Abstract
A 69-year-old Japanese woman was transferred to our hospital due to pancytopenia with a fever. She had Murphy's sign, and computed tomography showed pleural effusion and a swollen gallbladder without gallstones. We diagnosed her with systemic lupus erythematosus (SLE)-associated acute acalculous cholecystitis (AAC). Partly because her clinical and laboratory findings were not serious enough to warrant immediate surgical intervention, and partly because her poor general condition made her ineligible for surgery, surgical therapy was not selected. Corticosteroid therapy was performed with azathioprine, and the swelling in her gallbladder improved. As a conservative therapy for SLE-associated AAC, corticosteroid therapy combined with azathioprine might be beneficial.
Keywords: acute acalculous cholecystitis, systemic lupus erythematosus, corticosteroid, azathioprine
Introduction
Acute acalculous cholecystitis (AAC) is an acute inflammatory disease of the gallbladder - without gallstones - that occurs mainly in individuals with trauma, after surgery, or in other critically ill states (1). The clinical and laboratory findings of AAC are nonspecific and include abdominal pain (right upper-quadrant pain, epigastric pain, or diffuse pain), nausea, vomiting, a fever, jaundice, septic shock, leukocytosis, and the elevation of lactate dehydrogenase (LDH), bilirubin, and/or liver enzymes (i.e., aspartate aminotransferase or alkaline phosphatase) (2-4). AAC accounts for approximately 2-14% of all cases of acute cholecystitis (3-7). AAC occurs more often in men than women, with the male-to-female ratio of approximately 2-3:1 (4,6-8). The average age of patients with AAC is approximately 52-66 years old (3,4,6,8). The incidence of some critical complications is high: gangrene, 55-60%; empyema, 18%; and perforation, 7-13%. The mortality rate of AAC is approximately 6-47% (4,6-8).
Many possible precipitating factors have been reported for AAC (Table 1) (2,5-24). The etiology of AAC is not clear, but some pathogeneses have been suggested, such as ischemia, bile stasis, and abnormal epithelial permeability (25,26). As mentioned above, the clinical and laboratory findings in AAC are nonspecific, and most patients with AAC are critically ill. The diagnosis is therefore difficult. For the diagnosis of AAC, imaging techniques are mainly used, and some AAC criteria have been proposed for ultrasonography, computed tomography (CT), and hepatobiliary iminodiacetic acid (HIDA) scans (1,27). Regarding treatment, there are three prevailing treatment options: open cholecystectomy, laparoscopic cholecystectomy, and percutaneous cholecystostomy (1,28).
Table 1.
Possible Precipitating Factors for Acute Acalculous Cholecystitis.
| Physical damage | Disorders of metabolism and nutrition | |
| Surgical operations (particularly those not related to the biliary tract) (2, 5) | Diabetes mellitus (6, 7, 9) | |
| Trauma (7) | Hyperlipidemia (7) | |
| Burns (7) | Total parenteral nutrition (7, 8, 9) | |
| Disorders of the cardiovascular system and hemodynamics | Prolonged fasting (8) | |
| Hypertension (7, 6) | Obesity (5) | |
| Arteriosclerosis (5) | Infectious diseases | |
| Atrial fibrillation (7) | Sepsis (8) | |
| Congestive heart failure (7) | Bacterial infections | |
| Hypertrophic cardiomyopathy (7) | Hemolytic Streptococcus (5) | |
| Dilated cardiomyopathy (7) | Staphylococcus aureus (17) | |
| Phlebitis (5) | Salmonella enterica (5) | |
| Shock (7, 8) | Vibrio cholerae (18) | |
| Mesenteric ischemia (2) | Mycobacterium tuberculosis (19) | |
| Variceal hemorrhage (2) | Viral infections | |
| Cerebrovascular accident (2) | Human cytomegalovirus (20) | |
| Disorders of the gallbladder | Epstein-Barr virus (21) | |
| Gallbladder distension (9) | Fungal infections | |
| Gallbladder sludge (9) | Candida (22) | |
| Disorders of the immune system | Parasitic infections | |
| Systemic lupus erythematosus (10) | Cryptosporidium (20) | |
| Eosinophilic granulomatosis with polyangiitis (11) | Microsporidia (20) | |
| Polyarteritis nodosa (12) | Plasmodium falciparum (23) | |
| Cryoglobulinemic vasculitis (13) | Giardia lamblia (24) | |
| Antiphospholipid syndrome (14) | Clonorchis sinensis (6) | |
| Pemphigus vulgaris (2) | Others | |
| IgA vasculitis (15) | Progressive multiple organ failure (9) | |
| Macrophage activation syndrome (16) | Intravenous narcotics (8) | |
| Ethanol withdrawal (2) | ||
| Postpartum instances (5) |
Systemic lupus erythematosus (SLE) is a complex and heterogeneous autoimmune disease characterized by systemic clinical manifestations (29). SLE is a potential precipitating factor of AAC (10). In many reports, vasculitis is regarded as a possible pathogenesis of SLE-associated AAC (10,30,31). Serositis and mesenteric inflammatory veno-occlusive disease (MIVOD) are also reported as possible pathogeneses of AAC (32,33). Corticosteroids have been successfully used in some cases as a conservative therapy for SLE-associated AAC (34-38). However, only a few reports have examined effective drugs for use in combination with corticosteroids.
We herein report a patient with AAC and SLE that was successfully treated by corticosteroid combined with azathioprine. Written informed consent for the publication of the case was obtained from the patient by the attending physicians.
Case Report
A 69-year-old Japanese woman was taken to the emergency department of a local hospital because she had developed movement difficulty, loss of appetite, and consciousness disturbance. She had a 28-year history of rheumatoid arthritis and had been treated with prednisolone 2 mg/day and mizoribine 150 mg/day. After hospitalization, pancytopenia progressed gradually. Steroid pulse therapy was conducted with 1,000 mg methylprednisolone, and she was transferred to the Division of Hematology in our hospital for further examinations and treatment.
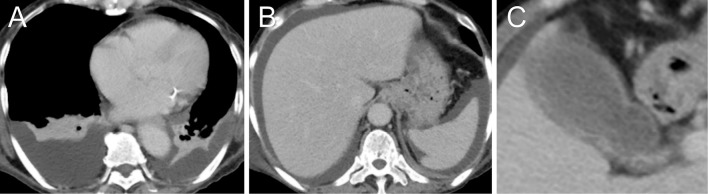
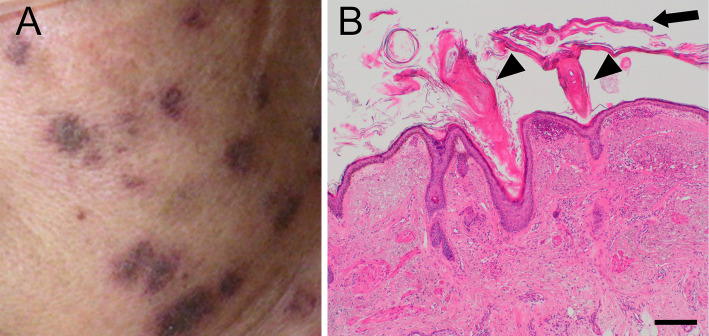
After transfer to our hospital, her temperature rose to 37.5℃. Her blood was drawn for culture, but the result was negative. Although there was no strong evidence of infection, antibiotic therapy was started with ceftriaxone 2 g/day because she also had pancytopenia. Laboratory tests showed a high anti-nuclear antibody titer (homogenous, ≥1:1,280) and low complements levels (CH50 11 CH50/mL, C3 26 mg/dL, C4 6 mg/dL). Contrast-enhanced CT showed pleural effusion, ascites, swelling gallbladder, and gross thickening of the gallbladder wall without gallstones (Fig. 1). Across the patient's left cheek from her forehead, there was a notable area of brownish round rash approximately 5 mm in diameter (Fig. 2A). The rash was compatible with that of discoid lupus erythematosus or subacute cutaneous lupus erythematosus. Based on these findings, SLE was suspected, and she was transferred to our hospital's Section of Internal Medicine and Rheumatology.
Figure 1.
A, B: Contrast-enhanced CT showed pleural effusion and ascites. C: The gallbladder was swollen, and gross thickening of the wall was observed. In the lumen, however, there were no gallstones.
Figure 2.
A: Some of the patient’s brownish round rash, which was present over the left cheek from the forehead (approximately 5 mm in diameter). B: Rash tissue, stained with Hematoxylin and Eosin staining. Hyperkeratosis (arrow) and keratotic plugs (arrowheads) were observed. Scale bar=200 μm.
On the first day after being transferred to our section, the patient's vital signs were as follows: respiratory rate 23 breaths/min, blood oxygen saturation by pulse oximetry 98% (nasal cannula, 1 L/min), pulse rate 90/min, blood pressure 90/48 mmHg, and body temperature 37.0℃. The physical examination revealed many spots of brownish round rash, as mentioned above, along with small painless oral ulcers on the left side of the patient's cheek, Murphy's sign, and pretibial pitting edema. Serum laboratory tests showed pancytopenia, hypoalbuminemia, elevation of alkaline phosphatase, lactate dehydrogenase, and C-reactive protein, hypocomplementemia, anti-ds-DNA IgG antibody, anti-RNP antibody, and anti-Sm antibody (Table 2). Urinary laboratory tests showed almost normal findings (Table 2).
Table 2.
Laboratory Data.
| Complete blood count | Immunology | |||||||
| White blood cell counts | 2,390 | /µL | C-reactive protein | 4.53 | mg/dL | |||
| Metamyelocytes | 1.0 | % | Serum amyloid A protein | 95.9 | ng/mL | |||
| Stab cells | 19.0 | % | CH50 | 11 | CH50/mL | |||
| Segmented cells | 74.0 | % | C3 | 26 | mg/dL | |||
| Lymphocytes | 4.0 | % | C4 | 6 | mg/dL | |||
| Monocytes | 1.0 | % | Brain natriuretic peptide | 85.3 | pg/mL | |||
| Atypical lymphocytes | 1.0 | % | Anti-ds-DNA IgG antibody | 23 | IU/mL | |||
| Hemoglobin | 8.3 | g/dL | Immune complex (C1q) | ≤1.5 | µg/mL | |||
| Mean corpuscular volume | 82.4 | fL | Anti-RNP antibody (CLEIA) | 169 | IU/mL | |||
| Mean corpuscular hemoglobin | 28.6 | pg | Anti-Smith antibody (CLEIA) | 10.0 | IU/mL | |||
| Mean corpuscular hemoglobin concentration | 34.7 | g/dL | Lupus anticoagulant (DRVVT) | 1.22 | ||||
| Platelets | 40,000 | /µL | Anti-CL-β2 GPI antibody | ≤1.2 | IU/mL | |||
| Blood chemistry | Anti-cardiolipin IgG antibody | ≤8 | IU/mL | |||||
| Total protein | 5.0 | g/dL | Anti-SS-A/Ro antibody (CLEIA) | 1.8 | IU/mL | |||
| Albumin | 1.4 | g/dL | Urinary | |||||
| Total bilirubin | 1.0 | mg/dL | pH | 6.5 | ||||
| Aspartate aminotransferase | 138 | IU/L | Specific gravity | 1.019 | ||||
| Alanine aminotransferase | 24 | IU/L | Bilirubin | (-) | ||||
| Alkaline phosphatase | 507 | IU/L | Urobilinogen | (±) | ||||
| Lactate dehydrogenase | 307 | IU/L | Acetone | (-) | ||||
| Urea nitrogen | 16.4 | mg/dL | Glucose | 0.00 | g/dL | |||
| Creatinine | 0.33 | mg/dL | Protein | 2.0 | mg/dL | |||
| Estimated glomerular filtration rate | 143.0 | mL/min/1.73 | Red blood cells | 4 - 9 | /HPF | |||
| Uric acid | 2.0 | mg/dL | White blood cells | 4 - 9 | /HPF | |||
| Na | 132 | mEq/L | Squamous cells | 1 - 3 | /HPF | |||
| K | 4.1 | mEq/L | Renal tubular epithelial cells | 1 - 3 | /HPF | |||
| Cl | 104 | mEq/L | Creatinine | 38.64 | mg/dL | |||
CH50: 50% hemolytic unit of complement, ds-DNA: double stranded deoxyribonucleic acid, RNP: ribonucleoprotein, CLEIA: chemiluminescent enzyme immunoassay, DRVVT: diluted Russell’s viper venom time, CL-β2 GPI: cardiolipin-β2 glycoprotein I complex, SS-A/Ro: Sjögren syndrome-A/Ro
In short, the patient had oral ulcers, serositis, hematologic disorders (leukopenia and thrombocytopenia), and immunologic disorders (anti-ds-DNA antibody, anti-Sm antibody). Given these findings, we diagnosed her with SLE based on the American College of Rheumatology (ACR) revised criteria in 1997 (39). Concerning the patient's antibiotic therapy, ceftriaxone 2 g/day was switched to cefoperazone 1.5 g/day with sulbactam 1.5 g/day, although ceftriaxone had already been administered for 5 days. This change was made because ceftriaxone can cause cholecystitis (40).
On the night of the first day, the patient's temperature rose to 37.7℃ again. Her cholecystitis was suspected to be SLE-associated AAC because of the negative blood culture and the patient's poor response to antibiotic therapy. Hydrocortisone 100 mg was administered via intravenous drip infusion. We chose this approach because, although AAC is known to have a high mortality rate, and the general condition of our patient was not very serious at that point; in addition, we considered it necessary to check the efficacy of corticosteroids before administering methylprednisolone. On the second day, we increased the prednisolone dose to 50 mg/day and stopped the cefoperazone with sulbactam.
For a further examination of the gallbladder, abdominal ultrasonography was performed. The gallbladder was swollen and tense. The short diameter was 41 mm, and the wall was entirely thickened to 5.6 mm at the thickest point. There was debris in the lumen, but no gallstones were noted. Although cholecystectomy was considered, we elected to treat the patient with conservative therapy alone because her general condition had worsened, making surgical intervention risky, while the clinical and laboratory findings of AAC were not so serious as to require immediate surgical intervention. On the fourth day after her transfer to our section, the patient complained of general malaise; hydrocortisone 100 mg was administered again on the fifth day. Mizoribine was stopped on the sixth day, and hydroxychloroquine 200 mg/day was started on the seventh day.
On the eighth day, in order to confirm the diagnosis of SLE, we performed a skin biopsy of the brownish round rash on the patient's left cheek. Hematoxylin-eosin staining revealed hyperkeratosis and keratotic plugs (Fig. 2B). There were also dermal-epidermal junction deposits of IgG, IgA, IgM, C3c, and C1q according to immunofluorescence staining. The results of the lupus band test were positive. These findings were compatible with those of chronic rash associated with SLE.
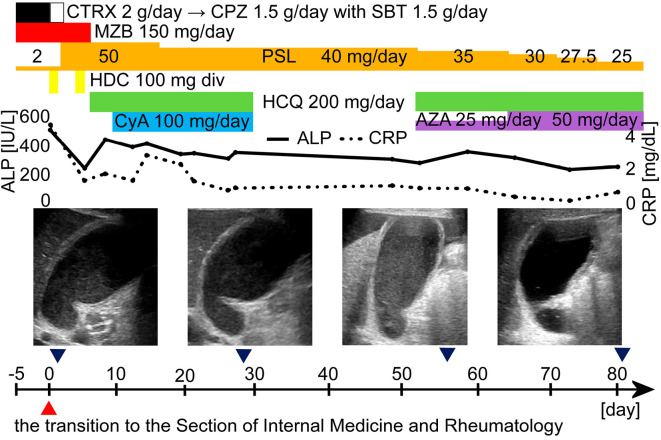
On the 10th day, cyclosporin 100 mg/day was started. The patient then became generally well, and prednisolone dose was reduced to 40 mg/day on the 17th day, although the gallbladder condition was not obviously improved on ultrasonography on the 27th day. On the 28th day, however, she developed uroseptic shock. The cyclosporin and hydroxychloroquine were stopped, and only the prednisolone was continued. Once the septic shock was controlled, hydroxychloroquine 200 mg/day was started, and the prednisolone was reduced to 35 mg/day on the 51st day. At the same time, cyclosporin 100 mg/day was switched to azathioprine 25 mg/day because vasculitis is considered the most common pathogenesis of SLE-associated AAC and because azathioprine has been recommended for the treatment of some vasculitises, such as primary small- and medium-vessel vasculitis and aortitis syndrome (32,41,42). On the 64th day, the azathioprine was increased to 50 mg/day, and the prednisolone was reduced to 30 mg/day. The patient became generally well again, and the 2.5 mg/day prednisolone was reduced every week from that day.
On the 82nd day, abdominal ultrasonography was performed again, and an improvement in the swelling and tension was seen in the patient's gallbladder (Fig. 3). The prednisolone was reduced to 20 mg/day on the 92nd day, and the patient was transferred to a hospital near her home for rehabilitation on the 97th day.
Figure 3.
The clinical course of the patient, a 69-year-old Japanese woman. Abdominal ultrasonography showed the thickening of the gallbladder wall and the swelling and tension of the gallbladder. Because SLE-associated AAC was suspected, antibiotics were stopped, and the PSL dose was increased to 50 mg/day. HCQ 200 mg/day and CyA 100 mg/day were added, and the patient’s general condition improved. After that, however, uroseptic shock occurred, and PSL alone was continued. After the septic shock was controlled, HCQ was started again, and the CyA was switched to AZA 25 mg/day. After the therapy, her general condition became good again, and the ALP and CRP values improved. The AZA dose was increased to 50 mg/day, and the PSL dose was reduced to 25 mg/day. After the introduction of azathioprine, the swelling and tension of the gallbladder also improved. ALP: alkaline phosphatase, AZA: azathioprine, CPZ: cefoperazone, CRP: C-reactive protein, CTRX: ceftriaxone, CyA: cyclosporin A, div: drip intravenous injection, HCQ: hydroxychloroquine, HDC: hydrocortisone, MZB: mizoribine, PSL: prednisolone, SBT: sulbactam
Discussion
In our patient's case, abdominal ultrasonography and contrast-enhanced CT showed a swollen and tense gallbladder with gross thickening of the wall but without gallstones. When she arrived at our hospital, she had a fever with pancytopenia. Two sets of blood cultures were negative, but infection was not completely denied. Antibiotic therapy was conducted with ceftriaxone 2 g/day for 5 days followed by cefoperazone 1.5 g/day for 2 days. The patient's response to antibiotic therapy was poor, and her body temperature rose to 37.7℃. She was diagnosed with SLE based on the 1997 ACR revised criteria (39). SLE-associated AAC was suspected. Antibiotic therapy was stopped, and prednisolone was increased to 50 mg/day after hydrocortisone 100 mg was administered via intravenous drip infusion. Corticosteroid therapy was continued with some immunosuppressive agents. After the introduction of azathioprine, improvement of the swelling and tension in her gallbladder was observed.
As a differential diagnosis of SLE-associated AAC, we considered ceftriaxone-associated cholecystitis. Ceftriaxone is excreted mainly in the urine, but part of the antibiotic is eliminated by the bile ducts (43). Ceftriaxone forms a ceftriaxone-calcium complex, which forms sludge in the gallbladder (44). This sludge sometimes causes cholecystitis (40), but the cholecystitis rapidly disappears after the discontinuation of the antibiotic (45). In our patient's case, ceftriaxone was used for five days. Before the ceftriaxone was introduced, however, the gallbladder had already been revealed by MRI at a previous hospital to be swollen and tense. Therefore, ceftriaxone did not seem to be the main cause of the cholecystitis in this case. In addition to the diagnosis of SLE, the patient's response to the antibiotic therapy was poor, while her response to the corticosteroid therapy was effective. In light of these findings, the cholecystitis in her case was finally diagnosed as SLE-associated AAC.
Several possible pathogeneses of SLE-associated AAC have been reported, including vasculitis, serositis, and MIVOD (10,30-33). Antiphospholipid syndrome has also been reported as a possible pathogenesis of AAC (14). Among these pathogeneses, vasculitis is considered the most common pathogenesis of SLE-associated AAC (32). In our patient's case, laboratory tests revealed anti-cardiolipin-β2 glycoprotein I complex antibody ≥1.2 IU/mL and anti-cardiolipin IgG antibody ≥8 IU/mL, showing anti-phospholipid antibody negativity. On contrast-enhanced CT, ischemia of the intestine was not detected. Therefore, antiphospholipid syndrome and MIVOD did not seem to be the pathogenesis of AAC in the present case.
Histologically, the gallbladder lacks serosa in the surface attached to the liver (46). In our patient's case, however, thickening of the gallbladder wall was also seen at the surface where there was no serosa on ultrasonography. For this reason, serositis was not actively considered. Based on these findings, vasculitis was deemed the most likely pathogenesis of the AAC in our patient's case.
Concerning the treatment, conservative therapy alone was conducted using a corticosteroid with immunosuppressive agents in this case. Generally, three prevailing therapies are considered for AAC: cholecystectomy, laparoscopic cholecystectomy, and percutaneous cholecystostomy (1,28). For SLE-associated AAC, corticosteroid therapy was successfully used in some reports as a conservative therapy (34-38). In previous reports of SLE-associated AAC conservatively treated with corticosteroids, antibiotics or immunosuppressive agents were used in combination with corticosteroids. (Table 3) (34-38,47). Among 13 previously reported cases treated with corticosteroid combined with antibiotics, 2 failed the treatment and required subsequent cholecystectomy (38,47). In other reports, however, two cases were treated successfully with corticosteroid combined with immunosuppressive agents (35,36). In our patient's case, cholecystectomy was also considered, but conservative therapy alone was ultimately selected, partly because her clinical and laboratory findings of AAC were not serious enough to warrant immediate surgical intervention, and partly because her poor general condition made her ineligible for surgery. After the introduction of azathioprine, the swelling and tension of the patient's gallbladder improved. If vasculitis was indeed the cause of the AAC in the present case, the azathioprine may have helped ameliorate this underlying condition.
Table 3.
Past Reports of SLE-associated AAC Conservatively Treated with Corticosteroid.
| Cases (Reference) | Number of patients | Age | Sex | Other collagen diseases complicated with SLE | Drugs combined with corticosteroids | Outcomes (number of patients) |
|---|---|---|---|---|---|---|
| Case 1 (34) | 1 | 39 | F | SjS | None | Success |
| Case 2 (47) | 1 | 10 | M | Not mentioned | CTRX, MTZ | Cholecystectomy |
| Case 3 (35) | 1 | 30 | F | None | AZA, MMF | Success |
| Case 4 (36) | 1 | 70 | F | Not mentioned | CPA | Success |
| Case 5 (37) | 1 | 32 | F | Not mentioned | None | Success |
| Cases 6-17 (38) | 12 | 34.7±11.0 | F | Not mentioned | MFLX | Success (11), Cholecystectomy (1) |
| Our case 2019 | 1 | 69 | F | RA | HCQ, CyA, AZA | Success |
AAC: acute acalculous cholecystitis, SLE: systemic lupus erythematosus, F: female, M: male, SjS: Sjögren’s syndrome, RA: rheumatoid arthritis, CTRX: ceftriaxone, MTZ: metronidazole, AZA: azathioprine, MMF: mycophenolate mofetil, CPA: cyclophosphamide, MFLX: moxifloxacin, HCQ: hydroxychloroquine, CyA: cyclosporin A
In conclusion, this was a case of the successful treatment of SLE-associated AAC with a corticosteroid combined with azathioprine. AAC has high mortality and is difficult to diagnose because of its nonspecific clinical and laboratory findings. It is important to consider AAC as a possible complication of SLE. As a treatment, corticosteroid with azathioprine might be beneficial, especially when the patient's general condition is not good and when the clinical and laboratory findings of AAC are not very serious.
The authors state that they have no Conflict of Interest (COI).
References
- 1. Huffman JL, Schenker S. Acute acalculous cholecystitis: a review. Clin Gastroenterol Hepatol 8: 15-22, 2010. [DOI] [PubMed] [Google Scholar]
- 2. Frazee RC, Nagorney DM, Mucha P Jr. Acute acalculous cholecystitis. Mayo Clin Proc 64: 163-167, 1989. [DOI] [PubMed] [Google Scholar]
- 3. Ganpathi IS, Diddapur RK, Eugene H, Karim M. Acute acalculous cholecystitis: challenging the myths. HPB (Oxford) 9: 131-134, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Savoca PE, Longo WE, Zucker KA, McMillen MM, Modlin IM. The increasing prevalence of acalculous cholecystitis in outpatients. Results of a 7-year study. Ann Surg 211: 433-437, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Glenn F. Acute acalculous cholecystitis. Ann Surg 189: 458-465, 1979. [PMC free article] [PubMed] [Google Scholar]
- 6. Ryu JK, Ryu KH, Kim KH. Clinical features of acute acalculous cholecystitis. J Clin Gastroenterol 36: 166-169, 2003. [DOI] [PubMed] [Google Scholar]
- 7. Wang A-J, Wang T-E, Lin C-C, Lin S-C, Shih S-C. Clinical predictors of severe gallbladder complications in acute acalculous cholecystitis. World J Gastroenterol 9: 2821-2823, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tsakayannis DE, Kozakewich HPW, Lillehei CW. Acalculous cholecystitis in children. J Pediatr Surg 31: 127-131, 1996. [DOI] [PubMed] [Google Scholar]
- 9. McChesney JA, Northup PG, Bickston SJ. Acute acalculous cholecystitis associated with systemic sepsis and visceral arterial hypoperfusion. A case series and review of pathophysiology. Dig Dis Sci 48: 1960-1967, 2003. [DOI] [PubMed] [Google Scholar]
- 10. Swanepoel CR, Floyd A, Allison H, Learmonth GM, Cassidy MJD, Pascoe MD. Acute acalculous cholecystitis complicating systemic lupus erythematosus: case report and review. Br Med J (Clin Res Ed) 286: 251-252, 1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lasser A, Ghofrany S. Necrotizing granulomatous vasculitis (allergic granulomatosis) of the gallbladder. Gastroenterology 71: 660-662, 1976. [PubMed] [Google Scholar]
- 12. Gorgun E, Ozmen V. Acalculous gangrenous cholecystitis in a young adult: a gastrointestinal manifestation of polyarteritis nodosa. Surg Laparosc Endosc Percutan Techs 12: 359-361, 2002. [DOI] [PubMed] [Google Scholar]
- 13. Meier M, Holl-Ulrich K, Perras B. A rare manifestation of cryoglobulinemic vasculitis: acalculous cholecystitis. Clin Gastroenterol Hepatol 3: 26, 2005. [DOI] [PubMed] [Google Scholar]
- 14. Dessatlloud R, Papo T, Vaneecloo S, Gamblin C, Vanhille P, Piette J-C. Acalculous ischemic gallbladder necrosis in the catastrophic antiphospholipid syndrome. Arthritis Rheum 41: 1318-1320, 1998. [DOI] [PubMed] [Google Scholar]
- 15. Viola S, Meyer M, Fabre M, et al. Ischemic necrosis of bile ducts complicating Schönlein-Henoch purpura. Gastroenterology 117: 211-214, 1999. [DOI] [PubMed] [Google Scholar]
- 16. Otsuka Y, Inoue Y. So-called acute acalculous cholecystitis in macrophage activation syndrome. Intern Med 55: 3043-3046, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Batra V, Ang JY, Asmar BI. Staphylococcal acalculous cholecystitis in a child. South Med J 96: 206-208, 2003. [DOI] [PubMed] [Google Scholar]
- 18. Gomez NA, Leon CJ, Gutierrez J. Acute acalculous cholecystitis due to Vibrio cholerae. Surg Endosc 9: 730-732, 1995. [DOI] [PubMed] [Google Scholar]
- 19. Vilaichone R-K, Mahachai V. Tuberculous acalculous cholecystitis: a case report. J Infect Dis Antimicrob Agents 22: 127-131, 2005. [Google Scholar]
- 20. French AL, Beaudet LM, Benator DA, Levy CS, Kass M, Orenstein JM. Cholecystectomy in patients with AIDS: clinicopathologic correlations in 107 cases. Clin Infect Dis 21: 852-858, 1995. [DOI] [PubMed] [Google Scholar]
- 21. Iaria C, Arena L, Maio GD, et al. Acute acalculous cholecystitis during the course of primary Epstein-Barr virus infection: a new case and a review of the literature. Int J Infect Dis 12: 391-395, 2008. [DOI] [PubMed] [Google Scholar]
- 22. Diebel LN, Raafat AM, Dulchavsky SA, Brown WJ. Gallbladder and biliary tract candidiasis. Surgery 120: 760-765, 1996. [DOI] [PubMed] [Google Scholar]
- 23. Yombi JC, Meuris CM, Van Gompel AM, Ben Younes M, Vandercam BC. Acalculous cholecystitis in a patient with Plasmodium falciparum infection: a case report and literature review. J Travel Med 13: 178-180, 2006. [DOI] [PubMed] [Google Scholar]
- 24. Araki H, Shimizu S, Hayashi K, et al. Acute acalculous cholecystitis caused by Giardia lamblia. Intern Med 56: 1657-1662, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Laurila JJ, Ala-Kokko TI, Laurila PA, et al. Histopathology of acute acalculous cholecystitis in critically ill patients. Histopathology 47: 485-492, 2005. [DOI] [PubMed] [Google Scholar]
- 26. Hakala T, Nuutinen PJO, Ruokonen ET, Alhava E. Microangiopathy in acute acalculous cholecystitis. Br J Surg 84: 1249-1252, 1997. [PubMed] [Google Scholar]
- 27. Mirvis SE, Vainright JR, Nelson AW, et al. The diagnosis of acute acalculous cholecystitis: a comparison of sonography, scintigraphy, and CT. AJR Am J Roentgenol 147: 1171-1175, 1986. [DOI] [PubMed] [Google Scholar]
- 28. Treinen C, Lomelin D, Krause C, Goede M, Oleynikov D. Acute acalculous cholecystitis in the critically ill: risk factors and surgical strategies. Langenbecks Arch Surg 400: 421-427, 2015. [DOI] [PubMed] [Google Scholar]
- 29. Di Battista M, Marcucci E, Elefante E, et al. One year in review 2018: systemic lupus erythematosus. Clin Exp Rheumatol 36: 763-777, 2018. [PubMed] [Google Scholar]
- 30. Basiratnia M, Vasei M, Bahador A, Ebrahimi E, Derakhshan A. Acute acalculous cholecystitis in a child with systemic lupus erythematosus. Pediatr Nephrol 21: 873-876, 2006. [DOI] [PubMed] [Google Scholar]
- 31. Newbold KM, Allum WH, Downing R, Symmons DPM, Oates GD. Vasculitis of the gall bladder in rheumatoid arthritis and systemic lupus erythematosus. Clin Rheumatol 6: 287-289, 1987. [DOI] [PubMed] [Google Scholar]
- 32. Hirase N, Arita Y, Watanabe H, et al. A case of systemic lupus erythematosus with acalculous cholecystitis and small intestinal perforation. Kyushu Riumachi (Kyushu J Rheumatol) 32: 45-51, 2012(in Japanese, Abstract in English). [Google Scholar]
- 33. Bando H, Kobayash S, Matsumoto T, et al. Acute acalculous cholecystitis induced by mesenteric inflammatory veno-occlusive disease (MIVOD) in systemic lupus erythematosus. Clin Rheumatol 22: 447-449, 2003. [DOI] [PubMed] [Google Scholar]
- 34. Shin SJ, Na KS, Jung SS, et al. Acute acalculous cholecystitis associated with systemic lupus erythematosus with Sjogren's syndrome. Korean J Intern Med 17: 61-64, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hegarty J, Chiu DYY, Middleton RJ, et al. Systemic lupus erythematosus, eosinophilic vasculitis and acalculous cholecystitis. Nephrol Dial Transplant 21: 792-795, 2006. [DOI] [PubMed] [Google Scholar]
- 36. Choi YJ, Yoon HY, Jang SA, et al. A case of systemic lupus erythematosus initially presented with acute acalculous cholecystitis. J Rheum Dis 21: 140-142, 2014. [Google Scholar]
- 37. Xu X, Li B, Zheng C. Hepatobiliary and pancreatic: acute acalculous cholecystitis in systemic lupus erythematosus, successfully treated with corticosteroid. J Gastroenterol Hepatol 31: 1673, 2016. [DOI] [PubMed] [Google Scholar]
- 38. Liu W, Chen W, He X, Qu Q, Hong T, Li B. Successful treatment using corticosteroid combined antibiotic for acute acalculous cholecystitis patients with systemic lupus erythematosus. Medicine 96: 27(e7478), 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hochberg MC. Updating the American College of Rheumatology Revised Criteria for the Classification of Systemic Lupus Erythematosus. Arthritis Rheum 40: 1725-1734, 1997. [DOI] [PubMed] [Google Scholar]
- 40. Jacobs RF. Ceftriaxone-associated cholecystitis. Pediatr Infect Dis J 7: 434-436, 1988. [DOI] [PubMed] [Google Scholar]
- 41. Mukhtyar C, Guillevin L, Cid MC, et al. EULAR recommendations for the management of primary small and medium vessel vasculitis. Ann Rheum Dis 68: 310-317, 2009. [DOI] [PubMed] [Google Scholar]
- 42. Mukhtyar C, Guillevin L, Cid MC, et al. EULAR recommendations for the management of large vessel vasculitis. Ann Rheum Dis 68: 318-323, 2009. [DOI] [PubMed] [Google Scholar]
- 43. Hayton WL, Schandlik R, Stoeckel K. Biliary excretion and pharmacokinetics of ceftriaxone after cholecystectomy. Eur J Clin Pharmacol 30: 445-451, 1986. [DOI] [PubMed] [Google Scholar]
- 44. Park HZ, Lee SP, Schy AL. Ceftriaxone-associated gallbladder sludge identification of calcium-ceftriaxone salt as a major component of gallbladder precipitate. Gastroenterology 100: 1665-1670, 1991. [DOI] [PubMed] [Google Scholar]
- 45. Palanduz A, Yalçın I, Tonguç E, et al. Sonographic assessment of ceftriaxone-associated biliary pseudolithiasis in children. J Clin Ultrasound 28: 166-168, 2000. [DOI] [PubMed] [Google Scholar]
- 46. Ross MH, Pawlina W. Histology: A text and atlas: With correlated cell and molecular biology. 6th ed Lippincott Williams & Wilkins, Philadelphia, PA, 2010: 646. [Google Scholar]
- 47. Basiratnia M, Vasei M, Bahador A, Ebrahimi E, Derakhshan A. Acute acalculous cholecystitis in a child with systemic lupus erythematosus. Pediatr Nephrol 21: 873-876, 2006. [DOI] [PubMed] [Google Scholar]