Abstract
We used baseline data from a sample of African-American women living with HIV who were recruited to participate in a stigma-reduction intervention in Chicago and Birmingham (2013–2015) to 1) evaluate the relationship between HIV-related stigma and viral suppression, and 2) assess the role of depression and nonadherence to antiretroviral therapy (ART) as mediators. Data from women were included in this secondary analysis if they were on ART, had viral load data collected within 8-weeks of study entry and had complete covariate data. We used logistic regression to estimate the total effect of HIV-related stigma (14-item Stigma Scale for Chronic Illness) on viral suppression (< 200 copies/mL), and serial mediation analysis to estimate indirect effects mediated by depressive symptoms (8-item Patient Health Questionnaire) and ART nonadherence (number of days with missed doses). Among 100 women who met study inclusion criteria, 95% reported some level of HIV-related stigma. In adjusted models, higher levels of HIV-related stigma were associated with lower odds of being virally suppressed (AOR = 0.93, 95% CI = [0.89, 0.98]). In mediation analysis, indirect effects through depression and ART nonadherence were not significant. Findings suggest that HIV-related stigma is common among African-American women living with HIV, and those who experience higher levels of stigma are less likely to be virally suppressed. However, the mechanisms remain unclear.
Keywords: HIV stigma, depression, ART adherence, viral suppression, African-American women
INTRODUCTION
African Americans are disproportionately burdened by HIV in the United States (U.S.) (1–11), and African-American women continue to be a particularly vulnerable subgroup. In 2015, African-American women who acquired HIV heterosexually accounted for 23% of new diagnoses among African Americans and 61% of new diagnoses among U.S. women – 5 times the rate of Hispanic women and 16 times the rate of White women (1). Studies also indicate that African-American women living with HIV are less likely to be on antiretroviral therapy (ART) (12, 13), experience more severe deterimental effects of late initiation and early discontinuation of ART (14), and have higher morbidity and mortality rates when compared to White women living with HIV (8, 15).
African-American women living with HIV are also vulnerable to HIV-related stigma. HIV-related stigma refers to the prejudice, discounting, discrediting and discrimination that is directed at people perceived as having HIV (16). Qualitative studies have documented that African-American women living with HIV experience debilitating HIV-related stigma (17–21). Furthermore, among these women, HIV-related stigma is associated with isolation (22), decreased psychological functioning (23), and other symptoms of depression (24, 25). In addition to poor psychosocial outcomes, HIV-related stigma has been consistently associated with diminished ART adherence (26–29). Research also suggests that associations between HIV-related stigma and ART nonadherence may be mediated through depressive symptoms (30–32).
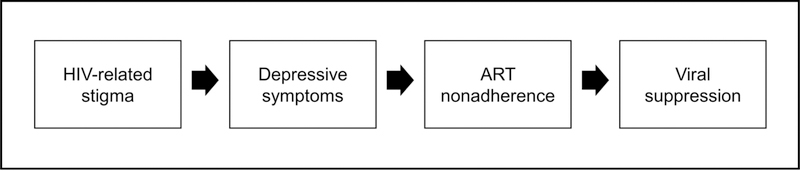
It is likely, therefore, that HIV-related stigma contributes to poor HIV viral load control among African-American women living with HIV (33). Specifically, we hypothesize that HIV-related stigma affects viral suppression through psychosocial pathways (e.g., depressive symptoms) which influence HIV health behaviors (e.g., ART nonadherence, see Figure 1). However, to date, no study has established an association between HIV-related stigma and viral suppression among African-American women living with HIV. Furthermore, no study has assessed whether associations between HIV-related stigma and viral suppression are mediated by depressive symptoms, and subsequently, by ART nonadherence.
Figure 1.
Hypothesized pathway from HIV-related stigma to viral suppression
ART – antiretroviral therapy
The purpose of this study was to 1) evaluate the relationship between HIV-related stigma and viral suppression in a sample of African-American women living with HIV, and 2) to assess the role of depressive symptoms and nonadherence to ART as potential mediators.
METHODS
Data source and study sample
This study is a secondary analysis of baseline data from the Unity Study, a multisite randomized controlled trial testing the effectiveness of a behavioral intervention to reduce HIV-related stigma among African-American women living with HIV (34). From May 2013 to October 2015, African-American women living with HIV were recruited from three clinical sites that provide HIV care in Chicago, Illinois and Birmingham, Alabama. Sites included the Northwestern University Infectious Diseases HIV clinic (NU) and the Ruth M. Rothstein CORE Center (CORE) in Chicago and the University of Alabama, Birmingham 1917 HIV Clinic (UAB) in Birmingham. To participate in the Unity Study, women needed to self-identify as African American, be at least 18 years old, and be currently receiving HIV services. Women were excluded from the Unity Study if they were foreign born and had lived in the US for less than 10 years.
Unity Study participants were included in the present secondary analysis if they reported being on ART during the baseline assessment, had HIV RNA viral load data collected within the relevant clinical window (defined below), and had complete data on covariates of interest.
Data collection
After being recruited into the study, Unity Study participants met with a research staff member for a baseline visit at which point they provided written consent to participate in the study, signed HIPAA authorizations to allow researchers to access their medical records for abstraction of clinical data, and completed baseline assessments. Baseline assessments were completed via tablet-based audio computer assisted self-interview (ACASI) and collected demographics and social-behavioral data. Relevant clinical data were abstracted from participant medical records.
Measures
Outcome
The outcome of interest was baseline viral suppression defined dichotomously as less than 200 vs. 200 or more copies/mL of plasma HIV RNA. Though a cutoff of 200 copies/mL reflects a threshold above undetectable, this cut point was chosen to accommodate potential differences in the sensitivity of viral load assays across study sites (35). A 200 copies/mL cutoff is also consistent with viral suppression as defined by the US Department of Health and Human Services (HHS) HIV/AIDS Bureau’s core performance indicators (36). Based on ART guidelines, anticipated changes in plasma viral load will occur within 8 weeks of changing an ART regimen (37). For this reason, we assessed baseline viral suppression using viral loads collected within 8-weeks of completing the baseline assessments (i.e., study entry).
Predictor
The predictor of interest was HIV-related stigma as measured by the 14-item Stigma Scale for Chronic Illness (SSCI) score. The SSCI consists of two sub-scales that assess enacted and internalized stigma. In a sample of African Americans living with HIV, the SSCI demonstrated good internal reliability (Cronbach’s alpha = 0.93) and excellent concurrent validity (38, 39). The scale includes statements such as, “Because of my illness, people were unkind to me,” and “Because of my illness, I felt left out of things.” Participants respond on a 5-point Likert-type scale ranging from 1 = “Never” to 5 = “Always”. For this study, participants were instructed to think of the past month and consider HIV as their “illness.” The responses were summed to create an index ranging from 14 to 70, with 14 indicating no reported HIV-related stigma, and higher scores reflecting greater HIV-related stigma.
Mediators
The first mediator of interest was depressive symptom severity as measured by continuous score from the 8-item Patient Health Questionnaire (PHQ-8) (40). The PHQ-8 is an abridged version of the PHQ-9 (suicidality item omitted under recommendation of the Institutional Review Board) and is widely used for screening, diagnosing, and monitoring depression (41). Regarding the preceding two weeks, participants respond to questions such as, “Have you been feeling down, depressed or hopeless?” and rank them on a 4-point Likert-type scale (from 0 = “Not at all” to 3 = “Nearly every day”). The responses were summed to create an index of depressive symptoms ranging from 0 to 24 with higher scores reflecting greater severity (0–4: minimal depression, 5–9: mild depression, 10–14: moderate depression, 15–19: moderately severe depression, 20–24: severe depression).
The second mediator of interest was nonadherence to ART, defined as the number of days in the past 30 in which the participant missed ART doses. Participants responded to the question, “In the last 30 days, on how many days did you miss at least one dose of any of your HIV medicines?” Self-reported adherence is less likely to be overreported and more likely to be associated with viral load when the recall period is longer (e.g., 30 days) (42, 43).
Covariates
Covariates were chosen a priori based on their potential for confounding. Primary covariates included Unity Study intervention arm (to account for early intervention effects among women who had their viral load collected after the intervention began), study site (NU, CORE, UAB), age in years (continuous), number of years living with HIV (continuous), and education (less than high school, high school degree or equivalent, some college, or college degree and beyond). Because alcohol use severity is strongly associated with both HIV-related stigma (44) and HIV viral suppression (45), but also strongly correlated with depression (46) and nonadherence (47) (and thus a potential co-mediator), alcohol use severity was considered a secondary covariate. Alcohol use severity was measured by the Alcohol Use Disorders Identification Test (AUDIT) Consumption (AUDIT-C) questionnaire which assesses quantity and frequency of average drinking as well as heavy episodic drinking (4 or more alcoholic drinks on one occasion) (48). AUDIT-C scores range from 0 to 12, and increased scores are associated with increased alcohol use disorder symptoms and consequences (49–53). For the present study, alcohol use severity was defined categorically (AUDIT-C = 0, 1–2, 3–7, and 8–12).
Analyses
Descriptive analyses were conducted to summarize participant characteristics. Missing items on scales were mean imputed before calculating total scores. Individuals missing entire scale responses were dropped from analysis. To test for differences across sites, we used χ2 test for independence for categorical variables and Kruskal-Wallis test (with ties) for continuous variables. A Kruskal-Wallis test is a non-parametric rank test that is appropriate when comparing medians (rather than means).
To evaluate the relationship between HIV-related stigma and viral suppression, we first estimated the total effect of HIV-related stigma on viral suppression using logistic regression. In these models, the viral suppression outcome was regressed on HIV-related stigma adjusted for primary covariates (intervention arm, study site, age, time living with HIV, and education). We calculated adjusted odds ratios (AOR) with 95% confidence intervals (95% CI). We also estimated and plotted predicted probabilties of viral suppression for all possible values of HIV-related stigma. Predicted probabilities were obtained separately for each site with the intervention arm set at control, education set at less than high school (the modal category) and age and time living with HIV set at their respective medians.
Then, to explore the roles of depressive symptom severity and ART nonadherence as mediators, we estimated indirect and direct effects along possible pathways using serial mediation analysis with 95% CI generated from bias-corrected bootstrapped standard errors. For indirect pathways of interest, we also estimated individual unstandardized coefficients.
Two secondary analyses were undertaken. First, we extended the primary models with additional adjustment for alcohol use severity. This measure was not included in primary analyses because alcohol use may represent a parallel mediating pathway. However, due to its complex associations with HIV-related stigma (44), depression (46), ART nonadherence (47), and viral suppression (45), it was also considered a potential confounder and assessed as such in secondary models.
Second, because limiting the primary study sample to women with viral loads collected within 8-weeks of study entry considerably reduced the sample size, and potentially our ability to detect effects of interest, we conducted a sensitivity analysis with a sample of women who had viral loads collected within 24-weeks of study entry (the sensitivity sample). Specifically, after assessing differences between characteristics of the primary sample and the sensitivity sample, we then repeated the primary analyses in the sensitivity sample.
All non-mediation analyses were conducted using Stata Version 13 (54). Serial mediation analyses were conducted in SPSS Version 25 (55), using the PROCESS macro, a regression-based approach that can accomodate serial mediation models with dichotomous outcomes (56). All Unity Study procedures were reviewed and approved by the Institutional Review Boards (IRB) at the University of Washington, NU, CORE, and UAB.
RESULTS
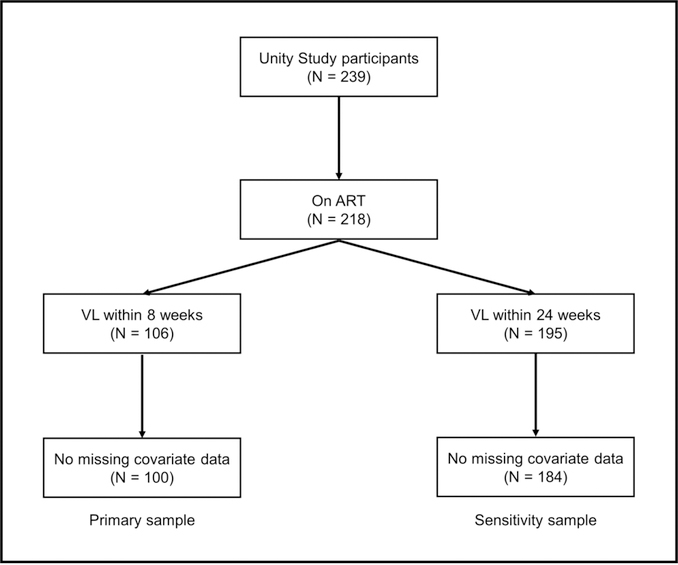
At baseline, 239 African-American women living with HIV were enrolled in the Unity Study. Among them, 218 women reported being on ART. Of those women on ART, 106 had viral loads collected within 8 weeks of study entry, and of those women, 100 had complete covariate data and were included in the primary sample. An additional 84 women on ART with complete covariate data had viral loads collected within 24 weeks of study entry, resulting in a sample of 184 women for sensitivity analyses (see Figure 2 for participant flow chart).
Figure 2.
Primary and sensitivity study samples: flow of participant study inclusion among African-American women living with HIV enrolled in an HIV-related stigma-reduction intervention (Unity Study)
ART – antiretroviral therapy, VL – viral load
Covariates of interest include age, number of years living with HIV, and education
Participant Characteristics
Table 1 summarizes participant characteristics for the primary sample (N = 100). Women in the sample were generally middle aged (median age = 45 years, interquartile range (IQR) = [38, 53]) and had been living with HIV for a median 14 years (IQR = [9, 20] years). In terms of education, most women (61%) had at least a high school education. Alcohol use was common (54%) and 24% of women reported unhealthy alcohol use (AUDIT-C ≥ 3) (57).
Table 1.
Baseline characteristics of African-American women living with HIV who participated in an HIV-related stigma-reduction intervention (Unity Study) and had viral loads collected within 8-weeks of study entry (N = 100)
| NU (N = 26) | CORE (N = 31) | UAB (N = 43) | Total (N = 100) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Median/N | (IQR/%) | Median/N | (IQR/%) | Median/N | (IQR/%) | Median/N | (IQR/%) | p-value* | |
| Age (years) | 36 years | (30–49) | 47 years | (41–54) | 45 years | (42–53) | 45 years | (38–53) | |
| Time living with HIV (years) | 15 years | (13–20) | 12 years | (7–18) | 13 years | (7–20) | 14 years | (9–20) | 0.03 |
| Education | 0.24 | ||||||||
| Less than high school | 5 | (19%) | 19 | (61%) | 15 | (35%) | 39 | (39%) | <0.01 |
| High school or equivalent | 5 | (19%) | 4 | (13%) | 9 | (21%) | 18 | (18%) | |
| College | 9 | (35%) | 7 | (23%) | 16 | (37%) | 32 | (32%) | |
| More than college | 7 | (27%) | 1 | (3%) | 3 | (7%) | 11 | (11%) | |
| Alcohol use severity (AUDIT-C score) | |||||||||
| 0 | 11 | (42%) | 21 | (68%) | 14 | (33%) | 46 | (46%) | 0.04 |
| 1–2 | 8 | (31%) | 5 | (16%) | 17 | (40%) | 30 | (30%) | |
| 3–7 | 7 | (27%) | 3 | (10%) | 11 | (26%) | 21 | (21%) | |
| 8–12 | 0 | (0%) | 2 | (6%) | 1 | (2%) | 3 | (3%) | |
| HIV-related stigma (SSCI) | 29 | (22–35) | 35 | (30–45) | 29 | (20–37) | 31 | (23–41) | |
| Depressive symptom severity (PHQ-8) | 5 | (2–13) | 7 | (3–15) | 8 | (4–12) | 7 | (3–13) | 0.07 |
| ART nonadherence (out of 30 days) | 2 | (0–4) | 0 | (0–2) | 1 | (0–2) | 1 | (0–2) | 0.53 |
| Viral suppression | 0.19 | ||||||||
| Yes | 18 | (69%) | 29 | (94%) | 32 | (74%) | 79 | (79%) | 0.05 |
| No | 8 | (31%) | 2 | (6%) | 11 | (26%) | 21 | (21%) | |
NU – Northwestern University, CORE – Ruth M. Rothstein CORE Center, UAB – University of Alabama, Birmingham
IQR – Interquartile range
p-values refer to χ2 test for independence for categorical variables and Kruskal-Wallis test (with ties) for continuous variables
AUDIT-C – Alcohol Use Disorders Identification Test - Consumption
SSCI – 14-item Stigma Scale for Chronic Illness
PHQ-8 – 8-item Patient Health Questionnaire
ART – antiretroviral therapy
The median HIV-related stigma (SSCI) score was 31 (IQR = [23, 41]), with 95% reporting at least some level of HIV-related stigma (i.e., SSCI > 14). The median PHQ-8 score was 7 (IQR = [3, 13]). Based on clinical cut points for depressive symptom severity, 64% reported minimal to mild symptoms, 29% reported moderate to moderately severe symptoms, and 7% reported severe symptoms. In this sample, about half of participants (52%) had missed at least one ART dose in the previous 30 days, and the majority of women (79%) were virally suppressed.
Participant characteristics differed slightly across sites. Compared to Ruth M. Rothstein CORE Center (CORE, N = 31) and University of Alabama, Birmingham (UAB, N = 43), participants from Northwestern University (NU, N = 26) tended to be younger (p = 0.03). Alternatively, participants from CORE were more likely to have less than a high school education (p < 0.01) and less likely to consume alcohol (p = 0.04). Participants from CORE also had slightly higher stigma scores (p = 0.07) and were more likely to be virally suppressed (p = 0.05) when compared to the other study sites.
Total effect of HIV-related stigma on viral suppression
In the primary analysis, HIV-related stigma had a statistically significantly association with viral suppression, such that higher levels of HIV-related stigma were associated with lower odds of being virally suppressed (p < .01). Specifically, a 1-unit higher SSCI score was associated with 7% lower odds of being virally suppressed (AOR = 0.93, 95% CI = [0.89, 0.98]). Results were similar with additional adjustment for alcohol use severity; a 1-unit higher SSCI score was associated with 9% lower odds of being virally suppressed (AOR = 0.91, 95% CI = [0.85, 0.97]).
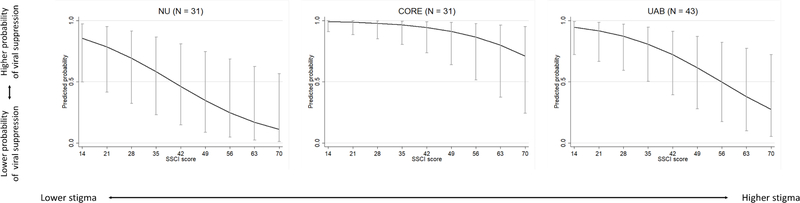
Using the primary analysis model, predicted probabilities of viral suppression at different levels of HIV-related stigma are presented for each site in Figure 3. Estimates reflect the predicted probability of viral suppression for a woman in the Unity Study control group, who is 45 years old, has lived with HIV for 14 years, and who has less than a high school education. For all sites, the predicted probability of viral suppression decreased with increased HIV-related stigma.
Figure 3.
Predicted probability of viral suppression and 95% confidence intervals for different levels of HIV-related stigma (SSCI), by study site, among a sample of African-American women living with HIV enrolled in the Unity Study (N = 100)
SSCI – 14-item Stigma Scale for Chronic Illness
NU – Northwestern University, CORE – Ruth M. Rothstein CORE Center, UAB – University of Alabama, Birmingham
Direct and indirect effects of HIV-related stigma on viral suppression
Table 2 presents results of serial mediation analyses including total effects expressed as difference in log-odds for comparison. In the primary model, after accounting for potential mediators, HIV-related stigma had a statistically significant negative direct effect on viral suppression (β = −0.085, 95% CI = [−0.145, −0.025]), and this direct effect was larger in magnitude than the estimated total effect of HIV-related stigma (β = −0.069, 95% CI = [−0.117, −0.021]). None of the possible indirect effects were statistically significant.
Table 2.
Direct and indirect effects of HIV-related stigma on viral suppression among a sample of African-American women living with HIV enrolled in the Unity Study (N = 100)
| β | (95% CI) | |
|---|---|---|
| Primary model* | ||
| Total effect (difference in log-odds) | −0.069 | (−0.117, −0.021) |
| Direct effect | −0.085 | (−0.145, −0.025) |
| Indirect effects | ||
| Stigma > depression > nonadherence > viral suppression | −0.006 | (−0.045, 0.029) |
| Stigma > depression > viral suppression | 0.010 | (−0.046, 0.054) |
| Stigma > nonadherence > viral suppression | 0.002 | (−0.087, 0.051) |
| Secondary model** | ||
| Total effect (difference in log-odds) | −0.096 | (−0.016, −0.032) |
| Direct effect | −0.238 | (−0.385, −0.091) |
| Indirect effects | ||
| Stigma > depression > nonadherence > viral suppression | −0.010 | (−0.072, 0.054) |
| Stigma > depression > viral suppression | 0.064 | (−0.140, 0.206) |
| Stigma > nonadherence > viral suppression | 0.009 | (−0.105, 0.107) |
Adjusted for treatment, site, age, education, time living with HIV
Adjusted for treatment, site, age, education, time living with HIV, alcohol use severity
Trends were similar in the secondary model; the direct effect of HIV-related stigma was larger than the total effect, and all indirect effects were statistically non-significant. Moreover, with the additional adjustment for alcohol use severity, the direct effect of HIV-related stigma was much larger in magnitude (β = −0.238, 95% CI = [−0.385, −0.091]) than in the primary model. Post hoc analyses demonstrated that alcohol use severity was not associated with HIV-related stigma in this sample.
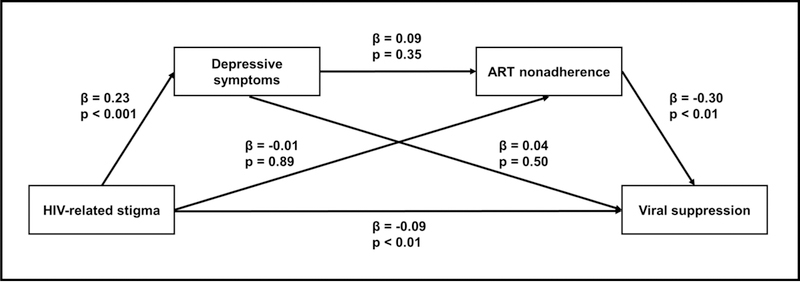
Figure 4 provides unstandardized path coefficients for the potential indirect pathways in the primary model. Looking first at the main indirect effect of interest (HIV-related stigma > depressive symptom severity > ART nonadherence > viral suppression), higher HIV-related stigma was significantly associated with higher depressive symptom severity (β = 0.23, p < .001) and ART nonadherence was significantly associated with lower likelihood of being virally suppressed (β = −0.30, p < .01). However, depressive symptom severity was not significantly associated with ART nonadherence after accounting for HIV-related stigma (p = .35) or viral suppression after accounting for ART nonadherence (p = .50). Similarly, HIV-related stigma was not significantly associated with ART nonadherence after accounting for depressive symptom severity (p =.89).
Figure 4.
Primary serial mediation model with unstandardized path coefficients estimating associations between HIV-related stigma, depressive symptoms, ART nonadherence and viral suppression among a sample of African-American women living with HIV enrolled in the Unity Study (N = 100)
ART – antiretroviral therapy
Sensitivity Analysis
Comparing the 84 additional women in the sensitivity sample to the primary sample, women were similar in terms of age, years living with HIV, education, alcohol use severity, HIV-related stigma, depressive symptom severity, and ART nonadherence (data not shown, see Appendix A). However, women who had viral loads collected within 24 weeks of study entry were slightly more likely to be virally suppressed than women who had viral loads collected within 8 weeks of study entry (χ2 [df = 1] = 4.15, p = 0.04).
In the sensitivity sample (N = 184), analysis of the total effect of HIV-related stigma on viral suppression produced estimates from both the primary and secondary models that were consistent with the primary analysis (data not shown, see Appendix B). However, trends from the serial mediation analysis in the sensitivity sample differed slightly from those in the primary sample. Namely, in the sensitivity analyses, the direct effect of HIV-related stigma was smaller than the total effect of HIV-related stigma in both the primary and secondary models (data not shown, see Appendix B).
DISCUSSION
In this sample of African-American women living with HIV, HIV-related stigma was commonly reported, and those who experienced greater levels of HIV-related stigma were less likely to be virally suppressed. In cross-sectional mediation analysis, depressive symptoms and ART nonadherence did not appear to account for this relationship. Specifically, we did not identify support for indirect pathways through depressive symptoms and ART nonadherence, and the negative association between HIV-related stigma and viral suppression remained even after accounting for these hypothesized mediators.
Almost all of the women in this sample reported some level of HIV-related stigma. This is consistent with myriad quantitative and qualitative studies of African-American women living with HIV (17, 18, 20, 21, 23, 25, 28, 58), and reiterates the importance of understanding and reducing HIV-related stigma in this population. Furthermore, in this study, we found a significant association between HIV-related stigma and lower likelihood of viral suppression, even when adjusting for potential confounders. This finding is in contrast with previous studies of people living with HIV (PLWH). For instance, in a sample of PLWH in the Netherlands, Sumari-Boer et al found no association between personalized HIV-related stigma and detectable viral load in univariate analysis (59). Similarly, in a nationally representative probability sample of PLWH in the U.S., Baugher et al found that stigma was no longer associated with viral suppression after adjusting for age (60). Some of the discrepancy in results may be attributable to key differences in study design (e.g., different stigma measures); however, it is also possible that the association observed in the current study is indicative of a particularly vulnerable subpopulation of PLWH. Specifically, because African-American women exist at the intersection of disadvantaged race and gender, the effects of HIV-related stigma may be magnified (61–63). Comparing the results from this study to analyses among other subgroups of PLWH in the U.S. could provide further insight into potential differences in the effects of HIV-related stigma across populations.
Interestingly, the results from this study did not support the hypothesis that depressive symptoms and ART nonadherence are the mechanisms through which HIV-related stigma is associated with viral suppression. As seen in Figure 3, two of the tested pathways (HIV-related stigma > depressive symptoms > ART nonadherence > viral suppression; HIV-related stigma > ART nonadherence > viral suppression) appear to break down at ART nonadherence. Although ART nonadherence was negatively associated with viral suppression as expected (64), neither HIV-related stigma nor depressive symptoms were associated with ART nonadherence in this sample; a finding inconsistent with the extant literature on ART adherence (65, 66). Possibly, we did not see these associations with ART nonadherence because women in the Unity Study were similarly adherent to their ART; almost 90% reported 4 or fewer missed doses in the past 30 days. In other words, there was minimal variability in ART nonadherence. Restricted ranges in variable values reduce statistical power and attenuate bivariate associations (67). As such, even if there were associations between ART nonadherence and other variables in the study, it would be difficult to detect in this sample. Repeating the analysis with a more sensitive measure of ART nonadherence or using a sample with greater variance in nonadherence may better elucidate the role of ART nonadherence in mediating the effects of HIV-related stigma on viral suppression among African-American women living with HIV.
Alternatively, the third pathway (HIV-related stigma > depressive symptoms > viral suppression) appears to break down between depressive symptoms and viral suppression; greater depressive symptom severity was not directly associated with likelihood of viral suppression after accounting for ART nonadherence. This finding is consistent with Leserman’s 2003 argument that direct biological effects of depression on HIV disease-related outcomes are slow and difficult to observe in the short-term (68). A longitudinal study of HIV-related stigma, depressive symptoms and viral suppression may be more appropriate for understanding this particular mechanism.
Finally, in this study, the association between HIV-related stigma and viral suppression remained after accounting for the two hypothesized mediators. Although HIV-related stigma may directly influence viral suppression, it is possible that other mediating variables, not examined in the present study, account for the observed association. For example, Turan et al found that social support was a mediating factor on the pathway between HIV-related stigma and ART adherence among a national sample of U.S. women living with HIV (32). Similarly, Helms et al found that attachment-related anxiety and concerns about inadvertent disclosure mediated an association between HIV-related stigma and ART adherence (69). Engagement in care (i.e. attended clinical visits) is another key component of effective HIV treatment that may be impacted by HIV-related stigma among African-American women living with HIV (21, 70). Finally, alcohol and other substance use may also play an important role.
Of these additional factors that may play a role in the association between HIV-related stigma and viral suppression, we assessed only alcohol use. Specifically, we fit secondary models controlling for alcohol use severity and found that alcohol use severity had a nuanced effect on the direct effect between HIV-related stigma and viral suppression. Exploration of other factors was limited by availability of relevant measures in the Unity Study and the scope of the primary hypothesis of the current study. Further research is needed to understand the many and complex roles of the myriad factors which may mediate the effect of HIV-related stigma on viral suppression in this population.
Limitations
This study had several important limitations. First, the data are cross-sectional. Though we limited our primary sample to women with viral loads collected within a clinically relevant window, we must be cautious in making causal interpretation of any observed associations. Second, there may have been limitations to our measures. Self-reported measures are subject to recall and social desirability bias. Additionally, the absence of certain measures (e.g., income, sexual orientation, binge drinking and prescribed ART dosage) may have left our analyses susceptible to residual confounding. Third, there were limitations to the sample in terms of generalizability. Namely, because the women in the Unity Study were all currently seeking HIV care and agreed to participate in a study that would require talking about living with HIV with researchers and other participants, findings may not be generalizable to the larger population of African-American women living with HIV, many of whom are not in care and are especially vulnerable to poor outcomes (13, 71). Finally, this study may have had inadequate statistical power. In serial mediation, the relationship between mediators can increase sampling variance and reduce power, especially when the sample size is small (56). Using a dichotomous outcome also reduces statistical power (72). While viral suppression has high clinical significance (and is recognized as the final stage of the HIV care continuum and considered the ultimate goal of HIV treatment) (37), using this particular measure may have contributed to insufficient power to detect the indirect effects of interest. Increasing the sample size from 100 to 184 in sensitivity analyses did not result in the detection of any additional effects; however, it is possible that an even larger sample may have been necessary.
Conclusions
In summary, the current study indicates that HIV-related stigma is common among African-American women living with HIV, and those who experience higher levels of HIV-related stigma are less likely to be virally suppressed. Though the mechanisms remain unclear, these findings suggest potential health-related consequences associated with HIV-related stigma and underscore the need for incorporating stigma-reduction programming into regular HIV clinical care, social services for PLWH, and HIV prevention efforts. Future research with African-American women living with HIV is needed to 1) better understand the roles of depressive symptoms and ART nonadherence and 2) clarify the roles of additional mediating factors such as social support, engagement in care and alcohol and other substance use. Longitudinal studies of large samples of African-American women living with HIV, including women outside of care, would be particularly valuable.
Acknowledgements
This study was made possible by funding from NIMH grant R01-MH98675 (PI: Rao) with additional support from Ms. Lipira’s AHRQ Health Services Training Award (T32 HS013853-13) and Dr. Williams’s VA Health Services Research & Development Career Development Award (CDA 12-276). The authors would also like to extend a special thank you to the women who participated in the Unity Study.
Appendix
Appendix A.
Baseline characteristics of African-American women living with HIV who participated in a trial of an HIV-related stigma-reduction intervention, comparing those who had viral loads collected within 8-weeks of study entry (primary sample) to those who had viral loads collected within 24-weeks of study entry (sensitivity sample)
| Primary sample (8-week viral load) | Sensitivity sample (24-week viral load) | |||
|---|---|---|---|---|
| N = 100 | N =184 | |||
| Median/N | (IQR/%) | Median/N | (IQR/%) | |
| Site | ||||
| NU | 26 | (26%) | 374 | (19%) |
| CORE | 31 | (31%) | 65 | (35%) |
| UAB | 43 | (43%) | 85 | (46%) |
| Age (years) | 45 years | (38–53) | 46 years | (39–54) |
| Time living with HIV (years) | 14 years | (9–20) | 14 years | (8–20) |
| Education | ||||
| Less than HS | 39 | (39%) | 68 | (37%) |
| HS or equivalent | 18 | (18%) | 41 | (22%) |
| College | 32 | (32%) | 59 | (32%) |
| More than college | 11 | (11%) | 16 | (9%) |
| Alcohol use severity (AUDIT-C score) | ||||
| 0 | 46 | (46%) | 81 | (44%) |
| 1–2 | 30 | (30%) | 63 | (34%) |
| 3–7 | 21 | (21%) | 36 | (20%) |
| 8–12 | 3 | (3%) | 4 | (2%) |
| HIV-related stigma (SSCI) | 31 | (23–41) | 30 | (22–41) |
| Depressive symptom severity (PHQ-8) | 7 | (3–13) | 6 | (2–12) |
| ART nonadherence (out of 30 days) | 1 | (0–2) | 1 | (0–2) |
| Viral suppression | ||||
| Yes | 79 | (79%) | 156 | (78%) |
| No | 21 | (21%) | 44 | (22%) |
NU – Northwestern University, CORE – Ruth M. Rothstein CORE Center, UAB – University of Alabama, Birmingham
IQR – Interquartile range
p-values refer to χ2 test for independence for categorical variables and Kruskal-Wallis test (with ties) for continuous variables
AUDIT-C – Alcohol Use Disorders Identification Test - Consumption
SSCI – 14-item Stigma Scale for Chronic Illness
PHQ-8 – 8-item Patient Health Questionnaire
ART – antiretroviral therapy
Appendix B.
Sensitivity analysis comparing direct and indirect effects of HIV-related stigma on viral suppression among two samples of African-American women living with HIV enrolled in the Unity Study, differing by viral load collection period
| Primary sample (8-week viral load) | Sensitivity sample (24-week viral load) | |||
|---|---|---|---|---|
| N = 100 | N = 184 | |||
| β | (95% CI) | β | (95% CI) | |
| Primary model* | ||||
| Total effect (difference in log-odds) | −0.069 | (−0.117, −0.021) | −0.052 | (−0.084, −0.020) |
| Direct effect | −0.085 | (−0.145, −0.025) | −0.043 | (−0.082, −0.004) |
| Indirect effects | ||||
| Stigma > depression > nonadherence > viral suppression | −0.006 | (−0.045, 0.029) | −0.003 | (−0.010, 0.002) |
| Stigma > depression > viral suppression | 0.010 | (−0.046, 0.054) | 0.010 | (−0.037, 0.014) |
| Stigma > nonadherence > viral suppression | 0.002 | (−0.087, 0.051) | 0.001 | (−0.012, 0.010) |
| Secondary model** | ||||
| Total effect (difference in log-odds) | −0.096 | (−0.016, −0.032) | −0.056 | (−0.089, −0.022) |
| Direct effect | −0.238 | (−0.385, −0.091) | −0.052 | (−0.092, −0.011) |
| Indirect effects | ||||
| Stigma > depression > nonadherence > viral suppression | −0.010 | (−0.072, 0.054) | −0.002 | (−0.011, 0.003) |
| Stigma > depression > viral suppression | 0.064 | (−0.140, 0.206) | 0.006 | (−0.034, 0.021) |
| Stigma > nonadherence > viral suppression | 0.009 | (−0.105, 0.107) | 0.001 | (−0.012, 0.011) |
Adjusted for treatment, site, age, education, time living with HIV
Adjusted for treatment, site, age, education, time living with HIV, alcohol use severity
References
- 1.CDC. Fact sheet: HIV among African Americans 2017 [updated February 2017 Available from: https://www.cdc.gov/nchhstp/newsroom/docs/factsheets/cdc-hiv-aa-508.pdf.
- 2.Torian LV, Wiewel EW, Liu KL, Sackoff JE, Frieden TR. Risk factors for delayed initiation of medical care after diagnosis of human immunodeficiency virus. Archives of internal medicine. 2008;168(11):1181–7. [DOI] [PubMed] [Google Scholar]
- 3.Novak RM, Hart RL, Chmiel JS, Brooks JT, Buchacz K. Disparities in Initiation of Combination Antiretroviral Treatment and in Virologic Suppression Among Patients in the HIV Outpatient Study, 2000–2013. J Acquir Immune Defic Syndr. 2015;70(1):23–32. [DOI] [PubMed] [Google Scholar]
- 4.CDC. Fact sheet: HIV in the United States: The Stages of Care: Centers for Disease Control and Prevention; 2014. [CDC Fact Sheet]. Available from: http://www.cdc.gov/nchhstp/newsroom/docs/HIV-Stages-of-Care-Factsheet-508.pdf.
- 5.Beer L, Mattson CL, Bradley H, Skarbinski J. Understanding Cross-Sectional Racial, Ethnic, and Gender Disparities in Antiretroviral Use and Viral Suppression Among HIV Patients in the United States. Medicine. 2016;95(13):e3171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ribaudo HJ, Smith KY, Robbins GK, Flexner C, Haubrich R, Chen Y, et al. Racial differences in response to antiretroviral therapy for HIV infection: an AIDS clinical trials group (ACTG) study analysis. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2013;57(11):1607–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beer L, Bradley H, Mattson CL, Johnson CH, Hoots B, Shouse RL. Trends in Racial and Ethnic Disparities in Antiretroviral Therapy Prescription and Viral Suppression in the United States, 2009–2013. J Acquir Immune Defic Syndr. 2016;73(4):446–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lesko CR, Cole SR, Miller WC, Westreich D, Eron JJ, Adimora AA, et al. Ten-year Survival by Race/Ethnicity and Sex Among Treated, HIV-infected Adults in the United States. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2015;60(11):1700–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lemly DC, Shepherd BE, Hulgan T, Rebeiro P, Stinnette S, Blackwell RB, et al. Race and sex differences in antiretroviral therapy use and mortality among HIV-infected persons in care. The Journal of infectious diseases. 2009;199(7):991–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murphy S, Xu J, Kochanek K. Deaths: Final data for 2010. 2013. May 8, 2013. Contract No.: 4. [PubMed] [Google Scholar]
- 11.Singh GK, Azuine RE, Siahpush M. Widening Socioeconomic, Racial, and Geographic Disparities in HIV/AIDS Mortality in the United States, 1987–2011. Advances in preventive medicine. 2013;2013:657961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lillie-Blanton M, Stone VE, Snow Jones A, Levi J, Golub ET, Cohen MH, et al. Association of race, substance abuse, and health insurance coverage with use of highly active antiretroviral therapy among HIV-infected women, 2005. American journal of public health. 2010;100(8):1493–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cohen MH, Cook JA, Grey D, Young M, Hanau LH, Tien P, et al. Medically eligible women who do not use HAART: the importance of abuse, drug use, and race. American journal of public health. 2004;94(7):1147–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Losina E, Schackman BR, Sadownik SN, Gebo KA, Walensky RP, Chiosi JJ, et al. Racial and sex disparities in life expectancy losses among HIV-infected persons in the united states: impact of risk behavior, late initiation, and early discontinuation of antiretroviral therapy. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2009;49(10):1570–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meditz AL, MaWhinney S, Allshouse A, Feser W, Markowitz M, Little S, et al. Sex, race, and geographic region influence clinical outcomes following primary HIV-1 infection. The Journal of infectious diseases. 2011;203(4):442–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Herek GM, Mitnick L, Burris S, Chesney M, Devine P, Fullilove MT, et al. Workshop report: AIDS and stigma: a conceptual framework and research agenda. AIDS Public Policy J. 1998;13(1):36–47. [PubMed] [Google Scholar]
- 17.Buseh AG, Stevens PE. Constrained but not determined by stigma: resistance by African American women living with HIV. Women & health. 2006;44(3):1–18. [DOI] [PubMed] [Google Scholar]
- 18.Sanicki A, Mannell J. HIV-positive African-American women’s perspectives on engaging communities in the response to HIV/AIDS in Washington, D.C. AIDS care. 2015;27(10):1213–9. [DOI] [PubMed] [Google Scholar]
- 19.Muturi N, An S. HIV/AIDS stigma and religiosity among African American women. Journal of health communication. 2010;15(4):388–401. [DOI] [PubMed] [Google Scholar]
- 20.Fletcher F, Ingram LA, Kerr J, Buchberg M, Bogdan-Lovis L, Philpott-Jones S. “She Told Them, Oh That Bitch Got AIDS”: Experiences of Multilevel HIV/AIDS-Related Stigma Among African American Women Living with HIV/AIDS in the South. AIDS patient care and STDs. 2016;30(7):349–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McDoom MM, Bokhour B, Sullivan M, Drainoni ML. How older black women perceive the effects of stigma and social support on engagement in HIV care. AIDS patient care and STDs. 2015;29(2):95–101. [DOI] [PubMed] [Google Scholar]
- 22.Grodensky CA, Golin CE, Jones C, Mamo M, Dennis AC, Abernethy MG, et al. “I should know better”: the roles of relationships, spirituality, disclosure, stigma, and shame for older women living with HIV seeking support in the South. The Journal of the Association of Nurses in AIDS Care: JANAC. 2015;26(1):12–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Clark HJ, Lindner G, Armistead L, Austin BJ. Stigma, disclosure, and psychological functioning among HIV-infected and non-infected African-American women. Women & health. 2003;38(4):57–71. [DOI] [PubMed] [Google Scholar]
- 24.Wingood GM, Diclemente RJ, Mikhail I, McCree DH, Davies SL, Hardin JW, et al. HIV discrimination and the health of women living with HIV. Women & health. 2007;46(2–3):99–112. [DOI] [PubMed] [Google Scholar]
- 25.Vyavaharkar M, Moneyham L, Corwin S, Saunders R, Annang L, Tavakoli A. Relationships between stigma, social support, and depression in HIV-infected African American women living in the rural Southeastern United States. Journal of the Association of Nurses in AIDS Care. 2010;21(2):144–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rao D, Kekwaletswe TC, Hosek S, Martinez J, Rodriguez F. Stigma and social barriers to medication adherence with urban youth living with HIV. AIDS care. 2007;19(1):28–33. [DOI] [PubMed] [Google Scholar]
- 27.Rintamaki LS, Davis TC, Skripkauskas S, Bennett CL, Wolf MS. Social stigma concerns and HIV medication adherence. AIDS patient care and STDs. 2006;20(5):359–68. [DOI] [PubMed] [Google Scholar]
- 28.Edwards LV. Perceived social support and HIV/AIDS medication adherence among African American women. Qualitative health research. 2006;16(5):679–91. [DOI] [PubMed] [Google Scholar]
- 29.Carr RL, Gramling LF. Stigma: a health barrier for women with HIV/AIDS. The Journal of the Association of Nurses in AIDS Care: JANAC. 2004;15(5):30–9. [DOI] [PubMed] [Google Scholar]
- 30.Rao D, Feldman BJ, Fredericksen RJ, Crane PK, Simoni JM, Kitahata MM, et al. A structural equation model of HIV-related stigma, depressive symptoms, and medication adherence. AIDS and behavior. 2012;16(3):711–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mitzel LD, Vanable PA, Brown JL, Bostwick RA, Sweeney SM, Carey MP. Depressive Symptoms Mediate the Effect of HIV-Related Stigmatization on Medication Adherence Among HIV-Infected Men Who Have Sex with Men. AIDS and behavior. 2015. [DOI] [PubMed] [Google Scholar]
- 32.Turan B, Smith W, Cohen MH, Wilson TE, Adimora AA, Merenstein D, et al. Mechanisms for the Negative Effects of Internalized HIV-Related Stigma on Antiretroviral Therapy Adherence in Women: The Mediating Roles of Social Isolation and Depression. J Acquir Immune Defic Syndr. 2016;72(2):198–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Turan B, Hatcher AM, Weiser SD, Johnson MO, Rice WS, Turan JM. Framing Mechanisms Linking HIV-Related Stigma, Adherence to Treatment, and Health Outcomes. American journal of public health. 2017;107(6):863–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rao D, Kemp CG, Huh D, Nevin PE, Turan J, Cohn SE, et al. Stigma Reduction Among African American Women with HIV: UNITY Health Study. J Acquir Immune Defic Syndr. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lalama CM, Jennings C, Johnson VA, Coombs RW, McKinnon JE, Bremer JW, et al. Comparison of Three Different FDA-Approved Plasma HIV-1 RNA Assay Platforms Confirms the Virologic Failure Endpoint of 200 Copies per Milliliter Despite Improved Assay Sensitivity. Journal of clinical microbiology. 2015;53(8):2659–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.U.S. Department of Health and Human Services. U.S. Department of Health and Human Services (HHS) Performance Indicators 2016. [updated 5/11/2016 Available from: https://hab.hrsa.gov/stateprofiles/HHS-Indicators.aspx.
- 37.Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. . 2016. [Google Scholar]
- 38.Rao D, Choi SW, Victorson D, Bode R, Peterman A, Heinemann A, et al. Measuring stigma across neurological conditions: the development of the stigma scale for chronic illness (SSCI). Quality of life research: an international journal of quality of life aspects of treatment, care and rehabilitation. 2009;18(5):585–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rao D, Molina Y, Lambert N, Cohn SE. Assessing Stigma among African Americans Living with HIV. Stigma and health. 2016;1(3):146–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kroenke K, Strine TW, Spitzer RL, Williams JB, Berry JT, Mokdad AH. The PHQ-8 as a measure of current depression in the general population. Journal of affective disorders. 2009;114(1–3):163–73. [DOI] [PubMed] [Google Scholar]
- 41.Center for quality assessment and improvement in mental health. The patient health questionnaire (PHQ-9) overview 1999. [Google Scholar]
- 42.Simoni JM, Kurth AE, Pearson CR, Pantalone DW, Merrill JO, Frick PA. Self-report measures of antiretroviral therapy adherence: A review with recommendations for HIV research and clinical management. AIDS and behavior. 2006;10(3):227–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lu M, Safren SA, Skolnik PR, Rogers WH, Coady W, Hardy H, et al. Optimal recall period and response task for self-reported HIV medication adherence. AIDS and behavior. 2008;12(1):86–94. [DOI] [PubMed] [Google Scholar]
- 44.Galvan FH, Davis EM, Banks D, Bing EG. HIV stigma and social support among African Americans. AIDS patient care and STDs. 2008;22(5):423–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hahn JA, Samet JH. Alcohol and HIV disease progression: weighing the evidence. Current HIV/AIDS reports. 2010;7(4):226–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sullivan LE, Fiellin DA, O’Connor PG. The prevalence and impact of alcohol problems in major depression: a systematic review. The American journal of medicine. 2005;118(4):330–41. [DOI] [PubMed] [Google Scholar]
- 47.Hendershot CS, Stoner SA, Pantalone DW, Simoni JM. Alcohol use and antiretroviral adherence: review and meta-analysis. J Acquir Immune Defic Syndr. 2009;52(2):180–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bush K, Kivlahan DR, McDonell MB, Fihn SD, Bradley KA. The AUDIT alcohol consumption questions (AUDIT-C): an effective brief screening test for problem drinking. Ambulatory Care Quality Improvement Project (ACQUIP). Alcohol Use Disorders Identification Test. Archives of internal medicine. 1998;158(16):1789–95. [DOI] [PubMed] [Google Scholar]
- 49.Rubinsky AD, Dawson DA, Williams EC, Kivlahan DR, Bradley KA. AUDIT-C scores as a scaled marker of mean daily drinking, alcohol use disorder severity, and probability of alcohol dependence in a U.S. general population sample of drinkers. Alcoholism, clinical and experimental research. 2013;37(8):1380–90. [DOI] [PubMed] [Google Scholar]
- 50.Chavez LJ, Williams EC, Lapham G, Bradley KA. Association between alcohol screening scores and alcohol-related risks among female veterans affairs patients. Journal of studies on alcohol and drugs. 2012;73(3):391–400. [DOI] [PubMed] [Google Scholar]
- 51.Bryson CL, Au DH, Sun H, Williams EC, Kivlahan DR, Bradley KA. Alcohol screening scores and medication nonadherence. Annals of internal medicine. 2008;149(11):795–804. [DOI] [PubMed] [Google Scholar]
- 52.Bradley KA, DeBenedetti AF, Volk RJ, Williams EC, Frank D, Kivlahan DR. AUDIT-C as a brief screen for alcohol misuse in primary care. Alcoholism, clinical and experimental research. 2007;31(7):1208–17. [DOI] [PubMed] [Google Scholar]
- 53.Bradley KA, Rubinsky AD, Lapham GT, Berger D, Bryson C, Achtmeyer C, et al. Predictive validity of clinical AUDIT-C alcohol screening scores and changes in scores for three objective alcohol-related outcomes in a Veterans Affairs population. Addiction (Abingdon, England). 2016;111(11):1975–84. [DOI] [PubMed] [Google Scholar]
- 54.StataCorp. Stata Statistical Software: Release 13. College Station, TX: StataCorp LP; 2013. [Google Scholar]
- 55.Corp I. SPSS Statistics for Windows, version 25. Armonk, NY: IBM Corp; 2017. [Google Scholar]
- 56.Hayes AF. Introduction to mediation, moderation, and conditional process analysis: A regression-based approach: Guilford Press; 2013. [Google Scholar]
- 57.Bradley KA, Bush KR, Epler AJ, Dobie DJ, Davis TM, Sporleder JL, et al. Two brief alcohol-screening tests From the Alcohol Use Disorders Identification Test (AUDIT): validation in a female Veterans Affairs patient population. Archives of internal medicine. 2003;163(7):821–9. [DOI] [PubMed] [Google Scholar]
- 58.Rao D, Desmond M, Andrasik M, Rasberry T, Lambert N, Cohn SE, et al. Feasibility, acceptability, and preliminary efficacy of the unity workshop: an internalized stigma reduction intervention for African American women living with HIV. AIDS patient care and STDs. 2012;26(10):614–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sumari-de Boer IM, Sprangers MA, Prins JM, Nieuwkerk PT. HIV stigma and depressive symptoms are related to adherence and virological response to antiretroviral treatment among immigrant and indigenous HIV infected patients. AIDS and behavior. 2012;16(6):1681–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Baugher AR, Beer L, Fagan JL, Mattson CL, Freedman M, Skarbinski J, et al. Prevalence of Internalized HIV-Related Stigma Among HIV-Infected Adults in Care, United States, 2011–2013. AIDS and behavior. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Parker R, Aggleton P. HIV and AIDS-related stigma and discrimination: a conceptual framework and implications for action. Social science & medicine (1982). 2003;57(1):13–24. [DOI] [PubMed] [Google Scholar]
- 62.Logie CH, James L, Tharao W, Loutfy MR. HIV, gender, race, sexual orientation, and sex work: a qualitative study of intersectional stigma experienced by HIV-positive women in Ontario, Canada. PLoS medicine. 2011;8(11):e1001124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rao D, Andrasik MP, Lipira L. HIV Stigma Among Black Women in the United States: Intersectionality, Support, Resilience. American journal of public health. 2018;108(4):446–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gross R, Bilker WB, Friedman HM, Strom BL. Effect of adherence to newly initiated antiretroviral therapy on plasma viral load. AIDS. 2001;15(16):2109–17. [DOI] [PubMed] [Google Scholar]
- 65.Katz IT, Ryu AE, Onuegbu AG, Psaros C, Weiser SD, Bangsberg DR, et al. Impact of HIV-related stigma on treatment adherence: systematic review and meta-synthesis. Journal of the International AIDS Society. 2013;16(3 Suppl 2):18640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gonzalez JS, Batchelder AW, Psaros C, Safren SA. Depression and HIV/AIDS treatment nonadherence: a review and meta-analysis. J Acquir Immune Defic Syndr. 2011;58(2):181–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shadish WR, Cook TD, Campbell DT. Experimental and quasi-experimental designs for generalized causal inference: Wadsworth Cengage learning; 2002. [Google Scholar]
- 68.Leserman J HIV disease progression: depression, stress, and possible mechanisms. Biological psychiatry. 2003;54(3):295–306. [DOI] [PubMed] [Google Scholar]
- 69.Helms BC, Turan JM, Atkins G, Kempf MC, Clay OJ, Raper JL, et al. Interpersonal Mechanisms Contributing to the Association Between HIV-Related Internalized Stigma and Medication Adherence. AIDS and behavior. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Walcott M, Kempf MC, Merlin JS, Turan JM. Structural community factors and sub-optimal engagement in HIV care among low-income women in the Deep South of the USA. Culture, health & sexuality. 2015:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kaiser Family Foundation. Fact sheet: Black Americans and HIV/AIDS 2017. [Available from: http://kff.org/hivaids/fact-sheet/black-americans-and-hiv-aids/.
- 72.Bhandari M, Lochner H, Tornetta P 3rd. Effect of continuous versus dichotomous outcome variables on study power when sample sizes of orthopaedic randomized trials are small. Archives of orthopaedic and trauma surgery. 2002;122(2):96–8. [DOI] [PubMed] [Google Scholar]