Abstract
A 77-year-old-man with renal cell carcinoma who was undergoing nivolumab treatment visited our department due to hyperglycemia; his plasma glucose level was 379 mg/dL. Although his serum C-peptide immunoreactivity (CPR) level was preserved (5.92 ng/mL), we suspected an onset of fulminant type 1 diabetes mellitus (FT1DM) and immediately started insulin therapy. His CPR levels gradually decreased and were depleted within 1 week. We later discovered that the patient's casual CPR level had been abnormally high (11.78 ng/mL) 2 weeks before his admission. Hence, the possibility of FT1DM in hyperglycemic patients undergoing nivolumab treatment should not be excluded, even with a preserved CPR level.
Keywords: nivolumab, fulminant type 1 diabetes mellitus, anti-PD-1 antibody
Introduction
Immune checkpoint inhibitors, such as anti-programmed cell death 1 (PD-1) antibodies, are increasingly being used as anticancer drugs. However, these antibodies can cause immune-related adverse events, including type 1 diabetes mellitus (T1DM) through their activation of autoreactive T cells (1). Nivolumab-related T1DM reportedly manifests as fulminant type 1 diabetes mellitus (FT1DM), which is an emergency condition because patients develop ketosis or ketoacidosis within approximately 1 week. The fasting serum C-peptide immunoreactivity (CPR) level of patients with FT1DM is usually <0.3 ng/mL (2) because the insulin secretion capability is destroyed immediately after the disease onset. Thus, clinicians may inadvertently rule out the possibility of FT1DM in hyperglycemic patients with preserved CPR levels. We herein report the case of a patient with nivolumab-related FT1DM who presented with a preserved serum CPR level at the onset of hyperglycemia.
Case Report
A 77-year-old Japanese man who was undergoing nivolumab treatment (3 mg/kg, once every 2 weeks) was referred to the endocrinology department of our hospital after developing hyperglycemia. He had no personal or family history of diabetes. He was being treated with nivolumab at our oncology department after previous courses of sunitinib, everolimus, axitinib, and pazopanib for renal cell carcinoma with lung metastasis. Despite receiving 4 lines of anti-cancer drugs, the patient developed progressive disease, which led to the prescription of nivolumab. No glucose intolerance was noted at that time; his casual blood glucose and glycated hemoglobin (HbA1c) levels were 112 mg/dL and 5.4%, respectively. On day 15 of the 6th cycle of nivolumab infusion, a blood test revealed hyperglycemia with a casual plasma glucose level of 379 mg/dL, whereupon he was referred to our department. Although his insulin secretion appeared to be preserved with a serum CPR level of 5.92 ng/mL, a diabetologist suspected the onset of FT1DM. The patient was hospitalized at our department the following day.
The patient's consciousness was clear. A physical examination revealed the following findings: body temperature, 36.5°C; blood pressure, 130/72 mmHg; pulse rate, 73 bpm; and respiratory rate, 16/min. He was 172.2 cm tall and his body weight was 65.5 kg (body mass index, 22.1 kg/m2). There were no abnormal findings on the patient's head, face, neck, chest, or abdomen and no neurological abnormalities were detected.
The patient's laboratory data are shown in Tables 1 and 2. A urinary glucose was strongly positive, while a urinary ketone test was negative. Although the patient's casual blood glucose level was markedly high (379 mg/dL), his HbA1c level was 6.2%; furthermore, elastase 1 was the only elevated pancreatic enzyme. While the patient's fasting serum CPR level was not depleted on the 2nd day of hospitalization (2.97 ng/mL), the secretion of additional insulin during meal tolerance and glucagon loading tests appeared to be impaired. All pancreatic islet-associated autoantibodies, including anti-GAD antibody, were negative, and endocrinological testing revealed no abnormal findings. Furthermore, there were no increases in virus titers. His HLA-DNA type was HLA-DRB1* 09:01:02/12:01:01, HLA-DQB1* 03:01:01/03:03:02, HLA-DPB1* 05:01:01, and HLA-DQA1* 03:02/05:05. Imaging examinations revealed no evidence of infection or morphological abnormalities in the pancreas.
Table 1.
The Patient’s Laboratory Data on Admission.
| Parameter | Value | Unit | Parameter | Value | Unit | |||
|---|---|---|---|---|---|---|---|---|
| TP | 8 | g/dL | WBC | 8,800 | /μL | |||
| ALB | 4.1 | g/dL | Neut | 42 | % | |||
| AST | 30 | U/L | Lymph | 24 | % | |||
| ALT | 27 | U/L | Mono | 7 | % | |||
| ALP | 291 | U/L | Basophil | 0 | % | |||
| LDH | 257 | U/L | Eosinophil | 19 | % | |||
| γ-GTP | 26 | U/L | HGB | 12.1 | g/dL | |||
| T-Bil | 0.8 | mg/dL | PLT | 24.3 | ×104/μL | |||
| UA | 6.6 | mg/dL | ||||||
| BUN | 25.1 | mg/dL | Venous blood gas analysis | |||||
| CK | 455 | U/L | pH | 7.42 | ||||
| CRE | 1.21 | mg/dL | PCO2 | 36.8 | mmHg | |||
| Na | 132 | mEq/L | pO2 | 49 | mmHg | |||
| K | 4.9 | mEq/L | HCO3- | 23.9 | mmol/L | |||
| Cl | 99 | mEq/L | ||||||
| Ca | 9.3 | mg/dL | Urinary analysis | |||||
| CRP | 0.25 | mg/dL | Protein | (±) | ||||
| AMY | 129 | U/L | Glucose | (4+) | ||||
| Elastase 1 | 406 | ng/dL | Ketone | (-) | ||||
| Lipase | 148.1 | U/L | ||||||
| PG | 379 | mg/dL | ||||||
| HbA1c | 6.2 | % | ||||||
| GA | 18.9 | % | ||||||
TP: total protein, ALB: albumin, AST: aspartate aminotransferase, ALT: alanine aminotransferase, ALP: alkaline phosphatase, LDH: lactate dehydrogenase, γ-GTP: γ-glutamyltranspeptidase, T-Bil: total bilirubin, UA: urine acid, BUN: blood urea nitrogen, CK: creatine kinase, CRE: creatinine, CRP: C-reactive protein, AMY: amylase, PG: plasma glucose, HbA1c: glycated hemoglobin, GA: glycated albumin, WBC: white blood cell count, HGB: hemoglobin, PLT: platelet
Table 2.
Specific Examinations.
| Examination | Value | Unit | Examination | Value | Unit | |
|---|---|---|---|---|---|---|
| Meal tolerance test (Day 2) | Endocrinological test | |||||
| Fasting PG | 227 | mg/dL | TSH | 0.728 | μIU/mL | |
| 2-h postprandial PG | 487 | mg/dL | FT3 | 2.3 | pg/mL | |
| Fasting CPR | 2.97 | ng/mL | FT4 | 1.6 | ng/dL | |
| 2-h postprandial CPR | 3.52 | ng/mL | GH | 0.48 | ng/mL | |
| IGF-1 | 153 | ng/mL | ||||
| Glucagon loading test (Day 3) | LH | 0.1 | mIU/mL | |||
| CPR 0 min | 2.64 | ng/mL | FSH | 7.48 | mIU/mL | |
| CPR 6 min | 3.31 | ng/mL | ACTH | 34.9 | pg/mL | |
| Cortisol | 18.4 | μg/dL | ||||
| Urinary CPR | PRL | 12.62 | ng/mL | |||
| Day 2 | 59.2 | μg/day | PRA | 1.3 | ng/mL/h | |
| Day 6 | 9.9 | μg/day | PAC | 152 | pg/mL | |
| Pancreatic islet-associated autoantibodies | Viral antibody titers | Day 1 | Day 14 | |||
| Anti-GAD antibody | <5.0 | U/mL | Mumps | ×16 | ×8 | |
| Anti-IA-2 antibody | <0.4 | U/mL | Coxsackie B4 | ×4 | ×8 | |
| Anti-IAA antibody | <0.4 | U/mL | Coxsackie B5 | ×4 | ×4 | |
| Anti-ZnT8 antibody | <10 | U/mL | EBVCA IgG | ×80 | ×80 | |
| EBVCA IgM | <×10 | <×10 | ||||
| HLA-typing analysis | VZV IgM | 1.27 | 1.93 | |||
| HLA-DRB1 | 09:01:02 12:01:01 |
CMV IgG | 71.8 | 38.4 | ||
| HLA-DQB1 | 03:01:01 03:03:02 |
CMV IgM | 0.54 | 0.56 | ||
| HLA-DPB1 | 05:01:01 | Rubella virus IgM | 0.12 | 0.14 | ||
| HLA-DQA1 | 03:02 05:05 |
|||||
PG: plasma glucose, CPR: C-peptide immunoreactivity, GAD: glutamic acid decarboxylase, IA-2: tyrosine phosphatase-related islet antigen 2, IAA: insulin autoantibody, ZnT8: zinc transporter 8, HLA: human leukocyte antigen, TSH: thyroid-stimulating hormone, FT3: free triiodothyronine, FT4: free thyroxine, GH: growth hormone, IGF-1, insulin-like growth factor-1, LH: luteinizing hormone, FSH: follicle stimulating hormone, ACTH: adrenocorticotropic hormone, PRL: prolactin, PRA plasma renin activity, PAC: plasma aldosterone concentration, EBVCA: Epstein-Barr virus capsid antigen, VZV: varicella zoster virus, CMV: cytomegalovirus
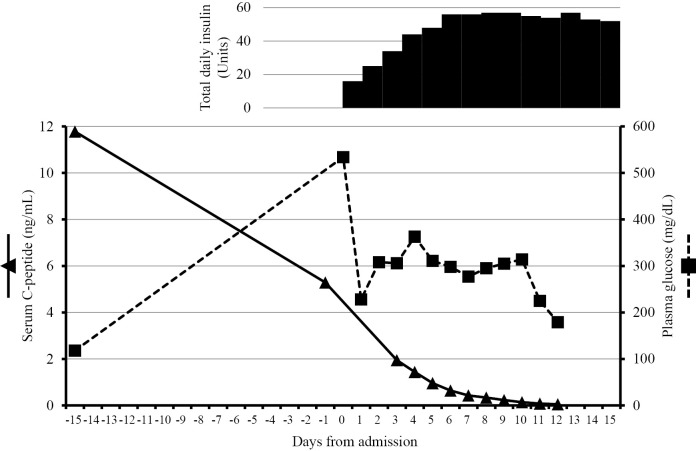
Although the serum CPR level appeared to be preserved, we considered the possibility of nivolumab-related FT1DM because there was no other explanation for the patient's hyperglycemia. Thus, we commenced intensive insulin therapy immediately after hospitalization. As shown in Figure, his serum CPR levels gradually fell to <0.3 ng/mL on the 8th day of hospitalization. Similarly, his urinary CPR levels decreased from 59.2 μg/day on day 2 to 9.9 μg/day on day 6. We subsequently discovered that his casual CPR level had been abnormally high (11.78 ng/mL with a blood glucose level of 118 mg/dL, at four hours after breakfast) 2 weeks before his hospitalization after examining a stored blood sample. By adjusting the amount of insulin according to his blood glucose levels, ketosis was averted. The patient was discharged from hospital on the 16th day of hospitalization with a prescription of 52 units of insulin daily. After his blood glucose level was stabilized, nivolumab treatment was resumed. Tumor regression continued to be observed at 6 months after this episode. The patient provided his written informed consent for the publication of this case report.
Figure.
Sequential changes of the serum C-peptide and plasma glucose levels before and after admission. The casual glucose or C-peptide levels were measured on day -15, day 0, and day 1. The fasting glucose or C-peptide levels were measured after day 2. Insulin was administered on day 0 with the dose increased daily, as shown.
Discussion
We encountered a patient with nivolumab-related FT1DM whose CPR levels were not depleted at the time of the initial diagnosis of hyperglycemia; moreover, his CPR level had been abnormally high 2 weeks before his hospitalization.
The type of diabetes in this case was nivolumab-related FT1DM. FT1DM is characterized by a markedly rapid onset of hyperglycemia with ketoacidosis, with near-normal HbA1c levels despite the presence of severe hyperglycemia. A negative islet-related autoantibody status, the absence of insulin-secretion capacity, even at the onset of disease, and elevated serum pancreatic enzyme levels are also noted. According to the Japanese Diabetes Society guideline (2), the following criteria are required to confirm the diagnosis of FT1DM: 1) the occurrence of diabetic ketosis or ketoacidosis soon (approximately 7 days) after the onset of hyperglycemic symptoms; 2) a plasma glucose level of ≥288 mg/dL and a glycated hemoglobin level of <8.7% (National Glycohemoglobin Standardization Program value) at first visit; 3) urinary C-peptide excretion <10 μg/day or a fasting serum C-peptide level of <0.3 ng/mL and <0.5 ng/mL after intravenous glucagon (or after meal) load at the onset of disease. Neither DKA nor the absence of insulin secretion was observed in our patient at the time of his first visit to our department; this was possibly due to the timing of the patient's examination being in the very early stage after the onset of FT1DM. His glycated albumin (GA) level (18.9%) and GA/HbA1c ratio (3.05) were relatively low in comparison to previously reports on patients with FT1DM (GA: 23.6±4.3%; GA/HbA1c ratio: 3.9±0.5) (3). Given that GA reflects short-term glycemic control (i.e., approximately 14 days), the patient's FT1DM may have been diagnosed before his GA levels rose and while his insulin secretion was still preserved. As such, he would likely have been diagnosed with DKA had he visited at a later time, given that his CPR level was exhausted within 1 week of hospitalization. That the patient was negative for islet-related autoantibodies and had elevated levels of elastase 1 supported the diagnosis of FT1DM. Additionally, his HLA type (HLA-DRB1* 09:01-HLA-DQB1* 03:03) was previously reported to be associated with acute-onset T1DM (4). After ruling out other causes, such as viral infection or drug-induced hypersensitivity syndrome, the cause of FT1DM was deemed to be nivolumab treatment (5).
Given the patient's CPR level of 5.92 ng/mL, his ability to secrete insulin appeared to be preserved at the time of his first visit, which might have caused us to overlook the onset of FT1DM. Table 3 summarizes previously published case reports on PD-1 inhibitor-related T1DM, including DKA and changes in the serum level of CPR (6-21). Most previously reported patients had depleted insulin secretion or DKA at their initial visit; 2 patients reported by Matsumura et al. and Saito et al. had preserved CPR levels without DKA (20,21). As with our patient, their subject's insulin secretion was gradually depleted. As mentioned above, the Japanese Diabetes Society criteria for the diagnosis of FT1DM includes the absence of insulin secretion capacity, and FT1DM may be overlooked if a patient's CPR level appears to be preserved. Clinicians should therefore consider the possibility of FT1DM if sudden hyperglycemia is detected, even if the CPR level is not depleted; DKA can be prevented with prompt insulin treatment (as was observed in the present case). Although the number of patients treated with PD-1 inhibitors is increasing, many of the clinicians who use these drugs will not be endocrinologists; as such, our patient represents an exceptional educational example of PD-1 inhibitor-related T1DM.
Table 3.
Summary of Reported Patients with PD-1 Inhibitor-related Type 1 Diabetes Mellitus.
| Ref. | Sex/Age | Cancer | Anti-PD-1Ab | DKA | PG | CPR | Autoantibodies | HLA type |
|---|---|---|---|---|---|---|---|---|
| 6 | F/54 | Melanoma | Pembrolizumab | (+) | n.r. | n.r. | GAD (+) | DRB1*04, DQB1*03:02 |
| 7 | M/60 | Melanoma | Pembrolizumab | (+) | 27 mmol/L (486 mg/dL) | 57 pmol/L (0.17 ng/mL) | GAD (-), IA-2(-) | n.r. |
| 8 | F/55 | Melanoma | Nivolumab | Ketonuria | 580 mg/dL | 1.0 ng/mL | Negative | DRB1*04:05, DQB1*04:01 |
| 9 | M/76 | NSCLC | Pembrolizumab | (-) | 40 mmol/L (721 mg/dL) | 0.81 ng/mL | GAD (+), IA-2 (+) | n.r. |
| 10 | M/51 | RCC | Nivolumab | (+) | 763 mg/dL | Undetectable | GAD (-), IA-2 (-) | n.r. |
| 11 | F/34 | NSCLC | Nivolumab | (+) | 739 mg/dL | <0.1 ng/mL | GAD (+), IA-2 (+) | A30:01,30:02 DR9 |
| 12 | M/31 | NSCLC | Nivolumab | (+) | n.r. | <0.03 ng/mL | GAD (+) | DRB1*04:05, DQB1*04:01 |
| 12 | F/62 | NSCLC | Nivolumab | Ketonuria | 246 mg/dL | n.r. | GAD (-) | DRB1*09:01 DQB1*03:03 |
| 13 | F/73 | NSCLC | Nivolumab | (+) | >1,000 mg/dL | 0.06 ng/mL | GAD (+) | DR3-DQ2, DR4-DQ8 |
| 14 | M/73 | NSCLC | Nivolumab | (-) | 708 mg/dL | 0.97 ng/mL | Negative | DRB1*09:01, DQB1*03:03, DRB1*01:01, DQB1*05:01 |
| 15 | M/73 | Melanoma | Nivolumab | (+) | 500 mg/dL | Undetectable | GAD (+), IA-2(+), ZnT8 (+) | n.r. |
| 16 | M/42 | Melanoma | Nivolumab | (+) | 728 mg/dL | n.r. | Negative | n.r. |
| 17 | F/74 | NSCLC | Nivolumab | (+) | 1,060 mg/dL | 0.2 ng/mL | GAD (+) | n.r. |
| 18 | F/68 | RCC | Nivolumab | (-) | 473 mg/dL | Undetectable | Negative | DRB1*09:01, DQB1*03:03 |
| 19 | F/63 | Melanoma | Nivolumab | (+) | 661 mg/dL | 0.08 ng/mL | n.r. | DRB1*09:01 |
| 20 | M/68 | NSCLC | Nivolumab | (-) | 330 mg/dL | 3.16 ng/mL | Negative | A*24:02, DRB1*09:01, DRB1*15:02 |
| 21 | M/82 | NSCLC | Pembrolizumab | (-) | 319 mg/dL | 2.03 ng/mL | Negative | DRB1* 12:01 |
| Our patient | M/77 | RCC | Nivolumab | (-) | 379 mg/dL | 5.92 ng/mL | Negative | DRB1* 09:01:02 12:01:01, DQB1* 03:01:01 03:03:02, DPB1* 05:01:01, DQA1 *03:02 05:05 |
PD-1: programed death-1, DKA: diabetic ketoacidosis, PG: plasma glucose, CPR: C-peptide immunoreactivity, HLA: human leukocyte antigen, NSCLC: non-small cell lung cancer, RCC: renal cell carcinoma, GAD: glutamic acid decarboxylase, IA-2: tyrosine phosphatase-related islet antigen 2, ZnT8: zinc transporter 8, n.r.: not reported
By examining a stored blood sample, we found that our patient's casual CPR level was abnormally high (11.78 ng/mL) 2 weeks before his hospitalization. Few patients have exhibited transient hyperinsulinemia and hypoglycemia before the onset of T1DM (22). As the etiology of nivolumab-related FT1DM is thought to be the invasion of PD-1-positive T cells into islet cells (23), hyperinsulinemia likely occurred as a result of pancreatic islet cell destruction. To the best of our knowledge, ours is the first patient reported to exhibit hyperinsulinemia before the onset of PD-1 inhibitor-related FT1DM. Our patient's course suggests that the destruction of pancreatic islet cells by nivolumab was already occurring 2 weeks before the detection of hyperglycemia. Although our patient did not experience hypoglycemia, our findings suggest that clinicians should consider the possibility of FT1DM when sudden hypoglycemia or hyperglycemia are detected, because the former may occur as a result of hyperinsulinemia due to the destruction of pancreatic islet cells.
Recently, the Japan Diabetes Society reported the characteristics and clinical course of anti-PD-1 antibody-related T1DM (24). According to this report, the insulin secretion capacity of most patients was exhausted at about 2-3 weeks after the onset of disease, which is slower than in patients with fulminant type 1 diabetes but faster than in patients with acute-onset type 1 diabetes. Our patient also had this characteristic. Because anti-PD-1 antibody-related T1DM varies from typical FT1DM to acute-onset T1DM, the authors proposed that it would be appropriate to consider anti-PD-1 antibody-related T1DM a distinct entity and to introduce a newly coined name for this entity (24). Thus, our case also would be classified as a new type of T1DM in the future.
In conclusion, we encountered a patient with nivolumab-related FT1DM in whom we observed a gradual change of serum CPR levels. Clinicians should not rule out the possibility of FT1DM in nivolumab-treated patients when detecting sudden hyperglycemia, even if the CPR level is not depleted.
The authors state that they have no Conflict of Interest (COI).
References
- 1. Torino F, Corsello SM, Salvatori R. Endocrinological side-effects of immune checkpoint inhibitors. Curr Opin Oncol 28: 278-287, 2016. [DOI] [PubMed] [Google Scholar]
- 2. Imagawa A, Hanafusa T, Awata T, et al. . Report of the Committee of the Japan Diabetes Society on the Research of Fulminant and Acute-onset Type 1 Diabetes Mellitus: New diagnostic criteria of fulminant type 1 diabetes mellitus (2012). J Diabetes Investig 3: 536-539, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Koga M, Murai J, Saito H, et al. . Serum glycated albumin to haemoglobin A1C ratio can distinguish fulminant type 1 diabetes mellitus from type 2 diabetes mellitus. Ann Clin Biochem 47: 313-317, 2010. [DOI] [PubMed] [Google Scholar]
- 4. Kawabata Y, Ikegami H, Awata T, et al. . Differential association of HLA with three subtypes of type 1 diabetes: fulminant, slowly progressive and acute-onset. Diabetologia 52: 2513-2521, 2009. [DOI] [PubMed] [Google Scholar]
- 5. Tohyama M, Hashimoto K. 2011 New aspects of drug-induced hypersensitivity syndrome. J Dermatol 38: 222-228, 2011. [DOI] [PubMed] [Google Scholar]
- 6. Martin-Liberal J, Furness AJ, Joshi K, Peggs KS, Quezada SA, Larkin J. Anti-programmed cell death-1 therapy and insulin-dependent diabetes: a case report. Cancer Immunol Immunother 64: 765-767, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Aleksova J, Lau PK, Soldatos G, McArthur G. Glucocorticoids did not reverse type 1 diabetes mellitus secondary to pembrolizumab in a patient with metastatic melanoma. BMJ Case Rep 2016: bcr2016217454, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Okamoto M, Okamoto M, Gotoh K, et al. . Fulminant type 1 diabetes mellitus with anti-programmed cell death-1 therapy. J Diabetes Investig 7: 915-918, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chae YK, Chiec L, Mohindra N, Gentzler R, Patel J, Giles F. A case of pembrolizumab-induced type-1 diabetes mellitus and discussion of immunecheckpoint inhibitor-induced type 1 diabetes. Cancer Immunol Immunother 66: 25-32, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Teló GH, Carvalhal GF, Cauduro CGS, Webber VS, Barrios CH, Fay AP. Fulminant type 1 diabetes caused by dual immune checkpoint blockade in metastatic renal cell carcinoma. Ann Oncol 28: 191-192, 2017. [DOI] [PubMed] [Google Scholar]
- 11. Godwin JL, Jaggi S, Sirisena I, et al. . Nivolumab-induced autoimmune diabetes mellitus presenting as diabetic ketoacidosis in a patient with metastatic lung cancer. J Immunother Cancer 5: 40, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Usui Y, Udagawa H, Matsumoto S, et al. . Association of serum anti-GAD antibody and HLA haplotypes with type 1 diabetes mellitus triggered by nivolumab in patients with non-small cell lung cancer. J Thorac Oncol 12: e41-e43, 2017. [DOI] [PubMed] [Google Scholar]
- 13. Araújo M, Ligeiro D, Costa L, et al. . A case of fulminant type 1 diabetes following anti-PD1 immunotherapy in a genetically susceptible patient. Immunotherapy 9: 531-535, 2017. [DOI] [PubMed] [Google Scholar]
- 14. Kumagai R, Muramatsu A, Nakajima R, et al. . Acute-onset type 1 diabetes mellitus caused by nivolumab in a patient with advanced pulmonary adenocarcinoma. J Diabetes Investig 8: 798-799, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gauci ML, Laly P, Vidal-Trecan T, et al. . Autoimmune diabetes induced by PD-1 inhibitor-retrospective analysis and pathogenesis: a case report and literature review. Cancer Immunol Immunother 66: 1399-1410, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Changizzadeh PN, Mukkamalla SKR, Armenio VA. Combined checkpoint inhibitor therapy causing diabetic ketoacidosis in metastatic melanoma. J Immunother Cancer 5: 97, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Capitao R, Bello C, Fonseca R, Saraiva C. New onset diabetes after nivolumab treatment. BMJ Case Rep 2018: bcr-2017-220999, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sakurai K, Niitsuma S, Sato R, Takahashi K, Arihara Z. Painless thyroiditis and fulminant type 1 diabetes mellitus in a patient treated with an immune checkpoint inhibitor, nivolumab. Tohoku J Exp Med 244: 33-40, 2018. [DOI] [PubMed] [Google Scholar]
- 19. Sakai G, Saito D, Nakajima R, et al. . Intrinsic insulin secretion capacity might be preserved by discontinuing anti-programmed cell death protein 1 antibody treatment in ‘anti-programmed cell death protein 1 antibody-induced’ fulminant type 1 diabetes. J Diabetes Investig 9: 448-449, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Matsumura K, Nagasawa K, Oshima Y, et al. . Aggravation of diabetes, and incompletely deficient insulin secretion in a case with type 1 diabetes-resistant human leukocyte antigen DRB1*15:02 treated with nivolumab. J Diabetes Investig 9: 438-441, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Saito D, Oikawa Y, Yano Y, et al. . Detailed time course of decline in serum C-peptide levels in anti-programmed cell death-1 therapy-induced fulminant type 1 diabetes. Diabetes Care 42: e40-e41, 2019. [DOI] [PubMed] [Google Scholar]
- 22. Hirota H, Tsutsumi C, Kimata H, et al. . A case of fulminant type 1 diabetes patient accompanied by hyperinsulinemic hypoglycemia prior to clinical diagnosis of diabetes. J Japan Diab Soc 59: 210-217, 2016(in Japanese, Abstract in English). [Google Scholar]
- 23. Ansari MJ, Salama AD, Chitnis T, et al. . The programmed death-1 (PD-1) pathway regulates autoimmune diabetes in nonobese diabetic (NOD) mice. J Exp Med 198: 63-69, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yamaguchi MB, Imagawa A, Abiru N, et al. . Characteristics and clinical course of type 1 diabetes mellitus related to anti-programmed cell death-1 therapy. Diabetol Int 10: 58-66, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]