Abstract
Immune checkpoint inhibitors (ICIs) are promising drugs for various cancers. However, immune activation by ICIs can lead to immune-related adverse events (irAEs). Autoimmune encephalitis is a rare irAE, and its clinical features remain unknown. We herein report two patients with ICI-associated autoimmune encephalitis who, saliently, showed elevated adenosine deaminase (ADA) levels in the cerebrospinal fluid (CSF). This is the first report of increased ADA levels in the CSF of patients with ICI-induced autoimmune encephalitis. Although the mechanism of the ADA increase is poorly understood, elevated ADA in the CSF may be informative in the diagnosis of this rare disorder.
Keywords: immune checkpoint inhibitor, immune-related adverse event, autoimmune encephalitis, adenosine deaminase, cerebrospinal fluid
Introduction
Immune checkpoint inhibitors (ICIs) enhance antitumor immunity by blocking negative regulatory components, such as programmed cell death protein-1 (PD-1) and cytotoxic T-lymphocyte-associated antigen-4 (CTLA-4). They improve the cancer-related outcomes in specific types of cancers and are being increasingly used in clinical practice. However, inappropriate immune activation by ICIs can lead to unique adverse effects, termed immune-related adverse events (irAEs). A recent review of case reports showed that about 10% of irAEs associated with CTLA-4 and PD-1 blockade therapies are neuromuscular complications (1). Among them, irAEs in the central nervous system (CNS), such as autoimmune encephalitis, meningitis, and myelitis, are especially rare; recent large-scale registration trials of ICIs showed that serious immune-related CNS toxicities occurred in 0.4%-1.0% of patients (2). Because of such a low incidence, clinical and laboratory features of CNS irAEs have not been elucidated in detail.
Adenosine deaminase (ADA) is an enzyme involved in purine metabolism, and the elevation of this enzyme in the cerebrospinal fluid (CSF) is well recognized as a diagnostic marker for tuberculous meningitis (TBM), although the detailed mechanism of the elevation remains unknown.
We herein report two patients with lung cancer who developed autoimmune encephalitis associated with ICIs. Both presented with unusually high levels of ADA in the CSF.
Case Reports
Case 1
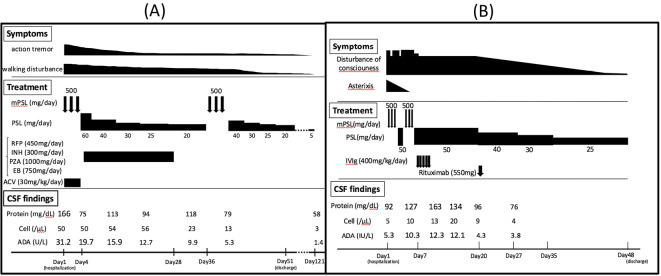
A 70-year-old man with combined pulmonary fibrosis and emphysema had lung adenocarcinoma, which had progressed despite a series of chemotherapy regimens: first with carboplatin, paclitaxel, and bevacizumab; and second with pemetrexed. He then received nivolumab biweekly (3 mg/kg), which was effective in shrinking the size of the lesion. However, four months after the first administration, his gustatory sense was impaired, and his dietary intake decreased. Shortly afterwards, he developed action tremor and difficulty walking. His symptoms worsened gradually, and he was admitted to our hospital. On admission, he had a slight fever, and a neurological examination revealed bilateral action tremor, limb ataxia, and hyperreflexia. He did not present with any typical meningeal irritation signs, such as headache, nausea, or neck stiffness. Brain magnetic resonance imaging (MRI) showed nonspecific T2-hyperintense lesions in the cerebral white matter without brain metastases. An electroencephalogram (EEG) on admission showed diffuse slow waves without epileptic discharges. A CSF analysis demonstrated pleocytosis (50 cells/μL), an elevated protein level (166 mg/dL), and a high ADA level (31.2 U/L) with a normal IgG index and no cytological evidence of cancer dissemination.
Due to concerns of autoimmune encephalitis, viral encephalitis, and tuberculosis meningitis, we administered high-dose intravenous methylprednisolone followed by oral prednisolone, acyclovir, and antituberculous medication empirically. After the confirmation of negative polymerase chain reaction (PCR) findings for herpes simplex virus, acyclovir was discontinued. The abnormal neurological findings and CSF pleocytosis, however, showed no significant change. Because of negative cultivation and nested PCR findings for tuberculosis, the antituberculous medication was discontinued. A few weeks after a second high-dose administration of intravenous methylprednisolone, his action tremor and walking disturbance improved, and the number of cells and protein level in the CSF decreased. The patient was thus discharged to another hospital with a slowly tapering regimen of oral prednisolone.
Four months later, his complaints were limited to minimal tremor, with completely normal CSF analysis results, including ADA levels (Figure A). He experienced a stable course for more than one year without neurological deterioration or tumor progression after the end of steroid treatment. The serum paraneoplastic autoantibody values were all negative, including P/Q-type calcium channel, N-type calcium channel, and voltage-gated potassium channel (VGKC) antibodies. Anti N-methyl-D-aspartate receptor (NMDAR) antibodies in the serum and the CSF were also negative.
Figure.
Clinical course and treatment. (A) Case 1, (B) Case 2. ACV: acyclovir, ADA: adenosine deaminase, CSF: cerebrospinal fluid, EB: ethambutol, INH: isoniazid, IVIg: intravenous immunoglobulin, mPSL: methylprednisolone, PSL: prednisolone, PZA: pyrazinamide, RFP: rifampicin
Case 2
We have previously described this patient in another case report, focusing on the efficacy of rituximab for the treatment of ICI-related encephalitis, without any CSF ADA data (3). We therefore present this case briefly. A 75-year-old man diagnosed with small-cell lung cancer and brain metastases in the right frontal lobe had received brain tumorectomy and whole-brain radiation therapy before the administration of ICIs. He then received nivolumab and ipilimumab combination therapy (nivolumab 1 mg/kg and ipilimumab 3 mg/kg every 3 weeks) in a clinical trial (NCT# NCT02538666). After four cycles, he developed sudden convulsions and unconsciousness and was transferred to our medical center. On admission, he showed a low-grade fever and asterixis without headache or nausea. The CSF analysis demonstrated pleocytosis (58 cells/μL), a high protein level (128.4 mg/dL), and a normal ADA level (5.3 U/L). An EEG showed intermittent diffuse slow waves without epileptic discharges, and brain MRI did not reveal new metastasis, bleeding, or infarction except for the old damage in the right frontal lobe. Bacterial culture, mycobacterium culture, cytology, and PCR findings for herpes simplex virus in the CSF were all negative. The values for anti-neural antibodies, including P/Q-type calcium channel, N-type calcium channel, VGKC antibody in the serum and anti NMDAR antibody, in the serum and CSF were also negative.
His neurological symptoms did not significantly improve despite methylprednisolone pulse therapy, so we administered intravenous immunoglobulin (IVIg) and rituximab sequentially. He recovered afterwards almost completely within 3 weeks from the onset of encephalitis and became neurologically stable with maintenance therapy of oral prednisone 25 mg daily after tapering down from 50 mg. However, his lung cancer progressed despite the combination therapy, and he died in a hospice from multiple-organ failure due to lung cancer four months later. His CSF ADA level increased to 12.3 U/L and normalized in accordance with the neurological symptoms (Figure B).
Discussion
We encountered two patients who developed acute encephalitis during the administration of ICIs. Based on their clinical course, laboratory findings, and response to the immunosuppressive therapy, we finally diagnosed them with autoimmune encephalitis caused by ICIs. There are only a few published case series of ICI-induced encephalitis; however, this clinical condition is expected to become more common as ICIs are administered to a growing number of cancer patients. In a recent case series (2), 8 out of 13 patients (62%) with ICI-induced encephalitis recovered completely following systemic immunosuppressive therapy within a median of 1 month. This finding suggests that the early diagnosis and therapeutic intervention are important to maintain the health-related quality of life of cancer patients with this irAE.
To our knowledge, this is the first report of ICI-related autoimmune encephalitis with increased CSF ADA levels. It is well known that elevated ADA in the CSF is a potential diagnostic marker for TBM, and a recent meta-analysis showed that CSF ADA levels >8 U/L suggested the diagnosis of TBM (sensitivity <59%, specificity >96%) (4). Both of our patients showed higher levels than the cut-off value, especially Case 1. Since that patient presented with subacute progressive neurological symptoms, we had difficulty distinguishing the case from TBM and administered anti-tuberculosis drugs until the nested PCR and cultivation of Mycobacterium tuberculosis provided negative findings.
The mechanism underlying the CSF ADA elevation in our patients remains unclear. High CSF ADA levels have also been reported in cases of Listeria meningitis (5), pneumococcal meningitis (6), and Cryptococcal meningitis (7), as well as leptomeningeal gliomatosis (8) and CNS lymphoma (9). A major function of ADA is related to lymphocyte proliferation and differentiation; ADA activity is found to be elevated with the cell-mediated immune response and is a marker of cellular immunity. Ipilimumab, a CTLA-4 antibody, and nivolumab, a PD-1 antibody, both activate antitumor immunity mediated by cytotoxic T cells. Therefore, we speculate that the CSF ADA elevation in our cases might be attributed to the proliferation of CD8+ T cells in the CNS caused by ICI-induced immune activation. Consistent with this hypothesis, a recent case report of myasthenic crisis plus polymyositis pathologically confirmed infiltrated CD8+ T cells in skeletal muscles, the target organ of irAEs (10).
In Case 2, as methylprednisolone pulse therapy followed by oral prednisolone did not achieve the remission of neurological symptoms, additional therapy with IVIg and rituximab, selected by referencing a previous study showing the efficacy of these drugs for ICI-induced encephalitis, had to be performed (11). Although little evidence is available at this time concerning the treatment of ICI-induced encephalitis, the clinical practice guideline recently advocated by the American Society of Clinical Oncology recommends additional IVIg therapy of corticosteroids, rituximab, or plasmapheresis be performed, depending on the severity or laboratory findings, such as oligoclonal bands or paraneoplastic antibodies (12).
Interestingly, in Case 2 and previously reported ICI-induced encephalitis cases (11), radiotherapy had been performed for brain metastasis. Williams et al. hypothesized that the disruption of the blood-brain barrier or damage resulting from stereotactic radiosurgery may facilitate T-cell and B-cell interactions with neuronal epitopes. They suggested that ICI-induced encephalitis may be associated with brain metastasis or previous radiation therapy; however, to our knowledge, no clinical studies have confirmed this.
Conclusion
In this report, we presented two lung cancer cases with ICI-induced autoimmune encephalitis that had increased ADA levels in the CSF. Elevated CSF ADA levels might be informative in the diagnosis of ICI-induced autoimmune encephalitis.
The authors state that they have no Conflict of Interest (COI).
Satoru Fujiwara and Naoya Miura contributed equally to this work.
References
- 1. Abdel-Wahab N, Shah M, Suarez-Almazor ME. Adverse events associated with immune checkpoint blockade in patients with cancer: a systematic review of case reports. PLoS One 11: e0160221, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Blackmon JT, Viator TM, Conry RM. Central nervous system toxicities of anti-cancer immune checkpoint blockade. J Neurol Neuromed 1: 39-45, 2016. [Google Scholar]
- 3. Ito M, Fujiwara S, Fujimoto D, et al. . Rituximab for nivolumab plus ipilimumab induced encephalitis in a small-cell lung cancer patient. Ann Oncol 28: 2318-2319, 2017. [DOI] [PubMed] [Google Scholar]
- 4. Tuon FF, Higashino HR, Lopes MIBF, et al. . Adenosine deaminase and tuberculous meningitis. A systematic review with meta-analysis. Scand J Infect Dis 42: 198-207, 2010. [DOI] [PubMed] [Google Scholar]
- 5. Nakae Y, Kuroiwa Y. A case of listeria meningitis showed high levels of adenosine deaminase in cerebrospinal fluid. Rinsho Shinkeigaku (Clin Neurol) 49: 590-593, 2014(in Japanese, Abstract in English). [DOI] [PubMed] [Google Scholar]
- 6. Dominguez Artiga MJ, Holanda Peña MS, Velasco Montes J, Orozco Abril RM, Teja Barbero JL. Bacterial meningitis with extraordinarily high ADA due to Streptococcus pneumoniae. Med Intensiva 34: 288-289, 2010. [DOI] [PubMed] [Google Scholar]
- 7. Tanaka Y, Satomi K. Cryptococcal meningitis associated with increased adenosine deaminase in the cerebrospinal fluid. Springerplus 5: 2093, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nishihara H, Omoto M, Ogasawara J, Koga M, Kawai M, Kanda T. Leptomeningeal gliomatosis with high levels of adenosine deaminase in the cerebrospinal fluid. Rinsho Shinkeigaku (Clin Neurol) 54: 308-312, 2014(in Japanese, Abstract in English). [DOI] [PubMed] [Google Scholar]
- 9. Taguchi S, Niwa J, Tokui K, et al. . Potent primary leptomeningeal lymphoma masquerading as tuberculous meningitis - a case report. Rinsho Shinkeigaku (Clin Neurol) 52: 416-420, 2012(in Japanese, Abstract in English). [DOI] [PubMed] [Google Scholar]
- 10. Kimura T, Fukushima S, Miyashita A, et al. . Myasthenic crisis and polymyositis induced by one dose of nivolumab. Cancer Sci 107: 1055-1058, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Williams Tenya J, Benavides David R, Patrice Kelly-Ann, et al. . Association of autoimmune encephalitis with combined immune checkpoint inhibitor treatment for metastatic cancer. JAMA Neurol 73: 928-933, 2016. [DOI] [PubMed] [Google Scholar]
- 12. Brahmer Julie R, Lacchetti Christina, Schneider Bryan J, et al. ; National Comprehensive Cancer Network.. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol 36: 1714-1768, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]