Abstract
Aims
Heart failure (HF) is classified into three types according to left ventricular ejection fraction (EF). The effect of blood pressure (BP) on the pathogenesis of each type is assumed to be different. However, the association between the prognosis of each type of HF and abnormal BP variations assessed by ambulatory BP monitoring (ABPM), such as nocturnal hypertension and the riser pattern, remains unclear.
Methods and results
We studied 325 consecutive patients with decompensated HF who were acutely admitted to our hospital and underwent ABPM at discharge. During a mean follow‐up of 30.0 months, 52 cardiovascular and 112 all‐cause deaths occurred. The Cox proportional hazards model showed that the mean values of 24 h, awake, and sleep‐time systolic BP (SBP), and abnormal 24 h ABPM patterns, such as nocturnal hypertension and non‐dipper pattern, were not associated with either all‐cause or cardiovascular mortality in patients with HF with reduced EF (HFrEF), HF with mid‐range EF (HFmrEF), or HF with preserved EF (HFpEF), except for sleep‐time SBP in HFrEF. However, the riser pattern was a significant and independent predictor of all‐cause and cardiovascular deaths in patients with HFpEF (hazard ratio, 2.01; 95% confidence interval, 1.12–3.62; 0.0200; and hazard ratio, 2.48; 95% confidence interval, 1.08–5.90; 0.0332, respectively). Sleep‐time pulse rate was similarly decreased in both the riser and non‐riser groups.
Conclusions
The riser pattern of SBP was associated with an increased risk of adverse outcomes among patients with HFpEF but not HFrEF or HFmrEF.
Keywords: Ambulatory blood pressure monitoring, Riser pattern, Heart failure with preserved ejection fraction, Heart failure with mid‐range ejection fraction, Heart failure with reduced ejection fraction
Introduction
Heart failure (HF) is a major public health issue worldwide. Recent guidelines on acute and chronic HF classified them into three groups according to left ventricular ejection fraction (LVEF): HF with reduced EF (HFrEF; EF <40%), HF with mid‐range EF (HFmrEF; 40% ≤ EF < 50%), and HF with preserved EF (HFpEF; 50% ≤ EF). Although treatment strategies for HFrEF have significantly improved over the past two decades,1, 2, 3 its prognosis remains poor. As well, previous randomized clinical trials in patients with HFpEF have failed to show a beneficial effect of the drug therapy being trialled. Of note, patients with HFmrEF were not included in some of these earlier clinical trials.
To further improve the treatment strategy for all types of HF, the treatable risk factors for HF should be investigated. Although hypertension is one of the well‐known major risk factors for the development of HF, once HF develops, the relationship between systolic blood pressure (SBP) and the recurrence of HF is unclear. Several earlier studies have reported that lower SBP at admission is associated with a higher incidence rate of cardiovascular events, including unexpected HF admission.4, 5, 6 However, none of the guidelines for the management of hypertension or HF have provided a target SBP for any of the HF types.
Ambulatory BP monitoring (ABPM) is a useful tool to investigate the circadian rhythm of BP in individuals on no medication and to assess the effect of antihypertensive drugs on 24 h BP control in patients undergoing treatment. Recently, serial out‐of‐office ABPM was recommended for better management of hypertension.7 ABPM can also provide novel information on risk factors for cardiovascular death and HF. Several earlier reports showed that abnormal variations in the 24 h BP, such as the morning surge and nocturnal increase in SBP, are important predictors of cardiovascular events in the general population and in patients with hypertension, irrespective of BP treatment.8, 9, 10, 11, 12, 13 However, there have been only a few reports on ABPM measurements in patients with HF.14, 15, 16 Recently, Komori et al.16 reported that the riser pattern of SBP is associated with worse prognosis among patients with HFpEF but not those with HFrEF. In this study, however, HFrEF was defined as EF ≤50%, which includes HFrEF (EF <40%) and HFmrEF (40% ≤ EF < 50%). Considering the limitation of current evidence regarding the relationship between BP and the three types of HF, our aim in this study was to investigated the prognostic impact of 24 h BP variation for the three types of HF (HFrEF, HFmrEF, and HFpEF), using the data from the Nara Registry and Analyses for Heart Failure cohort study (NARA‐HF study).17, 18, 19, 20, 21, 22
Methods
Study population and data collection
The NARA‐HF was designed as a dynamic cohort study.17, 18, 19, 20, 21, 22 The study recruited 1074 consecutive patients who were emergently admitted to our department or the coronary care unit at our hospital with documented acute decompensated HF (ADHF; either acute new‐onset or acute‐on‐chronic HF) between January 2007 and December 2016. The diagnosis of HF was based on the Framingham Criteria.23 The study population included patients with HFrEF, HFmrEF, and HFpEF. Patients with acute myocardial infarction, acute myocarditis, and acute HF with acute pulmonary embolism were excluded.
The ABPM measurements started in April 2011 as part of the NARA‐HF study, and 369 of 1074 patients had ABPM performed immediately before discharge. Among them, 44 patients were excluded from the analysis because of insufficient data. Consequently, we analysed the data of 325 patients (124 with HFrEF, 71 with HFmrEF, and 130 with HFpEF). For each patient, baseline data collected included age, sex, body mass index (BMI), HF aetiology, medical history, vital signs, laboratory and echocardiographic data, and medications on admission and at discharge.
The study was approved by the Ethics Committee of Nara Medical University, and written informed consent was obtained from all patients in accordance with the Declaration of Helsinki's Ethical Principles for Medical Research Involving Human Subjects.
Blood pressure measurement
Ambulatory blood pressure monitoring was performed immediately before discharge by an automatic system using electrical cuff inflation (FB‐270; Fukuda Denshi Co., Tokyo, Japan), which recorded SBP and diastolic BP (DBP) (by the oscillometric method) and pulse rate every 30 min during daytime (6 a.m. to 9:59 p.m.) and every 60 min during night‐time (10 p.m. to 5:59 a.m.). BP measurements were expressed in millimetres of mercury (mmHg). BP measurement was performed on the side opposite the dominant arm in this study.
The 24 h BP was defined as the average value of BP measured over an entire day. We defined the awake BP as the average value of BP measurements from 7 a.m. to 8:30 p.m. and the sleep‐time BP as the average value of BP measurements from 10 p.m. to 5 a.m., as awake‐time was 6 a.m. and lights‐out time was 9 p.m. at our hospital. A minimum of 20 valid awake readings and five valid sleep readings were made to define the awake and sleep‐time BP, but all patients had significantly more valid readings. Nocturnal hypertension was defined as a sleep‐time SBP ≥120 mmHg and/or sleep‐time DBP ≥70 mmHg, based on the 2014 guidelines for the management of hypertension published by The Japanese Society of Hypertension.7 The nocturnal BP fall (%) was calculated as (awake SBP − sleep‐time SBP)/awake SBP. We classified the patients' nocturnal BP fall into the following three patterns: the dipper pattern, if the nocturnal BP fall was higher than 10%; the non‐dipper pattern, if it was between 0% and 10%; and the riser pattern, if it was <0%. Patients with an extreme dipper pattern (nocturnal BP fall higher than 20%) were combined with those having the dipper pattern for analysis due to the limited number of cases (n = 8).
Outcomes
The primary endpoints were all‐cause and cardiovascular mortality. Cardiovascular death was defined as death due to HF, myocardial infarction, sudden death, stroke, and vascular disease such as aortic dissection. We checked medical records to determine the vital status and the cause of death. When this information was unavailable in the medical records, we telephoned the patients or their families.
Statistical analysis
Continuous variables were expressed as mean ± standard deviation or median (interquartile range), and inter‐group differences were compared using Student's t‐test. Categorical variables were summarized as percentages and analysed using the χ 2 test. A Cox proportional hazards model was used to investigate the hazard ratio (HR) for all‐cause and cardiovascular deaths. Results were reported as HR, 95% confidence interval (CI), and P values. The HR for outcomes in the riser group was compared with that for the non‐riser group, which served as the reference group. JMP version 12 for Windows (SAS Institute Inc., Cary, NC) was used for all statistical analyses. P values <0.05 were considered statistically significant.
Results
Baseline characteristics in patients with heart failure with reduced ejection fraction, heart failure with mid‐range ejection fraction, and heart failure with preserved ejection fraction
The mean age of all patients with HF registered in the NARA‐HF 3 study was 73.4 ± 12.3 years, and the proportion of female patients was 42.3%. As in previous reports, the proportion of elderly and female patients was as follows: HFpEF > HFmrEF > HFrEF (Supporting Information, Table S1 ). To investigate the differences in ABPM measurements between HFrEF, HFmrEF, and HFpEF, we studied 325 patients who underwent ABPM at discharge. We classified the 325 patients with HF into HFrEF, HFmrEF, and HFpEF groups based on their LVEF and compared their baseline clinical characteristics (Supporting Information, Table S2 ). The age was younger in the HFrEF group than in the HFmrEF and HFpEF group. The proportion of female patients was higher in the HFpEF group than in the HFrEF group, with the HFmrEF group falling in between. BMI was similar among the three groups. In terms of their echocardiographic data, the LV end‐diastolic diameter and LVEF in the HFmrEF group was also in between the values in the HFrEF and HFpEF groups. Haemoglobin levels was higher in the HFrEF group than in the HFmrEF and HFpEF groups, although sodium was higher and brain natriuretic peptide (BNP) level was lower in the HFpEF group than in the HFmrEF and HFrEF groups. The estimated glomerular filtration rate (eGFR) was higher in the HFrEF group than in the HFpEF group, with the HFmrEF group as the intermediate. The prescription rate of angiotensin‐converting enzyme inhibitors (ACEis)/angiotensin receptor blockers (ARBs), beta‐blockers, mineral corticoid receptor antagonists, and diuretics at discharge was higher in the HFrEF group than in the HFpEF group, although the prescription rate of calcium blockers was lower. The rate of the prescription of all drugs for the HFmrEF group was between that of the HFrEF and HFpEF groups.
Differences in vital signs and ambulatory blood pressure monitoring among the heart failure with reduced ejection fraction, heart failure with mid‐range ejection fraction, and heart failure with reduced ejection fraction groups
Table 1 presents the vital signs and ABPM data of all three groups. At discharge, patients with HFpEF had a significantly higher SBP compared with patients with HFrEF, and patients with HFmrEF were intermediate. However, the DBP and pulse rate at discharge were similar among the three groups.
Table 1.
Vital signs and ABPM in the HFpEF, HFmrEF, and HFrEF groups
| Characteristic | HFpEF (n = 130) | HFmrEF (n = 71) | HFrEF (n = 124) | P value |
|---|---|---|---|---|
| Vital signs on admission | ||||
| SBP, mmHg | 156.9 ± 39.9 | 153.0 ± 30.7 | 141.1 ± 34.0 | 0.0032 |
| DBP, mmHg | 85.1 ± 26.8 | 90.1 ± 21.2 | 87.2 ± 26.6 | 0.2614 |
| Pulse rate, b.p.m. | 96.2 ± 29.0 | 104.9 ± 26.9 | 105.7 ± 25.5 | 0.0034 |
| Vital signs at discharge | ||||
| SBP, mmHg | 116.7 ± 18.1 | 112.8 ± 17.0 | 105.1 ± 13.6 | <0.0001 |
| DBP, mmHg | 62.2 ± 10.1 | 61.2 ± 9.5 | 62.2 ± 10.5 | 0.7801 |
| Pulse rate, b.p.m. | 70.9 ± 12.0 | 68.8 ± 10.7 | 71.6 ± 10.8 | 0.2144 |
| ABPM | ||||
| The average SBP, mmHg | ||||
| 24 h (0:00–24:00) | 118.1 ± 18.1 | 111.5 ± 16.3 | 101.9 ± 14.1 | <0.0001 |
| Awake (7:00–20:30) | 118.8 ± 17.7 | 112.0 ± 16.7 | 102.6 ± 14.1 | <0.0001 |
| Sleep‐time (22:00–5:00) | 115.6 ± 21.4 | 109.6 ± 16.9 | 99.8 ± 16.5 | <0.0001 |
| The average pulse rate, b.p.m. | ||||
| 24 h (0:00–24:00) | 69.0 ± 8.9 | 70.5 ± 10.3 | 70.7 ± 8.3 | 0.2951 |
| Awake (7:00–20:30) | 69.9 ± 8.9 | 71.6 ± 9.9 | 72.0 ± 8.8 | 0.2203 |
| Sleep‐time (22:00–5:00) | 66.5 ± 12.4 | 68.3 ± 14.3 | 66.8 ± 10.3 | 0.8533 |
| Pattern of circadian rhythm, % | ||||
| Riser pattern | 21.5 | 18.3 | 14.5 | 0.3451 |
| Non‐dipper pattern | 40.0 | 43.7 | 52.4 | 0.1301 |
| Dipper pattern | 38.5 | 38.0 | 33.1 | 0.6310 |
| Nocturnal hypertension, % | 43.1 | 33.8 | 26.6 | 0.0217 |
ABPM, ambulatory blood pressure monitoring; DBP, diastolic blood pressure; HFmrEF, heart failure with mid‐range ejection fraction; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; SBP, systolic blood pressure.
With regard to ABPM, the mean 24 h, awake, and sleep‐time SBPs were significantly higher in the HFpEF group than in the HFrEF group, and all SBP measurements in the HFmrEF group were intermediate. In contrast, there was no significant between‐group difference with regard to mean DBP and pulse rate. With respect to the pattern of circadian rhythm, the proportion of patterns was similar among the three groups. The incidence of nocturnal hypertension was higher among patients in the HFpEF group than in the HFrEF group, and that in the HFmrEF group was intermediate.
Prognosis for all patients with acute decompensated heart failure
During the mean follow‐up period of 30.0 months, 112 (34.5%) of all patients with ADHF died, 52 (16.0%) of whom were caused by cardiovascular death. We constructed a univariate Cox proportional hazards model to investigate the HR for all‐cause death among all patients. None of the average SBPs measurements in all categories (24 h, awake, and sleep‐time) were related to all‐cause or cardiovascular death. Moreover, neither nocturnal hypertension nor the circadian rhythm pattern was related to all‐cause or cardiovascular death (Figure 1A ).
Figure 1.
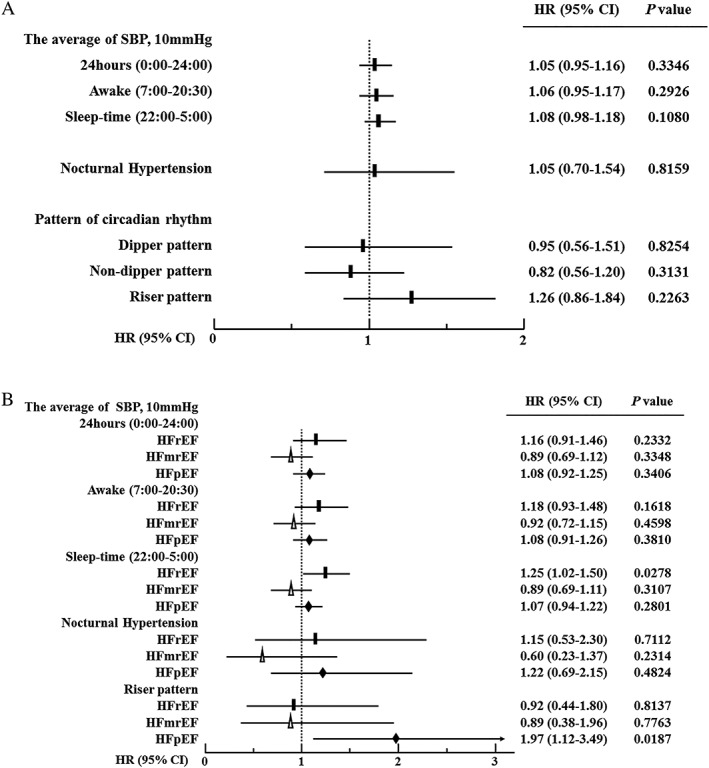
(A) Univariate HRs (95% CI) for all‐cause death in all patients. The black squares indicate the HRs for all‐cause death. The solid lines indicate the 95% CI. (B) Univariate HRs (95% CI) for all‐cause death in the HFpEF, HFmrEF, and HFrEF groups. The black rectangles, white triangles, and black diamonds indicate the HRs for all‐cause death in the HFpEF, HFmrEF, and HFrEF groups, respectively. Solid lines indicate the 95% CI. CI, confidence interval; HFmrEF, heart failure with mid‐range ejection fraction; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; HR, hazard ratio; SBP, systolic blood pressure.
Differences in prognosis among patients with heart failure with preserved ejection fraction, heart failure with mid‐range ejection fraction, and heart failure with reduced ejection fraction
Subsequently, we compared the HR for all‐cause death in the HFpEF, HFmrEF, and HFrEF groups (Figure 1B ). The mean SBPs in any category, except for night‐time in the HFrEF group, were not associated with all‐cause death. Nocturnal hypertension or non‐dipper patterns were also not related to all‐cause death in all groups. However, in the HFpEF group, the riser pattern was associated with all‐cause death but not in the HFrEF and HFmrEF groups. Similar results were observed with regard to cardiovascular death.
Comparison between the riser and non‐riser patterns
To further investigate the impact of the riser pattern on the prognosis of patients with ADHF, we divided patients in the HFpEF, HFmrEF, and HFrEF groups into two subgroups, according to their riser or non‐riser pattern (which includes the non‐dipper and dipper patterns).
We compared the baseline clinical characteristics between the riser and non‐riser groups (Table 2). Age, BMI, the proportion of female patients, and the cause of HF were similar between the two groups across all HF types. The proportions of clinical scenarios were equal between the two groups in all HF types. In terms of laboratory data at discharge, haemoglobin, eGFR, sodium, and BNP levels were similar in the two groups. With regard to antihypertensive drugs at discharge, the proportions of patients treated with ACEis/ARBs, beta‐blockers, diuretics, or calcium blockers were similar in the two groups across all HF types. In the HFpEF group, mineral corticoid receptor antagonists were more frequently administered in the non‐riser group than in the riser group.
Table 2.
Baseline characteristics in the riser and non‐riser groups
| Characteristic | HFpEF | HFmrEF | HFrEF | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Non‐riser (n = 80) | Riser (n = 50) | P value | Non‐riser (n = 44) | Riser (n = 27) | P value | Non‐riser (n = 83) | Riser (n = 41) | P value | |
| Demographic | |||||||||
| Age, years | 75.7 ± 11.0 | 75.9 ± 11.8 | 0.8368 | 75.5 ± 11.3 | 76.6 ± 10.5 | 0.7355 | 71.4 ± 12.7 | 69.0 ± 12.3 | 0.2542 |
| Female, % | 58.8 | 50.0 | 0.3292 | 50.0 | 33.3 | 0.1665 | 26.5 | 31.7 | 0.5472 |
| BMI, kg/m2 | 23.3 ± 3.4 | 23.6 ± 4.2 | 0.6677 | 22.0 ± 3.4 | 23.6 ± 4.2 | 0.6677 | 23.1 ± 3.1 | 24.1 ± 4.4 | 0.1221 |
| Cause of HF, % | |||||||||
| Ischaemic | 23.8 | 22.0 | 0.8174 | 65.9 | 51.9 | 0.2406 | 43.4 | 36.6 | 0.4684 |
| Valvular | 13.8 | 18.0 | 0.5166 | 11.4 | 11.1 | 0.9739 | 8.4 | 4.9 | 0.4585 |
| Dilated cardiomyopathy | 6.3 | 4.0 | 0.5729 | 15.9 | 18.5 | 0.7768 | 36.1 | 41.5 | 0.5668 |
| Medical history, % | |||||||||
| Hypertension | 77.5 | 86.0 | 0.2236 | 72.7 | 69.2 | 0.7550 | 74.1 | 85.4 | 0.1452 |
| Diabetes mellitus | 41.8 | 42.0 | 0.9796 | 52.3 | 40.7 | 0.3440 | 38.3 | 43.9 | 0.5498 |
| Dyslipidaemia | 37.3 | 48.0 | 0.2365 | 53.7 | 56.0 | 0.8529 | 40.0 | 31.7 | 0.3688 |
| Smoking | 16.3 | 22.0 | 0.4145 | 39.5 | 25.9 | 0.2381 | 31.7 | 27.5 | 0.6334 |
| Old myocardial infarction | 22.5 | 12.0 | 0.1241 | 45.5 | 29.6 | 0.1815 | 34.6 | 26.8 | 0.3827 |
| Clinical scenario (CS), % | 0.1701 | 0.3329 | 0.3191 | ||||||
| CS1 | 62.5 | 74.0 | 63.6 | 55.6 | 44.6 | 56.1 | |||
| CS2 | 28.8 | 24.0 | 36.4 | 40.7 | 45.8 | 31.7 | |||
| CS3 | 8.8 | 2.0 | 0.0 | 3.7 | 9.6 | 12.2 | |||
| NYHA class on admission, % | |||||||||
| III or IV | 90.0 | 84.0 | 0.3167 | 88.6 | 100 | 0.0251 | 90.4 | 90.2 | 0.9834 |
| Echocardiographic parameters | |||||||||
| LVEF, % | 63.5 ± 8.2 | 63.1 ± 6.8 | 0.7033 | 44.6 ± 2.9 | 44.7 ± 2.5 | 0.9430 | 29.4 ± 6.8 | 30.1 ± 6.7 | 0.5389 |
| LVEDD, mm | 46.4 ± 6.4 | 47.3 ± 6.0 | 0.6175 | 52.6 ± 6.8 | 53.1 ± 7.1 | 0.8708 | 59.7 ± 9.4 | 62.3 ± 8.7 | 0.3405 |
| Laboratory data at discharge | |||||||||
| Haemoglobin, g/dL | 11.3 ± 1.9 | 11.0 ± 1.9 | 0.2751 | 11.2 ± 1.7 | 11.1 ± 1.7 | 0.6826 | 12.3 ± 2.0 | 12.7 ± 2.4 | 0.3650 |
| eGFR, mL/min/1.73m2 | 40.7 ± 23.2 | 36.1 ± 22.6 | 0.3115 | 45.2 ± 18.9 | 40.6 ± 15.9 | 0.3255 | 47.2 ± 23.2 | 49.2 ± 27.0 | 0.8776 |
| Sodium, mmol/L | 138.0 ± 3.5 | 138.8 ± 3.6 | 0.0993 | 136.9 ± 3.2 | 138.0 ± 3.6 | 0.3467 | 137.5 ± 3.7 | 137.8 ± 3.1 | 0.8259 |
| Plasma BNP, pg/mLa | 196 (113–377) | 183 (114–345) | 0.9765 | 322 (104–574) | 335 (199–697) | 0.2469 | 288 (178–539) | 327 (209–513) | 0.9498 |
| Medication at discharge, % | |||||||||
| ACEi or ARBs | 77.5 | 80.0 | 0.7350 | 88.4 | 85.2 | 0.7002 | 92.7 | 97.6 | 0.2384 |
| MRAs | 37.5 | 18.0 | 0.0157 | 32.6 | 33.3 | 0.9464 | 47.7 | 51.2 | 0.7020 |
| Beta‐blockers | 61.3 | 62.0 | 0.9318 | 81.4 | 74.1 | 0.4707 | 90.2 | 92.7 | 0.6500 |
| Ca channel blockers | 41.8 | 56.0 | 0.1145 | 19.1 | 22.2 | 0.7499 | 7.4 | 2.4 | 0.2326 |
| Diuretics | 83.8 | 76.0 | 0.2797 | 79.1 | 92.6 | 0.1134 | 89.0 | 85.4 | 0.5638 |
ACEi, angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; beta‐blocker, beta‐adrenergic receptor blocker; BMI, body mass index; BNP, B‐type natriuretic peptide; Ca, calcium; EDD, end‐diastolic diameter; EF ejection fraction; eGFR, estimated glomerular filtration rate; HF, heart failure; HFmrEF, heart failure with mid‐range ejection fraction; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure reduced with ejection fraction; LV, left ventricular; MRA, mineralocorticoid receptor antagonist; NYHA, New York Heart Association; PRA, plasma renin activity.
Data are shown as percentages, means ± standard deviation, or medians (25th and 75th percentile).
The differences in ambulatory blood pressure monitoring between the riser and non‐riser patterns
Figure 2 and Table 3 show the BP profiles during the 24 h of ABPM in the HFpEF, HFmrEF, and HFrEF groups. Sleep‐time SBP was significantly higher in the riser group than in the non‐riser group across all HF types. The elevation of sleep‐time SBP was significantly higher among patients in the HFpEF group than either in the HFrEF or HFmrEF groups. However, the sleep‐time pulse rate was similarly decreased in both the riser and non‐riser groups across all HF types. Consequently, the sleep‐time pulse rate in the riser group was similar to that in the non‐riser group among all patients with HF.
Figure 2.
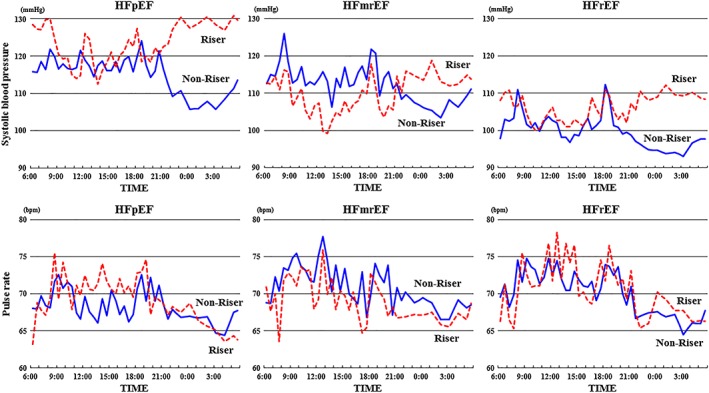
The SBP and pulse rate profiles over 24 h of ABPM in the HFpEF, HFmrEF, and HFrEF groups. The dotted line is the riser pattern, and the solid line is the non‐riser pattern. CI, confidence interval; HFmrEF, heart failure with mid‐range ejection fraction; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; HR, hazard ratio; SBP, systolic blood pressure.
Table 3.
Vital signs at discharge and ABPM in the riser and non‐riser groups
| Characteristic | HFpEF | HFmrEF | HFrEF | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Non‐riser (n = 80) | Riser (n = 50) | P value | Non‐riser (n = 44) | Riser (n = 27) | P value | Non‐riser (n = 83) | Riser (n = 41) | P value | |
| Vital signs on admission | |||||||||
| SBP, mmHg | 154.2 ± 41.0 | 161.1 ± 38.1 | 0.3549 | 154.8 ± 30.8 | 150.3 ± 30.8 | 0.4957 | 140.3 ± 35.2 | 142.8 ± 31.7 | 0.5662 |
| DBP, mmHg | 83.6 ± 25.3 | 87.5 ± 29.1 | 0.5092 | 91.7 ± 20.4 | 87.5 ± 22.6 | 0.3522 | 86.9 ± 28.7 | 87.9 ± 21.9 | 0.5412 |
| Pulse rate, b.p.m. | 95.4 ± 28.6 | 97.5 ± 29.9 | 0.4481 | 107.3 ± 27.3 | 101.0 ± 26.3 | 0.5339 | 104.6 ± 23.8 | 108.0 ± 28.8 | 0.9259 |
| Vital signs at discharge | |||||||||
| SBP, mmHg | 115.0 ± 17.5 | 119.4 ± 18.8 | 0.2435 | 114.3 ± 17.9 | 110.2 ± 15.4 | 0.3370 | 105.2 ± 13.8 | 105.0 ± 13.4 | 0.7249 |
| DBP, mmHg | 61.1 ± 9.2 | 64.1 ± 11.3 | 0.1046 | 61.8 ± 9.9 | 60.3 ± 9.0 | 0.4091 | 62.1 ± 10.6 | 62.4 ± 10.4 | 0.9336 |
| Pulse rate, b.p.m. | 69.5 ± 12.2 | 73.2 ± 11.5 | 0.0359 | 69.0 ± 11.2 | 68.4 ± 10.1 | 0.8122 | 72.3 ± 11.5 | 70.2 ± 9.1 | 0.3958 |
| ABPM | |||||||||
| Average SBP, mmHg | |||||||||
| 24 h (0:00–24:00) | 115.4 ± 17.8 | 122.5 ± 17.8 | 0.0171 | 112.8 ± 17.5 | 109.3 ± 14.2 | 0.4344 | 100.2 ± 13.7 | 105.4 ± 14.5 | 0.0422 |
| Awake (7:00–20:30) | 117.8 ± 18.2 | 120.3 ± 16.8 | 0.2935 | 114.8 ± 17.8 | 107.5 ± 14.0 | 0.0903 | 102.0 ± 14.2 | 103.8 ± 14.1 | 0.4966 |
| Sleep‐time (22:00–5:00) | 107.6 ± 17.9 | 128.4 ± 20.4 | <0.0001 | 106.8 ± 17.5 | 114.2 ± 15.0 | 0.0460 | 94.9 ± 13.8 | 109.9 ± 17.1 | <0.0001 |
| Average pulse rate, b.p.m. | |||||||||
| 24 h (0:00–24:00) | 68.6 ± 9.3 | 69.6 ± 8.3 | 0.4833 | 71.4 ± 10.2 | 69.1 ± 10.6 | 0.4379 | 70.6 ± 7.5 | 70.8 ± 9.8 | 0.9091 |
| Awake (7:00–20:30) | 69.3 ± 9.3 | 71.0 ± 8.2 | 0.2056 | 72.6 ± 9.5 | 70.0 ± 10.5 | 0.1653 | 72.0 ± 7.9 | 72.0 ± 10.5 | 0.8442 |
| Sleep‐time (22:00–5:00) | 66.5 ± 12.7 | 66.5 ± 12.0 | 0.9962 | 69.2 ± 15.7 | 66.8 ± 11.8 | 0.8590 | 66.6 ± 9.8 | 67.4 ± 11.4 | 0.9217 |
ABPM, ambulatory blood pressure monitoring; DBP, diastolic blood pressure; HFmrEF, heart failure with mid‐range ejection fraction; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; SBP, systolic blood pressure.
Data shown as mean ± standard deviation.
Prognosis and outcome
Figure 3 shows the Kaplan–Meier survival curves for the HFpEF, HFmrEF, and HFrEF groups. With regard to the HFpEF group, the survival rate was significantly lower in the riser group than in the non‐riser group, for all‐cause death (log‐rank 0.0159; Figure 3A ) and cardiovascular death (log‐rank 0.0172; Figure 3B ). In contrast, both all‐cause and cardiovascular deaths were similar between the riser group and the non‐riser group in patients with HFmrEF (log‐rank 0.7773 and log‐rank 0.2175; Figure 3C, D ) and patients with HFrEF (log‐rank 0.8145 and log‐rank 0.4147; Figure 3E, F ).
Figure 3.
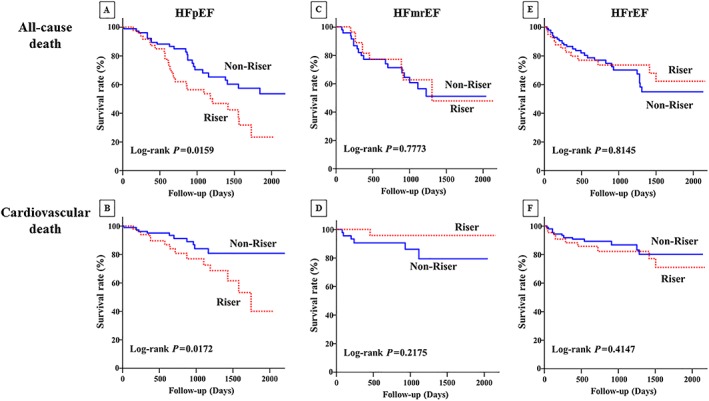
Kaplan–Meier event‐free survival curves for (A) all‐cause death and (B) cardiovascular death in patients with HFpEF, (C) all‐cause death and (D) cardiovascular death in patients with HFmrEF, and (E) all‐cause death and (F) cardiovascular death in patients with HFrEF in the riser group (dotted line) compared with the non‐riser group (solid line). HFmrEF, heart failure with mid‐range ejection fraction; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction.
Table 4 shows the unadjusted and adjusted HRs of outcomes for the riser and non‐riser groups. Among patients in the HFpEF group, the unadjusted HRs for all‐cause and cardiovascular deaths were significantly higher in the riser group than in the non‐riser group (HR, 1.97; 95% CI, 1.12–3.49; 0.0187; and HR, 2.60; 95% CI, 1.16–6.06; 0.0206, respectively). Even after adjustment for covariates (age, haemoglobin, eGFR, and BNP at discharge) in the multivariable Cox proportional hazards model, the riser pattern among patients in the HFpEF group remained an independent predictor of all‐cause and cardiovascular deaths (HR, 2.01; 95% CI, 1.12–3.62; 0.0200; and HR, 2.48; 95% CI, 1.08–5.90; 0.0332, respectively).
Table 4.
Unadjusted and adjusted HRs for adverse outcomes in the riser and non‐riser groups
| All‐cause death | Cardiovascular death | |||||
|---|---|---|---|---|---|---|
| Non‐riser | Riser | P value | Non‐riser | Riser | P value | |
| HFpEF | ||||||
| Unadjusted HR (95% CI) | 1 | 1.97 (1.12–3.49) | 0.0187 | 1 | 2.60 (1.16–6.06) | 0.0206 |
| Adjusted HR (95% CI) | 1 | 2.01 (1.12–3.62) | 0.0200 | 1 | 2.48 (1.08–5.90) | 0.0332 |
| HFmrEF | ||||||
| Unadjusted HR (95% CI) | 1 | 0.89 (0.38–1.96) | 0.7763 | 1 | 0.28 (0.02–1.68) | 0.1850 |
| Adjusted HR (95% CI) | 1 | 0.69 (0.28–1.64) | 0.4044 | 1 | 0.21 (0.01–1.95) | 0.2078 |
| HFrEF | ||||||
| Unadjusted HR (95% CI) | 1 | 0.92 (0.45–1.80) | 0.8137 | 1 | 1.43 (0.58–3.39) | 0.4224 |
| Adjusted HR (95% CI) | 1 | 1.21 (0.57–2.45) | 0.6048 | 1 | 2.16 (0.84–5.45) | 0.1085 |
CI, confidence interval; HFmrEF, heart failure with mid‐range ejection fraction; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; HR, hazard ratio.
Data shown as median (25th and 75th percentile). The Cox proportional hazards model adjusted for the following covariates: age, haemoglobin, estimated glomerular filtration rate, and B‐type natriuretic peptide at discharge.
Discussion
The present study investigated the 24 h profile of BP and pulse rate in patients with ADHF and demonstrated that the riser pattern is associated with both all‐cause and cardiovascular deaths only in patients with HFpEF but not in those with HFrEF or HFmrEF. The clinical significance of the riser pattern among patients with HFmrEF is closer to that of patients with HFrEF than those of patients with HFpEF, although most baseline characteristics for patients with HFmrEF fall between those for patients with HFrEF and HFpEF.
In earlier studies, high SBP was found to be a predictor of better prognosis among patients with HFrEF and HFpEF.24, 25 However, our study failed to show an association between average values of 24 h or awake‐time SBP and better outcomes for any type of HF. In the NARA‐HF 3 study, SBP higher than 100 mmHg at discharge was also a predictor of better outcomes among patients with HFrEF but not among those with HFmrEF or HFpEF (data not shown). More interestingly, abnormal 24 h variations of SBP, such as nocturnal hypertension or non‐dipper pattern, which are associated with cardiovascular events among patients with hypertension irrespective of whether they were being treated with antihypertensive drugs26, 27, 28, were not observed in any of the groups in our study. This indicates that the clinical significance of 24 h BP variation is different between patients with hypertension and those with HF.
The riser pattern was associated with all‐cause and cardiovascular deaths only among patients with HFpEF. This finding is consistent with the earlier report by Komori et al.,16 in which patients with HF were divided into two groups of patients, HFrEF and HFpEF, and patients with HFmrEF were included in the same group as patients with HFrEF. In accordance with their findings, our results showed that the riser pattern was not associated with outcomes among patients with either HFrEF or HFmrEF. Although the mechanism underlying the association between the riser pattern and a prognosis among patients with HFpEF is currently unclear, it may be related to the fact that hypertension is more closely involved in the aetiology of HFpEF than HFrEF. Because all patients in the current study were receiving medical therapy, the mechanism of the development of the riser pattern should be interpreted with caution. In short, our results could be indicative that sleep‐time SBP is simply not well controlled by the treatment or that the intrinsic pathophysiology of HFpEF is possibly related to the development of the riser pattern. Nevertheless, the pathology of HFpEF is different from that of HFrEF and probably from that of HFmrEF as well.
Although the mechanism of the development of the riser pattern has not been clearly understood yet, it has been suggested that the disturbances in the sympathetic nervous system, the baroreceptor reflex, and volume overload are involved in its development during sleep.12 Generally, the changes in pulse rate are also well correlated with the activation of the sympathetic nervous system. However, in this study, the sleep‐time pulse rate was similarly decreased in both the riser and non‐riser groups across all types of HFs, indicative of a decrease in sympathetic nervous activity during sleep in both the riser and non‐riser groups. Therefore, it may be possible that the riser pattern results from a volume overload than being related to sympathetic nervous activity. Considering that the pulse rate in patients with hypertension and the riser pattern is also lower during sleep‐time than during awake‐time,29 the development of the riser pattern seems to partly share a common mechanism in HFpEF and hypertension.
While therapeutic strategies with medical and non‐medical treatments have been established in patients with HFrEF,30, 31, 32 no effective therapies for HFpEF has been established. In patients with HFpEF, diuretics, ACEis, and ARBs are recommended to improve symptoms or reduce HF rehospitalization. Given that the riser pattern of SBP was associated with an increased risk of adverse outcomes among patients with HFpEF, the present study may provide a new treatment strategy to attempt to better control the circadian rhythm in patients with HFpEF.
Up to now, the association between HF and sleep‐disordered breathing (SDB) has been reported. Although SDB is broadly classified into two types, obstructive sleep apnoea and central sleep apnoea (CSA), CSA is more often associated with HF.33, 34, 35, 36 In SDB, the repeated episodes of apnoea, hypoxia, re‐oxygenation, and arousal throughout the night cause further sympathetic nervous system activation. In actually, the relationship between SDB and nocturnal hypertension has been reported. In our study, it is possible that SDB may affect the riser pattern, but the relationship is unknown because there is no record of respiratory frequency and SpO2 during sleep. Moreover, it was reported that continuous positive airway pressure (CPAP) and phrenic nerve stimulation therapy improved BP or the symptoms of patients with HF with CSA,30 but no one had used CPAP at the time of ABPM in our study.
Another interesting finding of our present study is that the clinical significance of the riser pattern in patients with HFmrEF is similar to those with HFrEF. The aetiology of HFmrEF is more closely associated with that of HFrEF than with that of HFpEF. For example, the rates of ischaemic and dilated cardiomyopathy were high, while the rate of hypertensive heart disease was low. Moreover, ~40% of patients with HFmrEF and 30% of those with HFrEF had a history of myocardial infarction, but only 18% of patients with HFpEF did. These similarities in aetiology and background between HFmrEF and HFrEF may have influenced the results in this study.
The event rate in this study was slightly higher compared with the recent meta‐analysis.37 Although the reason is unclear, it may be affect by lower administration rates of beta‐blockers or higher rate of co‐morbidities, such as hypertension and diabetes mellitus, and lower eGFR.
There are several limitations to this study. First, the analysis in this study was the low power because the sample size was small. In particular, in cardiovascular death, multiple analyses mean that the significance P about 0.03 could have been a chance finding. Therefore, a large‐scale prospective study will be necessary to confirm hypothesis in this study. Second, ABPM was performed under medications that have antihypertensive effects, because safety considerations prevented halting their use. Therefore, it was not clear whether the circadian BP rhythm observed in this study was due to self‐regulation, to the effect of the medications, or to both. Third, sleep‐time was pre‐determined based on the hospital hours and not individual patterns because we could not confirm accurate wake‐up time and bedtime for each subject, which might alter the results of the circadian BP rhythm in ABPM, including nocturnal and riser patterns. Fourth, the subject included paroxysmal atrial fibrillation patients, and it is unknown whether or not it was atrial fibrillation rhythm during measurement.
In summary, the riser pattern is associated with an increased risk of adverse outcomes among patients with HFpEF but not in patients with HFrEF or HFmrEF. This finding may be related to the pathology of HFpEF and may help us to develop better treatment strategies.
Conflict of interest
All authors declare no conflict of interest.
Funding
This work was supported in part by grants‐in‐aid from the Ministry of Education, Culture, Sports, Science, and Technology and the Ministry of Health, Labor, and Welfare of Japan (Comprehensive Research on Life‐Style Related Disease including Cardiovascular Disease and Diabetes Mellitus) and the Japan Agency for Medical Research and Development (Practical Research Project for Rare/Intractable Diseases, Practical Research Project for Life‐Style related Diseases including Cardiovascular Diseases and Diabetes Mellitus, and Project Promoting Clinical Trials for Development of New Drugs).
Supporting information
Table S1. Baseline characteristics of the HFpEF, HFmrEF, and HFrEF groups.
Table S2. Baseline characteristics in the HFpEF, HFmrEF, and HFrEF groups.
Acknowledgements
The authors thank R. Morishima, Y. Wada, and Y. Kamada for their support in the data collection process.
Ueda, T. , Kawakami, R. , Nakada, Y. , Nakano, T. , Nakagawa, H. , Matsui, M. , Nishida, T. , Onoue, K. , Soeda, T. , Okayama, S. , Watanabe, M. , Okura, H. , and Saito, Y. (2019) Differences in blood pressure riser pattern in patients with acute heart failure with reduced mid‐range and preserved ejection fraction. ESC Heart Failure, 6, 1057–1067. 10.1002/ehf2.12500.
References
- 1. Bhatia RS, Tu JV, Lee DS, Austin PC, Fang J, Haouzi A, Gong Y, Liu PP. Outcome of heart failure with preserved ejection fraction in a population‐based study. N Engl J Med 2006; 355: 260–269. [DOI] [PubMed] [Google Scholar]
- 2. Somaratne JB, Berry C, McMurray JJ, Poppe KK, Doughty RN, Whalley GA. The prognostic significance of heart failure with preserved left ventricular ejection fraction: a literature‐based meta‐analysis. Eur J Heart Fail 2009; 11: 855–862. [DOI] [PubMed] [Google Scholar]
- 3. Tsutsui H, Tsuchihashi‐Makaya M, Kinugawa S. Clinical characteristics and outcomes of heart failure with preserved ejection fraction: lessons from epidemiological studies. J Cardiol 2010; 55: 13–22. [DOI] [PubMed] [Google Scholar]
- 4. Gheorghiade M, Abraham WT, Albert NM, Greenberg BH, O'Connor CM, She L, Stough WG, Yancy CW, Young BJ, Fonarow GC. Systolic blood pressure at admission, clinical characteristics, and outcomes in patients hospitalized with acute heart failure. JAMA 2006; 296: 2217–2226. [DOI] [PubMed] [Google Scholar]
- 5. Simpson J, Jhund PS, Silva Cardoso J, Martinez F, Mosterd A, Ramires F, Rizkala AR, Senni M, Squire I, Gong J, Lefkowitz MP, Shi VC, Desai AS, Rouleau JL, Swedberg K, Zile MR, McMurray JJ, Packer M, Solomon SD. Comparing LCZ696 with enalapril according to baseline risk using the MAGGIC and EMPHASIS‐HF risk scores: an analysis of mortality and morbidity in PARADIGM‐HF. J Am Coll Cardiol 2015; 66: 2059–2071. [DOI] [PubMed] [Google Scholar]
- 6. Collier TJ, Pocock SJ, McMurray JJ, Zannad F, Krum H, van Veldhuisen DJ, Swedberg K, Shi H, Vincent J, Pitt B. The impact of eplerenone at different levels of risk in patients with systolic heart failure and mild symptoms: insight from a novel risk score for prognosis derived from the EMPHASIS‐HF trial. Eur Heart J 2013; 34: 2823–2829. [DOI] [PubMed] [Google Scholar]
- 7. Shimamoto K, Ando K, Fujita T, Hasebe N, Higaki J, Horiuchi M, Imai Y, Imaizumi T, Ishimitsu T, Ito M, Ito S, Itoh H, Iwao H, Kai H, Kario K, Kashihara N, Kawano Y, Kim‐Mitsuyama S, Kimura G, Kohara K, Komuro I, Kumagai H, Matsuura H, Miura K, Morishita R, Naruse M, Node K, Ohya Y, Rakugi H, Saito I, Saitoh S, Shimada K, Shimosawa T, Suzuki H, Tamura K, Tanahashi N, Tsuchihashi T, Uchiyama M, Ueda S, Umemura S. The Japanese Society of Hypertension Guidelines for the Management of Hypertension (JSH 2014). Hypertens Res 2014; 37: 253–390. [DOI] [PubMed] [Google Scholar]
- 8. Kikuya M, Ohkubo T, Asayama K, Metoki H, Obara T, Saito S, Hashimoto J, Tosune K, Hoshi H, Satoh H, Imai Y. Ambulatory blood pressure and 10‐year risk of cardiovascular and noncardiovascular mortality: the Ohasama study. Hypertension 2005; 45: 240–245. [DOI] [PubMed] [Google Scholar]
- 9. Sega R, Facchetti R, Bombelli M, Cesana G, Corrao G, Grassi G, Mancia G. Prognostic value of ambulatory and home blood pressures compared with office blood pressure in the general population: follow‐up results from the Pressioni Arteriose Monitorate e Loro Associazioni (PAMELA) study. Circulation 2005; 111: 1777–1783. [DOI] [PubMed] [Google Scholar]
- 10. Staessen JA, Thijs L, Fagard R, O'Brien ET, Clement D, de Leeuw PW, Mancia G, Nachev C, Palatini P, Parati G, Tuomilehto J, Webster J. Predicting cardiovascular risk using conventional vs ambulatory blood pressure in older patients with systolic hypertension. Systolic Hypertension in Europe Trial Investigators. JAMA 1999; 282: 539–546. [DOI] [PubMed] [Google Scholar]
- 11. Boggia J, Li Y, Thijs L, Hansen TW, Kikuya M, Björklund‐Bodegård K, Richart T, Ohkubo T, Kuznetsova T, Torp‐Pedersen C, Lind L, Ibsen H, Imai Y, Wang J, Sandoya E, O'Brien E, Staessen JA. Prognostic accuracy of day versus night ambulatory blood pressure: a cohort study. Lancet 2007; 370: 1219–1229. [DOI] [PubMed] [Google Scholar]
- 12. de la Sierra A, Gorostidi M, Banegas JR, Segura J, de la Cruz JJ, Ruilope LM. Nocturnal hypertension or nondipping: which is better associated with the cardiovascular risk profile? Am J Hypertens 2014; 27: 680–687. [DOI] [PubMed] [Google Scholar]
- 13. O'Flynn AM, Dolan E, Curtin RJ, O'Brien E, Perry IJ, Kearney PM. Night‐time blood pressure and target organ damage: a comparative analysis of absolute blood pressure and dipping status. J Hypertens 2015; 33: 2257–2264. [DOI] [PubMed] [Google Scholar]
- 14. Shin J, Kline S, Moore M, Gong Y, Bhanderi V, Schmalfuss CM, Johnson JA, Schofield RS. Association of diurnal blood pressure pattern with risk of hospitalization or death in men with heart failure. J Card Fail 2007; 13: 656–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Berry M, Lairez O, Fourcade J, Roncalli J, Carrie D, Pathak A, Chamontin B, Galinier M. Prognostic value of systolic short‐term blood pressure variability in systolic heart failure. Clin Hypertens 2016; 22: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Komori T, Eguchi K, Saito T, Hoshide S, Kario K. Riser pattern is a novel predictor of adverse events in heart failure patients with preserved ejection fraction. Circ J 2016; 81: 220–226. [DOI] [PubMed] [Google Scholar]
- 17. Nakada Y, Kawakami R, Nakano T, Takitsume A, Nakagawa H, Ueda T, Nishida T, Onoue K, Soeda T, Okayama S, Takeda Y, Watanabe M, Kawata H, Okura H, Saito Y. Sex differences in clinical characteristics and long‐term outcome in acute decompensated heart failure patients with preserved and reduced ejection fraction. Am J Physiol Heart Circ Physiol 2016; 310: H813–H820. [DOI] [PubMed] [Google Scholar]
- 18. Ueda T, Kawakami R, Horii M, Sugawara Y, Matsumoto T, Okada S, Nishida T, Soeda T, Okayama S, Somekawa S, Takeda Y, Watanabe M, Kawata H, Uemura S, Saito Y. Noncardiovascular death, especially infection, is a significant cause of death in elderly patients with acutely decompensated heart failure. J Card Fail 2014; 20: 174–180. [DOI] [PubMed] [Google Scholar]
- 19. Ueda T, Kawakami R, Sugawara Y, Okada S, Nishida T, Onoue K, Soeda T, Okayama S, Takeda Y, Watanabe M, Kawata H, Uemura S, Saito Y. Worsening of renal function during 1 year after hospital discharge is a strong and independent predictor of all‐cause mortality in acute decompensated heart failure. J Am Heart Assoc 2014; 3: e001174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ueda T, Kawakami R, Nishida T, Onoue K, Soeda T, Okayama S, Takeda Y, Watanabe M, Kawata H, Uemura S, Saito Y. Plasma renin activity is a strong and independent prognostic indicator in patients with acute decompensated heart failure treated with renin‐angiotensin system inhibitors. Circ J 2015; 79: 1307–1314. [DOI] [PubMed] [Google Scholar]
- 21. Ueda T, Kawakami R, Nishida T, Onoue K, Soeda T, Okayama S, Takeda Y, Watanabe M, Kawata H, Uemura S, Saito Y. Left ventricular ejection fraction (EF) of 55% as cutoff for late transition from heart failure (HF) with preserved EF to HF with mildly reduced EF. Circ J 2015; 79: 2209–2215. [DOI] [PubMed] [Google Scholar]
- 22. Ueda T, Kawakami R, Horii M, Sugawara Y, Matsumoto T, Okada S, Nishida T, Soeda T, Okayama S, Somekawa S, Takeda Y, Watanabe M, Kawata H, Uemura S, Saito Y. High mean corpuscular volume is a new indicator of prognosis in acute decompensated heart failure. Circ J 2013; 77: 2766–2771. [DOI] [PubMed] [Google Scholar]
- 23. Ho KK, Pinsky JL, Kannel WB, Levy D. The epidemiology of heart failure: the Framingham Study. J Am Coll Cardiol 1993; 22: 6A–13A. [DOI] [PubMed] [Google Scholar]
- 24. Regnault V, Lagrange J, Pizard A, Safar ME, Fay R, Pitt B, Challande P, Rossignol P, Zannad F, Lacolley P. Opposite predictive value of pulse pressure and aortic pulse wave velocity on heart failure with reduced left ventricular ejection fraction: insights from an Eplerenone Post‐Acute Myocardial Infarction Heart Failure Efficacy and Survival Study (EPHESUS) substudy. Hypertension 2014; 63: 105–111. [DOI] [PubMed] [Google Scholar]
- 25. Tsimploulis A, Lam PH, Arundel C, Singh SN, Morgan CJ, Faselis C, Deedwania P, Butler J, Aronow WS, Yancy CW, Fonarow GC, Ahmed A. Systolic blood pressure and outcomes in patients with heart failure with preserved ejection fraction. JAMA Cardiol 2018; 3: 288–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kario K, Pickering TG, Matsuo T, Hoshide S, Schwartz JE, Shimada K. Stroke prognosis and abnormal nocturnal blood pressure falls in older hypertensives. Hypertension 2001; 38: 852–857. [DOI] [PubMed] [Google Scholar]
- 27. Ohkubo T, Hozawa A, Yamaguchi J, Kikuya M, Ohmori K, Michimata M, Matsubara M, Hashimoto J, Hoshi H, Araki T, Tsuji I, Satoh H, Hisamichi S, Imai Y. Prognostic significance of the nocturnal decline in blood pressure in individuals with and without high 24‐h blood pressure: the Ohasama study. J Hypertens 2002; 20: 2183–2189. [DOI] [PubMed] [Google Scholar]
- 28. Hoshide S, Kario K, Hoshide Y, Umeda Y, Hashimoto T, Kunii O, Ojima T, Shimada K. Associations between nondipping of nocturnal blood pressure decrease and cardiovascular target organ damage in strictly selected community‐dwelling normotensives. Am J Hypertens 2003; 16: 434–438. [DOI] [PubMed] [Google Scholar]
- 29. Fujii T, Uzu T, Nishimura M, Takeji M, Kuroda S, Nakamura S, Inenaga T, Kimura G. Circadian rhythm of natriuresis is disturbed in nondipper type of essential hypertension. Am J Kidney Dis 1999; 33: 29–35. [DOI] [PubMed] [Google Scholar]
- 30. Coats AJS, Shewan LG, Abraham WT. The management of co‐morbidities in patients with heart failure central sleep apnea. Int Cardiovasc Forum J 2015; 2 10.17987/icfj.v10i0.456. [DOI] [Google Scholar]
- 31. Lopatin Y, Rosano GMC. Treatment of patients in the vulnerable phase (at discharge or early after discharge). Int Cardiovasc Forum J 2017; 10: 37–40. [Google Scholar]
- 32. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, Falk V, Gonzalez‐Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GM, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: The task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 2016; 18: 891–975. [DOI] [PubMed] [Google Scholar]
- 33. Valika A, Costanzo MR. Sleep‐disordered breathing during congestive heart failure: to intervene or not to intervene? Card Fail Rev 2017; 3: 134–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Abraham WT, Pleister A, Germany R. Identification and treatment of central sleep apnoea: beyond SERVE‐HF. Card Fail Rev 2018; 4: 50–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Suzuki S, Yoshihisa A, Sato Y, Watanabe S, Yokokawa T, Sato T, Oikawa M, Kobayashi A, Yamaki T, Kunii H, Nakazato K, Suzuki H, Saitoh SI, Ishida T, Takeishi Y. Association between sleep‐disordered breathing and arterial stiffness in heart failure patients with reduced or preserved ejection fraction. ESC Heart Failure 2018; 5: 284–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pietrock C, von Haehling S. Sleep‐disordered breathing in heart failure: facts and numbers. ESC Heart Failure 2017; 4: 198–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lauritsen J, Gustafsson F, Abdulla J. Characteristics and long‐term prognosis of patients with heart failure and mid‐range ejection fraction compared with reduced and preserved ejection fraction: a systematic review and meta‐analysis. ESC Heart Failure 2018; 5: 685–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Baseline characteristics of the HFpEF, HFmrEF, and HFrEF groups.
Table S2. Baseline characteristics in the HFpEF, HFmrEF, and HFrEF groups.


