Abstract
Myoferlin, a protein of the ferlin family, has seven C2 domains and exhibits activity in some cells, including myoblasts and endothelial cells. Recently, myoferlin was identified as a promising target and biomarker in non‐small‐cell lung cancer, breast cancer, pancreatic adenocarcinoma, hepatocellular carcinoma, colon cancer, melanoma, oropharyngeal squamous cell carcinoma, head and neck squamous cell carcinoma, clear cell renal cell carcinoma and endometrioid carcinoma. This evidence indicated that myoferlin was involved in the proliferation, invasion and migration of tumour cells, the mechanism of which mainly included promoting angiogenesis, vasculogenic mimicry, energy metabolism reprogramming, epithelial‐mesenchymal transition and modulating exosomes. The roles of myoferlin in both normal cells and cancer cells are of great significance to provide novel and efficient methods of tumour treatment. In this review, we summarize recent studies and findings of myoferlin and suggest that myoferlin is a novel potential candidate for clinical diagnosis and targeted cancer therapy.
Keywords: angiogenesis, cancer, metastasis, myoferlin, therapeutic target, vesicle trafficking
1. BACKGROUND
Myoferlin is a relatively novel membrane‐anchored protein within the ferlin family. The myoferlin gene is located at chromosome 10q23.33. It was initially found to have a high degree of homology to dysferlin, and the percentage of similarity between myoferlin and dysferlin sequences is 69%.1 Dysferlin gene mutations cause Miyoshi myopathy and limb girdle muscular dystrophy type 2B,2 while mutations in myoferlin are not correlated with human disease in previous studies. However, myoferlin functions have been revealed through animal and cytology experiments. Elimination of myoferlin in mice results in smaller myofibres and a dystrophic phenotype that has a decreased capacity to regenerate after injury.3 In the hindlimb muscles of resistance exercise‐trained rats, myoferlin mRNA was highly up‐regulated.4 In quadriceps biopsies from Duchenne muscular dystrophy patients, myoferlin mRNA was also up‐regulated 7.3‐fold.5 Of note, recent studies suggest that myoferlin participates in the proliferation, invasion and metastasis of multiple cancers. In addition, researchers regard myoferlin as a promising target and biomarker. In this review, we will present a summary of the functions and roles of myoferlin in normal and tumour cells with updated knowledge.
2. THE STRUCTURE OF MYOFERLIN
Myoferlin belongs to the ferlin family, which has a single pass transmembrane domain situated at the carboxy‐terminus in common.6 The ferlin family is composed of five different proteins, namely, dysferlin, myoferlin, otoferlin, Fer1L5 and Fer1L6.7 All of the ferlin family proteins contain various C2 domains (C2B, C2C, C2D, C2E and C2F) and a carboxy‐terminal transmembrane domain. Dysferlin, myoferlin and Fer1L5 contain the C2A and DYSF domains. Otoferlin also contains the C2A domain.7 In the ferlin family, myoferlin shares more similarities with dysferlin compared with other ferlin proteins.1 Myoferlin and dysferlin are both 230 kD proteins that contain seven C2 domains and one carboxy‐terminal transmembrane domain that is a membrane‐spanning protein domain. Myoferlin and dysferlin both contain a DYSF domain and are highly expressed in myoblasts. An interesting feature of the DYSF structure is that it contains a DYSF domain within another DYSF domain due to gene duplication, but the function of the DYSF domain still remains unknown.8 Mature myofibres express dysferlin and myoferlin at the plasma membrane.1, 3 Furthermore, myoferlin contains some positively charged residues that are important for allowing the transmembrane domain to anchor within the membrane.7 Myoferlin is highly expressed in skeletal muscle, heart muscle and endothelial cells and is also expressed in lungs and most other tissues at low levels.1 Myoferlin typically works with the help of C2 domains. Each C2 domain is formed by approximately 100 amino acids. Two C2‐domains (C2A and C2B) are found in the cytoplasmic domain of synaptotagmin I. Synaptotagmin I works as a Ca2+ sensor that facilitates membrane fusion. C2 domains typically function by two mechanisms: binding phospholipids and playing roles in protein‐protein interactions.9 The C2 domain at the amino terminus of myoferlin, C2A, binds negatively charged phospholipids in response to calcium and is highly expressed in myoblasts undergoing fusion. The C2A domain of myoferlin only binds to phospholipid mixtures that have a high fraction of phosphatidylserine.10 This domain is ideally located to mediate vesicle trafficking directly.11 The second domain of myoferlin, C2B, binds to EH‐domain‐containing 2 (EHD2) directly. Thus, myoferlin is implicated in both endocytic‐recycling and clathrin‐mediated endocytosis.12 Of note, the C2D domain is a target of WJ460, a novel small molecule compound, which exhibits high therapeutic significance in various tumours.13 The structure of myoferlin is shown in Figure 1.
Figure 1.
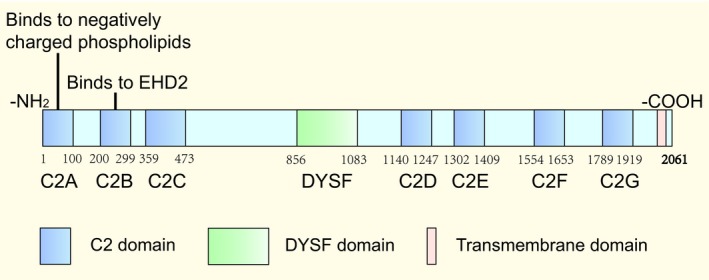
The structure of myoferlin. Myoferlin is a protein that is composed of 2061 amino acids and contains seven C2 domains (referred to C2A‐G), a DYSF domain and a carboxyl‐terminal transmembrane domain. C2A binds to negatively charged phospholipids; C2B binds to EHD2
3. FUNCTIONS OF MYOFERLIN IN NORMAL CELLS
3.1. Muscle cells
Skeletal muscle has inherent renewal properties that allow for regeneration and on‐going repair. The individual muscle cells are highly dependent on an effective and rapid vesicle trafficking system for efficient repair and growth.14 Vesicle trafficking system is crucial for many cellular functions, including cell division, cell migration and the regulation of signalling. In addition, the capacity to coordinate actin dynamics promoting cytoskeletal rearrangements plays an important role during myogenesis.15 Myoferlin functions in some of the above‐mentioned activities, and a summary of these mechanisms is presented in Figure 2.
Figure 2.
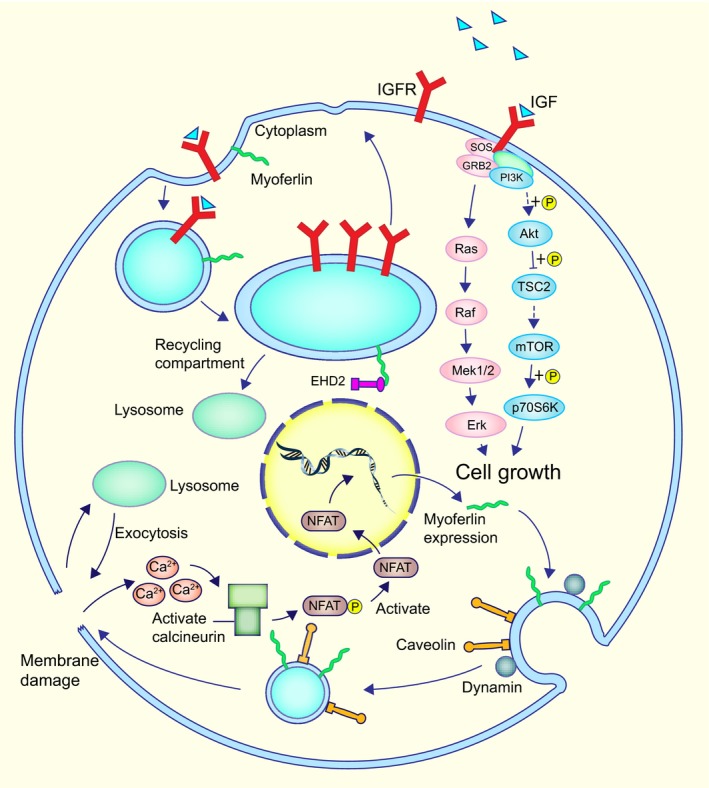
Models for myoferlin and EHD2 in vesicle cycling and myoferlin expression in damaged myofibres. Myoferlin and EHD2 are implicated in vesicle cycling. After endocytosis, some receptors and their ligands are internalized and then are shuttled to the endocytic‐recycling compartment. Finally, they are shuttled back to the membrane and work in another round. EHD2 binds directly to myoferlin in the endocytic‐recycling compartment and promotes the cyclic process of insulin‐like growth factor receptor (IGFR). IGF binds to IGFR directly to promote cell growth by activating the Akt/mTOR and MAPK pathway. In injured myofibres, Ca2+ flows into the cytoplasm and activates NFATs. NFAT then binds to the promoter of the myoferlin and increases the expression of myoferlin. Ca2+ influx is sensed by myoferlin and initiates dynamin‐dependent endocytosis, which cooperates with caveolin. The budded caveolae are then shuttled to the damaged area by an exocytic repair model
3.1.1. Essential for vesicle trafficking and muscle growth
Members of the EH‐domain‐containing (EHD) family act in the process of endocytosis and endocytic recycling, which mediate lipid and receptor recycling back to the plasma membrane.16 Myoferlin functions in this process as a critical participant with an asparagine‐proline‐phenylalanine motif in the myoferlin C2B domain and binds directly to EHD2, an EHD family molecule that is responsible for vesicular trafficking.
Insulin‐like growth factor (IGF) is an important protein in the regulation of muscle growth,17, 18 and its function is mediated by binding to the IGF1 receptor (IGF1R) then activating MAPK and AKT pathways.19 In developing myofibres, IGF1 stimulation induces muscle fibre hypertrophy.14 Myoferlin targets EHD2 to impact the translocation and recycling of IGF1R20 and is a modulator of muscle growth.21 In detail, myoferlin internalizes IGF1R and is subsequently shuttled back to the cell surface to bind more IGF1 in further rounds. In myoferlin‐null myoblasts, large vesicular structures are formed by the accumulation of IGF1R, and the receptors cannot be shuttled back to the membrane. In addition, EHD2 induces myotube formation,15 which is also affected by myoferlin.
3.1.2. Mediating the fusion of myoblasts
Myoferlin is highly expressed for myoblast fusion.11 Myoferlin plays an important role in the formation of the myotubes by the fusion of singly nucleated myoblasts during the regeneration of mature muscle and embryonic muscle growth.3 Mechanically, it has been demonstrated that myoferlin is necessary for calcium‐sensitive membrane resealing via the C2A domain, which can promote the fusion of two opposed lipid bilayers. Furthermore, myoferlin interacts with EHD2 to regulate myoblast fusion by regulating reorganization or disassembly of the cytoskeleton16 given that the C2B domain of myoferlin can directly bind to EHD2 and compete with EHD2‐binding protein 1 (EHBP1), the binding partner of EHD2.22
3.1.3. Promoting skeletal muscle repair
Skeletal muscle contains multinucleated myofibres, and myoferlin promotes their growth and repair by inducing mononucleated myoblasts to fuse with other mononucleated myoblasts and myoblasts to fuse to myotubes.23 Nuclear factor of activated T cells (NFAT) is expressed in muscle and works at different periods during the process of muscle growth and differentiation.24, 25, 26 NFAT targets a 1543‐bp fragment of the myoferlin promoter to induce the expression of myoferlin,23 which contributes significantly to the adaptation occurring in skeletal muscle during ground squirrel hibernation.27
3.2. Endothelial cells
3.2.1. Positively regulating VEGFR‐2
Myoferlin is highly expressed in vascular tissues and endothelial cells (ECs), which are especially enriched in CEM/LR (caveolae‐enriched buoyant membrane microdomains/lipid raft) microdomains. Myoferlin functions in migration, proliferation and nitric oxide (NO) release, which occur in response to vascular endothelial growth factor (VEGF).28 In addition, myoferlin is necessary in some plasma membrane events, such as signalling in specialized cells. The loss of myoferlin reduces the autophosphorylation and expression of VEGF receptor‐2 (VEGFR‐2) in native ECs. The transfection of myoferlin increases autophosphorylation in response to VEGF and VEGFR‐2 membrane expression in a reconstituted cell system. A complex is formed by myoferlin, which also contains dynamin‐2 and VEGFR‐2. This protein complex is necessary for the surface expression of VEGFR‐2.29 This complex prevents proteasomal degradation and CBL‐dependent VEGFR‐2 polyubiquitination, which increases functional signalling and VEGFR‐2 protein stability.28 Myoferlin depletion disrupts rapid VEGF‐mediated intracellular signalling pathways and attenuates VEGF‐mediated activation of key intracellular signalling cascades, that is, c‐Jun N‐terminal kinase (JNK), phospholipase Cγ (PLCγ) and extracellular signal‐regulated kinase‐1/2 (ERK‐1/2).30
3.2.2. Up‐regulating Tie‐2
Myoferlin is required for proper tyrosine kinase receptors expression at the plasma membrane. Myoferlin knockdown in ECs decreases the expression of a second tyrosine kinase receptor, Tie‐2, which is a well‐described angiogenic receptor.31 In addition, a study demonstrated that myoferlin gene silencing results in oedema formation and attenuates angiogenesis.32 Post‐translational regulation of many tyrosine kinase receptors requires myoferlin, including VEGFR‐2 and Tie‐2. Acute myoferlin knockdown may exhibit anti‐angiogenic effects and act as an anti‐angiogenesis target in the treatment of cancer or other angiogenesis‐related diseases.32
3.2.3. Modulating receptor‐dependent endocytosis
Myoferlin induces endomembrane fusion with the plasma membrane in endothelial cells. Myoferlin also regulates aspects of receptor‐dependent endocytosis. Myoferlin gene silencing decreases caveolae/raft‐dependent endocytosis, whereas ectopic myoferlin expression increases endocytosis in COS‐7 cells.29 Mechanistically, myoferlin partially colocalizes with caveolin‐1 (Cav‐1) and dynamin‐2 (Dyn‐2) to form a protein complex, which participates in membrane fusion and caveolae‐dependent endocytosis.29 Current research defines this complex as a molecular bandage that may be essential to the integrity of the cellular membrane. The molecular bandage may also provide a method to regulate various disease processes.33
4. ROLES OF MYOFERLIN IN CANCERS
Recently, myoferlin has roles in various cancers, and current studies suggest that myoferlin is a promising target and biomarker. Myoferlin is involved in the proliferation,34 invasion and migration35 of cancer cells via different mechanisms, mainly including promoting angiogenesis,36 vasculogenic mimicry,37 energy metabolism reprogramming,38, 39 epithelial‐mesenchymal transition (EMT)40 and affecting exosomes.41 Clinically, myoferlin levels correlate with histologic grade and prognosis in several types of cancers. Of note, the functions and mechanisms of myoferlin in cancers have not been thoroughly revealed to date. As myoferlin is considered a highly potential therapeutic target, further exploration is required. Myoferlin expression in cancers has been recently assessed and is presented in Table 1 below.
Table 1.
Expression of myoferlin in cancers
| Cancer | Total no. of samples | Positive rate | Correlation of myoferlin level and tumour stage or grade | References |
|---|---|---|---|---|
| NSCLC | 148 | 50.7% | Stage: No correlation observed (P = .632) | 42 |
| Breast cancer | 90 | No accurate data | Stage: Positive correlation | 13 |
| PAC | 154 | 41.6% | Histologic grade: Positive correlation | 43 |
| HCC | 138 | No accurate data | Stage or grade: No correlation observed | 44 |
| Melanoma | 52 | 42.3% | Pathological grade: No correlation observed (P = .190) | 37 |
| Colon cancer | 28 | 76% | Prognostic or TNM stage: No correlation observed | 38 |
| OPSCC | 211 | 78.2% | T stage: Positive correlationa | 45 |
| HNSCC | 20 | No accurate data | Not mentioned | 46 |
| ccRCC | 304b | No accurate data | Fuhrman nuclear grade: Positive correlation | 47 |
| Endometrioid carcinoma | 60 | 96.7% | FIGO stage: Negative correlationc | 48 |
Correlation between nuclear myoferlin expression and tumour stage.
304 samples were gathered from 152 patients.
Correlation between myoferlin and FIGO stage or FIGO histologic grading showed a negative correlation (opposite to the results in other cancers), the mechanism of which had not been fully revealed (see Section 4.2.7).
4.1. Overview
4.1.1. Promoting proliferation, invasion and migration
Unregulated proliferation is one of the major characteristics of tumour cells. Researchers observed that myoferlin protein is essential for the proliferation of breast cancer and pancreatic ductal adenocarcinoma (PDAC) cells.34, 36
Epithelial‐mesenchymal transition is a common phenomenon and a crucial step in the progression and dissemination of cancer metastasis.49 Functionally, myoferlin can induce EMT via up‐regulating mesenchymal cell markers, such as fibronectin and vimentin, and co‐ordinately down‐regulating epithelial markers, such as E‐cadherin.35, 40
A mathematical model prediction reveals that matrix metalloproteinases (MMPs) have a key function on cancer cell invasion.50 Myoferlin can also cause selective changes of MMPs in breast cancer40, 50 and melanoma.37
A summary of these mechanisms is shown in Figure 3.
Figure 3.
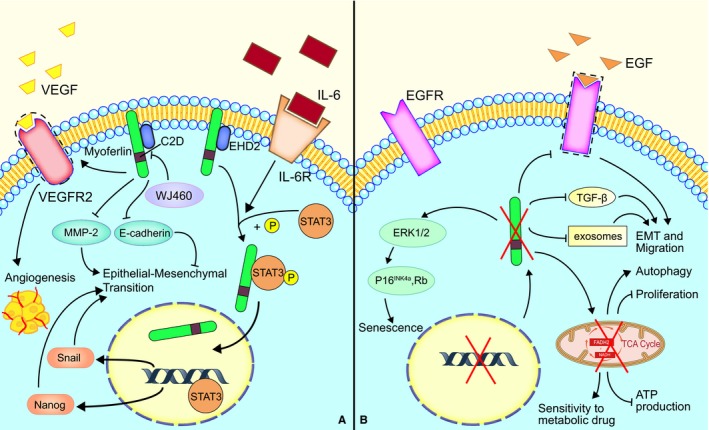
Myoferlin plays key roles in the development of cancer cells. A, Myoferlin is essential for the secretion of VEGFA and the function of VEGFR, which promotes angiogenesis and vasculogenic mimicry in cancer cells. Myoferlin has a significant function in IL‐6‐meditated tumour growth and tumour metastasis through promoting the nuclear translocation of STAT3. Furthermore, WJ460 binds to the C2D domain of myoferlin, which is considered a potential therapeutic target. B, Myoferlin depletion modulates MAPK and p16‐/Rb pathways and induces senescence. Myoferlin depletion decreases the capacity of exosomes and TGF‐β secretion, which has a negative function in EMT and migration. Myoferlin depletion also has an effect on endosomal and metabolism systems and inhibits cancer cell proliferation
4.1.2. Functions in exosomes
Exosomes, which are characterized by unique proteomic composition, participate in the intercellular exchange of metabolites, proteins and nucleic acids.51 Through proteomic analysis, myoferlin has been reported in several tumour‐derived exosomes, including prostate cancers,52 bladder cancers,53 colon cancers,54 ovary cancers,55 Hepatocellular carcinoma,56 squamous carcinoma cells57 and melanoma.58
Blomme et al41 revealed that myoferlin is an emerging oncogene that plays a functional role in exosome biology. Myoferlin is potentially involved in protein loading of exosome. The authors note that myoferlin is a general component of exosomes derived from pancreatic and breast cancer cell lines, and myoferlin‐depleted exosomes exhibit a reduced capacity to transfer nucleic acids to human endothelial cells, thus reducing the ability to induce proliferation and migration of human endothelial cells.
4.1.3. Promoting angiogenesis/vasculogenic mimicry
Myoferlin gene knockdown attenuates the expression of VEGFR‐2 and Tie‐2, well‐described angiogenic receptors, in endothelial cells.32 However, in several cancer cells, myoferlin also has another role. A recent study reported that myoferlin overexpression induced the formation of vasculogenic mimicry in melanoma.37 Concrete mechanisms are discussed in the next section. The mechanism and the exact regulatory system are presented in Figure 4.
Figure 4.
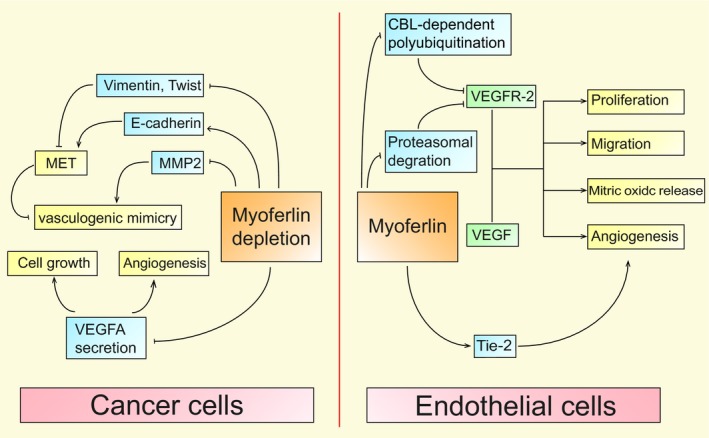
Myoferlin participates in angiogenesis in cancer and endothelial cells. In cancer cells, myoferlin depletion inhibits the secretion of VEGFA, which has a negative effect on proliferation and angiogenesis. Myoferlin depletion suppresses vasculogenic mimicry formation via down‐regulating MMP2 and induces the mesenchymal to epithelial transition (MET). In endothelial cells, myoferlin prevents proteasomal degradation and CBL‐dependent VEGFR‐2 polyubiquitination, which increases VEGFR‐2 protein stability and VEGF‐mediated intracellular signalling pathways. Myoferlin knockdown also down‐regulates the well‐described angiogenic receptor Tie‐2
4.1.4. Promoting energy metabolism reprogramming
Energy metabolism reprogramming is considered an emerging hallmark and a therapeutic target in cancers for cancer cells mostly prefer performed with glycolysis to produce energy.59 But cancer cells with more flexible metabolic phenotype have stronger resistance. In triple‐negative breast cancer,60 PDAC39 and colon cancer38 cells, myoferlin was described as an essential molecule to maintain a high oxidative phosphorylation activity. The mechanism will be discussed in the next section.
4.1.5. Clinical significance
Myoferlin plays an oncogenic role and promotes cancer cell metastasis; thus, it often exhibits a relationship with histological grade or clinical stage of cancers. In addition, overexpression of myoferlin often indicates a poor prognosis. Specifically, myoferlin is an independent prognostic factor in pancreatic adenocarcinoma,36, 43 oropharyngeal squamous cell carcinoma,45 head and neck squamous cell carcinoma patients,45, 61 clear cell renal cell carcinoma,47 colon cancer38 and non‐small‐cell lung carcinoma42 patients.
4.2. Specific roles of myoferlin in different cancers
4.2.1. Myoferlin in non‐small‐cell lung carcinoma
Lung cancer is a major cause of cancer‐related deaths worldwide.62 Non‐small‐cell lung cancer (NSCLC) accounts for approximately 85%‐90% of all lung cancers. Myoferlin is expressed in normal lung parenchyma and normal bronchial epithelium.63 A recent study found that myoferlin localizes to the cytoplasm of the cells in all NSCLC pathological subtypes, and adenocarcinomas exhibited the largest proportion.42 Moreover, in adenocarcinoma cases, myoferlin‐positive tumours may indicate a poor prognosis (odds ratio = 2.94; P = .339), while myoferlin and VEGFR‐2 expression exhibited a significant correlation in squamous cell carcinoma (P = .001), especially in stage I patients.42
4.2.2. Myoferlin in breast cancer
Female breast cancer is the second commonly diagnosed cancer in the world and the leading cause of cancer associated death in females.62 Studies have shown that myoferlin plays a critical role in cancer progression via various mechanisms. For migration, researchers found that myoferlin depletion enhances cell adhesion, enlarges focal adhesion,64 enhances cell‐matrix adhesion through elevating focal adhesion kinase and paxillin phosphorylation,65 redirects cancer cell motility35 and subsequently inhibits migration.
In molecular level, potential targets of myoferlin include MMPs, EGFR and TGF‐β. After knocking‐down myoferlin, mesenchymal to epithelial transition occurred,40 and selective changes in MMPs were observed (which included remarkable down‐regulation of MMP140, 50 and MMP3, 8, 12, 13, 14, 1640 and up‐regulation of MMP940), EGF‐induced cell migration and EMT were blocked66 (verified by impaired degradation of phosphorylated EGFR via dysfunctional plasma membrane caveolae and alteration of caveolin homo‐oligomerization) and bio‐mechanical properties were altered65 (cell stiffness decreased, cell‐substrate adhesion increased, and cells subsequently migrated directionally, collectively and slowly). In addition, vesicle traffic was impaired60 (saturated/unsaturated fatty acids were subsequently imbalanced, mitochondrial dysfunction occurred, metabolic reprogramming to glycolysis was triggered, the ability to balance between oxidative phosphorylation and glycolysis was reduced, and the sensitivity to metabolic drug increased), growth velocity was reduced34 (partly for myoferlin‐dependent plasma membrane fusion and fission events were inhibited). Moreover, the overall migration ability of the tumour decreased64 (due to the abovementioned mechanisms, and reduced autocrine TGF‐β production caused dysregulation of TGF‐β1 signalling). Of note, the author stated that mechanism by which myoferlin regulates TGF‐β1 secretion may occur altered gene expression or exocytosis, which deserves further study.64 Of note, Zhang et al13 found that myoferlin was directly targeted by WJ460 and suggest that targeting myoferlin by WJ460 may be a promising therapeutic strategy in myoferlin‐driven breast cancers.
4.2.3. Myoferlin in pancreatic adenocarcinoma
Pancreatic cancer is one of the deadliest cancers given its poor prognosis.62 Similarly, high myoferlin levels are a risk factor in pancreatic adenocarcinoma (PAC). Myoferlin expression significantly correlates with the degree of histological differentiation of PAC, and reduced myoferlin expression alleviated malignant phenotypes of both primary and metastatic PAC cells.43 Exosomes have various roles in PDAC, mainly including local invasion, migration, immune evasion and therapeutic resistance.67 Myoferlin is critical for producing functional exosomes with sufficient quantities of certain components.41
Autocrine and paracrine of vascular endothelial growth factor A (VEGFA) have pro‐proliferative effects independently.20, 68 Myoferlin knockdown down‐regulated VEGFA secretion, which is caused by impairment of VEGFA exocytosis, subsequently, tumours lacked VEGFA and VEGFA induced functional blood vessels thus exhibited a reduced volume.36 In addition, myoferlin is required to maintain a branched mitochondrial structure and high oxidative phosphorylation activity. Myoferlin depletion induces the phosphorylation of dynamin‐related protein (DRP)‐1 and increases its abundance, thus leading to mitochondrial fission and swelling.39 Interestingly, depletion of myoferlin led to a reduction in autophagy induction.39 Li et al reported lead compound 6y, one of 1,5‐diaryl‐1,2,4‐triazole derivatives, bound to myoferlin and inhibited pancreatic cancer metastasis. The anticancer activity of 6y is positively associated with the expression of myoferlin, and 6y is insensitive to myoferlin depletion. The specific mechanisms may be mediated by blocking the receptor tyrosine kinases, inhibiting the secretions of matrix metalloproteinase and reversing the EMT process.69 Overall, myoferlin may provide a novel therapeutic target in PAC.
4.2.4. Myoferlin in hepatocellular carcinoma
In total, 75%‐85% of liver cancer cases are hepatocellular carcinoma (HCC).62 HCC is often associated with poor prognosis.70 Hermanns et al44 demonstrated that myoferlin was necessary for invasion, proliferation and anchorage‐independent cell growth of HCC, and the myoferlin gene is targeted by MKL1/2. Furthermore, myoferlin inhibits EGFR and the downstream MAPK and p16‐/Rb pathways, thus affecting senescence phenotype. In detail, depletion of myoferlin in tumour cells from SRF‐VP16‐derived murine HCCs induced a senescence phenotype, which suggested that myoferlin might be a novel therapeutic target.
4.2.5. Myoferlin in colon cancer
Colorectal cancer ranks third in terms of incidence but second in terms of mortality worldwide,62 and the most common tumour location is the proximal colon (41%) in America.71 Myoferlin's functions are similar to those in PAC. Myoferlin is required for high oxidative phosphorylation activity and maintenance of an organized mitochondrial network.38 In addition, Rademaker et al38 also found that myoferlin silencing causes reactive oxygen species (ROS) accumulation, p53‐dependent reduction of cell growth, enhanced DNA damage response and increased apoptosis. These authors revealed that myoferlin could represent a suitable target for new anticancer therapies.
4.2.6. Myoferlin in melanoma
Melanoma is the most aggressive type of skin cancer, and the appearance of vasculogenic mimicry (VM) in this type of cancer always indicates a poor prognosis.72 In VM, tumour cells mimic true vascular endothelium cells and form microvascular channels.73 Myoferlin knockdown significantly impaired the capability of A375 cells to form VM structures and subsequently inhibit cell invasion and migration in vitro. At the molecular level, down‐regulation of myoferlin decreases MMP‐2 expression and induces MET.37 Thus, myoferlin may represent a biomarker for VM formation and a risk factor for poor prognosis of melanoma patients.
4.2.7. Myoferlin in other cancers
Kumar et al45 demonstrated that myoferlin could exist in the nucleus, cytosol or membrane of oropharyngeal squamous cell carcinoma (OPSCC) cells. These authors uncovered that nuclear myoferlin expression was directly associated with IL‐6 (P < .001), inversely associated with HPV status (P = .0014) and could predict poor clinical outcome in OPSCC patients independently. Yadav et al46 found that myoferlin is bound to EHD2 protein and modulates the IL‐6/STAT3 signalling pathway, which regulates the expression of IL‐6/STAT3 downstream genes, including snail and nanog, in head and neck squamous cell carcinoma (HNSCC) cell lines. Myoferlin knockdown significantly decreases tumour growth and metastasis of HNSCC. Song et al47 uncovered that high myoferlin levels correlate with unfavourable prognosis in clear cell renal cell carcinoma (ccRCC) patients. Amazingly, in endometrioid carcinoma, high expression of myoferlin was related to low‐grade carcinoma, while the loss of myoferlin expression was noted in high‐grade carcinoma, which was in contrast to findings in other cancers.48 The authors pointed out it was probably due to the fact that normal endometrial tissue underwent a continuous cycle of regeneration, in which myoferlin was implicated. As noncyclic continuous exposure to sex hormones contributes to the oncogenesis of endometrioid carcinoma, the authors predicted a potential correlation among cellular regeneration, hormonal effect and myoferlin expression.48 This finding may suggest that myoferlin plays a different role in endometrioid carcinoma, and the mechanism remains uncharacterized.
5. CONCLUSION AND PROSPECT
Myoferlin is a membrane‐anchored ferlin family protein. Myoferlin is highly expressed in skeletal muscle, heart muscle and endothelial cells and is essential for the growth, repair and normal function of cells as it modulates vesicle trafficking, cell fusion, receptor‐dependent endocytosis and the expression of certain receptors. Myoferlin is also expressed in most other tissues at low levels. However, in specific tumours, myoferlin is overexpressed and plays critical roles. Myoferlin functions in the proliferation, invasion and migration of cancer cells through various mechanisms. These findings identify myoferlin as a novel potential candidate for clinical diagnosis and targeted therapy.
The latest research shows that myoferlin regulates cancer‐derived exosomes and functions as a new player in exosome biology.41 As mentioned above, myoferlin impacts tumour‐associated angiogenesis by affecting VEGFA secretion and EGFR activity.36, 66 These findings suggest that myoferlin induces the malignant phenotype of cancers by altering the tumour microenvironment. Zhang et al13 found that WJ460 directly targets myoferlin, interacts with myoferlin C2 domain and hampers the proper function of myoferlin.13 WJ460 represents a potentially effective therapeutic molecule for preventing myoferlin‐related cancers and provides an opportunity for developing myoferlin‐targeted agents. Moreover, lead compound 6y, one of the 1,5‐diaryl‐1,2,4‐triazole derivatives, targets at myoferlin and prevents pancreatic cancer metastasis, which suggests 6y may also be a promising therapeutic strategy.69
CONFLICT OF INTERESTS
The authors declare that they have no competing interests.
AUTHORS' CONTRIBUTIONS
CR, WZ, BZ and CZ contributed to the study design. WZ, BZ and CZ drafted and critically revised the manuscript. CR, ZB, HX, XY, WL, BZ and LW discussed and revised the manuscript. All authors read and approved the final manuscript.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Not applicable.
ACKNOWLEDGEMENTS
The present study was supported by the National Natural Science Foundation of China (grant nos. 81773179 and 81272972 [C. Ren]), the National Basic Research Program of China (2010CB833605 [C. Ren]), Program for New Century Excellent Talents in University (NCET‐10‐0790 [C. Ren]), the Hunan Provincial Science and Technology Department (grant no. 2016JC2049 [C. Ren]), Provincial Natural Science Foundation of Hunan (grant no. 2016JJ2172 [W. Liu]) and the Undergraduate Training Programs for Innovation and Entrepreneurship (UTPIE, 502331018 [W. Zhu]).
Zhu W, Zhou B, Zhao C, et al. Myoferlin, a multifunctional protein in normal cells, has novel and key roles in various cancers. J Cell Mol Med. 2019;23:7180–7189. 10.1111/jcmm.14648
Wei Zhu, Bolun Zhou and Chenxuan Zhao contributed equally to this article.
REFERENCES
- 1. Davis DB, Delmonte AJ, Ly CT, McNally EM. Myoferlin, a candidate gene and potential modifier of muscular dystrophy. Hum Mol Genet. 2000;9:217‐226. [DOI] [PubMed] [Google Scholar]
- 2. Liu J, Aoki M, Illa I, et al. Dysferlin, a novel skeletal muscle gene, is mutated in Miyoshi myopathy and limb girdle muscular dystrophy. Nat Genet. 1998;20:31‐36. [DOI] [PubMed] [Google Scholar]
- 3. Doherty KR, Cave A, Davis DB, et al. Normal myoblast fusion requires myoferlin. Development. 2005;132:5565‐5575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Adams GR, Haddad F, Bodell PW, Tran PD, Baldwin KM. Combined isometric, concentric, and eccentric resistance exercise prevents unloading‐induced muscle atrophy in rats. J Appl Physiol (1985). 2007;103:1644‐1654. [DOI] [PubMed] [Google Scholar]
- 5. Haslett JN, Sanoudou D, Kho AT, et al. Gene expression comparison of biopsies from Duchenne muscular dystrophy (DMD) and normal skeletal muscle. Proc Natl Acad Sci USA. 2002;99:15000‐15005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jimenez JL, Bashir R. In silico functional and structural characterisation of ferlin proteins by mapping disease‐causing mutations and evolutionary information onto three‐dimensional models of their C2 domains. J Neurol Sci. 2007;260:114‐123. [DOI] [PubMed] [Google Scholar]
- 7. Posey AD Jr, Demonbreun A, McNally EM. Ferlin proteins in myoblast fusion and muscle growth. Curr Top Dev Biol. 2011;96:203‐230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sula A, Cole AR, Yeats C, Orengo C, Keep NH. Crystal structures of the human Dysferlin inner DysF domain. BMC Struct Biol. 2014;14:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hui E, Bai J, Chapman ER. Ca2+‐triggered simultaneous membrane penetration of the tandem C2‐domains of synaptotagmin I. Biophys J. 2006;91:1767‐1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Harsini FM, Bui AA, Rice AM, et al. Structural basis for the distinct membrane binding activity of the homologous C2A domains of myoferlin and dysferlin. J Mol Biol. 2019;431:2112‐2126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Davis DB, Doherty KR, Delmonte AJ, McNally EM. Calcium‐sensitive phospholipid binding properties of normal and mutant ferlin C2 domains. J Biol Chem. 2002;277:22883‐22888. [DOI] [PubMed] [Google Scholar]
- 12. Grant BD, Caplan S. Mechanisms of EHD/RME‐1 protein function in endocytic transport. Traffic. 2008;9:2043‐2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhang T, Li J, He Y, et al. A small molecule targeting myoferlin exerts promising anti‐tumor effects on breast cancer. Nat Commun. 2018;9:3726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Grounds MD, Shavlakadze T. Growing muscle has different sarcolemmal properties from adult muscle: a proposal with scientific and clinical implications: reasons to reassess skeletal muscle molecular dynamics, cellular responses and suitability of experimental models of muscle disorders. BioEssays. 2011;33:458‐468. [DOI] [PubMed] [Google Scholar]
- 15. Posey AD, Swanson KE, Alvarez MG, et al. EHD1 mediates vesicle trafficking required for normal muscle growth and transverse tubule development. Dev Biol. 2014;387:179‐190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Naslavsky N, Caplan S. EHD proteins: key conductors of endocytic transport. Trends Cell Biol. 2011;21:122‐131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yang SY, Goldspink G. Different roles of the IGF‐I Ec peptide (MGF) and mature IGF‐I in myoblast proliferation and differentiation. FEBS Lett. 2002;522:156‐160. [DOI] [PubMed] [Google Scholar]
- 18. Musarò A, McCullagh K, Paul A, et al. Localized Igf‐1 transgene expression sustains hypertrophy and regeneration in senescent skeletal muscle. Nat Genet. 2001;27:195‐200. [DOI] [PubMed] [Google Scholar]
- 19. Rommel C, Bodine SC, Clarke BA, et al. Mediation of IGF‐1‐induced skeletal myotube hypertrophy by PI(3)K/Akt/mTOR and PI(3)K/Akt/GSK3 pathways. Nat Cell Biol. 2001;3:1009‐1013. [DOI] [PubMed] [Google Scholar]
- 20. Doherty KR, Demonbreun AR, Wallace GQ, et al. The endocytic recycling protein EHD2 interacts with myoferlin to regulate myoblast fusion. J Biol Chem. 2008;283:20252‐20260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Demonbreun AR, Posey AD, Heretis K, et al. Myoferlin is required for insulin‐like growth factor response and muscle growth. FASEB J. 2010;24:1284‐1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Guilherme A, Soriano NA, Bose S, et al. EHD2 and the novel EH domain binding protein EHBP1 couple endocytosis to the actin cytoskeleton. J Biol Chem. 2004;279:10593‐10605. [DOI] [PubMed] [Google Scholar]
- 23. Demonbreun AR, Lapidos KA, Heretis K, et al. Myoferlin regulation by NFAT in muscle injury, regeneration and repair. J Cell Sci. 2010;123:2413‐2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Abbott KL, Friday BB, Thaloor D, Murphy TJ, Pavlath GK. Activation and cellular localization of the cyclosporine A‐sensitive transcription factor NF‐AT in skeletal muscle cells. Mol Biol Cell. 1998;9:2905‐2916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cho Y‐Y, Yao KE, Bode AM, et al. RSK2 mediates muscle cell differentiation through regulation of NFAT3. J Biol Chem. 2007;282:8380‐8392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. O'Connor RS, Mills ST, Jones KA, Ho SN, Pavlath GK. A combinatorial role for NFAT5 in both myoblast migration and differentiation during skeletal muscle myogenesis. J Cell Sci. 2007;120:149‐159. [DOI] [PubMed] [Google Scholar]
- 27. Zhang Y, Storey KB. Expression of nuclear factor of activated T cells (NFAT) and downstream muscle‐specific proteins in ground squirrel skeletal and heart muscle during hibernation. Mol Cell Biochem. 2016;412:27‐40. [DOI] [PubMed] [Google Scholar]
- 28. Bernatchez PN, Acevedo L, Fernandez‐Hernando C, et al. Myoferlin regulates vascular endothelial growth factor receptor‐2 stability and function. J Biol Chem. 2007;282:30745‐30753. [DOI] [PubMed] [Google Scholar]
- 29. Bernatchez PN, Sharma A, Kodaman P, Sessa WC. Myoferlin is critical for endocytosis in endothelial cells. Am J Physiol Cell Physiol. 2009;297:C484‐C492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bernatchez PN, Allen BG, Gélinas DS, Guillemette G, Sirois MG. Regulation of VEGF‐induced endothelial cell PAF synthesis: role of p42/44 MAPK, p38 MAPK and PI3K pathways. Br J Pharmacol. 2001;134:1253‐1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pan X, Liang L, Si RU, et al. Discovery of novel anti‐angiogenesis agents. Part 10: multi‐target inhibitors of VEGFR‐2, Tie‐2 and EphB4 incorporated with 1,2,3‐triazol. Eur J Med Chem. 2019;163:1‐9. [DOI] [PubMed] [Google Scholar]
- 32. Yu C, Sharma A, Trane A, Utokaparch S, Leung C, Bernatchez P. Myoferlin gene silencing decreases Tie‐2 expression in vitro and angiogenesis in vivo. Vascul Pharmacol. 2011;55:26‐33. [DOI] [PubMed] [Google Scholar]
- 33. Cipta S, Patel HH. Molecular bandages: inside‐out, outside‐in repair of cellular membranes. Focus on "Myoferlin is critical for endocytosis in endothelial cells". Am J Physiol Cell Physiol. 2009;297:C481‐C483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Leung C, Yu C, Lin MI, Tognon C, Bernatchez P. Expression of myoferlin in human and murine carcinoma tumors: role in membrane repair, cell proliferation, and tumorigenesis. Am J Pathol. 2013;182:1900‐1909. [DOI] [PubMed] [Google Scholar]
- 35. Volakis LI, Li R, Ackerman WE, et al. Loss of myoferlin redirects breast cancer cell motility towards collective migration. PLoS ONE. 2014;9:e86110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Fahmy K, Gonzalez A, Arafa M, et al. Myoferlin plays a key role in VEGFA secretion and impacts tumor‐associated angiogenesis in human pancreas cancer. Int J Cancer. 2016;138:652‐663. [DOI] [PubMed] [Google Scholar]
- 37. Zhang W, Zhou P, Meng A, et al. Down‐regulating myoferlin inhibits the vasculogenic mimicry of melanoma via decreasing MMP‐2 and inducing mesenchymal‐to‐epithelial transition. J Cell Mol Med. 2018;22:1743‐1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rademaker G, Costanza B, Bellier J, et al. Human colon cancer cells highly express myoferlin to maintain a fit mitochondrial network and escape p53‐driven apoptosis. Oncogenesis. 2019;8:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rademaker G, Hennequière V, Brohée L, et al. Myoferlin controls mitochondrial structure and activity in pancreatic ductal adenocarcinoma, and affects tumor aggressiveness. Oncogene. 2018;37:4398‐4412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Li R, Ackerman WE, Mihai C, Volakis LI, Ghadiali S, Kniss DA. Myoferlin depletion in breast cancer cells promotes mesenchymal to epithelial shape change and stalls invasion. PLoS ONE. 2012;7:e39766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Blomme A, Fahmy K, Peulen O, et al. Myoferlin is a novel exosomal protein and functional regulator of cancer‐derived exosomes. Oncotarget. 2016;7:83669‐83683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Song DH, Ko GH, Lee JH, et al. Myoferlin expression in non‐small cell lung cancer: prognostic role and correlation with VEGFR‐2 expression. Oncol Lett. 2016;11:998‐1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wang W‐S, Liu X‐H, Liu L‐X, et al. iTRAQ‐based quantitative proteomics reveals myoferlin as a novel prognostic predictor in pancreatic adenocarcinoma. J Proteomics. 2013;91:453‐465. [DOI] [PubMed] [Google Scholar]
- 44. Hermanns C, Hampl V, Holzer K, et al. The novel MKL target gene myoferlin modulates expansion and senescence of hepatocellular carcinoma. Oncogene. 2017;36:3464‐3476. [DOI] [PubMed] [Google Scholar]
- 45. Kumar B, Brown NV, Swanson BJ, et al. High expression of myoferlin is associated with poor outcome in oropharyngeal squamous cell carcinoma patients and is inversely associated with HPV‐status. Oncotarget. 2016;7:18665‐18677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Yadav A, Kumar B, Lang JC, Teknos TN, Kumar P. A muscle‐specific protein 'myoferlin' modulates IL‐6/STAT3 signaling by chaperoning activated STAT3 to nucleus. Oncogene. 2017;36:6374‐6382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Song DH, Ko GH, Lee JH, et al. Prognostic role of myoferlin expression in patients with clear cell renal cell carcinoma. Oncotarget. 2017;8:89033‐89039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kim MH, Song DH, Ko GH, et al. Myoferlin expression and its correlation with FIGO histologic grading in early‐stage endometrioid carcinoma. J Pathol Transl Med. 2018;52:93‐97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Mao X‐Y, Li Q‐Q, Gao Y‐F, Zhou H‐H, Liu Z‐Q, Jin W‐L. Gap junction as an intercellular glue: emerging roles in cancer EMT and metastasis. Cancer Lett. 2016;381:133‐137. [DOI] [PubMed] [Google Scholar]
- 50. Eisenberg MC, Kim Y, Li R, Ackerman WE, Kniss DA, Friedman A. Mechanistic modeling of the effects of myoferlin on tumor cell invasion. Proc Natl Acad Sci U S A. 2011;108:20078‐20083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Hu Y, Rao S‐S, Wang Z‐X, et al. Exosomes from human umbilical cord blood accelerate cutaneous wound healing through miR‐21‐3p‐mediated promotion of angiogenesis and fibroblast function. Theranostics. 2018;8:169‐184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kharaziha P, Chioureas D, Rutishauser D, et al. Molecular profiling of prostate cancer derived exosomes may reveal a predictive signature for response to docetaxel. Oncotarget. 2015;6:21740‐21754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Welton JL, Khanna S, Giles PJ, et al. Proteomics analysis of bladder cancer exosomes. Mol Cell Proteomics. 2010;9:1324‐1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Demory Beckler M, Higginbotham JN, Franklin JL, et al. Proteomic analysis of exosomes from mutant KRAS colon cancer cells identifies intercellular transfer of mutant KRAS. Mol Cell Proteomics. 2013;12:343‐355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Liang B, Peng P, Chen S, et al. Characterization and proteomic analysis of ovarian cancer‐derived exosomes. J Proteomics. 2013;80:171‐182. [DOI] [PubMed] [Google Scholar]
- 56. He M, Qin H, Poon T, et al. Hepatocellular carcinoma‐derived exosomes promote motility of immortalized hepatocyte through transfer of oncogenic proteins and RNAs. Carcinogenesis. 2015;36:1008‐1018. [DOI] [PubMed] [Google Scholar]
- 57. Park JE, Tan HS, Datta A, et al. Hypoxic tumor cell modulates its microenvironment to enhance angiogenic and metastatic potential by secretion of proteins and exosomes. Mol Cell Proteomics. 2010;9:1085‐1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Lazar I, Clement E, Ducoux‐Petit M, et al. Proteome characterization of melanoma exosomes reveals a specific signature for metastatic cell lines. Pigment Cell Melanoma Res. 2015;28:464‐475. [DOI] [PubMed] [Google Scholar]
- 59. Yuan LW, Yamashita H, Seto Y. Glucose metabolism in gastric cancer: the cutting‐edge. World J Gastroenterol. 2016;22:2046‐2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Blomme A, Costanza B, de Tullio P, et al. Myoferlin regulates cellular lipid metabolism and promotes metastases in triple‐negative breast cancer. Oncogene. 2017;36:2116‐2130. [DOI] [PubMed] [Google Scholar]
- 61. Yadav A, Kumar B, Datta J, Teknos TN, Kumar P. IL‐6 promotes head and neck tumor metastasis by inducing epithelial‐mesenchymal transition via the JAK‐STAT3‐SNAIL signaling pathway. Mol Cancer Res. 2011;9:1658‐1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394‐424. [DOI] [PubMed] [Google Scholar]
- 63. Leung C, Shaheen F, Bernatchez P, Hackett TL. Expression of myoferlin in human airway epithelium and its role in cell adhesion and zonula occludens‐1 expression. PLoS ONE. 2012;7:e40478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Barnhouse VR, Weist JL, Shukla VC, et al. Myoferlin regulates epithelial cancer cell plasticity and migration through autocrine TGF‐beta1 signaling. Oncotarget. 2018;9:19209‐19222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Blackstone BN, Li R, Ackerman WE, Ghadiali SN, Powell HM, Kniss DA. Myoferlin depletion elevates focal adhesion kinase and paxillin phosphorylation and enhances cell‐matrix adhesion in breast cancer cells. Am J Physiol Cell Physiol. 2015;308:C642‐C649. [DOI] [PubMed] [Google Scholar]
- 66. Turtoi A, Blomme A, Bellahcene A, et al. Myoferlin is a key regulator of EGFR activity in breast cancer. Cancer Res. 2013;73:5438‐5448. [DOI] [PubMed] [Google Scholar]
- 67. Massoumi RL, Hines OJ, Eibl G, King JC. Emerging evidence for the clinical relevance of pancreatic cancer exosomes. Pancreas. 2019;48:1‐8. [DOI] [PubMed] [Google Scholar]
- 68. von Marschall Z, Cramer T, Höcker M, et al. De novo expression of vascular endothelial growth factor in human pancreatic cancer: evidence for an autocrine mitogenic loop. Gastroenterology. 2000;119:1358‐1372. [DOI] [PubMed] [Google Scholar]
- 69. Li Y, He Y, Shao T, et al. Modification and biological evaluation of a series of 1,5‐diaryl‐1,2,4‐triazole compounds as novel agents against pancreatic cancer metastasis through targeting myoferlin. J Med Chem. 2019;62:4949‐4966. [DOI] [PubMed] [Google Scholar]
- 70. Yang YU, Wen F, Li J, et al. A high baseline HBV load and antiviral therapy affect the survival of patients with advanced HBV‐related HCC treated with sorafenib. Liver Int. 2015;35:2147‐2154. [DOI] [PubMed] [Google Scholar]
- 71. Siegel RL, Miller KD, Fedewa SA, et al. Colorectal cancer statistics, 2017. CA Cancer J Clin. 2017;67:177‐193. [DOI] [PubMed] [Google Scholar]
- 72. Yang JP, Liao YD, Mai DM, et al. Tumor vasculogenic mimicry predicts poor prognosis in cancer patients: a meta‐analysis. Angiogenesis. 2016;19:191‐200. [DOI] [PubMed] [Google Scholar]
- 73. Huang B, Xiao E, Huang M. MEK/ERK pathway is positively involved in hypoxia‐induced vasculogenic mimicry formation in hepatocellular carcinoma which is regulated negatively by protein kinase A. Med Oncol. 2015;32:408. [DOI] [PubMed] [Google Scholar]


