falsely elevated steroidal assay levels related to heterophile antibodies against various animal species. Gynecol Obstet Invest 1995; 40: 139–140. . [DOI] [PubMed] [Google Scholar]
- 15.Patterson DJ, Kojima FN, Smith MF. Methods to Synchronize Estrous Cycles of Postpartum Beef Cows with Melengestrol Acetate. Prof Anim Sci 2003; 19: 109–115. [Google Scholar]
- 16.Mercadante VR, Kozicki LE, Ciriaco FM, Henry DD, Dahlen CR, Crosswhite MR, Larson JE, Voelz BE, Patterson DJ, Perry GA, Funston RN, Steckler TL, Hill SL, Stevenson JS, Lamb GC. Effects of administration of prostaglandin F at initiation of the seven-day CO-Synch+controlled internal drug release ovulation synchronization protocol for suckled beef cows and replacement beef heifers. J Anim Sci 2015; 93: 5204–5213. [DOI] [PubMed] [Google Scholar]
- 17.Islam R. Synchronization of estrus in cattle: A review. Vet World 2011; 4: 136–141. [Google Scholar]
- 18.Bigelow KL, Fortune JE. Characteristics of prolonged dominant versus control follicles: follicle cell numbers, steroidogenic capabilities, and messenger ribonucleic acid for steroidogenic enzymes. Biol Reprod 1998; 58: 1241–1249. [DOI] [PubMed] [Google Scholar]
- 19.De Rensis F, Ronci G, Guarneri P, Nguyen BX, Presicce GA, Huszenicza G, Scaramuzzi RJ. Conception rate after fixed time insemination following ovsynch protocol with and without progesterone supplementation in cyclic and non-cyclic Mediterranean Italian buffaloes (Bubalus bubalis). Theriogenology 2005; 63: 1824–1831. [DOI] [PubMed] [Google Scholar]
- 20.Taylor PD, Hillier SG, Fraser HM. Effects of GnRH antagonist treatment on follicular development and angiogenesis in the primate ovary. J Endocrinol 2004; 183: 1–17. [DOI] [PubMed] [Google Scholar]
- 21.Yamada O, Abe M, Takehana K, Iwasa K, Hiraga T, Hiratsuka T. Microvasculature of mature bovine follicles and its changes with ovulation. J Reprod Dev 1994; 40: 307–315. [Google Scholar]
- 22.Acosta TJ, Hayashi KG, Ohtani M, Miyamoto A. Local changes in blood flow within the preovulatory follicle wall and early corpus luteum in cows. Reproduction 2003; 125: 759–767. [PubMed] [Google Scholar]
- 23.Acosta TJ, Miyamoto A. Vascular control of ovarian function: ovulation, corpus luteum formation and regression. Anim Reprod Sci 2004; 82–83: 127–140. . [DOI] [PubMed] [Google Scholar]
- 24.Castro T, Oliveira FA, Siddiqui MA, Baldrighi JM, Wolf CA, Ginther OJ. Stimulation of LH, FSH, and luteal blood flow by GnRH during the luteal phase in mares. Theriogenology 2016; 85: 740–746. [DOI] [PubMed] [Google Scholar]
- 25.Janssens RM, Brus L, Cahill DJ, Huirne JA, Schoemaker J, Lambalk CB. Direct ovarian effects and safety aspects of GnRH agonists and antagonists. Hum Reprod Update 2000; 6: 505–518. [DOI] [PubMed] [Google Scholar]
- 26.Kitajima Y, Endo T, Manase K, Nishikawa A, Shibuya M, Kudo R. Gonadotropin-releasing hormone agonist administration reduced vascular endothelial growth factor (VEGF), VEGF receptors, and vascular permeability of the ovaries of hyperstimulated rats. Fertil Steril 2004; 81 (Suppl 1): 842–849. [DOI] [PubMed] [Google Scholar]
- 27.Siddiqui MA, Almamun M, Ginther OJ. Blood flow in the wall of the preovulatory follicle and its relationship to pregnancy establishment in heifers. Anim Reprod Sci 2009; 113: 287–292. [DOI] [PubMed] [Google Scholar]