Abstract
The treatment landscape of advanced prostate cancer continues to evolve rapidly, with newer and more active drugs being used in earlier phases of the disease based on improved overall survival. After adoption of docetaxel for metastatic castration-sensitive disease, large trials with next-generation androgen receptor-signaling inhibitors (abiraterone, enzalutamide and apalutamide) have demonstrate significant improvements in survival and important secondary endpoints. For non-metastatic castration-resistant prostate cancer, recent phase III placebo-controlled trials with enzalutamide, apalutamide and darolutamide all demonstrated benefits in improving metastasis-free survival. This review aims to summarize the clinical development of darolutamide, a novel next-generation androgen receptor antagonist, including preclinical data, clinical studies and the potential of darolutamide for the treatment of advanced prostate cancer. To date, darolutamide efficacy and tolerability has been demonstrated in the ARAMIS trial, which demonstrated an improvement in metastasis-free survival compared to placebo for non-metastatic castration-resistant prostate cancer patients with a rapid PSA doubling time. Ongoing studies will further evaluate the role of darolutamide in metastatic castration-sensitive prostate cancer in combination with docetaxel (ARASENS trial) and also in other stages of the disease.
Keywords: darolutamide, androgen receptor antagonist, prostate cancer, castration-resistant
Introduction
During the past few years we have witnessed significant improvements in the understanding of prostate cancer biology, which have led to marked changes in the treatment landscape of advanced prostate cancer, for both castration-sensitive and castration-resistant disease.1 After docetaxel’s approval in 2004, the United States Food and Drug Administration (FDA) approved several drugs from 2010 to 2013 for the treatment of metastatic castration-resistant prostate cancer (mCRPC): abiraterone2,3 and enzalutamide,4,5 both novel androgen-receptor (AR) signaling axis inhibitors; cabazitaxel, an anti-microtubule cytotoxic chemotherapy agent;6 an immunotherapy agent (sipuleucel-T)7 and the α-emitting radiopharmaceutical drug, radium-223.8 These compounds added new and active treatment options for patients with mCRPC, leading to improvements in overall survival (OS), radiographic progression-free survival and quality of life (QoL), as demonstrated in the multiple positive controlled phase III clinical trials conducted with these agents.2–8
As intensive research continued to evolve, important areas demonstrated significant advances: increasing knowledge of novel biomarkers in mCRPC, including AR splice variant 7 (AR-V7),9–14 AR and DNA damage repair (DDR) gene alterations,15–18 DNA mismatch repair defects (dMMR),19,20 among others;21–23 and also incorporation of docetaxel24–26 and next-generation AR-signaling axis inhibitors (ARSi)27–31 earlier in the disease spectrum, with significant overall survival benefits observed in castration-sensitive prostate cancer (CSPC). In addition, advances have also been made for non-metastatic castration-resistant prostate cancer (nmCRPC), with the FDA approval of the next-generation AR antagonists enzalutamide,32 apalutamide;33 and darolutamide34 in this space based on metastasis-free survival benefits.
In this manuscript, we will review the clinical development and perspectives of darolutamide (formerly known as ODM-201), a next-generation AR antagonist, for the treatment of CRPC. We will also speculate of the future role of darolutamide in this disease.
Current Treatment Landscape Of Recurrent And Advanced Prostate Cancer
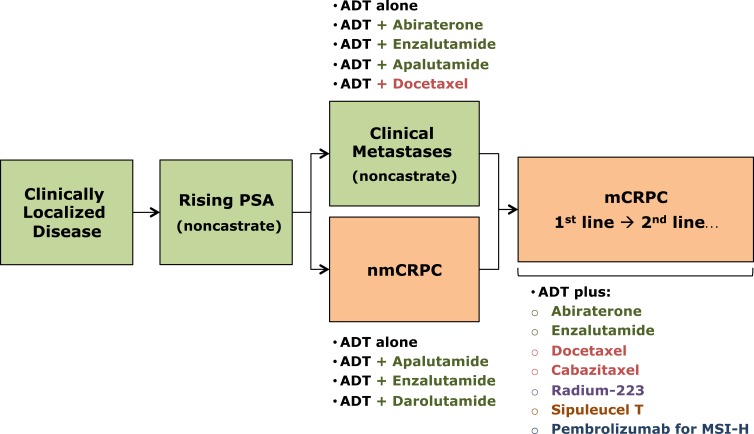
As previously discussed, the treatment options for CRPC have expanded in the last decade and new and more active therapies are currently available. Figure 1 depicts the FDA approved life-prolonging or metastasis-prolonging therapies for advanced prostate cancer, including the recently approved drugs for nmCRPC (enzalutamide, apalutamide, darolutamide). Notably, while enzalutamide also has an FDA label for mCRPC, apalutamide and darolutamide will only have labels for nmCRPC initially. This schematic also includes pembrolizumab, an anti-programed cell death-1 receptor inhibitor, for patients with MMR-deficient or microsatellite-high (MSI-H) tumors. It is important to emphasize that several clinical trials are ongoing in the different prostate cancer clinical states, testing novel agents (e.g. PARP inhibitors, PSMA-ligand therapies) and/or combinations, which may further modify the treatment landscape of this disease in the near future and lead to improved outcomes for patients with advanced prostate cancer.
Figure 1.
Prostate cancer clinical states model.
Notes: Data created to include the corresponding agents that are FDA approved in each state. Data from Scher et al.35 Green boxes refer to the castration-sensitive state whereas the orange boxes refer to castration-resistant state. Green texts refer to castration-sensitive state whereas orange texts refer to castration-resistant state.
Abbreviations: ADT, androgen deprivation therapy; nmCRPC, non-metastatic castration-resistant prostate cancer; mCRPC, metastatic castration-resistant prostate cancer; MSI-H, microsatellite instability.
Darolutamide For mCRPC: Early Drug Development
Drug Characteristics
Darolutamide, formerly known as ODM-201, is a synthetic nonsteroidal androgen receptor (AR) antagonist with a distinct molecular structure compared to the other next-generation AR antagonists: enzalutamide and apalutamide. It is composed of 2 pharmacologically active compounds ([S,R]-darolutamide and [S,S]-darolutamide) which convert into the metabolically active metabolite keto-darolutamide.36,37 As a next-generation AR antagonists, darolutamide is a full AR inhibitor that blocks the AR translocation into the cell nucleus and testosterone-induced downstream effects of DNA activation, cell growth and survival. However, there are important differences between darolutamide and other AR antagonists beyond the described structural features (Table 1). These include: 1) higher AR inhibition potency in preclinical studies as demonstrated by the lower inhibition constant (Ki) and maximal inhibitory concentration (IC50) values compared to enzalutamide and apalutamide; 2) darolutamide does not activate mutant AR such as AR(F877L), AR(W742L) and AR(T878A) which lead to promiscuous activation; and 3) darolutamide has negligible blood-brain barrier penetration as demonstrated in mouse PK studies, with a brain/plasma ratio of about 2% compared to 25% for enzalutamide,36 which may theoretically lead to improved central nervous system (CNS) potential adverse events. However, it is important to note that despite these differences, current available data do not clearly demonstrate better clinical outcomes with darolutamide compared to enzalutamide or apalutamide.
Table 1.
Key Differences Of Selected Next-Generation AR Antagonists
| Bicalutamide | Enzalutamide | Apalutamide | Darolutamide | |
|---|---|---|---|---|
| AR inhibition | Yes, also a partial agonist | Full antagonist (IC50 219 nM)* | Full antagonist (IC50 200 nM)* | Full antagonist (IC50 26 nM)* |
| Inhibition of Nuclear Translocation | No | Yes | Yes | Yes |
| Activation of mutant AR | Yes (W742L) | Yes (F877L) | Yes (F877L) | None described |
| Increase of serum testosterone levels | Yes | Yes | Yes | No |
| Blood-brain barrier penetration | Negligible | Yes | Yes | Negligible |
Note: *Transactivation assays in AR-HEK293 cells stably expressing full-length AR. Data from Moilanen et al.36
Abbreviations: AR, androgen receptor; IC50, half maximal inhibitory concentration.
Clinical Development And Early-Phase Studies
The initial clinical development of darolutamide included two important early-phase studies that assessed the safety and preliminary efficacy of the drug in patients with mCRPC: i) the ARADES study: activity and safety of ODM-201 in patients with progressive metastatic castration-resistant prostate cancer: an open-label phase 1 dose-escalation and randomized phase 2 dose expansion trial38 and ii) the ARAFOR study: pharmacokinetics, antitumor activity, and safety of ODM-201 in patients with chemotherapy-naive metastatic castration-resistant prostate cancer: an open-label phase 1 study.39 These will be discussed in turn.
ARADES was a phase 1/2 open label study of darolutamide for patients with progressive mCRPC with 2 components: a phase I component using oral darolutamide starting from 200 mg up to 1800 mg daily (non-randomized dose-escalation cohort), with a primary endpoint of safety and tolerability; and a phase II component where patients with mCRPC were randomized to receive darolutamide 200 mg, 400 mg or 1400 mg daily, with the primary endpoint of PSA response rate (decline ≥50%).38 Twenty-four patients were included in the phase I component and no dose-limiting toxic effects were reported; the maximum tolerated dose was not reached. Importantly, no patient developed drug-related grade 3-4 adverse events. Of the 21 patients with PSA data available, 17 (81%) demonstrated a PSA response. Regarding the pharmacokinetic analysis, darolutamide was rapidly absorbed (median time to maximum plasma concentrations (Cmax) of 3.0–5.1 hours) and reached steady-state plasma concentration after 1 week of continuous therapy. Plasma concentration of darolutamide increased linearly depending on the dose level up to 1400 mg, with a plateau affect thereafter. The mean half-live of darolutamide was 15.8 hours. In the ARADES phase II component, 110 patients were included and PSA declines of ≥50% were achieved in 29%, 33% and 33% in the 200mg, 400mg and 1400 mg groups, respectively. Treatment was well tolerated and the most common reported adverse events were fatigue (12%), hot flashes (5%], and anorexia (4%).38
In the ARADES study, darolutamide was administred in 100 mg capsules, and therefore patients on higher doses needed to take several capsules per day, amounting to a large pill burden. As an attempt to improve patient’s convenience during therapy, 300 mg tablets of darolutamide were developed and tested in the ARAFOR study. The ARAFOR trial was a 2-part multicenter phase I study to compare the pharmacokinetics between the 300 mg tablet compared to the 100 mg capsule formulation and to evaluate the effect of food on darolutamide absorption (part 1), and also to assess safety and antitumor activity of darolutamide 600 mg twice-daily in 30 patients with chemotherapy-naive mCRPC (part 2).39 This study demonstrated a clear food interaction with darolutamide absorption, with nearly double the area under the curve and Cmax vaues after a high-fat meal compared to the fasting state after a single dose of darolutamide 600 mg. The 12-week PSA decline ≥50% rate in the ARAFOR study with darolutamide 600 mg twice daily was 83% (25 of 30 patients), and the median time to PSA progression and radiographic progression were 54 and 66 weeks, respectively. In terms of safety, although adverse events (AEs) were reported by 73% of patients (91% grade 1-2), only 20% presented with treatment-related AEs, demonstrating a good tolerability with the 600 mg b.i.d regimen. The reported AEs were all grade 1 and included fatigue, anorexia, dysgeusia, headache, abdominal pain, tinnitus, and solar dermatitis. Of note, no patient discontinued therapy due to treatment-related AEs and no seizures were reported.39 A subsequent analysis of the ARADES and ARAFOR trials with longer follow-up did not demonstrate any new toxicity signals, and a sustained efficacy of darolutamide in mCRPC was confirmed.40 Based on these results, darolutamide 600 mg (two 300 mg tablets) given twice daily in fed state was the selected regimen for the subsequent randomized placebo-controlled trial evaluating darolutamide in high-risk nmCRPC (the ARAMIS study).
Non-Metastatic CRPC
Non-metastatic CRPC is a prostate cancer disease state defined by a rising PSA level in the setting of “castrate” levels of serum testosterone (< 50 ng/dL) and no metastatic disease identified on conventional imaging such as bone scan, computed tomography (CT) and/or magnetic resonance imaging (MRI). Until 2018, there were no FDA approved drugs in this space and clinicians used to face the challenge of observing such patients with rising PSA and no available therapies to delay the development of metastasis and prolonging survival. In this setting, a common previous practice was the use of the so-called vintage hormonal therapy with bicalutamide, flutamide or nilutamide, although no randomized trials had demonstrated the benefit of this approach.
Recently, three large placebo-controlled randomized trials were conducted for patients with nmCRPC using enzalutamide,32 apalutamide,33 and darolutamide,34 which led to FDA approval based on improvements in metastasis-free survival (MFS). Table 2 highlights the main characteristics and results of the three phase III trials for nmCRPC.
Table 2.
Comparison Of Main Characteristics And Outcomes Of The Pivotal Phase III Trials For nmCRPC
| Drug Name | PROSPER Trial32 | SPARTAN Trial33 | ARAMIS Trial34 |
|---|---|---|---|
| Enzalutamide | Apalutamide | Darolutamide | |
| Design |
|
|
|
| Sample size | 1,401 | 1,207 | 1,509 |
| Population | High-risk nmCRPC (PSAdt < 10 months) | High-risk nmCRPC (PSAdt < 10 months) | High-risk nmCRPC (PSAdt < 10 months) |
| N1 disease | Not included | Included (< 2 cm) | Included (< 2 cm) |
| 1 endpoint | Metastasis-free survival | Metastasis-free survival | Metastasis-free survival |
| Median time to PSA progression (months) | 37.2 vs 3.9 mo (P<0.001) | NR vs 3.7 mo | 33.2 vs 7.3 mo (P<0.001) |
| Median MFS (months) | 36.6 vs 14.7 mo (P<0.001) | 40.5 vs 16.2 mo (P<0.001) | 40.4 vs 18.4 mo (P<0.001) |
| Overall Survival (months) | NR vs NR (P>0.05*) | NR vs 39 (P=0.07*) | NR vs NR (P= 0.045*) |
Note: *not statistically significant with current follow-up.
Abbreviations: N1, loco-regional (pelvic) lymph node metastasis; MFS, metastasis-free survival; AE, adverse events; nmCRPC, non-metastatic castration-resistant prostate cancer; NR, not reached.
The PROSPER study was a double-blind placebo controlled study which randomized 1,401 patients in a 2:1 ratio to receive enzalutamide 160 mg daily or matching placebo. The primary endpoint of the study was met with a median MFS of 36.6 versus 14.7 months favoring the enzalutamide arm (hazard ratio [HR] 0.29; 95% confidence interval [CI], 0.24 to 0.35; P<0.001). Moreover, time to PSA progression (37.2 vs. 3.9 months; HR 0.07; P<0.001) and time to subsequent systemic therapy (39.6 vs. 17.7 months; hazard ratio, 0.21; P<0.001) were longer in the enzalutamide arm, confirming its meaningful clinical activity in the nmCRPC space. The safety profile of enzalutamide was consistent with previous phase III studies in the mCRPC setting, mainly grade 1-2 fatigue (33%), hypertension (12%), falls (11%) and nausea (11%). In the first interim analysis, the OS data was still immature and not different between the treatment groups although longer follow-up is planned.32
The SPARTAN trial had a very similar design compared to the PROSPER study, and randomized patients to receive apalutamide 240 mg daily versus placebo in a 2:1 fashion. This study also met its primary endpoint, with a significant increase in the median MFS favoring the apalutamide group: 40.5 versus 16.2 months (HR 0.28; 95% confidence interval [CI], 0.23 to 0.35; P<0.001). Apalutamide demonstrated impressive antitumor activity, with significant improvements in PSA responses (89.7% vs 2.2%) and median time to symptomatic progression. Also, the exploratory endpoint second-progression-free survival (time between randomization to disease progression after subsequent therapy) was longer in the apalutamide arm, suggesting that early use of apalutamide does not preclude response to subsequent therapies for mCRPC. In terms of safety, apalutamide was generally well tolerated and main AEs compared to placebo included fatigue (30.4% vs 21.1%), hypertension (24.8% vs 19.8%), skin rash (23.8% vs 5.5%), falls (15.6% vs 9%), and weight loss (16.1% vs 6.3%), among others (Table 3). Of note, 2 patients (0.2%) who received apalutamide developed seizures, and 8.1% developed hypothyroidism.33
Table 3.
Main Adverse Events Of Enzalutamide, Apalutamide And Darolutamide In The Pivotal Phase III Trials For nmCRPC
| Drug Name | PROSPER Trial32 | SPARTAN Trial33 | ARAMIS Trial34 |
|---|---|---|---|
| Enzalutamide | Apalutamide | Darolutamide | |
| AEs leading to drug discontinuation | 9% vs 6% | 10.7% vs 7% | 8.9% vs 8.7% |
| Grade ≥3 AEs | 31% vs 23% | 24.8% vs 23.1% | 24.7% vs 19.5% |
| AEs (Any grade): | |||
| Fatigue | 33.0% | 30.4% | 12.1% |
| Hypertension | 12.0% | 24.8% | 6.6% |
| Rash | NR | 23.8% | 2.9% |
| Diarrhea | 10.0% | 20.3% | 6.9% |
| Weight loss | 6.0% | 16.1% | 3.6% |
| Falls | 11.0% | 15.6% | 4.2% |
| Mental impairment | 5.0% | 5.1% | 0.9% |
| Seizures | <1% | 0.2% | 0.2% |
Abbreviations: AE, adverse events; NR, not reported.
Darolutamide was then tested for nmCRPC in the phase III ARAMIS trial, which randomized 1,509 patients (also 2:1) to receive darolutamide 600 mg twice daily versus placebo.34 This study also met its primary endpoint and demonstrated a significant improvement in median MFS using darolutamide versus placebo (40.4 vs 18.4 months; HR 0.41; 95% CI, 0.34 to 0.50; P<0.001). Darolutamide also significantly improved all secondary endpoints including time to pain progression, time to cytotoxic chemotherapy, and time to a symptomatic skeletal event; with a trend also towards improved overall survival (although the statistical boundary was crossed). In terms of tolerability, darolutamide was very well tolerated, with similar AEs compared to placebo, with the exception of fatigue, which was slightly greater in the darolutamide arm (12.1% vs 8.7%). Of note, there were no AE differences between darolutamide and placebo regarding the rates of hypertension, falls, cognitive disorders, or seizures. Moreover, the drug discontinuation rates of darolutamide and placebo were 8.9% and 8.7%, respectively, demonstrating the low toxicity potential of darolutamide. Table 3 depicts the adverse event (AE) profile of each AR antagonist in the phase III trials of nmCRPC.
Ongoing Studies With Darolutamide
Beyond the initial clinical development of darolutamide and the published ARAMIS trial that demonstrated significant efficacy in nmCRPC, several ongoing trials are being conducted testing darolutamide in different prostate cancer scenarios.
The most notable ongoing study with darolutamide is the ARASENS trial, a placebo-controlled randomized phase III trial with a target population of 1,300 patients with metastatic castration-sensitive prostate cancer (mCSPC). In this trial (which has completed enrolment), patients have been randomized to receive darolutamide 600 mg or placebo twice daily with food, in addition to standard androgen ADT and up-front docetaxel. Importantly, this is the first study in mCSPC that uses chemo-hormonal therapy as the backbone for both study arms; therefore it aims to determine whether the incorporation of darolutamide will produce additional benefit beyond the backbone of ADT plus docetaxel. The primary endpoint of the ARASENS trial is overall survival, and several secondary endpoints include time to CRPC, time to initiation of subsequent therapy, symptomatic skeletal event-free survival, and others including QoL. The top-line results of this study are eagerly awaited, and have not been reported or presented yet. To date, the available data from randomized trials support ADT plus docetaxel OR a next-generation ARSi (abiraterone, enzalutamide, apalutamide) for the treatment of mCSPC, but not both at the same time in the opinion of the authors.
There are also ongoing phase II trials such as an EORTC trial comparing ADT versus darolutamide monotherapy in men with hormone-naïve prostate cancer, with a primary endpoint of 6-month PSA response rate, and secondary endpoints including quality of life. Additionally, based on the impression of improved tolerability and favorable adverse event profile similar to placebo in the ARAMIS trial, the ODENZA trial will compare darolutamide versus enzalutamide in the mCRPC space with a primary endpoint of patient preference. Table 4 summarizes the ongoing trials with darolutamide across prostate cancer disease states.
Table 4.
Selected Ongoing Trials With Darolutamide In Prostate Cancer
| Study/Sponsor | Setting | Target Accrual | Design | Study Arms | Primay Endpoint | ClinicalTrials.gov Identifier |
|---|---|---|---|---|---|---|
| ARASENS | mCSPC | 1,300 | Phase III | ADT+Docetaxel + Darolutamide vs ADT + Docetaxel + Placebo | OS | NCT02799602 |
| EORTC-1532 | CSPC | 250 | Phase II | ADT vs Darolutamide | PSA response | NCT02972060 |
| SGCCR | mCRPC | 88 | Phase II | Maintanance after docetaxel: Darolutamide vs Placebo | rPFS | NCT02933801 |
| ODENZA | mCRPC | 250 | Phase II | Enzalutamide vs Darolutamide | Patient preference | NCT03314324 |
| INTREPId | Localized | 220 | Phase II | RT + ADT + Bicalutamide vs RT + darolutamide | PSA nadir ≤ 0.5 | NCT04025372 |
Abbreviations: EORTC, European Organisation for Research and Treatment of Cancer; SGCCR, Swiss Group for Clinical Cancer Research; mCSPC, metastatic castration-sensitive prostate cancer; mCRPC, metastatic castration-resistant prostate cancer; ADT, androgen deprivation therapy; OS, overall survival; PSA, prostate specific antigen; rPFS, radiographic progression-free survival; RT, radiation therapy.
Perspectives And Conclusions
Androgen receptor signaling inhibitors (ARSi) have demonstrated significant improvements in survival, metastasis-free survival and quality of life in different clinical states of advanced prostate cancer, including metastatic castration-sensitive and non-metastatic as well as metastatic castration-resistant disease. Darolutamide is a next-generation AR antagonist with a distinct molecular structure compared to enzalutamide and apalutamide, and preclinical data suggest more potent AR inhibition, negligible blood-brain barrier penetration and no activation of AR mutations such as AR(F877L). Moreover, phase II and III data demonstrate high anti-tumor activity and a very safe toxicity profile, similar to placebo in the ARAMIS study. Ongoing studies are underway with the goal of expanding the potential use of darolutamide for advanced prostate cancer, including the ARASENS trial in mCSPC, the results of which are eagerly awaited.
One potential advantage of darolutamide (over enzalutamide and apalutamide) is the lower risk of cytochrome P450 (CYP450) drug-drug interactions, since several patients with advanced prostate cancer have significant comorbidities and are usually receiving multiple concurrent medications. Differently from enzalutamide and apalutamide, which are strong CYP3A4 inducers, a recent study with darolutamide has shown no clinically relevant inhibition of CYP1A2, 2A6, 2B6, 2C8, 2C9, 2C19, 2D6, 2E1, or 3A4.41 It is possible that this difference regarding drug-drug interactions may be the reason why there were no significant differences in terms of toxicity between darolutamide and placebo in the ARAMIS trial, considering that 98% of the patients on the trial were receiving at least one concurrent medication. For this reason, darolutamide might be the most attractive partner for combination with other AR-directed and non-AR agents in future clinical trials. Furthermore, due to less CNS penetration and central adverse events, darolutamide may theoretically cause less additive side effects when combined with other agents that may cross the blood-brain barrier. In addition, this agent could potentially be tested in patients with CNS disorders (e.g. cognitive impairment, dementia, ataxia, apraxia) without worsening such conditions, while enzalutamide and apalutamide would generally be discouraged in these settings.
However, darolutamide also faces certain challenges moving forward. First, it is the third drug to market for the nmCRPC indication, and it may have trouble capturing a market share due to the existing familiarity of the other two AR-directed agents. Second, there is a concern that the ARASENS trial might be a negative study. To this end, in the ENZAMET trial, the added value of enzalutamide in men who also received up-front docetaxel chemotherapy was more questionable in terms of overall survival gains.30 So it is possible that the use of darolutamide in a population of patients all of whom are already getting chemo-hormonal therapy may not translate into a survival benefit. Finally, it is unlikely that darolutamide will obtain an indication for use in the mCRPC space any time soon, as there are currently no ongoing pivotal trials testing this agent in the mCRPC population. Indeed, such trials would be difficult to conduct, and would probably require some type of head-to-head comparison against apalutamide or enzalutamide.
Nevertheless, the availability of one more AR-targeting agent in our therapeutic armamentarium is a welcome addition, and may lead to cost-reductions in a competitive market place. The ongoing trials with darolutamide and the future combination strategies are likely to shed additional light on other uses for this drug as well as the optimal combinations for synergy. These and other studies will keep the field busy for many years to come.
Funding Statement
ESA has received funding from NIH grants R01 CA185297 and P30 CA006973, and DOD grant W81XWH-16-PCRP-CCRSA.
Disclosure
Dr Diogo A Bastos reports grants and personal fees from Janssen, Astellas, and Bayer. He also received personal fees from Bristol Myers Squib, MSD, and AztraZeneca, during the conduct of the study. Dr Emmanuel S Antonarakis reports grants and personal fees from Janssen, Bayer, and Sanofi, during the conduct of the study. He also received grants and/or personal fees from Pfizer, Dendreon, Bristol Myers Squibb, Amgen, Merck, AstraZeneca, Celgene, and Clovis, outside the submitted work. In addition, Dr Antonarakis has a patent inventor of a biomarker technology licensed to Qiagen. The authors report no other conflicts of interest in this work.
References
- 1.Sartor O, de Bono JS. Metastatic prostate cancer. N Engl J Med. 2018;378:645–657. doi: 10.1056/NEJMra1701695 [DOI] [PubMed] [Google Scholar]
- 2.de Bono JS, Logothetis CJ, Molina A, et al. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011;364:1995–2005. doi: 10.1056/NEJMoa1014618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ryan CJ, Smith MR, de Bono JS, et al. Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med. 2013;368:138–148. doi: 10.1056/NEJMoa1209096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scher HI, Fizazi K, Saad F, et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012;367:1187–1197. doi: 10.1056/NEJMoa1207506 [DOI] [PubMed] [Google Scholar]
- 5.Beer TM, Armstrong AJ, Rathkopf DE, et al. Enzalutamide in metastatic prostate cancer before chemotherapy. N Engl J Med. 2014;371:424–433. doi: 10.1056/NEJMoa1405095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Bono JS, Oudard S, Ozguroglu M, et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet. 2010;376:1147–1154. doi: 10.1016/S0140-6736(10)61389-X [DOI] [PubMed] [Google Scholar]
- 7.Kantoff PW, Higano CS, Shore ND, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363:411–422. doi: 10.1056/NEJMoa1001294 [DOI] [PubMed] [Google Scholar]
- 8.Parker C, Nilsson S, Heinrich D, et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med. 2013;369:213–223. doi: 10.1056/NEJMoa1213755 [DOI] [PubMed] [Google Scholar]
- 9.Antonarakis ES, Lu C, Wang H, et al. AR-V7 and resistance to enzalutamide and abiraterone in prostate cancer. N Engl J Med. 2014;371:1028–1038. doi: 10.1056/NEJMoa1315815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Antonarakis ES, Lu C, Luber B, et al. Androgen receptor splice variant 7 and efficacy of taxane chemotherapy in patients with metastatic castration-resistant prostate cancer. JAMA Oncol. 2015;1:582–591. doi: 10.1001/jamaoncol.2015.1341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scher HI, Lu D, Schreiber NA, et al. Association of AR-V7 on circulating tumor cells as a treatment-specific biomarker with outcomes and survival in castration-resistant prostate cancer. JAMA Oncol. 2016;2:1441. doi: 10.1001/jamaoncol.2016.1828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Antonarakis ES, Lu C, Luber B, et al. Clinical significance of androgen receptor splice variant-7 mrna detection in circulating tumor cells of men with metastatic castration-resistant prostate cancer treated with first- and second-line abiraterone and enzalutamide. J Clin Oncol. 2017;35:2149–2156. doi: 10.1200/JCO.2016.70.1961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Armstrong AJ, Halabi S, Luo J, et al. The PROPHECY trial: multicenter prospective trial of circulating tumor cell (CTC) AR-V7 detection in men with mCRPC receiving abiraterone (A) or enzalutamide (E). J Clin Oncol. 2018;36(15_suppl):5004. doi: 10.1200/JCO.2018.36.15_suppl.5004 [DOI] [Google Scholar]
- 14.Bernemann C, Schnoeller TJ, Luedeke M, et al. Expression of AR-V7 in circulating tumour cells does not preclude response to next generation androgen deprivation therapy in patients with castration resistant prostate cancer. Eur Urol. 2017;71:1–3. doi: 10.1016/j.eururo.2016.07.021 [DOI] [PubMed] [Google Scholar]
- 15.Azad AA, Volik SV, Wyatt AW, et al. Androgen receptor gene aberrations in circulating cell-free DNA: biomarkers of therapeutic resistance in castration-resistant prostate cancer. Clin Cancer Res. 2015;21:2315–2324. doi: 10.1158/1078-0432.CCR-14-2666 [DOI] [PubMed] [Google Scholar]
- 16.Romanel A, Gasi Tandefelt D, Conteduca V, et al. Plasma AR and abiraterone-resistant prostate cancer. Sci Transl Med. 2015;7:312re310. doi: 10.1126/scitranslmed.aad3106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goodall J, Mateo J, Yuan W, et al. Circulating cell-free DNA to guide prostate cancer treatment with PARP inhibition. Cancer Discov. 2017;7:1006–1017. doi: 10.1158/2159-8290.CD-17-0261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mateo A, Porta N, McGovern UB, et al. TOPARP-B: a phase II randomized trial of the poly(ADP)-ribose polymerase (PARP) inhibitor olaparib for metastatic castration resistant prostate cancers (mCRPC) with DNA damage repair (DDR) alterations. J Clin Oncol. 2019;37(suppl; abstr 5005):5005. doi: 10.1200/JCO.2019.37.15_suppl.5005 [DOI] [Google Scholar]
- 19.Abida W, Cheng ML, Armenia J, et al. Analysis of the prevalence of microsatellite instability in prostate cancer and response to immune checkpoint blockade. JAMA Oncol. 2019;5(4):471–478. doi: 10.1001/jamaoncol.2018.5801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nava Rodrigues D, Rescigno P, Liu D, et al. Immunogenomic analyses associate immunological alterations with mismatch repair defects in prostate cancer. J Clin Invest. 2018;128:4441–4453. doi: 10.1172/JCI121924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abida W, Cyrta J, Heller G, et al. Genomic correlates of clinical outcome in advanced prostate cancer. Proc Natl Acad Sci U S A. 2019;116:11428–11436. doi: 10.1073/pnas.1902651116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boysen G, Rodrigues DN, Rescigno P, et al. SPOP-mutated/CHD1-deleted lethal prostate cancer and abiraterone sensitivity. Clin Cancer Res. 2018;24:5585–5593. doi: 10.1158/1078-0432.CCR-18-0937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chung JH, Dewal N, Sokol E, et al. Prospective comprehensive genomic profiling of primary and metastatic prostate tumors. JCO Precis Oncol. 2019;3:1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sweeney CJ, Chen YH, Carducci M, et al. Chemohormonal therapy in metastatic hormone-sensitive prostate cancer. N Engl J Med. 2015;373:737–746. doi: 10.1056/NEJMoa1503747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.James ND, Sydes MR, Clarke NW, et al. Addition of docetaxel, zoledronic acid, or both to first-line long-term hormone therapy in prostate cancer (STAMPEDE): survival results from an adaptive, multiarm, multistage, platform randomised controlled trial. Lancet. 2016;387:1163–1177. doi: 10.1016/S0140-6736(15)01037-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vale CL, Burdett S, Rydzewska LH, et al. Addition of docetaxel or bisphosphonates to standard of care in men with localised or metastatic, hormone-sensitive prostate cancer: a systematic review and meta-analyses of aggregate data. Lancet Oncol. 2016;17:243–256. doi: 10.1016/S1470-2045(15)00489-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fizazi K, Tran N, Fein L, et al. Abiraterone plus prednisone in metastatic, castration-sensitive prostate cancer. N Engl J Med. 2017;377:352–360. doi: 10.1056/NEJMoa1704174 [DOI] [PubMed] [Google Scholar]
- 28.Fizazi K, Tran N, Fein L, et al. Abiraterone acetate plus prednisone in patients with newly diagnosed high-risk metastatic castration-sensitive prostate cancer (LATITUDE): final overall survival analysis of a randomised, double-blind, phase 3 trial. Lancet Oncol. 2019;20:686–700. doi: 10.1016/S1470-2045(19)30082-8 [DOI] [PubMed] [Google Scholar]
- 29.James ND, de Bono JS, Spears MR, et al. Abiraterone for prostate cancer not previously treated with hormone therapy. N Engl J Med. 2017;377:338–351. doi: 10.1056/NEJMoa1702900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Davis ID, Martin AJ, Stockler MR, et al. Enzalutamide with standard first-line therapy in metastatic prostate cancer. N Engl J Med. 2019;381:121–131. doi: 10.1056/NEJMoa1903835 [DOI] [PubMed] [Google Scholar]
- 31.Chi KN, Agarwal N, Bjartell A, et al. Apalutamide for metastatic, castration-sensitive prostate cancer. N Engl J Med. 2019;381:13–24. doi: 10.1056/NEJMoa1903307 [DOI] [PubMed] [Google Scholar]
- 32.Hussain M, Fizazi K, Saad F, et al. Enzalutamide in men with nonmetastatic, castration-resistant prostate cancer. N Engl J Med. 2018;378:2465–2474. doi: 10.1056/NEJMoa1800536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smith MR, Saad F, Chowdhury S, et al. Apalutamide treatment and metastasis-free survival in prostate cancer. N Engl J Med. 2018;378:1408–1418. doi: 10.1056/NEJMoa1715546 [DOI] [PubMed] [Google Scholar]
- 34.Fizazi K, Shore N, Tammela TL, et al. Darolutamide in nonmetastatic, castration-resistant prostate cancer. N Engl J Med. 2019;380:1235–1246. doi: 10.1056/NEJMoa1815671 [DOI] [PubMed] [Google Scholar]
- 35.Scher HI, Morris MJ, Stadler WM, et al. Trial design and objectives for castration-resistant prostate cancer: updated recommendations from the prostate cancer clinical trials working group 3. J Clin Oncol. 2016;34:1402–1418. doi: 10.1200/JCO.2015.64.2702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moilanen AM, Riikonen R, Oksala R, et al. Discovery of ODM-201, a new-generation androgen receptor inhibitor targeting resistance mechanisms to androgen signaling-directed prostate cancer therapies. Sci Rep. 2015;5:12007. doi: 10.1038/srep12007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fizazi K, Smith MR, Tombal B. Clinical development of darolutamide: a novel androgen receptor antagonist for the treatment of prostate cancer. Clin Genitourin Cancer. 2018;16:332–340. doi: 10.1016/j.clgc.2018.07.017 [DOI] [PubMed] [Google Scholar]
- 38.Fizazi K, Massard C, Bono P, et al. Activity and safety of ODM-201 in patients with progressive metastatic castration-resistant prostate cancer (ARADES): an open-label phase 1 dose-escalation and randomised phase 2 dose expansion trial. Lancet Oncol. 2014;15:975–985. doi: 10.1016/S1470-2045(14)70240-2 [DOI] [PubMed] [Google Scholar]
- 39.Massard C, Penttinen HM, Vjaters E, et al. Pharmacokinetics, antitumor activity, and safety of ODM-201 in Patients with Chemotherapy-naive Metastatic Castration-resistant Prostate Cancer: an open-label phase 1 study. Eur Urol. 2016;69:834–840. doi: 10.1016/j.eururo.2015.09.046 [DOI] [PubMed] [Google Scholar]
- 40.Shore ND, Tammela TL, Massard C, et al. Safety and antitumour activity of ODM-201 (BAY-1841788) in Chemotherapy-naive and CYP17 inhibitor-naive patients: follow-up from the ARADES and ARAFOR trials. Eur Urol Focus. 2018;4:547–553. doi: 10.1016/j.euf.2017.01.015 [DOI] [PubMed] [Google Scholar]
- 41.Zurth C, Graudenz K, Denner K, et al. Drug-drug interaction (DDI) of darolutamide with cytochrome P450 (CYP) and P-glycoprotein (P-gp) substrates: results from clinical and in vitro studies. J Clin Oncol. 2019;37(suppl 7S; abstr 297):297. doi: 10.1200/JCO.2019.37.7_suppl.297 [DOI] [Google Scholar]