Abstract
Abnormal Ca homeostasis has been associated with impaired glucose metabolism. However, the epidemiological evidence is controversial. We aimed to assess the association between circulating Ca levels and the risk of type 2 diabetes mellitus (T2DM) or abnormal glucose homeostasis through conducting a systematic review and meta-analysis. Eligible studies were identified by searching electronic database (PubMed, Embase and Google Scholar) and related references with de novo results from primary studies up to December 2018. A random-effects meta-analysis was performed to estimate the weighted relative risks (RR) and 95 % CI for the associations. The search yielded twenty eligible publications with eight cohort studies identified for the meta-analysis, which included a total of 89 165 participants. Comparing the highest with the lowest category of albumin-adjusted serum Ca, the pooled RR was 1·14 (95 % CI 1·05, 1·24) for T2DM (n 51 489). Similarly, serum total Ca was associated with incident T2DM (RR 1·25; 95 % CI 1·10, 1·42) (n 64 502). Additionally, the adjusted RR for 1 mg/dl increments in albumin-adjusted serum Ca or serum total Ca levels was 1·16 (95 % CI 1·07, 1·27) and 1·19 (95 % CI 1·11, 1·28), respectively. The observed associations remained with the inclusion of a cohort study with ionised Ca as the exposure. However, data pooled from neither case–control (n 4) nor cross-sectional (n 8) studies manifested a significant correlation between circulating Ca and glucose homeostasis. In conclusion, accumulated data from the cohort studies suggest that higher circulating Ca levels are associated with an augmented risk of T2DM.
Keywords: Blood calcium, Serum calcium, Diabetes, Insulin, Glucose
Type 2 diabetes mellitus (T2DM), a major metabolic disorder characterised by hyperglycaemia, is associated with an increased risk of multiple chronic conditions(1,2), such as CVD(3) and nephropathy(4,5). T2DM has been recognised as a leading cause of morbidity and mortality(1,6).
Ca, an essential mineral, exerts a wide range of biological functions, including bone and tooth mineralisation, blood coagulation, muscle contraction, nerve impulse transmission and cellular signalling transduction(4,6–9). Additionally, Ca plays a pivotal role in insulin secretion and glucose homeostasis(4,10,11). Glucose-dependent insulin secretion is a Ca-regulated process, which depends on intracellular Ca concentration in pancreatic β-cells(10,12). Moreover, increased cytosolic Ca also affects glucose uptake in the myocyte(10,13,14). Consequently, abnormal Ca homeostasis could potentially be involved with defects in insulin action and disorders in glucose homeostasis, contributing to T2DM development(4,10,11,15,16).
Findings from epidemiological studies are inconsistent. Some observational studies have indicated that increased serum Ca concentration may be directly associated with the risk of developing T2DM(3,4,6,7,17,18–21), whereas others reported the null correlation between circulating Ca and prevalent T2DM(7,10,22). Also, it has been observed that elevated serum Ca levels were related to insulin resistance(4,20,21), reduced insulin sensitivity(4,18,23,24) and impaired glucose tolerance(4,16,17,23,24), but not a decrease in insulin secretion(4,23,24). These contradictory results may be partially attributable to diverse Ca assessments, for example, total serum Ca and albumin-adjusted serum Ca, which are different biomarkers of Ca status(10,23). Because approximate 40 % of Ca in the serum is bound to albumin(8), it is vital to know the serum albumin level when evaluating the total serum Ca. Moreover, circulating Ca homeostasis is exquisitely regulated by multiple negative feedback loops which involve several integrated hormonal responses and target organs(8). Thus, each of these components may mediate the possible Ca-related risk of diabetes. Interestingly, two double-blind, placebo-controlled, randomised clinical trials reported non-significant effects of Ca supplementation on insulin resistance in obese adults(25), as well as insulin secretion, insulin sensitivity and glycaemia in adults at risk of T2DM(26). Of note, these studies have relatively short follow-up periods, insufficient numbers of participants and heterogeneous characteristics between populations(25,26).
One early meta-analysis(9) was conducted by Sing et al. based only on four cohort studies(3,4,9,17), which reported a positive association of both serum total Ca and albumin-adjusted Ca with risk of diabetes. Since then, four additional cohort studies(6,10,22,27) with larger sample sizes have been published. Moreover, the linear association between circulating Ca levels and the T2DM risk has not been investigated.
Therefore, in the present study, we aimed to quantitatively assess the overall association between circulating Ca levels and the risk of T2DM or abnormal glucose homeostasis by performing an up-to-date systematic review and meta-analysis.
Methods
The present study was conducted according to the guidelines of Meta-analysis of Observational Studies in Epidemiology (MOOSE)(28). The present study was registered with the International Prospective Register of Systematic Reviews (PROSPERO) (https://www.crd.york.ac.uk/PROSPERO/) with the registration number CRD42018092835.
Study search strategy
We performed a systematic search of published studies in English in PubMed from inception to December 2018 using the terms ‘calcium or serum calcium or blood calcium’, ‘diabetes or insulin resistance or impaired glucose tolerance or impaired fasting glucose’, ‘epidemiological studies’, ‘cohort/prospective longitudinal/follow-up/cross-sectional/case-control studies’ and ‘survival analysis or proportional hazard model or hazard ratio Cox or hazards ratio or odds ratio’. In addition, we searched Embase, Google Scholar and reference lists of narrative and systematic reviews to identify missing studies. Importantly, to standardise results specifically for this meta-analysis and to obtain additional information, we requested de novo data from two original studies(22,27).
Selection criteria
Studies were considered for inclusion if they met the following criteria: (a) published in English; (b) had a cohort, case–control or cross-sectional design; (c) provided the exposure information of total serum Ca, albumin-adjusted serum Ca or serum ionised Ca and (d) had a relative risk (RR), hazard ratio (HR) or OR with 95 % CI for T2DM in relation to Ca exposure or a correlation coefficient between Ca exposure and T2DM related variables (e.g. fasting glucose, fasting insulin, insulin sensitivity and insulin resistance), or such information could be derived from the published results.
Data extraction
Two investigators (J. Z. and P. C. X.) reviewed all relevant literature and assessed the eligibility of each study independently. Discrepancies were resolved with consensus reached by group discussion. From each retrieved study, the following information was extracted: author name, year of publication, study region, total number of participants and events (or total number of cases and controls), proportion of males, age of participants, exposure evaluation method, exposure classification, adjusted variables and HR, RR or OR estimates with corresponding 95 % CI for all corresponding Ca exposure categories and/or for continuous exposure, compared with the lowest exposure group. OR reported in a cohort study(17) was transformed to RR by adopting the following formula: OR = ((1−P0) × RR/(1−RR × P0)), where P0 is the incidence rate in the reference group, according to the published methods(9).
Quality assessment
The Newcastle–Ottawa Scale (NOS) system(29) was employed to evaluate the quality of each selected cohort study or case– control study. The NOS system has a maximum score of nine points. Studies with a sum score of 0–4, 5–7 and 8–9 were considered as low, moderate and high quality, respectively. Also, the original version of the NOS system was modified for assessing the quality of the included cross-sectional studies for the systematic review based on the existing literature(30–32). The modified NOS system has a maximum score of eight points. Studies with a sum score of 0–4, 5–7 and 8 were considered as low, moderate and high quality, respectively. Two co-authors(J. Z. and P. C. X.) independently conducted the quality assessment. Any unconformity was resolved with consensus reached by group discussion with the involvement of a third investigator.
Statistical analysis
We pooled the cohort studies’ data for each outcome of interest using a random-effects model in Stata 13.0 (STATA Corp). P values ≤ 0·05 were considered statistically significant if not otherwise specified. For the comparisons, we used the multivariable-adjusted associations (RR) for the highest v. lowest category of albumin-adjusted serum Ca or total Ca levels, and for the linear associations, we used standardised Ca levels with 1 mg/dl increments. Because of an insufficient number of studies measuring ionised Ca, we did not pool the data separately for this subgroup. Heterogeneity was estimated quantitatively using I2 and tested by Cochran’s Q-test. An I2 value of >75, 51–75, 26–50 or 0–25 % corresponds to high, moderate, low or very low heterogeneity, respectively. We evaluated publication bias through both Egger’s asymmetry test and Begg’s non-parametric test with the α level set as 0·10. The Duval and Tweedie nonparametric ‘trim and fill’ method was adopted, if publication bias existed(33).
We also conducted sensitivity analyses to assess the effect of replacing a random-effects model with a fixed-effects model and the influence of an individual study on the overall association by excluding one study each time from the analysis. We also evaluated the impact of one study measuring ionised Ca on the overall association by combining it with other studies.
Results
Literature search
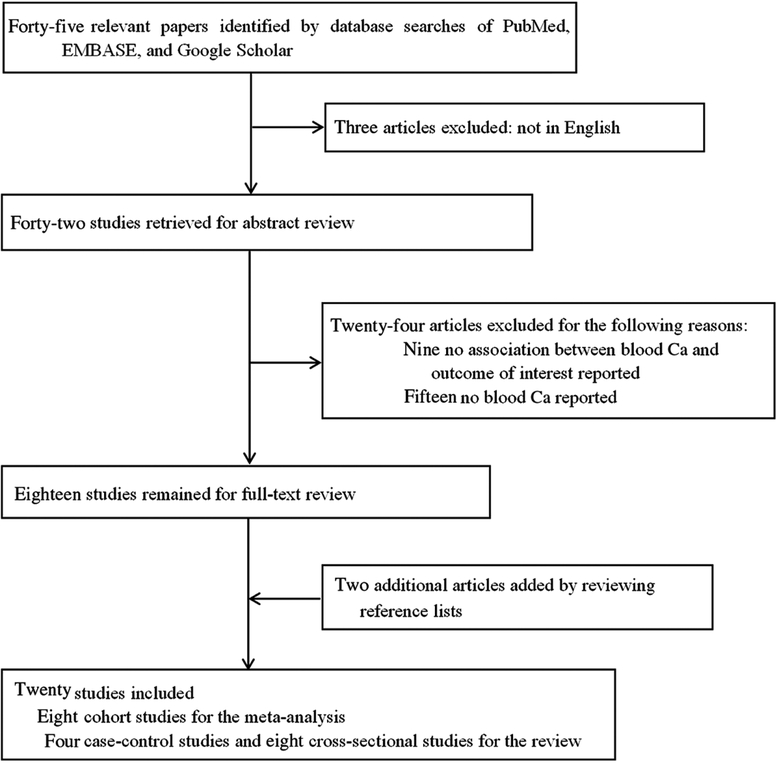
The literature selection process is shown in Fig. 1. A total of forty-five related articles were retrieved from PubMed, Embase and Google Scholar. Of these, three articles were excluded since they were not issued in English; nine publications were further excluded because they did not associate blood Ca with an outcome of interest and additional fifteen articles were excluded because they did not report blood Ca as either a categorical variable or a continuous variable on the original scale. Furthermore, we also identified two articles by searching the reference lists of relevant publications. Thus, the search revealed eight cohort studies(3,4,6,9,10,17,22,27) for the meta-analysis and another twelve eligible studies (four case–control studies(7,18,34,35) and eight cross-sectional studies(19–21,23,24,36–38)) for the systematic review.
Fig. 1.
Flow chart of study selection.
Study characteristics
The information from the eligible studies was extracted (Tables 1–3). The total number of participants of all the included studies was 89 165 (48·6 % males). For eight cohort studies(3,4,6,9,10,17,22,27), there were 80 359 individuals (49·9 % males) with 6447 incidents of diabetes. For four case–control studies(7,18,34,35), there were 858 individuals (35·9 % males) with 324 diabetes cases and 534 controls. For eight cross-sectional studies(19–21,23,24,36–38), there were 7948 participants (37·2 % males) including 740 diabetes cases. The mean age of participants across primary studies was from 25·7 to 80 years.
Table 1.
Characteristics of eight included cohort studies on the association between blood calcium concentrations and incidence of type 2 diabetes mellitus (T2DM)
| Source | Participants (n) | Age (years) | Males (%) | Duration of follow-up (years) | Exposure assessment and categories | Number of cases | Case identification methods | Adjusted variables |
|---|---|---|---|---|---|---|---|---|
| Jorde et al.(3), The Tromsø Study, Norway | 25 657 | Range 31·5–62·4 | 48·2 | 13 | Non-fasting blood Ca (mmol/l): 2·20–2·29; 2·30–2·39; 2·40–2·49; 2·50–2·60 |
705 | Self-reported T2DM in questionnaires and the record linkage to the national hospital discharge registry | Age, sex, BMI, smoking status, SBP and serum cholesterol |
| Becerra-Tomas et al.(4), The Prevencion con Dieta Mediterranea (PREDIMED) study, spain | 707 | 67 (sd 6) | 40 | 4·78 | Fasting albumin-adjusted serum Ca (mg/dl): 9·01 (sd 0·28); 9·60 (sd 0 ·13); 10·20 (sd 0·29) |
77 | Diagnosed by clinical examination according to ADA 2010 criteria | Age, sex, intervention group, BMI, smoking status, educational level, prevalence of hypertension, prevalence of hypercholesterolaemia, use of antihypertensive medication, use of statins, alcohol intake, leisuretime physical activity and FPG at baseline |
| Lorenzo et al.(17), The Insulin Resistance Atherosclerosis Study (IRAS), USA | 863 | Non-DM, 54·0 (se 0·3) DM, 56·5 (se 0·4) |
Non-DM, 39·3 DM, 45·8 |
5·2 | Fasting serum Ca (mmol/l): <2·13; 2·13–2·24; 2·25–2·37 2·38–2·49; ≥2·50 |
140 | Diagnosed by clinical examination and defined as fasting glucose ≥7·0 mmol/l and/or 2 h glucose ≥11·1 mmol/l, and impaired glucose tolerance as 2 h glucose ≥7·8–11·0 mmol/l. | Age, sex, ethnicity, clinic site, BMI, family history of diabetes, fasting and 2-h glucose concentrations, logeSI, loge AIR, eGFR and use of diuretic drugs |
| Rooney et al.(6), The AtherosclerosisRisk in Communities (ARIC) study, USA | 12 800 | 53·9 (sd 5·7) | 44·5 | 8·8 | Albumin-adjusted serum Ca (mg/dl): 7·28–9·54; 9·56–9·76; 9·78–9·96; 9·98–10·20; 10·22–13·28 |
1516 | Defined as fasting (≥8 h) blood glucose ≥126 mg/dl, nonfasting glucose ≥200 mg/dl; self–reported physician diagnosis of diabetes or ‘sugar in the blood’ or current medication use for diabetes | Age, sex, race, centre, education, physical activity, smoking status, alcohol use, waist circumference, BMI, parathyroid hormone, 25-hydroxyvitamin D and P |
| Zaccardi et al.(10), The Kuopio Ischaemic Heart Disease Risk Factor (KIHDRF) study, Finland | 2350 | 52·9 (sd 5·2) | 100 | 23·1 | Fasting serum ionised Ca (mmol/l): 0·88–1·15; 1·16–1·18; 1·19–1·21; 1·22–1·50 |
140 | Defined as a self–reported physician–set diagnosis and/or FPG ≥7·0 mmol/l or 2-h oral glucose tolerance test plasma glucose ≥11·1 mmol/l, and by record linkage to the national hospital discharge registry and to the social Insurance Institution of Finland register | Age, BMI, SBP, serum HDL-C, family history of T2DM, C-reactive protein, physical activity, serum TAG and FPG |
| Sing et al.(9), The Hong Kong Osteoporosis study (HKOS), China | 6096 | Non-DM, 51·4 (sd 16·3) DM, 62·1 (sd 11·2) |
27 | 10·2 | Albumin-adjusted serum Ca (mmol/l): <2·25; 2·25–2·29; 2·30–2·35; >2·35 Serum total Ca (mmol/l): <2·33; 2·33–2·38; 2·39–2·44; >2·44 |
631 | Ascertained from the EMR in several ways: (1) having a diagnosis of diabetes (ICD–9 code 250); (2) having a prescription record of diabetic medications; (3) having a laboratory record of HbA1c ≥6·5 % or FPG >7·0 mmol/l and (4) having enrolled in a diabetic complication screening programme | Age, sex, BMI, smoking status, drinking status, physical activity, serum albumin, serum phosphate, parathyroid hormone, alkaline phosphatase, femoral neck BMD T-score and season |
| Suh et al.(27), A retrospective longitudinal cohort study, Korea | 23 086 | 50·7 (sd 8·2) | 57·4 | 4·8 | Albumin-adjusted serum Ca (mg/dl): 7·58–8·54; 8·56–8·76; 8·78–9·00; 9·02–10·40 Serum total Ca (mg/dl): 8·20–8·70; 8·80–8·90; 9·00–9·20; 9·30–10·40 |
1922 | Determined as FPG ≥ 126 mg/dl or HbA1 c ≥ 6·5 %; subjects who reported a history of diabetes or current use of insulin or oral antidiabetic drugs | Age, sex, BMI, LDL, TAG, SBP and smoking status |
| Kim et al.(22), The Korean Genome and Epidemiology Study (KoGES), Korea | 8800 | 51·8 (sd 8·8) | 47 | 10 | Albumin-adjusted serum Ca (mg/dl): 5·64–9·10; 9·12–9·48; 9·48–9·76; 9·78–13·64 Serum total Ca (mg/dl): 5·80–9·20; 9·30–9·60; 9·70–9·90; 10·00–13·80 |
1316 | Defined as fasting glucose concentration ≥ 126 mg/dl, post–load 2-h glucose concentration ≥ 200 mg/dl or antidiabetic medication use | Age, sex, residential area, monthly family income, tobacco smoking, alcohol intake, physical activity, BMI, SBP, DBP, serum creatinine level and dietary Ca intake |
SBP, systolic blood pressure; ADA, American Diabetes Association; FPG, fasting plasma glucose; DM, diabetes mellitus; SI, insulin sensitivity index; AIR, acute insulin response; eGFR, estimated glomerular filtration rate; HDL-C, HDL-cholesterol; EMR, electronic medical record; HbA1c, glycated Hb; BMD, bone mineral density; LDL, LDL-cholesterol; DBP, diastolic blood pressure.
Table 3.
Characteristics of eight cross-sectional studies on the association between blood calcium concentrations and incidence of diabetes or impaired glucose tolerance
| Source | Participants (n) |
Age (years) | Males (%) | Exposure categories | No. of cases | Case identification methods | Adjusted variables | OR* | 95 % CI | r/β |
|---|---|---|---|---|---|---|---|---|---|---|
| Wareham et al.(23), UK | 1071 | 40–65 | 42·8 | Fasting albumin-adjusted serum Ca (mmol/l): <2·20; 2·20–2·25; 2·25–2·31; 2·31–2·38; >2·38 |
188 with IGT | IGT by WHO criteria | Age, BMI, sex, season and 25(OH)D | 1·63 | 1·42, 1·88 | - |
| Sun et al.(21), Canada | 1182 | Men, 39·47 (sd 12·65) Women, 42·93 (sd 9·98) |
18·4–20·1 | Fasting albumin-adjusted serum Ca (mmol/l): men, 2·31 (sd 0·12); women, 2·31 (sd 0·12) Fasting total serum Ca (mmol/l): men, 2·36 (sd 0·12); women, 2·33 (sd 0·12) |
- | - | Age, trunk fat percentage, P and Mg | - | - | Correlation between serum Ca and fasting glucose†: r = 0·31 for women; r = 0·22 for men Correlation between serum Ca and insulin resistance‡: r = 0·13 for women; r = 0·14 for men |
| Hagström et al.(24), Sweden | 961 | 71·0 (sd 0·58) | 100 | Albumin-adjusted serum Ca (mmol/l): 1·62–2·28; 2·29–2·33; 2·34–2·39; 2·40–2·85 |
- | DM was diagnosed as FPG ≥7·0 mmol/l, 2-h post-load glucose level ≥11·1 mmol/l, or by the use of oral hypoglycaemic agents or insulin | BMI, physical activity, smoking, consumption of tea, alcohol, coffee and dietary Ca, serum phosphate and serum creatinine | - | - | Linear regression analysis on M/I against serum Ca levels in total cohort§: β = –0·17 |
| Kim et al.(19), Korea | 1329 | 65.8 | 38–1 | Fasting albumin-adjusted serum Ca (mmol/l): <2·24; 2·24–2·30; 2·30–2·36; 2·37–2·44; >2·45 |
260 with DM | FPG ≥7·0 mmol/l and a 2-h post–load glucose level ≥11·1 mmol/l | Age, sex, BMI, season of blood sampling, and serum creatinine, P, PTH, 25(OH)D levels, smoking, alcohol drinking, exercise and total energy, Ca and Na intake | 3·32 | 1·87, 5·88 | - |
| Yamaguchi et al.(20), Japan | 480 | Men,60·8 (sd 13·0); Women,65·5 (sd 11·4) |
56·5 | Fasting albumin-adjusted serum Ca (mg/dl): men, 9·2 (sd 0·4); women, 9·2 (sd 0·4) |
480 with T2DM | - | Age, body weight, height, creatinine, albumin, phosphate, intact PTH, BAP, osteocalcin, uNTX and oestradiol | - | - | Correlations between serum Ca and FPG: r = 0·215 for men; 0·118 for women Correlations between serum Ca and HOMA-IR: r = 0·184 for men; −0·026 for women |
| Cho et al.(36), Korea | 1941 | 65·16 (sd 4·58) | 0 | Fasting serum Ca (mg/dl) 8·40–< 9·00; 9·00–< 9·20; 9·20–< 9·40; 9·40–10·20 |
648 with high fasting glucose | The criteria for high glucose by ADA 2010 criteria | Age, BMI, alcohol intake, cigarette smoking and exercise | 2·77 | 2·07, 3·72 | Correlations between serum Ca and fasting glucose: r = 0·148 |
| Guasch et al.(38), Spain | 316 | 46·85 (95 % Cl 45·44, 48·26)‡ | 24·1 | Fasting albumin-adjusted plasma Ca (mmol/l) tertile (with no specific data) | - | From the medical records of T2DM | PTH, 25(OH)D, age, sex, season and current smoking, BMI, uCRP and leucocyte count | 2·17 | 0·92,5·26 | - |
| Shimodaira et al.(37), Japan | 668 | NGT men, 48·8 (sd 9·1) NGT women, 51·6 (sd 6·7) pre-DM men, 52·5 (sd 8·6); pre-DM women, 54·2 (sd 8·0) |
66·8 | Fasting albumin-adjusted serum Ca (mg/dl): NGT men, 9·0 (sd 0·3); NGT women, 9·1 (sd 0·3); pre-DM men, 9·0 (sd 0·3); pre-DM women, 9·1 (sd 0·3) |
316 with pre-DM | Based on the criteria of ADA (2014): NGT = FPG, <100 mg/dl and 2-h PG, <140 mg/dl and pre-DM = FPG, 100–125 mg/dl or 2-h PG, 140–199 mg/dl |
Age, BMI, HDL-C and HbA1c | - | - | Multiple linear regression analyses on IGI against adjusted Ca: β= 0·236 for NGT men; 0·128 for pre-DM men |
IGI, insulinogenic index; 25(OH)D, 25-hydroxyvitaminD3; DM, diabetes mellitus; FPG, fasting plasma glucose; M/I, glucose disposal rate [M] divided by mean insulin concentration [I]; PTH, parathyroid hormone; T2DM, type 2 diabetes mellitus; BAP, bone-specific alkaline phosphatase; uNTX, urinary N-terminal cross-linked telopeptide of type-I collagen; HOMA-IR, homeostatic model assessment for insulin resistance; ADA, American Diabetes Association; uCRP, ultrasensitive C-reactive protein; NGT, normal glucose tolerance; Pre-DM, pre-diabetes mellitus; PG, plasma glucose; HDL-C, HDL-cholesterol; HbA1c, glycated Hb; IGT, impaired glucose tolerance.
The highest quintile of blood Ca was compared with the lowest quintile.
Partial correlations between total serum Ca and fasting serum glucose.
Partial correlations between total serum Ca and insulin resistance.
Standard deviation increase in serum Ca was associated with 0·17 mg·kg−1·min−1 (mU/l)−1 × 100 (0·024 mg·kg−1·min−1 (pmol/l) × 100) decrease in M/I.
Regarding the quality of the included eight cohort studies(3,4,6,9,10,17,22,27), six studies(3,4,6,17,22,27) were assessed as high quality and two studies(9,10) as moderate quality (online Supplementary Table S1). Of the included four case–control studies(7,18,34,35), two studies(18,34) were assessed as moderate quality and two studies(7,35) as low quality. For the included eight cross-sectional studies(19–21,23,24,36–38), two studies(19,23) were assessed as high quality and six studies(20,21,24,36–38) as moderate quality.
Meta-analysis of the cohort studies
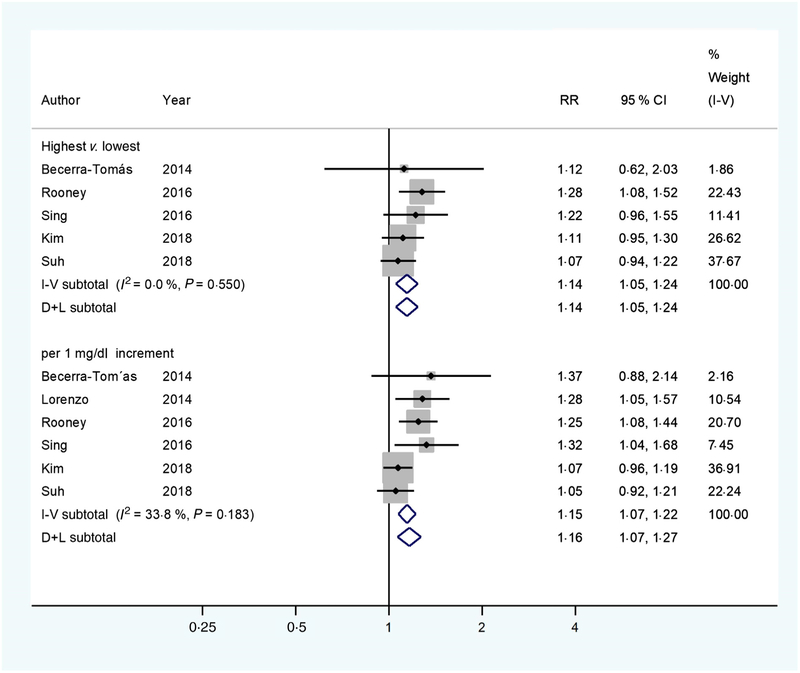
A significant association between albumin-adjusted serum Ca levels and T2DM incidence was found (RR 1·14; 95 % CI 1·05, 1·24) (Fig. 2), comparing the highest with the lowest Ca levels based on available data from five cohort studies(4,6,9,22,27). Neither heterogeneity (I2 = 0·0 %, P = 0·550) nor publication bias (Egger’s test: P = 0·631; Begg’s test: P = 0·462) existed. Consistently, a significant linear association was found (RR 1·16; 95 % CI 1·07, 1·27) with 1 mg/dl increments in albumin-adjusted serum Ca levels. Low heterogeneity (I2 = 33·8 %, P = 0·183) was observed, but no publication bias (Egger’s test: P = 0·104; Begg’s test: P = 0·452) was evidenced. When adding the cohort study with ionised Ca as the exposure(10), the afore-mentioned results of pooled RR remained (the highest v. the lowest: 1·13 (95 % CI 1·04, 1·22); per 1 mg/dl increment: 1·16 (95 % CI 1·06, 1·27)).
Fig. 2.
Multivariable-adjusted relative risks (RR) (◆) and 95 % confidence intervals (—) of type 2 diabetes mellitus (T2DM) in relation to albumin-adjusted serum calcium levels. The summary assessments (◇) were obtained by adopting a random-effects model. Values are adjusted RR comparing the highest with the lowest category levels or per 1 mg/dl increase in albumin-adjusted serum calcium. The size of the shaded square is proportional to the weight of each study. We requested de novo data from the authors of Kim et al.(22) and Suh et al.(27), respectively.
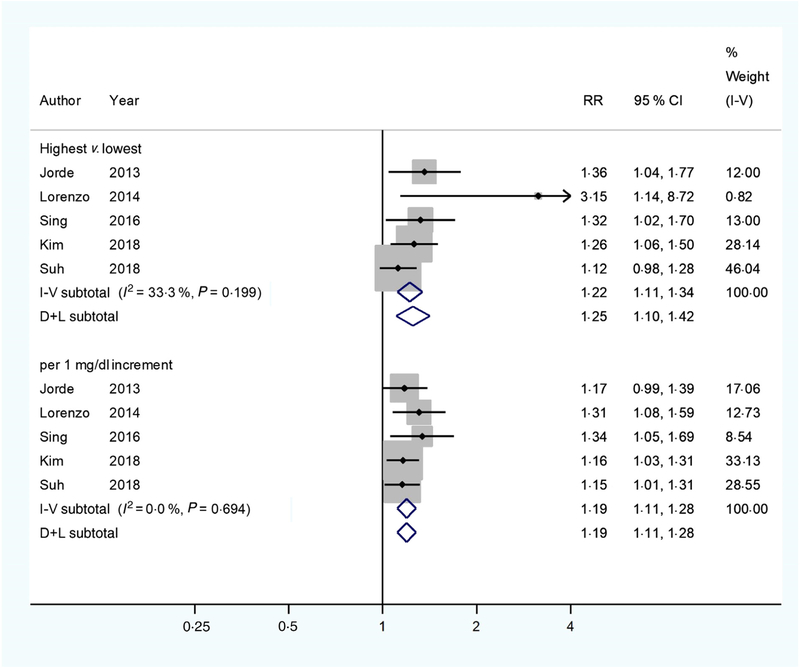
Similar results (Fig. 3) were found when the pooled analysis was restricted to studies(3,9,17,22,27) that measured serum total Ca (RR 1·25; 95 % CI 1·10, 1·42), comparing the highest with the lowest serum total Ca levels. However, low heterogeneity (I2 = 33·3 %, P = 0·199) and potential publication bias (Egger’s test: P = 0·008; Begg’s test: P = 0·027) were observed. We then used the Duval and Tweedie non-parametric ‘trim and fill’ method and observed the overall RR was 1·21 (95 % CI 1·06, 1·38). A significant linear association was also found (RR 1·19 (95 % CI 1·11, 1 28) with 1 mg/dl increments in serum total Ca levels). No heterogeneity (I2 = 0 %, P = 0·694) was observed, but potential publication bias (Egger’s test: P = 0·031; Begg’s test: P = 0·221) was evidenced. After using the Duval and Tweedie non-parametric ‘trim and fill’ method, we observed the overall RR was 1·18 (95 % CI 1·10, 1·26). When further including the study that assayed ionised Ca(10), the results of pooled RR persisted (the highest v. the lowest: 1·21 (95 % CI 1·06, 1·39) and 1·19 (95 % CI 1·11, 1·27) with 1 mg/dl increments).
Fig. 3.
Multivariable-adjusted relative risks (RR) (◆) and 95 % confidence intervals (—) of type 2 diabetes mellitus (T2DM) in relation to serum total calcium levels. The summary assessments (◇) were obtained by adopting a random-effects model. Values are adjusted RR comparing the highest with the lowest category levels or per 1 mg/dl increase in serum total calcium. The size of the shaded square is proportional to the weight of each study. We requested de novo data from the authors of Kim et al.(22) and Suh et al.(27), respectively.
The sensitivity analyses revealed that replacing the random-effects model with a fixed-effects model or any single study did not appreciably influence the pooled results or conclusions (online Supplementary Tables S4–S7).
Systematic review of the case–control and cross-sectional studies
The included case–control studies reported inconsistent results on the association between Ca homeostasis and diabetic risk. Higher serum total Ca levels(7) or higher plasma Ca levels(18) were reported in the middle-aged and elderly diabetic patients. Contrarily, normal serum total Ca(34) and ionised Ca levels(7,34) were observed, respectively, in the middle-aged and elderly diabetic patients, and decreased serum ionised Ca concentrations(35) were also observed in the young diabetic patients, respectively, compared with the non-diabetic individuals (Table 2).
Table 2.
Characteristics of four case–control studies on the association between blood calcium concentrations and incidence of diabetes
| Source | Participants (n) |
Age (years) | Males (%) | Exposure assessment | Cases | Controls | Case identification methods | Adjusted variables | ||
|---|---|---|---|---|---|---|---|---|---|---|
| n | Blood Ca | n | Blood Ca | |||||||
| Heath et al.(34), USA | 122 | Untreated, 58 (se 1) Treated A*, 43 (se 5); Treated B†, 51 (se 4); Control 44 (se 2) |
53·3 | Fasting serum total Ca | Total, 82, Untreated, 47 Treated A*, 19 Treated B†, 16 |
Untreated, 9·4 (se 0·1) mg/dl Treated A*, 9·2 (se 0·1) mg/dl Treated B†, 9·4 (se 0·1) mg/dl |
40 | 9·4 (se 0·1) mg/dl | All were judged by endocrinologist | - |
| Fasting serum ionised Ca | 7 | Untreated, 4·22 (se 0·6) mg/dl | 11 | 4·2 (se 0·1) mg/dl | ||||||
| McNair et al.(35), Denmark | 48 | Case, 28·5 (range 16–41); Control, 25·7 (range 16–40) |
Case, 47·8; Control, 64·0 |
Fasting pH-adjusted serum ionic Ca | 25 insulin-treated DM | 1·16 (se 0·01) mmol/l | 23 | 1·20 (se 0·01) mmol/l | - | - |
| Levy et al.(18), Israel | 184 | Case, 48 (sd 4); Control 48 (sd 4)‡ |
Case, 52·2; Control, 52·2‡ |
Fasting plasma Ca | 92 | 2·48 (se 0·004) mmol/l | 92‡ | 2·38 (se 0·006) mmol/l | - | Age, sex and mode of treatment |
| Sorva & Tilvis(7), Finland | 504 | 80 (sd 9) | Case, 28·8; Control, 19·0 |
Fasting serum total/ionised Ca concentrations | 125 | CaT, 2·31 mmol/lCal, 1·24 mmol/l | 379 | CaT, 2·27 mmol/l Cal, 1·23 mmol/l |
Use antidiabetic medication or fasting plasma glucose exceeded 7 mmol/l | Albumin |
DM, diabetes mellitus; CaT, total Ca; Cal, ionised, actual Ca.
Treated A: fasting plasma glucose > 200 mg/dl.
Treated B: fasting plasma glucose < 200 mg/dl.
Matched with the case group.
The included cross-sectional studies reported that serum Ca was directly correlated to glucose intolerance(21,23,36), insulin resistance(21), impaired glucose metabolism(20), early phase insulin secretion(37) and diabetes(19,38) mainly in middle-aged and elderly individuals. However, serum Ca was found inversely correlated to β-cell function in women(21) and insulin sensitivity in older men(24) (Table 3).
Discussion
The accumulated evidence from the cohort studies suggests that either albumin-adjusted serum Ca or serum total Ca was directly associated with T2DM risk, though data from case–control and cross-sectional studies were inconsistent and inconclusive. The present study provided updated and robust data to the literature by including additional and de novo results from a few newly published large cohort studies. Also, the present study investigated both categorical and linear associations between circulating Ca and T2DM incidence, which strengthened our conclusion.
The results in case–control and cross-sectional studies were not consistent, which may be partially explained by the different Ca measurements, such as total serum, albumin-corrected serum, serum ionised or plasma levels or heterogeneous study populations with various outcomes.
The results of the present study are inconsistent with findings from studies on dietary Ca intake and the T2DM risk(22,39–42). One possible explanation is that dietary estimation and blood Ca are different assessments, which are subject to different measurement errors. Another explanation is that the dietary Ca and diabetes association may be confounded by Mg intake, which is highly correlated with Ca intake and associated with diabetes risk(43). Moreover, circulating Ca concentrations could reflect not only the exogenous Ca intake but also the endogenous capability of maintaining homeostasis(22), which is tightly regulated by multiple negative feedback loops involving several target organs and hormones(3,6,8,22). Ca intake may lead to an elevation of serum Ca that activates the Ca-sensing receptor (CaR) in the parathyroid glands to reduce parathyroid hormone (PTH) secretion(8). The reduced PTH inactivates the PTH receptor (PTHR) in kidney to decrease tubular Ca reabsorption, and PTHR in bone to decrease net bone Ca resorption(8). The reduced PTH also results in a decreased secretion of 1,25-dihydroxyvitamin D (1,25(OH)2D), which inactivates the vitamin D receptor in intestine to reduce Ca absorption, in the parathyroid glands to augment PTH secretion and in bone to reduce Ca resorption(8). The rise in serum Ca may also activate the CaR in kidney to decrease Ca reabsorption(8). When a decrease in serum Ca occurs, this integrated hormonal response is reversed with serum Ca increased, which helps to maintain total serum Ca levels within a physiological range of approximately 10 %(8). However, emerging evidence showed that hormones (such as PTH, 1,25(OH)2D) that participate in this complex homeostatic system have themselves been related to diabetes(44,45). Thus, abnormalities of these pivotal physiological factors may affect the Ca–diabetes association, which warrants further investigation.
The potential mechanisms underlying the role of circulating Ca in T2DM incidence remain unclear. However, growing evidence implies that Ca may mediate insulin secretion via activation of the CaR(46,47), which is wildly expressed in tissues, such as pancreatic islets of Langerhans(46). Glucose-dependent insulin secretion from the pancreatic β-cells is a Ca-regulated process(11,12,48), which requires the influx of Ca through voltage-gated Ca channels to the secretory granules in β-cells(49–51). Thus, high Ca concentration may induce β-cell dysfunction(21). Moreover, abnormal Ca status impacts insulin sensitivity and glucose transport in adipocytes and skeletal muscle(14,52,53) through regulating GLUT4 expression, which is a passive transporter necessary for glucose uptake in peripheral tissues(13,14). Chronic exposure to increased cytosolic Ca levels has been shown to prohibit GLUT4 expression in skeletal muscle(54). Furthermore, elevated or sustained cytosolic Ca levels have been revealed to reduce GLUT4 expression and consequently decrease insulin receptor activity and reduce glucose uptake in adipocytes(4). Because of the pivotal role of insulin in modulating blood glucose(55), abnormalities in circulating Ca levels could potentially impair β-cell secretory function, glucose intolerance and insulin sensitivity. Thus, it may consequently lead to T2DM development(17). Notably, it has been observed that abnormal intracellular Ca may cause pancreatic β-cell apoptosis(56,57). Intracellular Ca increases may accompany the activation of endoplasmic reticulum stress and dysfunction of organelles, including mitochondria and the nucleus, which may lead to destruction of pancreatic β-cells(58). Therefore, future studies investigating such targets might help elucidate mechanisms relevant to the Ca–diabetes association and progression to T2DM.
Several limitations need to be acknowledged when interpreting the findings from this systematic review and meta-analysis. First, most observational studies adopted albumin-adjusted serum Ca as exposure assessment(2,6,9,17,19–21,23,24,37,38), which is prone to errors because of the assumption that Ca binds to albumin steadily(59,60). In fact, it may neglect other relevant ligands and Ca fractions(61). Hence, the results of the risk prediction based on measuring albumin-adjusted serum Ca need to be interpreted with caution. Ionised Ca is considered as the physiologically active form(61) and the ‘gold standard’ of Ca homeostasis assessment(4,62,63). However, only one available cohort study measured ionised Ca and reported a null association with incident T2DM(10), though it did not appreciably alter the overall association. The association of ionised Ca levels with T2DM risk merits further investigation. Second, serum Ca is highly modulated by some other factors, such as PTH or vitamin D. However, not all primary studies adjusted for these potential confounding variables. This inherent limitation may somehow affect the combined association. Third, low heterogeneity was evidenced for studies with some serum Ca measurements. The impact should be reduced using a random-effects model in the analyses. Fourth, a potential publication bias could not be completely excluded in some pooled analyses. Nevertheless, the associations were not substantially changed after using the ‘trim and fill’ method to account for the publication bias. Thus, our conclusion should remain.
In summary, data from this systematic review and meta-analysis provide accumulated evidence supporting the conclusion that circulating Ca levels are associated with the incidence of T2DM. Further studies are needed to establish the causal inference and elucidate the underlying mechanism of action.
Supplementary Material
Acknowledgements
The present study was supported by the National Natural Science Foundation of China (81573140); the US National Institutes of Health (R01DK116603); the Shanghai Key Laboratory of Pediatric Gastroenterology and Nutrition (14DZ2272400); the Danone Nutrition Research and Education Grant (DIC2017–09) and the Shanghai Municipal Commission of Health and Family Planning (20134196, 2013ZYJB0017).
Abbreviations:
- NOS
Newcastle–Ottawa Scale
- PTH
parathyroid hormone
- RR
relative risk
- T2DM
type 2 diabetes mellitus
Footnotes
The authors declare that there are no conflicts of interest.
Supplementary material
For supplementary materials referred to in this article, please visit https://doi.org/10.1017/S0007114519001430
References
- 1.World Health Organization (2014) Global status report on noncom-municable diseases. https://www.who.int/nmh/publications/ncd-status-report-2014/en/ (accessed January 2019).
- 2.Seshasai SR, Kaptoge S, Thompson A, et al. (2011) Diabetes mellitus, fasting glucose, and risk of cause-specific death. N Engl J Med 364, 829–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jorde R, Schirmer H, Njølstad I, et al. (2013) Serum calcium and the calcium-sensing receptor polymorphism rs17251221 in relation to coronary heart disease, type 2 diabetes, cancer and mortality: the Tromsø Study. Eur J Epidemiol 28, 569–578. [DOI] [PubMed] [Google Scholar]
- 4.Becerra-Tomás N, Estruch R, Bulló M, et al. (2014) Increased serum calcium levels and risk of type 2 diabetes in individuals at high cardiovascular risk. Diabetes Care 37, 3084–3091. [DOI] [PubMed] [Google Scholar]
- 5.American Diabetes Association (2013) Standards of medical care in diabetes. Diabetes Care 36, S11–S66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rooney MR, Pankow JS, Sibley SD, et al. (2016) Serum calcium and incident type 2 diabetes: the Atherosclerosis Risk in Communities (ARIC) study. Am J Clin Nutr 104, 1023–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sorva A & Tilvis RS (1990) Low serum ionized to total calcium ratio: association with geriatric diabetes mellitus and with other cardiovascular risk factors. Gerontology 36, 212–216. [DOI] [PubMed] [Google Scholar]
- 8.Peacock M (2010) Calcium metabolism in health and disease. Clin J Am Soc Nephrol 5, S23–S30. [DOI] [PubMed] [Google Scholar]
- 9.Sing CW, Cheng VK, Ho DK, et al. (2016) Serum calcium and incident diabetes: an observational study and meta-analysis. Osteoporos Int 27, 1747–1754. [DOI] [PubMed] [Google Scholar]
- 10.Zaccardi F, Webb DR, Carter P, et al. (2015) Association between direct measurement of active serum calcium and risk of type 2 diabetes mellitus: a prospective study. Nutr Metab Cardiovasc Dis 25, 562–568. [DOI] [PubMed] [Google Scholar]
- 11.Mears D (2004) Regulation of insulin secretion in islets of Langerhans by Ca(2+) channels. J Membr Biol 200, 57–66. [DOI] [PubMed] [Google Scholar]
- 12.Wollheim CB & Sharp GW (1981) Regulation of insulin release by calcium. Physiol Rev 61, 914–973. [DOI] [PubMed] [Google Scholar]
- 13.Ojuka EO, Jones TE, Nolte LA, et al. (2002) Regulation of GLUT4 biogenesis in muscle: evidence for involvement of AMPK and Ca(2+). Am J Physiol Endocrinol Metab 282, E1008–E1013. [DOI] [PubMed] [Google Scholar]
- 14.Begum N, Leitner W, Reusch JE, et al. (1993) GLUT-4 phosphorylation and its intrinsic activity. Mechanism of Ca(2+)-induced inhibition of insulin-stimulated glucose transport. J Biol Chem 268, 3352–3356. [PubMed] [Google Scholar]
- 15.American Diabetes Association (2010) Diagnosis and classification of diabetes mellitus. Diabetes Care 33, S62–S69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Procopio M, Magro G, Cesario F, et al. (2002) The oral glucose tolerance test reveals a high frequency of both impaired glucose tolerance and undiagnosed type 2 diabetes mellitus in primary hyperparathyroidism. Diabet Med 19, 958–961. [DOI] [PubMed] [Google Scholar]
- 17.Lorenzo C, Hanley AJ, Rewers MJ, et al. (2014) Calcium and phosphate concentrations and future development of type 2 diabetes: the insulin resistance atherosclerosis study. Diabetologia 57, 1366–1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Levy J, Stern Z, Gutman A, et al. (1986) Plasma calcium and phosphate levels in an adult noninsulin-dependent diabetic population. Calcif Tissue Int 39, 316–318. [DOI] [PubMed] [Google Scholar]
- 19.Kim MK, Kim G, Jang EH, et al. (2010) Altered calcium homeostasis is correlated with the presence of metabolic syndrome and diabetes in middle-aged and elderly Korean subjects: the Chungju Metabolic Disease Cohort study (CMC study). Atherosclerosis 212, 674–681. [DOI] [PubMed] [Google Scholar]
- 20.Yamaguchi T, Kanazawa I, Takaoka S, et al. (2011) Serum calcium is positively correlated with fasting plasma glucose and insulin resistance, independent of parathyroid hormone, in male patients with type 2 diabetes mellitus. Metabolism 60, 1334–1339. [DOI] [PubMed] [Google Scholar]
- 21.Sun G, Vasdev S, Martin GR, et al. (2005) Altered calcium homeostasis is correlated with abnormalities of fasting serum glucose, insulin resistance, and beta-cell function in the Newfoundland population. Diabetes 54, 3336–3339. [DOI] [PubMed] [Google Scholar]
- 22.Kim KN, Oh SY & Hong YC (2018) Associations of serum calcium levels and dietary calcium intake with incident type 2 diabetes over 10 years: the Korean Genome and Epidemiology Study (KoGES). Diabetol Metab Syndr 10, 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wareham NJ, Byrne CD, Carr C, et al. (1997) Glucose intolerance is associated with altered calcium homeostasis: a possible link between increased serum calcium concentration and cardiovascular disease mortality. Metabolism 46, 1171–1177. [DOI] [PubMed] [Google Scholar]
- 24.Hagström E, Hellman P, Lundgren E, et al. (2007) Serum calcium is independently associated with insulin sensitivity measured with euglycaemic–hyperinsulinaemic clamp in a community-based cohort. Diabetologia 50, 317–324. [DOI] [PubMed] [Google Scholar]
- 25.Shalileh M, Shidfar F, Haghani H, et al. (2010) The influence of calcium supplement on body composition, weight loss and insulin resistance in obese adults receiving low calorie diet. J Res Med Sci 15, 191–201. [PMC free article] [PubMed] [Google Scholar]
- 26.Mitri J, Dawson-Hughes B, Hu FB, et al. (2011) Effects of vitamin D and calcium supplementation on pancreatic β cell function, insulin sensitivity, and glycemia in adults at high risk of diabetes: the Calcium and Vitamin D for Diabetes Mellitus (CaDDM) randomized controlled trial. Am J Clin Nutr 94, 486–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Suh S, Bae JC, Jin SM, et al. (2017) Serum calcium changes and risk of type 2 diabetes mellitus in Asian population. Diabetes Res Clin Pract 133, 109–114. [DOI] [PubMed] [Google Scholar]
- 28.Williamson GD, Rennie D, Moher D, et al. (2000) Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of Observational Studies in Epidemiology (MOOSE) group. JAMA 283, 2008–2012. [DOI] [PubMed] [Google Scholar]
- 29.Wells GA, Shea B, O’Connell D, et al. (2019) The Newcastle–Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed January 2019).
- 30.Zhao D, Khawaja AT, Jin L, et al. (2018) The directional and non-directional associations of periodontitis with chronic kidney disease: a systematic review and meta-analysis of observational studies. J Periodontal Res 53, 682–704. [DOI] [PubMed] [Google Scholar]
- 31.Elyasi M, Abreu LG, Badri P, et al. (2015) Impact of sense of coherence on oral health behaviors: a systematic review. PLOS ONE 10, e0133918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ferreira MC, Dias-Pereira AC, Branco-de-Almeida LS, et al. (2017) Impact of periodontal disease on quality of life: a systematic review. J Periodontal Res 52, 651–665. [DOI] [PubMed] [Google Scholar]
- 33.Duval S & Tweedie R (2000) Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics 56, 455–463. [DOI] [PubMed] [Google Scholar]
- 34.Heath H III, Lambert PW, Service FJ, et al. (1979) Calcium homeostasis in diabetes mellitus. J Clin Endocrinol Metab 49, 462–466. [DOI] [PubMed] [Google Scholar]
- 35.McNair P, Fogh-Andersen N, Madsbad S, et al. (1983) Decreased serum concentration of ionized calcium in insulin-dependent human diabetes mellitus. Eur J Clin Invest 13, 267–270. [DOI] [PubMed] [Google Scholar]
- 36.Cho GJ, Shin JH, Yi KW, et al. (2011) Serum calcium level is associated with metabolic syndrome in elderly women. Maturitas 68, 382–386. [DOI] [PubMed] [Google Scholar]
- 37.Shimodaira M, Niwa T, Nakajima K, et al. (2015) The relationship between serum calcium level and early-phase insulin secretion in normoglycemic and pre-diabetic individuals. Exp Clin Endocrinol Diabetes 123, 165–169. [DOI] [PubMed] [Google Scholar]
- 38.Guasch A, Bulló M, Rabassa A, et al. (2012) Plasma vitamin D and parathormone are associated with obesity and atherogenic dyslipidemia: a cross-sectional study. Cardiovasc Diabetol 11, 149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pittas AG, Dawson-Hughes B, Li T, et al. (2006) Vitamin D and calcium intake in relation to type 2 diabetes in women. Diabetes Care 29, 650–656. [DOI] [PubMed] [Google Scholar]
- 40.Villegas R, Gao YT, Dai Q, et al. (2009) Dietary calcium and magnesium intakes and the risk of type 2 diabetes: the Shanghai women’s health study. Am J Clin Nutr 89, 1059–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van Dam RM, Hu FB, Rosenberg L, et al. (2006) Dietary calcium and magnesium, major food sources, and risk of type 2 diabetes in U.S. black women. Diabetes Care 29, 2238–2243. [DOI] [PubMed] [Google Scholar]
- 42.Kirii K, Mizoue T, Iso H, et al. (2009) Calcium, vitamin D and dairy intake in relation to type 2 diabetes risk in a Japanese cohort. Diabetologia 52, 2542–2550. [DOI] [PubMed] [Google Scholar]
- 43.Dong JY & Qin LQ (2012) Dietary calcium intake and risk of type 2 diabetes: possible confounding by magnesium. Eur J Clin Nutr 66, 408–410. [DOI] [PubMed] [Google Scholar]
- 44.Reis JP, Selvin E, Pankow JS, et al. (2016) Parathyroid hormone is associated with incident diabetes in white, but not black adults: the Atherosclerosis Risk in Communities (ARIC) Study. Diabetes Metab 42, 162–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Song Y, Wang L, Pittas AG, et al. (2013) Blood 25-hydroxy vitamin D levels and incident type 2 diabetes: a meta-analysis of prospective studies. Diabetes Care 36, 1422–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gray E, Muller D, Squires PE, et al. (2006) Activation of the extracellular calcium-sensing receptor initiates insulin secretion from human islets of Langerhans: involvement of protein kinases. J Endocrinol 190, 703–710. [DOI] [PubMed] [Google Scholar]
- 47.Jones PM, Kitsou-Mylona I, Gray E, et al. (2007) Expression and function of the extracellular calcium-sensing receptor in pancreatic beta-cells. Arch Physiol Biochem 113, 98–103. [DOI] [PubMed] [Google Scholar]
- 48.Henquin JC (2000) Triggering and amplifying pathways of regulation of insulin secretion by glucose. Diabetes 49, 1751–1760. [DOI] [PubMed] [Google Scholar]
- 49.Jing X, Li DQ, Olofsson CS, et al. (2005) CaV2.3 calcium channels control second-phase insulin release. J Clin Invest 115, 146–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pittas AG, Lau J, Hu FB, et al. (2007) The role of vitamin D and calcium in type 2 diabetes. A systematic review and metaanalysis. J Clin Endocrinol Metab 92, 2017–2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ko SH, Lee GS & Vo TT (2009) Dietary calcium and 1, 25-dihydroxyvitamin D3 regulate transcription of calcium transporter genes in calbindin-D9k knockout mice. J Reprod Dev 55, 137–142. [DOI] [PubMed] [Google Scholar]
- 52.Draznin B, Lewis D, Houlder N, et al. (1989) Mechanism of insulin resistance induced by sustained levels of cytosolic free calcium in rat adipocytes. Endocrinology 125, 2341–2349. [DOI] [PubMed] [Google Scholar]
- 53.Draznin B, Sussman KE, Eckel RH, et al. (1988) Possible role of cytosolic free calcium concentrations in mediating insulin resistance of obesity and hyperinsulinemia. J Clin Invest 82, 1848–1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Park S, Scheffler TL, Gunawan AM, et al. (2009) Chronic elevated calcium blocks AMPK-induced GLUT-4 expression in skeletal muscle. Am J Physiol Cell Physiol 296, C106–C115. [DOI] [PubMed] [Google Scholar]
- 55.Khan AH & Pessin JE (2002) Insulin regulation of glucose uptake: a complex interplay of intracellular signalling pathways. Diabetologia 45, 1475–1483. [DOI] [PubMed] [Google Scholar]
- 56.Chandra J, Zhivotovsky B, Zaitsev S, et al. (2001) Role of apoptosis in pancreatic beta-cell death in diabetes. Diabetes 50, S44–S47. [DOI] [PubMed] [Google Scholar]
- 57.Wang L, Bhattacharjee A, Zuo Z, et al. (1999) A low voltage-activated Ca2+ current mediates cytokine-induced pancreatic beta-cell death. Endocrinology 140, 1200–1204. [DOI] [PubMed] [Google Scholar]
- 58.Ramadan JW, Steiner SR, O’Neill CM, et al. (2011) The central role of calcium in the effects of cytokines on beta-cell function: implications for type 1 and type 2 diabetes. Cell Calcium 50, 481–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Calvi LM & Bushinsky DA (2008) When is it appropriate to order an ionized calcium? J Am Soc Nephrol 19, 1257–1260. [DOI] [PubMed] [Google Scholar]
- 60.Zhao G, Ford ES & Li C (2010) Associations of serum concentrations of 25-hydroxyvitamin D and parathyroid hormone with surrogate markers of insulin resistance among U.S. adults with-out physician-diagnosed diabetes: NHANES, 2003–2006. Diabetes Care 33, 344–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Guynn RW, Veloso D & Veech RL (1972) Enzymic determination of inorganic phosphate in the presence of creatine phosphate. Anal Biochem 45, 277–285. [DOI] [PubMed] [Google Scholar]
- 62.Baird GS (2011) Ionized calcium. Clin Chim Acta 412, 696–701. [DOI] [PubMed] [Google Scholar]
- 63.Bushinsky DA & Monk RD (1998) Electrolyte quintet: calcium. Lancet 352, 306–311. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.