Abstract
Background
Diabetic nephropathy (DN) is a common complication of diabetes mellitus (DM) and also a major cause of end-stage renal disease (ESRD). Olmesartan medoxomil (OM) is an angiotensin II receptor blocker (ARB) and has been shown to exhibit renoprotective effects on a streptozotocin (STZ)-induced diabetic rat model. Yet, whether OM affects DN progression and renal injury in db/db mice, a type 2 diabetic murine model, has not been established.
Methods
Wild-type (n = 15) and db/db mice (n = 15) were treated with control saline or OM via oral gavage. The physiological and biochemical parameters were evaluated and histological examinations of kidney specimens were performed.
Results
Compared with saline-treated db/db mice, db/db mice administered with OM showed ameliorated diabetic physiological and biochemical parameters. In addition, OM decreased urinary albumin excretion and plasma creatinine level in db/db mice. Moreover, histologically, OM reduced glomerular hypertrophy and injury, and also ameliorated tubular injury, thus suggesting that OM improves renal function and minimizes renal pathological deterioration in db/db mice.
Conclusion
Our study reveals a beneficial role of OM in ameliorating DN in db/db mice, which is associated with its renoprotective function.
Keywords: olmesartan medoxomil, angiotensin II, renal injury, diabetic nephropathy, db/db mice
Introduction
Diabetes mellitus (DM) is a major metabolic disorder that endangers the health of over 350 million people in the world currently, with its prevalence rapidly increasing in both developed and developing countries.1 Besides, the chronic structural and functional impairment of vascular systems resulting from DM leads to a number of complications, including diabetic nephropathy (DN), the most common cause of end-stage renal disease (ESRD) that is a globally public health problem with poor clinical prognosis,2,3 for which novel therapeutic modalities are urgently needed.4
It has long been known that the progressive renal injury associated with the overactive renin-angiotensin-aldosterone system (RAAS) and hypertension accelerates the progression of DN and ESRD.5–8 Indeed, controlling blood pressure with inhibitors of RAAS, such as angiotensin converting enzyme inhibitors (ACEIs) and angiotensin II receptor blockers (ARBs), causes renoprotection and brings clinical benefits, and remains a focus of current therapeutic approaches for DN.6,9–13
Olmesartan medoxomil (OM) is an orally administered ARB that is rapidly converted to active metabolite olmesartan in vivo.14 Recent studies have shown that OM exerts renoprotective effects and ameliorates the progression of streptozotocin (STZ)-induced DN in rats,15 and also that OM has beneficial effects on STZ-induced DN in mice through the modulation of AT-1R/MAPK pathway,16 in both of which the rodent models of type 1 diabetes were investigated.17 In two early clinical studies, two ARBs, including irbesartan13 and losartan,18 were reported to preserve renal function and reduce proteinuria and ESRD incidence in patients with type 2 diabetes. In addition, a clinical trial suggested that OM delays the onset of microalbuminuria in patients with type 2 diabetes.19 However, as far as we know, whether OM elicits renoprotective effects and ameliorates DN progression in db/db mice, a type 2 diabetic murine model,20 have not yet been investigated. In this study, we explored the effects of an oral administration of OM on DN progression as well as renal function and injury in db/db mice.
Materials And Methods
Animals
The male wild-type littermates and db/db mice (BKS.Cg-Dock7m+/+Leprdb/JNju) were obtained from the Model Animal Research Center of Nanjing University. Mice were maintained in a humidity-controlled environment with constant room temperature (22°C) and 12-h light/12-h dark cycle and were allowed free access to water and a standard chow diet. All animal studies were conducted in accordance with the guidelines and approved by the ethics committee of The Fifth Affiliated Hospital of Sun Yat-sen University.
Experimental Design
Following acclimatization, 8-week-old male db/db mice were randomly divided into the following three groups (n= 15 mice per group): db/db mice received olmesartan medoxomil (OM, Shanghai Sankyo Pharmaceutical Co., Ltd) at a dose of 10 mg/kg/day via oral gavage; db/db mice received OM at a dose of 20 mg/kg/day via oral gavage; db/db mice received equal volume of saline. The treatment lasted for 8 weeks. The wild-type littermates (n= 15 mice per group) receiving equal volume of saline were used as controls for db/db mice.
Physiological And Biochemical Parameters
The blood glucose was measured every week using a blood glucose meter (Roche) to confirm diabetes. Following the last day of treatment with OM or equal volume of vehicle saline, mice were fed a known volume of water and amount of food for 24 h, and the remaining volume of liquid and amount of chow, feces in the cages, and body weight were measured. The 12-h urine samples were collected using metabolic cages (Tecniplast) and its volume was measured. Mice were euthanized and sacrificed, and blood samples were collected. The urinary biochemical parameters including albumin and creatinine, and blood biochemical parameters including glucose, creatinine, cholesterol, triglycerides, urea and uric acid were detected using an ultraviolet-visible spectrophotometer with commercial reagents. HbA1C was measured using an Ultra2 HbA1C Analyzer. The creatinine clearance was calculated using urinary creatinine × urine volume × 1000/serum creatinine/1440 and expressed as microliters per minute.
Tissue Preparation And Histological Evaluation
After renal perfusion with ice-cold phosphate-buffered saline (PBS) through renal artery, mouse kidneys were removed, weighed, and rinsed in PBS, and were then fixed in 4% formalin and embedded in paraffin. Kidney slices with 4-µM-thickness were sectioned and stained with periodic acid-Schiff (PAS) for light microscopic examination. The glomerular and tubular injury was evaluated by an investigator in a blinded manner. In detail, the mean area was obtained by calculating the mean value of 35 random fields of glomerular tuft area, 30 random fields of glomerular mesangial matrix area, 40 random fields of renal tubular area and tubular lumen area using Image J software. The glomerular tuft volume was calculated by (β/k) (GA) 3/2 as previously described,21 in which A represents the mean value of glomerular random cross-sectional area, β represents 1.38 pertains to spheres, and K represents a distribution coefficient set at 1.10. The tubular wall area was calculated by renal tubular area-tubular lumen area.
Western Blot Analysis
The renal cortex samples from removed kidneys were homogenized in RIPA lysis buffer (150 mM NaCl, 50 mM Tris, 1 mM EDTA, 1 mM Na3VO4, 0.5% NP-40, and 0.25% sodium-deoxycholate, pH7.4) supplemented with the protease inhibitor cocktail (Roche) on ice for 20 min to extract total proteins. Equal amount of proteins was loaded onto 8% SDS-PAGE gel and separated by electrophoresis, and then transferred to PVDF membranes (Millipore). Membranes were blocked with 5% non-fat milk in Tris-buffered saline supplemented with 0.1% Tween-20 (TBST) at room temperature (RT) for 1 h, and then incubated overnight at 4°C with primary antibodies against fibronectin (1:1000, Proteintech, 66042-1-Ig). β-actin (1:5000, Proteintech, 60008-1-Ig) was probed and used as a loading control for each sample. After washing with TBST for 30 min, membranes were incubated with horseradish peroxidase-conjugated secondary antibodies for 1 h at RT. After similar washing step, protein bands were developed by enhanced chemiluminescence (ECL) reagent (Amersham Biosciences). The density of protein bands was analyzed by Image J software. Results of fibronectin expression were normalized to β-actin.
Statistical Analysis
Data are shown as means ± standard deviation (SD). The two-tailed unpaired Student’s test was applied for comparisons using SPSS statistical software 16.0 (SPSS Inc., Chicago, IL, USA). Differences with P < 0.05 were considered to be statistically significant.
Results
OM Improves Diabetic Physiological Characteristics In db/db Mice
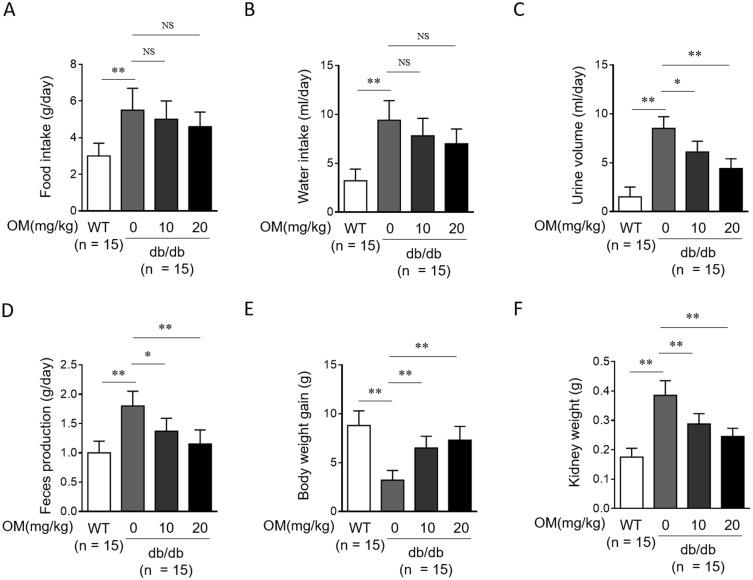
The db/db mouse is a genetic murine obese hyperinsulinaemic model of diabetes which develops nephropathy paralleling that in type 2 diabetic patients.20 Besides, olmesartan medoxomil (OM) is an orally active angiotensin II type-1 receptor blocker (ARB), possessing good antihypertensive activity and little adverse effects, with a recommended dose of 20 mg daily.14 In order to examine whether OM affects disease progression in type 2 diabetes in an in vivo setting, we first monitored the diabetic physiological parameters in db/db mice administered with or without 10 mg/kg or 20 mg/kg OM via oral gavage for 8 weeks. As shown in statistical graphs of the general physiological indices, compared with wild-type littermates (WT), db/db mice indeed manifested obvious diabetic characteristics, such as significantly increased amount of food intake (Figure 1A), water intake (Figure 1B), urine volume (Figure 1C), feces production (Figure 1D), as well as decreased gain of body weight (Figure 1E) and elevated kidney weight (Figure 1F). Following OM treatment, although the amount of food intake (Figure 1A) and water intake (Figure 1B) were decreased to some extent, no statistical significance was obtained. In addition, in contrast to vehicle saline treatment, db/db mice with OM treatment displayed significantly decreased levels of urine volume (Figure 1C), feces production (Figure 1D), and kidney weight (Figure 1F), and meanwhile, the gain of body weight was significantly increased in OM-treated db/db mice (Figure 1E) at the end of the treatment. This shows that OM moderately improves hyperphagia and polydipsia, and remarkably attenuates polyuria, body weight loss, and kidney hyperplasia in db/db mice, together suggesting that OM could alleviate the typical symptoms of type 2 diabetes.
Figure 1.
OM ameliorates diabetic physiological parameters in db/db mice. (A–F) Eight-week-old male db/db mice were intragastrically administered with 10 mg/kg OM, 20 mg/kg OM or equal volume of vehicle saline (0 mg/kg OM) per day for 8 weeks (n = 15). The wild-type littermates (WT, n = 15) were administered with equal volume of saline and used as controls for db/db mice. The physiological parameters including food intake (A), water intake (B), urine volume (C), feces production (D), gain of body weight (E), and kidney weight (F) were recorded at the last day of experiment. Data are mean ± SD. *P < 0.05; **P < 0.01.
Abbreviation: NS, not significant.
OM Ameliorates Diabetic Biochemical Parameters In db/db Mice
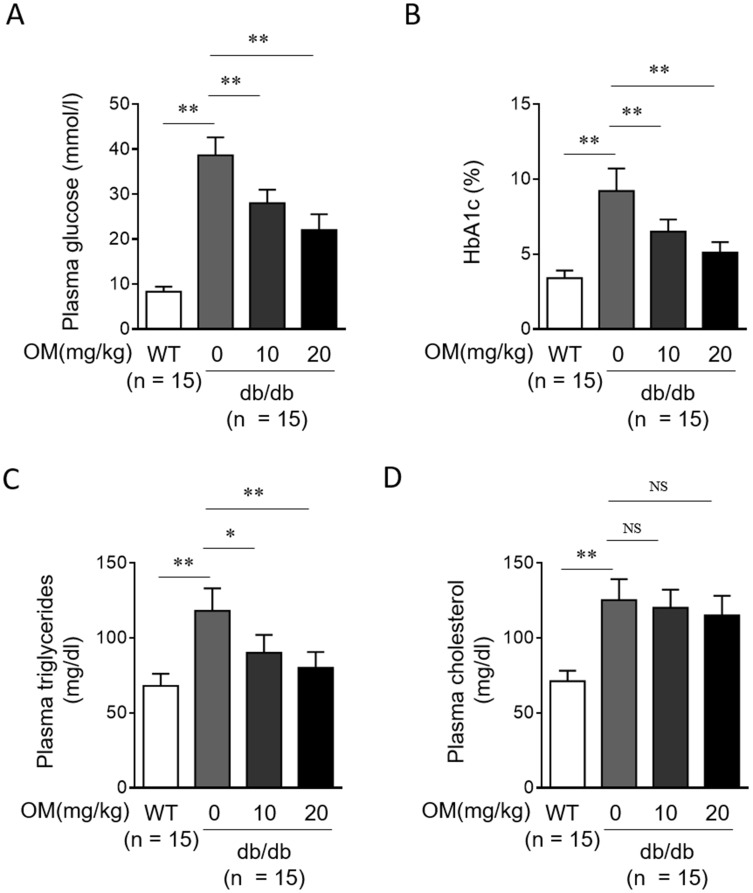
Further, we checked whether OM ameliorates diabetic biochemical parameters by analyzing plasma samples. Expectedly, db/db mice showed a prominent increase in levels of blood glucose (Figure 2A), HbA1c (Figure 2B), triglycerides (Figure 2C), and cholesterol (Figure 2D) compared with WT mice. Whereas, in comparison with db/db mice without OM treatment, the levels of blood glucose (Figure 2A) and HbA1c (Figure 2B) were significantly lower in OM-treated db/db mice. Moreover, a remarkable reduction in the level of blood triglycerides was also observed in db/db mice treated with OM (Figure 2C). Whereas, OM treatment failed to reduce the level of blood cholesterol in db/db mice (Figure 2D). These results of blood indices indicate that OM could also improve symptoms of hyperglycemia and hypertriglyceridemia, two abnormalities of glucose and lipid metabolism in type 2 diabetic mice.22
Figure 2.
OM ameliorates diabetic metabolic parameters in db/db mice. (A–D) Eight-week-old male db/db mice were intragastrically administered with 10 mg/kg OM, 20 mg/kg OM or equal volume of vehicle saline (0 mg/kg OM) per day for 8 weeks (n = 15). The wild-type littermates (WT, n = 15) were administered with equal volume of saline and used as controls for db/db mice. The metabolic parameters including plasma glucose (A), HbA1c (B), plasma triglycerides (C), and plasma total cholesterol (D) were measured at the last day of experiment. Data are mean ± SD. *P < 0.05; **P < 0.01.
Abbreviation: NS, not significant.
OM Preserves Renal Function In db/db Mice
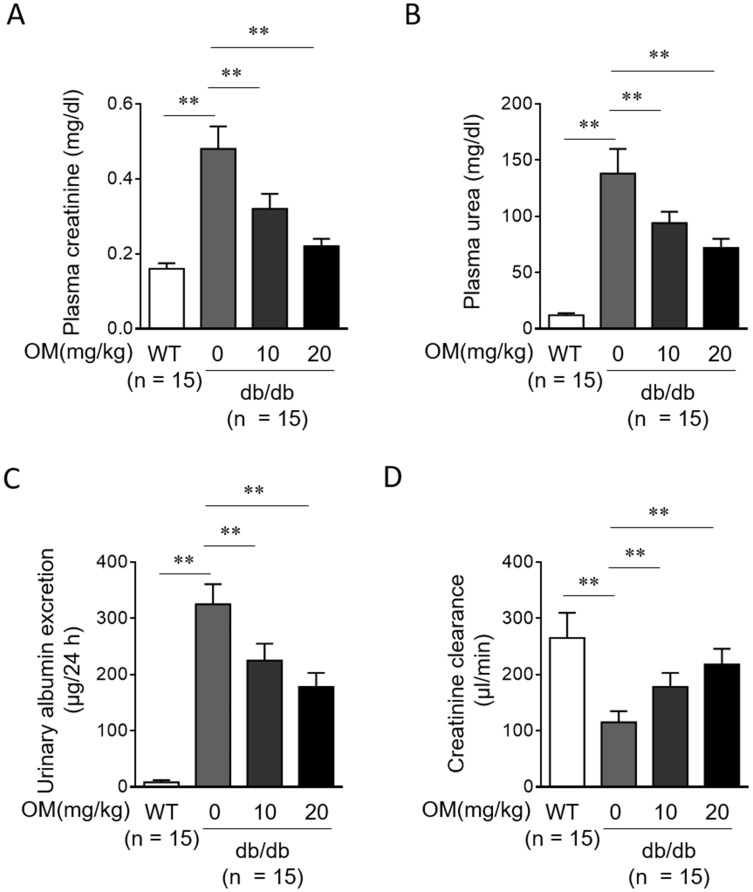
Although OM protects renal function in STZ-induced rat DN,15 whether this is reproducible in db/db mice is uncertain. To address this issue, we evaluated parameters of renal function using plasma and urine samples. We found that compared with WT mice, the concentrations of plasma creatinine (Figure 3A) and plasma urea (Figure 3B), and urinary albumin excretion (Figure 3C) were all markedly increased in db/db mice, while the clearance of creatinine (Figure 3D) was reduced accordingly, hence indicating an impaired renal function when DN was developed in db/db mice. Moreover, following OM treatment, the concentrations of plasma creatinine (Figure 3A) and plasma urea (Figure 3B), and urinary albumin excretion (Figure 3C) were decreased, and the clearance of creatinine (Figure 3D) was markedly recovered in db/db mice. These findings illustrate that OM preserves renal function and ameliorates albuminuria in type 2 diabetic mice. Combined with the improved physiological (Figure 1) and biochemical parameters (Figure 2), we conclude that the oral administration of OM ameliorates DN progression in db/db mice.
Figure 3.
OM preserves renal function in db/db mice. (A-D) Eight-week-old male db/db mice were intragastrically administered with 10 mg/kg OM, 20 mg/kg OM or equal volume of vehicle saline (0 mg/kg OM) per day for 8 weeks (n = 15). The wild-type littermates (WT, n = 15) were administered with equal volume of saline and used as controls for db/db mice. The parameters which reflect renal function, including plasma creatinine (A), plasma urea nitrogen (B), urinary albumin excretion (C), and creatinine clearance (D) were evaluated at the last day of experiment. Data are mean ± SD. **P < 0.01.
OM Reduces Glomerular Injury In db/db Mice
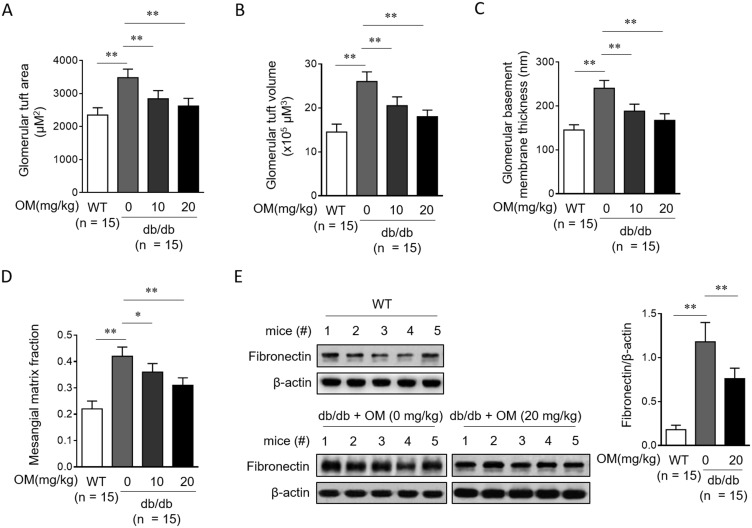
Previous histological evidence has reported that OM minimizes renal lesions and decreases glomerular hypertrophy in STZ-induced rat DN.15 Therefore, to learn more about the renoprotection of OM in type 2 diabetic db/db mice, we next investigated whether OM prevents renal injury. The periodic acid-Schiff (PAS) staining assay on renal biopsies revealed that compared with vehicle control, OM treatment significantly reversed the increase in the glomerular tuft area (Figure 4A), glomerular tuft volume (Figure 4B), glomerular basement membrane thickness (Figure 4C) as well as mesangial matrix fraction (Figure 4D) in db/db mice. Furthermore, Western blotting analysis showed that the fibronectin level in renal cortex was also decreased in db/db mice following OM treatment (Figure 4E). Thus, these data suggest that OM could reduce glomerular hypertrophy and injury in db/db mice.
Figure 4.
OM reduces glomerular hypertrophy and injury in db/db mice. (A–D) Eight-week-old male db/db mice were intragastrically administered with 10 mg/kg OM, 20 mg/kg OM or equal volume of vehicle saline (0 mg/kg OM) per day for 8 weeks (n = 15). The wild-type littermates (WT, n = 15) were administered with equal volume of saline and used as controls for db/db mice. The glomerular tuft area (A), glomerular tuft volume (B), glomerular basement membrane thickness (C), and mesangial matrix fraction (D) were analyzed by the periodic acid-Schiff (PAS) staining on kidney sections. The fibronectin expression (E) in the renal cortex from 5 representative mice in each group was analyzed by Western blotting assay. β-Actin was used as a loading control. The representative images (left) and analysis of fibronectin expression (right) are shown. Data are mean ± SD. *P < 0.05; **P < 0.01.
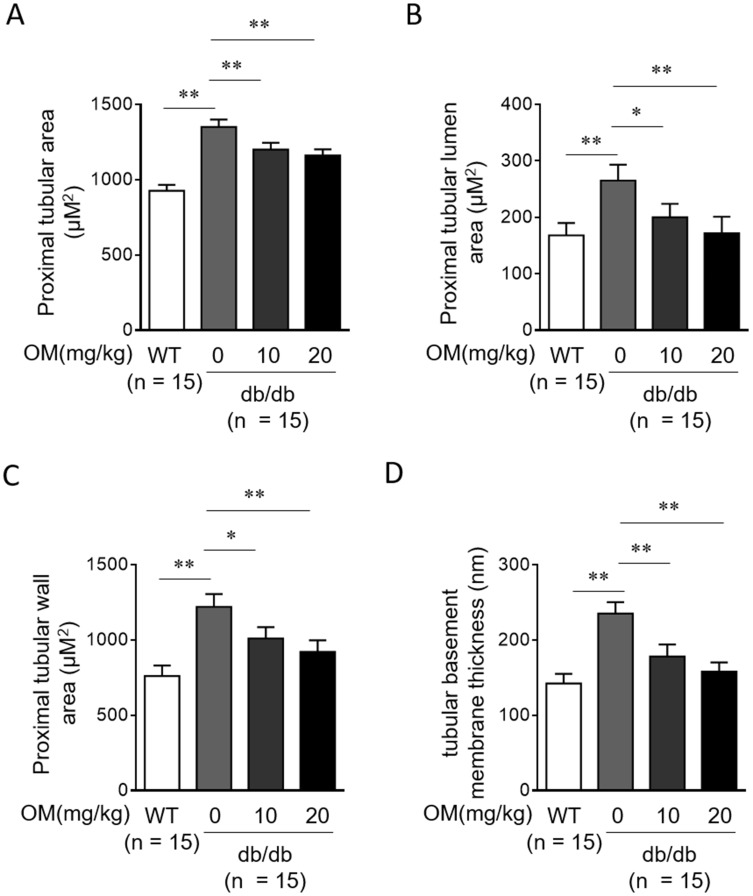
OM Reduces Tubular Injury In db/db Mice
In addition to glomerular injury, the injury of renal tubules also contributes greatly to the pathogenesis of DN.23 We found that in db/db mice, the proximal tubular area (Figure 5A), proximal tubular lumen area (Figure 5B), proximal tubular wall area (Figure 5C), and tubular basement membrane thickness (Figure 5D) were all significantly reduced by OM treatment compared with vehicle control treatment, suggesting that OM not only reduces glomerular injury but also prevents tubular injury which would otherwise deteriorate in type 2 diabetic db/db mice. Taken as a whole, these lines of evidence obtained from histologic examinations combined with the preserved renal function in db/db mice treated with OM, may provide these renoprotective roles as reasonable explanations, at least in part, for the aforementioned alleviating effects of OM treatment on DN progression in db/db mice (Figures 1 and 2).
Figure 5.
OM reduces tubular injury in db/db mice. (A-D) Eight-week-old male db/db mice were intragastrically administered with 10 mg/kg OM, 20 mg/kg OM or equal volume of vehicle saline (0 mg/kg OM) per day for 8 weeks (n = 15). The wild-type littermates (WT, n = 15) were administered with equal volume of saline and used as controls for db/db mice. The proximal tubular area (A), proximal tubular lumen area (B), proximal tubular wall area (C), and tubular basement membrane thickness (D) were analyzed by periodic acid-Schiff (PAS) staining on kidney sections. Data are mean ± SD. *P < 0.05; **P < 0.01.
Discussion
DN occurs as a common progressive renal microangiopathy in DM patients due to chronic hyperglycemia, which is mainly characterized by mesangial expansion and nodular sclerosis, along with clinical symptoms including proteinuria, hypertension and edema, and ultimately, renal failure.24,25 To prevent and interfere with the progression of DN, the management of hyperglycaemia, hypertension, and proteinuria in patients is of vital importance.26,27 Some large-scale clinical trials using antihypertensive strategy via blockade of the RAAS have proven that ARBs, such as OM, are clearly effective in delaying DN progression, as shown by the prevented microalbuminuria, delayed progression from microalbuminuria to overt proteinuria, and reduced incidence of ESRD in type 2 diabetic patients.19,28,29 Whereas, it has also been reported that the protection of ARBs against DN progression is independent of their blood pressure-lowering effect, and instead, their renoprotective role should be considered as an important contributor.13,30 More supporting evidence was obtained from preclinical studies using different animal models, such as in rats injected with the advanced glycation end products (AGEs),31 Zucker diabetic fatty (ZDF) rats,32 and SHR rats,32 where OM was found to reduce renal damage independently from a reduction in blood pressure. Therefore, this collection of evidence may highlight the importance of renoprotective effects other than antihypertensive activity of OM in modifying the disease progression of DN.
On the other hand, however, to better understand the mechanisms of action and exploit OM for DN treatment, its therapeutic function in DN and the underlying renoprotective effects need to be investigated and validated in other diabetic animal models. In the current study, we investigated the roles and mechanisms of OM in affecting DN progression through taking advantage of type 2 diabetic db/db mice, which has always been a knowledge gap in this research area. We found that in these mice, several physiological and biochemical diabetic parameters, such as body weight, plasma glucose, HbA1c and plasma triglycerides, were improved following OM administration, suggesting that OM ameliorates the major characteristic symptoms of DM. Besides, as the reflection of renal function, the indices of plasma creatinine, plasma urea, urinary albumin excretion, and the clearance of creatinine were all improved by OM as well. These support a protective role of OM against renal dysfunction and proteinuria development in type 2 diabetic mice. Subsequent histological evidence demonstrated that OM attenuated glomerular injury and tubular injury in the kidneys of db/db mice. Based on these observations from studying OM treatment in type 2 diabetic db/db mice, we reached a conclusion that the alleviating effects of OM on DN progression rely on, at least in part, its renoprotective activities, through which OM reduces renal injury and preserves renal function. Thus, our study may provide another layer of theoretical foundation for exploiting OM as a confirmative renoprotective agent in DN therapy.
The beneficial effects of OM treatment on DN progression described in our study are consistent with previous findings obtained in STZ-induced type 1 diabetic rats,15 therefore together suggesting that these effects of OM are very likely reproducible in a broad range of experimental models with diabetes. In db/db mice, DN with obvious histological changes will be developed at 16-weeks after birth due to sustained hyperglycemia, and these changes include multiple forms of renal lesions combined with striking mesangial extracellular matrix accumulation and increased thickness of glomerular and tubular basement membranes.33,34 We designed our animal experiments based on this discipline, in which we continuously treated 8-week-old db/db mice with OM for 8 weeks, and at the end of treatment, DN and histological changes were evaluated. In line with these reports, OM exerted effectiveness to improve these DN parameters in 16-week-old db/db mice, such as proteinuria accompanied by glomerular injury and tubular injury, including: 1) enlarged glomerular tuft area and glomerular tuft volume; 2) increased glomerular basement membrane thickness, 3) mesangial matrix fraction; 4) accumulation of fibronectin; 5) expanded proximal tubular area, proximal tubular lumen area, proximal tubular wall area; 6) tubular basement membrane thickness.
Hyperglycemia is a driving force for DN development.35 We noticed that OM reduced plasma glucose in db/db mice. We speculate that the alleviated DN syndromes by OM should be, at least partly, attributed to the decreased level of hyperglycemia. Notably, proteinuria serves as an important indicator for early-stage DN development, and is also able to accelerate the incidence of renal damage.36,37 We suppose that the attenuated proteinuria contributed to OM-improved glomerular injury and tubular injury in the kidneys of db/db mice. As we have mentioned previously, some studies have shown that the protective effect of OM against DN is irrespective of its function in decreasing blood pressure. Nevertheless, the renoprotective effects of ACEIs and ARBs are associated with the blockade of RAAS.38 Therefore, due to its potent inhibitory activity for RAAS,39 the blocked RAAS is possibly one of the molecular mechanisms by which OM reduces renal injury and preserves renal function in db/db mice. Moreover, in recent studies, OM has been found to possess anti-inflammatory and anti-oxidant activities.40,41 It is known that oxidative stress and inflammatory cytokines contribute to the development and progression of DN.42 Hence, it is possible that the inhibition of oxidative stress and inflammation may also participate in the protection of OM against DN. In conclusion, we believe that OM ameliorates DN progression and renal injury in db/db mice through distinct mechanisms. More in-depth studies are required in the future to fully elucidate how OM fulfils the diverse renoprotective effects.
Funding
This study was supported in part by grants of the National Natural Science Foundation of China (NSFC No.81600529), the Fundamental Research Funds for the Central Universities (17ykpy64), and Science and Technology Program of Zhuhai, China (20181117E030069).
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Lotfy M, Adeghate J, Kalasz H, Singh J, Adeghate E. Chronic complications of diabetes mellitus: a mini review. Curr Diabetes Rev. 2017;13(1):3–10. doi: 10.2174/1573399812666151016101622 [DOI] [PubMed] [Google Scholar]
- 2.Ghaderian SB, Hayati F, Shayanpour S, Beladi Mousavi SS. Diabetes and end-stage renal disease; a review article on new concepts. J Renal Inj Prev. 2015;4(2):28–33. doi: 10.12861/jrip.2015.07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haneda M, Utsunomiya K, Koya D, et al. A new classification of diabetic nephropathy 2014: a report from joint committee on diabetic nephropathy. J Diabetes Investig. 2015;6(2):242–246. doi: 10.1111/jdi.12319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gnudi L, Coward RJM, Long DA. Diabetic nephropathy: perspective on novel molecular mechanisms. Trends Endocrinol Metab. 2016;27(11):820–830. doi: 10.1016/j.tem.2016.07.002 [DOI] [PubMed] [Google Scholar]
- 5.Pugsley MK. The angiotensin-II (AT-II) receptor blocker olmesartan reduces renal damage in animal models of hypertension and diabetes. Proc West Pharmacol Soc. 2005;48:35–38. [PubMed] [Google Scholar]
- 6.Lozano-Maneiro L, Puente-Garcia A. Renin-angiotensin-aldosterone system blockade in diabetic nephropathy. Present evidences. J Clin Med. 2015;4(11):1908–1937. doi: 10.3390/jcm4111908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lewis EJ, Hunsicker LG, Bain RP, Rohde RD. The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. The Collaborative Study Group. N Engl J Med. 1993;329(20):1456–1462. doi: 10.1056/NEJM199311113292004 [DOI] [PubMed] [Google Scholar]
- 8.Klag MJ, Whelton PK, Randall BL, et al. Blood pressure and end-stage renal disease in men. N Engl J Med. 1996;334(1):13–18. doi: 10.1056/NEJM199601043340103 [DOI] [PubMed] [Google Scholar]
- 9.Bangalore S, Fakheri R, Toklu B, Messerli FH. Diabetes mellitus as a compelling indication for use of renin angiotensin system blockers: systematic review and meta-analysis of randomized trials. BMJ. 2016;352:i438. doi: 10.1136/bmj.i1717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fernandez-Fernandez B, Ortiz A, Gomez-Guerrero C, Egido J. Therapeutic approaches to diabetic nephropathy–beyond the RAS. Nat Rev Nephrol. 2014;10(6):325–346. doi: 10.1038/nrneph.2014.74 [DOI] [PubMed] [Google Scholar]
- 11.Schievink B, Kropelin T, Mulder S, et al. Early renin-angiotensin system intervention is more beneficial than late intervention in delaying end-stage renal disease in patients with type 2 diabetes. Diabetes Obes Metab. 2016;18(1):64–71. doi: 10.1111/dom.12583 [DOI] [PubMed] [Google Scholar]
- 12.Roscioni SS, Heerspink HJ, de Zeeuw D. The effect of RAAS blockade on the progression of diabetic nephropathy. Nat Rev Nephrol. 2014;10(2):77–87. doi: 10.1038/nrneph.2013.251 [DOI] [PubMed] [Google Scholar]
- 13.Lewis EJ, Hunsicker LG, Clarke WR, et al. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med. 2001;345(12):851–860. doi: 10.1056/NEJMoa011303 [DOI] [PubMed] [Google Scholar]
- 14.Brunner HR. The new oral angiotensin II antagonist olmesartan medoxomil: a concise overview. J Hum Hypertens. 2002;16 Suppl 2:S13–S16. doi: 10.1038/sj.jhh.1001391 [DOI] [PubMed] [Google Scholar]
- 15.Si X, Li P, Zhang Y, Zhang Y, Lv W, Qi D. Renoprotective effects of olmesartan medoxomil on diabetic nephropathy in streptozotocin-induced diabetes in rats. Biomed Rep. 2014;2(1):24–28. doi: 10.3892/br.2013.183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lakshmanan AP, Thandavarayan RA, Watanabe K, et al. Modulation of AT-1R/MAPK cascade by an olmesartan treatment attenuates diabetic nephropathy in streptozotocin-induced diabetic mice. Mol Cell Endocrinol. 2012;348(1):104–111. doi: 10.1016/j.mce.2011.07.041 [DOI] [PubMed] [Google Scholar]
- 17.Tesch GH, Allen TJ. Rodent models of streptozotocin-induced diabetic nephropathy. Nephrology. 2007;12(3):261–266. doi: 10.1111/j.1440-1797.2007.00796.x [DOI] [PubMed] [Google Scholar]
- 18.Brenner BM, Cooper ME, de Zeeuw D, et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345(12):861–869. doi: 10.1056/NEJMoa011161 [DOI] [PubMed] [Google Scholar]
- 19.Haller H, Ito S, Izzo JL Jr., et al. Olmesartan for the delay or prevention of microalbuminuria in type 2 diabetes. N Engl J Med. 2011;364(10):907–917. doi: 10.1056/NEJMoa1007994 [DOI] [PubMed] [Google Scholar]
- 20.Sharma K, McCue P, Dunn SR. Diabetic kidney disease in the db/db mouse. Am J Physiol Renal Physiol. 2003;284(6):F1138–F1144. doi: 10.1152/ajprenal.00315.2002 [DOI] [PubMed] [Google Scholar]
- 21.Advani A, Huang Q, Thai K, et al. Long-term administration of the histone deacetylase inhibitor vorinostat attenuates renal injury in experimental diabetes through an endothelial nitric oxide synthase-dependent mechanism. Am J Pathol. 2011;178(5):2205–2214. doi: 10.1016/j.ajpath.2011.01.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jeong SM, Kang MJ, Choi HN, Kim JH, Kim JI. Quercetin ameliorates hyperglycemia and dyslipidemia and improves antioxidant status in type 2 diabetic db/db mice. Nutr Res Pract. 2012;6(3):201–207. doi: 10.4162/nrp.2012.6.3.201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fiseha T, Tamir Z. Urinary markers of tubular injury in early diabetic nephropathy. Int J Nephrol. 2016;2016:4647685. doi: 10.1155/2016/4647685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schena FP, Gesualdo L. Pathogenetic mechanisms of diabetic nephropathy. J Am Soc Nephrol. 2005;16 Suppl 1:S30–S33. doi: 10.1681/asn.2004110970 [DOI] [PubMed] [Google Scholar]
- 25.Dronavalli S, Duka I, Bakris GL. The pathogenesis of diabetic nephropathy. Nat Clin Pract Endocrinol Metab. 2008;4(8):444–452. doi: 10.1038/ncpendmet0894 [DOI] [PubMed] [Google Scholar]
- 26.Ahmad J. Management of diabetic nephropathy: recent progress and future perspective. Diabetes Metab Syndr. 2015;9(4):343–358. doi: 10.1016/j.dsx.2015.02.008 [DOI] [PubMed] [Google Scholar]
- 27.Wolf G, Ritz E. Diabetic nephropathy in type 2 diabetes prevention and patient management. J Am Soc Nephrol. 2003;14(5):1396–1405. doi: 10.1097/01.asn.0000065639.19190.cf [DOI] [PubMed] [Google Scholar]
- 28.Haller H, Viberti GC, Mimran A, et al. Preventing microalbuminuria in patients with diabetes: rationale and design of the Randomised Olmesartan and Diabetes Microalbuminuria Prevention (ROADMAP) study. J Hypertens. 2006;24(2):403–408. doi: 10.1097/01.hjh.0000202820.56201.e6 [DOI] [PubMed] [Google Scholar]
- 29.Imai E, Ito S, Haneda M, Chan JC, Makino H, Investigators O. Olmesartan reducing incidence of endstage renal disease in diabetic nephropathy trial (ORIENT): rationale and study design. Hypertens Res. 2006;29(9):703–709. doi: 10.1291/hypres.29.703 [DOI] [PubMed] [Google Scholar]
- 30.Lewis EJ. The role of angiotensin II receptor blockers in preventing the progression of renal disease in patients with type 2 diabetes. Am J Hypertens. 2002;15(10 Pt 2):123S–128S. doi: 10.1016/s0895-7061(02)03007-8 [DOI] [PubMed] [Google Scholar]
- 31.Yamagishi S, Takeuchi M, Inoue H. Olmesartan medoxomil, a newly developed angiotensin II type 1 receptor antagonist, protects against renal damage in advanced glycation end product (age)-injected rats. Drugs Exp Clin Res. 2005;31(2):45–51. [PubMed] [Google Scholar]
- 32.Mizuno M, Sada T, Kato M, Koike H. Renoprotective effects of blockade of angiotensin II AT1 receptors in an animal model of type 2 diabetes. Hypertens Res. 2002;25(2):271–278. [DOI] [PubMed] [Google Scholar]
- 33.Cohen MP, Clements RS, Hud E, Cohen JA, Ziyadeh FN. Evolution of renal function abnormalities in the db/db mouse that parallels the development of human diabetic nephropathy. Exp Nephrol. 1996;4(3):166–171. [PubMed] [Google Scholar]
- 34.Mori H, Inoki K, Masutani K, et al. The mTOR pathway is highly activated in diabetic nephropathy and rapamycin has a strong therapeutic potential. Biochem Biophys Res Commun. 2009;384(4):471–475. doi: 10.1016/j.bbrc.2009.04.136 [DOI] [PubMed] [Google Scholar]
- 35.Sun YM, Su Y, Li J, Wang LF. Recent advances in understanding the biochemical and molecular mechanism of diabetic nephropathy. Biochem Biophys Res Commun. 2013;433(4):359–361. doi: 10.1016/j.bbrc.2013.02.120 [DOI] [PubMed] [Google Scholar]
- 36.Oktem F, Ozguner F, Yilmaz HR, Uz E, Dundar B. Melatonin reduces urinary excretion of N-acetyl-beta-D-glucosaminidase, albumin and renal oxidative markers in diabetic rats. Clin Exp Pharmacol Physiol. 2006;33(1–2):95–101. doi: 10.1111/cep.2006.33.issue-1-2 [DOI] [PubMed] [Google Scholar]
- 37.Abbate M, Zoja C, Remuzzi G. How does proteinuria cause progressive renal damage? J Am Soc Nephrol. 2006;17(11):2974–2984. doi: 10.1681/ASN.2006040377 [DOI] [PubMed] [Google Scholar]
- 38.Zhou A, Yu L, Li J, Zhang J, Wang H. Renal protective effects of blocking the intrarenal renin-angiotensin system: angiotensin II type I receptor antagonist compared with angiotensin-converting enzyme inhibitor. Hypertens Res. 2000;23(4):391–397. [DOI] [PubMed] [Google Scholar]
- 39.Scott LJ, McCormack PL. Olmesartan medoxomil: a review of its use in the management of hypertension. Drugs. 2008;68(9):1239–1272. [DOI] [PubMed] [Google Scholar]
- 40.Gounder VK, Arumugam S, Arozal W, et al. Olmesartan protects against oxidative stress possibly through the Nrf2 signaling pathway and inhibits inflammation in daunorubicin-induced nephrotoxicity in rats. Int Immunopharmacol. 2014;18(2):282–289. doi: 10.1016/j.intimp.2013.11.018 [DOI] [PubMed] [Google Scholar]
- 41.Nagib MM, Tadros MG, ElSayed MI, Khalifa AE. Anti-inflammatory and anti-oxidant activities of olmesartan medoxomil ameliorate experimental colitis in rats. Toxicol Appl Pharmacol. 2013;271(1):106–113. doi: 10.1016/j.taap.2013.04.026 [DOI] [PubMed] [Google Scholar]
- 42.Elmarakby AA, Sullivan JC. Relationship between oxidative stress and inflammatory cytokines in diabetic nephropathy. Cardiovasc Ther. 2012;30(1):49–59. doi: 10.1111/j.1755-5922.2010.00218.x [DOI] [PubMed] [Google Scholar]