Abstract
Aims
Epidemiological heart failure (HF) data in the era of natriuretic peptides and echocardiography are scarce. The primary aim of this study is to evaluate the HF prevalence in the general population. We will also investigate natriuretic peptide cut‐off for diagnosis of HF. Finally, we will be able to identify left ventricular function phenotypes and study relations between cardiac function, clinical presentation, and health‐related quality of life.
Methods and results
Screening Of adult urBan pOpulation To diAgnose Heart Failure (SOBOTA‐HF) is a cross‐sectional prevalence study in a representative sample of Murska Sobota residents aged 55 years or more. Individuals will be invited to attend screening visit with point‐of‐care N‐terminal pro‐b‐type natriuretic peptide (NT‐proBNP) testing. All subjects with NT‐proBNP ≥ 125 pg/mL will be invited for a diagnostic visit that will include history and physical examination, electrocardiogram, echocardiography, blood and urine sampling, ankle brachial index, pulmonary function tests, body composition measurement, physical performance tests, and questionnaires. To validate the screening procedure, a control group (NT‐proBNP < 125 pg/mL) will undergo the same diagnostic evaluation. An external centre will validate echocardiography results, and the HF diagnosis will be adjudicated within an international HF expert panel. Overall and age‐specific HF prevalence will be calculated in individuals ≥ 55 years and extrapolated to the whole population.
Conclusions
The SOBOTA‐HF study will test the latest HF guideline diagnostic criteria in the general population sample. Next to HF prevalence, it will provide insight into left ventricular function and general patient phenotype; we will also extend current understanding of natriuretic peptides for HF screening.
Keywords: Heart failure, Prevalence, Epidemiology, Rationale and Design, Natriuretic peptide
Introduction
Chronic diseases are major public health problem and represent an ever increasing burden for the healthcare systems. Heart failure (HF) is one of the leading chronic diseases that is estimated to affect 26 million people worldwide.1, 2, 3 HF is not only an issue in the developed societies but affects world globally.4, 5, 6, 7 Patients with HF have co‐morbidities, are frequently hospitalized, and have poor survival.8, 9, 10, 11, 12, 13, 14, 15, 16
It is postulated that overall HF prevalence in developed countries is 1–2% and increases with age to more than 10% in people over 85 years, while being extremely rare in young.1, 2 Through better management of acute cardiac conditions and due to population ageing, HF is the only cardiovascular condition with increasing prevalence.1 Despite HF is an unmet medical need, the epidemiology remains under‐investigated. Most studies focused on hospitalization burden, while only few studies evaluated the prevalence and/or incidence of HF. Furthermore, most studies were performed in an era when natriuretic peptide screening was not established and left ventricular phenotypes were different than today. The available data therefore can be challenged for diagnostic reliability as per the latest guidelines and thus for current daily practice representativeness. To the best of our knowledge, only one study that evaluated the prevalence of HF in the general population in Europe was published in the last decade.17 In this study, a random sample of 5940 residents aged 65–84 years was invited for physical examination, N‐terminal pro‐b‐type natriuretic peptide (NT‐proBNP) assessment, electrocardiography, and echocardiography. Within responders (2001—34% of the total sample), HF prevalence was 6.7%, and majority (4.9%) had HF with preserved ejection fraction (HFpEF).
With limited information about key epidemiological aspects of HF, the Screening Of adult urBan pOpulation To diAgnose Heart Failure (SOBOTA‐HF) study was conceived. The primary aim is to evaluate the prevalence of HF in the general population using the latest European Society of Cardiology (ESC) guidelines on HF. We will also identify left ventricular function phenotypes and perform comprehensive patient evaluation to study relations between cardiac function, clinical presentation, and health‐related quality of life. Finally, we will get insight into NT‐proBNP distribution in the general population and potential correlations with renal function, co‐morbidity and medications as well as diagnostic performance of different cut‐offs for HF diagnosis.
Methods
Study design
SOBOTA‐HF is a cross‐sectional observational study in a representative sample of urban population in Murska Sobota, Slovenia, aged 55 years or more. Individuals will be invited to participate in a screening for NT‐proBNP concentration and collection of basic medical history data. All subjects with NT‐proBNP ≥ 125 pg/mL will be invited for a diagnostic visit that will include history and physical examination, electrocardiogram (ECG), echocardiography, ankle brachial index, pulmonary function tests, body composition measurement, physical performance tests, and questionnaires. To validate the screening procedure, a control group (NT‐proBNP < 125 pg/mL) will undergo the same diagnostic evaluation (Figure 1 ). An external centre will validate echocardiography exams, and the HF diagnosis will be adjudicated within an independent international HF expert panel.
Figure 1.
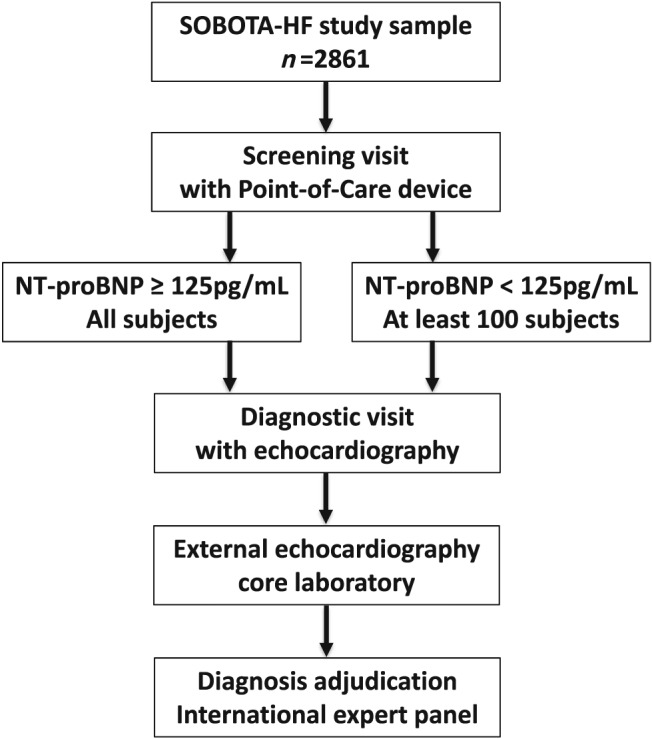
Study flow and plan. NT‐proBNP, N‐terminal pro‐b‐type natriuretic peptide; SOBOTA‐HF, Screening Of adult urBan pOpulation To diAgnose Heart Failure.
All participants in the SOBOTA‐HF study will give their written informed consent for participation in the study prior to study‐related procedures. The study protocol was evaluated and approved by the National Medical Ethics Committee (Approval No. 0120‐656/2016), and the study will be conducted in accordance with the Declaration of Helsinki. SOBOTA‐HF study is registered with ClinicalTrials.gov (NCT03526601).
Study population and enrolment
The Statistical Office of the Republic of Slovenia provided a 60% representative sample of permanent residents aged 55 years or more in Murska Sobota, Slovenia. The SOBOTA‐HF study will consider 2861 individuals for study‐related procedures using the information as provided after the standard sampling protocol that used January 2017 population data.
Personalized invitation letters for enrolment in the screening visits were sent to all individuals from the sample. In case of non‐response, two additional invitation letters were sent for the screening visit. Invitation process was supported by an extensive local media campaigns and advertisement.
Screening visit
After being invited for study participation, screening visits could be attended during workdays throughout the study duration in the Community Health Centre Murska Sobota or the General Hospital Murska Sobota.
At the screening visit, a venous blood sample was drawn to measure NT‐proBNP using point‐of‐care device (Cobas h 232 POC System; Roche CARDIAC proBNP+). In addition, participants completed a questionnaire about HF symptoms, diseases, and medication. Plasma samples were prepared and stored at −80 °C.
Overall, 1851 individuals or 64.7% of the study sample attended the screening visit; this corresponds to 38.8% of all Murska Sobota inhabitants aged 55 years or more. They were comparable with the overall study sample in terms of age (68.1 ± 8.0 vs. 68.4 ± 8.9 years) and proportion of women (60 vs. 58%). Figure 2 presents proportions of subjects in the study sample and among the screening visit participants per age and sex. Within respondents, we recorded lower proportion in the oldest age category and in the youngest men category than in the overall study sample; nonetheless, with high response rate and sample stratification per age and sex, respondents remain representative of the study population.
Figure 2.
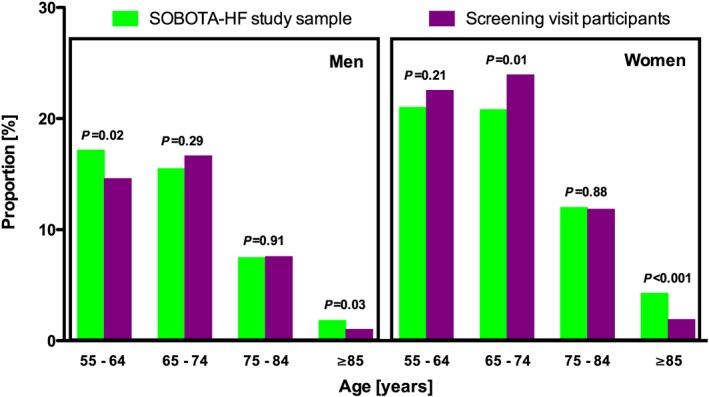
Screening Of adult urBan pOpulation To diAgnose Heart Failure (SOBOTA‐HF) study sample and screening visit participants.
Diagnostic visit
All participants with NT‐proBNP ≥ 125 pg/mL will be individually contacted and invited to attend the diagnostic visit in the General Hospital Murska Sobota. Additionally, at least 100 randomly selected subjects with normal levels of NT‐proBNP (< 125 pg/mL) will be invited to undergo the same evaluation. During the diagnostic visit, participants' blood and urine will be collected. Detailed examination including history and physical examination, echocardiography, other tests (ECG, ankle brachial index, pulmonary function tests, and body composition measurement), physical performance tests, and questionnaires will be performed (Table 1).
Table 1.
Test and procedures performed at a diagnostic visit
| Blood and urine sample collection and storage at −80 °C |
|---|
| History and physical examination |
| ‐History of diseases and interventions |
| ‐Symptoms and signs of heart failure |
| ‐Standard measurements (blood pressure, heart rate, height, weight, and waist and hip circumference) |
| ‐Medication |
| Transthoracic echocardiography |
|---|
| Other tests |
| ‐12‐lead ECG and high‐resolution ECG |
| ‐Ankle brachial index measurement |
| ‐Pulmonary function tests |
| ‐Body composition measurement |
| Physical activity tests |
| ‐6MWT |
| ‐SPPB |
| ‐Handgrip test |
| Questionnaires |
| ‐Health‐related quality of life (SF‐12 and EQ‐5D) |
| ‐Depression assessment (PHQ‐9) |
| ‐Nutritional assessment (Mini Nutritional Assessment) |
| ‐General well‐being (SWLS) |
6MWT, 6 min walk test; ECG, electrocardiogram; EQ‐5D, EuroQol‐5 Dimension; PHQ‐9, Patient Health Questionnaire 9 Item; SF‐12, 12‐Item Short Form Survey; SPPB, Short Physical Performance Battery; SWLS, Satisfaction With Life Scale.
Blood and urine collection and storage
Blood and urine samples will be collected for all patients for immediate analysis in local laboratory. Biological samples (full blood, serum, plasma, and urine) will be stored at −80 °C within 2 h of collection and will be analysed in reference laboratories.
History and physical examination
All participants will be examined by a physician who will conduct the standardized interview protocol. Age, sex, positive family history for ischaemic heart disease, history of disease (myocardial infarction, angina pectoris, arterial hypertension, HF, stroke or transient ischaemic attack, chronic obstructive pulmonary disease, diabetes mellitus, chronic kidney failure, anaemia, iron deficiency, malignant disease, and others), history of interventions (coronary artery bypass surgery, percutaneous coronary intervention, and implanted pacemaker or cardioverter–defibrillator), symptoms for HF (dyspnoea scale, New York Heart Association class, tiredness, orthopnoea, paroxysmal nocturnal dyspnoea, oedema of lower extremities, nicturia, angina pectoris, and claudication), medication (diuretic, nitrates, beta‐blocker, angiotensin‐converting enzyme inhibitor or angiotensin receptor blocker, mineralocorticoid receptor antagonist, digoxin, anti‐platelet therapy, anticoagulation therapy, calcium antagonist, statins, pulmonary disease therapy, and antidiabetic therapy), and HF signs (skin pallor, central venous pressure, heart and lung auscultation, heart apex palpation, palpation of pulses, congestive hepatopathy, and the presence of oedema) will be evaluated, and blood pressure, heart rate, height, weight, waist, and hip circumference will be measured. Likelihood for HF will be evaluated twice during the patient assessment: with findings of history and physical examination and with results of NT‐proBNP. Assessment will be performed in accordance with the ESC guidelines.3
Transthoracic echocardiography
Echocardiography is the standard tool for heart function assessment and is the key for the diagnosis of HF. The ESC and European Association of Cardiovascular Imaging standards18 will be applied as appropriate. Echocardiography will be performed using GE Vivid S70 (GE Healthcare, Chicago, IL, USA) by experienced cardiologist using standardized protocol (Supporting Information, Table S1 ). Participants will be in left lateral decubitus position with left arm resting under the head and standard views (parasternal long and short axes in different sections, apical four‐chamber, five‐chamber, two‐chamber, and three‐chamber views and substernal views), and loops will be recorded using cardiac probe. Size of cardiac chambers, valve function, left ventricular systolic and diastolic function, and Doppler measurements across valves will be assessed and saved on work station. All measurements and loops will be analysed offline, and findings will be sent to core echocardiographic laboratory (Department of Cardiology, University Clinical Hospital Centre Zemun, Zemun, Serbia) for validity analysis. Left ventricular systolic function phenotypes will be based on left ventricular ejection fraction (LVEF) determined by biplane Simpson rule to group patients in those with HF with reduced ejection fraction (< 40%), mid‐range ejection fraction (40–49%), and preserved ejection fraction (≥ 50%). Relevant structural heart disease (left ventricular mass index and left atrial enlargement) and diastolic dysfunction and left ventricular diastolic function (evaluated with transmittal flow and tissue Doppler imaging) will be performed according to the ESC guidelines.3
Other tests
Electrocardiogram
Standard and high‐resolution 12‐lead ECG using Cardiax PC ECG (IMED Ltd., Budapest, Hungary) will be recorded after 15 min rest in supine position, and subjects will be asked to relax, breathe normally, refrain from moving and talking, but to keep awake during the procedure. ECG electrodes will be placed in standard positions, and 8, 32, and 300 s recordings will be saved for processing and analysis as described previously.19
Ankle brachial index
Blood pressure from right arm and both ankles will be measured using appropriate cuffs, and ankle brachial index will be calculated for each extremity. The automated oscillometric device will be used (MESI ABP MD, MESI, Ljubljana, Slovenia) according to the manufacturer's instructions. This device is based on simultaneous plethysmographic measurements and uses one conical cuff placed on the right or left arm and two conical cuffs placed on each of the ankles. Simultaneous cuff inflation and deflation at the same pressure level is performed, and heart beat is detected based on plethysmographic signal oscillations. The respective blood pressures and ankle brachial index are then calculated using specific proprietary algorithms. The ankle cuffs measure oscillometric signals over the whole ankle, and hence, the highest systolic blood pressure of all arteries within the ankle is detected.20
Pulmonary function test
Forced vital capacity and forced expiratory volume in 1 s (FEV1) will be measured (Micro Lab, Micro Medical Ltd, Rochester, England). Up to three tests will be performed or until two measurements of FEV1 are within 150 mL. The forced vital capacity and FEV1 from the best measurement will be used, and FEV1/FEV ratio will be calculated.
Body composition measurement
Body composition will be assessed using bioimpendance measurement with Bodystat 1500 (Bodystat Ltd, Douglas, Isle of Man, British Isles, UK) in supine position after 10 min rest, without shoes and stockings. Electrodes are connected to the hands (wrist and middle finger) and feet (ankle and above the knuckle of the toe), after the areas are cleaned with alcohol. Using height, weight, and sex, body composition (body fat, lean body mass, and total body water) will be calculated from impedance at different voltage.21
Physical performance tests
We will perform 6 min walk test, short physical performance battery, and handgrip test. The 6 min walk test will be performed on a flat surface. We will measure the distance walked in 6 min and Borg dyspnoea score (from 0 to 10), heart rate, and blood oxygen saturation at test start and end. Short physical performance battery tests consist of balance test, gait speed, and multiple chair standing that is rated using the standard scale. Handgrip force will be measured in both hands, using the JAMAR® Hydraulic Hand Dynamometer (Patterson Medical Ltd, Nottinghamshire, UK), and the best of three measurements will be used as already performed in HF patients.22
Questionnaires
Participants will complete questionnaires regarding health‐related quality of life (12‐Item Short Form Survey and EuroQol‐5 Dimension), depression (Patient Health Questionnaire 9 Item), nutrition (Mini Nutritional Assessment), and general well‐being (Satisfaction With Life Scale).21, 23, 24, 25, 26
Diagnosis adjudication
An international expert panel composed of members unrelated to study procedures will have access to study information but will be blinded to patient identity. The ESC guidelines criteria will be used for HF diagnosis adjudication.3
Data storage and statistical analysis plan
All participants' data will be saved anonymously using study IDs in an appropriately secured server. Primary endpoint will be HF diagnosis. The prevalence of HF will thereafter be calculated in the study respondents; next to population prevalence, age‐specific and sex‐specific prevalence will be calculated. With representative sample of 60% of total population aged 55 years or more, no specific sample size calculation for primary endpoint has been performed. In overall population and in each age and sex subgroup, we will calculate the prevalence of three HF phenotypes: HF with reduced ejection fraction, HF with mid‐range ejection fraction, and HF with preserved ejection fraction. These groups will be compared per patient clinical, echocardiographic, and other characteristics. Furthermore, we will extrapolate the study population prevalence to all inhabitants of Murska Sobota. Finally, the burden of co‐morbidities and their impact on health‐related quality of life will be assessed as well as diagnostic accuracy of different signs, symptoms, tests, and procedures for the diagnosis of HF with receiver operating characteristic curves, and C‐statistics and area under the curve will be calculated. Sensitivity, specificity, and negative and positive prediction values for different procedures and tests and their combinations will be calculated.
Study management
The study steering committee supervises the study execution and takes academic responsibility for the study. An independent core laboratory will validate the echocardiography investigations. An independent international expert panel will adjudicate study primary endpoint.
Discussion
Even though our understanding of HF is growing and the treatment for HF is improving, there are almost no recent reliable data regarding HF prevalence. In view of population ageing, improvements in management of acute conditions and changed strategy to diagnose HF, epidemiology figures we are usually referring to1, 2 are likely outdated. The SOBOTA‐HF study is to the best of our knowledge the first epidemiological study to apply the latest ESC HF guidelines in the general population. Also, it is one of very few general population epidemiological studies in the last decade. Therefore, it will provide contemporary information about HF prevalence and HF phenotypes using the latest definitions and current state‐of‐the art clinical approach.
In the general population, HF may remain undiagnosed in about half of HF patients and in some hospitalization, or even death is the first presentation.27, 28, 29 Because typical signs and symptoms have low specificity for diagnosing HF,30 natriuretic peptides have established their role as reliable diagnostic test to rule out HF or to refer patients for echocardiography.3 Over time, the cut‐off value had some dynamics, and in this study, we apply low value to minimize false negative cases. Nonetheless, it needs to be emphasized that natriuretic peptides are highly dependent on sex, age, and renal function; thus, it might be applicable to personalize the cut‐off value to decrease the proportion of false positives and reduce referral for echocardiography.31 In conjunction to that, it needs to be acknowledged that point‐of‐care testing may be used for decision making during the diagnostic work‐up in daily practice, which is not limited only to primary care.32, 33 The SOBOTA‐HF study database will have capacity, on top of available reports, to analyse these issues and give suggestions for early diagnostic management in suspected HF of non‐acute onset.
Our understanding of left ventricular function phenotypes has been revised with the latest HF guidelines.3, 34 A new patient group according to LVEF has been established, the HF with mid‐regional ejection fraction (40–49%) that is now interposed between those with reduced ejection fraction and those with preserved ejection fraction. The current literature lacks the validated prevalence figures in general population, but we may extrapolate figures from studies in hospitalized and specific populations that would give an estimated range between 10 and 15%. SOBOTA‐HF study will, with comprehensive transthoracic echocardiography and external independent core echocardiography laboratory centre, be able to investigate the prevalence of three HF phenotypes per LVEF. For the purposes of the primary endpoint, we will apply the ESC guidelines criteria; nonetheless, we will also adopt other criteria for diagnosis of diastolic dysfunction,35, 36 which will allow the comparison of different approaches, also in conjunction with different natriuretic peptide cut‐offs to suggest the optimal practice to be adopted in daily routine.37, 38, 39, 40, 41, 42
Such a detailed analysis of echocardiography data in subjects without HF (with both negative and positive NT‐proBNP screening) and in those with HF of different phenotypes may increase our knowledge about cardiac ageing, which is particularly relevant for diastolic function.38 Today, the natriuretic peptide cut‐off that guides further diagnostic evaluation in non‐acute onset HF is the same over entire span of LVEF.3 Herein, we need to acknowledge that underlying mechanisms of ventricular dysfunction are different as well as patient general characteristics and co‐morbidity profile. Previously, different (and generally higher) natriuretic peptide cut‐offs increased referrals for imaging. SOBOTA‐HF study will give the capacity to investigate whether there are differences between various patient phenotypes and different natriuretic peptide levels that should indicate the need for echocardiography.43, 44, 45, 46
The SOBOTA‐HF study will enable investigations of body composition, nutritional status, and physical performance in patients with HF as compared with healthy individuals. HF is frequently associated with body wasting and reduced exercise capacity22; herein, the characteristics of various HF phenotypes per LVEF are under‐investigated as well as the relations to healthy individuals of the same age; thus, our findings will be complimentary to the existing recent evidence.47, 48, 49, 50
Strengths and limitations
With almost two‐thirds of sample attending (corresponds to about 39% of the Murska Sobota inhabitants aged 55 years or more), the screening visit, and with age and sex distribution as described, the results will be representative for the studied population. Also, we will be able to extrapolate the findings to whole population. Of particular note are external echocardiography laboratory and an independent expert panel; both give reassurance about HF diagnosis validity.
A comprehensive data from the screening and diagnostic visits as well as stored blood and urine samples obtained during the SOBOTA‐HF study will be important for understanding the many presentations and phenotypes of patients with HF. Diagnosing the previously undiagnosed HF patients will allow us to evaluate the diagnostic procedure and the management of HF patients and in the future, with an additional follow‐up visits, expanding the cross‐sectional into observational cohort study.
Study committees
M.L. (chair), J.F., D.O., N.S., and S.D.A. are the SOBOTA‐HF study steering committee members.
Giuseppe Rosano (chair), Rudolf de Boer, and Dipak Kotecha are the international expert panel for heart failure diagnosis adjudication.
Conflict of interest
None declared.
Funding
The authors acknowledge the project (Epidemiology of heart failure in Slovenia: prevalence, hospitalizations and mortality, ID J3‐7405) that was financially supported by the Slovenian Research Agency. D.O. received research fellowship from the Slovenian Research Agency (Grant No. 630‐84/2015‐1). The study was also supported through an independent research grant by Roche Diagnostics International Ltd, Rotkreuz, Switzerland.
Supporting information
Supporting info item.
Table S1. Standardised protocol.
Lainscak M., Omersa D., Sedlar N., Anker S. D., and Farkas J. (2019) Heart failure prevalence in the general population: SOBOTA‐HF study rationale and design, ESC Heart Failure, 6, 1077–1084. 10.1002/ehf2.12496.
References
- 1. Bui AL, Horwich TB, Fonarow GC. Epidemiology and risk profile of heart failure. Nat Rev Cardiol 2011; 8: 30–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ponikowski P, Anker SD, AlHabib KF, Cowie MR, Force TL, Hu S, Jaarsma T, Krum H, Rastogi V, Rohde LE, Samal UC, Shimokawa H, Budi Siswanto B, Sliwa K, Filippatos G. Heart failure: preventing disease and death worldwide. ESC Heart Fail 2014; 1: 4–25. [DOI] [PubMed] [Google Scholar]
- 3. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, Falk V, González‐Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GM, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P, Authors/Task Force Members; Document Reviewers . ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 2016; 18: 891–975. [DOI] [PubMed] [Google Scholar]
- 4. Hassanein M, Abdelhamid M, Ibrahim B, Elshazly A, Aboleineen MW, Sobhy H, Nasr G, Elmesseiry F, Abdelmoniem A, Ashmawy M, Farag N, Youssef A, Elbahry A, Elrakshy Y, Sobhy M, Khairy Abdel Dayem TM, Ebeid H, Reda A, Boshra H, Saleh A, Maggioni AP. Clinical characteristics and management of hospitalized and ambulatory patients with heart failure—results from ESC heart failure long‐term registry—Egyptian cohort. ESC Heart Fail 2015; 2: 159–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Konishi M, Ishida J, Springer J, von Haehling S, Akashi YJ, Shimokawa H, Anker SD. Heart failure epidemiology and novel treatments in Japan: facts and numbers. ESC Heart Fail 2016; 3: 145–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lam CSP. Heart failure in Southeast Asia: facts and numbers. ESC Heart Fail 2015; 2: 46–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sliwa K, Davison BA, Mayosi BM, Damasceno A, Sani M, Ogah OS, Mondo C, Ojji D, Dzudie A, KouamKouam C, Suliman A, Schrueder N, Yonga G, Ba SA, Maru F, Alemayehu B, Edwards C, Cotter G. Readmission and death after an acute heart failure event: predictors and outcomes in sub‐Saharan Africa: results from the THESUS‐HF registry. Eur Heart J 2013; 34: 3151–3159. [DOI] [PubMed] [Google Scholar]
- 8. van Riet EE, Hoes AW, Wagenaar KP, Limburg A, Landman MA, Rutten FH. Epidemiology of heart failure: the prevalence of heart failure and ventricular dysfunction in older adults over time. A systematic review. Eur J Heart Fail 2016; 18: 242–252. [DOI] [PubMed] [Google Scholar]
- 9. Omersa D, Farkas J, Erzen I, Lainscak M. National trends in heart failure hospitalization rates in Slovenia 2004–2012. Eur J Heart Fail 2016; 18: 1321–1328. [DOI] [PubMed] [Google Scholar]
- 10. Gabet A, Juillière Y, Lamarche‐Vadel A, Vernay M, Olié V. National trends in rate of patients hospitalized for heart failure and heart failure mortality in France, 2000–2012. Eur J Heart Fail 2015; 17: 583–590. [DOI] [PubMed] [Google Scholar]
- 11. Dinatolo E, Sciatti E, Anker MS, Lombardi C, Dasseni N, Metra M. Updates in heart failure: what last year brought to us. ESC Heart Fail 2018; 5: 989–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Crespo‐Leiro MG, Anker SD, Maggioni AP, Coats AJ, Filippatos G, Ruschitzka F, Ferrari R, Piepoli MF, Delgado Jimenez JF, Metra M, Fonseca C, Hradec J, Amir O, Logeart D, Dahlström U, Merkely B, Drozdz J, Goncalvesova E, Hassanein M, Chioncel O, Lainscak M, Seferovic PM, Tousoulis D, Kavoliuniene A, Fruhwald F, Fazlibegovic E, Temizhan A, Gatzov P, Erglis A, Laroche C, Mebazaa A, Heart Failure Association (HFA) of the European Society of Cardiology (ESC) . European Society of Cardiology Heart Failure Long‐Term Registry (ESC‐HF‐LT): 1‐year follow‐up outcomes and differences across regions. Eur J Heart Fail 2016; 18: 613–625. [DOI] [PubMed] [Google Scholar]
- 13. Chioncel O, Lainscak M, Seferovic PM, Anker SD, Crespo‐Leiro MG, Harjola VP, Parissis J, Laroche C, Piepoli MF, Fonseca C, Mebazaa A, Lund L, Ambrosio GA, Coats AJ, Ferrari R, Ruschitzka F, Maggioni AP, Filippatos G. Epidemiology and one‐year outcomes in patients with chronic heart failure and preserved, mid‐range and reduced ejection fraction: an analysis of the ESC Heart Failure Long‐Term Registry. Eur J Heart Fail 2017; 19: 1574–1585. [DOI] [PubMed] [Google Scholar]
- 14. Canepa M, Straburzynska‐Migaj E, Drozdz J, Fernandez‐Vivancos C, Pinilla JGM, Nyolczas N, Temporelli PL, Mebazaa A, Lainscak M, Laroche C, Maggioni AP, Piepoli MF, Coats AJS, Ferrari R, Tavazzi L, ESC‐HFA Heart Failure Long‐Term Registry Investigators . Characteristics, treatments and 1‐year prognosis of hospitalized and ambulatory heart failure patients with chronic obstructive pulmonary disease in the European Society of Cardiology Heart Failure Long‐Term Registry. Eur J Heart Fail 2018; 20: 100–110. [DOI] [PubMed] [Google Scholar]
- 15. Böhm M, Robertson M, Ford I, Borer JS, Komajda M, Kindermann I, Maack C, Lainscak M, Swedberg K, Tavazzi L. Influence of cardiovascular and noncardiovascular co‐morbidities on outcomes and treatment effect of heart rate reduction with ivabradine in stable heart failure (from the SHIFT trial). Am J Cardiol 2015; 116: 1890–1897. [DOI] [PubMed] [Google Scholar]
- 16. Seferović PM, Petrie MC, Filippatos GS, Anker SD, Rosano G, Bauersachs J, Paulus WJ, Komajda M, Cosentino F, de Boer RA, Farmakis D, Doehner W, Lambrinou E, Lopatin Y, Piepoli MF, Theodorakis MJ, Wiggers H, Lekakis J, Mebazaa A, Mamas MA, Tschöpe C, Hoes AW, Seferović JP, Logue J, McDonagh T, Riley JP, Milinković I, Polovina M, van Veldhuisen DJ, Lainscak M, Maggioni AP, Ruschitzka F, McMurray JJV. Type 2 diabetes mellitus and heart failure: a position statement from the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail 2018; 20: 853–872. [DOI] [PubMed] [Google Scholar]
- 17. Mureddu GF, Agabiti N, Rizzello V, Forastiere F, Latini R, Cesaroni G, Masson S, Cacciatore G, Colivicchi F, Uguccioni M, Perucci CA, Boccanelli A, PREDICTOR Study Group . Prevalence of preclinical and clinical heart failure in the elderly. A population‐based study in Central Italy. Eur J Heart Fail 2012; 14: 718–729. [DOI] [PubMed] [Google Scholar]
- 18. Lang RM, Badano LP, Mor‐Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2015; 16: 233–270. [DOI] [PubMed] [Google Scholar]
- 19. Zupanic E, Zivanovic I, Kalisnik JM, Avbelj V, Lainscak M. The effect of 4‐week rehabilitation on heart rate variability and QTc interval in patients with chronic obstructive pulmonary disease. COPD 2014; 11: 659–669. [DOI] [PubMed] [Google Scholar]
- 20. Span M, Gersak G, Millasseau SC, Meza M, Kosir A. Detection of peripheral arterial disease with an improved automated device: comparison of a new oscillometric device and the standard Doppler method. Vasc Health Risk Manag 2016; 12: 305–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Benedik B, Farkas J, Kosnik M, Kadivec S, Lainscak M. Mini nutritional assessment, body composition, and hospitalisations in patients with chronic obstructive pulmonary disease. Respir Med 2011; 105: S38–S43. [DOI] [PubMed] [Google Scholar]
- 22. Emami A, Saitoh M, Valentova M, Sandek A, Evertz R, Ebner N, Loncar G, Springer J, Doehner W, Lainscak M, Hasenfuß G, Anker SD, von Haehling S. Comparison of sarcopenia and cachexia in men with chronic heart failure: results from the Studies Investigating Co‐morbidities Aggravating Heart Failure (SICA‐HF). Eur J Heart Fail 2018; 20: 1580–1587. [DOI] [PubMed] [Google Scholar]
- 23. Ware J, Kosinski M, Keller SD. A 12‐Item Short‐Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care 1996; 34: 220–233. [DOI] [PubMed] [Google Scholar]
- 24. EuroQol Group . EuroQol—a new facility for the measurement of health‐related quality of life. Health Policy 1990; 16: 199–208. [DOI] [PubMed] [Google Scholar]
- 25. Kroenke K, Spitzer RL, Williams JB. The PHQ‐9: validity of a brief depression severity measure. J Gen Intern Med 2001; 16: 606–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Diener E, Emmons RA, Larsen RJ, Griffin S. The Satisfaction With Life Scale. J Pers Assess 1985; 49: 71–75. [DOI] [PubMed] [Google Scholar]
- 27. Koudstaal S, Pujades‐Rodriguez M, Denaxas S, Gho JMIH, Shah AD, Yu N, Patel RS, Gale CP, Hoes AW, Cleland JG, Asselbergs FW, Hemingway H. Prognostic burden of heart failure recorded in primary care, acute hospital admissions, or both: a population‐based linked electronic health record cohort study in 2.1 million people. Eur J Heart Fail 2017; 19: 1119–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fonseca C. Diagnosis of heart failure in primary care. Heart Fail Rev 2006; 11: 95–107. [DOI] [PubMed] [Google Scholar]
- 29. Davies M, Hobbs F, Davis R, Kenkre J, Roalfe AK, Hare R, Wosornu D, Lancashire RJ. Prevalence of left‐ventricular systolic dysfunction and heart failure in the Echocardiographic Heart of England Screening study: a population based study. Lancet 2001; 358: 439–444. [DOI] [PubMed] [Google Scholar]
- 30. Fonseca C, Morais H, Mota T, Matias F, Costa C, Gouveia‐Oliveira A, Ceia F, EPICA Investigators . The diagnosis of heart failure in primary care: value of symptoms and signs. Eur J Heart Fail 2004; 6: 795–800. [DOI] [PubMed] [Google Scholar]
- 31. McDonagh TA, Holmer S, Raymond I, Luchner A, Hildebrant P, Dargie HJ. NT‐proBNP and the diagnosis of heart failure: a pooled analysis of three European epidemiological studies. Eur J Heart Fail 2004; 6: 269–273. [DOI] [PubMed] [Google Scholar]
- 32. Taylor KS, Verbakel JY, Feakins BG, Price CP, Perera R, Bankhead C, Plüddemann A. Diagnostic accuracy of point‐of‐care natriuretic peptide testing for chronic heart failure in ambulatory care: systematic review and meta‐analysis. BMJ 2018; 361: k1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gils C, Ramanathan R, Breindahl T, Brokner M, Christiansen AL, Eng Ø, Hammer IJ, Herrera CB, Jansen A, Langsjøen EC, Løkkebo ES, Osestad T, Schrøder AD, Walther L. NT‐proBNP on Cobas h 232 in point‐of‐care testing: performance in the primary health care versus in the hospital laboratory. Scand J Clin Invest 2015; 75: 602–609. [DOI] [PubMed] [Google Scholar]
- 34. Nauta JF, Hummel YM, van Melle JP, van der Meer P, Lam CSP, Ponikowski P, Voors AA. What have we learned about heart failure with mid‐range ejection fraction one year after its introduction? Eur J Heart Fail 2017; 19: 1569–1573. [DOI] [PubMed] [Google Scholar]
- 35. Nagueh SF, Smiseth OA, Appleton CP, Byrd BF 3rd, Dokainish H, Edvardsen T, Flachskampf FA, Gillebert TC, Klein AL, Lancellotti P, Marino P, Oh JK, Popescu BA, Waggoner AD. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2016; 17: 1321–1360. [DOI] [PubMed] [Google Scholar]
- 36. Reddy YNV, Carter RE, Obokata M, Redfield MM, Borlaug BA. A simple, evidence‐based approach to help guide diagnosis of heart failure with preserved ejection fraction. Circulation 2018; 138: 861–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Nauta JF, Hummel YM, van der Meer P, Lam CSP, Voors AA, van Melle JP. Correlation with invasive left ventricular filling pressures and prognostic relevance of the echocardiographic diastolic parameters used in the 2016 ESC heart failure guidelines and in the 2016 ASE/EACVI recommendations: a systematic review in patients with heart failure with preserved ejection fraction. Eur J Heart Fail 2018; 20: 1303–1311. [DOI] [PubMed] [Google Scholar]
- 38. Obokata M, Reddy YNV, Borlaug BA. The role of echocardiography in heart failure with preserved ejection fraction: what do we want from imaging? Heart Fail Clin 2019; 15: 241–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Buggey J, Alenezi F, Yoon HJ, Phelan M, DeVore AD, Khouri MG, Schulte PJ, Velazquez EJ. Left ventricular global longitudinal strain in patients with heart failure with preserved ejection fraction: outcomes following an acute heart failure hospitalization. ESC Heart Fail 2017; 4: 432–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kitada S, Kikuchi S, Tsujino T, Masuyama T, Ohte N, J‐MELODIC Study Investigators . The prognostic value of brain natriuretic peptide in patients with heart failure and left ventricular ejection fraction higher than 60%: a sub‐analysis of the J‐MELODIC study. ESC Heart Fail 2018; 5: 36–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Biering‐Sørensen T, Querejeta Roca G, Hegde SM, Shah AM, Claggett B, Mosley TH Jr, Butler KR Jr, Solomon SD. Left ventricular ejection time is an independent predictor of incident heart failure in a community‐based cohort. Eur J Heart Fail 2018; 20: 1106–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Howlett J, Comin‐Colet J, Dickstein K, Fuat A, Pölzl G, Delaney S. Clinical practices and attitudes regarding the diagnosis and management of heart failure: findings from the CORE Needs Assessment Survey. ESC Heart Fail 2018; 5: 172–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sanders‐van Wijk S, van Empel V, Davarzani N, Maeder MT, Handschin R, Pfisterer ME. Brunner‐La Rocca HP; TIME‐CHF Investigators. Circulating biomarkers of distinct pathophysiological pathways in heart failure with preserved vs. reduced left ventricular ejection fraction. Eur J Heart Fail 2015; 17: 1006–1014. [DOI] [PubMed] [Google Scholar]
- 44. D'Elia E, Vaduganathan M, Gori M, Gavazzi A, Butler J, Senni M. Role of biomarkers in cardiac structure phenotyping in heart failure with preserved ejection fraction: critical appraisal and practical use. Eur J Heart Fail 2015; 17: 1231–1239. [DOI] [PubMed] [Google Scholar]
- 45. Bettencourt P, Ferreira‐Coimbra J, Rodrigues P, Marques P, Moreira H, Pinto MJ, Guimarães JT, Lourenço P. Towards a multi‐marker prognostic strategy in acute heart failure: a role for GDF‐15. ESC Heart Fail 2018; 5: 1017–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Delepaul B, Robin G, Delmas C, Moine T, Blanc A, Fournier P, Roger‐Rollé A, Domain G, Delon C, Uzan C, Boudjellil R, Carrié D, Roncalli J, Galinier M, Lairez O. Who are patients classified within the new terminology of heart failure from the 2016 ESC guidelines? ESC Heart Fail 2017; 4: 99–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Saitoh M, Dos Santos MR, Emami A, Ishida J, Ebner N, Valentova M, Bekfani T, Sandek A, Lainscak M, Doehner W, Anker SD, von Haehling S. Anorexia, functional capacity, and clinical outcome in patients with chronic heart failure: results from the Studies Investigating Co‐morbidities Aggravating Heart Failure (SICA‐HF). ESC Heart Fail 2017; 4: 448–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Calvani R, Marini F, Cesari M, Buford TW, Manini TM, Pahor M, Leeuwenburgh C, Bernabei R, Landi F, Marzetti E. Systemic inflammation, body composition, and physical performance in old community‐dwellers. J Cachexia Sarcopenia Muscle 2017; 8: 69–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Gonzalez MC, Heymsfield SB. Bioelectrical impedance analysis for diagnosing sarcopenia and cachexia: what are we really estimating? J Cachexia Sarcopenia Muscle 2017; 8: 187–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Reijnierse EM, de Jong N, Trappenburg MC, Blauw GJ, Butler‐Browne G, Gapeyeva H, Hogrel JY, McPhee JS, Narici MV, Sipilä S, Stenroth L, van Lummel RC, Pijnappels M, Meskers CGM, Maier AB. Assessment of maximal handgrip strength: how many attempts are needed? J Cachexia Sarcopenia Muscle 2017; 8: 466–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting info item.
Table S1. Standardised protocol.


