Abstract
BACKGROUND
In pancreatic cancer, acute pancreatitis (AP) is a serious morbidity, but its negative effect on long-term outcomes remains to be elucidated.
AIM
To investigate the effects of AP on the tumor recurrence pattern of pancreatic ductal adenocarcinoma (PDAC) and tumor-specific survival.
METHODS
The medical records of 219 patients with curative pancreatectomy for pancreatic cancer at the Pancreatic Surgery Center of West China Hospital from July 2012 to December 2016 were analyzed retrospectively. The severity of acute pancreatitis was classified according to the Atlanta classification of AP. The patient demographics and tumor characteristics were assessed. Early recurrence was defined as a relapse within 12 mo after surgery. Overall and disease-free survival and recurrence patterns were analyzed. Mild acute pancreatitis was excluded because its negative effects can be negligible.
RESULTS
Early recurrence in AP group was significantly higher than in non-AP group (71.4% vs 41.2%; P = 0.009). Multivariate analysis of postoperative early recurrence showed that moderate or severe AP was an independent risk factor for an early recurrence [odds ratio (OR): 4.13; 95% confidence interval (CI): 1.41-12.10; P = 0.01]. The median time to recurrence was shorter in patients with AP than in those without (8.4 vs 12.8 mo; P = 0.003). Multivariate analysis identified AP as an independent prognostic factor for overall survival [relative risk (RR): 2.35; 95%CI: 1.45-3.83] and disease-free survival (RR: 2.24; 95%CI: 1.31-3.85) in patients with PDAC.
CONCLUSION
Patients with moderate or severe acute pancreatitis developed recurrences earlier than those without. Moderate or severe AP is associated with shorter overall and disease-free survival of patients with PDAC.
Keywords: Acute pancreatitis, Pancreatic ductal adenocarcinoma, Recurrence, Prognosis, Overall survival, Disease-free survival
Core tip: This study investigated the effects of acute pancreatitis (AP) on the tumor recurrence pattern of pancreatic ductal adenocarcinoma (PDAC) and tumor-specific survival. We found that moderate or severe AP was significantly related to early recurrence, and patients with moderate or severe AP developed recurrence earlier than those without. Moderate or severe AP was adversely correlated with overall and disease-free survival of patients with PDAC. Surgeons are thus urged to reduce the complications of endoscopic retrograde cholangiopancreatography and make efforts to diagnose PDAC timely in patients who present with AP in order to improve the oncological outcomes of PDAC.
INTRODUCTION
There are multiple etiological factors involved in the development of acute pancreatitis (AP). Excluding common etiologies, such as alcohol and gallstones, the onset of AP may be due to cancer progression itself or to complications of the diagnostic and therapeutic interventional procedures used in pancreatic ductal adenocarcinoma (PDAC) treatment, such as endoscopic retrograde cholangiopancreatography (ERCP), surgery and chemotherapy[1-3]. PDAC often presents initially as AP[4-6]. Moreover, the diagnosis of AP may precede PDAC by weeks or even months[7,8]. Approximately 2.6% to 13.8% of pancreatic cancer patients present with AP, according to previous studies[7,9]. Overwhelming evidence suggests that patients with a history of AP have an increased risk of pancreatic cancer compared with non-AP subjects. Even after 12 mo, the incidence of pancreatic cancer was reported to be 3.84-fold higher in the AP group than in the non-AP group[4,10,11].
Moreover, as the most common complication of ERCP[1], pancreatitis occurs with an incidence of 2%-20% in association with ERCP according to the 2002 NIH State-of-the-Science statement on ERCP[12]. In these patients complicated with pancreatitis after ERCP, about one-third had moderate or severe pancreatitis[13].
Many studies have shown that AP has significant negative effects on prognosis and outcome of PDAC[14,15]. Our group recently demonstrated that the short-term outcome of pancreatic cancer patients after surgery is adversely affected by AP[15]. Adverse effects of perioperative AP include delayed surgical treatment, longer hospital stay, higher cost of treatment and a reduced quality of life, and may be related to premature mortality[14,16,17].
Only a few studies have evaluated the overall survival of patients with pancreatic cancer and AP, and the impact of AP on tumor-specific survival has not been thoroughly understood. Mujica et al[16] showed that in pancreatic cancer patients with pancreatitis as their initial symptom, their overall survival rates at six and 12 months were 51% and 28%, respectively. This finding suggests that PDAC patients with concomitant underlying pancreatitis may have a poor prognosis. In contrast, a recent study investigated whether initial clinical presentation with AP affected the survival of patients with PDAC, and the results did not show any survival difference[5]. Hence, the purpose of our study was to investigate the relationship of AP with the tumor recurrence pattern of PDAC and tumor-specific survival.
MATERIALS AND METHODS
Study population and data collection
From July 2012 to December 2016, 274 patients underwent curative resection for PDAC at the Pancreatic Surgery Center of West China Hospital, China. This study followed the ethical guidelines of the Helsinki Declaration of 1975 (revised in 1983) and was approved by the Medical Ethics Committee of West China Hospital at Sichuan University. Patients who died within 90 d after surgery or had incomplete records were not included. The resected pancreatic tumors were pathologically confirmed to be PDAC. We retrospectively assessed the patients’ tumor status, plexus invasion, resection margin, lymph node characteristics, tumor recurrence, and adjuvant chemotherapy. The AJCC Cancer Staging System (7th edition) was applied.
The Atlanta classification was utilized to grade the severity of AP[18]. Patients with mild AP were excluded because they experience a mild clinical course. The remaining 219 cases were subdivided into two groups. Group AP [n = 21, (9.6%)] included those patients with moderate or severe AP and group non-AP [n = 198, (90.4%)] included those without acute pancreatitis. To explore the relationship of moderate and severe AP with the recurrence pattern and patient outcomes, we assessed the tumor recurrence and tumor-specific survival of PDAC patients who underwent curative resection. Overall survival was defined as the time from the date of surgery to death or last follow-up. Postoperative tumor recurrence within 12 mo was regarded as early recurrence. Patients without early recurrence were defined as a relapse more than 12 mo after surgery or without recurrence after surgery. The median follow-up time was 18.3 mo. Postoperative recurrence was diagnosed by imaging studies. We obtained postoperative recurrence data for only 186 of 219 patients.
Statistical analysis
Results were analyzed using SPSS version 23.0 (SPSS Inc., Chicago, IL, United States), and the data are shown as the mean ± standard deviation. Clinical characteristics and baseline demographics were compared between group AP and group non-AP using Student’s t test or the Mann–Whitney U test. Univariate and multivariate logistic regression analysis was used to analyze the predictive factors related to recurrence. Overall survival and disease-free survival were used to measure the prognostic role of AP. Total mortality was the primary endpoint of the trial. The associations of AP and other clinical and demographic characteristics with overall survival and disease-free survival were analyzed using univariable Cox proportional hazards regression models. A forward elimination procedure was used to remove terms until all variables had P < 0.10. An association was considered statistically significant if P < 0.05.
RESULTS
Of the 219 patients (78 women and 141 men), 32 underwent distal pancreatectomy, 152 underwent pancreatic head resection, and 4 underwent total pancreatectomy. The tumor characteristics and patient demographics in the patients with and without AP were roughly equivalent (Table 1). Table 1 shows that 5 of 21 AP were caused by ERCP . Nine of 21 AP were due to alcoholic factor. The etiology of remaining 8 cases was unknown. Most of the tumors in the two groups were stage II (90.5% in group AP, 90.9% in group non-AP) and moderately differentiated (76.2% in group AP, 73.2% in group non-AP). Positive lymph nodes, vascular invasion, perineural invasion and microscopic residual tumor (R1 resections) were similar between the two groups. Medical complications of AP group were higher than non-AP group(33.3% vs 29.8; P = 0.737). Grade B/C pancreatic fistula occurrence rate of AP group was higher than non-AP group(14.3% vs 4.5%; P = 0.174). Less than half of the cases underwent adjuvant chemotherapy (group AP, 33.3% and group non-AP, 24.7%; P = NS). However, early recurrence in group AP was significantly higher than in group non-AP (71.4% vs 41.2%; P = 0.009).
Table 1.
Patient characteristics
| Parameters | AP (n = 21) | No AP (n = 198) | P value |
| Age (yr), mean ± SD | 55.6 ± 16.4 | 61.2 ± 10.5 | 0.137 |
| Male, n (%) | 13 (61.9) | 128 (64.6) | 0.803 |
| Cause of acute pancreatitis n (%) | |||
| ERCP | 5 (23.8) | ||
| Alcoholic | 9 (42.9) | ||
| Unknown | 8 (33.3) | ||
| Time to surgery (d), mean ± SD | 41.4 ± 10.7 | 10.8 ± 5.8 | < 0.001 |
| Tumor site, (head) n (%) | 14 (66.7) | 149 (75.3) | 0.391 |
| Positive lymph nodes, n (%) | 9 (42.9) | 82 (41.4) | 0.898 |
| TNM Stage, n (%) | 1.000 | ||
| I | 2 (9.5) | 18 (9.1) | |
| II | 19 (90.5) | 180 (90.9) | |
| Surgical margin (R1), n (%) | 2 (9.5) | 13 (6.6) | 0.955 |
| Perineural invasion, n (%) | 5 (23.8) | 65 (32.8) | 0.399 |
| Vascular invasion, n (%) | 2 (9.5) | 20 (10.1) | 1.000 |
| Differentiation, n (%) | 0.283 | ||
| Well | 0 (0.0) | 12 (6.1) | |
| Moderate | 16 (76.2) | 145 (73.2) | |
| Poor | 5 (23.8) | 41 (20.7) | |
| Medical complications n (%) | 7 (33.3) | 59 (29.8) | 0.737 |
| Pancreatic fstula n (%) | |||
| biochemical leak | 3 (14.3) | 10 (5.1) | 0.223 |
| Grade B/C | 3 (14.3) | 9 (4.5) | 0.174 |
| Grade B | 2 (9.5) | 7 (3.5) | 0.461 |
| Grade C | 1 (4.8) | 2 (1.0) | 0.675 |
| Adjuvant Chemotherapy, n (%) | 7 (33.3) | 49 (24.7) | 0.391 |
| Early recurrence, n (%) | 15/21 (71.4) | 68/165 (41.2) | 0.009 |
| DFS, median (95%CI) | 8.4 (6.7-10.1) | 14.6 (10.7-18.5) | 0.007 |
| OS, median (95%CI) | 14.9 (12.5-17.3) | 22.3 (17.9-26.7) | < 0.001 |
TNM: Tumor node metastasis; DFS: Disease-free survival; OS: Overall survival; AP: Acute pancreatitis; SD: Standard deviation; ERCP: Endoscopic retrograde cholangiopancreatography.
Table 2 shows the predictive factors of postoperative early recurrence. A total of 83 patients experienced an early recurrence. According to the univariate analysis, the presence of AP was significantly correlated with early recurrence (P = 0.012). In multivariate analysis, the presence of AP was proven to be an independent risk factor for early recurrence [odds ratio (OR): 4.13; P = 0.01] (Table 2). In addition, other independent risk factors for early recurrence included R1 resection, positive lymph nodes, tumor site, and the absence of adjuvant chemotherapy.
Table 2.
Predictive factors of postoperative early recurrence (within 12 mo)
| Parameters | P value | Univariate OR (95%CI) | Multivariate OR (95%CI) | P value |
| Acute pancreatitis, (yes vs no) | 0.012 | 3.57 (1.32-9.66) | 4.13 (1.41-12.10) | 0.010 |
| Adjuvant chemotherapy, (yes vs no) | 0.027 | 0.46 (0.24-0.92) | 0.42 (0.20-0.90) | 0.025 |
| Positive lymph nodes, (yes vs no) | 0.001 | 2.74 (1.49-5.04) | 3.02 (1.54-5.90) | 0.001 |
| Surgical margin, (R1 vs R0) | 0.015 | 5.09 (1.37-18.91) | 4.78 (1.16-19.74) | 0.030 |
| Vascular invasion, (yes vs no) | 0.093 | 2.32 (0.87-6.18) | 0.463 | |
| Tumor site, (distal vs head) | 0.036 | 1.96 (1.05-3.65) | 2.70 (1.33-5.47) | 0.006 |
| TNM (II/III vs I) | 0.038 | 3.37 (1.07-10.57) | 0.188 |
Patient characteristics and pathologic feature, including age, sex, differentiation, and prineural invasion were analyzed as well, and the differences did not reach statistical significance in these factors (data not shown). TNM: Tumor node metastasis; OR: Odd ratio; CI: Confidence interval.
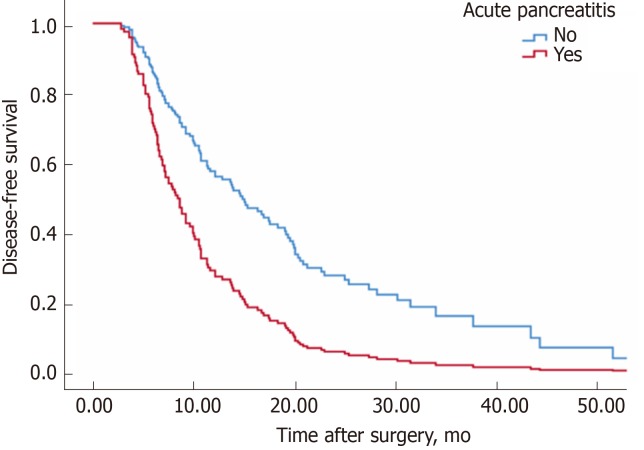
The median disease-free survival time of group non-AP was 12.8 mo, while that of group AP was 8.6 mo (P = 0.003, log rank test) (Figure 1). Correspondingly, 12-month disease-free survival rates of the non-AP group were superior to the AP group (58.8% vs 28.6%, P = 0.003). Table 3 shows the factors related to postoperative disease-free survival among all patients. Patients with AP were associated with a shorter disease-free survival interval [relative risk (RR): 2.0; P = 0.009].
Figure 1.
Disease-free survival in patients without acute pancreatitis and with moderate or severe acute pancreatitis. Median disease-free survival times were 12.8 and 8.4 mo for patients without acute pancreatitis (n = 165) and with moderate or severe acute pancreatitis (n = 21), respectively (P = 0.003, log rank test).
Table 3.
Cox regression analysis for disease-free survival
| Parameters | Univariate RR (95%CI) | P value | Multivariate RR(95%CI) | P value |
| Acute pancreatitis, (yes vs no) | 2.00 (1.19-3.36) | 0.009 | 2.24 (1.31-3.85) | 0.003 |
| Chemotherapy, (yes vs no) | 0.47 (0.31-0.72) | < 0.001 | 0.45 (0.29-0.70) | < 0.001 |
| Positive lymph nodes, (yes vs no) | 2.02 (1.40-2.91) | < 0.001 | 2.10 (1.41-3.14) | < 0.001 |
| Vascular invasion, (yes vs no) | 1.72(1.00-2.97) | 0.051 | 0.063 | |
| Surgical margin, (R1 vs R0) | 3.00(1.68-5.35) | < 0.001 | 2.55 (1.40-4.64) | 0.002 |
| Differentiation | 0.016 | 0.019 | ||
| Moderate vs Well | 7.81 (1.92-31.84) | 0.004 | 5.84 (1.42-24.01) | 0.014 |
| Poor vs Well | 7.13 (1.67-30.38) | 0.008 | 7.84 (1.82-33.74) | 0.006 |
| T stage, (T1/T2 vs T3/T4) | 0.76 (0.47-1.22) | 0.254 | 0.832 | |
| Perineural invasion, (yes vs no) | 1.53 (1.07-2.19) | 0.020 | 0.454 | |
| TNM stage, (II/III vs I) | 2.25 (1.21-4.21) | 0.011 | 0.516 | |
| tumor site, (distal vs head) | 1.45 (1.00-2.11) | 0.051 | 1.74 (1.15-2.64) | 0.009 |
| Age, (< 65 yr vs ≥ 65 yr) | 0.82 (0.57-1.16) | 0.261 | 0.404 | |
| Sex, (male vs female) | 1.07 (0.74-1.55) | 0.732 | 0.951 |
TNM: Tumor node metastasis; RR: Relative risk; CI: Confidence interval.
The final multivariable analysis included AP, positive lymph nodes, adjuvant chemotherapy, surgical margin, tumor differentiation and location of the tumor. The occurrence of AP was regarded as an independent risk factor after adjustment for other covariates (RR: 2.24; P = 0.003).
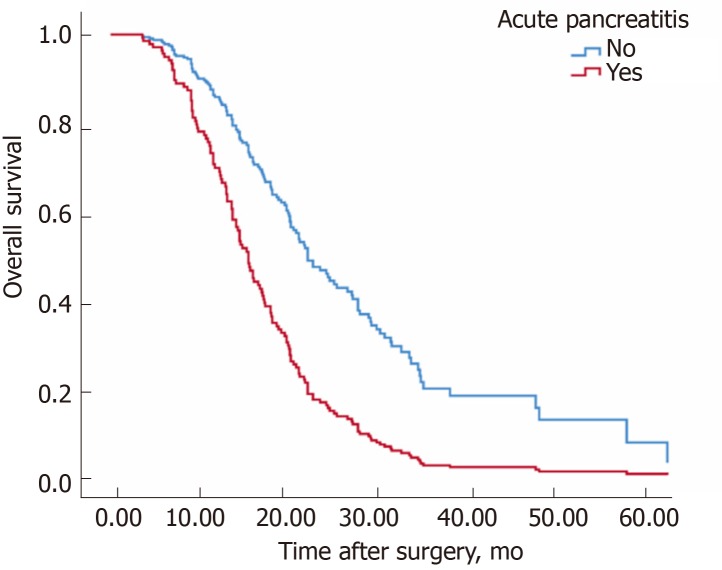
Table 4 shows the factors related to overall survival among all patients. The median overall survival time in patients with AP was shorter than in those without AP (22.3 mo for the group non-AP, 14.9 mo for the group AP, P < 0.001, log rank test) (Figure 2). Similar to disease-free survival, the presence of AP had a significant negative impact on overall survival (Figure 1). Positive lymph nodes, stage, the absence of adjuvant chemotherapy, differentiation and tumor site were also factors significantly related to decreased overall survival. In the multivariable analysis, the presence of AP (RR: 2.35; P = 0.001), tumor site (head) (RR: 1.90; P = 0.003), adjuvant chemotherapy (RR: 0.52; P = 0.002), well-differentiated tumors vs moderately differentiated (RR: 3.07; P = 0.059), and positive lymph nodes (RR: 2.68; P < 0.001) were the independent factors related to overall survival.
Table 4.
Overall survival for the entire patient cohort
| Parameters | Univariate RR (95%CI) | P value | Multivariate RR (95%CI) | P value |
| Acute pancreatitis (yes vs no) | 2.47 (1.55-3.96) | < 0.001 | 2.35 (1.45-3.83) | 0.001 |
| Chemotherapy, (yes vs no) | 0.63 (0.43-0.94) | 0.024 | 0.52 (0.34-0.79) | 0.002 |
| Differentiation | 0.002 | < 0.001 | ||
| Moderate vs Well | 4.65 (1.47-14.72) | 0.009 | 3.07 (0.96-9.84) | 0.059 |
| Poor vs Well | 7.25 (2.21-23.74) | 0.001 | 6.70 (2.02-22.17) | 0.002 |
| T stage, T12 vs T34 | 0.51 (0.27-0.98) | 0.043 | 0.781 | |
| Positive lymph nodes, (yes vs no) | 2.32 (1.64-3.29) | < 0.001 | 2.68 (1.83-3.92) | < 0.001 |
| Perineural invasion, (yes vs no) | 1.10 (0.77-1.57) | 0.611 | 0.667 | |
| Vascular invasion, (yes vs no) | 1.44 (0.86-2.39) | 0.164 | 0.430 | |
| Surgical margin, (R1 vs R0) | 1.41 (0.69-2.89) | 0.346 | 0.728 | |
| TNM stage, (II/III vs I) | 2.35 (1.15-4.82) | 0.0 | 0.378 | |
| Sex, (male vs female) | 1.15 (0.81-1.62) | 0.443 | 0.568 | |
| Age (< 65 yr vs ≥ 65 yr ) | 0.96 (0.69-1.34) | 0.803 | 0.694 | |
| tumor site, (distal vs head) | 1.52 (1.03-2.24) | 0.035 | 1.90 (1.24-2.90) | 0.003 |
TNM: Tumor node metastasis; RR: Relative risk; CI: Confidence interval.
Figure 2.
Overall survival of patients without acute pancreatitis and with moderate or severe acute pancreatitis. Survival of patients without acute pancreatitis (n = 198) and with moderate or severe acute pancreatitis (n = 21) was compared. Median survival time was 22.3 mo for the non-acute pancreatitis group, and 14.9 mo for the moderate or severe acute pancreatitis group. The difference was statistically significant (P < 0.001, log rank test).
DISCUSSION
Our study found that AP was negatively associated with survival time and early recurrence (within 12 mo). This trend was also perceived even after stratification by pathological stage. Only a few studies mentioned the overall survival of patients with pancreatic cancer complicated by AP, and as a result, the impact of AP on tumor-specific survival has not been sufficiently elucidated. A recent study investigated whether an initial clinical presentation with AP was associated with the survival of patients with PDAC, but the results did not show any survival difference[3]. However, it is still difficult to draw firm conclusions. In their study, patients with mild AP (more than 90% of the patients) were included in the analysis. Because patients with mild AP experience a minor clinical course, their treatment and outcomes differ from those with moderate or severe AP, which might have confounded the results. Patients with mild AP are managed conservatively for a short period , leading to timely resection of their tumors with a relatively good prognosis.
In another study, the median overall survival after surgery was 17.8 mo in the patients who presented with pancreatitis as the initial sign of PDAC compared to 15.7 mo in patients without pancreatitis[19]. In this study, patients with AP and chronic pancreatitis were grouped together, which may have confounded the conclusion.
In contrast, other studies have reported that the prognosis of PDAC patients with AP is poor. Mujicaet reported that the overall survival rates at six months and 12 months in PDAC patients who presented initially with AP were 51% (23 cases) and 28% (13 cases), respectively[16]. A recent study at our medical center reported a survival rate of 23.4%(11 cases) at one year in PDAC patients with AP[17]. Consistent with this report, our previous study found that AP was negatively correlated with both overall survival and early recurrence (within 12 mo). This trend was also perceived even after stratification by pathological stage.
It is unclear why AP is correlated with the prognosis of patients with PDAC. Currently, it is recognized that early diagnosis is a key factor for a better oncological prognosis[20,21]. Although the reasons for the delayed diagnosis of PDAC are unclear in patients presenting with AP, it is suspected that whether the patient was evaluated for an associated malignancy or not, diffuse pancreatic inflammation might have masked the localized mass effect of the underlying cancer[7]. In a recent study, among a total of 76 patients pathologically diagnosed with PDAC within 24 mo after an attack of pancreatitis, 42 patients experienced a delay of 3-24 mo before a diagnosis of PDAC was made[7]. Omid Sadr-Azodi et al[4] also reported that the diagnosis of pre-existing pancreatic cancer was delayed if it clinically presented as AP. Inflammation may aggravate tissue adhesion and edema, that increased the difficulty of operation and postoperative complications (e.g., pancreatic fistula), thus affecting the therapeutic results of surgical treatment of PDAC.
The timing of surgical intervention in patients with PDAC and AP has yet to be defined, but a recent study at our medical center suggested that 24.5 d may be the best cutoff point of surgery for PDAC with mild or moderate AP[17]. In patients who underwent resection, a longer delay before surgery may be associated with poor survival. Erkan et al[14] reported that because of the accompanying mild to moderate AP, the pancreaticoduodenectomy of 4 patients with periampullary tumors was delayed, and the median interval from the diagnosis of AP to the operation was 34 d. Consistent with this report, our previous cohort demonstrated that the average time between the cancer diagnosis and the surgery was 33.4 d[15]. In the present study, timing of surgical intervention in patients with PDAC and moderate or severe AP was 41.4 d, and the average interval time of non-AP group was 10.8 d. Although there was no statistical difference due to our small sample, the incidence of grade B/C pancreatic fistula was obviously higher in AP group. In addition, our previous study suggested that AP was an independent risk factor for pancreatic fistula after pancreaticoduodenectomy[15].
Since PDAC is characterized by rapid growth and aggressive invasive capabilities, a prolonged preoperative waiting time may result in cancer progression or even losing the opportunity for radical resection of PDAC[16]. It has been reported that whenever pancreatic carcinoma is associated with pancreatitis, the surgery is often delayed, resulting in a poorer prognosis. Some scholars have also considered that genetic factors may play a role in contributing to inflammation and oncogenesis and thus affect prognosis[22]. The exact mechanism of PDAC evolution affected by AP is unclear and needs to be further investigated[23].
The potential adverse effects of AP should be taken into consideration in PDAC. Surgeons should work on decreasing the complications of ERCP and strive for timely diagnoses in order to improve the oncological outcomes of PDAC. A new meta-analysis of prospective cohort studies showed that patients with AP had an increased risk of pancreatic cancer compared with a feature-matched group[8]. However, Rijkers et al[24] suggested that chronic pancreatitis that progressed from AP was associated with a higher risk of pancreatic cancer. The incidence of pancreatic cancer in patients with chronic pancreatitis was almost 9 times that in the non-AP group.
Nevertheless, doctors should pay closer attention to active screening for underlying PDAC after AP[4,7,25]. Evaluation of these patients with endoscopic ultrasound for an underlying PDAC could help with timely cancer diagnosis[9]. In Tummala’s study[9], among 218 patients with non-alcoholic and non-gallstone-related AP who underwent endoscopic ultrasound-guided fine-needle aspiration, 38 PDAC patients were diagnosed and their resection rate was 39%. These data remind us that early cancer detection and medical intervention is particularly important for PDAC with AP, as it may be associated with postoperative complications and prognosis[9,16]. Countermeasures to the dissemination of tumor cells due to AP are still unclear. Prompt postoperative chemotherapy represents a possible option.
This study had limitations due to its retrospective nature. Although we used CT or MRI to detect recurrences with diligence, it is conceivable that not all recurrence events were captured timely. Another limitation is that the sample size was small. However, despite the relatively low incidence of moderate or severe AP of PDAC and the low radical resection rate, even a relatively small simple size would be indicative. Hence, further prospective studies are necessary.
In conclusion, moderate or severe AP was significantly related to early recurrence, and patients with moderate or severe AP developed recurrence earlier than those without. Moderate or severe AP was adversely correlated with overall and disease-free survival of patients with PDAC. Surgeons are thus urged to reduce the complications of ERCP and make efforts to diagnose PDAC timely in patients who present with AP in order to improve the oncological outcomes of PDAC.
ARTICLE HIGHLIGHTS
Research background
Excluding common etiologies, the onset of acute pancreatitis (AP) may be due to cancer progression itself or to complications of the diagnostic and therapeutic interventional procedures used in pancreatic ductal adenocarcinoma (PDAC) treatment, such as endoscopic retrograde cholangiopancreatography (ERCP), surgery and chemotherapy. Generally, PDAC patients with AP should initially be managed conservatively. However, surgical resection is the sole curative measure for pancreatic cancer, and conservative treatments can significantly delay the need for the cancer operation.
Research motivation
Many studies have shown that AP has significant negative effects on prognosis and outcome of PDAC. However, only a few studies have evaluated the overall survival of patients with pancreatic cancer and AP, and the impact of AP on tumor-specific survival has not been thoroughly understood.
Research objectives
The main objectives of this study were to investigate the effect of AP on the tumor recurrence pattern of PDAC and tumor-specific survival.
Research methods
The clinical data of 274 patients with PDAC who underwent curative resection at our center from July 2012 to December 2016 were analyzed retrospectively. Patients with mild AP were excluded because they experience a mild clinical course. The remaining 219 cases were subdivided into two groups. Group AP [n = 21 (9.6%)] included those patients with moderate or severe acute pancreatitis and group non-AP [n = 198 (90.4%)] included those without acute pancreatitis. Overall survival was defined as the time from the date of surgery to death or last follow-up. Postoperative tumor recurrence within 12 mo was regarded as early recurrence. The median follow-up time was 18.3 mo. Postoperative recurrence was diagnosed by imaging studies. We obtained postoperative recurrence data for only 186 of 219 patients.
Research results
Moderate or severe AP was an independent risk factor for an early recurrence of PDAC. Moderate or severe AP was an independent prognostic factor for overall survival and disease-free survival in patients with PDAC.
Research conclusions
PDAC patients with moderate or severe AP developed recurrences earlier and had shorter tumor-specific survival than those without.
Research perspectives
We should reduce the incidence of ERCP related to AP of PDAC . When accompanied with AP, PDAC should be timely diagnosed and surgical intervention should be performed in order to improve the oncological outcomes. Further prospective and multi-center studies are needed to verify our conclusions.
Footnotes
Institutional review board statement: The study was reviewed and approved by the Medical Ethics Committee of West China Hospital at Sichuan University.
Informed consent statement: All study participants or their legal guardian provided informed written consent about personal and medical data collection prior to study enrolment.
Conflict-of-interest statement: All authors declare no conflicts of interest related to this article.
Data sharing statement: No additional data are available.
Manuscript source: Unsolicited manuscript
Peer-review started: August 4, 2019
First decision: August 27, 2019
Article in press: September 27, 2019
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Neri V, Sikiric P S-Editor: Wang J L-Editor: Ma JY E-Editor: Ma YJ
Contributor Information
Qian Feng, Department of Pancreatic Surgery, West China Hospital, Sichuan University, Chengdu 610041, Sichuan Province, China.
Cheng Li, Rehabilitation Medicine Center, West China Hospital, Sichuan University, Chengdu 610041, Sichuan Province, China.
Sheng Zhang, Department of Hepatobiliary Surgery, The First Affiliated Hospital of Xinxiang Medical University, Xinxiang 453100, Henan Province, China.
Chun-Lu Tan, Department of Pancreatic Surgery, West China Hospital, Sichuan University, Chengdu 610041, Sichuan Province, China.
Gang Mai, Department of Hepatobiliary Surgery, Deyang People's Hospital, Deyang 618000, Sichuan Province, China.
Xu-Bao Liu, Department of Pancreatic Surgery, West China Hospital, Sichuan University, Chengdu 610041, Sichuan Province, China.
Yong-Hua Chen, Department of Pancreatic Surgery, West China Hospital, Sichuan University, Chengdu 610041, Sichuan Province, China. chenyonghua2007@163.com.
References
- 1.Asari S, Matsumoto I, Ajiki T, Shinzeki M, Goto T, Fukumoto T, Ku Y. Perioperative management for pancreatoduodenectomy following severe acute pancreatitis in patients with periampullary cancer: our experience with six consecutive cases. Surg Today. 2015;45:181–188. doi: 10.1007/s00595-014-0900-x. [DOI] [PubMed] [Google Scholar]
- 2.She WH, Chan AC, Cheung TT, Chok KSh, Chan SC, Poon RT, Lo CM. Acute pancreatitis induced by transarterial chemoembolization: a single-center experience of over 1500 cases. Hepatobiliary Pancreat Dis Int. 2016;15:93–98. doi: 10.1016/s1499-3872(15)60034-0. [DOI] [PubMed] [Google Scholar]
- 3.Zitinic I, Plavsic I, Poropat G, Hauser G. ERCP induced and non-ERCP-induced acute pancreatitis: Two distinct clinical entities? Med Hypotheses. 2018;113:42–44. doi: 10.1016/j.mehy.2018.02.017. [DOI] [PubMed] [Google Scholar]
- 4.Sadr-Azodi O, Oskarsson V, Discacciati A, Videhult P, Askling J, Ekbom A. Pancreatic Cancer Following Acute Pancreatitis: A Population-based Matched Cohort Study. Am J Gastroenterol. 2018;113:1711–1719. doi: 10.1038/s41395-018-0255-9. [DOI] [PubMed] [Google Scholar]
- 5.Minato Y, Kamisawa T, Tabata T, Hara S, Kuruma S, Chiba K, Kuwata G, Fujiwara T, Egashira H, Koizumi K, Saito I, Endo Y, Koizumi S, Fujiwara J, Arakawa T, Momma K, Kurata M, Honda G. Pancreatic cancer causing acute pancreatitis: a comparative study with cancer patients without pancreatitis and pancreatitis patients without cancer. J Hepatobiliary Pancreat Sci. 2013;20:628–633. doi: 10.1007/s00534-013-0598-y. [DOI] [PubMed] [Google Scholar]
- 6.Kimura Y, Kikuyama M, Kodama Y. Acute Pancreatitis as a Possible Indicator of Pancreatic Cancer: The Importance of Mass Detection. Intern Med. 2015;54:2109–2114. doi: 10.2169/internalmedicine.54.4068. [DOI] [PubMed] [Google Scholar]
- 7.Munigala S, Kanwal F, Xian H, Scherrer JF, Agarwal B. Increased risk of pancreatic adenocarcinoma after acute pancreatitis. Clin Gastroenterol Hepatol. 2014;12:1143–1150.e1. doi: 10.1016/j.cgh.2013.12.033. [DOI] [PubMed] [Google Scholar]
- 8.Kirkegård J, Cronin-Fenton D, Heide-Jørgensen U, Mortensen FV. Acute Pancreatitis and Pancreatic Cancer Risk: A Nationwide Matched-Cohort Study in Denmark. Gastroenterology. 2018;154:1729–1736. doi: 10.1053/j.gastro.2018.02.011. [DOI] [PubMed] [Google Scholar]
- 9.Tummala P, Tariq SH, Chibnall JT, Agarwal B. Clinical predictors of pancreatic carcinoma causing acute pancreatitis. Pancreas. 2013;42:108–113. doi: 10.1097/MPA.0b013e318254f473. [DOI] [PubMed] [Google Scholar]
- 10.Zhang X, An R, Tian H, Zhao J. Increased risk of pancreatic cancer after acute pancreatitis: A meta-analysis of prospective cohort studies. Clin Res Hepatol Gastroenterol. 2019;43:e39–e41. doi: 10.1016/j.clinre.2018.09.008. [DOI] [PubMed] [Google Scholar]
- 11.Lai SW, Lin CL, Chang Ou KC, Liao KF. Association between acute pancreatitis and pancreatic cancer. Eur J Gastroenterol Hepatol. 2019;31:896–897. doi: 10.1097/MEG.0000000000001411. [DOI] [PubMed] [Google Scholar]
- 12.Cohen S, Bacon BR, Berlin JA, Fleischer D, Hecht GA, Loehrer PJ Sr, McNair AE Jr, Mulholland M, Norton NJ, Rabeneck L, Ransohoff DF, Sonnenberg A, Vannier MW. National Institutes of Health State-of-the-Science Conference Statement: ERCP for diagnosis and therapy, January 14-16, 2002. Gastrointest Endosc. 2002;56:803–809. doi: 10.1067/mge.2002.129875. [DOI] [PubMed] [Google Scholar]
- 13.Cheng CL, Sherman S, Watkins JL, Barnett J, Freeman M, Geenen J, Ryan M, Parker H, Frakes JT, Fogel EL, Silverman WB, Dua KS, Aliperti G, Yakshe P, Uzer M, Jones W, Goff J, Lazzell-Pannell L, Rashdan A, Temkit M, Lehman GA. Risk factors for post-ERCP pancreatitis: a prospective multicenter study. Am J Gastroenterol. 2006;101:139–147. doi: 10.1111/j.1572-0241.2006.00380.x. [DOI] [PubMed] [Google Scholar]
- 14.Erkan M, Kleeff J, Reiser C, Hinz U, Esposito I, Friess H, Büchler MW. Preoperative acute pancreatitis in periampullary tumors: implications for surgical management. Digestion. 2007;75:165–171. doi: 10.1159/000106799. [DOI] [PubMed] [Google Scholar]
- 15.Chen YH, Xie SM, Zhang H, Tan CL, Ke NW, Mai G, Liu XB. Clinical impact of preoperative acute pancreatitis in patients who undergo pancreaticoduodenectomy for periampullary tumors. World J Gastroenterol. 2015;21:6937–6943. doi: 10.3748/wjg.v21.i22.6937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mujica VR, Barkin JS, Go VL. Acute pancreatitis secondary to pancreatic carcinoma. Study Group Participants. Pancreas. 2000;21:329–332. doi: 10.1097/00006676-200011000-00001. [DOI] [PubMed] [Google Scholar]
- 17.Li S, Tian B. Acute pancreatitis in patients with pancreatic cancer: Timing of surgery and survival duration. Medicine (Baltimore) 2017;96:e5908. doi: 10.1097/MD.0000000000005908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bradley EL., 3rd A clinically based classification system for acute pancreatitis. Summary of the International Symposium on Acute Pancreatitis, Atlanta, Ga, September 11 through 13, 1992. Arch Surg. 1993;128:586–590. doi: 10.1001/archsurg.1993.01420170122019. [DOI] [PubMed] [Google Scholar]
- 19.Thorat A, Huang WH, Yeh TS, Jan YY, Hwang TL. Pancreatic ductal adenocarcinoma presenting with acute and chronic pancreatitis as initial presentation: is prognosis better? A comparison study. Hepatogastroenterology. 2014;61:2110–2116. [PubMed] [Google Scholar]
- 20.Bruenderman EH, Martin RC 2nd. High-risk population in sporadic pancreatic adenocarcinoma: guidelines for screening. J Surg Res. 2015;194:212–219. doi: 10.1016/j.jss.2014.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Driedger MR, Dixon E, Mohamed R, Sutherland FR, Bathe OF, Ball CG. The diagnostic pathway for solid pancreatic neoplasms: are we applying too many tests? J Surg Res. 2015;199:39–43. doi: 10.1016/j.jss.2015.04.026. [DOI] [PubMed] [Google Scholar]
- 22.Shelton CA, Umapathy C, Stello K, Yadav D, Whitcomb DC. Hereditary Pancreatitis in the United States: Survival and Rates of Pancreatic Cancer. Am J Gastroenterol. 2018;113:1376. doi: 10.1038/s41395-018-0194-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Macinga P, Pulkertova A, Bajer L, Maluskova J, Oliverius M, Smejkal M, Heczkova M, Spicak J, Hucl T. Simultaneous occurrence of autoimmune pancreatitis and pancreatic cancer in patients resected for focal pancreatic mass. World J Gastroenterol. 2017;23:2185–2193. doi: 10.3748/wjg.v23.i12.2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rijkers AP, Bakker OJ, Ahmed Ali U, Hagenaars JCJP, van Santvoort HC, Besselink MG, Bollen TL, van Eijck CH Dutch Pancreatitis Study Group. Risk of Pancreatic Cancer After a Primary Episode of Acute Pancreatitis. Pancreas. 2017;46:1018–1022. doi: 10.1097/MPA.0000000000000879. [DOI] [PubMed] [Google Scholar]
- 25.Phillips AE, Shah N, Borhani AA, Yadav D, Brand RE. Prior History of Pancreatitis Accelerates the Development of Pancreatic Adenocarcinoma. Pancreas. 2018;47:1262–1266. doi: 10.1097/MPA.0000000000001160. [DOI] [PMC free article] [PubMed] [Google Scholar]