Abstract
Exogenous anabolic androgen steroid use is associated with adverse cardiovascular outcomes. A 53‐year‐old bodybuilder presented with 3 months of exertional dyspnoea. Physical examination showed tachycardia and pan‐systolic murmur; an echocardiogram showed a left ventricular ejection fraction (EF) of 15%. Evaluations included normal coronary angiogram, iron panel and thyroid studies, a negative viral panel (human immunodeficiency virus, Lyme disease, and hepatitis), and urine toxicology. He admitted to intramuscular anabolic steroid use; his testosterone level was 30 160.0 ng/dL (normal 280–1100 ng/dL). In addition to discontinuation of anabolic steroid use, he was treated with guideline‐directed heart failure medical therapy. Repeat echocardiogram at 6 months showed an EF of 54% and normalized testosterone level of 603.7 ng/dL. Anabolic steroid use is a rare, reversible cause of cardiomyopathy in young, otherwise healthy athletes; a high index of suspicion is required to prevent potentially fatal side effects.
Keywords: Testosterone, Cardiomyopathy, Anabolic hormones
Introduction
Gender‐based differences in cardiovascular disease incidence, prevalence, and severity may, in part, be related to the difference in endogenous sex hormones. The use of exogenous anabolic androgen steroids (AASs) for bodybuilding is increasing among young athletes in the USA, and some reports link AAS use to adverse cardiovascular outcomes.1, 2, 3 In this report, we highlight the anabolic steroid as a potential cause of cardiomyopathy that resolved upon discontinuation.
Case report
A 53‐year‐old, otherwise healthy bodybuilder was seen in the emergency department with 3 months of progressive dyspnoea, palpitations, headache, and an episode of syncope. He admitted to a history of fatigue, decreased libido, and erectile dysfunction for which he had been prescribed topical androgen and sildenafil. He is employed as a firefighter, does not smoke tobacco, and uses <6 oz of alcohol per week. He is married with two children and denies any family history of cardiomyopathy. He enjoys 60–90 min of vigorous exercise daily, which predominantly involves heavy weightlifting.
Vital signs were remarkable for a regular heart rate of 90 b.p.m. and a blood pressure of 167/95 mmHg. The physical examination demonstrated a muscular man, elevated central venous pressure (12 cm H2O), normal symmetric pulses, a slightly enlarged and laterally displaced cardiac point of maximal impulse, a grade 3/6 holosystolic murmur at the apex, and a positive third heart sound. Chest X‐ray revealed cardiomegaly, and a Doppler echocardiogram demonstrated a dilated left ventricle (6.9 cm) with severe global hypokinesis, left ventricular septal thickness in diastole (LVSthd) of 1.0 cm (normal < 1.2 cm), and a calculated left ventricular ejection fraction (LVEF) of 15% (Figure 1A). Colour Doppler images demonstrated severe mitral regurgitation (Figure 1 B); myocardial speckle tracking showed a severely abnormal global longitudinal strain pattern (Figure 2). Of note, 3 years prior, his LVEF had been 57%.
Figure 1.
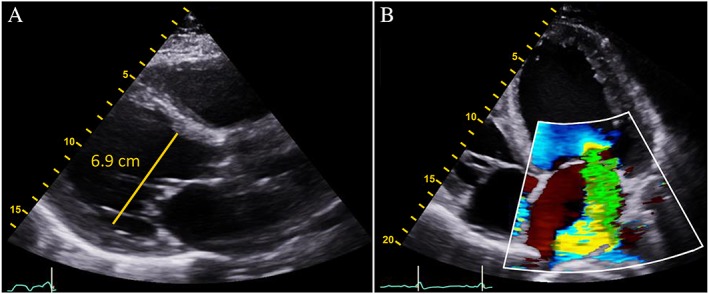
(A) Parasternal long‐axis view shows the dilated left ventricle (6.9 cm) and severe global hypokinesis with a left ventricular ejection fraction of 15%. (B) Colour Doppler apical four‐chamber view demonstrates severe functional mitral regurgitation.
Figure 2.
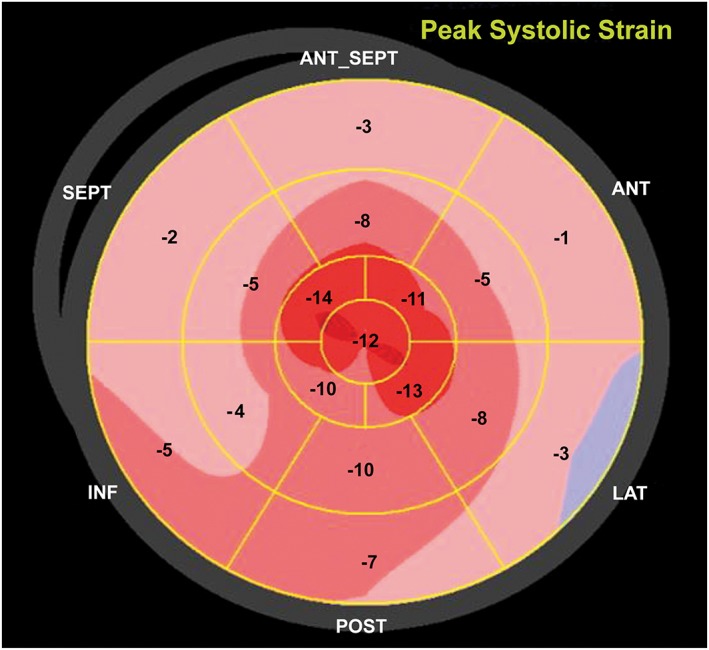
Myocardial strain assessed with two‐dimensional speckle‐tracking echocardiography shows severe peak systolic longitudinal strain abnormality (−6.7%).
The patient was admitted for diagnosis of new‐onset heart failure, and subsequent evaluation revealed normal coronary angiogram, iron panel, and thyroid studies and negative blood viral panel (human immunodeficiency virus, Lyme disease, and hepatitis) and urine toxicology panel for illicit drugs. B‐type natriuretic peptide was 303 pg/mL (normal < 100 pg/mL), and high‐sensitivity troponin was 0.05 ng/mL (normal < 0.05 ng/mL) on initial presentation.
The patient was referred to the Advanced Heart Failure Therapy program at Aurora St. Luke's Hospital, Milwaukee, WI. Upon further questioning, the patient admitted to a 3 year history of routine intramuscular androgen administration for bodybuilding. A testosterone level was obtained and documented at 30 160.0 ng/dL (normal 280–1100 ng/dL). The patient was counselled extensively about the cardiovascular risks associated with AAS use. AAS use was discontinued, and he was started on guideline‐directed medical therapy (GDMT) with comprehensive neurohormonal blockade with a focus on maximum tolerated doses of carvedilol; final doses of therapy were lisinopril 5 mg daily, spironolactone 25 mg daily, and carvedilol 25 mg twice daily. Subsequent outpatient care documented resolution of the patient's symptoms by 3 months. A repeat Doppler echocardiogram at 6 months indicated a normal LV dimension (4.5 cm) with improved cardiac function (LVEF of 53%) and LVSthd of 0.9 cm (normal < 1.2 cm), resolution of mitral regurgitation (Figure 3 A,B), and improvement in, but not normalization of, the global longitudinal strain pattern (Figure 4). Subsequent clinical testing revealed testosterone level of 603.7 ng/dL and normalization of B‐type natriuretic peptide (19 pg/mL).
Figure 3.
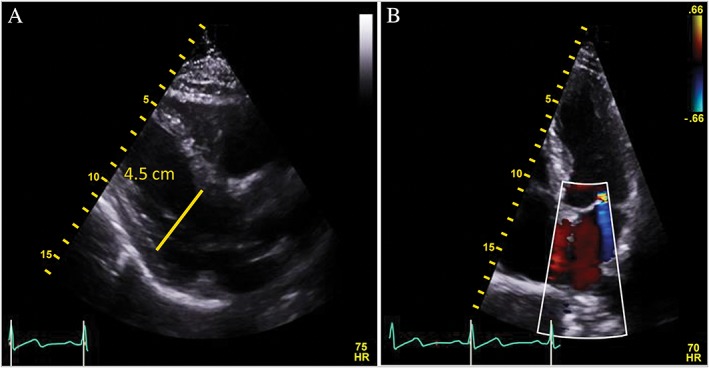
(A) Parasternal long‐axis view shows normal left ventricular size (4.5 cm) and normal left ventricular ejection fraction of 53%. (B) Colour Doppler apical four‐chamber view demonstrates trace mitral regurgitation.
Figure 4.
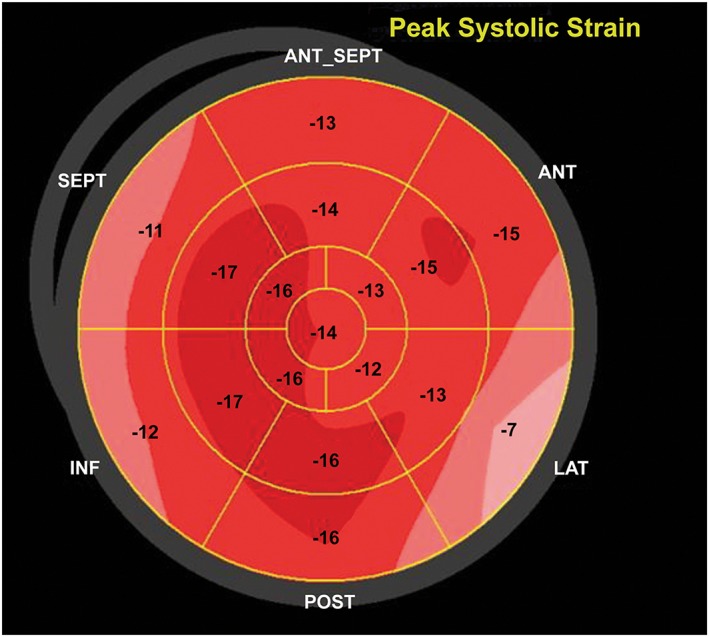
Myocardial strain assessed with two‐dimensional speckle‐tracking echocardiography shows persistent mild peak systolic longitudinal strain abnormality (−13.9%).
Discussion
Normal male physiologic levels of testosterone have been documented to be between 280 and 1100 ng/dL and may vary on the basis of age,4 race, physiologic or emotional stress, incurrent illness,5 and even time of day.6 Experimental and indirect human evidence presumes that testosterone is beneficial for cardiovascular health, as several studies have shown an inverse association of endogenous testosterone level and cardiovascular outcome independent of the traditional risk factors.7 Amid growing public concern and after the US Food and Drug Administration's release of a warning statement about testosterone therapy, a published literature review showed that the normal physiologic testosterone level is beneficial to the cardiovascular health in men and that its deficiency is associated with unfavourable metabolic profile and increased cardiovascular disease events.8
Exogenous AAS use is a growing public health concern among young athletes; estimates indicate that 3–4 million Americans use AAS, some of which include testosterone preparations.1 Access to various AAS formulations is unregulated, and these formulations can have active metabolites that are 5–15 times more potent than standard testosterone replacement therapies. Animal studies have demonstrated that exogenous AAS administration has deleterious cardiovascular effects including indirect neurohormonal activation and direct androgenic receptor stimulation resulting in hypertension, myocyte hypertrophy and extracellular fibrosis, apoptotic cell death, premature coronary artery disease, and arrhythmogenesis.9 Multiple reports of exogenous AAS have been linked with adverse cardiovascular outcome in humans, and long‐term testosterone use may lead to hypertension10 and stroke, cardiac diastolic and systolic dysfunction,2 coronary artery disease, arrhythmias, and sudden death.3
The diagnosis of AAS‐induced cardiomyopathy requires a thorough history and physical, and exclusion of other common causes of cardiomyopathy. Finally, a high index of suspicion regarding conditioned athletes with cardiovascular disease should prompt questions about exogenous AAS use, and the measurement of blood levels may be diagnostic. Discontinuation of AAS use and the initiation of GDMT are advocated and, as demonstrated in our case, may normalize cardiac structure and function. Further studies are warranted to fully investigate the long‐term use of AAS and its cardiovascular adverse effects.
We advocate medical societies to raise public awareness of the detrimental cardiovascular effects of AAS use.
Conflict of interest
None declared.
Funding
None.
Doleeb, S. , Kratz, A. , Salter, M. , and Thohan, V. (2019) Strong muscles, weak heart: testosterone‐induced cardiomyopathy. ESC Heart Failure, 6, 1000–1004. 10.1002/ehf2.12494.
References
- 1. Pope HG Jr, Wood RI, Rogol A, Nyberg F, Bowers L, Bhasin S. Adverse health consequences of performance‐enhancing drugs: an endocrine society scientific statement. Endocr Rev 2014; 35: 341–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Baggish AL, Weiner RB, Kanayama G, Hudson JI, Lu MT, Hoffmann U, Pope HG Jr. Cardiovascular toxicity of illicit anabolic‐androgenic steroid use. Circulation 2017; 135: 1991–2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lichtenfeld J, Deal BJ, Crawford S. Sudden cardiac arrest following ventricular fibrillation attributed to anabolic steroid use in an adolescent. Cardiol Young 2016; 26: 996–998. [DOI] [PubMed] [Google Scholar]
- 4. Harman SM, Metter EJ, Tobin JD, Pearson J, Blackman MR. Longitudinal effects of aging on serum total and free testosterone levels in healthy men. Baltimore longitudinal study of aging. J Clin Endocrinol Metab 2001; 86: 724–731. [DOI] [PubMed] [Google Scholar]
- 5. Woolf PD, Hamill RW, McDonald JV, Lee LA, Kelly M. Transient hypogonadotropic hypogonadism caused by critical illness. J Clin Endocrinol Metab 1985; 60: 444–450. [DOI] [PubMed] [Google Scholar]
- 6. Bremner WJ, Vitiello MV, Prinz PN. Loss of circadian rhythmicity in blood testosterone levels with aging in normal men. J Clin Endocrinol Metab 1983; 56: 1278–1281. [DOI] [PubMed] [Google Scholar]
- 7. Budoff MJ, Ellenberg SS, Lewis CE, Mohler ER 3rd, Wenger NK, Bhasin S, Barrett‐Connor E, Swerdloff RS, Stephens‐Shields A, Cauley JA, Crandall JP, Cunningham GR, Ensrud KE, Gill TM, Matsumoto AM, Molitch ME, Nakanishi R, Nezarat N, Matsumoto S, Hou X, Basaria S, Diem SJ, Wang C, Cifelli D, Snyder PJ. Testosterone treatment and coronary artery plaque volume in older men with low testosterone. JAMA 2017; 317: 708–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Elagizi A, Kohler TS, Lavie CJ. Testosterone and cardiovascular health. Mayo Clin Proc 2018; 93: 83–100. [DOI] [PubMed] [Google Scholar]
- 9. Rieder RF, Wolf DJ, Clegg JB, Lee SL. Rapid postsynthetic destruction of unstable haemoglobin Bushwick. Nature 1975; 254: 725–727. [DOI] [PubMed] [Google Scholar]
- 10. Kuipers H, Wijnen JA, Hartgens F, Willems SM. Influence of anabolic steroids on body composition, blood pressure, lipid profile and liver functions in body builders. Int J Sports Med 1991; 12: 413–418. [DOI] [PubMed] [Google Scholar]


