Abstract
Aims
The risk of HeartMate II (HMII) left ventricular assist device (LVAD) thrombosis has been reported, and serum lactate dehydrogenase (LDH), a biomarker of haemolysis, increases secondary to LVAD thrombosis. This study evaluated longitudinal measurements of LDH post‐LVAD implantation, hypothesizing that LDH trends could timely predict future LVAD thrombosis.
Methods and results
From October 2004 to October 2014, 350 HMIIs were implanted in 323 patients at Cleveland Clinic. Of these, patients on 339 HMIIs had at least one post‐implant LDH value (7996 total measurements). A two‐step joint model combining longitudinal biomarker data and pump thrombosis events was generated to assess the effect of changing LDH on thrombosis risk. Device‐specific LDH trends were first smoothed using multivariate boosted trees, and then used as a time‐varying covariate function in a multiphase hazard model to analyse time to thrombosis. Pre‐implant variables associated with time‐varying LDH values post‐implant using boostmtree were also investigated. Standardized variable importance for each variable was estimated as the difference between model‐based prediction error of LDH when the variable was randomly permuted and prediction error without permuting the values. The larger this difference, the more important a variable is for predicting the trajectory of post‐implant LDH. Thirty‐five HMIIs (10%) had either confirmed (18) or suspected (17) thrombosis, with 15 (43%) occurring within 3 months of implant. LDH was associated with thrombosis occurring both early and late after implant (P < 0.0001 for both hazard phases). The model demonstrated increased probability of HMII thrombosis as LDH trended upward, with steep changes in LDH trajectory paralleling trajectories in probability of pump thrombosis. The most important baseline variables predictive of the longitudinal pattern of LDH were higher bilirubin, higher pre‐implant LDH, and older age. The effect of some pre‐implant variables such as sodium on the post‐implant LDH longitudinal pattern differed across time.
Conclusions
Longitudinal trends in surveillance LDH for patients on HMII support are useful for dynamic prediction of pump thrombosis, both early after implant and late. Incorporating upward and downward trends in LDH that dynamically update a model of LVAD thrombosis risk provides a useful tool for clinical management and decisions.
Keywords: Pump thrombosis, Left ventricular assist device, Lactate dehydrogenase, Biomarker
Introduction
Left ventricular assist devices (LVADs) are integral to managing patients with advanced heart failure, and the number of patients receiving continuous‐flow LVADs for end‐stage heart failure is rising.1 Recent investigations into an increase in HeartMate II (HMII) pump thrombosis indicated an upward trend in the rate of HMII thrombosis at three major academic medical centres at 3 months following implantation, culminating at a rate of 8.4% by January 2013.2 This major complication was considerably higher than the 2–4% found in initial HMII pivotal clinical trials.3 Analysis of Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) data confirmed these findings.4, 5 Although the recent 2 year prespecified analysis from the MagLev Technology in Patients Undergoing Mechanical Circulatory Support Therapy with HeartMate 3 trial6 demonstrated the suspected or confirmed pump thrombosis was lower with the centrifugal‐flow HeartMate 3 compared with the axial‐flow HMII (1.1 vs. 15.7%, respectively, P < 0.001), LVAD thrombosis is associated with substantial mortality and morbidity, including stroke, worsening heart failure, need for LVAD exchange or urgent transplantation, and peripheral embolism.2, 4 Therefore, there is an urgent need for earlier diagnosis to permit timely decision making and effective intervention that may avert adverse outcomes.
Patients with continuous‐flow LVADs are known to have low‐grade haemolysis, albeit less severe than haemolysis occurring in earlier pulsatile‐flow pumps.7 Increases in values of laboratory tests associated with haemolysis, notably lactate dehydrogenase (LDH), may be useful in diagnosing already developed LVAD thrombosis.5, 8
We thus hypothesized that serial values of LDH can be used to predict patients who are in highest risk of developing pump thrombosis in the future. Our objective was to construct and test a dynamic prediction model that uses LDH trends during LVAD support and accordingly stratify the potential risk of LVAD thrombosis.
Methods
Study population
This was a single‐centre retrospective cohort study of 323 consecutive adults (age ≥ 18 years) who received 350 HMII devices at Cleveland Clinic from 1 October 2004, to 1 October 2014; 297 received one device, 25 two devices, and 1 three devices. Of these 323 patients, 79% were White, 20% were female, and mean age was 55 ± 14 years (Table 1). The unit of study was each HMII device, censored at pump exchange or removal, transplantation, death, or end of follow‐up while still supported by the device without evidence of pump thrombosis.
Table 1.
Characteristics of patients at first left ventricular assist device implant (n = 323)
| Characteristic | No. (%) or Mean ± SD |
|---|---|
| Demographics | |
| Age (years) | 55 ± 14 |
| Female | 65 (20) |
| Height (cm) | 176 ± 10.1 |
| Weight (kg) | 87 ± 20 |
| Body surface area (m2) | 2.08 ± 0.28 |
| Body mass index (kg/m2) | 28 ± 5.6 |
| Race | |
| White | 256 (79) |
| Black | 63 (20) |
| Other | 4 (1.2) |
| Preoperative diagnosis | |
| Ischaemic cardiomyopathy | 146 (45) |
| Non‐ischaemic cardiomyopathy | 156 (48) |
| Restrictive myopathy | 16 (5) |
| Valvular heart disease | 5 (1.5) |
| Treatment intention | |
| Bridge to transplant | 105 (33) |
| Bridge to decision | 82 (25) |
| Bridge to recovery | 1 (0.31) |
| Combined bridge to transplant/decision | 187 (58) |
| Destination therapy | 135 (42) |
SD, standard deviation.
Lactate dehydrogenase values
In accordance with institutional standard‐of‐care follow‐up, patients returned to the Clinic and underwent prescheduled blood draws, which included LDH levels, weekly for the first month, biweekly during the second month, monthly for the remainder of the first year, and every 3 months thereafter. A total of 396 person‐years of follow‐up were available for analysis. Median follow‐up time for survivors on HMII support was 0.89 years, with 10% of patients followed more than 2.9 years.
Pre‐implant and post‐implant LDH values were extracted by a direct electronic feed from the Cleveland Clinic electronic health record system through a common closing date of 16 September 2014. Values were available for 339 devices, totalling 7996 longitudinal measures with a median of 20 per HMII device (Supporting Information, Figure S1 ). Distribution of blood draws for LDH level was not perceptibly different among patients who eventually developed pump thrombosis and those that did not (Supporting Information, Figure S2 ).
Endpoints
The study endpoint was suspected or confirmed pump thrombosis. Confirmed pump thrombosis was defined by visualization of a thrombus on the blood‐device interface of the pump or within the inflow cannula or outflow conduit at pump explantation or autopsy. Suspected pump thrombosis was defined as pump malfunction with a clinical picture consistent with thrombus formation within the device, cannulae, or grafts. Although not available prospectively for the duration of this study, suspected pump thrombosis events were adjudicated using the current INTERMACS definition of major haemolysis: a plasma‐free haemoglobin value >20 mg/dL or a serum LDH greater than 2.5 times the institution's upper limit of normal at least 72 h after implantation that is associated with accompanying signs and symptoms of haemolysis, including haemoglobinuria, anaemia, hyperbilirubinemia, and pump malfunction or abnormal values of pump variables (Supporting Information). A team including 1 cardiac surgeon, 1 cardiologist, and 1 nurse coordinator reviewed each case and all clinical, laboratory, and pathology materials and assigned each event as confirmed or suspected thrombosis based upon the definitions mentioned.
Clinical data
Clinical data were extracted electronically from the Cleveland Clinic EDIT database, a prospectively maintained registry of all solid organ transplants and candidates including all patients receiving durable mechanic circulatory support devices. The Institutional Review Board approved use of all data used in this study for research, with patient consent waved (IRB 13‐629).
Data analysis
All statistical analyses were performed using SAS software, version 9.2 (SAS Institute, Cary, NC) and R software, versions 3.1.3 and 3.5.0 (R Foundation for Statistical Computing, Vienna, Austria).
The main goal of the analysis was to assess the association between longitudinal LDH measurements after HMII implant and future risk of pump thrombosis, a joint time‐to‐event analysis informed by longitudinal data. However, the longitudinal process and time‐to‐event process likely change with time. The most straightforward modelling approach would be to treat the longitudinal LDH data as a time‐varying covariate function in a time‐to‐event model with last value carried forward.9 However, LDH measurements exhibit some noise because of measurement error and short‐term biological variations. Therefore, we used a two‐step modelling approach.10, 11, 12 First, we estimated the device‐specific ‘true’ longitudinal process by removing or reducing noise (smoothing). Second, we used the resulting smoothed longitudinal process as a time‐varying covariate function in a joint longitudinal and time‐to‐event analysis of pump thrombosis.
Step 1: Device‐specific lactate dehydrogenase trends
Multivariate boosted trees, an ensemble statistical method that can improve weak learning algorithms by growing small regression trees through repeated sampling, was used for noise reduction.13, 14 A series of multivariate boosted trees were grown and weighted based on computed error, with final smoothed functions being calculated from the weighted average of entire ensemble trees (Supporting Information). The boosted multivariate tree method cost function was optimized to minimize prediction error.
Observed LDH values for each device were compared with smoothed continuous LDH functions resulting from the multivariate boosting trees (Figure 1). The smoothed LDH function for each HMII device was thus informed by both the observed LDH values for each unique device in conjunction with trends from the entire data set.
Figure 1.
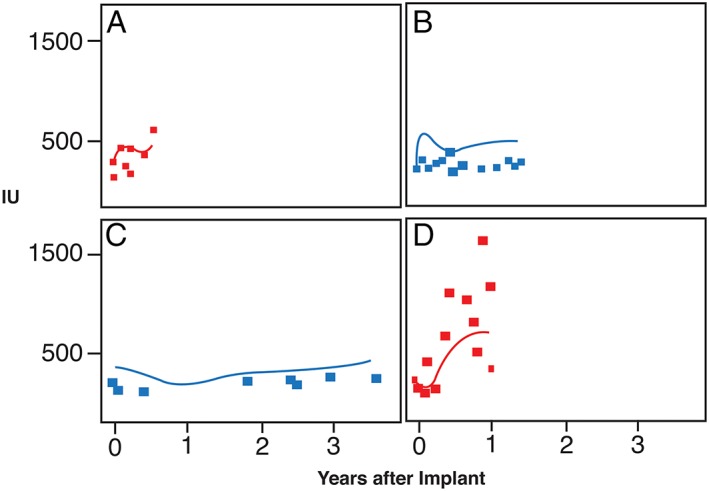
Continuous smoothed functions of lactate dehydrogenase (LDH) overlaid on measured LDH values. A and D developed pump thrombosis (red); B and C did not (blue).
Step 2: Lactate dehydrogenase trends and pump thrombosis
In the second step, the time‐varying effect of smoothed longitudinal LDH on the risk of HMII thrombosis was analysed. Pump thrombosis was assessed parametrically using a multiphase temporal decomposition hazard model.15 This parametric model is useful because of the nonproportional hazard nature of the risk of pump thrombosis.2, 4
Because there were few thrombotic events and date of implant was strongly associated with risk of thrombosis, the analysis was adjusted only for the date of surgery. To allow for a possible non‐linear relationship between smoothed LDH values and risk of thrombosis, two models with different transformations were used: (i) the common logarithmic, inverse, squared, and inverse squared transformations of smoothed LDH and (ii) quadratic splines, with knots at the quartile values of smoothed LDH. Forward selection was performed to select the appropriate transformation or spline variable(s) to optimize the model. Further, it was unclear whether changing LDH values or rate of change, or both, influenced the risk of thrombosis, so we performed two time‐to‐event analyses with smoothed LDH trends or the rate of change in the trends as time‐varying covariate functions.
Discrimination ability of the survival model was assessed at multiple time points by the concordance index—the probability of concordance between predicted and observed survival.16 The relationship among time after implant, temporal trend of LDH, and likelihood of pump thrombosis is depicted both on a patient‐specific level and overall using a filled‐contour plot.
We also investigated pre‐implant variables (Supporting Information, Appendix S1 ) associated with time‐varying LDH values post‐implant using boostmtree.14 Standardized variable importance for each variable was estimated as the difference between model‐based prediction error of LDH when the variable was randomly permuted and prediction error without permuting the values. The larger this difference, the more important a variable is for predicting the trajectory of post‐implant LDH. The risk‐adjusted relationship between post‐implant LDH and baseline characteristics is depicted by partial dependency plots.17
Results
Pump thrombosis
Of the 350 HMII devices, 35 experienced confirmed (n = 18) or suspected (n = 17) pump thrombosis. Fifteen occurred within 3 months of implantation, of which seven were confirmed. The instantaneous risk of suspected or confirmed pump thrombosis illustrates two clear phases, with early risk decreasing to a near constant value within approximately 12 months after implant ( Figure 2 ).
Figure 2.
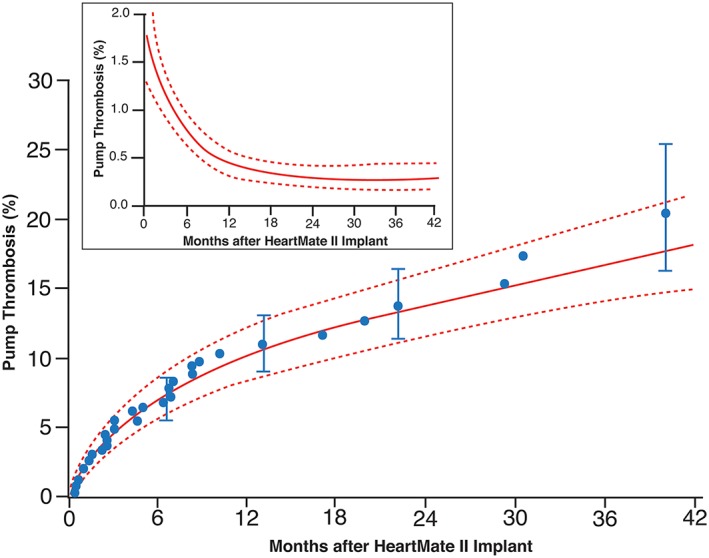
Overall probability of pump thrombosis. Each symbol represents a pump thrombosis positioned on the vertical axis by the Kaplan–Meier estimator. Vertical bars are confidence limits equivalent to ±1 standard error. Solid lines are parametric hazard estimates enclosed within dashed 68% confidence bands equivalent to ±1 standard error. The inset shows instantaneous risk of pump thrombosis (hazard function) after HeartMate II implantation. Solid lines are parametric hazard estimates enclosed within dashed 68% confidence bands equivalent to ±1 standard error.
Lactate dehydrogenase predicting pump thrombosis
Higher LDH was associated with pump thrombosis in both the early and late hazard phases (P < 0.0001) (Table 2). Absolute LDH across time, not the rate of LDH change across time (P > 0.9), was associated with risk of pump thrombosis. Probability of thrombosis closely paralleled fluctuations in smoothed LDH for each device. For two devices that never experienced pump thrombosis, the model illustrates low LDH values and corresponding low probability of pump thrombosis (low risk patients) (Figure 3 A). For two devices that did experience thrombosis, the model illustrates a steep increase in LDH and corresponding steep increase in probability of pump thrombosis (high risk patients) (Figure 3 B). Figure 4 depicts the overall relationship of time on LVAD support, LDH level post‐implant, and pump thrombosis.
Table 2.
Multiphase hazard model of pump thrombosis
| Factor | Coefficient ± SE | P |
|---|---|---|
| Early phase | ||
| Higher LDHa | −13 ± 2.0 | <0.0001 |
| Recent date of implantb | 0.64 ± 0.25 | 0.009 |
| Late phase | ||
| Higher LDHc | 0.45 ± 0.081 | <0.0001 |
| Earlier date of implantd | −2.6 ± 1.3 | 0.06 |
The hazard model separates early phase, the time of implant to approximately 12 months post‐implant, and late phase, approximately 12 months post‐implant to removal of the device for any reason or death. Date of implant refers to the calendar date of surgery, with more recent years increasing risk of pump thrombosis in the early phase. LDH, lactate dehydrogenase; SE, standard error.
[440/(LDH + 352)], inverse transformation.
[(Date of implant, expressed as interval between 01/01/2004 and implant)/7]3, cubed transformation.
[(LDH + 352)/440]2, squared transformation.
Log[(date of implant, expressed as interval between 01/01/2004 and implant)], logarithmic transformation.
Figure 3.
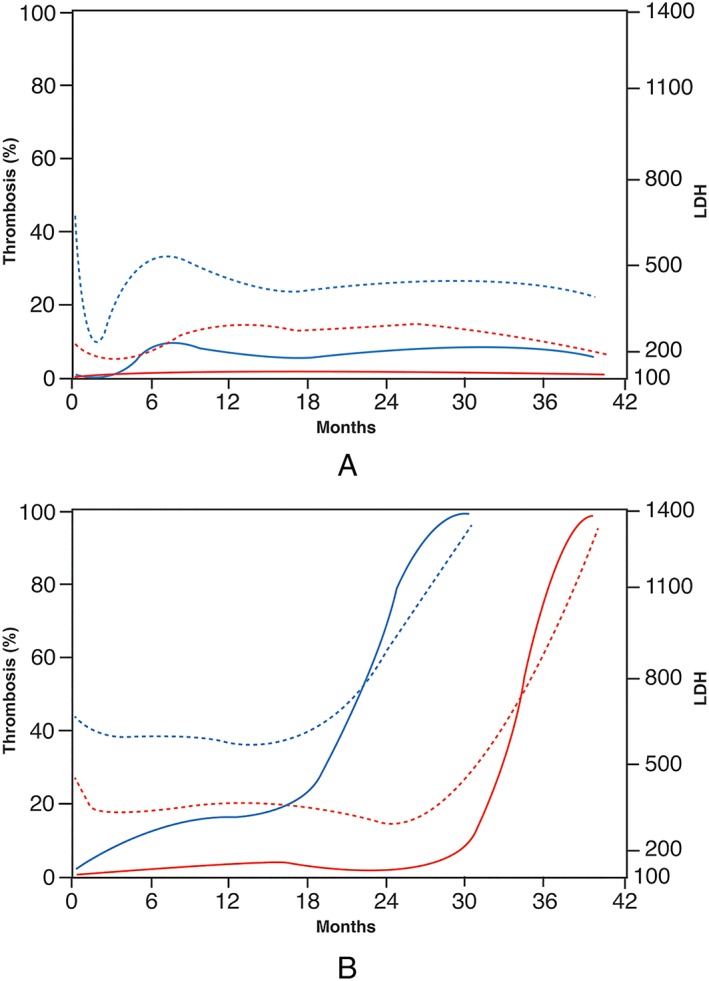
Smoothed lactate dehydrogenase (LDH) trends and modelled probability of pump thrombosis for two pairs of HeartMate II devices. One device is illustrated in blue, the other in red. Dashed lines represent LDH trends and solid lines the corresponding probability of pump thrombosis. (A) Two devices that did not experience pump thrombosis. The device in red illustrates the very low probability of thrombosis with continuous low LDH values, while the device in blue shows a mild increase coinciding with uptrending in LDH around 3 months post‐implant. Notably, the model did not respond to increased LDH values in the first month post‐implant. (B) Two devices that experienced pump thrombosis. The device in red shows a low probability of pump thrombosis with stable LDH values, with sharply increasing probability matching a steep increase in LDH values beginning at 30 months. The device in blue shows moderate risk of pump thrombosis while LDH trends around 600 IU/L, with increasing probability matching a steep increase at 18 months post‐implant.
Figure 4.
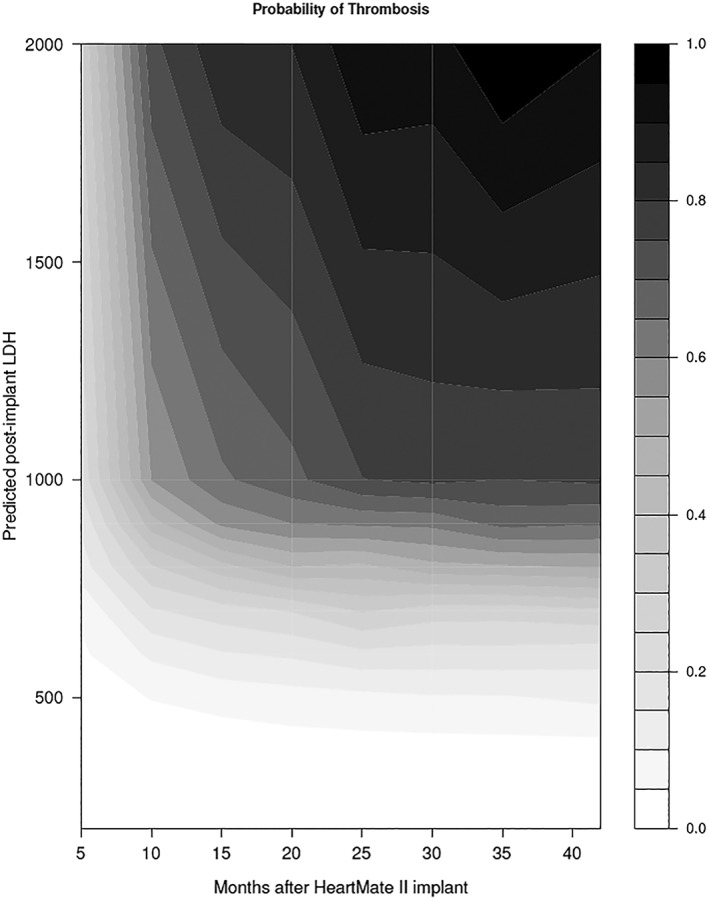
Relationship between time after HeartMate II implant, temporal trend of LDH, and likelihood of pump thrombosis. As LDH increases, the probability of pump thrombosis increases as time progresses (darker shade). LDH, lactate dehydrogenase.
The joint model incorporating longitudinal LDH values and date of implant was most useful beyond 1 month after implantation, particularly between 3 months and 2 years, according to the time‐related concordance index (Supporting Information, Table S1 ).
Baseline variables predictive of post‐implant lactate dehydrogenase
The most important baseline variables predictive of the longitudinal pattern of LDH were higher bilirubin, higher pre‐implant LDH, and older age (Figures 5 and 6). Effect of some variables differed across time, such as sodium, depicted in Figure 6 . We used this pre‐implant model to predict the expected pattern of LDH post‐implant for each patient (Figure 7).
Figure 5.
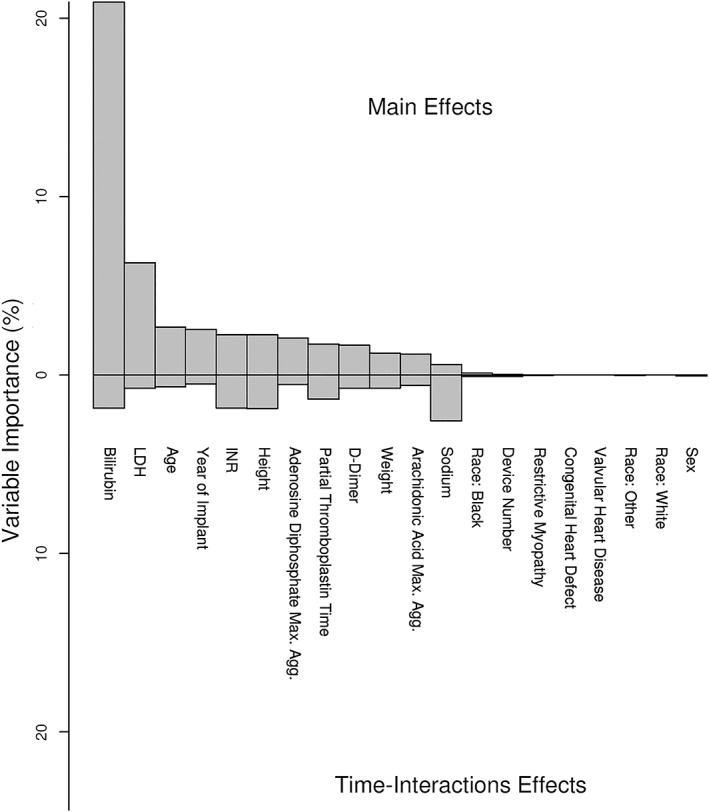
Importance of association longitudinal trajectory of LDH values with baseline variables. Standardized variable importance (VIMP) is shown on the vertical axis. VIMP above the horizontal line at zero depict values for the main effects of variables. Values below this horizontal axis depicts importance of variable–time interactions. LDH, lactate dehydrogenase.
Figure 6.
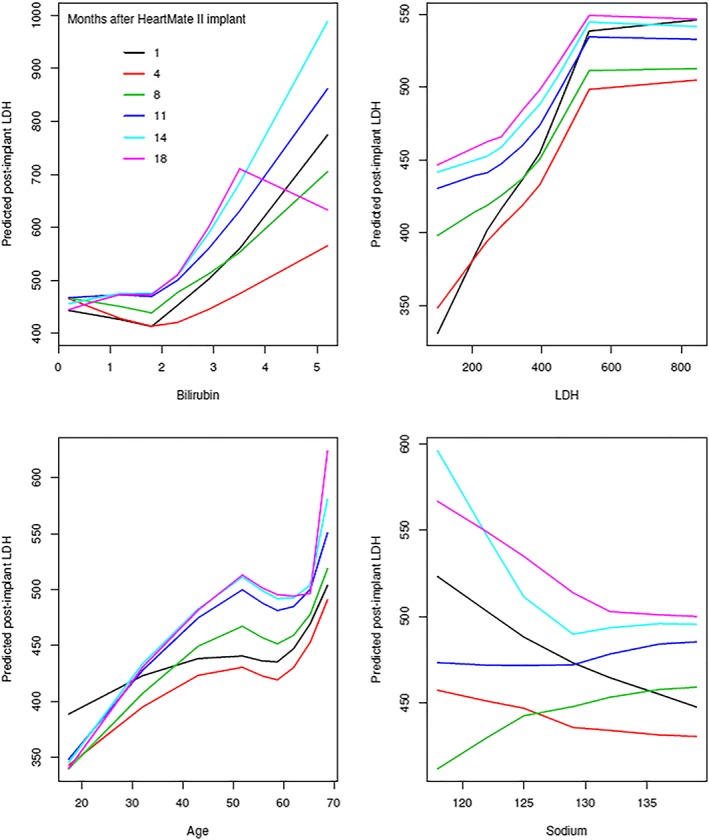
Risk‐adjusted relationship of predicted post‐implant LDH level with baseline variables, chosen from among the variables with highest variable importance (shown in Figure 5 ): bilirubin, LDH, age, and sodium level. These are partial dependency plots from the boosting analysis depicted in Figure 5 . These generally show increasing LDH with elevated baseline values. A non‐linear relationship with longer implant time is shown with sodium. LDH, lactate dehydrogenase.
Figure 7.
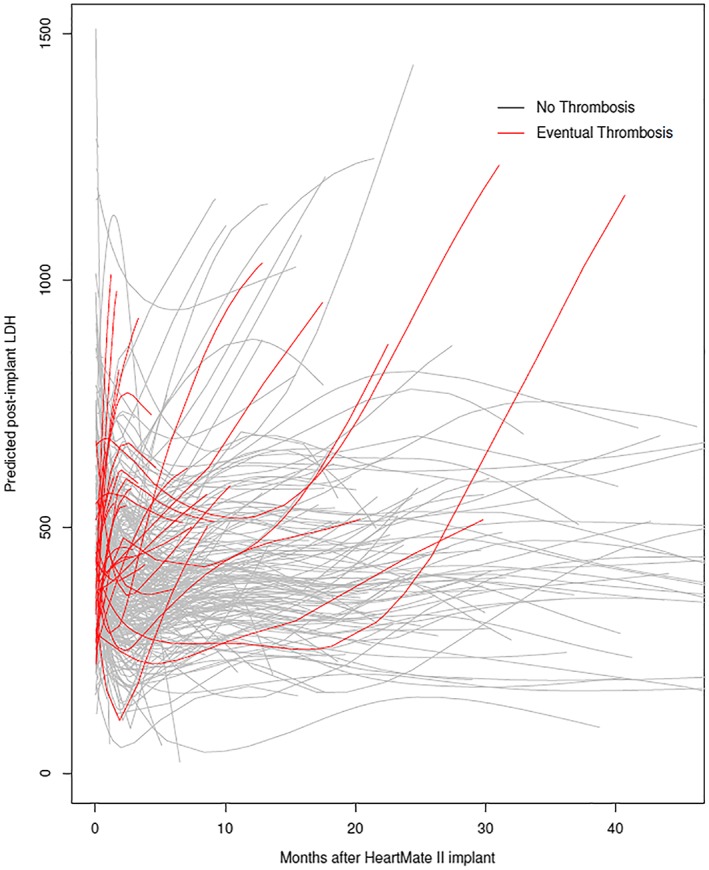
Predicted temporal trend of LDH post‐implant predicted from pre‐implant variables for all patients in the study. Grey lines are predicted lines for patients who did not exhibit pump thrombosis; red lines are for patients who eventually experienced pump thrombosis. LDH, lactate dehydrogenase.
Discussion
Thrombosis is a serious complication of mechanical circulatory devices; however, its causative factors remain unresolved.18 In this study, we have illustrated the utility of monitoring LDH trends for those on HMII support as an inexpensive and effective method to timely assess the risk of pump thrombosis. The model is potentially generalizable to any type of LVAD with clinically relevant pump thrombosis. LDH has become a standard biomarker used in surveilling patients with LVADs.5, 8, 19, 20 This study provides evidence to support the clinical application of LDH monitoring as standard clinical practice in all LVAD patients.
Assessment for suspected pump thrombosis has included serum biomarkers as well as echocardiography.21 Echocardiographic ramp testing, initially created for speed optimization in patients with LVADs, is used at many centres to identify suspected LVAD thrombosis.22, 23 Slope of left ventricular end‐diastolic diameter measurements in ramp testing, in conjunction with serum LDH values, has been shown to provide excellent sensitivity for LVAD thrombosis, but with limitations.24 Further, some data suggest that serum biomarkers may become elevated earlier than abnormalities appreciated on ramp testing.25
When comparing serum biomarkers, LDH has been shown to be a more sensitive and specific marker of haemolysis and device thrombosis than serum free haemoglobin.26 INTERMACS data from 2006 to 2012 showed haemolysis, as defined by elevation in serum free haemoglobin >40 mg/dL and clinical signs, occurred in 3% of individuals on HMII support at 6 months post‐ implant, 6% at 1 year, and 9% at 2 years, indicating that haemolysis was not a rare event.27 Cowger et al. illustrated that a cohort of HMII patients with haemolysis as defined by the same INTERMACS criteria had a 1 year event‐free survival of 16%, compared with 85% in patients who did not experience haemolysis.28 In comparison, when this same cohort was categorized by LDH, with a cut‐off of >600 IU/L (2.5 times the upper limit of normal), event‐free survival at 1 year was 32% for patients meeting this LDH haemolysis criteria, and 89% for those not meeting it. Further, LDH appeared to provide earlier diagnosis of suspected HMII thrombosis.
Others have provided differing LDH cut‐offs than Cowger et al. for identifying pump thrombosis, including 1550 IU/L based on receiver‐operating‐characteristic analysis, with 89% sensitivity and 100% specificity.29 However, at present, these analyses have focused on LDH values typically obtained during hospitalization for the onset of a pump thrombosis event. Less is known concerning the usefulness of LDH monitoring for the duration of HMII support.20
Using novel statistical methods, we assessed LDH as a harbinger of HMII pump thrombosis from a different vantage point, integrating every post‐implant LDH value to assess its value as a monitoring laboratory study while on HMII support, not just as an indicator for the presence of thrombosis. It is anticipated that these findings will apply to all devices with a predisposition to thrombosis, perhaps with unique relationships to the time of thrombosis. For example, LDH fluctuations in patients with centrifugal‐flow LVADs may be less pronounced but could remain clinically useful.19, 30
Importantly, LDH monitoring can be used dynamically for the duration of LVAD support, providing an updated probability estimate of pump thrombosis from measurement to measurement. In the clinical setting, serial monitoring of LDH could help identify patients who are at highest risk of pump thrombosis and merit closer follow‐up with clinical, device, and biomarker evaluations. The outcome of early intervention in those patients deemed at higher risk of pump thrombosis requires further investigation. Although antithrombotic agents may dissipate early thrombus formation within the LVAD and limit the need for pump exchange, they have been associated with concerning risks of bleeding and recurrence of pump thrombosis.31
The recently published PREVENT study (PREVENtion of Heart Mate II Pump Thrombosis Through Clinical Management) was a prospective multicentre non‐randomized trial that reported a low range of confirmed pump thrombosis in the participating centres that adopted specific surgical (size and location of the pump pocket, position of the inflow cannula, the outflow graft, and the pump and fixation of the pump) and clinical (heparin bridging, pump speeds, and blood pressure management) recommendations.32 An analysis of the PREVENT study20 showed that early diagnosis of LVAD thrombosis on the basis of LDH patterns may provide clinical benefit. In particular, acute and sustained medical resolution defined as ‘elevated LDH treated successfully with intensified medical therapy alone without subsequent surgical intervention or occurrence of suspected pump thrombosis’ at 3 and 6 months post‐treatment, respectively,20 was observed more frequently in the group of moderate elevated LDH patients (2,5‐3,2 × Upper Limit of Normal) compared with the groups with higher LDH values (>3,2 × Upper Limit of Normal). Hence, it is possible that early diagnosis based on LDH trends may improve the efficacy of medical management and reduce the need for pump exchange. Taken all together, the key message from PREVENT study comes to an agreement with our work and highlights the importance of LDH longitudinal monitoring as far as the prediction of pump thrombosis concerns. However, the above‐mentioned findings of PREVENT study are limited by the short follow‐up (up to 6 months post‐implantation) contrary to our study that provides both short‐term and long‐term evidence, starting 1 month after implantation and continuing for the duration of LVAD support.
Currently, medical management of pump thrombosis has significant mortality (~48% at 6 months), and pump replacement carries risks exceeding those of primary implants.2, 4, 5 An INTERMACS report showed lower survival at 2 years, as well as increased incidence of infection and stroke in patients undergoing pump exchange for thrombosis compared with those receiving a primary LVAD implant.26 Intraoperative mortality for HMII replacement is <7%.33 There are also concerns about substantial rates of repeat thrombosis following device exchange,4, 5 which may be due in part to the fact that only the pump itself is exchanged, not the inflow and outflow cannulae. Interestingly, Levin et al. stated that LVAD patients who presented with haemolysis, which was refractory to intensification of antithrombotic therapy, exhibited major risk for cerebrovascular events and deaths, and suggested that they should be considered for early device exchange.34
Lastly, the current analysis revealed the important pre‐implant variables (i.e. bilirubin, pre‐implant LDH, and age) for the prediction of the trajectory of post‐implant LDH. These pre‐implant predictions are not as powerful as identifying the actual trend of post‐implant LDH, but in many cases could have alerted physicians to the high probability of anticipated pump thrombosis.
Study limitations
This analysis was performed using single‐centre data with a limited effective sample size of 35 thrombosis cases, of which 18 were confirmed thromboses. When analysing serial biomarkers, consideration must be paid to laboratory reference values, which may vary among institutions. Although LVAD thrombosis can develop in many devices, this analysis is only directly applicable to the HMII device. Elevated LDH alone does not indicate pump thrombosis; clinical judgement, exam findings, echo findings, and haemodynamics remain essential diagnosing and treating this adverse event.
Future directions
The statistical techniques described for the joint predictive model are novel and may require refinement. It remains to be seen how these results would compare to a simpler predictive approach, such as with simpler smoothing algorithms or even raw data with post‐processing smoothing. Importantly, any of these methods can provide near instantaneous calculation of these trends on hand‐held smart devices.
Conclusions
Lactate dehydrogenase has been illustrated to be a valuable prognostic marker at the time of presentation with suspected pump thrombosis. This work illustrates that continuous monitoring of LDH values in patients on HMII support proves to be an effective tool for the ongoing assessment of the risk of pump thrombosis.
Conflict of interest
None declared.
Funding
The statistical analysis for this work was supported in part by the National Heart, Lung, and Blood Institute through grant number R01HL103552 to E.H.B. This work was funded by the Cleveland Clinic Foundation.
Supporting information
Appendix S1. List of Random Survival Forest Variables for Multivariate Boosting Tree Analysis. List of Pre‐Implant Variables for Boosting Tree Analysis of Longitudinal LDH Values.
Figure S1. Frequency histogram of number of postoperative lactate dehydrogenase measures per HeartMate II device.
Figure S2. Timing of LDH measurements post‐implant stratified by patients with no eventual thrombosis and those who eventually had thrombosis. Histograms depict the distributions of time of LDH measurements.
Table S1. Concordance Indices for LDH Model of Pump Thrombosis.
Acknowledgements
We would like to thank Mrs. Citraro Mary Ann (Cleveland Clinic illustrator) for her valuable assistance regarding the artwork.
Hurst, T. E. , Xanthopoulos, A. , Ehrlinger, J. , Rajeswaran, J. , Pande, A. , Thuita, L. , Smedira, N. G. , Moazami, N. , Blackstone, E. H. , and Starling, R. C. (2019) Dynamic prediction of left ventricular assist device pump thrombosis based on lactate dehydrogenase trends. ESC Heart Failure, 6, 1005–1014. 10.1002/ehf2.12473.
References
- 1. Kirklin JK, Pagani FD, Kormos RL, Stevenson LW, Blume ED, Myers SL, Miller MA, Baldwin JT, Young JB, Naftel DC. Eighth annual INTERMACS report: Special focus on framing the impact of adverse events. J Heart Lung Transplant 2017; 36: 1080–1086. [DOI] [PubMed] [Google Scholar]
- 2. Starling RC, Moazami N, Silvestry SC, Ewald G, Rogers JG, Milano CA, Rame JE, Acker MA, Blackstone EH, Ehrlinger J, Thuita L, Mountis MM, Soltesz EG, Lytle BW, Smedira NG. Unexpected abrupt increase in left ventricular assist device thrombosis. N Engl J Med 2014; 370: 33–40. [DOI] [PubMed] [Google Scholar]
- 3. Slaughter MS, Rogers JG, Milano CA, Russell SD, Conte JV, Feldman D, Sun B, Tatooles AJ, Delgado RM 3rd, Long JW, Wozniak TC, Ghumman W, Farrar DJ, Frazier OH. Advanced heart failure treated with continuous‐flow left ventricular assist device. N Engl J Med 2009; 361: 2241–2251. [DOI] [PubMed] [Google Scholar]
- 4. Smedira NG, Blackstone EH, Ehrlinger J, Thuita L, Pierce CD, Moazami N, Starling RC. Current risks of HeartMate II pump thrombosis: Non‐parametric analysis of Interagency Registry for Mechanically Assisted Circulatory Support data. J Heart Lung Transplant 2015; 34: 1527–1534. [DOI] [PubMed] [Google Scholar]
- 5. Kirklin JK, Naftel DC, Pagani FD, Kormos RL, Myers S, Acker MA, Rogers J, Slaughter MS, Stevenson LW. Pump thrombosis in the Thoratec HeartMate II device: An update analysis of the INTERMACS Registry. J Heart Lung Transplant 2015; 34: 1515–1526. [DOI] [PubMed] [Google Scholar]
- 6. Mehra MR, Goldstein DJ, Uriel N, Cleveland JC Jr, Yuzefpolskaya M, Salerno C, Walsh MN, Milano CA, Patel CB, Ewald GA, Itoh A, Dean D, Krishnamoorthy A, Cotts WG, Tatooles AJ, Jorde UP, Bruckner BA, Estep JD, Jeevanandam V, Sayer G, Horstmanshof D, Long JW, Gulati S, Skipper ER, O'Connell JB, Heatley G, Sood P, Naka Y. Two‐year outcomes with a magnetically levitated cardiac pump in heart failure. N Engl J Med 2018; 378: 1386–1395. [DOI] [PubMed] [Google Scholar]
- 7. Heilmann C, Geisen U, Benk C, Berchtold‐Herz M, Trummer G, Schlensak C, Zieger B, Beyersdorf F. Haemolysis in patients with ventricular assist devices: Major differences between systems. Eur J Cardiothorac Surg 2009; 36: 580–584. [DOI] [PubMed] [Google Scholar]
- 8. Starling RC, Blackstone EH, Smedira NG. Increase in left ventricular assist device thrombosis. N Engl J Med 2014; 370: 1465–1466. [DOI] [PubMed] [Google Scholar]
- 9. Kalbfleisch JD, Prentice RL. The statistical analysis of failure time data. Hoboken, N.J: Wiley; 2002. [Google Scholar]
- 10. Raboud J, Reid N, Coates RA, Farewell VT. Estimating risks of progressing to aids when covariates are measured with error. J R Stat Soc A Stat Soc 1993; 156: 393. [Google Scholar]
- 11. Tsiatis AA, Davidian M. Joint modeling of longitudinal and time‐to‐event data: An overview. Stat Sin 2004; 14: 809–834. [Google Scholar]
- 12. Bycott P, Taylor J. A comparison of smoothing techniques for CD4 data measured with error in a time‐dependent cox proportional hazards model. Stat Med 1998; 17: 2061–2077. [DOI] [PubMed] [Google Scholar]
- 13. Sain R, Carmack PS. Boosting multi‐objective regression trees. Comput Sci Stat 2002; 34: 232–241. [Google Scholar]
- 14. Pande A, Li L, Rajeswaran J, Ehrlinger J, Kogalur UB, Blackstone EH, Ishwaran H. Boosted multivariate trees for longitudinal data. Mach Learn 2017; 106: 277–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Blackstone EH, Naftel DC, Turner ME. The decomposition of time‐varying hazard into phases, each incorporating a separate stream of concomitant information. J Am Stat Assoc 1986; 81: 615–624. [Google Scholar]
- 16. Heagerty PJ, Zheng Y. Survival model predictive accuracy and ROC curves. Biometrics 2005; 61: 92–105. [DOI] [PubMed] [Google Scholar]
- 17. Friedman JH. Greedy function approximation: A gradient boosting machine. Ann Stat 2001; 29: 1189–1232. [Google Scholar]
- 18. Mehra MR, Stewart GC, Uber PA. The vexing problem of thrombosis in long‐term mechanical circulatory support. J Heart Lung Transplant 2014; 33: 1–11. [DOI] [PubMed] [Google Scholar]
- 19. Uriel N, Colombo PC, Cleveland JC, Long JW, Salerno C, Goldstein DJ, Patel CB, Ewald GA, Tatooles AJ, Silvestry SC, John R, Caldeira C, Jeevanandam V, Boyle AJ, Sundareswaran KS, Sood P, Mehra MR. Hemocompatibility‐related outcomes in the MOMENTUM 3 trial at 6 months: A randomized controlled study of a fully magnetically levitated pump in advanced heart failure. Circulation 2017; 135: 2003–2012. [DOI] [PubMed] [Google Scholar]
- 20. Thenappan T, Stulak JM, Agarwal R, Maltais S, Shah P, Eckman P, Emani S, Katz JN, Gregoric I, Keebler ME, Uriel N, Adler E, Chuang J, Farrar DJ, Sundareswaran KS, John R. Early intervention for lactate dehydrogenase elevation improves clinical outcomes in patients with the HeartMate II left ventricular assist device: Insights from the PREVENT study. J Heart Lung Transplant 2018; 37: 25–32. [DOI] [PubMed] [Google Scholar]
- 21. Estep JD, Vivo RP, Krim SR, Cordero‐Reyes AM, Elias B, Loebe M, Bruckner BA, Bhimaraj A, Trachtenberg BH, Ashrith G, Torre‐Amione G, Nagueh SF. Echocardiographic evaluation of hemodynamics in patients with systolic heart failure supported by a continuous‐flow LVAD. J Am Coll Cardiol 2014; 64: 1231–1241. [DOI] [PubMed] [Google Scholar]
- 22. Uriel N, Morrison KA, Garan AR, Kato TS, Yuzefpolskaya M, Latif F, Restaino SW, Mancini DM, Flannery M, Takayama H, John R, Colombo PC, Naka Y, Jorde UP. Development of a novel echocardiography ramp test for speed optimization and diagnosis of device thrombosis in continuous‐flow left ventricular assist devices: The Columbia ramp study. J Am Coll Cardiol 2012; 60: 1764–1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Estep JD, Vivo RP, Cordero‐Reyes AM, Bhimaraj A, Trachtenberg BH, Torre‐Amione G, Chang SM, Elias B, Bruckner BA, Suarez EE, Loebe M. A simplified echocardiographic technique for detecting continuous‐flow left ventricular assist device malfunction due to pump thrombosis. J Heart Lung Transplant 2014; 33: 575–586. [DOI] [PubMed] [Google Scholar]
- 24. Adatya S, Holley CT, Roy SS, Yarmohammadi H, Feng A, Eckman P, Colvin‐Adams M, John R, Masri C. Echocardiographic ramp test for continuous‐flow left ventricular assist devices: Do loading conditions matter? JACC Heart Fail 2015; 3: 291–299. [DOI] [PubMed] [Google Scholar]
- 25. Bartoli CR, Ghotra AS, Pachika AR, Birks EJ, McCants KC. Hematologic markers better predict left ventricular assist device thrombosis than echocardiographic or pump parameters. Thorac Cardiovasc Surg 2014; 62: 414–418. [DOI] [PubMed] [Google Scholar]
- 26. Shah P, Mehta VM, Cowger JA, Aaronson KD, Pagani FD. Diagnosis of hemolysis and device thrombosis with lactate dehydrogenase during left ventricular assist device support. J Heart Lung Transplant 2014; 33: 102–104. [DOI] [PubMed] [Google Scholar]
- 27. Katz JN, Jensen BC, Chang PP, Myers SL, Pagani FD, Kirklin JK. A multicenter analysis of clinical hemolysis in patients supported with durable, long‐term left ventricular assist device therapy. J Heart Lung Transplant 2015; 34: 701–709. [DOI] [PubMed] [Google Scholar]
- 28. Cowger JA, Romano MA, Shah P, Shah N, Mehta V, Haft JW, Aaronson KD, Pagani FD. Hemolysis: A harbinger of adverse outcome after left ventricular assist device implant. J Heart Lung Transplant 2014; 33: 35–43. [DOI] [PubMed] [Google Scholar]
- 29. Ravichandran AK, Parker J, Novak E, Joseph SM, Schilling JD, Ewald GA, Silvestry S. Hemolysis in left ventricular assist device: A retrospective analysis of outcomes. J Heart Lung Transplant 2014; 33: 44–50. [DOI] [PubMed] [Google Scholar]
- 30. Stulak JM, Sharma S, Maltais S. Management of pump thrombosis in patients with left ventricular assist devices. Am J Cardiovasc Drugs 2015; 15: 89–94. [DOI] [PubMed] [Google Scholar]
- 31. Schlendorf K, Patel CB, Gehrig T, Kiefer TL, Felker GM, Hernandez AF, Blue LJ, Milano CA, Rogers JG. Thrombolytic therapy for thrombosis of continuous flow ventricular assist devices. J Card Fail 2014; 20: 91–97. [DOI] [PubMed] [Google Scholar]
- 32. Maltais S, Kilic A, Nathan S, Keebler M, Emani S, Ransom J, Katz JN, Sheridan B, Brieke A, Egnaczyk G, Entwistle JW 3rd, Adamson R, Stulak J, Uriel N, O'Connell JB, Farrar DJ, Sundareswaran KS, Gregoric I. PREVENtion of HeartMate II Pump Thrombosis Through Clinical Management: The PREVENT multi‐center study. J Heart Lung Transplant 2017; 36: 1–12. [DOI] [PubMed] [Google Scholar]
- 33. Moazami N, Milano CA, John R, Sun B, Adamson RM, Pagani FD, Smedira N, Slaughter MS, Farrar DJ, Frazier OH. Pump replacement for left ventricular assist device failure can be done safely and is associated with low mortality. Ann Thorac Surg 2013; 95: 500–505. [DOI] [PubMed] [Google Scholar]
- 34. Levin AP, Saeed O, Willey JZ, Levin CJ, Fried JA, Patel SR, Sims DB, Nguyen JD, Shin JJ, Topkara VK, Colombo PC, Goldstein DJ, Naka Y, Takayama H, Uriel N, Jorde UP. Watchful waiting in continuous‐flow left ventricular assist device patients with ongoing hemolysis is associated with an increased risk for cerebrovascular accident or death. Circ Heart Fail 2016; 9. pii: e002896. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. List of Random Survival Forest Variables for Multivariate Boosting Tree Analysis. List of Pre‐Implant Variables for Boosting Tree Analysis of Longitudinal LDH Values.
Figure S1. Frequency histogram of number of postoperative lactate dehydrogenase measures per HeartMate II device.
Figure S2. Timing of LDH measurements post‐implant stratified by patients with no eventual thrombosis and those who eventually had thrombosis. Histograms depict the distributions of time of LDH measurements.
Table S1. Concordance Indices for LDH Model of Pump Thrombosis.


