Abstract
Purpose
This study was designed to determine the incidence of venous and arterial thromboembolic events (TEEs) in patients treated with cisplatin-based chemotherapy and to analyze the prognostic value of patients' baseline and treatment characteristics in predicting TEE occurrence.
Patients and Methods
We performed a large retrospective analysis of all patients treated with cisplatin-based chemotherapy for any type of malignancy at Memorial Sloan-Kettering Cancer Center in 2008. A TEE was cisplatin-associated if it occurred between the time of the first dose of cisplatin and 4 weeks after the last dose.
Results
Among 932 patients, 169 (18.1%) experienced a TEE during treatment or within 4 weeks of the last dose. TEEs included deep vein thrombosis (DVT) alone in 49.7%, pulmonary embolus (PE) alone in 25.4%, DVT plus PE in 13.6%, arterial TEE alone in 8.3%, or DVT plus arterial TEE in 3.0%. TEEs occurred within 100 days of initiation of treatment in 88% of patients. By univariate analysis, sex, age, race, Karnofsky performance status (KPS), exposure to erythropoiesis-stimulating agents, presence of central venous catheter (CVC), site of cancer, stage of cancer, leukocyte and hemoglobin levels, and Khorana score were all identified as risk factors. However, by multivariate analysis, only age, KPS, presence of CVC, and Khorana score retained significance.
Conclusion
This large retrospective analysis confirms the unacceptable incidence of TEEs in patients receiving cisplatin-based chemotherapy. In view of the controversy associated with prophylactic anticoagulation in patients with cancer treated with chemotherapy, randomized studies are urgently needed in this specific cancer population treated with cisplatin-based regimens.
INTRODUCTION
Cancer is associated with an increased risk in venous and arterial thromboembolic events (TEEs), including deep venous thrombosis (DVT), pulmonary embolus (PE), cerebrovascular accident, and unstable angina/myocardial infarction (MI). Although the average annual incidence rate of venous thromboembolism in the general population is approximately 117 per 100,000, the incidence in patients with cancer is approximately one in 200.1,2 On the basis of a large cohort study,3 cancer alone is associated with a 4.1-fold increased risk of TEE. As a leading cause of death, TEEs significantly reduce survival of patients with cancer.4–6 In addition, TEEs result in a substantial economic burden because of the need for hospitalization, with a mean estimated cost of more than $20,000, which is probably an underestimate because 25% of patients with cancer who have a TEE potentially need to be readmitted as a result of bleeding or a recurrent event.7
Several factors are known to influence the incidence of TEEs in patients with cancer, including but not limited to age, type/site/extent of cancer, hospitalization, indwelling catheters, and hereditary predisposition. Besides these factors, active treatment with several agents, including chemotherapy, imparts a significant risk. Several controlled clinical trials of systemic chemotherapy in women with breast cancer have shown a clear link between chemotherapy and increased incidence of TEEs.8–15 A large cohort study3 has shown that although cancer alone is associated with a 4.1-fold increase in the risk of thrombosis, the addition of chemotherapy enhances that risk to 6.5-fold.
Among chemotherapeutic agents, cisplatin-based regimens particularly have been associated with a wide range of thromboembolic complications.16–25 In one retrospective study of patients with urothelial cancer, 35 (12.9%) of 271 patients receiving multiagent cisplatin-based chemotherapy experienced a TEE, with three events directly resulting in death.26 Another retrospective study of patients with germ cell cancer receiving cisplatin-based chemotherapy found that 15 (8.4%) of 179 patients experienced a TEE, with one fatality.27 In a prospective study of 108 patients with stage III to IV non–small-cell lung cancer treated with cisplatin and gemcitabine, 19 (17.6%) of 108 patients experienced a TEE. Four of those 19 patients died as a result of the event.28 The direct role of cisplatin in thromboembolic toxicity is also suggested in a prospective, randomized, double-blind trial comparing cisplatin-based chemotherapy with oxaliplatin-based chemotherapy in 214 patients with locally advanced or metastatic adenocarcinoma of the stomach or gastroesophageal junction. This study found a statistically significant difference in the incidence of TEEs between the two groups: 7.8% in the cisplatin group versus 0.9% in the oxaliplatin group.29 Similar observations were reported in an analysis of the Randomised ECF for Advanced and Locally Advanced Esophagogastric Cancer 2 (REAL-2) study,30 which showed a statistically significant difference in the incidence of TEEs in the cisplatin-containing regimens compared with the oxaliplatin-containing regimens in patients with advanced gastroesophageal cancer (15.1% v 7.6%, respectively; P < .001).
The results of these studies as well as our clinical experience prompted us to conduct a large retrospective analysis of all patients treated with cisplatin-based chemotherapy in 2008 for any type of malignancy at Memorial Sloan-Kettering Cancer Center (MSKCC). The primary objective of this study was to determine the overall incidence of TEEs in these patients.
PATIENTS AND METHODS
Patient Population
In this retrospective study, we have included all adult patients treated with cisplatin-based chemotherapy who received the first cisplatin dose between January 1, 2008, and December 31, 2008, and had at least 4 weeks of follow-up after their last cisplatin dose. Cisplatin treatment into 2009 was allowed as long as they had at least 4 weeks of follow-up after their last cisplatin dose as of July 31, 2009, when the data were censored for analysis. This study was approved by the institutional review board at MSKCC.
Retrospective Data Collection
Electronic medical records were reviewed to identify patients who experienced a venous or arterial TEE. As initial screening, all reports of imaging studies were reviewed. A TEE occurrence was recorded only if an imaging study documented such an event. Because events diagnosed in other institutions could be missed by this initial screening, the patients' electronic medication profiles were scrutinized searching for the addition of an anticoagulant or antiplatelet agent, which would suggest that a TEE had occurred. If such an addition was noted, further investigation was conducted to confirm the occurrence of a TEE. TEE occurrence was based only on results of angiography, magnetic resonance imaging, computed tomography, venous Doppler ultrasound, ventilation/perfusion scan, or clinical and laboratory documentation of MI by ECG or troponin. A TEE was considered cisplatin-associated if it was diagnosed between the time of the first dose of cisplatin and 4 weeks after the last dose and was determined to be a new event not seen on a previous recent imaging study or, for MI, a new event as determined by clinical presentation, ECG, or troponin.
Baseline characteristics were collected by using electronic medical records and included hemoglobin level, platelet and WBC counts, site of cancer, extent of disease, and thrombotic risk factors. The latter included surgery within 2 months of beginning cisplatin-based treatment, cardiac disease, obesity (body mass index [BMI] ≥ 30 kg/m2), diabetes, Karnofsky performance status (KPS), history of previous TEE, presence of central venous catheter (CVC), presence of an inferior vena cava filter, and administration of an erythropoiesis-stimulating agent (ESA) within 6 weeks before cisplatin initiation, during cisplatin treatment, or within 4 weeks after the last cisplatin dose. Concurrent anticoagulation/antiplatelet therapy during the cisplatin-based chemotherapy was also documented.
Treatment characteristics were collected, including chemotherapy regimens, number of cisplatin doses, mean cisplatin dose, and cumulative cisplatin dose. The mean cisplatin dose was calculated by dividing the cumulative cisplatin dose by the number of doses.
Statistical Analysis
The primary objective of this study was to determine the overall incidence, timing, and characteristics of TEEs (venous or arterial) in adult patients treated with cisplatin-based chemotherapy. Secondary objectives were to analyze the prognostic value of baseline and treatment characteristics in predicting the occurrence of a TEE in patients treated with cisplatin-based chemotherapy. The association between each baseline and treatment characteristic detailed in the previous section and the development of a TEE during the defined treatment period was evaluated by using the χ2 test for categorical variables, the Mantel-Haenszel test for trend for ordinal variables, and the Wilcoxon rank sum test for continuous variables. Variables found to be significant (P ≤ .05) by univariate analysis were subsequently entered into a multivariate logistic regression model.
RESULTS
Baseline Characteristics and Treatment Characteristics of the Study Population
Overall, 1,093 patients received at least one dose of cisplatin in 2008, of which 932 met the inclusion criteria. Patients' baseline and treatment characteristics are detailed in Table 1. Also included in Table 1 is the Khorana risk score.31 The following were reasons for exclusion: age was younger than 18 years (n = 1), an accurate cisplatin start date in 2008 could not be determined (n = 1), patient was lost to follow-up/insufficient follow-up after last cisplatin dose/still receiving cisplatin as of July 31, 2009 (n = 11), and the first cisplatin dose was received in 2007 (n = 148).
Table 1.
Baseline and Treatment Characteristics of the Total Study Population (N = 932)
| Characteristic | No. of Patients | % |
|---|---|---|
| Age, years | ||
| Median | 60 | |
| Range | 19-87 | |
| Sex | ||
| Male | 500 | 53.6 |
| Female | 432 | 46.4 |
| Race/ethnicity | ||
| White | 791 | 84.9 |
| Asian | 62 | 6.7 |
| African American | 60 | 6.4 |
| Other/unknown | 19 | 2.0 |
| Thrombosis risk factors | ||
| Hypertension | 295 | 31.7 |
| Coronary artery disease | 62 | 6.7 |
| Atrial fibrillation/arrhythmias | 26 | 2.8 |
| Prior strokes | 4 | 0.4 |
| Central venous catheter | 266 | 28.5 |
| Surgery within 2 months | 169 | 18.1 |
| ESA 6 weeks before/4 weeks after | 167 | 17.9 |
| Karnofsky performance status, % | ||
| Median | 80 | |
| IQR | 80-90 | |
| Obesity (BMI ≥ 30 kg/m2) | 157 | 16.8 |
| Diabetes | 99 | 10.6 |
| Previous TEE | 90 | 9.7 |
| IVC filter | 11 | 1.2 |
| Khorana variables | ||
| Site of cancer | ||
| Very high risk | 193 | 21.7 |
| High risk | 319 | 34.2 |
| Prechemotherapy platelet count ≥ 350,000/μL | 230 | 24.7 |
| Prechemotherapy leukocyte count > 11,000/μL | 156 | 16.7 |
| Prechemotherapy hemoglobin < 10 g/dL or use of ESA | 214 | 23.0 |
| BMI ≥ 35 kg/m2 | 35 | 3.8 |
| Khorana risk score | ||
| 0 | 224 | 24.0 |
| 1 | 293 | 31.4 |
| 2 | 245 | 26.3 |
| 3 | 129 | 13.8 |
| 4 | 35 | 3.8 |
| 5 | 6 | 0.6 |
| Khorana risk group | ||
| Low | 224 | 24.0 |
| Intermediate | 538 | 57.7 |
| High | 170 | 18.2 |
| Cancer diagnosis | ||
| Lung | 204 | 21.9 |
| Gastric/GE junction | 114 | 12.2 |
| Head and neck | 94 | 10.1 |
| Pancreatic | 79 | 8.5 |
| Melanoma | 69 | 7.4 |
| Ovarian | 57 | 6.1 |
| Esophageal | 46 | 4.9 |
| Germ cell | 39 | 4.2 |
| Cervical/uterine/vulvar | 39 | 4.2 |
| Bladder | 33 | 3.5 |
| Endometrial | 22 | 2.4 |
| Cholangiocarcinoma | 18 | 1.9 |
| Mesothelioma | 15 | 1.6 |
| Colorectal/anal/small bowel | 13 | 1.4 |
| Other | 90 | 9.7 |
| Stage of disease | ||
| Early | 66 | 7.1 |
| Locally advanced | 415 | 44.5 |
| Metastatic | 426 | 45.7 |
| Undocumented | 19 | 2 |
| Other | 6 | 0.6 |
| Chemotherapy regimens | ||
| Cisplatin + gemcitabine | 134 | 14.4 |
| Cisplatin + radiation | 94 | 10.1 |
| Cisplatin + irinotecan | 89 | 9.6 |
| Cisplatin + etoposide | 80 | 8.6 |
| Cisplatin + vinblastine + temozolomide | 57 | 6.1 |
| Cisplatin + pemetrexed | 43 | 4.6 |
| Cisplatin IP + paclitaxel IV/IP | 33 | 3.5 |
| Cisplatin + pemetrexed + bevacizumab | 33 | 3.5 |
| Other cisplatin-based regimens | 369 | 39.6 |
| Mean cisplatin dose, mg/m2 | ||
| Median | 40 | |
| IQR | 25-65 | |
| Cumulative cisplatin dose, mg/m2 | ||
| Median | 200 | |
| IQR | 120-300 | |
| No. of cisplatin doses | ||
| Median | 5 | |
| IQR | 3-7 | |
| Entire time on cisplatin, days | ||
| Median | 61 | |
| IQR | 28-91 | |
Abbreviations: BMI, body mass index; ESA, erythropoiesis-stimulating agent; GE, gastroesophageal; IP, intraperitoneal; IQR, interquartile range; IV, intravenous; IVC, inferior vena cava; TEE, thromboembolic event.
Incidence, Timing, and Characteristics of TEEs
Among 932 patients included in the study, 169 (18.1%; 95% CI, 15.7% to 20.8%) patients developed a TEE within 4 weeks of their last cisplatin dose (Table 2). Patients who experienced two or more TEEs either simultaneously or at different times were counted as one event. The majority of events were DVTs alone (n = 84), followed by PEs alone (n = 43), DVT plus PE (n = 23), arterial thromboses alone (n = 14), and DVT plus an arterial thrombosis (n = 5). Details of events in Table 2 show that 95 events were symptomatic, and 74 were found incidentally. We consider that most, if not all, events would warrant therapeutic intervention.
Table 2.
Overall Incidence of Thromboembolic Events (N = 932)
| Thromboembolic Event | No. of Patients | % |
|---|---|---|
| Thrombosis | 169 | 18.1 |
| Types of thromboses (n = 169) | ||
| DVT alone | 84 | 49.7 |
| PE alone | 43 | 25.4 |
| DVT + PE | 23 | 13.6 |
| Arterial thrombosis alone | 14 | 8.3 |
| DVT + arterial thrombosis | 5 | 3.0 |
| Subtypes of DVTs (n = 112) | ||
| Proximal lower extremity | 22 | 19.6 |
| Proximal lower and distal lower extremity | 18 | 16.1 |
| Proximal lower extremity and central* | 5 | 4.4 |
| Proximal lower extremity and central* and distal lower extremity | 1 | 0.9 |
| Proximal upper extremity | 2 | 1.8 |
| Proximal upper and distal upper extremity | 3 | 2.7 |
| Proximal upper and internal jugular vein and distal upper extremity | 4 | 3.6 |
| Internal jugular vein | 5 | 4.4 |
| Internal jugular vein and distal upper extremity | 1 | 0.9 |
| Central* | 27 | 24.1 |
| Distal lower extremity | 20 | 17.9 |
| Distal lower and distal upper extremity | 1 | 0.9 |
| Distal upper extremity | 3 | 2.7 |
| Subtypes of arterial events (n = 19) | ||
| Central† | 6 | 31.6 |
| Myocardial infarction | 2 | 10.5 |
| Cerebrovascular accident | 10 | 52.6 |
| Transient ischemic attack | 1 | 5.3 |
| Symptomatic or incidental event (n = 169) | ||
| Symptomatic | 95 | 56.2 |
| Incidental | 74 | 43.8 |
Abbreviations: DVT, deep venous thrombosis; PE, pulmonary embolus.
Central venous thromboses sites include brachiocephalic vein (n = 1), gonadal vein (n = 7), hepatic vein (n = 1), inferior vena cava (n = 5), pelvic vein (n = 4), portal vein (n = 6), renal vein (n = 4), splenic vein (n = 3), superior mesenteric vein (n = 6), and superior vena cava (n = 3).
Central arterial thromboses sites include aortic arch (n = 1), infrarenal aorta (n = 3), internal carotid (n = 1), splenic artery (n = 1), and superior mesenteric artery (n = 1).
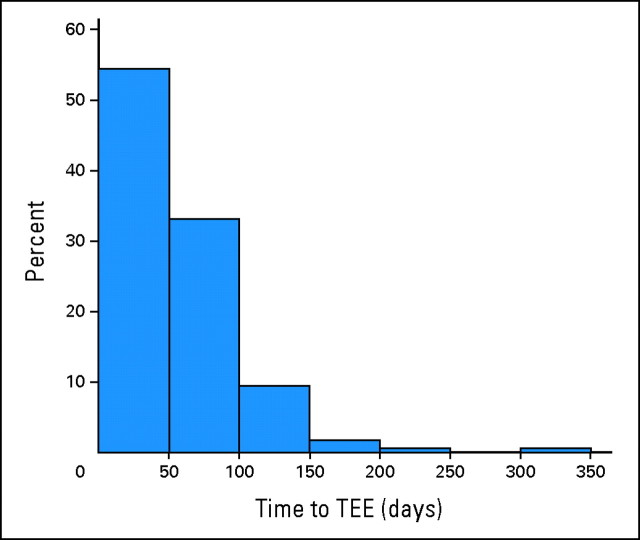
Figure 1 represents the time to TEE from cisplatin start among patients who developed a TEE. The majority of events (88%) occurred within the first 100 days of starting cisplatin. Overall, the median time until TEE occurrence was 48 days (interquartile range, 26 to 73 days).
Fig 1.
Time to thrombosis in patients who developed a thromboembolic event (TEE).
Thirty-one patients (3.3%) died during cisplatin treatment or within 4 weeks of the last cisplatin dose. Among these 31 patients, 13 had a known major TEE that occurred within days of death during the hospitalization that culminated in death, two had events highly suggestive of catastrophic TEE although unproven, and the remaining patients had advanced disease during their last hospitalization but immediate cause of death was not investigated.
Univariate and Multivariate Analysis of Baseline and Treatment Characteristics
To examine the association of each baseline and treatment variable with the development of TEE, a univariate analysis was conducted. Sex, age, race, KPS, ESA use, presence of CVC, site and stage of cancer, leukocyte and hemoglobin level, and Khorana score were all associated with a significant increase in risk of TEE (Table 3). Since more than 20 cancers were represented (Table 4), we examined the subset of cancer types with at least 50 patients (pancreatic, gastric/gastroesophageal (GE) junction, ovarian, melanoma, head and neck, and lung). The incidence of thromboses was significantly different by cancer type (P < .001), with cancers of the pancreas and gastric/GE junction having the highest incidence of TEEs: 36.7% and 27.2%, respectively (Table 3). Regarding disease stage, the incidence of TEEs was higher in patients with metastatic disease (21.6%) compared with patients who had early-stage disease (16.7%) or locally advanced disease (15.2%), with P = .05 on univariate analysis (Table 3).
Table 3.
Univariate Analysis of Patient and Treatment Variables
| Variable | Rate of TEE |
P | |
|---|---|---|---|
| No.* | % | ||
| Sex | .05 | ||
| Male | 79/500 | 15.8 | |
| Female | 90/432 | 20.8 | |
| Age, years (patients with TEE v patients with no TEE) | .02 | ||
| Median | 62 v 59 | ||
| Range | 23-83 v 19-87 | ||
| Race/ethnicity | .05 | ||
| Asian | 10/62 | 16.1 | |
| African American | 14/60 | 23.3 | |
| White | 141/791 | 17.8 | |
| Obese (BMI ≥ 30 kg/m2) | .43 | ||
| No | 144/775 | 18.6 | |
| Yes | 25/157 | 15.9 | |
| Surgery within 2 months | .76 | ||
| No | 137/763 | 18.0 | |
| Yes | 32/169 | 18.9 | |
| Previous TEE | .63 | ||
| No | 151/842 | 17.9 | |
| Yes | 18/90 | 20.0 | |
| Anticoagulation/antiplatelet | .50 | ||
| No | 144/776 | 18.6 | |
| Yes | 25/156 | 16.0 | |
| IVC filter | .43 | ||
| No | 166/921 | 18.0 | |
| Yes | 3/11 | 27.3 | |
| Karnofsky performance status (patients with TEE v patients with no TEE) | .01 | ||
| Mean | 71 v 79 | ||
| SD | 30 v 21 | ||
| Diabetes | .99 | ||
| No | 151/833 | 18.1 | |
| Yes | 18/99 | 18.2 | |
| ESA 6 weeks before/4 weeks after | .002 | ||
| No | 125/765 | 16.3 | |
| Yes | 44/167 | 26.3 | |
| Central venous catheter/pacemaker | .002 | ||
| No | 104/666 | 15.6 | |
| Yes | 65/266 | 24.4 | |
| Hypertension | .65 | ||
| No | 113/637 | 17.7 | |
| Yes | 56/295 | 19.0 | |
| Coronary artery disease | .67 | ||
| No | 159/870 | 18.3 | |
| Yes | 10/62 | 16.1 | |
| Atrial fibrillation/cardiac arrhythmias | .38 | ||
| No | 166/906 | 18.3 | |
| Yes | 3/26 | 11.5 | |
| Prior strokes | .72 | ||
| No | 168/928 | 18.1 | |
| Yes | 1/4 | 25.0 | |
| Stage | .05 | ||
| Early | 11/66 | 16.7 | |
| Locally advanced | 63/415 | 15.2 | |
| Metastatic | 92/426 | 21.6 | |
| Disease (restricted to groups with > 50 patients) | < .001 | ||
| Lung | 24/204 | 11.8 | |
| Gastric and GE junction | 31/114 | 27.2 | |
| Head and neck | 12/94 | 12.8 | |
| Pancreatic | 29/79 | 36.7 | |
| Melanoma | 9/69 | 13.0 | |
| Ovarian | 12/57 | 21.1 | |
| Mean cisplatin dose, mg/m2 (patients with TEE v patients with no TEE) | .07 | ||
| Median | 45 v 40 | ||
| IQR | 25-68 v 25-60 | ||
| Khorana variables | |||
| Site of cancer | < .001 | ||
| Very high risk | 60/193 | 31.1 | |
| High risk | 41/319 | 12.8 | |
| Other | 68/420 | 16.2 | |
| Platelet count ≥ 350,000/μL† | .17 | ||
| No | 120/702 | 17.1 | |
| Yes | 49/230 | 21.3 | |
| Leukocyte count > 11,000/μL† | .03 | ||
| No | 131/776 | 16.9 | |
| Yes | 38/156 | 24.4 | |
| Hemoglobin < 10 g/dL or use of ESA† | .03 | ||
| No | 119/718 | 16.6 | |
| Yes | 50/214 | 23.4 | |
| Body mass index ≥ 35 kg/m2 | .82 | ||
| No | 162/897 | 18.1 | |
| Yes | 7/35 | 20 | |
| Khorana risk group | < .001 | ||
| Low | 29/224 | 13.0 | |
| Intermediate | 91/537 | 17.1 | |
| High | 48/170 | 28.2 | |
Abbreviations: BMI, body mass index; ESA, erythropoiesis-stimulating agent; GE, gastroesophageal; IQR, interquartile range; IVC, inferior vena cava; SD, standard deviation; TEE, thromboembolic event.
Ratios represent No. of patients with TEE over the total No. of patients in respective categories.
Prechemotherapy hematologic parameters.
Table 4.
Thromboembolic Rate According to Underlying Cancer and Treatment Regimen
| Cancer Type and Treatment Regimen | No. of Patients* | % |
|---|---|---|
| Cancer diagnosis | ||
| Colorectal/anal/small bowel | 5/13 | 38.5 |
| Pancreatic | 29/79 | 36.7 |
| Gallbladder/ampullary | 3/10 | 30.0 |
| Gastric/GE junction | 31/114 | 27.2 |
| Cholangiocarcinoma | 5/18 | 27.8 |
| Ovarian | 12/57 | 21.0 |
| Bladder | 6/33 | 18.2 |
| Sarcoma | 2/11 | 18.2 |
| Germ cell/seminoma | 7/39 | 18.0 |
| Esophageal | 8/46 | 17.4 |
| Endometrial | 3/22 | 13.6 |
| Melanoma | 9/69 | 13.0 |
| Head and neck | 12/94 | 12.8 |
| Lung | 24/204 | 11.8 |
| Cervical/uterine/vulvar | 4/39 | 10.3 |
| Hematologic malignancies | 1/11 | 9.1 |
| Neuroendocrine/carcinoid | 0/11 | 0 |
| Other | 8/62 | 12.9 |
| Chemotherapy regimens | ||
| Cisplatin + docetaxel + fluorouracil/leucovorin + bevacizumab | 11/16 | 68.8 |
| Cisplatin + docetaxel + fluorouracil/leucovorin | 7/11 | 63.6 |
| Cisplatin IP + paclitaxel IV/IP + bevacizumab | 6/13 | 46.2 |
| Cisplatin + docetaxel + fluorouracil | 5/17 | 29.4 |
| Cisplatin + gemcitabine | 38/134 | 28.4 |
| Cisplatin + paclitaxel + ifosfamide | 2/11 | 18.2 |
| Cisplatin + irinotecan | 15/89 | 16.9 |
| Cisplatin + pemetrexed | 6/43 | 14 |
| Cisplatin + etoposide | 10/80 | 12.5 |
| Cisplatin + fluorouracil + epirubicin | 2/16 | 12.5 |
| Cisplatin + vinblastine + temozolomide | 7/57 | 12.3 |
| Cisplatin + pemetrexed + bevacizumab | 4/33 | 12.1 |
| Cisplatin + radiation | 9/94 | 9.6 |
| Cisplatin IP + paclitaxel IV/IP | 3/33 | 9.1 |
| Other cisplatin-based regimens | 44/285 | 15.4 |
Abbreviations: GE, gastroesophageal; IP, intraperitoneal; IV, intravenous.
Ratios represent No. of patients with TEE over the total No. of patients in respective categories.
Of the five predictive variables included in the previously validated Khorana risk model, only three were significantly associated with TEE occurrence in our study: site of cancer (P < .001), prechemotherapy leukocyte count more than 11,000/μL (P = .03), and prechemotherapy hemoglobin level less than 10 g/dL or use of an ESA (P = .03). Despite the lack of association between the remaining two variables, the overall Khorana risk score was significantly associated with TEE occurrence (P < .001).
When analyzing the data by treatment variables, some cisplatin-based chemotherapy regimens were associated with a higher incidence of TEEs; however, reliable statistical analysis was not possible because of the multiplicity of the regimens and small sample sizes (Table 4). Univariate analysis revealed that mean cisplatin dose did not correlate with the incidence of TEE (Table 3).
Variables with a P value of ≤ .05 on univariate analysis were subsequently entered into the multivariate logistic regression model. On multivariate analysis, variables significantly associated with TEE occurrence included age (odds ratio [OR], 1.19 per 10-year increase; 95% CI, 1.02 to 1.39; P = .03), KPS (odds ratio, 0.92 per 10-unit increase; 95% CI, 0.86 to 0.98; P = .02), presence of a CVC (OR, 1.61; 95% CI, 1.10 to 2.36; P = .01), and the Khorana risk group (P = .04; Table 5).
Table 5.
Multivariate Analysis of Baseline and Treatment Variables
| Variable | Odds Ratio | 95% CI | Adjusted P |
|---|---|---|---|
| Sex | .15 | ||
| Male | 1 | ||
| Female | 1.31 | 0.91 to 1.88 | |
| Age (per 10-year increase) | 1.19 | 1.02 to 1.39 | .03 |
| Race/ethnicity | .51 | ||
| White | 1 | ||
| Asian | 0.87 | 0.41 to 1.85 | |
| African American | 1.43 | 0.74 to 2.76 | |
| KPS (per 10-unit increase) | 0.92 | 0.86 to 0.98 | .02 |
| Central venous catheter/pacemaker | 1.61 | 1.10 to 2.36 | .01 |
| Stage | .57 | ||
| Early | 1 | ||
| Locally advanced | 0.84 | 0.41 to 1.72 | |
| Metastatic | 1.03 | 0.50 to 2.13 | |
| Khorana risk group | .04 | ||
| Low | 1 | ||
| Intermediate | 1.33 | 0.81 to 2.16 | |
| High | 2.06 | 1.16 to 3.65 |
Abbreviation: KPS, Karnofsky performance status.
DISCUSSION
In this large retrospective analysis of 932 patients treated with cisplatin-based chemotherapy for a variety of cancers, we have observed an unacceptably high incidence of TEEs (18.1%) during the period of administration or within 4 weeks of completion of treatment. We believe that the true incidence may even be higher, since we may have failed to capture every patient with a TEE because of the retrospective nature of this analysis. We also note a death rate of 3.3% during the same observation period with a strong probability of a TEE contributing to or causing death in the majority of these patients. This large retrospective study confirms the high incidence of TEEs in patients treated with cisplatin-based chemotherapy.
Few small retrospective studies26,27 have reported a lower incidence of TEEs in cisplatin-treated patients, ranging from 8.4% to 12.9%. Such a discrepancy may be ascribed to several factors. The systematic strategy used in this study, which included a thorough review of patients' medications that triggered further investigations, may have allowed capturing additional patients with a TEE who would otherwise have been missed. In contrast, previous retrospective studies26,27 often did not clearly define their strategy for capturing events. In addition, some studies included only symptomatic events, whereas we included all events regardless of presentation.26 It is also worth noting that some retrospective (and even prospective) studies fail to include cerebrovascular events and MIs, possibly because they are viewed as cardiovascular rather than TEEs and are derived from different physiopathologic mechanisms.32,33 The inadequate reporting of arterial events related to cisplatin chemotherapy has indeed been previously highlighted.34 In contrast, arterial events represent a significant percentage of TEEs in this article and probably contribute to the higher incidence observed.
Conversely, and in accordance with our results, few small prospective trials28,30 indicate an incidence of TEEs in cisplatin-treated patients ranging up to 17.6%. Importantly, in these studies, arterial events including cerebrovascular accidents and MIs account for a substantial percentage of TEEs. The pathogenesis of cisplatin-associated vascular toxicity, which may include hypomagnesemia, increased von Willebrand factor, and damage to endothelial cells via increased formation of procoagulant endothelial microparticles,17,23 is likely to involve a pathway affecting both venous and arterial systems. This is in contrast with most other thrombosis risk factors that often involve mostly the venous compartment.
The timing of the TEEs in relation to initiation of therapy further suggests a relation between cisplatin administration and TEE occurrence. In this study, 88% of TEEs occurred within the first 100 days of starting cisplatin. A similar finding was noted by Numico et al,28 in which 45% of the vascular events occurred during the first two courses of cisplatin and gemcitabine. Likewise, in the study by Weijl et al,27 the median time interval between initiation of cisplatin-based treatment and first TEE was 52 days.
In this study, we have attempted to identify risk factors associated with the development of TEEs in patients receiving cisplatin. Several patient baseline and treatment characteristics previously associated with thrombosis were analyzed by univariate and multivariate analysis. Sex, age, race, KPS, exposure to ESA, presence of CVC, type and stage of cancer, prechemotherapy hemoglobin level and leukocyte count, and the Khorana score were all associated with significantly increased risk of TEE by univariate analysis. It is interesting to note that among the five Khorana variables, platelet number and BMI were not identified as independent risk factors, although the overall Khorana score including these two variables was. This discrepancy is probably due to the fact that the patient population under consideration in this study (exclusively cisplatin-exposed patients) carries a much higher risk of thrombosis than the general cancer population analyzed in the Khorana study. In this regard, it is interesting to highlight that when we used the five Khorana variables that segregate outpatients undergoing chemotherapy into low-, intermediate-, and high-risk groups, the incidence of TEE was much higher in this study for each specified risk group in comparison to Khorana's original study: 13% versus 0.3% among patients with a score of 0, 17.1% versus 2% among those with a score of 1 or 2, and 28.2% versus 6.7% among those with a score of 3 or higher.31 It is therefore likely that exposure to cisplatin may dwarf the added risk associated with BMI and platelet count, which would explain the lack of prognostic significance for these variables by univariate analysis. The same rationale may underlie the lack of statistical significance of a history of previous TEEs and recent surgery, both strong risk factors for thrombosis in previous studies. Interestingly, by multivariate analysis, only age, KPS, presence of CVC, and Khorana score retained statistical significance.
It is important to note that in this study, several treatment characteristics were difficult to analyze because of the retrospective nature of the study and the inclusion of many different cancers and chemotherapeutic regimens resulting in small sample sizes within these groups. Likewise, the strong association of specific regimens with specific diseases, such as cisplatin plus docetaxel plus fluorouracil/leucovorin with bevacizumab and gastric/GE junction cancer, hindered the statistical analysis. A comparison between various regimens was therefore difficult to perform.
Considering the high incidence of TEE seen in this study and acknowledging its impact on hospital cost, morbidity, and mortality, the question of thromboprophylaxis needs to be addressed. Established guidelines recommend prophylactic anticoagulation for all oncology patients in high-risk settings, such as hospitalization, major surgery, or postoperatively.35 However, in the ambulatory setting, prophylactic anticoagulation is not currently recommended except for patients with multiple myeloma receiving thalidomide- lenalidomide–based combinations, in whom the incidence of TEE is similar to that observed in this study.35 Data on primary prevention of thrombosis in ambulatory patients with cancer receiving chemotherapy are limited with mixed results.36–40 Levine et al38 showed a significant reduction in the rate of thrombosis in women with metastatic breast cancer receiving low-dose warfarin; however, the TOPIC I and II trials37 showed no benefit with low-molecular-weight heparin prophylaxis in metastatic breast cancer and advanced stages of non–small-cell lung cancer, respectively. More recently, the CONKO 004 trial41,42 (A Prospective, Randomized Trial of Simultaneous Pancreatic Cancer Treatment With Enoxaparin and Chemotherapy) showed a significant decrease in symptomatic TEEs that used enoxaparin in advanced pancreatic cancer after 3 months (1.3% v 9.9%) and after 12 months (5.0% v 15.1%). Similarly, the United Kingdom FRAGEM (Gemcitabine With or Without Prophylactic Weight-Adjusted Dalteparin in Patients With Advanced or Metastatic Pancreatic Cancer) study43 reported benefit from using dalteparin in advanced pancreatic cancer (12% v 31%; P = .02), and the randomized PROTECT (Prophylaxis of Thromboembolism during Chemotherapy) Trial44 showed benefit from using nadroparin in metastatic or locally advanced cancers (2.0% v 3.9%; P = .02) compared with placebo. Overall, although acknowledging that the question of prophylactic anticoagulation remains controversial in patients with cancer, we believe that prospective randomized studies evaluating the benefit of prophylactic anticoagulation are warranted in patients with cancer who are receiving cisplatin on the basis of their high risk. We also speculate, since the risk of TEE was 13%, even in the low-risk category as specified by the Khorana predictive model, that all patients receiving cisplatin-based chemotherapy should be considered for thromboprophylaxis, unless there is a contraindication, at least until a more applicable predictive model is available for this group of patients.
In summary, this large retrospective analysis confirms the high incidence of TEEs in patients treated with cisplatin-based chemotherapy, which in our opinion, is unacceptable. The vast majority of events occur early in the treatment course. As expected, the incidence of TEEs varied according to the type of underlying primary malignancy and stage of disease, with age, KPS, presence of CVC, and Khorana score being significant predictors by multivariate analysis. On the basis of the results of this and previous studies, TEE prophylaxis may be advisable for patients receiving cisplatin-based chemotherapy. Prospective studies are urgently needed.
Footnotes
Presented at the 51st Annual Meeting of the American Society of Hematology December 5-8, 2009, New Orleans, LA.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Russell A. Moore, Nelly Adel, Darren R. Feldman, Hani Hassoun
Provision of study materials or patients: Russell A. Moore, Darren R. Feldman, Hani Hassoun
Collection and assembly of data: Russell A. Moore, Manisha Bhutani, Nour Elise Tabbara, Hani Hassoun
Data analysis and interpretation: Russell A. Moore, Nelly Adel, Elyn Riedel, Darren R. Feldman, Gerald Soff, Rekha Parameswaran, Hani Hassoun
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1. Silverstein MD Heit JA Mohr DN , etal : Trends in the incidence of deep vein thrombosis and pulmonary embolism: A 25-year population-based study Arch Intern Med 158: 585– 593,1998. [DOI] [PubMed] [Google Scholar]
- 2. Lee AY, Levine MN: Venous thromboembolism and cancer: Risks and outcomes Circulation 107: I17– 21,2003. suppl 1 [DOI] [PubMed] [Google Scholar]
- 3. Heit JA Silverstein MD Mohr DN , etal : Risk factors for deep vein thrombosis and pulmonary embolism: A population-based case-control study Arch Intern Med 160: 809– 815,2000. [DOI] [PubMed] [Google Scholar]
- 4. Khorana AA Francis CW Culakova E , etal : Thromboembolism is a leading cause of death in cancer patients receiving outpatient chemotherapy J Thromb Haemost 5: 632– 634,2007. [DOI] [PubMed] [Google Scholar]
- 5. Chew HK Wun T Harvey D , etal : Incidence of venous thromboembolism and its effect on survival among patients with common cancers Arch Intern Med 166: 458– 464,2006. [DOI] [PubMed] [Google Scholar]
- 6. Sørensen HT Mellemkjaer L Olsen JH , etal : Prognosis of cancers associated with venous thromboembolism. N Engl J Med 343: 1846– 1850,2000. [DOI] [PubMed] [Google Scholar]
- 7. Lyman GH, Khorana AA: Cancer, clots and consensus: New understanding of an old problem J Clin Oncol 27: 4821– 4826,2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Clahsen PC van de Velde CJ Julien JP , etal : Thromboembolic complications after perioperative chemotherapy in women with early breast cancer: A European Organization for Research and Treatment of Cancer Breast Cancer Cooperative Group study J Clin Oncol 12: 1266– 1271,1994. [DOI] [PubMed] [Google Scholar]
- 9. Fisher B Costantino J Redmond C , etal : A randomized clinical trial evaluating tamoxifen in the treatment of patients with node-negative breast cancer who have estrogen-receptor-positive tumors N Engl J Med 320: 479– 484,1989. [DOI] [PubMed] [Google Scholar]
- 10. Fisher B Dignam J Wolmark N , etal : Tamoxifen and chemotherapy for lymph node-negative, estrogen receptor-positive breast cancer J Natl Cancer Inst 89: 1673– 1682,1997. [DOI] [PubMed] [Google Scholar]
- 11. Fisher B Redmond C Legault-Poisson S , etal : Postoperative chemotherapy and tamoxifen compared with tamoxifen alone in the treatment of positive-node breast cancer patients aged 50 years and older with tumors responsive to tamoxifen: Results from the National Surgical Adjuvant Breast and Bowel Project B-16 J Clin Oncol 8: 1005– 1018,1990. [DOI] [PubMed] [Google Scholar]
- 12. Levine MN Gent M Hirsh J , etal : The thrombogenic effect of anticancer drug therapy in women with stage II breast cancer N Engl J Med 318: 404– 407,1988. [DOI] [PubMed] [Google Scholar]
- 13. Pritchard KI Paterson AH Paul NA , etal : Increased thromboembolic complications with concurrent tamoxifen and chemotherapy in a randomized trial of adjuvant therapy for women with breast cancer: National Cancer Institute of Canada Clinical Trials Group Breast Cancer Site Group J Clin Oncol 14: 2731– 2737,1996 [DOI] [PubMed] [Google Scholar]
- 14. Rivkin SE Green S Metch B , etal : Adjuvant CMFVP versus tamoxifen versus concurrent CMFVP and tamoxifen for postmenopausal, node-positive, and estrogen receptor-positive breast cancer patients: A Southwest Oncology Group study. J Clin Oncol 12: 2078– 2085,1994. [DOI] [PubMed] [Google Scholar]
- 15. Saphner T, Tormey DC, Gray R: Venous and arterial thrombosis in patients who received adjuvant therapy for breast cancer J Clin Oncol 9: 286– 294,1991. [DOI] [PubMed] [Google Scholar]
- 16. Azak A Oksüzoğlu B Deren T , etal : Cerebrovascular accident during cisplatin-based combination chemotherapy of testicular germ cell tumor: An unusual case report Anticancer Drugs 19: 97– 98,2008. [DOI] [PubMed] [Google Scholar]
- 17. Doll DC, Ringenberg QS, Yarbro JW: Vascular toxicity associated with antineoplastic agents J Clin Oncol 4: 1405– 1417,1986. [DOI] [PubMed] [Google Scholar]
- 18. Gerl A: Vascular toxicity associated with chemotherapy for testicular cancer Anticancer Drugs 5: 607– 614,1994. [DOI] [PubMed] [Google Scholar]
- 19. Grenader T Shavit L Ospovat I , etal : Aortic occlusion in patients treated with cisplatin-based chemotherapy Mt Sinai J Med 73: 810– 812,2006. [PubMed] [Google Scholar]
- 20. Içli F Karaoğuz H Dinçol D , etal : Severe vascular toxicity associated with cisplatin-based chemotherapy Cancer 72: 587– 593,1993. [DOI] [PubMed] [Google Scholar]
- 21. Illarramendi JJ, Gallego J: Cisplatin-based chemotherapy and acute cerebrovascular events. Lancet 338: 705,1991 [DOI] [PubMed] [Google Scholar]
- 22. Jafri M, Protheroe A: Cisplatin-associated thrombosis Anticancer Drugs 19: 927– 929,2008. [DOI] [PubMed] [Google Scholar]
- 23. Lechner D Kollars M Gleiss A , etal : Chemotherapy-induced thrombin generation via procoagulant endothelial microparticles is independent of tissue factor activity J Thromb Haemost 5: 2445– 2452,2007. [DOI] [PubMed] [Google Scholar]
- 24. Pretnar-Oblak J Zaletel M Jagodic M , etal : Thrombosis of internal carotid artery after cisplatin-based chemotherapy Eur Neurol 57: 109– 110,2007. [DOI] [PubMed] [Google Scholar]
- 25. Karam C, Koussa S: Cerebral dural sinus thrombosis following cisplatin chemotherapy J Clin Neurosci 15: 1274– 1275,2008. [DOI] [PubMed] [Google Scholar]
- 26. Czaykowski PM, Moore MJ, Tannock IF: High risk of vascular events in patients with urothelial transitional cell carcinoma treated with cisplatin based chemotherapy. J Urol 160: 2021– 2024,1998. [DOI] [PubMed] [Google Scholar]
- 27. Weijl NI Rutten MF Zwinderman AH , etal : Thromboembolic events during chemotherapy for germ cell cancer: A cohort study and review of the literature J Clin Oncol 18: 2169– 2178,2000. [DOI] [PubMed] [Google Scholar]
- 28. Numico G Garrone O Dongiovanni V , etal : Prospective evaluation of major vascular events in patients with nonsmall cell lung carcinoma treated with cisplatin and gemcitabine Cancer 103: 994– 999,2005. [DOI] [PubMed] [Google Scholar]
- 29. Al-Batran SE Hartmann JT Probst S , etal : Phase III trial in metastatic gastroesophageal adenocarcinoma with fluorouracil, leucovorin plus either oxaliplatin or cisplatin: A study of the Arbeitsgemeinschaft Internistische Onkologie J Clin Oncol 26: 1435– 1442,2008. [DOI] [PubMed] [Google Scholar]
- 30. Starling N Rao S Cunningham D , etal : Thromboembolism in patients with advanced gastroesophageal cancer treated with anthracycline, platinum, and fluoropyrimidine combination chemotherapy: A report from the UK National Cancer Research Institute Upper Gastrointestinal Clinical Studies Group J Clin Oncol 27: 3786– 3793,2009. [DOI] [PubMed] [Google Scholar]
- 31. Khorana AA Kuderer NM Culakova E , etal : Development and validation of a predictive model for chemotherapy-associated thrombosis Blood 111: 4902– 4907,2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Behrendt CE, Ruiz RB: Venous thromboembolism among patients with advanced lung cancer randomized to prinomastat or placebo, plus chemotherapy Thromb Haemost 90: 734– 737,2003. [DOI] [PubMed] [Google Scholar]
- 33. Fotopoulou C duBois A Karavas AN , etal : Incidence of venous thromboembolism in patients with ovarian cancer undergoing platinum/paclitaxel-containing first-line chemotherapy: An exploratory analysis by the Arbeitsgemeinschaft Gynaekologische Onkologie Ovarian Cancer Study Group J Clin Oncol 26: 2683– 2689,2008. [DOI] [PubMed] [Google Scholar]
- 34. Anders JC, Grigsby PW, Singh AK: Cisplatin chemotherapy (without erythropoietin) and risk of life-threatening thromboembolic events in carcinoma of the uterine cervix: The tip of the iceberg? A review of the literature Radiat Oncol 1: 14,2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lyman GH Khorana AA Falanga A , etal : American Society of Clinical Oncology guideline: Recommendations for venous thromboembolism prophylaxis and treatment in patients with cancer J Clin Oncol 25: 5490– 5505,2007. [DOI] [PubMed] [Google Scholar]
- 36. Couban S Goodyear M Burnell M , etal : Randomized placebo-controlled study of low-dose warfarin for the prevention of central venous catheter-associated thrombosis in patients with cancer J Clin Oncol 23: 4063– 4069,2005. [DOI] [PubMed] [Google Scholar]
- 37. Haas SK Kakkar A Kemkes-Matthes B , etal: Prevention of venous thromboembolism with low-molecular-weight heparin in patients with metastatic breast or lung cancer: Results of the TOPIC studies J Thromb Haemost 2005. 3: suppl 1 abstr OR059 [Google Scholar]
- 38. Levine M Hirsh J Gent M , etal : Double-blind randomised trial of a very-low-dose warfarin for prevention of thromboembolism in stage IV breast cancer Lancet 343: 886– 889,1994. [DOI] [PubMed] [Google Scholar]
- 39. Perry JR Rogers L Laperriere N , etal: PRODIGE: A phase III randomized placebo-controlled trial of thromboprophylaxis using dalteparin low molecular weight heparin (LMWH) in patients with newly diagnosed malignant glioma J Clin Oncol 25: 77s,2007. suppl abstr 2011 [DOI] [PubMed] [Google Scholar]
- 40. Young AM Billingham LJ Begum G , etal: Warfarin thromboprophylaxis in cancer patients with central venous catheters (WARP): An open-label randomised trial Lancet 373: 567– 574,2009 [DOI] [PubMed] [Google Scholar]
- 41. Riess H Pelzer U Hilbig A , etal: Rationale and design of PROSPECT-CONKO 004: A prospective, randomized trial of simultaneous pancreatic cancer treatment with enoxaparin and chemotherapy BMC Cancer 8: 361,2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Riess H Pelzer U Opitz B , etal: A prospective, randomized trial of simultaneous pancreatic cancer treatment with enoxaparin and chemotherapy: Final results of the CONKO-004 trial J Clin Oncol 28: 309s,2010. suppl abstr 4033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Maraveyas A Waters J Roy R , etal: Gemcitabine with or without prophylactic weight-adjusted dalteparin in patients with advanced or metastatic pancreatic cancer (APC): A multicentre, randomised phase IIB trial (the UK FRAGEM study) Eur J Cancer Supplements 7: 362,2009. abstr 6503 [Google Scholar]
- 44. Agnelli G Gussoni G Bianchini C , etal: Nadroparin for the prevention of thromboembolic events in ambulatory patients with metastatic or locally advanced solid cancer receiving chemotherapy: A randomised, placebo-controlled, double-blind study Lancet Oncol 10: 943– 949,2009 [DOI] [PubMed] [Google Scholar]