Abstract
Purpose
Immune checkpoint inhibition has been demonstrated to be an effective anticancer strategy. Several lines of evidence support the study of immunotherapy in triple-negative breast cancer (TNBC). We assessed the safety and antitumor activity of the programmed cell death protein 1 (PD-1) inhibitor pembrolizumab in patients with advanced TNBC.
Methods
KEYNOTE-012 (ClinicalTrials.gov identifier: NCT01848834) was a multicenter, nonrandomized phase Ib trial of single-agent pembrolizumab given intravenously at 10 mg/kg every 2 weeks to patients with advanced PD-L1–positive (expression in stroma or ≥ 1% of tumor cells by immunohistochemistry) TNBC, gastric cancer, urothelial cancer, and head and neck cancer. This report focuses on the TNBC cohort.
Results
Among 111 patients with TNBC whose tumor samples were screened for PD-L1 expression, 58.6% had PD-L1–positive tumors. Thirty-two women (median age, 50.5 years; range, 29 to 72 years) were enrolled and assessed for safety and antitumor activity. The median number of doses administered was five (range, 1 to 36 doses). Common toxicities were mild and similar to those observed in other tumor cohorts (eg, arthralgia, fatigue, myalgia, and nausea), and included five (15.6%) patients with grade ≥ 3 toxicity and one treatment-related death. Among the 27 patients who were evaluable for antitumor activity, the overall response rate was 18.5%, the median time to response was 17.9 weeks (range, 7.3 to 32.4 weeks), and the median duration of response was not yet reached (range, 15.0 to ≥ 47.3 weeks).
Conclusion
This phase Ib study describes preliminary evidence of clinical activity and a potentially acceptable safety profile of pembrolizumab given every 2 weeks to patients with heavily pretreated, advanced TNBC. A single-agent phase II study examining a 200-mg dose given once every 3 weeks (ClinicalTrials.gov identifier: NCT02447003) is ongoing.
INTRODUCTION
Triple-negative breast cancer (TNBC) is histologically defined by a lack of estrogen receptor and progesterone receptor expression and the absence of human epidermal growth factor receptor 2 (HER2) overexpression and/or amplification.1,2 TNBC represents up to 20% of all breast cancers.3 Although not synonymous with the basal-like subtype defined by gene expression profiling, approximately 70% of TNBCs have basal-like characteristics.4,5 TNBC is more common in younger women,2,6,7 those of African descent,6,7 and those with BRCA1 germline mutations.1,4,8
TNBC tumors are frequently of high histologic grade,7,9 present at an advanced stage,7,9 are typically more aggressive and difficult to treat than hormone receptor–positive tumors,2,10 and are associated with a higher risk of early relapse.2,10 The lack of estrogen receptor, progesterone receptor, and HER2 expression precludes the use of targeted therapies, and the only approved systemic treatment option is chemotherapy. Responses to chemotherapy occur, but are often short lived and are frequently accompanied by considerable toxicity.5,10-13 Given the suboptimal outcomes with chemotherapy, new targeted therapies for TNBC are urgently needed.
The programmed death receptor 1 (PD-1) pathway plays a critical role in regulating the immune response. PD-1, an inhibitory immune checkpoint receptor expressed on activated T cells, B cells, natural killer cells, activated monocytes, dendritic cells, myeloid cells, and a subset of thymocytes,14-16 limits autoimmunity by regulating the activity of effector T cells in the periphery in response to an inflammatory stimulus.14,17 PD-L1, a PD-1 ligand, is an immunosuppressive signal that is upregulated in response to proinflammatory signals such as interferon-γ.15,17,18 Abundant research has shown that PD-L1 is expressed in multiple solid malignancies, including melanoma and cancers of the lung, bladder, colon, liver, and head and neck,15,18 and may be a predictor of response to PD-1 pathway inhibition.19 Primary breast cancers also express PD-L1, with expression generally higher in TNBC.20-23 Through adaptive immune resistance, tumors are able to co-opt the PD-1 pathway via T-cell exhaustion and immunosuppression, thereby evading destruction by the antitumor immune response.14,17
Pembrolizumab is a high-affinity, highly selective, humanized monoclonal IgG4-κ antibody against PD-1. Pembrolizumab is approved in several countries for the treatment of advanced melanoma. Additionally, clinical studies with pembrolizumab have demonstrated promising efficacy with durable responses and a manageable safety profile in many advanced malignancies, including non–small-cell lung cancer (NSCLC),24 head and neck cancer,25 gastric cancer,26 and urothelial cancer.27
Several lines of evidence support the study of immunotherapy in TNBC. Gene expression profiling demonstrated an association between expression of immunomodulatory genes and better clinical outcomes in TNBC.28 In addition, significant infiltration of TNBC tumors with tumor-infiltrating lymphocytes, which have been shown to have prognostic significance in TNBC, has been reported.29-33 A direct link to the PD-1 pathway came from The Cancer Genome Atlas34; using RNA expression data from The Cancer Genome Atlas, higher PD-L1 mRNA expression was demonstrated in TNBC versus non–TNBC samples (P < .001).20 Subsequent studies reported that PD-L1 is expressed in approximately 20% to 30% of TNBC,20,35 is associated with infiltrating lymphocytes,23 and correlates with higher histologic grade.20,35 Mutations or deletions in the PTEN/PI3K pathway have been implicated in breast cancer34,36; loss of PTEN specifically correlates with hormone receptor–negative breast cancer,36 leads to the upregulation of PD-L1, and suppresses T-cell proliferation and survival.20 Given the need for new targeted therapies that will improve outcomes, coupled with the strong association between hormone receptor–negative tumors and immune cell infiltration, we examined the safety and antitumor activity of pembrolizumab in advanced TNBC.
METHODS
Study Design and Population
KEYNOTE-012 (ClinicalTrials.gov identifier: NCT01848834) was a nonrandomized, multicohort, phase Ib study designed to evaluate the safety, tolerability, and antitumor activity of pembrolizumab in patients with advanced TNBC, gastric cancer, urothelial cancer, and head and neck cancer. For the TNBC cohort, key eligibility requirements included: age 18 years or older; estrogen receptor–negative, progesterone receptor–negative, HER2-negative, recurrent or metastatic breast cancer; measurable disease per Response Evaluation Criteria in Solid Tumors (RECIST) v1.1; an Eastern Cooperative Oncology Group performance status of 0 to 1; any number of prior systemic treatments; and PD-L1–positive tumors. Key exclusion criteria included: use of systemic steroids within 7 days of study entry; chemotherapy within 2 weeks of first pembrolizumab dose; active brain metastases (treated and stable brain metastases were allowed); prior therapy with anti–PD-1/anti–PD-L1/anti–CLTA-4 antibody; and active autoimmune disease.
Treatment
Patients received pembrolizumab 10 mg/kg intravenously every 2 weeks until unacceptable toxicity, disease progression, or investigator decision. If clinically stable, patients with first radiologic evidence of disease progression per RECIST v1.1 were permitted to continue on pembrolizumab until a second scan performed ≥ 4 weeks later confirmed progression.
Assessments
PD-L1 was assessed in formalin-fixed, paraffin-embedded archival tumor samples at a central laboratory using a prototype immunohistochemistry assay and the 22C3 antihuman PD-1 antibody (Merck & Co., Kenilworth, NJ).24 Positivity was defined as PD-L1 expression in the stroma or in ≥ 1% of tumor cells. Adverse events (AEs) were graded per the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.0, during study treatment and for up to 30 days thereafter. Serious AEs were collected for up to 90 days after the last pembrolizumab dose. Other safety end points included regular monitoring of laboratory assessments, vital signs, and physical examinations. Imaging was performed every 8 weeks, and response was based on RECIST v1.1 as assessed by central radiology review.
The primary efficacy end point was overall response rate (ORR), defined as percentage of patients with a best overall response of complete response (CR) or partial response (PR). Patients evaluable for response were those with measurable disease by central review at baseline, who received at least one pembrolizumab dose, and who had at least one postbaseline scan or discontinued therapy before the first scan as a result of progressive disease (PD) or a treatment-related AE. Secondary efficacy end points were progression-free survival (PFS), defined as time from enrollment to disease progression or death, whichever occurred first; duration of response, defined as time from first RECIST v1.1 response to disease progression in patients who achieved a PR or better; and overall survival (OS), defined as time from enrollment to death from any cause.
Study Oversight
The study protocol and all amendments were approved by the appropriate institutional review boards and ethics committees at each institution. The study was conducted in accordance with the protocol, Good Clinical Practice guidelines, and the provisions outlined in the Declaration of Helsinki. All patients provided written informed consent.
Statistical Analysis
Antitumor activity was assessed in all patients who received at least one pembrolizumab dose, had measurable disease at baseline per RECIST v1.1 as assessed by central review, and had either at least one postbaseline scan or discontinued the trial as a result of progressive disease or a treatment-related AE before the first scan. Safety was assessed in all patients who received at least one pembrolizumab dose. With 26 evaluable patients, the study had approximately 80% power to detect a 25% difference in ORR under the null hypothesis of ORR = 20% with a type I error rate of 2.5% if the true ORR was 45%. PFS, OS, and duration of response were estimated using the Kaplan-Meier method. Ninety-five percent CIs for ORR were calculated using the binomial exact method. The association between PD-L1 expression and clinical outcomes was tested using logistic and Cox regressions for ORR and PFS, respectively.
RESULTS
Study Patients
Of the 111 patients with metastatic (m)TNBC whose tumor samples were screened for PD-L1 expression, 65 (58.6%) had PD-L1–positive tumors; of these, 32 patients enrolled in the study. Baseline characteristics were as expected for patients with advanced TNBC (Table 1). The median age was 50.5 years (range, 29 to 72 years). All patients had mTNBC at study entry and most were heavily pretreated, having received therapy in both the early and advanced disease settings. Twenty-eight patients (87.5%) were initially diagnosed with early-stage disease and had received (neo)adjuvant treatment. The remaining four patients (12.5%) presented with de novo metastatic disease and had received at least one line of therapy in the metastatic setting. The median number of prior lines of systemic therapy for metastatic disease was two, with 46.9% of patients having received at least three lines of therapy for metastatic disease and 25.0% having received at least five. Elevated lactate dehydrogenase (LDH) levels were observed in 40.6% of patients, and 78.1% of patients had visceral metastases. As of the data cutoff date of March 23, 2015, the median duration of follow up was 10.0 months (range, 0.4 to 19.5 months).
Table 1.
Baseline Patient Demographics and Clinical Characteristics
| Characteristic | Value |
|---|---|
| Age, years, median (range) | 50.5 (29-72) |
| Female, No. (%) | 32 (100) |
| Race, No. (%) | |
| White | 25 (78.1) |
| Black or African American | 7 (21.9) |
| ECOG performance status, No. (%) | |
| 0 | 14 (43.8) |
| 1 | 18 (56.3) |
| Location of metastases | |
| Brain | 3 (9.4) |
| Visceral | 25 (78.1) |
| Nonvisceral | 7 (21.9) |
| LDH level, No. (%) | |
| > ULN | 13 (40.6) |
| ≥ 2× ULN | 5 (15.6) |
| No. of prior therapies for metastatic disease | |
| Median (range) | 2 (0-9) |
| 0, No. (%) | 5 (15.6) |
| 1, No. (%) | 6 (18.8) |
| 2, No. (%) | 6 (18.8) |
| 3, No. (%) | 5 (15.6) |
| 4, No. (%) | 2 (6.3) |
| ≥ 5, No. (%) | 8 (25.0) |
| Previous neoadjuvant or adjuvant therapy, No. (%) | 28 (87.5) |
| Previous chemotherapy exposure, No. (%) | |
| Taxane | 32 (100.0) |
| Anthracycline | 23 (71.9) |
| Capecitabine | 21 (65.6) |
| Platinum | 19 (59.4) |
| Eribulin | 8 (25.0) |
NOTE. Data included a total of 32 patients.
Abbreviations: ECOG, Eastern Cooperative Oncology Group; LDH, lactate dehydrogenase; ULN, upper limit of normal.
Safety and Tolerability
The median duration of treatment was 59.5 days (range, 1 to 530 days), and the median number of pembrolizumab doses administered was five (range, 1 to 36 doses). Overall, 56.3% of patients experienced at least one treatment-related toxicity, including 15.6% who experienced at least one grade 3 to 5 event. The most common treatment-related AEs of any grade included arthralgia (18.8%), fatigue (18.8%), myalgia (18.8%), and nausea (15.6%; Table 2). Five grade 3 treatment-related AEs were observed: anemia, aseptic meningitis, lymphopenia, headache, and pyrexia (Table 2). The patient with drug-related aseptic meningitis was successfully treated with pembrolizumab interruption and steroids for 6 weeks, and was subsequently able to resume the study drug at a reduced dose. This patient exhibited long-lasting PR to pembrolizumab and has remained on study treatment for more than 17 months.
Table 2.
Treatment-Related Adverse Events*
| Grade | No. (%) |
|---|---|
| Any grade occurring in at least two patients | |
| Arthralgia | 6 (18.8) |
| Fatigue | 6 (18.8) |
| Myalgia | 6 (18.8) |
| Nausea | 5 (15.6) |
| Diarrhea | 4 (12.5) |
| ALT increased | 2 (6.3) |
| AST increased | 2 (6.3) |
| Erythema | 2 (6.3) |
| Headache | 2 (6.3) |
| Pruritus | 2 (6.3) |
| Grade 3-5 occurring in at least one patient | |
| Anemia (grade 3) | 1 (3.1) |
| Aseptic meningitis (grade 3) | 1 (3.1) |
| Blood fibrinogen decreased (grade 4)† | 1 (3.1) |
| Disseminated intravascular coagulation (grade 5)† | 1 (3.1) |
| Headache (grade 3) | 1 (3.1) |
| Lymphopenia (grade 3) | 1 (3.1) |
| Pyrexia (grade 3) | 1 (3.1) |
NOTE. Data included a total of 32 patients.
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase.
Reported during or within 30 days of study treatment (90 days for serious adverse events and events of clinical interest).
Reported in the same patient.
One patient died as a result of disseminated intravascular coagulation (DIC) accompanied by grade 4 decreased blood fibrinogen, both of which were considered by the investigator to be treatment related. The onset of DIC was 10 days after the first pembrolizumab dose, and death occurred 4 days later. This patient was diagnosed with de novo metastatic disease, and before enrollment had rapid disease progression on three lines of prior therapy. Nine days before the first pembrolizumab dose, the patient’s platelet count was 151 × 109/L, and results of liver function tests were within normal limits. Six days before the first pembrolizumab dose, the platelet count had decreased to 115 × 109/L. At the time of DIC presentation, the platelet count was 8 × 109/L, blood fibrinogen was 0.46 g/L, the international normalized ratio was 1.67, and results of liver function tests were elevated; these findings are suggestive of hepatic decompensation in the background of rapidly progressing metastatic disease.
Possible immune-mediated AEs (regardless of attribution to pembrolizumab by the investigator) included one case each of grade 3 colitis, grade 3 hepatitis, and grade 2 hypothyroidism. The colitis was reported 40 days after the last pembrolizumab dose and after the patient had started subsequent therapy with capecitabine. Another patient experienced grade 2 enterocolitis accompanied by grade 3 diarrhea 136 days after the last pembrolizumab dose and 94 days after the start of eribulin. Both colitis cases were attributed to pembrolizumab by the investigator, were diagnosed by biopsy and exclusion of infectious etiology, and responded to steroids.
Antitumor Activity
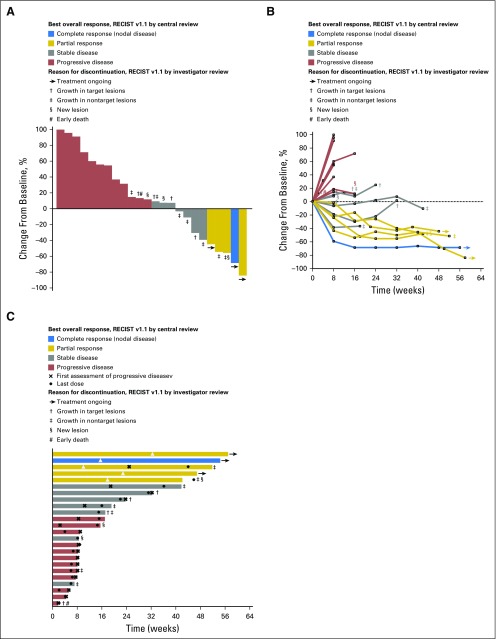
Overall, 37.5% of patients experienced a decrease from baseline in tumor burden (Fig 1A); decreases were maintained over time (Fig 1B). Of the 32 patients enrolled, 27 met the protocol-specified criteria for inclusion in the efficacy analysis population based on centrally assessed RECIST v1.1. In these 27 patients, the ORR was 18.5% (95% CI, 6.3 to 38.1; Table 3). Best overall responses were CR in one (3.7%) patient, PR in four (14.8%) patients, stable disease (SD) in seven (25.9%) patients, and PD in 13 (48.1%) patients (Table 3). The disease control rate (ie, percentage of patients with best response of CR, PR, or SD for ≥ 24 weeks) was 25.9% (95% CI, 11.1% to 46.3%). The patient who experienced a CR had previously received eight lines of therapy for metastatic disease, including anthracycline-, taxane-, and platinum-based regimens, capecitabine, and eribulin. Of the four patients who experienced a PR, one patient received one line of prior therapy, one patient received three lines of prior therapy, and two patients received six lines of prior therapy in the metastatic setting. Two of four (50.0%) patients with nonvisceral disease and 10 of 23 (43.5%) patients with visceral disease experienced response or SD.
Fig 1.
Antitumor activity of pembrolizumab based on RECIST v1.1 assessed by central review. (A) Best percentage change from baseline in the sum of the longest diameters of target lesions. (B) Longitudinal change from baseline in the sum of the longest diameters of target lesions. (C) Time to and durability of response. For each panel, only patients who received at least one pembrolizumab dose and who had evaluable tumor measurements based on RECIST v1.1 assessed by central review at baseline and at least one postbaseline assessment were included (n = 24). Reasons for treatment discontinuation in patients whose change from baseline per RECIST v1.1 by central review was ≤ 20% are indicated; because patients were managed by investigator assessment, reasons for discontinuation are based on RECIST v1.1 by investigator review. In panel C, length of bars equals time to last imaging assessment by central review. RECIST, Response Evaluation Criteria in Solid Tumors.
Table 3.
Best Overall Response Based on Response Evaluation Criteria in Solid Tumors v1.1 as Assessed by Central Review
| Response Type | Patients Evaluable for Response, N = 27* |
|---|---|
| Overall response rate, % (95% CI) | 18.5 (6.3 to 38.1) |
| Best overall response, No. (%) | |
| Complete response† | 1 (3.7) |
| Partial response† | 4 (14.8) |
| Stable disease | 7 (25.9) |
| Progressive disease | 13 (48.1) |
| No assessment‡ | 2 (7.4) |
Includes patients with measurable disease at baseline, based on Response Evaluation Criteria in Solid Tumors v1.1 as assessed by central review, who received at least one pembrolizumab dose. Five patients were excluded because they did not have centrally confirmed measurable disease at baseline.
Confirmed responses only.
Signifies patients who discontinued therapy before the first postbaseline scan because of progressive disease or a treatment-related adverse event.
Elevated LDH has been recognized as a poor prognostic factor in solid malignancies, including breast cancer.37 Among the 27 patients with known LDH level at baseline, greater than two-fold elevations in baseline LDH levels were associated with rapid disease progression: five of five (100%) patients with LDH ≥ 800 IU/L experienced PD within 8 weeks of study entry compared with 11 of 22 (50.0%) patients with LDH < 800 IU/L. This suggests that patients with mTNBC and high LDH may not have the opportunity to derive benefit from pembrolizumab, which in TNBC is associated with a longer median time to response (18 weeks; range, 7 to 32 weeks) than cytotoxic chemotherapy.
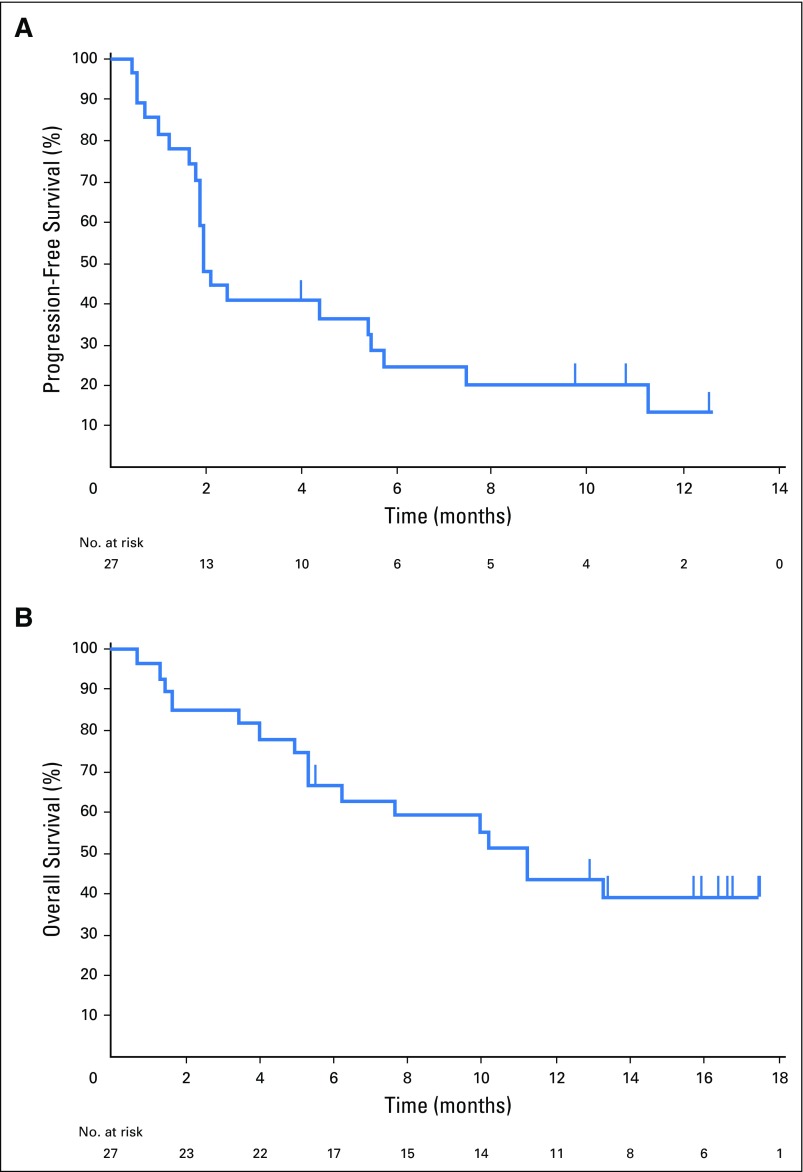
The median duration of SD was 17.0 weeks (range, 7.1 to 32.1 weeks), and two patients had SD ≥ 24 weeks duration. The median time to response was 17.9 weeks (range, 7.3 to 32.4 weeks; Fig 1C). Of the five responders, two discontinued treatment: one for progression in nontarget lesions and one for appearance of new lesions. Three responders remain on study and have received pembrolizumab for ≥ 1 year, with response durations of 24.1, 24.7, and 47.3 weeks as of data cutoff. The median duration of response was not reached at the time of data cutoff (range, 15.0 to ≥ 47.3 weeks). Twenty-two PFS events were observed, and the median PFS was 1.9 months (95% CI, 1.7 to 5.5), with a 6-month PFS rate of 24.4% (Fig 2A). The median OS was 11.2 months (95% CI, 5.3 to [not reached]), with 6-month and 12-month OS rates of 66.7% and 43.1%, respectively (Fig 2B).
Fig 2.
Kaplan-Meier estimates of (A) progression-free survival based on Response Evaluation Criteria in Solid Tumors v1.1 assessed by central review and (B) overall survival.
In this small population, using a prototype assay scoring PD-L1 expression as the percentage of inflammatory and tumor cells staining for PD-L1, there was evidence of an increasing probability of response (one-sided P = .028 for ORR) and a reduction in the hazard (one-sided P = .012 for PFS) with increasing expression of PD-L1.
DISCUSSION
In KEYNOTE-012, we investigated the safety and antitumor activity of single-agent pembrolizumab in PD-L1–expressing, advanced TNBC; all patients were exposed to chemotherapy in the (neo)adjuvant and/or metastatic setting. We found that pembrolizumab demonstrated an acceptable safety and tolerability profile, with an incidence of grade 3 to 5 treatment-related AEs (15.6%) comparable to that seen with its use in other advanced malignancies. Importantly, pembrolizumab demonstrated an ORR of 18.5%, including one CR. To our knowledge, this makes it the first published report showing clinical activity for an immune checkpoint inhibitor in a heavily pretreated mTNBC population. The 18.5% ORR is comparable to that reported for the head and neck (21.4%)25 and gastric (22.2%)26 cancer cohorts of KEYNOTE-012, and only slightly lower than that of the urothelial cancer cohort (27.6%).27
All 32 enrolled patients had previously received chemotherapy, including 27 who received chemotherapy for both early and advanced disease. All patients had previously received a taxane, and the majority had also been exposed to anthracyclines, platinum agents, and capecitabine. Four of the five responders had visceral disease, suggesting that immune checkpoint inhibition is an effective therapy even in the setting of heavily pretreated, advanced disease. However, in patients with baseline LDH levels > 2× the upper limit of normal (suggestive of rapid disease progression), no responses were seen. Combining immune checkpoint inhibition with cytotoxic therapy may be a reasonable strategy in these patients, to enable more rapid disease control while waiting for immune checkpoint blockade to take effect.
The durability of response observed with pembrolizumab monotherapy in heavily pretreated mTNBC (median not reached; range, 15.0 to > 47.3 weeks), including three responders who remain on study and have received treatment for ≥ 1 year, is promising, given that the duration of response to standard chemotherapy in this population is, at best, 4 to 12 weeks.38 The 25.9% disease control rate, 24.4% 6-month PFS rate, and 66.7% 6-month OS rate support the durable benefit of pembrolizumab in patients with mTNBC. Using a prototype assay, there was a trend toward clinical benefit with pembrolizumab and increasing PD-L1 expression. Although these data are hypothesis generating, we were unable to conclude whether PD-L1 expression was predictive of response, given the small sample size and the enrollment of only patients with PD-L1–positive tumors. In general, use of PD-L1 as a biomarker has been controversial. Further studies are therefore needed to identify immune biomarkers to select patients who would most likely benefit from immunotherapies.
One limitation of this study was the small sample size, which makes broad generalizations regarding efficacy challenging. The 18.5% response rate may have been dampened by the heavily pretreated population studied and the inclusion of patients with high LDH levels. These patients appeared to have aggressive and rapidly proliferating tumors, given that all of them experienced disease progression within 60 days of study entry. However, it is notable that in KEYNOTE-012, the ORR associated with single-agent pembrolizumab (18.5%) was approximately double that reported for capecitabine (9%) as second or higher line (2L+) therapy for mTNBC in a prespecified subgroup analysis of a phase III clinical trial.39
The high dose administered in this study potentially led to greater incidence of toxicities. However, in patients with advanced melanoma40 and advanced NSCLC41 enrolled in the pivotal KEYNOTE-001 study, pembrolizumab given at 10 mg/kg every 2 or every 3 weeks showed similar incidence of grade 3 to 4 treatment-related toxicities (15% v 12% in melanoma, 9% v 10% in NSCLC). Based on the similar safety profiles, the highest dose, 10 mg/kg every 2 weeks, was chosen to assess initial tumor activity in the TNBC cohort. Subsequent pharmacokinetic/pharmacodynamic analyses found no exposure-response relationship for efficacy or safety between pembrolizumab doses and schedules, and the approved dose of pembrolizumab was accordingly changed to once every 3 weeks.42
Overall, these results support further development of pembrolizumab for the treatment of mTNBC. The phase II KEYNOTE-086 trial (ClinicalTrials.gov identifier: NCT02447003) evaluating the efficacy and safety of single-agent pembrolizumab in mTNBC is currently enrolling patients. Combination studies of pembrolizumab with other anticancer therapies are in development.
Acknowledgment
The authors thank the patients and their families and caregivers for participating in the study, Marisa Dolled-Filhart and Kenneth Emancipator (Merck & Co., Kenilworth, NJ) for immunohistochemistry expertise and study support, Roger Dansey (Merck & Co.) for critical manuscript review and study support, Karl Heath (Merck & Co.) for study support, and QualTek Molecular Laboratories (Santa Barbara, CA) for PD-L1 immunohistochemistry assay testing. Medical writing and editorial assistance was provided by Tricia Brown and Melanie Leiby, of the ApotheCom Merck oncology team (Yardley, PA); this assistance was funded by Merck & Co.
Footnotes
Supported by Merck & Co., Kenilworth, NJ.
Presented in part at the San Antonio Breast Cancer Symposium, San Antonio, TX, December 9-13, 2014 and at the IMPAKT Breast Cancer Conference, Brussels, Belgium, May 7-9, 2015.
Authors' disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
Clinical trial information: NCT01848834.
AUTHOR CONTRIBUTIONS
Conception and design: Rita Nanda, Gursel Aktan, Jonathan D. Cheng
Provision of study materials or patients: Rita Nanda, Laura Q.M. Chow, E. Claire Dees, Shilpa Gupta, Lajos Pusztai, Laurence Buisseret
Collection and assembly of data: Rita Nanda, Laura Q.M. Chow, E. Claire Dees, Raanan Berger, Lajos Pusztai, Gursel Aktan, Laurence Buisseret
Data analysis and interpretation: Rita Nanda, Laura Q.M. Chow, Shilpa Gupta, Ravit Geva, Lajos Pusztai, Kumudu Pathiraja, Gursel Aktan, Jonathan D. Cheng, Vassiliki Karantza, Laurence Buisseret
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Pembrolizumab in Patients With Advanced Triple-Negative Breast Cancer: Phase Ib KEYNOTE-012 Study
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
Rita Nanda
Consulting or Advisory Role: Corcept Therapeutics, Genentech, Merck, Novartis
Research Funding: Celgene, Corcept Therapeutics, Merck, Synta
Laura Q.M. Chow
Honoraria: Merck, Novartis, Emergent BioSolutions, Amgen
Consulting or Advisory Role: Merck, Novartis, Amgen, Emergent BioSolutions
Research Funding: Merck (Inst), Novartis (Inst), Genentech (Inst), Bristol-Myers Squibb (Inst), Pfizer (Inst), VentiRx (Inst), AstraZeneca/MedImmune (Inst), GlaxoSmithKline (Inst), Incyte (Inst), Lilly/ImClone (Inst)
Travel, Accommodations, Expenses: Merck, Novartis
E. Claire Dees
Consulting or Advisory Role: Novartis (I), Amgen (I)
Research Funding: Novartis (Inst), Genentech (Inst), Bayer (Inst), Lilly/ImClone (Inst), Pfizer (Inst), Millennium (Inst), Agensys (Inst), Merck (Inst), Aveo Pharmaceuticals, New Link Genetics
Raanan Berger
No relationship to disclose
Shilpa Gupta
Honoraria: Pfizer, Genentech, Seattle Genetics
Consulting or Advisory Role: Pfizer, Seattle Genetics, Genentech
Speakers' Bureau: Genentech
Research Funding: Astellas Medivation (Inst), Innocrin Pharma (Inst), Pfizer (Inst), MedImmune (Inst), Merck (Inst)
Ravit Geva
No relationship to disclose
Lajos Pusztai
Honoraria: BioTheranostics, Pfizer, Merck
Consulting or Advisory Role: Clovis Oncology, Celgene, Merck
Research Funding: Foundation Medicine, Merck, Genentech
Kumudu Pathiraja
Employment: Merck
Gursel Aktan
Employment: Merck, GlaxoSmithKline, Novartis (I), Merck (I), GlaxoSmithKline (I)
Leadership: Merck
Stock or Other Ownership: Merck, GlaxoSmithKline, ImmunoCellular Therapeutics (I), TapImmune Inc. (I), OncoSec
Jonathan D. Cheng
Employment: Merck & Co.
Stock or Other Ownership: Merck & Co.
Vassiliki Karantza
Employment: Merck
Stock or Other Ownership: Merck
Laurence Buisseret
No relationship to disclose
REFERENCES
- 1.Foulkes WD, Smith IE, Reis-Filho JS: Triple-negative breast cancer. N Engl J Med 363:1938–1948,2010 [DOI] [PubMed] [Google Scholar]
- 2.Dent R Trudeau M Pritchard KI, etal: Triple-negative breast cancer: Clinical features and patterns of recurrence. Clin Cancer Res 13:4429–4434,2007 [DOI] [PubMed] [Google Scholar]
- 3.American Cancer Society : American Cancer Society Breast Cancer Facts and Figures 2013-2014 2013. Atlanta, GA: American Cancer Society [Google Scholar]
- 4.Rakha EA Elsheikh SE Aleskandarany MA, etal: Triple-negative breast cancer: Distinguishing between basal and nonbasal subtypes. Clin Cancer Res 15:2302–2310,2009 [DOI] [PubMed] [Google Scholar]
- 5.Gucalp A, Traina TA: Triple-negative breast cancer: Adjuvant therapeutic options. Chemother Res Pract 2011:696208,2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carey LA Perou CM Livasy CA, etal: Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA 295:2492–2502,2006 [DOI] [PubMed] [Google Scholar]
- 7.Bauer KR Brown M Cress RD, etal: Descriptive analysis of estrogen receptor (ER)-negative, progesterone receptor (PR)-negative, and HER2-negative invasive breast cancer, the so-called triple-negative phenotype: A population-based study from the California Cancer Registry. Cancer 109:1721–1728,2007 [DOI] [PubMed] [Google Scholar]
- 8.Peshkin BN, Alabek ML, Isaacs C: BRCA1/2 mutations and triple negative breast cancers. Breast Dis 32:25–33,2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Couch FJ Hart SN Sharma P, etal: Inherited mutations in 17 breast cancer susceptibility genes among a large triple-negative breast cancer cohort unselected for family history of breast cancer. J Clin Oncol 33:304–311,2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haffty BG Yang Q Reiss M, etal: Locoregional relapse and distant metastasis in conservatively managed triple negative early-stage breast cancer. J Clin Oncol 24:5652–5657,2006 [DOI] [PubMed] [Google Scholar]
- 11.Carey LA Rugo HS Marcom PK, etal: TBCRC 001: Randomized phase II study of cetuximab in combination with carboplatin in stage IV triple-negative breast cancer. J Clin Oncol 30:2615–2623,2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rouzier R Perou CM Symmans WF, etal: Breast cancer molecular subtypes respond differently to preoperative chemotherapy. Clin Cancer Res 11:5678–5685,2005 [DOI] [PubMed] [Google Scholar]
- 13.Harris LN Broadwater G Lin NU, etal: Molecular subtypes of breast cancer in relation to paclitaxel response and outcomes in women with metastatic disease: Results from CALGB 9342. Breast Cancer Res 8:R66,2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pardoll DM: The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 12:252–264,2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Keir ME Butte MJ Freeman GJ, etal: PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol 26:677–704,2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nishimura H Agata Y Kawasaki A, etal: Developmentally regulated expression of the PD-1 protein on the surface of double-negative (CD4-CD8-) thymocytes. Int Immunol 8:773–780,1996 [DOI] [PubMed] [Google Scholar]
- 17.Topalian SL, Drake CG, Pardoll DM: Targeting the PD-1/B7-H1(PD-L1) pathway to activate anti-tumor immunity. Curr Opin Immunol 24:207–212,2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Taube JM Anders RA Young GD, etal: Colocalization of inflammatory response with B7-h1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci Transl Med 4:127ra37,2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Topalian SL Hodi FS Brahmer JR, etal: Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 366:2443–2454,2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mittendorf EA Philips AV Meric-Bernstam F, etal: PD-L1 expression in triple-negative breast cancer. Cancer Immunol Res 2:361–370,2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schalper KA Velcheti V Carvajal D, etal: In situ tumor PD-L1 mRNA expression is associated with increased TILs and better outcome in breast carcinomas. Clin Cancer Res 20:2773–2782,2014 [DOI] [PubMed] [Google Scholar]
- 22.Wimberly H Brown JR Schalper K, etal: PD-L1 expression correlates with tumor-infiltrating lymphocytes and response to neoadjuvant chemotherapy in breast cancer. Cancer Immunol Res 3:326–332,2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ali HR Glont SE Blows FM, etal: PD-L1 protein expression in breast cancer is rare, enriched in basal-like tumours and associated with infiltrating lymphocytes. Ann Oncol 26:1488–1493,2015 [DOI] [PubMed] [Google Scholar]
- 24.Garon EB Rizvi NA Hui R, etal: Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med 372:2018–2028,2015 [DOI] [PubMed] [Google Scholar]
- 25. Seiwert TY, Burtness B, Weiss J: A phase Ib study of MK-3475 in patients with human papillomavirus (HPV)-associated and non-HPV-associated head and neck (H/N) cancer. J Clin Oncol 32:5s, 2014 (suppl; abstr 6011)
- 26. Bang YJ, Chung HC, Shankaran S, et al: Relationship between PD-L1 expression and clinical outcomes in patients with advanced gastric cancer treated with the anti-PD-1 monoclonal antibody pembrolizumab (MK-3475) in KEYNOTE-012. Presented at the 2015 American Society of Clinical Oncology Annual Meeting, Chicago, IL, May 29-June 2, 2015. [Google Scholar]
- 27.Plimack ER Gupta S Bellmunt J, etal: A phase 1b study of pembrolizumab (pembro; MK-3475) in patients with advanced urothelial cancer Ann Oncol 201425: [Google Scholar]
- 28.Desmedt C Haibe-Kains B Wirapati P, etal: Biological processes associated with breast cancer clinical outcome depend on the molecular subtypes Clin Cancer Res 14:5158–5165,2008 [DOI] [PubMed] [Google Scholar]
- 29.Loi S Sirtaine N Piette F, etal: Prognostic and predictive value of tumor-infiltrating lymphocytes in a phase III randomized adjuvant breast cancer trial in node-positive breast cancer comparing the addition of docetaxel to doxorubicin with doxorubicin-based chemotherapy: BIG 02-98 J Clin Oncol 31:860–867,2013 [DOI] [PubMed] [Google Scholar]
- 30.Liu S Lachapelle J Leung S, etal: CD8+ lymphocyte infiltration is an independent favorable prognostic indicator in basal-like breast cancer Breast Cancer Res 14:R48, 2012, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liyanage UK Moore TT Joo HG, etal: Prevalence of regulatory T cells is increased in peripheral blood and tumor microenvironment of patients with pancreas or breast adenocarcinoma J Immunol 169:2756–2761,2002 [DOI] [PubMed] [Google Scholar]
- 32.Bates GJ Fox SB Han C, etal: Quantification of regulatory T cells enables the identification of high-risk breast cancer patients and those at risk of late relapse. J Clin Oncol 24:5373–5380,2006 [DOI] [PubMed] [Google Scholar]
- 33.Adams S Gray RJ Demaria S, etal: Prognostic value of tumor-infiltrating lymphocytes in triple-negative breast cancers from two phase III randomized adjuvant breast cancer trials: ECOG 2197 and ECOG 1199. J Clin Oncol 32:2959–2966,2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.The Cancer Genome Atlas Network : Comprehensive molecular portraits of human breast tumours Nature 490:61–70,2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ghebeh H Mohammed S Al-Omair A, etal: The B7-H1 (PD-L1) T lymphocyte-inhibitory molecule is expressed in breast cancer patients with infiltrating ductal carcinoma: Correlation with important high-risk prognostic factors. Neoplasia 8:190–198,2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Saal LH Holm K Maurer M, etal: PIK3CA mutations correlate with hormone receptors, node metastasis, and ERBB2, and are mutually exclusive with PTEN loss in human breast carcinoma. Cancer Res 65:2554–2559,2005 [DOI] [PubMed] [Google Scholar]
- 37.Zhang J Yao YH Li BG, etal: Prognostic value of pretreatment serum lactate dehydrogenase level in patients with solid tumors: A systematic review and meta-analysis. Sci Rep 5:9800,2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Senkus E, Cardoso F, Pagani O: Time for more optimism in metastatic breast cancer? Cancer Treat Rev 40:220–228,2014 [DOI] [PubMed] [Google Scholar]
- 39.Pivot XB Li RK Thomas ES, etal: Activity of ixabepilone in oestrogen receptor-negative and oestrogen receptor-progesterone receptor-human epidermal growth factor receptor 2-negative metastatic breast cancer Eur J Cancer 45:2940–2946,2009 [DOI] [PubMed] [Google Scholar]
- 40.Robert C Ribas A Wolchok JD, etal: Anti-programmed-death-receptor-1 treatment with pembrolizumab in ipilimumab-refractory advanced melanoma: A randomised dose-comparison cohort of a phase 1 trial Lancet 384:1109–1117,2014 [DOI] [PubMed] [Google Scholar]
- 41. Garon EB, Leighl NA, Rizvi NA: Safety and clinical activity of MK-3475 in previously treated patients (pts) with non-small cell lung cancer (NSCLC). J Clin Oncol 32:5s, 2014 (suppl; abstr 8020)
- 42.Kang SP Chatterjee M Ahamadi M, etal: Relationship between pembrolizumab exposure and efficacy and safety in patients with advanced or metastatic melanoma. Presented at The European Cancer Congress 2015, Vienna, Austria, September 25-29, 2015