Abstract
Background:
The protozoan Giardia and Cryptosporidium are responsible for most water-borne diseases all over the world. The extent and number of outbreaks of waterborne diseases suggests a significant risk of their potential transmission via drinking water. This study aimed to document the prevalence and viability of Giardia and Cryptosporidium (oo) cysts in tap water samples in Beni-Suef Governorate, Egypt and to detect the predominant Giardia and Cryptosporidium assemblages/species using nested PCR/ Restriction Fragment Length Polymorphism (RFLP) confirmed by further sequencing of positive samples.
Methods:
A total of 80 tap water samples were collected throughout a year from four big centers and filtered using the membrane filtration method. Samples were stained by Lugol's iodine, Modified Zeihl-Neelsen (MZN) (to detect prevalence) and trypan blue stain (to detect viability). Nested PCR-RFLP and sequencing were used for molecular characterizations and genotyping of the detected Giardia and Cryptosporidium.
Results:
Giardia and Cryptosporidium DNA was detected in 20 (25%) and 29 (36.3%) samples respectively, with predominance of Giardia assemblage B (85%) and C. hominis (75.9%). The prevalence and viability of both parasites (oo) cysts showed seasonality which peaked in summer and were greater in Beba center and in rural areas.
Conclusion:
To our knowledge, no studies have been done in these areas before. The anthroponotic transmission has an important role in giardiasis and cryptosporidiosis epidemiology in this studied area.
Keywords: Cryptosporidium spp., Tap water, Egypt, Giardia
Introduction
Water plays an important role in the transmission of many different pathogenic microorganisms such as bacteria, viruses, fungi, protozoa, and helminthes. Water-borne pathogenic protozoa including Cryptosporidium spp., Cystoisospora belli, Cyclospora cayetanensis, Microsporidia, Giardia lamblia, Entamoeba histolytica and free-living amoebae are responsible for emerging cases of waterborne diseases (1).
Both Giardia and Cryptosporidium are the most common waterborne and foodborne parasites all over the world (2). They are transmitted by sustained anthroponotic and zoonotic cycles including many species and genotypes (3, 4). The robust form ((oo) cyst), is resistant to common disinfectants at the exposure times and the concentrations usually applied in water treatment processes. The infectious doses of both parasites are as low as 10 cysts (3) and 30 oocysts (5) for Giardia and Cryptosporidium, respectively. This problem is potentiated by the wide range of infected hosts shedding large number of infective (oo) cysts causing environmental contamination, particularly in water sources (4).
Outbreaks of water borne diseases showed a great increase not only in number but also in extent (4). These outbreaks are attributed to contamination of water sources by soil or dead animal's thrown into them, agricultural runoffs, snowmelts, biosolids and heavy rainfall (6, 7). Exposure of uncovered water tanks to excreta of infected rodents and birds and inadequate treatment of drinking water may be additional factors (8, 9).
The Cryptosporidium genus is comprised of 30 species and more than 40 genotypes (10). Twelve species were reported to infect mammals, of which C. hominis and C. parvum account for over 90% of human infections (11).
G. lamblia is a parasite of mammals as well as humans. Six species are reported in the Giardia genera according to its morphological characteristics and infected hosts (12). It is generally accepted that G. lamblia is a complex of eight distinct genetic groups (designated A–H). These groups are identical in morphology but differ in genomic mutations (13). Genetic groups A and B which are subdivided into five sub-groups (named AI–III and BIII–BIV), mainly infect humans (14).
In Egypt, waterborne diseases represent a major public health problem. The Nile River, the main source of drinking water in Egypt, is polluted by human activities, reservoir animal hosts, sewage and industrial discharge, and run-off from agricultural fields. The problem is augmented in some rural Egyptian villages which obtain their water supply from unprotected streams and ground water (15).
This problem highlights the need to determine prevailing protozoa species and genotypes contaminating water sources to evaluate the risks on human and animal health, and outline proper control measures. However, some of the protozoa (oo) cysts contaminating water are non-viable and have no threat to the public health. Consequently, there is a great interest in developing in-vitro techniques capable of determining (oo) cyst viability (16).
Materials and Methods
Study design and water samples collection
The present work is a descriptive analytical study conducted in Beni-Suef Governorate, Egypt. A total of 80 tap water samples (10 L/sample) were collected in sterile containers over one year, from April 2016 to April 2017. Samples were collected from four different big centers representing different communities in Beni-Suef Governorate, namely Beni-Suef, Naser, El Wasta and Beba centers. From each center, 20 tap water samples were collected (10 each from urban and rural areas). Date and place were labeled on the containers. Distribution of the collected 80 samples in different seasons was as follows: 17, 21, 12 and 30 samples in spring, summer, autumn and winter seasons, respectively.
Water samples filtration and processing
Water samples were transferred immediately after collection to the laboratory of the Medical Parasitology Department, Faculty of Medicine, Beni-Suef University, stored at 4°C until processed on the same day. Each water sample was filtered using a stainless steel filtration unit with an oil free pump according to the manufacturer's instructions through 47-mm diameter sterile nylon membrane filter with 1 μm pore size. The membrane filter was folded twice lengthwise with the upper surface facing out, soaked in phosphate buffered saline (PBS) in a 15-ml conical centrifuge tubes for two hours and then centrifuged at 6000 rpm for 10 min. (17). The supernatant was decanted. Part of the sediment was examined parasitologically and another part was kept at −20°C for further molecular assays.
Parasitological examination
A drop of the pellet was put on a slide and examined using saline and iodine wet mount smears using 40X objective lens for detection of Giardia cysts (17, 18). Part of the pellet was preserved in 10% buffered formalin solution, stained by MZN and examined by 40X and 100X objectives to detect Cryptosporidium (oo) cysts (9,17).
Trypan Blue vital stain (Euromedex, France) was used for detecting the viability of (oo) cysts in fresh positive water samples following the manufacturer instructions (9, 17, 19).
Molecular assays
Genomic DNA was extracted from fresh frozen pellets using Stool DNA Mini Kit of FavorPrep (Favorgen Biotech corporation ping-Tung, 908 Taiwan) following the instructions of the manufacturer. The concentration and purity of extracted DNA was determined.
Extracted DNA was amplified by nested PCR-RFLP for detection and typing of Cryptosporodium and Giardia. Nested PCR targeting Cryptosporidium oocyst wall protein (COWP) gene was done as reported earlier (20, 21). Another nested PCR targeting Giardia beta (β) giardin gene was performed (22, 23).
Positive Cryptosporidium and Giardia DNA produced by nested PCRs were cleaved by RsaI restrictive enzyme (Fermentas UAB, V. Graiciuno 8, LT-02241 Vilnius, Lithuania) and HaeIII restrictive enzyme (Fermentas UAB, V. Graiciuno 8, and LT-02241 Vilnius, Lithuania), respectively. Digested nested PCR fragments were visualized by 3.2% agarose gels electrophoresis to determine the G. lamblia assemblages and Cryptosporidium genotypes.
Species/assemblage identification of all positive PCR-products was purified by PCR purification kit. Purified PCR products were bidirectionally sequenced using the big Dye Terminator version 3.1 cycle sequencing kit (Applied Biosystems) and the nested PCR primers for each parasite on an ABI 310 sequencer (Applied Biosystems) according to the manufacturer's instructions. The obtained sequences were compared to the GenBank reference sequences using nucleotide BLAST search at NCBI website (http://www.ncbi.nlm.nih.gov) to determine the Cryptosporidium and Giardia species/assemblages. Sequencing of PCR products and phylogenetic analysis were aligned by the BioEdit alignment program (24)
Statistical analysis
Results were displayed in tables and analyzed statistically using SPSS-23 (IBM, Somers, NY, USA) software. Descriptive data were expressed as numbers and percentages. Differences in discrete variables were compared and assessed for significance by Chi square-test. Diagnostic yield (accuracy, sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV) and kappa agreement) of conventional microscopy and staining results was measured compared to nested PCR results as a reference standard. The final model included all variables with significant P at <0.05.
Ethical consideration
The protocol of this study was approved by Beni-Suef University, Scientific Research Development Unit, Projects Funding and Granting Unit. This article does not contain any studies with human or animal subjects. Individuals in contaminated areas were informed about the obtained results of the research for subsequent precautions.
Results
Out of the 80 collected tap water samples, Giardia cysts were detected in 6 (7.5%) samples by lugol's iodine while Cryptosporidium oo-cysts were detected only by MZN stain in 13 (16.3%) samples. According to nested PCR results, Cryptosporidium and Giardia (oo) cysts were identified in 29 (36.3%) and 20 (25%) samples, respectively with statistical significance (P <0.001) (Table 1). Eleven samples (13.7%) had mixed infection of both parasites by nested PCR. Diagnostic yields of microscopic examination of water samples by iodine and MZN in relation to nested PCR are presented in (Table 2).
Table 1:
Giardia and Cryptosporidium (oo) cysts detection in examined water samples by microscopy (lugol's iodine and MZN stain) and nested PCR
| Diagnostic technique | Nested PCR | P | ||||
|---|---|---|---|---|---|---|
| Positive n. (%) | Negative n. (%) | Total n. (%) | ||||
| Lugol's Iodine | Giardia cysts | Positive | 6 (7.5) | 0 (0.0) | 6 (7.5) | <0.001 * |
| Negative | 14 (17.5) | 60 (75) | 74 (92.5) | |||
| Total | 20 (25) | 60 (75) | 80 (100) | |||
| MZN | Cryptosporidium oocysts | Positive | 13 (16.3) | 0 (6.2) | 13 (16.3) | <0.001 * |
| Negative | 16 (20) | 51 (63.8) | 67 (83.8) | |||
| Total | 29 (36.3) | 51 (63.8) | 80 (100) | |||
Significant (P < 0.05)
Table 2:
Diagnostic yield of iodine and MZN stained smears to detect Giardia and Cryptosporidium (oo) cysts in water samples considering nested PCR as a reference test
| Variable | Iodine stained smears Giardia cyst (%) | MZN stain Cryptosporidium oocyst (%) |
|---|---|---|
| Sensitivity | 30 | 44.8 |
| Specificity | 100 | 100 |
| PPV | 100 | 100 |
| NPV | 81.1 | 76.1 |
| Accuracy | 82. 5 | 80 |
RFLP results revealed the predominance of Giardia assemblage B (85%) and Cryptosporidium hominis (75.9%) with high statistical significance (P<0.001) (Table 3).
Table 3:
Giardia assemblages and Cryptosporidium species identified in tested water samples using nested PCR-RFLP
| Protozoa | Nested PCR-RFLP n. (%) | P | |
|---|---|---|---|
| Giardia assemblages | Assemblage A | 3 (15) | <0.001 * |
| Assemblage B | 17 (85) | ||
| Total | 20 (100) | ||
| Cryptosporidium genotypes | C. hominis | 22 (75.9) | <0.001 * |
| C. parvum | 6 (20.7) | ||
| C. hominis + C. parvum | 1 (3.4) | ||
| Total | 29 (100) |
Significant (P < 0.05)
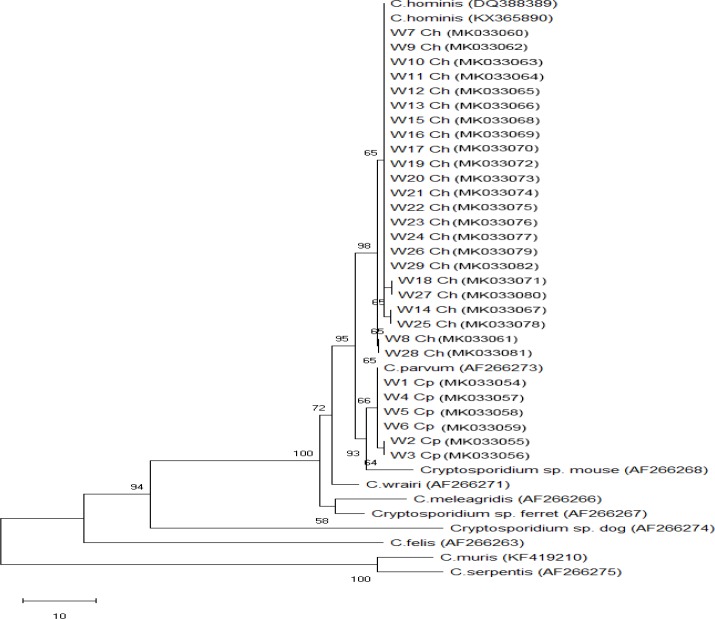
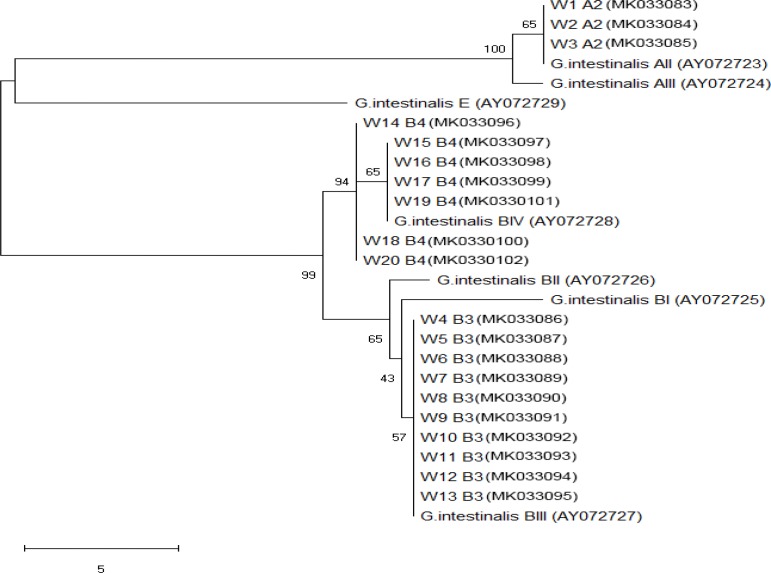
Phylogenetic analyses of the SSU rDNA showed that analysis of Giardia assemblages revealed that all three assemblage A samples by nested PCR were of AII, while 17 assemblage B samples were of BIII (10 samples.) and BIV (7 samples) representing 58.8% and 41.2%, respectively. Subgenotyping of Cryptosporidium species confirmed detection of C. parvum and C. hominis. All species were matched with the same cluster of Giardia/Cryptosporidium subtypes in the NCBI database with no genetic variability. Cryptosporidium/Giardia isolates sequence data were placed in GenBank with accession numbers (MK033054-MK033102) (Figs. 1, 2).
Fig. 1:
Phylogenetic tree of COWP sequences of Cryptosporidium from water samples and reference sequences from the GenBank. Neighborjoining tree showing the evolutionary history of Cryptosporidium isolates, inferred by distance-based analysis of Cryptosporidium COWP gene sequence. Bootstrap value is 500 with the sum of the branch length =0.1. The monophyletic clades of Cryptosporidium parvum (samples W1–W6) and Cryptosporidium hominis (samples W7–W29) were supported by high bootstrap values
Fig. 2:
Phylogenetic tree of Beta giardin sequences of Giardia intestinalis from water samples and reference sequences from the GenBank. Neighborjoining tree showing the evolutionary history of Giardia isolates, inferred by distance-based analysis of Giardia intestinalis Beta giardin gene sequence. Bootstrap value is 500 with the sum of the branch length =0.1. The monophyletic clades of Giardia intestinalis AII (samples W1–W3), Giardia intestinalis BIII (samples W4–W13) and Giardia intestinalis BIV (samples W14–W20) were supported by high bootstrap values
Beba center had the highest positive rate of Giardia and Cryptosporidium water contamination (40% and 37.9% respectively), while El Wasta center had the lowest rate (15% and 13.8 %) without statistical significance. Water contamination was higher in rural areas (75% for Giardia and 58.6% for Cryptosporidium) than urban areas. These data showed statistical significance for Giardia only (P =0.009) (Table 4).
Table 4:
Distribution of Giardia and Cryptosporidium using nPCR according to geographic area and represented communities
| Variables | Giardia (nested PCR) | P | Cryptosporidium (nested PCR) | P | ||
|---|---|---|---|---|---|---|
| Positive n. (%) | Negative n. (%) | Positive n. (%) | Negative n. (%) | |||
| Geographic area (20 samples/center) | ||||||
| Beni-Suef | 4 (20) | 16 (26.7) | 0.2 | 7 (24.1) | 13 (25.5) | 0.1 |
| El Wasta | 3 (15) | 17 (28.3) | 4 (13.8) | 16 (31.4) | ||
| Nasser | 5 (25) | 15 (25) | 7 (24.1) | 13 (25.5) | ||
| Beba | 8 (40) | 12 (20) | 11(37.9) | 9 (17.6) | ||
| Representing community (40/point) | ||||||
| Urban | 5 (25) | 35 (58.3) | 0.009* | 12 (41.4) | 28 (54.9) | 0.1 |
| Rural | 15 (75) | 25 (41.7) | 17 (58.6) | 23 (45.1) | ||
| Total | 20 (100) | 60 (100) | 29 (100) | 51 (100) | ||
Significant (P < 0.05)
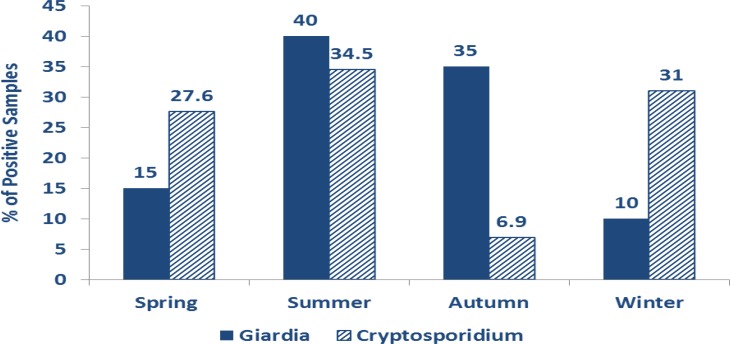
Both parasites were detected in all seasons with a seasonal fluctuation and peak in summer (40% for Giardia and 34.5% for Cryptosporidium). The lowest rate of Giardia cysts was in winter and the lowest rate of Cryptosporidium oocysts was in the autumn season with statistical significance regarding Giardia only (Fig. 3).
Fig. 3:
Seasonal distribution of positive Giardia cysts (P =0.002) and Cryptosporidium oocyst (P =0.2)
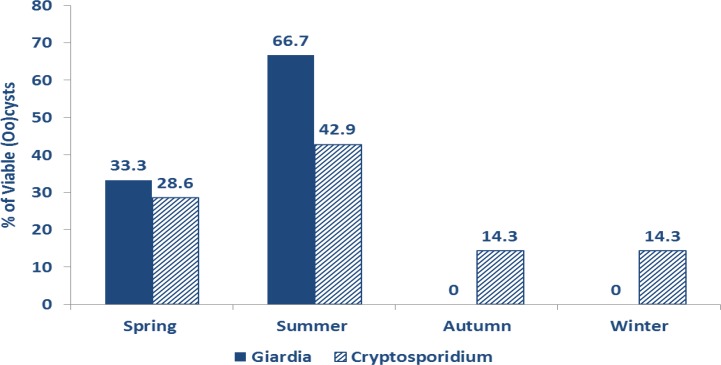
Cryptosporidium and Giardia (oo) cysts were viable in 24.1% and 15% respectively, without statistical significance between sampled areas in different centers. Viability was higher in rural areas and in summer season with statistical-significance with Giardia only (Table 5 and Fig. 4).
Table 5:
Detection of viability of Giardia cysts and Cryptosporidium oocysts using trypan blue stain according to different geographic areas and represented communities
| Variables | Giardia using nPCR (n.=20) | P | Cryptosporidium using nPCR (n.=29) | P | ||
|---|---|---|---|---|---|---|
| Viable No. (%) | Non-viable No. (%) | Viable No. (%) | Non-viable No. (%) | |||
| Geographic area (20 samples/center) | ||||||
| Beni-Suef | 0 (0) | 4 (23.5) | 0.5 | 1 (14.3) | 6 (27.3) | 0.4 |
| El Wasta | 1 (33.3) | 2 (11.8) | 1 (14.3) | 3 (13.6) | ||
| Nasser | 1 (33.3) | 4 (23.5) | 2 (28.6) | 5 (22.7) | ||
| Beba | 1(33.3) | 7 (41.2) | 3 (42.9) | 8 (36.4) | ||
| Representing community (40/point) | ||||||
| Urban | 1 (33.3) | 4 (23.5) | 0.03* | 3 (42.9) | 9 (40.9) | 0.5 |
| Rural | 2 (66.7) | 13 (76.5) | 4 (57.1) | 13 (59.1) | ||
| Total | 3 (15) | 17 (85) | 7 (24.1) | 22 (75.9) | ||
Significant (P < 0.05)
Fig. 4:
Seasonal distribution of viable Giardia cysts (P =0.004) and Cryptosporidium oocyst (P =0.4)
Discussion
Water is considered the main environmental route for transmitting Giardia cysts and Cryptosporidium oocysts (25). In Egypt, surface water is the main drinking water source with an absence of mandatory programs for monitoring pathogenic protozoan parasites in water.
Prevalence of Giardia and Cryptosporidium showed varied results in Egypt. In Alexandria Governorate, Giardia and Cryptosporidium (oo) cysts were identified in 36.7% and 100%, respectively in tank water samples (9). In Giza Governorate, Giardia cysts were detected in 50% and 33% of tap water in Abo-El Nomros and El Hawamdia, respectively (26). In Assuit Governorate, Cryptosporidium oocysts were detected in 50% of drinking water samples (27) and 79% of drinking water supply of Assiut university hospitals (28). Recent study at Fayoum Governorate had detected 52.6%, 13.7% of Cryptosporidium spp. and G. lamblia, respectively in tap water and storage water tanks (18). In El-Minia Governorate, Giardia and Cryptosporidium were detected in 0% and 12.5%, respectively in tap water (29). In Gharbiya Governorate, Giardia and Cryptosporidium were detected in 13% and 7.4%, respectively in tap water samples (17).
Similar results were reported worldwide. Cryptosporidium was detected in 51% and 25%, while G. lamblia was detected in 0.62% and 2.4% of tap water samples in Jeddah and Makkah, respectively (30). On the other hand, in Iran Feiz Hadad et al. detected 0% of both parasites in filter system household tap water samples (31).
In Spain, both parasites were detected in 26.8% of examined water samples (32). In the UK Nichols et al. detected Cryptosporidium in 100% of drinking water samples using PCR (33). Hashimoto et al. found Giardia and Cryptosporidium in 12% and 35%, respectively of filtered water samples from a water plant in Japan (34). Lower detection rates were reported in the north of Portugal, Giardia was detected in 8.4% and Cryptosporidium was detected in 10.2% in drinking water samples (25).
In our study, there was seasonality of prevalence and viability of both Giardia and Cryptosporidium (oo) cysts which were higher in summer season. Seasonality of Cryptosporidium and Giardia prevalence in water had been recorded worldwide (35) and in Egypt (17, 28, 29) and was confirmed by seasonality of human infection (36, 37).
Peaking of Cryptosporidium and Giardia (oo) cysts prevalence in water in summer may be attributed to warm temperatures, humidity and stagnation of water that could increase incidence of parasites, prolong the infective period and the transmission of (oo) cysts, and promote more cyst contact with populations (38). Possibly, the key determinant of distinct seasonality is the increase of human outdoor activity during the summer season, which fosters more transmission of Cryptosporidium/Giardia (oo) cysts (39).
Our study showed seasonal variation in viability of detected Giardia cysts (15%) and Cryptosporidium oocysts (24.1%). This obtained viability may be attributed to their ability to persist in the environment and resist the conventional disinfection process and chlorination practices generally applied in drinking water treatment (32, 15).
The fact that flow cytometry accurately estimates the viability and parasite load in water samples with more sensitivity than trypan blue stain (9,17), may mean that the obtained viability percentage in our study is perhaps lower than what we expected.
Predominance of anthroponotic Cryptosporidium species (C. hominis) and Giardia assemblage B was reported in both urban and rural areas which suggest that human activities with person-to-person transmission rather than zoonotic transmission are the main source of water contamination.
Even in humans, there are nearly 12 species/genotype of Cryptosporidium have been reported, though C. hominis, C. parvum, C. ubiquitum, and C. meleagridis are the most common causative agents (11). In the case of giardiasis, although A and B are the main etiologies for human infections (14), it is worth noting that humans infections by assemblages C, D, E, and F have been sporadically reported particularly in immunocompromised patients and children (40).
In the present study, microscopy of MZN stained smear improved Cryposporidium oocyst detection by 13.6%. Although it stills a method of limited sensitivity (44.8 %) compared to nested PCR results, it was used in our study due to its safety, accuracy and simplicity than other stains for identifying Cryptosporidium species in water samples as confirmed by previous studies (9,17,28, 41).
In the present study, Giardia and Cryptosporidium (oo) cysts were detected at higher prevalence rate in rural areas than urban area. This may be attributed to the fact that rural populations in Egypt obtain their water supply from unprotected streams and ground water. In addition, there is an increase in water contamination due to the lack of proper infrastructure and inefficient water treatment procedures in rural areas (42). To our knowledge no studies have been done in these areas before.
Conclusion
Detection of the seasonal prevalence of parasites in drinking water system aids to establish efficient control measures that should be applied in high-risk seasons to reduce the rate of infection. The obtained results highlighted the compromised water sanitation in Beni-Suef Governorate, Egypt and the need for proper control measures and effective water sanitation programs. The predominance of Cryptosporidium and Giardia species/assemblages emphasizes that anthroponotic transmission has an important role in cryptosporidiosis and giardiasis epidemiology in this studied area.
Acknowledgements
This study was financially supported by a grant from Beni-Suef University, Scientific Research Development Unit, Projects Funding and Granting unit.
Footnotes
Conflict of interest
The authors declare that they have no conflict of interest to disclose.
References
- 1.Mons C, Dumètre A, Gosselin S, et al. Monitoring of Cryptosporidium and Giardia river contamination in Paris area. Water Res. 2009;43(1):211–7. [DOI] [PubMed] [Google Scholar]
- 2.Baldursson S, Karanis P. Waterborne transmission of protozoan parasites: review of worldwide outbreaks - an update 2004–2010. Water Res. 2011;45(20):6603–14. [DOI] [PubMed] [Google Scholar]
- 3.Adam RD. Biology of Giardia lamblia. Clin Microbiol Rev. 2001;14(3):447–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fayer R. Cryptosporidium a water-borne zoonotic parasite. Vet Parasitol. 2004;126(1–2):37–56. [DOI] [PubMed] [Google Scholar]
- 5.DuPont HL, Chappell CL, Sterling CR, et al. The infectivity of Cryptosporidium parvum in healthy volunteers. N Engl J Med. 1995;332(13):855–9. [DOI] [PubMed] [Google Scholar]
- 6.Mac Kenzie WR, Hoxie NJ, Proctor ME, et al. A massive outbreak in Milwaukee of Cryptosporidium infection transmitted through the Public Water Supply. N Engl J Med. 1994;331(3):161–7. [DOI] [PubMed] [Google Scholar]
- 7.Hunter PR, Thompson RC. The zoonotic transmission of Giardia and Cryptosporidium. Int J Parasitol. 2005;35(11–12):1181–90. [DOI] [PubMed] [Google Scholar]
- 8.Karanis P, Kourenti C, Smith H. Waterborne outbreaks of protozoan parasites: A worldwide review of outbreaks and lessons learnt. J Water Health. 2007;5(1):1–38. [DOI] [PubMed] [Google Scholar]
- 9.Khalifa AM, Ibrahim IR, Said DE, Aleem EA, Nabil RA. Cryptosporidium and Giardia in Water in Alexandria: Detection and Evaluation of Viability by Flow Cytometry and Different Stains. PUJ. 2011; 4(2): 155–164. [Google Scholar]
- 10.Ryan U, Hijjawi N. New developments in Cryptosporidium research. Int J Parasitol. 2015;45(6):367–73. [DOI] [PubMed] [Google Scholar]
- 11.Rossle N, Latif B. Cryptosporidiosis as threatening health problem: a review. Asian Pac J Trop Biomed. 2013; 3: 916–924. [Google Scholar]
- 12.Thompson RCA, Monis PT. Taxonomy of Giardia species. Luján HD, Svard S, eds. Giardia: a model organism. Springer; 2011; 3–15. [Google Scholar]
- 13.Durigan M, Abreu AG, Zucchi MI, et al. Genetic diversity of Giardia duodenalis: Multilocus genotyping reveals zoonotic potential between clinical and environmental sources in a metropolitan region of Brazil. PLoS One. 2014; 9(12):e115489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feng Y, Xiao L. Zoonotic potential and molecular epidemiology of Giardia species and giardiasis. Clin Microbiol Rev. 2011; 24: 110–140. Dechesne M, Soyeux EJ. Assessment of source water pathogen contamination. Clin Microbiol Rev. 2011;24(1): 110–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Elshazly AM, Elsheikha HM, Soltan DM, et al. Protozoal pollution of surface water sources in Dakahlia Governorate. J Egypt Soc Parasitol. 2007;37(1):51–64. [PubMed] [Google Scholar]
- 16.Dechesne M, Soyeux E. Assessment of source water pathogen contamination. J Water Health. 2007;5 Suppl 1:39–50. [DOI] [PubMed] [Google Scholar]
- 17.El-Kowrany SI, El-Zamarany EA, El-Nouby KA, et al. Water pollution in the Middle Nile Delta, Egypt: An environmental study. J Adv Res. 2016; 7 (5):781–794. [Google Scholar]
- 18.Sakran TF, El-Shahawy GA, Shalaby MA, Sabry HY, Matooq PM, Elmallah AM. Detection rates of waterborne protozoa in water sources from Fayoum Governorate. PUJ, 2017; 10 (1): 30–38. [Google Scholar]
- 19.Giacometti A, Cirioni O, Barchiesi F, et al. Activity of nitazoxanide alone and in combination with azithromycin and rifabutin against Cryptosporidium parvum in cell culture. J Antimicrob Chemother. 2000;45(4):453–6. [DOI] [PubMed] [Google Scholar]
- 20.Spano F, Putignani L, McLauchlin J, et al. PCR-RFLP analysis of the Cryptosporidium oocyst wall protein (COWP) gene discriminates between C. wrairi and C. parvum, and between C. parvum isolates of human and animal origin. FEMS Microbiol Lett. 1997;150(2):209–17. [DOI] [PubMed] [Google Scholar]
- 21.Pedraza-Díaz S, Amar C, Nichols GL, McLauchlin J. Nested polymerase chain reaction for amplification of the Cryptosporidium oo-cyst wall protein gene. Emerg Infect Dis. 2001;7(1):49–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cacciò SM, De Giacomo M, Pozio E. Sequence analysis of the β-giardin gene and development of a PCR-RFLP assay to genotype Giardia duodenalis cysts from human faecal samples. Int J Parasitol. 2002;32(8):1023–30. [DOI] [PubMed] [Google Scholar]
- 23.Lalle M, Pozio E, Capelli G, et al. Genetic heterogeneity at the b-giardin locus among human and animal isolates of Giardia duodenalis and identification of potentially zoonotic subgenotypes. Int J Parasitol. 2005;35(2):207–13. [DOI] [PubMed] [Google Scholar]
- 24.Hall TA. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucl. Acids Symp Se. 1999; 41:95–98. [Google Scholar]
- 25.Almeida A, Moreira MJ, Soares S, et al. Presence of Cryptosporidium spp. and Giardia duodenalis in Drinking Water Samples in the North of Portugal. Korean J Parasitol. 2010;48(1):43–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ali MA, Al-Herrawy AZ, El-Hawaary SE. Detection of enteric viruses, Giardia and Cryptosporidium in two different types of drinking water treatment facilities. Water Res. 2004; 38(18):3931–9. [DOI] [PubMed] [Google Scholar]
- 27.Khalifa AM, Yacout MA, Sadek AA. Genetical and electron microscopical studies on Cryptosporidia. J Egypt Soc Parasitol. 2001;31(3):799–814. [PubMed] [Google Scholar]
- 28.Sayed FG, Hamza AI, Galal LA, Sayed DM, Gaber M. Detection of Cryptosporidium parvum oocysts contaminating hospitals drinking water supply using different techniques during winter/summer season. Glo Adv Res J Microbiol. 2016; 5(6):068–079. [Google Scholar]
- 29.Khalifa RM, Ahmad AK, Abdel-Hafeez EH, Mosllem FA. Present Status Of Protozoan Pathogens Causing Water Borne Disease In Northen Part Of El-Minia Governorate, Egypt. J Egypt Soc Parasitol. 2014;44(3):559–66. [DOI] [PubMed] [Google Scholar]
- 30.Zakai HA, Barnawi HI. Prevalence of Cryptosporidium and Giardia lamblia in Water Samples from Jeddah and Makkah Cities. J. Adv. Lab. Res. Biol. 2014; V (I): 12–17. [Google Scholar]
- 31.Feiz Hadad MH, Karamkhani A, Haddad R F. Waterborne Parasites: A Recent Status of Occurrence, Source and Human Intestinal Parasites in Sources and Tap Water; Dehloran, South West, Iran. Allergy Drugs Clin Immunol, 2016; 1(1): 18–21. [Google Scholar]
- 32.Carmena D, Aguinagalde X, Zigorraga C, Fernandez-Crespo JC, Ocio JA. Presence of Giardia cysts and Cryptosporidium oocysts in drinking water supplies in northern Spain. J Appl Microbiol. 2007; 102 619–629. [DOI] [PubMed] [Google Scholar]
- 33.Nichols RA, Campbell BM, Smith HV. Identification of Cryptosporidium spp. oocysts in United Kingdom noncarbonated natural mineral waters and drinking waters by using a modified nested PCR-restriction fragment length polymorphism assay. Appl Environ Microbiol. 2003;69(7):4183–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hashimoto A, Kunikane S, Hirata T. Prevalence of Cryptosporidium oocysts and Giardia cysts in the drinking water supply in Japan. Water Res. 2002;36(3):519–26. [DOI] [PubMed] [Google Scholar]
- 35.Koompapong K, Sukthana Y. Seasonal variation and potential sources of Cryptosporidium contamination in surface waters of Chao Phraya River and Bang Pu Nature Reserve Pier, Thailand. Southeast Asian J Trop Med Public Health. 2012;43(4):832–40. [PubMed] [Google Scholar]
- 36.El-Badry AA, Al-Antably AS, Hassan MA, et al. Molecular seasonal, age and gender distributions of Cryptosporidium in diarrhoeic Egyptians: distinct endemicity. Eur J Clin Microbiol Infect Dis. 2015;34(12):2447–53. [DOI] [PubMed] [Google Scholar]
- 37.Ismail MA, El-Akkad DM, Rizk EM, et al. Molecular seasonality of Giardia lamblia in a cohort of Egyptian children: a circannual pattern. Parasitol Res. 2016;115(11):4221–4227. [DOI] [PubMed] [Google Scholar]
- 38.Lal A, Baker MG, Hales S, French NP. Potential effects of global environmental changes on cryptosporidiosis and giardiasis transmission. Trends Parasitol. 2013;29(2):83–90. [DOI] [PubMed] [Google Scholar]
- 39.Cama VA, Bern C, Roberts J, et al. Cryptosporidium species and subtypes and clinical manifestations in children, Peru. Emerg Infect Dis. 2008;14(10):1567–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ryan U, Cacciò SM. Zoonotic potential of Giardia. Int J Parasitol. 2013;43(12–13):943–56. [DOI] [PubMed] [Google Scholar]
- 41.Mossallam SF. Detection of some intestinal protozoa in commercial fresh juices. J Egypt Soc Parasitol. 2010;40(1):135–49. [PubMed] [Google Scholar]
- 42.El-Sherbini GT, Abosdera MM. Risk factors associated with intestinal parasitic infections among children. J Egypt Soc Parasitol. 2013;43(1):287–94. [DOI] [PubMed] [Google Scholar]