Abstract
BACKGROUND
Mixed neuroendocrine non-neuroendocrine neoplasm (MiNEN) is a rare diagnosis, mainly encountered in the gastro-entero-pancreatic tract. There is limited knowledge of its epidemiology, prognosis and biology, and the best management for affected patients is still to be defined.
AIM
To investigate clinical-pathological characteristics, treatment modalities and survival outcomes of a retrospective cohort of patients with a diagnosis of MiNEN.
METHODS
Consecutive patients with a histologically proven diagnosis of MiNEN were identified at 5 European centres. Patient data were retrospectively collected from medical records. Pathological samples were reviewed to ascertain compliance with the 2017 World Health Organisation definition of MiNEN. Tumour responses to systemic treatment were assessed according to the Response Evaluation Criteria in Solid Tumours 1.1. Kaplan-Meier analysis was applied to estimate survival outcomes. Associations between clinical-pathological characteristics and survival outcomes were explored using Log-rank test for equality of survivors functions (univariate) and Cox-regression analysis (multivariable).
RESULTS
Sixty-nine consecutive patients identified; Median age at diagnosis: 64 years. Males: 63.8%. Localised disease (curable): 53.6%. Commonest sites of origin: colon-rectum (43.5%) and oesophagus/oesophagogastric junction (15.9%). The neuroendocrine component was; predominant in 58.6%, poorly differentiated in 86.3%, and large cell in 81.25%, of cases analysed. Most distant metastases analysed (73.4%) were occupied only by a poorly differentiated neuroendocrine component. Ninety-four percent of patients with localised disease underwent curative surgery; 53% also received perioperative treatment, most often in line with protocols for adenocarcinomas from the same sites of origin. Chemotherapy was offered to most patients (68.1%) with advanced disease, and followed protocols for pure neuroendocrine carcinomas or adenocarcinomas in equal proportion. In localised cases, median recurrence free survival (RFS); 14.0 mo (95%CI: 9.2-24.4), and median overall survival (OS): 28.6 mo (95%CI: 18.3-41.1). On univariate analysis, receipt of perioperative treatment (vs surgery alone) did not improve RFS (P = 0.375), or OS (P = 0.240). In advanced cases, median progression free survival (PFS); 5.6 mo (95%CI: 4.4-7.4), and median OS; 9.0 mo (95%CI: 5.2-13.4). On univariate analysis, receipt of palliative active treatment (vs best supportive care) prolonged PFS and OS (both, P < 0.001).
CONCLUSION
MiNEN is most commonly driven by a poorly differentiated neuroendocrine component, and has poor prognosis. Advances in its biological understanding are needed to identify effective treatments and improve patient outcomes.
Keywords: Mixed neuroendocrine non-neuroendocrine neoplasm, 2017 World Health Organisation classification, Mixed adeno-neuroendocrine carcinoma, Gastro-entero-pancreatic tract, Digestive system, Neuroendocrine neoplasms, Survival outcomes
Core tip: Mixed neuroendocrine non-neuroendocrine neoplasm (MiNEN) is a rare, albeit aggressive diagnosis. Evidence from literature is limited and inconsistent. This study reports on one of the largest cohorts of patients with a diagnosis of MiNEN in the current literature, and aims to provide useful suggestions for clinical management, in the absence of data from clinical trials. Potentially curable cases are most commonly offered surgery alone or in combination with chemotherapy or chemo-radiotherapy (predominantly according to the “standard of care” for adenocarcinomas). Advanced cases are most often treated with palliative chemotherapy and protocols follow either the “standard of care” for adenocarcinomas or neuroendocrine carcinomas.
INTRODUCTION
Mixed tumours exhibiting both exocrine and neuroendocrine morphological features are frequently encountered by pathologists in routine practice, and can originate in all organs. Over the years, these tumours have been assigned variable designations, giving rise to huge inconsistency within the literature[1]. Since 2000, tumours from the gastro-entero-pancreatic (GEP) tract consisting of an exocrine and a neuroendocrine component, accounting for at least a third or 30% of the tumour mass, have been classified by the World Health Organisation (WHO) as separate entities from their pure counterparts[2-5]. In 2010, the WHO classification of tumours of the digestive system[4] named those mixed tumours mixed adeno-neuroendocrine carcinomas (MANECs).
MANEC is a rare and controversial diagnosis; data from the current literature are limited, almost exclusively derived from case reports and small retrospective series, and inconsistent, mainly due to differences across studies in patient inclusion criteria (e.g., disease stage, grade of differentiation of the two components, sites of origin), population size and interpretation of the 2010 WHO definition of MANEC. In fact, there is still large disagreement among authors on whether to include goblet cell carcinoids/carcinomas of the appendix[6] under the diagnosis of MANEC, and whether to consider mixed tumours composed by an adenoma and a well differentiated neuroendocrine component separately from MANECs with more aggressive histological features[7]. The median overall survival (OS) of affected patients varies greatly across the retrospective series, ranging between 10 to 78 months (any disease stage or disease stage not specified)[8-13].
The European Neuroendocrine Tumour Society (ENETS) clinical practice guidelines recommend that the management of MANEC should follow the standard of care for pure, grade 3, neuroendocrine carcinoma (NEC)[14], since the neuroendocrine component in MANEC is most commonly poorly differentiated and predominant, both in the primary tumours and in distant metastatic sites[15]. However, other authors suggest treating MANEC according to the clinical practice guidelines for adenocarcinomas (ADCs) from the same site of origin, when the ADC component is prevalent and/or the least differentiated[7].
In 2017, the WHO classification of tumours of endocrine organs has renamed MANECs from the pancreas “mixed neuroendocrine non-neuroendocrine neoplasms” (MiNENs)[5], in order to better convey the variety of possible combinations between neuroendocrine and non-neuroendocrine histologies, extending the spectrum of the latter to incorporate “non-gland-forming” variants (e.g., squamous cell carcinoma or sarcoma) and precancerous lesions (e.g., adenoma). The term MiNEN currently only appears in the nomenclature for endocrine neoplasms arising from the pancreas. However, pathologists commonly extend the use of the phrase and apply it to mixed tumours meeting the diagnostic criteria and originating from any organ site[16].
It is worth noting that, since the diagnosis of MiNEN is based on a quantitative threshold, tissue biopsies may not be able to accurately discriminate between MiNENs and neuroendocrine neoplasms with a minor non-neuroendocrine differentiation (< 30%), or vice versa, potentially accounting for underestimation of the frequency of this diagnosis. As a result of the limitations of diagnostic methods, as well as the paucity and inconsistency of available evidence, the actual epidemiology and prognosis of MiNEN remains unknown.
Furthermore, identifying effective therapeutic strategies for MiNEN represents a major challenge, which can be mainly explained by; (1) the lack of high-quality evidence from large prospective trials, due to the rareness and limited awareness of this diagnosis outside the community of clinicians and researchers with interest in neuroendocrine neoplasms; and (2) the different sensitivity of the two histologies to conventional systemic treatments and radiotherapy; the selective treatment of one of the two components can favour the clonal expansion of the other, leading to the rapid development of resistance.
The present study aimed to collect data from a large, retrospective, multi-centre, series of patients with a diagnosis of MiNEN, for whom there was compliance with the 2017 WHO classification[5], confirmed by pathologists with expertise in neuroendocrine neoplasms, to inform clinicians on the clinical-pathological characteristics, biological behaviour and management of this poorly understood disease.
MATERIALS AND METHODS
Patient and tumour samples
Consecutive patients with a diagnosis of MiNEN, as per the 2017 WHO classification[5], were retrospectively identified from 5 European Institutions, 4 of which are ENETS Centres of Excellence; The Christie National Health Service (NHS) Foundation Trust in Manchester (United Kingdom), The Royal Free London NHS Foundation Trust in London (United Kingdom), University Hospitals Birmingham NHS Foundation Trust (United Kingdom), Edouard Herriot Hospital (Hospices Civils de Lyon) (France), and Cardiff and Vale University Health Board (United Kingdom).
All pathological samples, obtained by either surgical resection or tissue biopsy from primary tumours or metastatic sites, were reviewed by pathologists with expertise in neuroendocrine neoplasms, and fulfilled the 2017 WHO diagnostic criteria for MiNEN (each component accounting for at least 30% of the tumour mass)[5]. Only patients with a primary tumour from the GEP tract, or of unknown origin, where other primary sites were excluded, were considered eligible for inclusion in this study. Diagnosis by cytology (e.g., brushing or fine needle aspiration) was not permitted, as deemed not informative enough to allow an accurate diagnosis of MiNEN. Other exclusion criteria included; goblet cell carcinoids Tang subtype A, ADC ex-goblet cell carcinoids Tang subtype B and C[6], and amphicrine tumours. In fact, studies reporting on goblet cell carcinoids (any Tang subtype) indicate that these tumours rarely exhibit a neuroendocrine component exceeding 30% of the tumour mass[7,17], and seem to have a more favourable prognosis than patients with MiNENs[11,12].
Demographic characteristics, treatment modalities and clinical outcomes of eligible patients, and morphological data of corresponding tumour samples were collected from local medical records (approved by local audit committees). This study was approved by the Christie NHS Foundation Trust Audit committee (16/1806).
Due to differences in staging systems among tumours from different sites of origin, the disease stage was classified as follows; localised (Loc), if the tumour was amenable to curative treatment, whether or not loco-regional nodes were affected, and in the absence of distant metastases; advanced (Adv), if the tumour was not amenable to curative treatment, either because locally infiltrative or because of the presence of distant metastases.
Chemotherapy and chemo-radiotherapy regimens, used either in the Loc or Adv setting, were defined as “ADC-like” or “NEC-like” according to whether they were in keeping with the “standard-of-care” for the treatment of ADCs from the same site of origin or NECs, respectively.
The following endpoints were used to investigate patient and treatment outcomes; recurrence-free-survival (RFS) (defined as the time from the beginning of the initial curative treatment to radiological or clinical evidence of recurrence of the tumour or tumour-related death), progression-free-survival (PFS) (defined as the time from the diagnosis of Adv disease or the beginning of active palliative treatment to radiological/clinical evidence of progression of the tumour or death from any cause), and OS (defined as the time from the initial pathological diagnosis to death from any cause). For patients with Loc MiNEN who developed recurrent Adv disease, PFS and OS were also calculated from the time of radiological diagnosis of Adv disease or the beginning of active palliative treatment to the time of radiological/clinical evidence of further progression or death from any cause; these PFS and OS data were combined with those from patients with Adv disease “ab initio”, in order to increase the sample size of the Adv subgroup.
The date of data cut-off was the 28th of February 2018. The follow-up time was estimated from the date of the first contact by the patient with the institution and the date of last follow-up visit, or contact, or death from any cause. The response to chemotherapy or chemo-radiotherapy was assessed according to the Response Evaluation Criteria In Solid Tumors (RECIST) version 1.1[18]. Frequency of imaging was performed as per institutional and ENETS guidelines[14] (every 3 mo).
Statistical analysis
Microsoft excel was used for descriptive statistics, and “R” software was used for inferential statistics. Survival outcomes (median RFS, PFS and OS) were estimated by using Kaplan-Meier analysis (patients lost to follow-up were censored at the time of last follow-up visit or contact). Associations between clinical and pathological characteristics and survival outcomes were investigated by applying Log-rank test for equality of survivors function (univariate), and Cox-regression analysis (multivariable). Probability values (P) were considered to be statistically significant at a level < 0.05.
RESULTS
Clinical characteristics of patients and pathological data of tumour samples
A total of 69 consecutive patients with a diagnosis of MiNEN (2017 WHO definition)[5] were eligible for inclusion in the study. The date of diagnosis ranged from the 1st of September 1980 to the 1st of August 2017. The median follow-up time was 11.5 months [95% confidence interval (CI); 6.5-13.5]. Baseline demographic and clinical/pathological characteristics are summarised in Table 1 and Supplementary Table 1. The median age of patients at diagnosis was 64 years (range: 34-89).
Table 1.
Demographic and clinical-pathological characteristics in patients with a diagnosis of mixed neuroendocrine non-neuroendocrine neoplasm
| Variable | Category | n (%) | Variable | Category | n (%) |
| Gender | Predominant component | ||||
| Female | 25 (36.2) | NE | 27 (39.1) | ||
| Male | 44 (63.8) | Non-NE | 12 (17.4) | ||
| Equal proportion | 7 (10.1) | ||||
| NA | 23 (33.3) | ||||
| Disease stage | Morphology of NE component | ||||
| Localised | 37 (53.6) | Large cell | 13 (18.8) | ||
| Advanced | 30 (43.5) | Small cell | 5 (7.2) | ||
| NA | 2 (2.9) | Others | 1 (1.4) | ||
| NA | 50 (72.4) | ||||
| Primary tumour site | Grading of NE component | ||||
| Colon | 22 (31.9) | Grade 1 | 3 (4.3) | ||
| Oesophagus/OGJ | 11 (15.9) | Grade 2 | 14 (20.3) | ||
| Rectum | 8 (11.6) | Grade 3 | 57 (82.6) | ||
| Small bowel/ICJ | 8 (11.6) | NA | 3 (4.3) | ||
| Pancreas | 6 (8.7) | ||||
| Stomach | 4 (5.8) | Ki-67 of NE component | |||
| Anus | 4 (5.8) | Median | 70% (55-68) | ||
| Biliary tract | 3 (4.3%) | < 55% | 19 (27.5) | ||
| Appendix | 2 (1.9) | ≥ 55% | 37 (53.6) | ||
| Unknown | 1 (1.5) | NA | 13 (18.8) | ||
| Histology of non-NE component | |||||
| Lower GI | 4 (63.8) | ADC | 51 (39.1) | ||
| Upper GI | 15 (21.7) | Adenoma | 2 (17.4) | ||
| PB | 9 (13) | Carcinoma/SCC | 1 (10.1) | ||
| Unknown | 1 (1.5) | NA | 15 (33.3) | ||
| Lower GI | 4 (63.8) | ||||
| Grading of non- NE component | |||||
| Grade 1 | 10 (14.5) | ||||
| Grade 2 | 14 (20.3) | ||||
| Grade 3 | 20 (29.0) | ||||
| Adenoma | 2 (17.4) | ||||
| NA | 23 (33.3) |
Note: the sum of the percentages might not reach 100% due to rounding. ECOG: Eastern Cooperative Oncology Group; PS: Performance status; Loc: Localised stage; Adv: Advanced stage; NE: Neuroendocrine; ADC: Adenocarcinoma; SCC: Squamous cell carcinoma; OE: Oesophageal; OGJ: Oesophago-gastric junction; ICJ: Ileo-caecal junction; Lower GI: Lower gastrointestinal tract; Upper GI: Upper gastrointestinal tract; PB: Pancreatico-biliary tract; NA: Not available.
Pathological material for diagnosis (collection procedure; surgery 58%, biopsy 36.2%, unknown but not fine needle aspiration or brushings in 5.8%) was obtained from primary tumours in 50 cases (72.5%) and metastatic sites in 5 cases (7.2%), whereas the site from where the tumour tissue was retrieved could be ascertained in 14 cases (20.3%). The neuroendocrine component was predominant and poorly differentiated, grade 3, in 58.7% and 86.4% of cases for which this information was available, respectively. The median Ki-67 index of the neuroendocrine component (recorded for 56 patients) was 70% (range: 2%-95%). The predominant histology in MiNEN, and the morphological subtype of the neuroendocrine component was not available from pathological reports in 33.3% and 72.4% of cases, respectively. Immunohistochemical data on diagnostic samples of MiNEN are presented in Table 2.
Table 2.
Immunohistochemical data on diagnostic samples of mixed neuroendocrine non-neuroendocrine neoplasm
| Variable | Category | Number | % |
| Synaptophysin | |||
| Positive | 60 | 87.0 | |
| Negative | 0 | 0 | |
| NA | 9 | 13.0 | |
| Chromogranin A | |||
| Positive | 37 | 53.6 | |
| Negative | 16 | 23.2 | |
| NA | 16 | 23.2 | |
| CD56 | |||
| Positive | 26 | 37.7 | |
| Negative | 10 | 14.5 | |
| NA | 33 | 47.8 | |
| CK-20 | |||
| Positive | 22 | 31.9 | |
| Negative | 11 | 15.9 | |
| NA | 36 | 52.2 | |
| CK-7 | |||
| Positive | 24 | 34.7 | |
| Negative | 10 | 14.5 | |
| NA | 35 | 50.8 | |
| CDX-2 | |||
| Positive | 33 | 47.8 | |
| Negative | 4 | 5.8 | |
| NA | 32 | 46.4 |
Note: The sum of the percentages may not reach 100% due to rounding. NA: Not available.
Additional pathological material from synchronous or metachronous metastatic sites was available for 15 patients and consisted of a pure NEC in 11 (73.4%) cases, a pure ADC in 1 (6.6%), and an admixture of both histologies in 3 (20%).
Management and clinical outcomes of patients
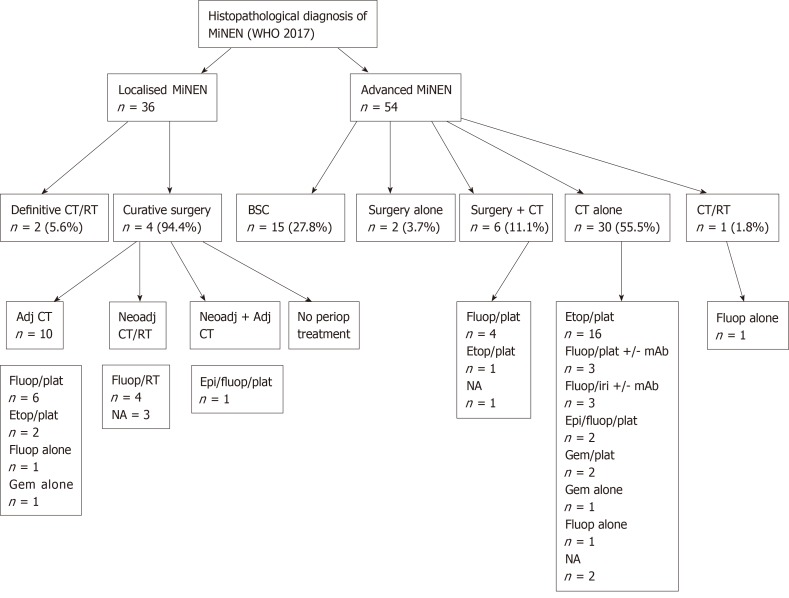
Treatment modalities of MiNEN was available for 36 patients with Loc disease and 54 patients with Adv disease, and are illustrated in Figure 1. The Adv subgroup included both patients who presented with Adv disease at diagnosis (n = 29) and patients who developed recurrent metastatic disease after initial curative treatment (n = 25).
Figure 1.
Treatment modalities of patients with a diagnosis of mixed neuroendocrine non-neuroendocrine neoplasm according to disease stage.
Chemotherapy and chemo-radiotherapy regimens administered with curative intent in the Loc setting followed ADC-like protocols in 13/18 (72.2%) cases and NEC-like protocols in 2/18 (11.1%) cases [for 3/18 (16.7%) patients, the chemotherapy regimen used was unknown]. Regimens of systemic treatment administered with palliative intent in the Adv setting were in keeping with ADC-like protocols in 17/37 (45.9%) cases and NEC-like protocols in 17/37 (45.9%) cases [for 3/37 (8.1%) patients, the chemotherapy regimen used was unknown]. Descriptive associations between the predominant and/or most aggressive component in diagnostic samples, or second biopsies obtained at the time of diagnosis of Adv disease (pre-treatment), and chemotherapy or chemo-radiotherapy regimens used were possible for 30 patients (Supplementary Table 2); the choice of regimen (either NEC-like or ADC-like) was in line with the predominant or most aggressive component in 20 (67%) cases.
In the Adv setting, the response to first-line chemotherapy or chemo-radiotherapy was assessed for 30 patients; 2 (6.7%) had complete response (CR), 4 (13.3%) had partial response (PR), 13 (43.3%) had stable disease (SD) (disease control rate; 63.3%), and 11 (36.6%) had progressive disease (PD). Correlations between treatment response and predominant/most aggressive histology or chemotherapy/chemo-radiotherapy protocols used were not interrogated, as individual subgroups were too small to allow reliable statistical analyses.
Eleven patients received second-line active treatment; 9 chemotherapy (5-fluorouracil-based in 6, gemcitabine-based in 1, cisplatin/etoposide in 1, cyclophosphamide/adriamycin/vincristine in 1), 1 chemo-radiotherapy and 1 de-bulking surgery with intraperitoneal chemotherapy.
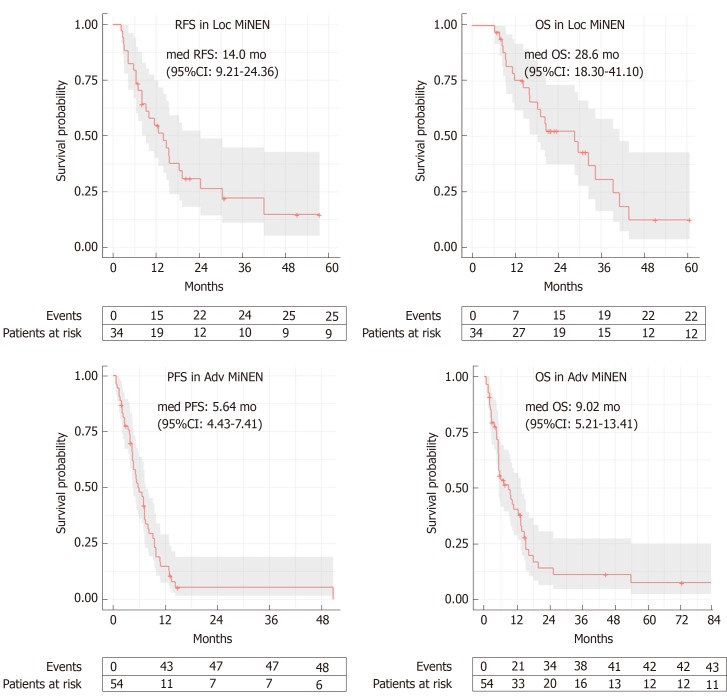
At the time of data cut-off, the median OS in the whole population was 15.9 mo (95%CI: 12.4-29.6) (whether or not death had occurred was unknown in 6 patients). In the Loc subgroup, 25 (67.6%) patients relapsed and the median RFS was 14.03 mo (95%CI: 9.21-24.36) (whether or not disease relapse occurred was unknown for 4 patients), and 22 (59.5%) died. The median OS was 28.6 mo (95%CI: 18.30-41.10) (whether or not death occurred was unknown for 3 patients) (Figure 2). Univariate analyses for RFS and OS in the Loc setting are presented in Table 3. The primary tumour site (lower gastro-intestinal, upper gastro-intestinal or pancreatico-biliary) significantly impacted on OS (P < 0.001), with MiNENs of pancreatico-biliary origin seeming to have the worst outcomes. An age at diagnosis below 70 years (vs ≥ 70) was a positive prognostic factor for both RFS (P = 0.023) and OS (P = 0.017). Female gender was prognostic for worse OS (P = 0.037) and was associated with a trend towards worse RFS, although not statistically significant (P = 0.081). The absence of local-regional lymph node metastases in post-operative specimens was prognostic for longer RFS (P = 0.015), and was associated with a trend towards improved OS, although not statistically significant (P = 0.069). Interestingly, neither the predominant component nor the receipt of perioperative treatment (vs surgery alone) impacted on PFS or OS. Multivariable analysis was considered but due to lack of complete data, the number of analysable cases for each subgroup was too small (n = 1-11) to enable reliable comparisons.
Figure 2.
Kaplan-Meier curves for recurrence-free survival and overall survival in the localised stage subgroup, and for progression free survival and overall survival in the advanced stage subgroup. The confidence interval gets wider with time mainly due to smaller sample sizes. The wider confidence intervals indicate more uncertainty. MiNEN: Mixed neuroendocrine non-neuroendocrine neoplasm; med: Median; 95%CI: 95% confidence interval.
Table 3.
Univariate analyses for recurrence-free-survival and overall survival in patients with a diagnosis of localised mixed neuroendocrine non-neuroendocrine neoplasm
|
RFS |
OS |
|||||||
| n | Median (mo) | 95%CI | P value | n | Median (mo) | 95%CI | P value | |
| Primary tumour site | ||||||||
| Lower GI | 25 | 15.70 | 10.1-NR | 0.28 | 25 | 29.6 | 19.1-NR | < 0.001 |
| PB | 4 | 8.11 | 2.9-NR | 4 | 8.6 | 7.6-NR | ||
| Upper GI | 5 | 14.89 | 8.0-NR | 5 | 20.4 | 9.4-NR | ||
| Unknown | 0 | 0 | ||||||
| Age at diagnosis | ||||||||
| < 70 yr | 22 | 15.5 | 11.5-NR | 0.023 | 22 | 32.6 | 20.7-NR | 0.017 |
| ≥ 70 yr | 12 | 9.2 | 6.5-NR | 12 | 16.1 | 8.6-NR | ||
| ECOG PS | ||||||||
| 0-1 | 27 | 12.7 | 9.2-NR | 0.29 | 27 | 29.6 | 18.3-NR | 0.34 |
| ≥ 2 | 5 | 18.5 | 5.7-NR | 5 | 28.6 | 14.3-NR | ||
| Gender | ||||||||
| Female | 14 | 7.0 | 4.2-NR | 0.081 | 14 | 11.9 | 8.6-NR | 0.037 |
| Male | 20 | 15.5 | 11.5-NR | 20 | 32.6 | 19.1-NR | ||
| Predominant component | ||||||||
| ADC | 9 | 11.5 | 6.4-NR | 0.99 | 9 | 20.7 | 15.9-NR | 0.66 |
| EQUAL | 2 | 8.1 | - | 2 | - | - | ||
| NE | 14 | 12.7 | 7.0-NR | 14 | 20.4 | 18.3-NR | ||
| Grading NE component | ||||||||
| G1 | 2 | - | - | 0.16 | 2 | - | - | 0.24 |
| G2 | 2 | 33.7 | 24.4-NR | 2 | 36.7 | 29.6-NR | ||
| G3 | 29 | 11.5 | 8.0-18.5 | 29 | 20.7 | 15.9-NR | ||
| Ki-67 of NE component | ||||||||
| < 55% | 9 | 17.1 | 2.9-NR | 0.67 | 9 | 28.6 | 8.6-NR | 0.83 |
| ≥ 55% | 17 | 9.2 | 7.0-NR | 17 | 19.1 | 14.3-NR | ||
| pN+ | ||||||||
| No | 4 | - | - | 0.015 | 4 | - | - | 0.069 |
| Yes | 16 | 10.1 | 6.5 | 16 | 28.6 | 14.3-NR | ||
| Periop-treatment | ||||||||
| No | 13 | 14.0 | 7.0-NR | 0.375 | 13 | 28.6 | 16.1-NR | 0.24 |
| Yes | 19 | 14.9 | 8.0-NR | 19 | 20.4 | 14.3-NR | ||
RFS: Recurrent free survival; OS: Overall survival; 95%CI: 95% confidence interval; NR: Not reached; Lower GI: Lower gastrointestinal tract; Upper GI: Upper gastrointestinal tract; PB: Pancreatico-biliary tract; PS: Performance status; ECOG: Eastern Cooperative Oncology Group; ADC: Adenocarcinoma; EQUAL: Equal proportions of the two histologies; NE: Neuroendocrine component; pN+: Presence of metastatic loco-regional lymph nodes on post-operative pathological examination; Periop: Perioperative.
In the Adv subgroup, 29 (53.7%) patients had progressed and 43 (78.2%) had died, and the median PFS and OS were 5.64 months (95%CI: 4.43-7.41) and 9.02 months (95%CI: 5.21-13.41), respectively (whether or not disease progression or death occurred was unknown for 1 patient) (Figure 2). Univariate analyses for PFS and OS in the Adv setting are presented in Table 4. The primary tumour site (lower gastro-intestinal, upper gastro-intestinal or pancreatico-biliary) significantly impacted on both PFS (P = 0.008) and OS (P < 0.001), with MiNENs of pancreatico-biliary origin seeming to have the worst outcomes. The receipt of first-line active treatment (vs best supportive care alone) was associated with significantly better PFS (P < 0.001) and OS (P < 0.001), whereas the predominant component did not impact on either survival outcomes. On multivariable analysis (Supplementary Table 3), the primary tumour site retained prognostic significance for OS (P = 0.016), and had “borderline” prognostic significance for PFS (P = 0.057). In contrast, the administration of first line active treatment lost significance for both PFS (P = 0.237) and OS (P = 0.523).
Table 4.
Univariate analysis of progression-free survival and overall survival in patients with advanced mixed neuroendocrine non-neuroendocrine neoplasm
|
PFS |
OS |
|||||||
| n | Median (mo) | 95%CI | P value | n | Median (mo) | 95%CI | P value | |
| Primary tumour site | ||||||||
| Lower GI | 35 | 6.6 | 4.6-7.4 | 0.008 | 37 | 9.6 | 5.21-15.2 | < 0.001 |
| PB | 7 | 4.4 | 0.8-NR | 7 | 4.4 | 2-NR | ||
| Upper GI | 11 | 9.5 | 5.1-NR | 11 | 9.5 | 5.1-NR | ||
| Unknown | 1 | 1.3 | - | 1 | 1.3 | - | ||
| Age at diagnosis | ||||||||
| < 70 yr | 32 | 5.9 | 4.3-9.5 | 0.19 | 32 | 9.5 | 5.2-16.3 | 0.23 |
| ≥ 70 yr | 22 | 5.6 | 3.8-8.0 | 22 | 9.0 | 4.6-14.7 | ||
| ECOG PS | ||||||||
| 0-1 | 36 | 6.9 | 4.6-8.2 | 0.9 | 36 | 9.6 | 5.216.3 | 0.45 |
| ≥ 2 | 13 | 5.9 | 3.4-NR | 13 | 5.9 | 4.6-NR | ||
| Gender | ||||||||
| Female | 20 | 5.2 | 2.6-9.1 | 0.5 | 20 | 7.9 | 3.7-19.8 | 0.96 |
| Male | 34 | 6.9 | 4.6-9.5 | 34 | 9.0 | 5.2-15.2 | ||
| Predominant component | ||||||||
| ADC | 11 | 4.4 | 2.6-NR | 0.08 | 11 | 4.4 | 2.6-NR | 0.14 |
| EQUAL | 6 | 9.5 | 4.4-NR | 6 | 34.2 | 12.4-NR | ||
| NE | 23 | 5.6 | 4.3-7.2 | 23 | 7.2 | 5.2-13.7 | ||
| Grading NE component | ||||||||
| G1 | 0 | 0 | ||||||
| G2 | 5 | 6.9 | 5.2-NR | 0.65 | 5 | 53.8 | 5.2-NR | 0.34 |
| G3 | 49 | 5.6 | 4.4-7.4 | 49 | 9.0 | 5.2-13.4 | ||
| Ki-67 of NE component | ||||||||
| < 55% | 14 | 7.0 | 5.3-12.9 | 0.78 | 10.7 | 5.6-NR | 0.6 | |
| ≥ 55% | 32 | 4.7 | 3.9-8.0 | 7.2 | 5.15-13.4 | |||
| First line active treatment | ||||||||
| No | 13 | 2.2 | 1.6 | < 0.001 | 3.7 | 2.0 | < 0.001 | |
| Yes | 40 | 7.1 | 5.2-9.8 | 13.4 | 9.0-16.3 | |||
Note: Advanced mixed neuroendocrine non-neuroendocrine neoplasms include both patients who presented with advanced disease at diagnosis and those who developed advanced recurrent disease after initial curative treatment. CT: Chemotherapy; RT: Radiotherapy; BSC: Best supportive care; neoadj: Neoadjuvant; adj: Adjuvant; periop: Perioperative; fluop: Fluopyrimidine; etop: Etoposide; plat: Platinum; epi: Epirubicin; iri: Irinotecan; gem: Gemcitabine; mAB: Monoclonal antibody; NA: Not available.
Univariate analysis for RFS, PFS and OS according to immunohistochemical data from tumour samples at diagnosis was also performed, and significant results can be summarised as follows; in the Loc setting, CK-7 positive staining was associated with shorter RFS (P = 0.021) and OS (P = 0.035), and CDX-2 positive staining was associated with improved OS (P = 0.009). In the Adv setting, Chromogranin A positive staining was associated with shorter PFS (P = 0.039).
DISCUSSION
This current study is one of the largest reporting on a population of patients with a diagnosis of MiNEN in the literature, with expert pathological review confirming the diagnosis. The results of this study indicate that, most commonly, MiNEN is diagnosed in men and arises from the colon-rectum or oesophagus/oesophago-gastric junction. These data closely mirror those from a German, single-centre, retrospective study by Apostolidis et al[19] which included 58 patients with a diagnosis of MiNEN. In addition, MiNEN has an aggressive biological behaviour, usually driven by a poorly differentiated neuroendocrine component. In nearly all cases, the non-neuroendocrine component is of ADC histology. These findings are in line with those from other retrospective cohorts of MiNENs where the neuroendocrine component was predominant in 50%-66.7% of tumour samples analysed[15,20-23] and poorly differentiated in 59%-100% of tumour samples analysed[12,15,19-26], and the non-neuroendocrine component was of ADC or adenoma histology in 66.7-100% of tumour samples analysed[10,15,20-27]. In addition, in the present study, the NEC component of MiNEN was predominantly of large cell morphology. Although this information was available only for a small proportion of cases (16; 23%), two other studies reported similar findings; a large cell NEC component in 41/42 and 10/10 MiNEN samples, respectively[20,24].
Interestingly, the present study suggests that, in MiNEN, the metastatic process is dominated by a single component, which is usually of NEC histology. This is corroborated by similar findings from an Italian and two Asian studies reporting on patients with GEP MiNENs[15,20,23], and carries an important implication; biopsies of metastatic sites may not capture both the tumour components, deceptively leading to a diagnosis of pure NEC or ADC, especially in Adv cases when surgical material for full sampling of the primary tumour is not available. This may also explain why the majority of cases, in the present cohort of MiNENs, were diagnosed at a Loc stage, which is unexpected for an aggressive disease; a proportion of Adv MiNENs may be misdiagnosed due to limitations of tissue biopsies.
Consistent with other studies from the literature[8,11,19,20,22], curative surgery was the preferred treatment for Loc MiNEN, and pre-operative and/or post-operative treatment was delivered to between a half and three quarters of resected cases. Chemotherapy and chemo-radiotherapy regimens in the Loc setting were most commonly adherent to the clinical practice guidelines for pure ADCs from the same sites of origin; this might be explained by the lack of solid evidence advocating the use of peri-operative chemotherapy or chemo-radiotherapy for potentially curable pure neuroendocrine neoplasms.
In the present study, palliative chemotherapy was the most common treatment offered to patients with Adv disease, whereas in the majority of reported studies on MiNEN from the literature, surgery, either alone or in combination with palliative chemotherapy, is the preferred management for this subgroup of patients[8,20-22,26,28,29]. This discrepancy might be explained by a selection bias, since most of those studies were conducted in surgical cohorts, only including cases of MiNEN diagnosed by surgical excision[8,20-22].
There is variability within the literature with regard to chemotherapy and chemo-radiotherapy regimens (ADC-like or NEC-like) used in the palliative setting[8,19,20,22,29], and the criteria driving the choice of the regimen remains unexplained. Similar to the study by Apostolidis et al[19], in the present series, first-line chemotherapy and chemoradiotherapy regimens were in line with ADC-like or NEC-like protocols in equal proportion. In addition, the choice of the regimen seemed to be based on the predominant or most aggressive histology. Noticeably, in a proportion of cases, ADC-like platinum-based regimens (e.g., 5-fluorouracil/oxaliplatin or 5-fluorou-racil/irinotecan) were preferred, despite a predominant or more aggressive neuroendocrine component; a possible explanation is that clinicians opted for such combinations, which have shown anti-tumour activity in small retrospective series of NECs[30,31], in an attempt to target both the components.
The results of the present study indicate that MiNEN has a poor prognosis, with a high likelihood of recurrence or progression after a short period of time from the initial management. Survival outcomes of MiNEN were close to those of pure GEP-NECs[32-34], and such a similarity was more evident in the Adv setting. Similarly, disease control rates to first-line palliative chemotherapy, which were consistent with those from other series of MiNENs (60%-68%)[19,20], were close to those of pure GEP-NECs (64%-100%)[32-34].
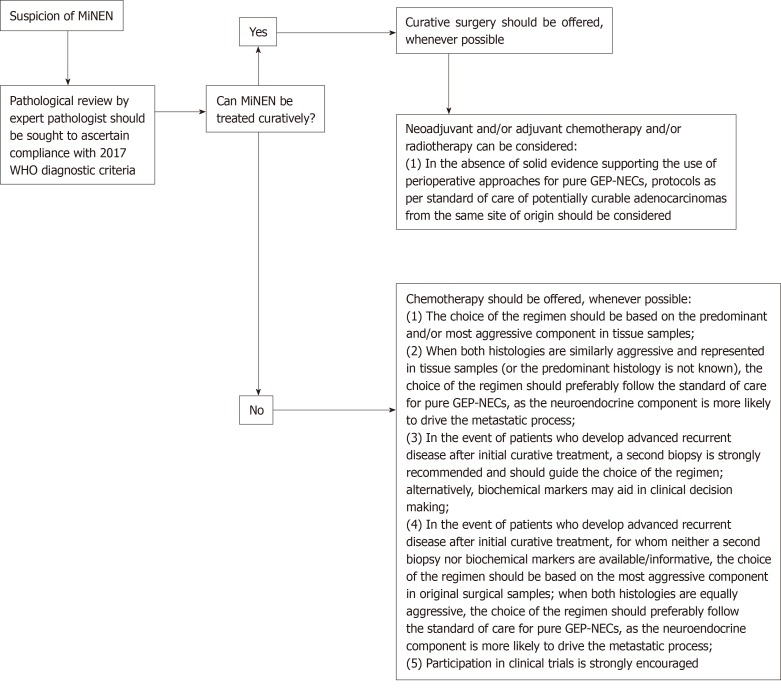
The present study summarises real-world data on an under-researched diagnosis, providing suggestions for clinical practice (Figure 3). However, there are limitations which are worth acknowledging. Firstly, it is a retrospective study and, therefore, the data reported may not be as accurate as in prospective clinical trials; information on a number of key pathological features (e.g., predominant component, histology of non-neuroendocrine component, morphological subtype and Ki-67 value of the neuroendocrine component, and immunohistochemical data) could not be retrieved from medical records or pathological reports in non-negligible proportions of patients, and further pathological review of the samples was not feasible. Although it would be interesting from a scientific standpoint to obtain the Ki-67 value and the morphological subtype (small cell vs large cell) of the neuroendocrine component from a larger number of patients, neither are proven prognostic or predictive factors in this disease subgroup and, therefore, currently, this information is of limited relevance for clinical practice. Secondly, the results of univariate and multivariable analyses have to be interpreted with caution, as the small sample size of comparator subgroups would not permit reliable deductions. Furthermore, survival outcomes of the Adv subgroup might be biased by the inclusion of patients who developed metastatic recurrent disease after initial curative treatment for Loc MiNENs; some of those patients who had received previous perioperative treatment, might have developed more chemo- and/or radiotherapy-resistant phenotypes, that might have negatively influenced the outcomes of the whole Adv subgroup. Lastly, there may be inaccuracies in diagnosis for those patients (around a third) having biopsy samples as the only diagnostic material, since verifying the 30% threshold for each component based on a limited amount of tissue might be challenging. However, biopsy samples were reviewed by expert pathologists, who based their conclusion on either the presence of an admixture of equal proportions of the two components within the tumour sample, or the evidence of mixed exocrine/neuroendocrine features on further tumour samples from the same patient.
Figure 3.
Algorithm of suggestions for clinical management of patients with a diagnosis of mixed neuroendocrine non-neuroendocrine neoplasm. NEC: Neuroendocrine carcinoma; GEP: Gastro-entero-pancreatic tract; WHO: World Health Organisation.
To conclude, due to limitations in diagnostic methods (insufficient amount of tumour tissue provided by biopsies) and criteria (a quantitative arbitrary 30% threshold for each component), the actual incidence of MiNEN may be underestimated in general clinical practice. To pursue an accurate diagnosis of MiNEN, and therefore deliver the most appropriate management, in the presence of a tumour from the GEP tract exhibiting a mixed histology and/or an unconventional behaviour on standard treatment, the tumour sample should be reviewed by pathologists with expertise in neuroendocrine neoplasms. Collection of additional tumour material from metastatic sites is also advisable to optimise the choice of treatment.
Recent molecular and genetic studies on MiNEN have uncovered shared molecular vulnerabilities between the neuroendocrine and non-neuroendocrine components[24,25,35], suggesting a potential role for targeted treatments against both the components, overcoming the long-standing problem of their differential sensitivity to conventional chemotherapy and chemo-radiotherapy. Sample biobanking and wider molecular analyses are paramount to forward the biological understanding of this rare disease, to inform novel drug development treatments and patient allocation to early-phase biomarker driven clinical trials. Liquid biopsies may aid in overcoming the limitations of tissue biopsies.
ARTICLE HIGHLIGHTS
Research background
Mixed neuroendocrine non-neuroendocrine neoplasm (MiNEN) is characterised by the coexistence of two histologies and is a rare disease, most commonly originating in the digestive system. Evidence from the literature is limited and inconsistent.
Research motivation
Although rare, when encountered, MiNEN represents a therapeutic riddle for clinicians, as there is still uncertainty as to how patients with this diagnosis should be managed, in the absence of data from clinical trials. In addition, the actual incidence of MiNEN remains unknown. Therefore, data from large retrospective studies is warranted.
Research objectives
The present study was designed to provide insights on the epidemiology and prognosis of MiNEN from the gastro-entero-pancreatic tract (GEP), as well as on commonly applied therapeutic approaches, with the ultimate aim of providing some guidance for clinical practice.
Research methods
To this end, a large retrospective, multicentre collection of clinical-pathological and survival data, and treatment modalities from patients with a diagnosis of GEP-MiNEN was carried out. Original diagnostic samples were reviewed by pathologists with expertise in neuroendocrine neoplasms to ascertain compliance with the most recent diagnostic criteria for MiNEN (WHO classification 2017). Potential differences in survival outcomes between subgroups with distinct clinical-pathological characteristics were also investigated.
Research results
MiNEN is most commonly diagnosed in the colon-rectum and oesophagus/oesophago-gastric junction. The neuroendocrine component is almost always grade 3, and is most commonly the predominant histology in both the primary tumour and distant metastatic sites. The non-neuroendocrine component is of adenocarcinoma histology in most cases. Patients with potentially curable MiNEN usually receive curative surgery, in combination or not with pre- and/or post-operative chemotherapy and/or radiotherapy as per standard of care for pure adenocarcinomas form the same sites of origin. Patients with advanced MiNEN most commonly receive chemotherapy following protocols for pure neuroendocrine carcinomas or adenocarcinomas from the same sites of origin. The prognosis of MiNEN is poor; patients with initially curative MiNEN have a high likelihood of recurrence (around 2/3 of cases), with half of cases developing disease recurrence within the first 12 months from curative treatment. Patients with advanced stage MiNEN progress soon after the beginning of palliative treatment and have survival outcomes very close to those of pure GEP-NECs.
Research conclusions
The incidence of MiNEN is likely underestimated, as tissue biopsies may not be able to capture both the histologies; it is a predominantly metastatic disease, and metastatic sites are usually occupied by a single component (most frequently G3, neuroendocrine). A pathological review of the samples by pathologists with expertise in neuroendocrine neoplasms is strongly recommended. A second biopsy from metastatic sites is encouraged, whenever possible, especially on disease progression. Systemic treatments directed against one of the two components have limited efficacy. Novel drug development should exploit common biological vulnerabilities between the two components. Biological studies and liquid biopsies may aid in unveiling the molecular landscape of MiNEN, and informing drug development and clinical trial design.
Footnotes
Institutional review board statement: This study was approved by the Christie NHS Foundation Trust Audit committee (16/1806), and by local audit committees from the other centres involved.
Informed consent statement: Patients were not required to give informed consent to the study because the analysis used anonymous clinical data.
Conflict-of-interest statement: The authors have declared no conflict of interest.
Peer-review started: May 4, 2019
First decision: May 30, 2019
Article in press: September 11, 2019
Specialty type: Gastroenterology and hepatology
Country of origin: United Kingdom
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Annibale B, Aosasa S, Tsolakis AV S-Editor: Ma RY L-Editor: A E-Editor: Ma YJ
Contributor Information
Melissa Frizziero, Department of Medical Oncology, The Christie NHS Foundation Trust, Manchester M20 4BX, United Kingdom.
Xin Wang, Department of Analytics and Development, The Christie NHS Foundation Trust, Manchester M20 4BX, United Kingdom.
Bipasha Chakrabarty, Department of Pathology, The Christie NHS Foundation Trust, Manchester M20 4BX, United Kingdom.
Alexa Childs, Department of Medical Oncology, Royal Free London NHS Foundation Trust, London NW3 2QG, United Kingdom; UCL Cancer Institute, University College London, London WC1E 6AG, United Kingdom.
Tu V Luong, Department of Histopathology, Royal Free London NHS Foundation Trust, London NW3 2QG, United Kingdom.
Thomas Walter, Department of Gastroenterology and Medical Oncology, Edouard Herriot Hospital, Hospices Civils de Lyon, Lyon 69003, France.
Mohid S Khan, Department of Gastroenterology, Cardiff and Vale University Health Board, University Hospital of Wales, Cardiff CF14 4XW, United Kingdom.
Meleri Morgan, Department of Cellular Pathology, Cardiff and Vale University Health Board, University Hospital of Wales, Cardiff CF14 4XW, United Kingdom.
Adam Christian, Department of Cellular Pathology, Cardiff and Vale University Health Board, University Hospital of Wales, Cardiff CF14 4XW, United Kingdom.
Mona Elshafie, Department of Pathology, University Hospitals Birmingham NHS Foundation Trust, Birmingham B15 2TH, United Kingdom.
Tahir Shah, Department of Hepatology, University Hospitals Birmingham NHS Foundation Trust, Birmingham B15 2TH, United Kingdom.
Annamaria Minicozzi, Department of Surgical Oncology, The Christie NHS Foundation Trust, Manchester M20 4BX, United Kingdom.
Wasat Mansoor, Department of Medical Oncology, The Christie NHS Foundation Trust, Manchester M20 4BX, United Kingdom.
Tim Meyer, Department of Medical Oncology, Royal Free London NHS Foundation Trust, London NW3 2QG, United Kingdom; UCL Cancer Institute, University College London, London WC1E 6AG, United Kingdom; UCL Cancer Institute, University College London, London WC1E 6AG, United Kingdom.
Angela Lamarca, Department of Medical Oncology, The Christie NHS Foundation Trust, Manchester M20 4BX, United Kingdom.
Richard A Hubner, Department of Medical Oncology, The Christie NHS Foundation Trust, Manchester M20 4BX, United Kingdom.
Juan W Valle, Department of Medical Oncology, The Christie NHS Foundation Trust, Manchester M20 4BX, United Kingdom; Division of Cancer Sciences, University of Manchester, Manchester M13 9PL, United Kingdom.
Mairéad G McNamara, Department of Medical Oncology, The Christie NHS Foundation Trust, Manchester M20 4BX, United Kingdom. mairead.mcnamara@christie.nhs.uk; Division of Cancer Sciences, University of Manchester, Manchester M13 9PL, United Kingdom.
References
- 1.Volante M, Rindi G, Papotti M. The grey zone between pure (neuro)endocrine and non-(neuro)endocrine tumours: a comment on concepts and classification of mixed exocrine-endocrine neoplasms. Virchows Arch. 2006;449:499–506. doi: 10.1007/s00428-006-0306-2. [DOI] [PubMed] [Google Scholar]
- 2.Hamilton SR, Aaltonen LA. Lyon, France: International Agency fo Research on Cancer (IARC) Press; 2000. World Health Organization Classification of Tumours. Pathology and Genetics of Tumours of the Digestive System. [Google Scholar]
- 3.DeLellis RA, Lloyd RV, Heitz PU. Lyon, France: Internationl Agency for Research on Cancer (IARC) Press; 2004. World Health Organization of Tumours. Pathology and Genetics of Tumours of Endocrine Organs. [Google Scholar]
- 4.Bosman T, Carneiro F, Hruban ND. Lyon, France: International Agency for Research on Cancer (IARC) Press; 2010. World Health Organization of Tumours. WHO Classification of Tumours of the Digestive System. [Google Scholar]
- 5.Lloyd RV, Osamura RY, Klöppel G. Lyon, France: International Agency for Research on Cancer (IARC) Press; 2017. World Health Organization of Tumours. WHO Classification of Tumours of Endocrine Organs. 4th ed. [Google Scholar]
- 6.Tang LH, Shia J, Soslow RA, Dhall D, Wong WD, O'Reilly E, Qin J, Paty P, Weiser MR, Guillem J, Temple L, Sobin LH, Klimstra DS. Pathologic classification and clinical behavior of the spectrum of goblet cell carcinoid tumors of the appendix. Am J Surg Pathol. 2008;32:1429–1443. doi: 10.1097/PAS.0b013e31817f1816. [DOI] [PubMed] [Google Scholar]
- 7.La Rosa S, Marando A, Sessa F, Capella C. Mixed Adenoneuroendocrine Carcinomas (MANECs) of the Gastrointestinal Tract: An Update. Cancers (Basel) 2012;4:11–30. doi: 10.3390/cancers4010011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shen C, Chen H, Chen H, Yin Y, Han L, Chen J, Tang S, Yin X, Zhou Z, Zhang B, Chen Z. Surgical treatment and prognosis of gastric neuroendocrine neoplasms: a single-center experience. BMC Gastroenterol. 2016;16:111. doi: 10.1186/s12876-016-0505-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Milione M, Spada F, Maisonneuve P. Clinico-pathological and survival analysis of 211 gastroenteropancreatic G3 neuroendocrine carcinomas. Neuroendocrinology. 2015;102:102–103, 0028-3835. [Google Scholar]
- 10.Basturk O, Adsay V, Hruban EH. Pancreatic acinar cell carcinomas with prominent neuroendocrine differentiation: Clinicaopathological analysis of a distinct and diagnostically challenging neoplasm. Laboratory Investigation. 2014;94:447A. [Google Scholar]
- 11.Brathwaite S, Rock J, Yearsley MM, Bekaii-Saab T, Wei L, Frankel WL, Hays J, Wu C, Abdel-Misih S. Mixed Adeno-neuroendocrine Carcinoma: An Aggressive Clinical Entity. Ann Surg Oncol. 2016;23:2281–2286. doi: 10.1245/s10434-016-5179-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brathwaite S, Yearsley MM, Bekaii-Saab T, Wei L, Schmidt CR, Dillhoff ME, Frankel WL, Hays JL, Wu C, Abdel-Misih S. Appendiceal Mixed Adeno-Neuroendocrine Carcinoma: A Population-Based Study of the Surveillance, Epidemiology, and End Results Registry. Front Oncol. 2016;6:148. doi: 10.3389/fonc.2016.00148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lim SM, Kim H, Kang B, Kim HS, Rha SY, Noh SH, Hyung WJ, Cheong JH, Kim HI, Chung HC, Yun M, Cho A, Jung M. Prognostic value of (18)F-fluorodeoxyglucose positron emission tomography in patients with gastric neuroendocrine carcinoma and mixed adenoneuroendocrine carcinoma. Ann Nucl Med. 2016;30:279–286. doi: 10.1007/s12149-016-1059-x. [DOI] [PubMed] [Google Scholar]
- 14.Garcia-Carbonero R, Sorbye H, Baudin E, Raymond E, Wiedenmann B, Niederle B, Sedlackova E, Toumpanakis C, Anlauf M, Cwikla JB, Caplin M, O'Toole D, Perren A Vienna Consensus Conference participants. ENETS Consensus Guidelines for High-Grade Gastroenteropancreatic Neuroendocrine Tumors and Neuroendocrine Carcinomas. Neuroendocrinology. 2016;103:186–194. doi: 10.1159/000443172. [DOI] [PubMed] [Google Scholar]
- 15.Sahnane N, Furlan D, Monti M, Romualdi C, Vanoli A, Vicari E, Solcia E, Capella C, Sessa F, La Rosa S. Microsatellite unstable gastrointestinal neuroendocrine carcinomas: a new clinicopathologic entity. Endocr Relat Cancer. 2015;22:35–45. doi: 10.1530/ERC-14-0410. [DOI] [PubMed] [Google Scholar]
- 16.La Rosa S, Sessa F, Uccella S. Mixed Neuroendocrine-Nonneuroendocrine Neoplasms (MiNENs): Unifying the Concept of a Heterogeneous Group of Neoplasms. Endocr Pathol. 2016;27:284–311. doi: 10.1007/s12022-016-9432-9. [DOI] [PubMed] [Google Scholar]
- 17.Volante M, Righi L, Asioli S, Bussolati G, Papotti M. Goblet cell carcinoids and other mixed neuroendocrine/nonneuroendocrine neoplasms. Virchows Arch. 2007;451 Suppl 1:S61–S69. doi: 10.1007/s00428-007-0447-y. [DOI] [PubMed] [Google Scholar]
- 18.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 19.Apostolidis L, Bergmann F, Jäger DC. Prognosis and Treatment Outcomes of Patients with Mixed Adenoneuroendocrine carcinoma - a Single Cancer Center Experience. Neuroendocrinology. 2017:(abstract). Available from: https://www.enets.org/prognosis-and-treatment-outcomes-of-patients-with-mixed-adenoneuroendocrine-carcinoma-manec-r-a-single-cancer-center-experience.html. [Google Scholar]
- 20.Watanabe J, Suwa Y, Ota M, Ishibe A, Masui H, Nagahori K, Tsuura Y, Endo I. Clinicopathological and Prognostic Evaluations of Mixed Adenoneuroendocrine Carcinoma of the Colon and Rectum: A Case-Matched Study. Dis Colon Rectum. 2016;59:1160–1167. doi: 10.1097/DCR.0000000000000702. [DOI] [PubMed] [Google Scholar]
- 21.Nie L, Li M, He X, Feng A, Wu H, Fan X. Gastric mixed adenoneuroendocrine carcinoma: correlation of histologic characteristics with prognosis. Ann Diagn Pathol. 2016;25:48–53. doi: 10.1016/j.anndiagpath.2016.09.004. [DOI] [PubMed] [Google Scholar]
- 22.Komatsubara T, Koinuma K, Miyakura Y, Horie H, Morimoto M, Ito H, Lefor AK, Sata N, Fukushima N. Endocrine cell carcinomas of the colon and rectum: a clinicopathological evaluation. Clin J Gastroenterol. 2016;9:1–6. doi: 10.1007/s12328-015-0623-6. [DOI] [PubMed] [Google Scholar]
- 23.Chen MH, Kuo YJ, Yeh YC, Lin YC, Tzeng CH, Liu CY, Chang PM, Chen MH, Jeng YM, Chao Y. High neuroendocrine component is a factor for poor prognosis in gastrointestinal high-grade malignant mixed adenoneuroendocrine neoplasms. J Chin Med Assoc. 2015;78:454–459. doi: 10.1016/j.jcma.2015.04.002. [DOI] [PubMed] [Google Scholar]
- 24.Olevian DC, Nikiforova MN, Chiosea S, Sun W, Bahary N, Kuan SF, Pai RK. Colorectal poorly differentiated neuroendocrine carcinomas frequently exhibit BRAF mutations and are associated with poor overall survival. Hum Pathol. 2016;49:124–134. doi: 10.1016/j.humpath.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 25.Jesinghaus M, Konukiewitz B, Keller G, Kloor M, Steiger K, Reiche M, Penzel R, Endris V, Arsenic R, Hermann G, Stenzinger A, Weichert W, Pfarr N, Klöppel G. Colorectal mixed adenoneuroendocrine carcinomas and neuroendocrine carcinomas are genetically closely related to colorectal adenocarcinomas. Mod Pathol. 2017;30:610–619. doi: 10.1038/modpathol.2016.220. [DOI] [PubMed] [Google Scholar]
- 26.Acosta AM, Wiley EL. Primary Biliary Mixed Adenoneuroendocrine Carcinoma (MANEC): A Short Review. Arch Pathol Lab Med. 2016;140:1157–1162. doi: 10.5858/arpa.2015-0102-RS. [DOI] [PubMed] [Google Scholar]
- 27.La Rosa S, Marando A, Furlan D, Sahnane N, Capella C. Colorectal poorly differentiated neuroendocrine carcinomas and mixed adenoneuroendocrine carcinomas: insights into the diagnostic immunophenotype, assessment of methylation profile, and search for prognostic markers. Am J Surg Pathol. 2012;36:601–611. doi: 10.1097/PAS.0b013e318242e21c. [DOI] [PubMed] [Google Scholar]
- 28.La Rosa S, Inzani F, Vanoli A, Klersy C, Dainese L, Rindi G, Capella C, Bordi C, Solcia E. Histologic characterization and improved prognostic evaluation of 209 gastric neuroendocrine neoplasms. Hum Pathol. 2011;42:1373–1384. doi: 10.1016/j.humpath.2011.01.018. [DOI] [PubMed] [Google Scholar]
- 29.Yamaguchi T, Machida N, Morizane C, Kasuga A, Takahashi H, Sudo K, Nishina T, Tobimatsu K, Ishido K, Furuse J, Boku N, Okusaka T. Multicenter retrospective analysis of systemic chemotherapy for advanced neuroendocrine carcinoma of the digestive system. Cancer Sci. 2014;105:1176–1181. doi: 10.1111/cas.12473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hentic O, Hammel P, Couvelard A, Rebours V, Zappa M, Palazzo M, Maire F, Goujon G, Gillet A, Lévy P, Ruszniewski P. FOLFIRI regimen: an effective second-line chemotherapy after failure of etoposide-platinum combination in patients with neuroendocrine carcinomas grade 3. Endocr Relat Cancer. 2012;19:751–757. doi: 10.1530/ERC-12-0002. [DOI] [PubMed] [Google Scholar]
- 31.Hadoux J, Malka D, Planchard D, Scoazec JY, Caramella C, Guigay J, Boige V, Leboulleux S, Burtin P, Berdelou A, Loriot Y, Duvillard P, Chougnet CN, Déandréis D, Schlumberger M, Borget I, Ducreux M, Baudin E. Post-first-line FOLFOX chemotherapy for grade 3 neuroendocrine carcinoma. Endocr Relat Cancer. 2015;22:289–298. doi: 10.1530/ERC-15-0075. [DOI] [PubMed] [Google Scholar]
- 32.Sorbye H, Strosberg J, Baudin E, Klimstra DS, Yao JC. Gastroenteropancreatic high-grade neuroendocrine carcinoma. Cancer. 2014;120:2814–2823. doi: 10.1002/cncr.28721. [DOI] [PubMed] [Google Scholar]
- 33.Sorbye H, Welin S, Langer SW, Vestermark LW, Holt N, Osterlund P, Dueland S, Hofsli E, Guren MG, Ohrling K, Birkemeyer E, Thiis-Evensen E, Biagini M, Gronbaek H, Soveri LM, Olsen IH, Federspiel B, Assmus J, Janson ET, Knigge U. Predictive and prognostic factors for treatment and survival in 305 patients with advanced gastrointestinal neuroendocrine carcinoma (WHO G3): the NORDIC NEC study. Ann Oncol. 2013;24:152–160. doi: 10.1093/annonc/mds276. [DOI] [PubMed] [Google Scholar]
- 34.Walter T, Tougeron D, Baudin E, Le Malicot K, Lecomte T, Malka D, Hentic O, Manfredi S, Bonnet I, Guimbaud R, Coriat R, Lepère C, Desauw C, Thirot-Bidault A, Dahan L, Roquin G, Aparicio T, Legoux JL, Lombard-Bohas C, Scoazec JY, Lepage C, Cadiot G CEPD investigators. Poorly differentiated gastro-entero-pancreatic neuroendocrine carcinomas: Are they really heterogeneous? Insights from the FFCD-GTE national cohort. Eur J Cancer. 2017;79:158–165. doi: 10.1016/j.ejca.2017.04.009. [DOI] [PubMed] [Google Scholar]
- 35.Yang H-M, Schaaf C, Schaeffer D. Molecular Analysis of Mixed Adenoneuroendocrine carcinomas (MANEC) Signifies a Common Clonal Origin of Adeno and Neuroendocrine components. Laboratory Investigation. 2015;95:199A–200A. [Google Scholar]