Abstract
The genus Thielavia is morphologically defined by having non-ostiolate ascomata with a thin peridium composed of textura epidermoidea, and smooth, single-celled, pigmented ascospores with one germ pore. Thielavia is typified with Th. basicola that grows in close association with a hyphomycete which was traditionally identified as Thielaviopsis basicola. Besides Th. basicola exhibiting the mycoparasitic nature, the majority of the described Thielavia species are from soil, and some have economic and ecological importance. Unfortunately, no living type material of Th. basicola exists, hindering a proper understanding of the classification of Thielavia. Therefore, Thielavia basicola was neotypified by material of a mycoparasite presenting the same ecology and morphology as described in the original description. We subsequently performed a multi-gene phylogenetic analyses (rpb2, tub2, ITS and LSU) to resolve the phylogenetic relationships of the species currently recognised in Thielavia. Our results demonstrate that Thielavia is highly polyphyletic, being related to three family-level lineages in two orders. The redefined genus Thielavia is restricted to its type species, Th. basicola, which belongs to the Ceratostomataceae (Melanosporales) and its host is demonstrated to be Berkeleyomyces rouxiae, one of the two species in the “Thielaviopsis basicola” species complex. The new family Podosporaceae is sister to the Chaetomiaceae in the Sordariales and accommodates the re-defined genera Podospora, Trangularia and Cladorrhinum, with the last genus including two former Thielavia species (Th. hyalocarpa and Th. intermedia). This family also includes the genetic model species Podospora anserina, which was combined in Triangularia (as Triangularia anserina). The remaining Thielavia species fall in ten unrelated clades in the Chaetomiaceae, leading to the proposal of nine new genera (Carteria, Chrysanthotrichum, Condenascus, Hyalosphaerella, Microthielavia, Parathielavia, Pseudothielavia, Stolonocarpus and Thermothielavioides). The genus Canariomyces is transferred from Microascaceae (Microascales) to Chaetomiaceae based on its type species Can. notabilis. Canariomyces is closely related to the human-pathogenic genus Madurella, and includes three thielavia-like species and one novel species. Three monotypic genera with a chaetomium-like morph (Brachychaeta, Chrysocorona and Floropilus) are introduced to better resolve the Chaetomiaceae and the thielavia-like species in the family. Chrysocorona lucknowensis and Brachychaeta variospora are closely related to Acrophialophora and three newly introduced genera containing thielavia-like species; Floropilus chiversii is closely related to the industrially important and thermophilic species Thermothielavioides terrestris (syn. Th. terrestris). This study shows that the thielavia-like morph is a homoplastic form that originates from several separate evolutionary events. Furthermore, our results provide new insights into the taxonomy of Sordariales and the polyphyletic Lasiosphaeriaceae.
Key words: Ceratostomataceae, Chaetomiaceae, Multi-gene phylogeny, Non-ostiolate ascomycetes, Taxonomy, 54 Taxonomic novelties
Taxonomic novelties: new family: Podosporaceae X. Wei Wang & Houbraken
New genera: Brachychaeta X. Wei Wang & Houbraken, Carteria X. Wei Wang & Houbraken, Chrysanthotrichum X. Wei Wang & Houbraken, Chrysocorona X. Wei Wang & Houbraken, Condenascus X. Wei Wang & Houbraken, Floropilus X. Wei Wang & Houbraken, Hyalosphaerella X. Wei Wang & Houbraken, Microthielavia X. Wei Wang & Houbraken, Parathielavia X. Wei Wang & Houbraken, Pseudothielavia X. Wei Wang & Houbraken, Stolonocarpus X. Wei Wang & Houbraken, Thermothielavioides X. Wei Wang & Houbraken
New species: Acrophialophora teleoafricana X. Wei Wang & Houbraken, Canariomyces vonarxii X. Wei Wang & Houbraken, Carteria arctostaphyli X. Wei Wang & Houbraken, Chrysanthotrichum allolentum X. Wei Wang & Houbraken, Chrysanthotrichum leptolentum X. Wei Wang & Houbraken, Pseudothielavia subhyaloderma X. Wei Wang & Houbraken
New combination: Acrophialophora jodhpurensis (Lodha) X. Wei Wang & Houbraken, Brachychaeta variospora (Udagawa & Y. Horie) X. Wei Wang & Houbraken, Canariomyces arenarius (Mouch.) X. Wei Wang & Houbraken, Canariomyces microsporus (Mouch.) X. Wei Wang & Houbraken, Canariomyces subthermophilus (Mouch.) X. Wei Wang & Houbraken, Chrysanthotrichum lentum (van Warmelo) X. Wei Wang & Houbraken, Chrysanthotrichum peruvianum (Goch.) X. Wei Wang & Houbraken, Chrysocorona lucknowensis (J.N. Rai & J.P. Tewari) X. Wei Wang & Houbraken, Cladorrhinum hyalocarpum (Arx) X. Wei Wang & Houbraken, Cladorrhinum intermedium (Stchigel & Guarro) X. Wei Wang & Houbraken, Condenascus tortuosus (Udagawa & Y. Sugiy.) X. Wei Wang & Houbraken, Floropilus chiversii (J.C. Cooke) X. Wei Wang & Houbraken, Hyalosphaerella fragilis (Natarajan) X. Wei Wang & Houbraken, Microthielavia ovispora (Pidopl. et al.) X. Wei Wang & Houbraken, Parathielavia appendiculata (M.P. Srivast. et al.) X. Wei Wang & Houbraken, Parathielavia hyrcaniae (Nicot) X. Wei Wang & Houbraken, Parathielavia kuwaitensis (Moustafa) X. Wei Wang & Houbraken, Podospora bulbillosa (W. Gams & Mouch.) X. Wei Wang & Houbraken, Pseudothielavia arxii (Stchigel & Guarro) X. Wei Wang & Houbraken, Pseudothielavia hamadae (Udagawa) X. Wei Wang & Houbraken, Pseudothielavia terricola (J.C. Gilman & E.V. Abbott) X. Wei Wang & Houbraken, Stolonocarpus gigasporus (Moustafa & Abdel-Azeem) X. Wei Wang & Houbraken, Thermothielavioides terrestris (Apinis) X. Wei Wang & Houbraken, Triangularia anserina (Rabenh.) X. Wei Wang & Houbraken, Triangularia allahabadensis (M.P. Srivast. et al.) X. Wei Wang & Houbraken, Triangularia bellae-mahoneyi (C. Boucher et al.) X. Wei Wang & Houbraken, Triangularia comata (Milovtz.) X. Wei Wang & Houbraken, Triangularia longicaudata (Cain) X. Wei Wang & Houbraken, Triangularia pauciseta (Ces.) X. Wei Wang & Houbraken, Triangularia phialophoroides (Mouch. & W. Gams) X. Wei Wang & Houbraken, Triangularia pseudoanserina (C. Boucher et al.) X. Wei Wang & Houbraken, Triangularia pseudocomata (C. Boucher et al.) X. Wei Wang & Houbraken, Triangularia pseudopauciseta (C. Boucher et al.) X. Wei Wang & Houbraken, Triangularia setosa (G. Winter) X. Wei Wang & Houbraken, Triangularia verruculosa (C.N. Jensen) X. Wei Wang & Houbraken
Typifications: Neotypifications: Thielavia basicola Zopf, Chaetomium trilaterale var. chiversii J.C. Cooke
Lectotypifications & Epitypifications: Chaetomium jodhpurense Lodha, Chaetomium lucknowense J.N. Rai & J.P. Tewari, Coniothyrium terricola J.C. Gilman & E.V. Abbott, Schizothecium fimicola Corda
Introduction
The genus Thielavia (Th.) was established by Zopf (1876) based on Th. basicola, which was found in association with the hyphomycete Thielaviopsis basicola, and the former was once considered to be the sexual morph of the latter. McCormick (1925) showed that Th. basicola was not genetically connected to Thielaviopsis basicola, and that they represented two different organisms. Historically, Thielavia was once assigned in Eurotiales (Saccardo, 1882-1931, Booth, 1961). Booth (1961) defined Thielavia as having dark spherical ascocarps, with a thin pseudoparenchymatous wall without sutures or an ostiole; broadly clavate to ovate, unitunicate asci with 4–8 ascospores, which usually become deliquescent as spores mature; oval to fusoid, brown to black ascospores with one or more germ pores.
The advances in the studies of ontogenetic characters challenged the position of Thielavia in Eurotiales (Luttrell 1951). With the increasing number of species described in the genus, the similarity between Thielavia and Chaetomidium became gradually clear. Chaetomidium was considered to be the non-ostiolate counterpart of Chaetomium (Ch.) and was consistently placed in the Chaetomiaceae (Zopf, 1881, Saccardo, 1882-1931). Chaetomidium was characterised by non-ostiolate ascomata with well-developed ascomatal hairs, while species with glabrous ascomata were placed in Thielavia (Zopf, 1881, Saccardo, 1882-1931, Whiteside, 1962, von Arx, 1975). In 1967, von Arx classified Thielavia in his newly proposed family Thielaviaceae in Pyrenomycetes (Sordariomycetes), followed by von Arx & Mahmood (1968) and von Arx et al. (1988). Thielaviaceae is, however, an invalidly introduced family because no Latin description was provided in the original paper (Art. 39.1, Melbourne). Malloch & Cain (1973) suggested the presence or absence of setae or hairs on the ascomata as a criterion of insufficient taxonomic value at genus level. In their broad concept, Chaetomidium was treated as a synonym of Thielavia and nearly all Chaetomidium species were transferred to the latter genus. They also considered Thielavia to be closely related to Chaetomium, possessing a similar morphology in colony, ascospores, ascocarp initials, ascocarp vestiture and conidial morphs, but differing in having ascocarps without ostioles. In contrast to Malloch and Cain, 1973, von Arx, 1975 maintained both genera: Thielavia for the species with non-ostiolate, glabrous, setose or tomentose ascomata having a thin wall composed of textura epidermoidea, and Chaetomidium for species with non-ostiolate ascomata having a pseudoparenchymatous wall covered by straight, undulate or circinate and pigmented hairs.
Thielavia sensu Booth (1961) was considered to be a heterogeneous group even without the addition of Chaetomidium species (Mouchacca, 1973, von Arx, 1973, von Arx, 1975), and there were several attempts to homogenise this genus. Coniochaetidium (= Coniochaeta) was proposed in Coniochaetaceae to accommodate species producing ascospores with a germ slit (Malloch & Cain 1971). Corynascus was established for species characterised by ascospores with two germ pores, one at each end, and by the formation of a chrysosporium-like conidial morph (von Arx 1973). Species producing ascospores with two germ pores, but lacking a chrysosporium-like conidial morph, were classified in the genus Corynascella (von Arx 1975). Thielavia was then restricted to those species having non-ostiolate ascomata with a wall of textura epidermoidea and ascospores with a single distinct germ pore (von Arx 1975).
Thielavia sensu von Arx represented the contemporary concept of the genus in which over 40 species have been described. Based on further studies of morphological and ontogenetic characters, Thielavia was transferred to Sordariales (Hawksworth et al. 1983). Following this assignment, the invalid Thielaviaceae was not accepted and the genus was widely accepted in the Chaetomiaceae (Sordariales) (Barr, 1990, Eriksson and Hawksworth, 1993, Alexopoulos et al., 1996, Kirk et al., 2008).
Thielavia species exhibit a diverse ecology. The type species is usually associated with Thielaviopsis basicola, appearing fungicolous in culture, while almost all the other taxa are saprobes. Most of the species in the genus are found in soil, including desert or soda soils at pH levels up to 11 (von Arx, 1975, von Arx et al., 1988, Guarro et al., 2012, Grum-Grzhimaylo et al., 2016). Some species can be coprophilous, endophytes, lichenicolous and even marine-derived (Stchigel et al., 2003, Moustafa and Abdel-Azeem, 2008, Qadri et al., 2014, Han et al., 2017). Different species present different growth temperature in culture. Although the majority of species are mesophilic, the genus also includes psychrotolerant, thermotolerant and thermophilic species (Apinis, 1963, von Arx et al., 1988, Stchigel et al., 2002, Stchigel et al., 2003, Moustafa and Abdel-Azeem, 2008, van den Brink et al., 2015). The thermotolerant and thermophilic species represent a potential reservoir of industrial-relevant enzymes (Kallio et al., 2011, Lu et al., 2013, van den Brink et al., 2015). Among them, Th. terrestris is the most eminent species. Genome analyses and enzymological studies showed that this thermophilic species is able to produce enzymes with biotechnological applications, including the hydrolysis of all major polysaccharides found in biomass (Berka et al., 2011, de Vries et al., 2011, Syed et al., 2014, Xu et al., 2015, Woon et al., 2016, Gao et al., 2017, García-Huante et al., 2017). The capacity of Th. arenaria to degrade bisphenol A and reduce its acute toxicity presents its bioremediation potential in pollutant removal processes (Mtibaà et al. 2018). Several species were found to produce bioactive metabolites, such as inhibitors of prostaglandin biosynthesis in Th. terricola (Kitahara et al. 1981), antifungal compounds active against Candida albicans in Th. subthermophila (Qadri et al. 2014), and antifouling activities in a Thielavia sp. (Han et al. 2017). On the other hand, the thermotolerant species Th. subthermophila has been reported as the causal agent of keratitis (Theoulakis et al. 2009) and fatal cerebral mycoses (Badali et al. 2011).
Molecular data have proved that the morphologically-defined Chaetomidium was polyphyletic with no evidence showing a relationship with Thielavia species (Greif et al., 2009, Wang et al., 2016b). The genus Chaetomidium now has been synonymised with Chaetomium (Wang et al. 2016b). The separation of Coniochaeta, Corynascus and Corynascella from Thielavia was also phylogenetically supported (van den Brink et al., 2012, van den Brink et al., 2015, Maharachchikumbura et al., 2016, Wang et al., 2016a). Stchigel et al. (2002) performed the first phylogenetic study of Thielavia on the basis of ITS-5.8S rDNA sequences, including 17 representative species. They selected isolate CBS 229.82 as the representative of the type species, Th. basicola, although the pure culture of this isolate grows well on the medium and has no association with Thielaviopsis basicola, and its morphology was not compared with the original description in the protologue of Thielavia basicola. In their phylogenetic tree, 16 Thielavia species and Melanocarpus thermophilus (the only reference that is not a Thielavia species in their study) formed a moderately supported clade, which was distant from Th. intermedia. Based on a five-locus phylogeny of 45 isolates representing 32 species of seven morphologically defined genera in Chaetomiaceae, van den Brink et al. (2015) showed that ten representative Thielavia species clustered in six distant clades throughout the Chaetomiaceae. However, the type species, Th. basicola was not included in their study, and no taxonomic conclusions were made. In our recent studies of the Chaetomiaceae, Thielavia antarctica was located in the genus Trichocladium (Wang et al. 2019), and one isolate (CBS 541.76) that was mentioned as Th. minuta by Stchigel et al. (2002) was transferred to the genus Melanocarpus (Wang et al. 2016a). These studies demonstrated that the morphologically-defined Thielavia are not monophyletic.
The phylogenetic location of the type species is the key step in the taxonomic studies of Thielavia. Since neither a holotype specimen nor ex-type culture of Th. basicola were available, our first priority was to obtain suitable material in order to typify the type species. Isolate CBS 178.82 has been used as a representative strain for Th. basicola. It presented a morphology well agreeing with the original description of Th. basicola (Zopf 1876) and grew in close association with a hyphomycete fungus (its host) possessing the typical morphology of Thielaviopsis basicola in culture (von Arx et al. 1988). Thus, we targeted the strain CBS 178.82 as a candidate for a neotype. Our research started in identifying its host.
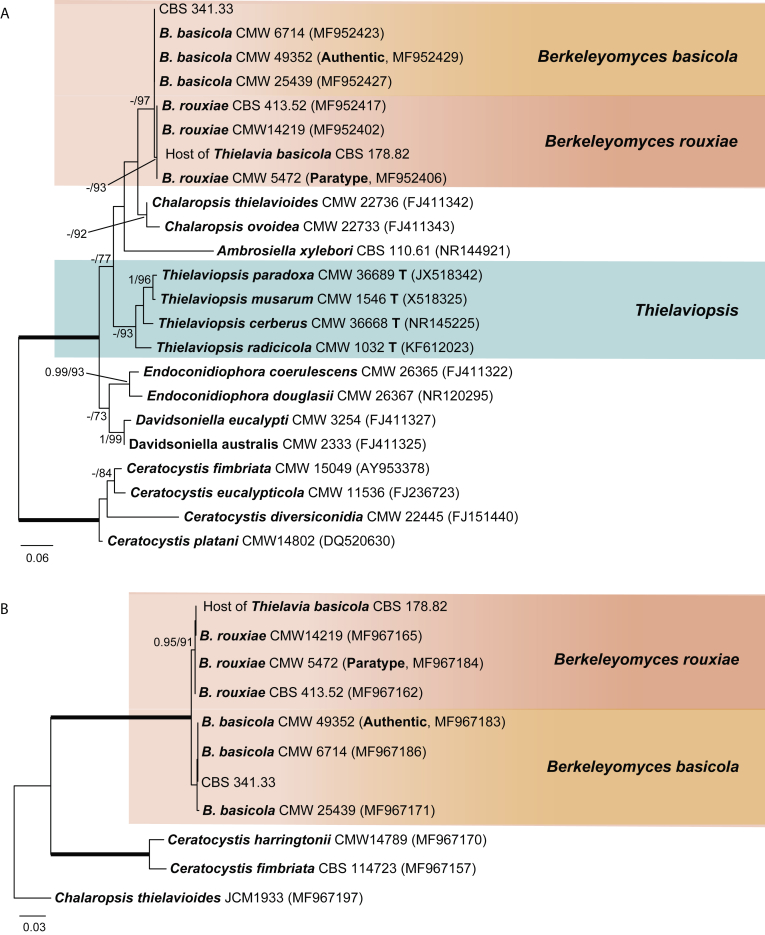
Traditionally defined Thielaviopsis basicola has been reclassified in a newly proposed genus Berkeleyomyces separate from Thielaviopsis sensu stricto (Nel et al. 2018). Thielaviopsis has a tumultuous taxonomic history. Several recent studies resolved the taxonomy of Thielaviopsis basicola and allied genera/species (Mbenoun et al., 2014, de Beer et al., 2014, Nel et al., 2018). Mbenoun et al. (2014) epitypified the type species of Thielaviopsis, Thielaviopsis ethacetica, and transferred it to Ceratocystis sensu lato. De Beer et al. (2014) phylogenetically resurrected and redefined the genus Thielaviopsis. The genus was considered to produce both sexual and asexual morphs, and characterised by the distinctly digitate or stellate appendages on the globose basal part of the ascomata. Thielaviopsis basicola, however, was not included in their study. Nel et al. (2018) introduced Berkeleyomyces to accommodate the traditionally defined Thielaviopsis basicola, in which two species were included: B. basicola and a new cryptic species, B. rouxiae. In their study, no sexual morph was observed in the two Berkeleyomyces species, and these two species were morphologically undistinguishable. There is no information, however, which of the two Berkeleyomyces species is associated with the holotype of Thielavia basicola described by Zopf (1876).
The success in molecular identification of both the sexual Thielavia member and its asexual host in the culture of CBS 178.82 enabled us to resolve the taxonomy of Thielavia. The aims of the present study were: (i) to resolve the phylogenetic placement of each of the species available in Thielavia; (ii) to re-evaluate the phylogenetic relationships between Thielavia species and their related taxa; (iii) to re-describe and illustrate the species available in culture that were formerly described in Thielavia and those that are related to the studied Thielavia species.
Materials and methods
Isolates
Thirty-three Thielavia strains were obtained from the CBS culture collection (CBS) housed at the Westerdijk Fungal Biodiversity Institute (WI), Utrecht, the Netherlands. More isolates potentially related to the obtained Thielavia strains were selected based on a preliminary phylogenetic analysis of LSU sequences from the in-house database of WI (Vu et al. 2019), as well as several cultures of the Chaetomiaceae maintained in the Institute of Fungus Resources housed at Guizhou University in China. All the strains used in this study are listed in Table 1.
Table 1.
Details of strains used in this study.
| Current name | Culture accession number1 | previous identification (if different) | Origin | GenBank accession numbers2 |
||
|---|---|---|---|---|---|---|
| ITS & LSU | rpb2 | tub2 | ||||
| Ceratostomataceae | ||||||
| Microthecium fimicola | CBS 967.97 | Sphaerodes fimicola | Coriolus flabelliformis, Papua New Guinea | MK926777 | MK876739 | MK926877 |
| M. quandrangulatum | CBS 112763 T | Sphaerodes quandrangularis | Soil, Spain | MK926778 | MK876740 | MK926878 |
| M. retisporum | CBS 995.72 | Sphaerodes retispora var.inferior | Soil, Japan | MK926779 | MK876741 | MK926879 |
| M. tenuissimum | CBS 112764 T | Sphaerodes tenuissima | Soil, Spain | MK926780 | MK876742 | MK926880 |
| M. zobelii | CBS 268.62 | Melanospora zobelii | Dung of roe, Netherlands | MK926781 | MK876743 | MK926881 |
| M. zobelii | CBS 341.73 | Melanospora zobelii | Coriolus flabelliformis, Papua New Guinea | MK926782 | MK876744 | MK926882 |
| Thielavia basicola | CBS 178.82 NeoT | n/a3 | MK926783 | MK876745 | MK926883 | |
| Ceratocystidaceae | ||||||
| Berkeleyomyces basicola | CBS 341.33 | Thielaviopsis basicola | Pathogenic Primula sp., Netherlands | MK926784 | MK876746 | MK926884 |
| B. rouxiae | CBS 178.82 | Thielaviopsis basicola | Diseased root of Phaseolus vulgaris, Host of Thielavia basicola | MK926785 | MK876747 | MK926885 |
| Chaetomiaceae | ||||||
| Acrophialophora ellipsoidea | CBS 102.61 | Acrophialophora levis | Soil, Belgium | MK926786 | MK876748 | MK926886 |
| CGMCC 3.17487 | n/a | Compost, China | MK926787 | MK876749 | MK926887 | |
| Acro. fusispora | CBS 380.55 T | n/a | Forest soil, India | MK926788 | MK876750 | MK926888 |
| Acro. hechuanensis | GZUIFR-H08-1 T | n/a | Soil, Chongqing City, China | MK926789 | MK876751 | MK926889 |
| Acro. jodhpurensiscomb. nov. | CBS 602.69 eT | Chaetomium jodhpurense | Substrate unknown, Pakistan | MK926790 | MK876752 | MK926890 |
| CBS 509.84 | Chaetomium jodhpurense | Soil, Kenya | MK926791 | MK876753 | MK926891 | |
| Acro. major | GZUIFR-H57-2 T | n/a | Soil, | MK926792 | MK876754 | MK926892 |
| Acro. nainiana | CBS 100.60 T | n/a | Farm soil,India | MK926793 | MK876755 | MK926893 |
| CBS 417.67 | n/a | Soil, India | MK926794 | MK876756 | MK926894 | |
| Acro. teleoafricanasp. nov. | CBS 281.79 T | Chaetomium jodhpurense | Soil, Sudan | MK926795 | MK876757 | MK926895 |
| CBS 280.79 | Chaetomium jodhpurense | Soil, Sudan | MK926796 | MK876758 | MK926896 | |
| Brachychaeta variosporagen. et comb. nov. | CBS 414.73 T | Chaetomium variosporum | Soil, Thailand | MK926797 | MK876759 | MK926897 |
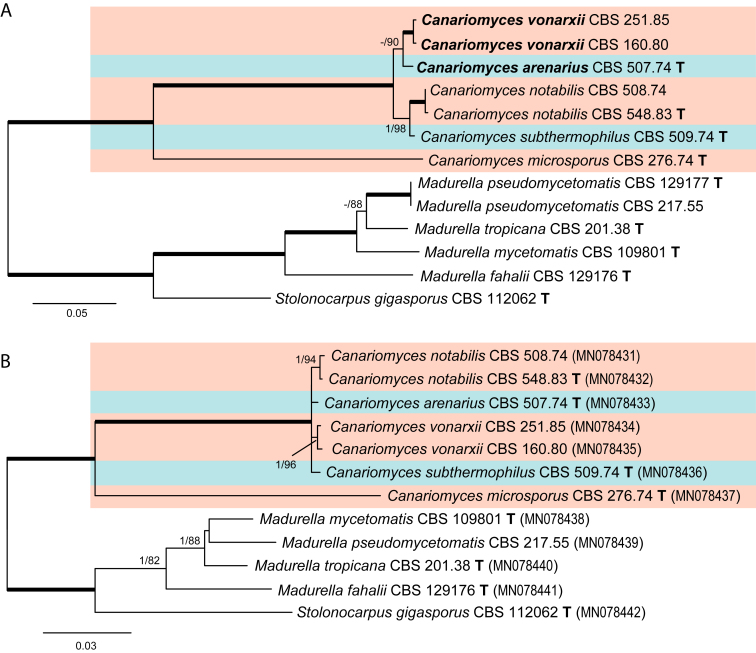
| Canariomyces arenariuscomb. nov. | CBS 507.74 T | Thielavia arenaria | Desert soil, Egypt | MK926798 | KM655438 | MK926898 |
| Can. microsporuscomb. nov. | CBS 276.74 T | Thielavia microspora | Desert soil, Egypt | MK926799 | MK876760 | MK926899 |
| CBS 161.80 | Thielavia microspora | Leaf of Thymus, Japan | MK926800 | MK876761 | MK926900 | |
| CBS 808.73 | Thielavia microspora | Saline desert soil, Kuwait | MK926801 | MK876762 | MK926901 | |
| Can. notabilis | CBS 548.83 T | n/a | Litter of Phoenix canariensis, Spain | MK926802 | MK876763 | MK926902 |
| CBS 508.74 | Thielavia arenaria | Desert soil, Egypt | MK926803 | KM655439 | MK926903 | |
| Can. subthermophiluscomb. nov. | CBS 509.74 T | Thielavia subthermophila | Desert soil, Egypt | MK926804 | MK876764 | MK926904 |
| Can. vonarxiisp. nov. | CBS 160.80 T | Thielavia subthermophila | Dried flower of Hibiscus, Sudan | MK926805 | MK876765 | MK926905 |
| CBS 251.85 | Canariomyces notabilis | Substrate unknown, Nigeria | MK926806 | MK876766 | MK926906 | |
| Carteria arctostaphyligen. et sp. nov. | CBS 229.82 T | Thielavia basicola | Arctostaphylos uva-ursi, Switzerland | MK926807 | MK876767 | MK926907 |
| Chrysanthotrichum allolentumgen. et sp. nov. | CBS 644.83 T | Chaetomium lentum | Soil, USA | MK926808 | MK876768 | MK926908 |
| Chrysan. lentumcomb. nov. | CBS 339.67 T | Chaetomium lentum | Soil, South Africa | MK926809 | MK876769 | MK926909 |
| Chrysan. leptolentumsp. nov. | CBS 126.85 T | Chaetomium lentum | Dung of elephant, Kenya | MK926810 | MK876770 | MK926910 |
| CBS 127.85 | Chaetomium lentum | Dung of moose or deer, Canada | MK926811 | MK876771 | MK926911 | |
| Chrysan. peruvianumcomb. nov. | CBS 732.68 T | Thielavia peruviana | High mountain tundra soil, Peru | MK926812 | MK876772 | MK926912 |
| Chrysocorona lucknowensisgen. et comb. nov. | CBS 727.71 eT | Chaetomium lucknowense | Dung of deer, India | MK926813 | MK876773 | MK926913 |
| CBS 124.85 | Chaetomium lucknowense | Dung of burro, Venezuela | MK926814 | MK876774 | MK926914 | |
| CBS 562.67 | Chaetomium lucknowense | Dung of rabbit, Germany | MK926815 | MK876775 | MK926915 | |
| CBS 385.66 | Chaetomium lucknowense | Soil and vegetable detritus, Venezuela | MK926816 | MK876776 | MK926916 | |
| Condenascus tortuosusgen. et comb. nov. | CBS 610.97 | Thielavia tortuosa | Soil, India | MK926817 | MK876777 | MK926917 |
| Floropilus chiversiigen. et comb. nov. | CBS 558.80 NeoT | Chaetomium chiversii | Dung of moose, Canada | MK926818 | MK876778 | MK926918 |
| Hyalosphaerella fragilisgen. et comb. nov. | CBS 456.73 T | Thielavia fragilis | Rhizosphere of Pennisetum typhoideum in garden soil, India | KX976693 + KX976791 | MK876779 | KX977042 |
| Madurella fahalii | CBS 129176 T | n/a | Mycetoma of a man's foot, Sudan | MK926819 | MK876780 | MK926919 |
| Mad. mycetomatis | CBS 109801 T | n/a | Foot mycetoma of a woman, Sudan | MK926820 | MK876781 | MK926920 |
| Mad. pseudomycetomatis | CBS 129177 T | n/a | Mycetoma of a man's lower jaw, China | MK926821 | MK876782 | MK926921 |
| CBS 217.55 | n/a | Man hand, Argentina | MK926822 | MK876783 | MK926922 | |
| CBS 124574 | n/a | Black grains discharged by a case of mycetoma, China | MK926823 | MK876784 | MK926923 | |
| Mad. tropicana | CBS 201.38 T | n/a | Man foot, Indonesia | MK926824 | MK876785 | MK926924 |
| CBS 206.47 | n/a | Man foot, Netherlands | MK926825 | MK876786 | MK926925 | |
| Microthielavia ovisporagen. et comb. nov. | CBS 165.75 T | Thielavia ovispora | Root of Avena sativa, Ukraine | MK926826 | MK876787 | MK926926 |
| Parathielavia appendiculatagen. et comb. nov. | CBS 723.68 T | Thielavia appendiculata | Leaf of Punica granatum, India | MK926827 | MK876788 | MK926927 |
| CBS 731.68 | Thielavia appendiculata | Dung of rabbit, Wales | KM655330 + KM655369 | KM655402 | KX977041 | |
| CBS 417.73 | Thielavia appendiculata | Unknown substrat and country | MK926828 | MK876789 | MK926928 | |
| Par. hyrcaniaecomb. nov. | CBS 353.62 T | Thielavia hyrcaniae | Sand dune soil, Iran | KM655329 + KM655368 | KM655401 | KX977043 |
| Par. kuwaitensiscomb. nov. | CBS 945.72 T | Thielavia kuwaitensis | Desert soil, Kuwait | KM655332 + KM655371 | KM655404 | KX977044 |
| CBS 119771 | Thielavia kuwaitensis | Soil, China | MK926829 | MK876790 | MK926929 | |
| Pseudothielavia arxiigen. et comb. nov. | CBS 603.97 T | Thielavia arxii | Soil, Chile | MK926830 | MK876791 | MK926930 |
| CBS 102199 | Thielavia arxii | Soil, Chile | MK926831 | MK876792 | MK926931 | |
| Pse. hamadaecomb. nov. | CBS 499.83 T | Chaetomium hamadae | Soil, Japan | MK926832 | MK876793 | MK926932 |
| Pse. subhyalodermasp. nov. | CBS 473.86 T | n/a | Forest soil, Papua New Guinea | MK926833 | MK876794 | MK926933 |
| Pse. terricolacomb. nov. | CBS 165.88 eT | Thielavia terricola | Soil, USA | KX976694 + KX976792 | MK876795 | KX977045 |
| CBS 487.74 | Thielavia terricola | Kernel of Arachis hypogaea, USA | MK926834 | MK876796 | MK926934 | |
| Stellatospora terricola | CBS 811.95 T | n/a | Paddy soil, Japan | MK926835 | MK876797 | MK926935 |
| Stolonocarpus gigasporusgen. et comb. nov. | CBS 112062 T | Thielavia gigaspora | Dung of Camelus dromedarius, Egypt | MK926836 | MK876798 | MK926936 |
| Thermothielavioides terrestrisgen. et comb. nov. | CBS 117535 T | Thielavia terrestris | Soil, UK | MK926837 | MK876799 | MK926937 |
| CBS 492.74 | Thielavia terrestris | Soil, Japan | MK926838 | MK876800 | MK926938 | |
| CBS 546.86 | Thielavia terrestris | Unknown substrat and country | MK926839 | MK876801 | MK926939 | |
| CBS 351.90 | Thielavia terrestris | Cellulose in soil, Malaysia | MK926840 | MK876802 | MK926940 | |
| Lasiosphaeriaceae | ||||||
| Apiosordaria microcarpa* | CBS 692.82 T | n/a | Rice-field soil, Japan | MK926841 | MK876803 | MK926941 |
| Podospora decidua* | CBS 254.71 T | n/a | Dung of hare, Birao | MK926842 | MK876804 | MK926942 |
| Pod. fabiformis* | CBS 112043 T | n/a | Animal dung, Australia | MK926843 | MK876805 | MK926943 |
| Pod. fibrinocaudata* | CBS 315.91 T | n/a | Dung of dusky footed wood rat, USA | MK926844 | MK876806 | MK926944 |
| Pod. glutinoides* | CBS 116865 | n/a | MK926845 | MK876807 | MK926945 | |
| Pod. inaequalis* | CBS 356.49 T | n/a | Daucus carot seed, USA | MK926846 | MK876808 | MK926946 |
| Pod. longicollis* | CBS 368.52 T | n/a | Deteriorating material, Panama | MK926847 | MK876809 | MK926947 |
| Pod. prolifica* | CBS 250.71 T | n/a | Dung of Cobus defassa, Central African Republic | MK926848 | MK876810 | MK926948 |
| Pod. selenospora* | CBS 109403 T | n/a | Forest soil, Chile | MK926849 | MK876811 | MK926949 |
| Zopfiella karachiesis | CBS 657.74 | n/a | Arid soil, Egypt | MK926850 | MK876812 | MK926950 |
| Z. marina | CBS 155.77 T | n/a | Marine mud, Taiwan, China | MK926851 | MK876813 | MK926951 |
| Z. pilifera | CBS 413.73 T | n/a | Soil, Japan | MK926852 | MK876814 | MK926952 |
| Z. submersa | CBS 698.96 T | n/a | Submerged dead culms, Iraq | MK926853 | MK876815 | MK926953 |
| Z. tabulata | CBS 230.78 | n/a | Dung of porcupine, Quebec, Canada | MK926854 | MK876816 | MK926954 |
| Z. tardifaciens | CBS 670.82 T | n/a | Dung of cow, Argentina | MK926855 | MK876817 | MK926955 |
| Podosporaceaefam. nov. | ||||||
| Cladorrhinum foecundissimum | CBS 180.66 T | n/a | Soil, Netherlands | MK926856 | MK876818 | MK926956 |
| Clad. hyalocarpumcomb. nov. | CBS 322.70 T | Thielavia hyalocarpa | Soil, Netherlands | MK926857 | MK876819 | MK926957 |
| CBS 102198 | Thielavia hyalocarpa | Forest soil, Spain | MK926858 | MK876820 | MK926958 | |
| Clad. intermediumcomb. nov. | CBS 433.96 T | Thielavia intermedia | Soil, India | MK926859 | MK876821 | MK926959 |
| CBS 100257 | Thielavia sp. | Soil, Tunisia | MK926860 | MK876822 | MK926960 | |
| Podospora bulbillosacomb. nov. | CBS 304.90 T | Cladorrhinum bulbillosum | Desert soil, Egypt | MK926861 | MK876823 | MK926961 |
| Pod. fimicola | CBS 482.64 eT | n/a | Dung of cow, Switzerland | MK926862 | MK876824 | MK926962 |
| CBS 990.96 | n/a | Dung of horse, New Zealand | MK926863 | MK876825 | MK926963 | |
| Triangularia anserinacomb. nov.** | S mat+ T | Podospora anserina | ||||
| CBS 433.50 | Podospora pauciseta | Dung of cow, Canada | MK926864 | MK876826 | MK926964 | |
| Trian. allahabadensiscomb. nov. | CBS 724.68 T | Sordaria allahabadensis | Flower of Carica papaya, India | MK926865 | MK876827 | MK926965 |
| Trian. backusii | CBS 539.89 T | Apiosordaria backusii | Soil, USA | MK926866 | MK876828 | MK926966 |
| CBS 106.77 | n/a | Sandy soil, Japan | MK926867 | MK876829 | MK926967 | |
| Trian. bambusae | CBS 352.33 T | Triangularia bambusae | Shoot of Bambusa sp., unknown country | MK926868 | MK876830 | MK926968 |
| Trian. longicaudatacomb. nov. | CBS 252.57 T | Zopfiella longicaudata | Dung of horse, Canada | MK926869 | MK876831 | MK926969 |
| Trian. paucisetacomb. nov. | CBS 451.62 | Podospora pauciseta | Dung of cow, Argentina | MK926870 | MK876832 | MK926970 |
| Trian. phialophoroidescomb. nov. | CBS 301.90 T | Cladorrhinum phialophoroides | Desert sandy soil, Egypt | MK926871 | MK876833 | MK926971 |
| Trian. setosacomb. nov. | CBS 311.58 | Podospora setosa | Soil, UK | MK926872 | MK876834 | MK926972 |
| CBS 369.59 | Podospora setosa | Dung of horse, UK | MK926873 | MK876835 | MK926973 | |
| Trian. verruculosacomb. nov. | CBS 148.77 | Apiosordaria verruculosa | Soil, Japan | MK926874 | MK876836 | MK926974 |
| Sordariaceae | ||||||
| Boothiella tetraspora | CBS 887.97 | n/a | Sand, Spain | MK926875 | MK876837 | MK926975 |
| CBS 334.67 | n/a | Soil, Pakistan | MK926876 | MK876838 | MK926976 | |
*: Generic affiliation remains to be re-evaluated.
**: Sequences obtained from genome.
T: ex-type strains; eT: ex-epitype strain designated in this study; NeoT: ex-neotype strain designated in this study.
Sequences generated in this study indicated in bold.
n/a: No name changed.
DNA isolation and sequencing
Genomic DNA was extracted from fungal mycelium grown on oatmeal agar (OA) using the DNeasy® UltraClean® Microbial Kit (Qiagen, Germany) following the manufacturer's instructions. The internal transcribed spacer 1 and 2 including the intervening 5.8S nrDNA (ITS), the D1/D2 domains of the 28S nrDNA (LSU) and a part of the DNA-directed RNA polymerase II second largest subunit gene (rpb2) and the β-tubulin gene (tub2) were selected for phylogenetic inference. In addition, the partial translation elongation factor 1-α (tef1-α) gene region was used to delimit species in the Th. arenaria species complex, which was amplified with the primers EF1-728 & EF1-2Rd combined with EF1-983F & EF1-2218R (Carbone & Kohn 1999, S. Rehner, AFTOL, http://aftol.org/). The PCR conditions and primers used for PCR amplification and sequencing were the same as those described by Wang et al. (2019). Each amplicon was sequenced in both directions using the same set of primers and the BigDye Terminator v. 3.1 Cycle Sequencing Kit (Applied Biosystems, Foster City, CA, USA) following the manufacturer's instructions. Sequencing was performed with an ABI PRISM 3730XL DNA Analyzer (Applied Biosystems, CA, USA).
Ascomatal formation of the fungicolous Th. basicola was induced by growing the culture CBS 178.82 on cornmeal agar (CMA) covered with a cellophane membrane. The young ascomata formed along the edge of the colony (Fig. 7A) were collected for genomic DNA extraction. DNA extraction and PCR amplification were performed as described above. Each of the ITS, LSU rpb2 and tub2 amplicons was purified and cloned into pGEM-T vector (Promega, USA). The recombinant plasmids were transformed into E. coli DH5α cells (Sambrook et al. 1989). About 20–30 recombinant colonies containing the positive plasmids were randomly picked up for each of the amplicons. The colonies were heated at 100 °C for 10 min to release the recombinant DNA fragments, and then were used for sequencing as described above.
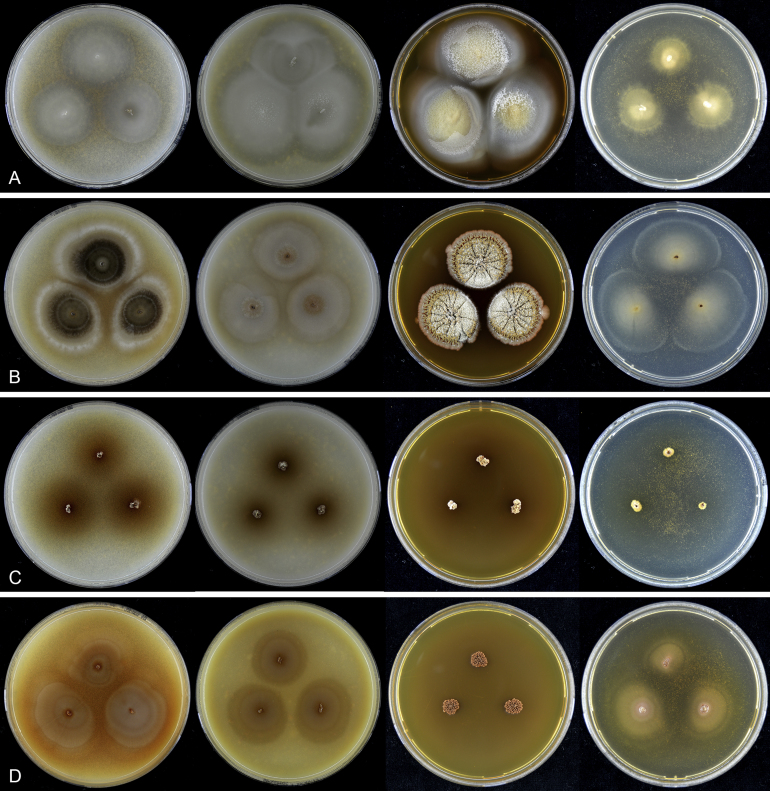
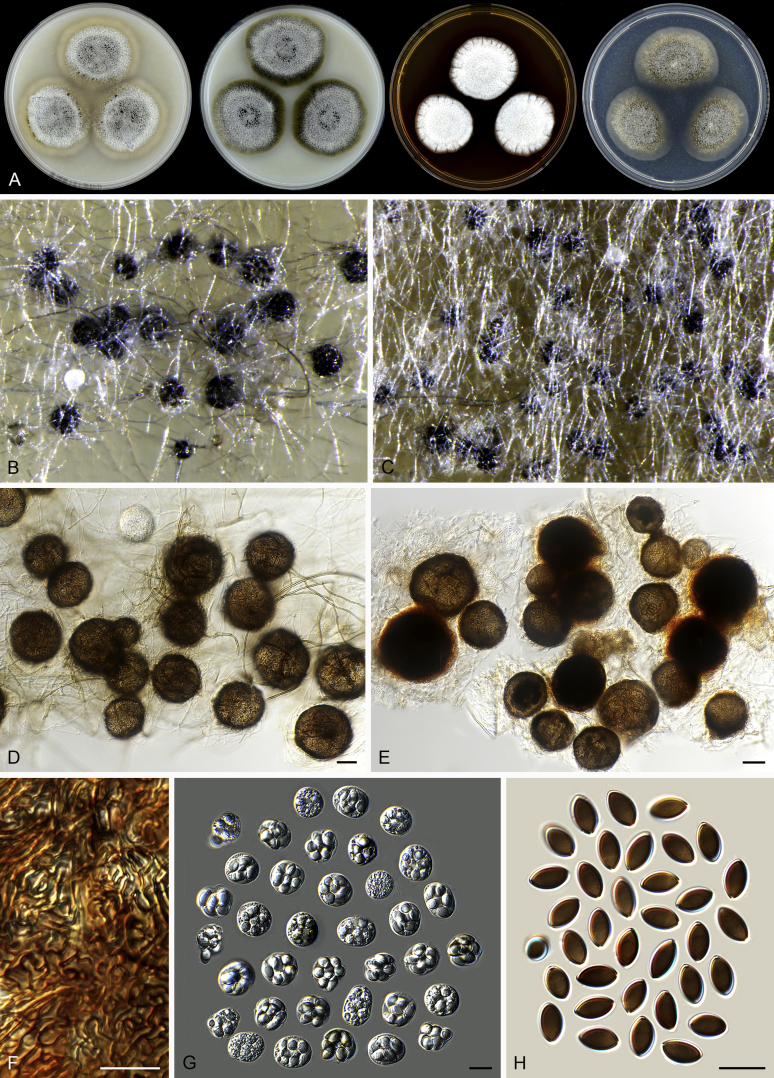
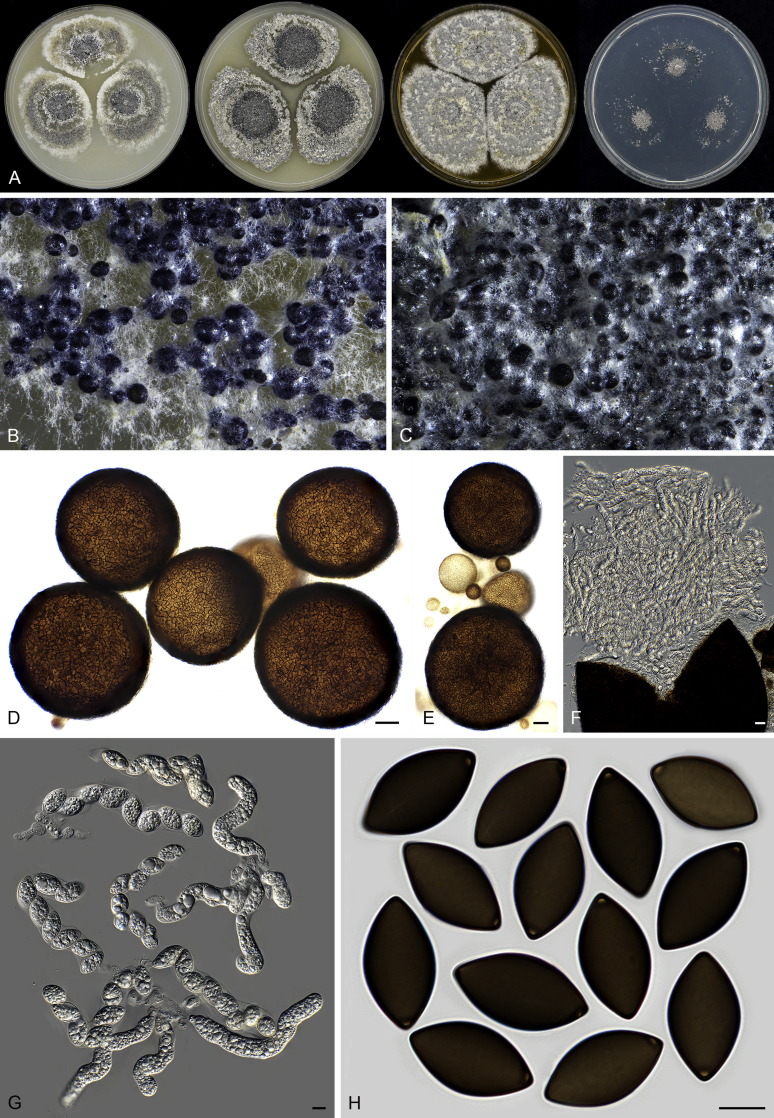
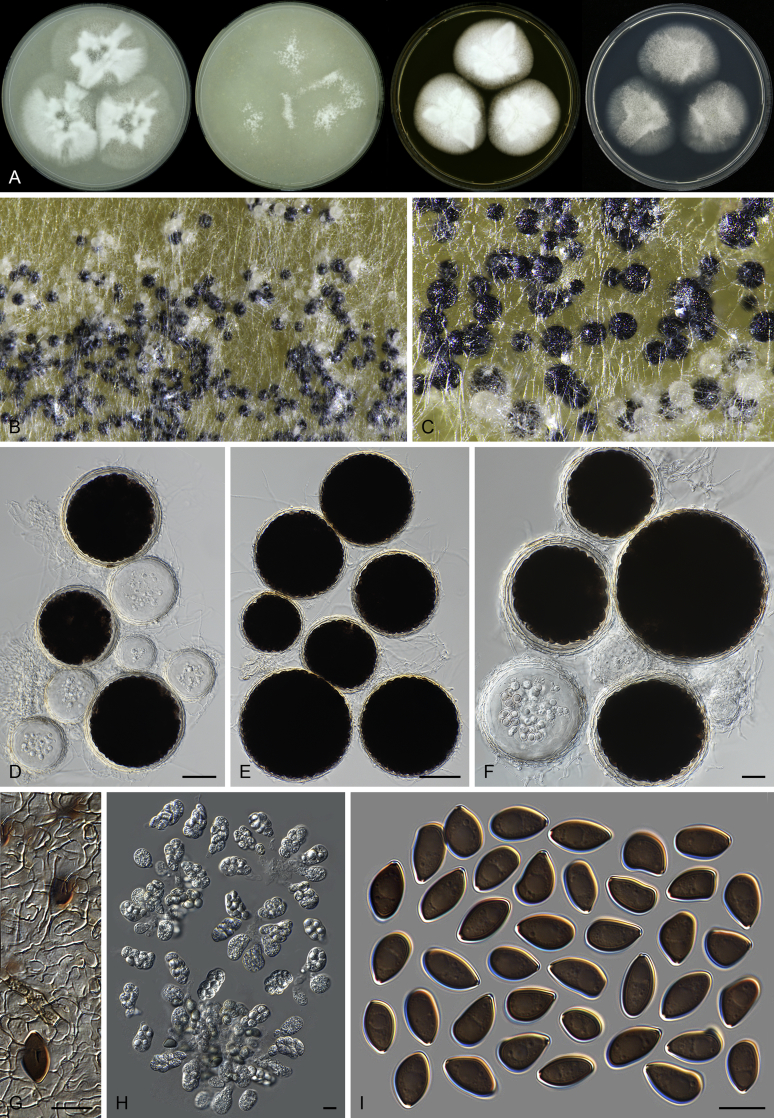
Fig. 7.
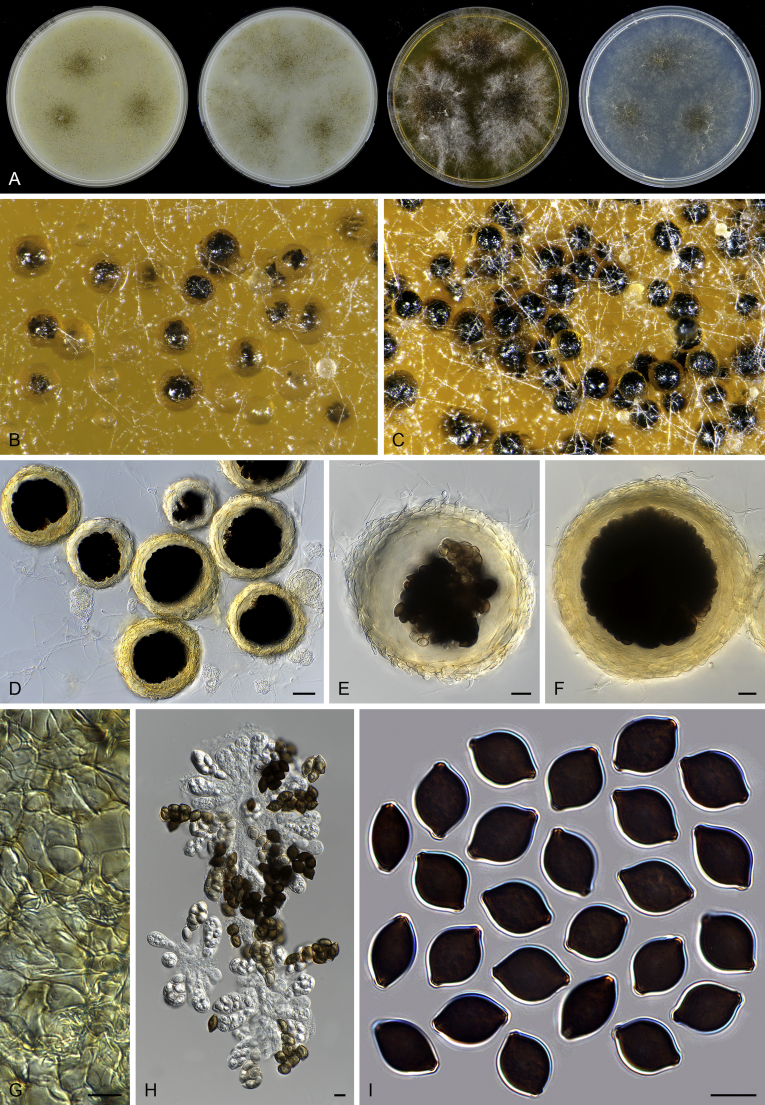
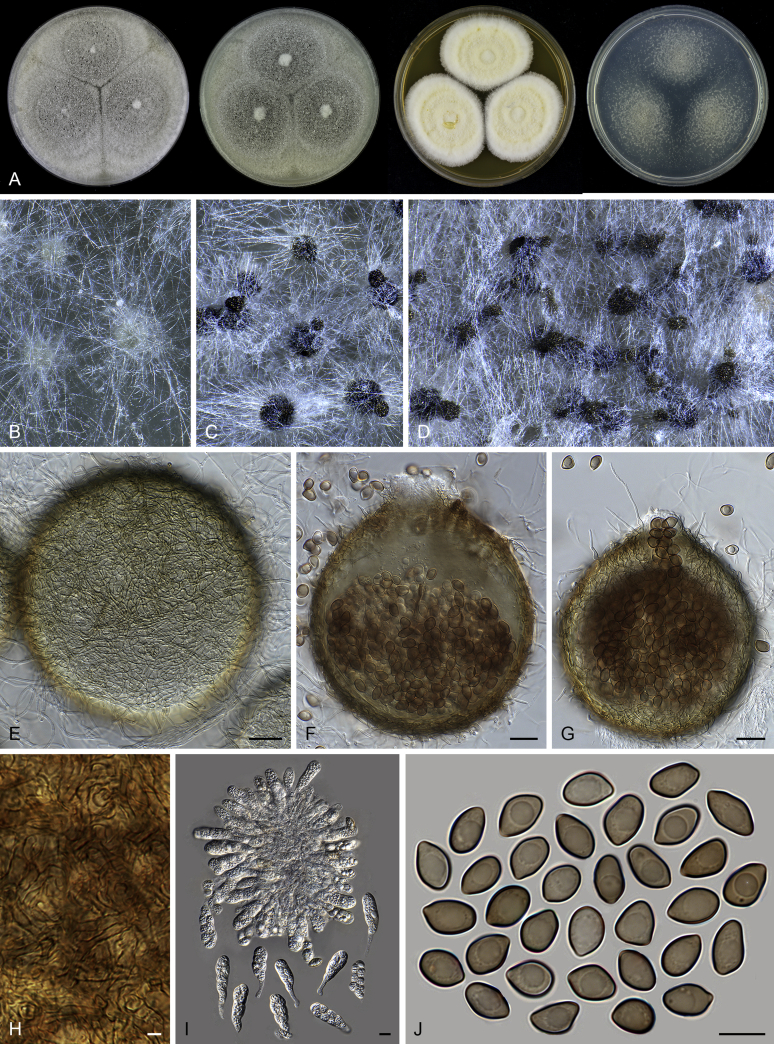
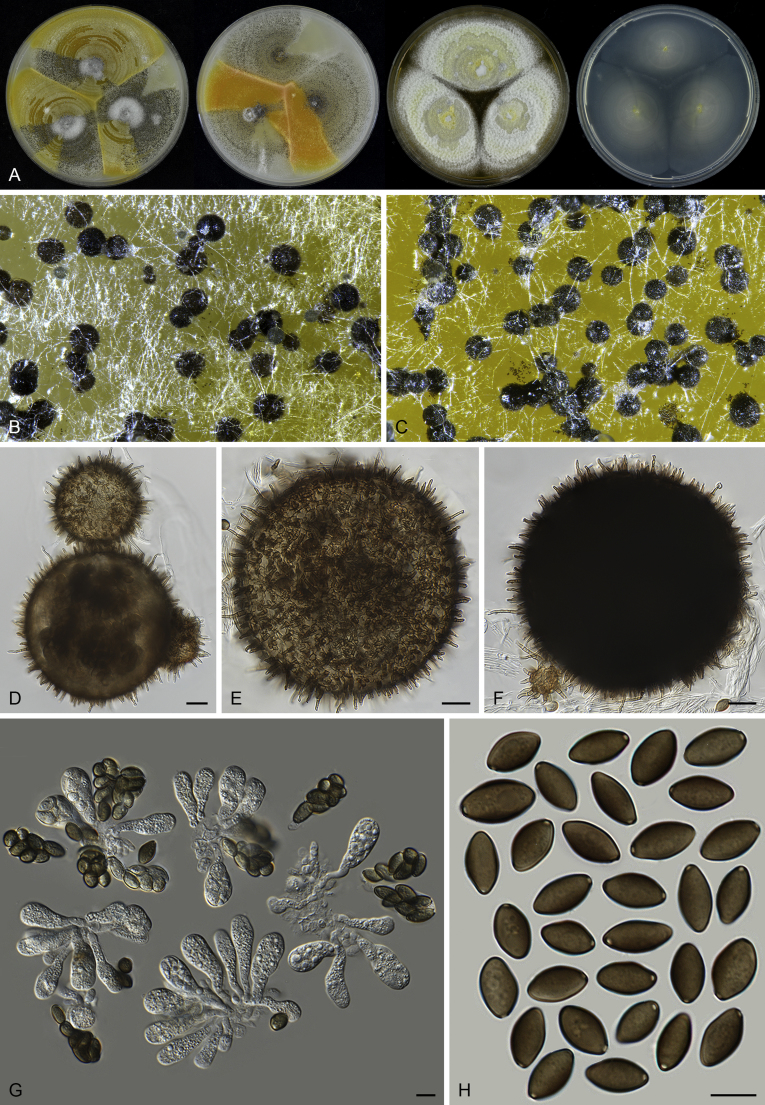
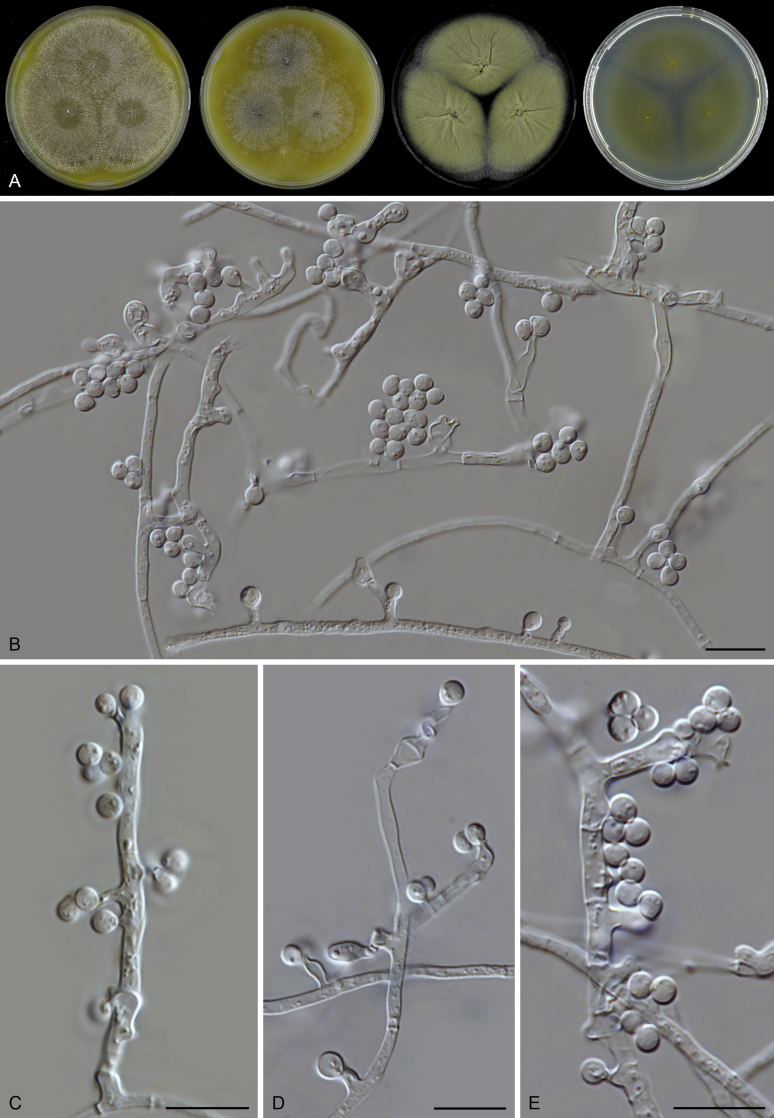
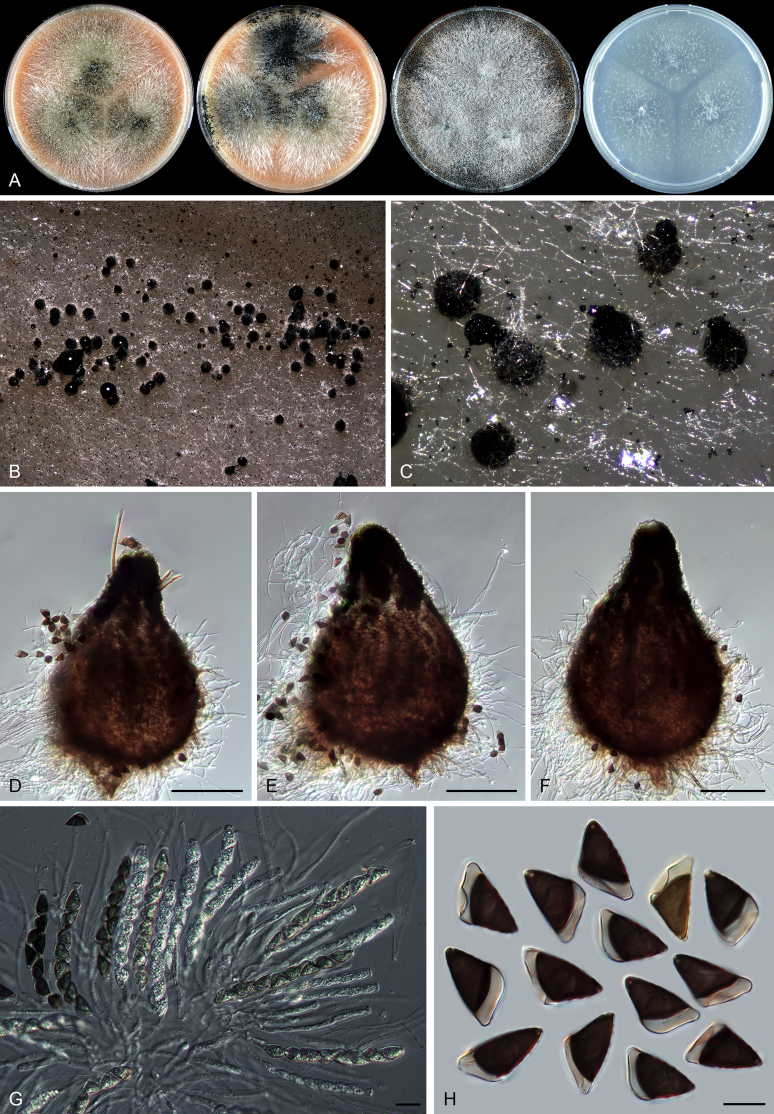
Thielavia basicola growing with its host Berkeleyomyces rouxiae (CBS 178.82, ex-neotype culture). A. Colonies from left to right on OA, CMA, MEA and PCA after 10 d incubation. B. Part of the 2-wk old colony on CMA covered with a cellophane membrane to show ascomata, top view. C. Part of the 5-wk old colony on CMA covered with a cellophane membrane to show ascomata surrounded by host fungus, top view. D–G. Ascomata surrounded by asexual structures of the host, mounted in lactic acid. H–I. Asci. J. Ascospores. Scale bars: D–G = 20 μm; H–J = 10 μm.
Data sets and phylogeny
Novel sequences generated in this study were deposited in GenBank (http://www. ncbi.nlm.nih.gov, Table 1, tef1-α marked directly in the phylogenetic tree, Fig. 5). Additional sequences of representative species belonging to the Chaetomiaceae were obtained from previous studies (Wang et al., 2016a, Wang et al., 2016b, Wang et al., 2019). Rpb2 reference sequences of other Sordariales members were obtained from GenBank and added to our rpb2 dataset to delimit the family Chaetomiaceae and to study the relationship between Chaetomiaceae and non-chaetomiaceae species. Those reference sequences retrieved from GenBank database or from the released genomic data were marked directly in the phylogenetic trees (Fig. 1, Fig. 2, Fig. 4, Fig. 6; in parentheses behind the strain numbers). The phylogenetic relationships of Thielavia species with related taxa were further studied in a combined ITS, LSU, rpb2 and tub2 data set. Additionally, several small datasets were made to identify the host member in the culture CBS 178.82 (ITS and rpb2), determining the taxonomic placement of Th. basicola (LSU), and to delimit species in the Th. arenaria species complex (tub2 and tef1-α) and the Podospora anserina/pauciseta/comata species complex (ITS, Rchr3, Rchr4, and Rchr6). Alignments were made using the web interface MAFFT v. 7 (Katoh & Standley 2013), followed by manual adjustments with MEGA v. 6 (Tamura et al. 2013). Phylogenetic analyses were performed using Maximum-Likelihood (ML) and Bayesian Inference (BI) approaches under RAxML-HPC2 on XSEDE 8.2.10 (Stamatakis 2014) using the Cipres Science gateway portal (Miller et al. 2010) and MrBayes v. 3.2.6 (Ronquist et al. 2012), respectively. For BI, the best evolutionary model for each locus was determined using MrModeltest v. 2.0 (Nylander 2004). The Maximum-Likelihood analysis used the GTRGAMMA model. Obtained trees were viewed in FigTree v. 1.1.2 (Rambaut 2009) and subsequently visually prepared and edited in Adobe® Illustrator® CS6. Confident branch support is defined as Bayesian posterior probabilities (PP) ≥ 0.95 and maximum likelihood bootstrap values (ML-BS) ≥ 70 %.
Fig. 5.
Delimitation of species in the Canariomyces clade based on the separate analyses of partial gene sequences of tub2 (A) and tef1-α (B). Maximum-Likelihood (ML) trees are showed with the confidence values indicated at the notes same to Fig. 1. Recognised species are discriminated with boxes in different colours. Type strains are marked with “T” after the culture number. The scale bar shows the expected number of changes per site. The tree is rooted with the four species of Madurela together with the type species of Stolonocarpus.
Fig. 1.
Phylogenetic identification of the host partner in the culture CBS 178.82 based on the separate analyses of partial gene sequences of ITS (A) and rpb2 (B). Maximum-Likelihood (ML) trees are showed with the confidence values indicated at the notes: the posterior probabilities from the Bayesian analysis before the backslash, bootstrap proportions from the ML analysis after the backslash. The “-” means lacking statistical support (<70 % for bootstrap proportions from ML analysis; <0.95 for posterior probabilities from Bayesian analysis). The branches with full statistical support (PP = 1.0; ML-BS = 100 %) are highlighted by thickened branches. Targeted genus/species clades are discriminated with boxes in different colours. The scale bar shows the expected number of changes per site. Type strains are marked with “T” after the culture number. The ITS tree is rooted with the Ceratocystis clade containing four species. The rpb2 tree is rooted with Chalaropsis thielavioides JCM1933.
Fig. 2.
Phylogenetic tree inferred from ML analysis of the partial rpb2 gene region alignment. The confidence values are indicated at the notes same to Fig. 1. Genus/species clades in the Chaetomiaceae and potential family/genus lineages in the other traditional family are discriminated with boxes in different colours. Type strains are marked with “T” after the culture number. “eT” or “NeoT” represents the ex-epitype or ex-neotype designated in the this study. Thielavia-like strains are indicated with a red star on its right side. The reference sequences retrieved from GenBank database or from the released genomic data were marked behind the strain numbers. The scale bar shows the expected number of changes per site. The tree is rooted with three species in the Microascales.
Fig. 4.
Phylogenetic confirmation of the placement of Thielavia basicola in the Ceratostomataceae based on the analysis of the D1/D2 domains of LSU sequences with the confidence values indicated at the notes same to Fig. 1. Targeted genus/species clades are discriminated with boxes in different colours. The scale bar shows the expected number of changes per site. The tree is rooted with two Berkeleyomyces species and one Microascus species in the Microascales.
Fig. 6.
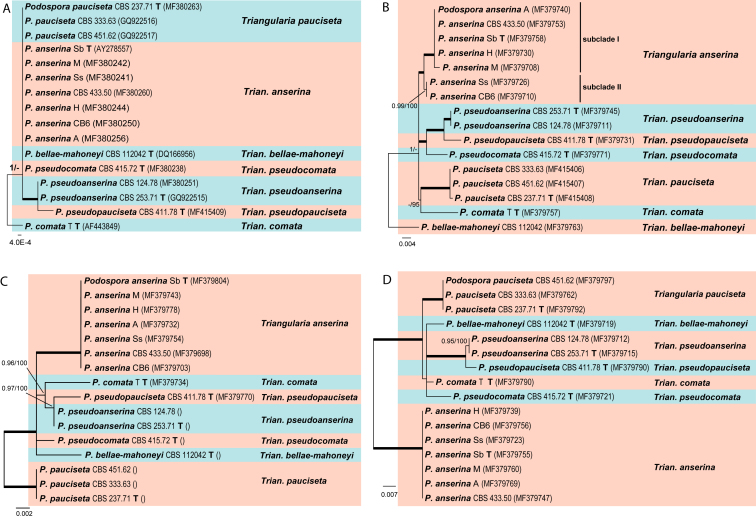
Delimitation of Podospora anserina and its closely related species based on the separate analyses of four loci from Boucher et al. (2017): ITS (A), Rchr3 on chromosome 3 (B), Rchr4 on chromosome 4 (C) and Rchr6 on chromosome 6 (D). Unrooted ML trees are shown with the confidence values indicated at the notes as in Fig. 1. Recognised species are discriminated with boxes in different colours. Type strains are marked with “T” after the culture number. The scale bar shows the expected number of changes per site.
Morphology
Colony morphology and microscopic morphology were examined as described by Wang et al. (2019). In short, strains were grown on OA, cornmeal agar (CMA), malt extract agar (MEA) and potato carrot agar (PCA) at 25 °C (or 37 °C for Thermothielavioides terrestris) in darkness. After 7 d incubation, colony diameters on the various media were measured. Incubation on OA continued and growth was monitored until informative structures such as ascomata, asci, ascospores and/or an asexual morph were observed. Morphological data on those structures were obtained from microscopic slides under a Nikon Eclipse 80i compound microscope equipped with differential interference contrast (DIC) illumination, and from observation under Nikon SMZ 1500 dissecting-microscope. At least 30 measurements were made for all morphologically informative features.
Results
Phylogeny
The matrix statistics and related characters resulting from the phylogenetic analyses of all the datasets in the present study are summarised in Table 2.
Table 2.
A summary of matrix statistics for each alignment analysed phylogenetically in this study.
| Analyses | ITS |
rpb2 |
4-locus combined |
LSU |
tub2 |
tef1-α |
Rchr3 |
Rchr4 |
Rchr6 |
||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Berkeleyomyces1 | P. anserina5 | Berkeleyomyces1 | Thielavia2 | Thielavia2 | Ceratostomataceae3 | Canariomyces4 | Canariomyces4 | P. anserina5 | P. anserina5 | P. anserina5 | |
| Characters of alignments | |||||||||||
| Number of ingroup taxa | 19 | 16 | 10 | 280 | 221 | 16 | 7 | 7 | 16 | 16 | 16 |
| Number of outgroup taxa | 4 | 0∗ | 1 | 3 | 3 | 3 | 6 | 5 | 0∗ | 0∗ | 0∗ |
| Number of nucleotide characters including gaps | 530 | 518 | 1016 | 900 | 3648 | 577 | 703 | 1440 | 838 | 755 | 524 |
| Number of constant characters | 345 | 514 | 830 | 315 | 1355 | 365 | 424 | 1088 | 756 | 727 | 467 |
| Number of parsimony-informative characters | 111 | 1 | 128 | 551 | 2009 | 168 | 190 | 201 | 30 | 13 | 42 |
| Number of parsimony-uninformative characters | 74 | 3 | 58 | 34 | 284 | 44 | 89 | 151 | 52 | 15 | 15 |
| Statistics for the Bayesian analyses | |||||||||||
| Substitution model | GTR+I+G | HKY | K80 | GTR+I+G | GTR+I+G for each | GTR+G | HKY+G | GTR+G | SYM | HKY | K80 |
| Number of generated trees | 2385 | 168 | 84 | 31878 | 13053 | 1513 | 5 | 45 | 1109 | 1500 | 1180 |
| Number of trees discarded as the ‘‘burn-in’’ phase | 596 | 42 | 21 | 7969 | 3263 | 378 | 1 | 11 | 277 | 375 | 295 |
| Number of trees used for final tree | 1789 | 126 | 63 | 23909 | 9790 | 1135 | 4 | 34 | 832 | 1125 | 885 |
∗: indicating an unrooted tree.
Dataset for identification of the host (asexual partner) in the culture of CBS 178,82.
Dataset for taxonomic study of morphologically identified Thielavia and potentially related taxa.
Dataset for determining the phylogenetic placement of Thielavia basicola in the family Ceratostomataceae.
Dataset for delimitation of species in the Canariomyces clade.
Dataset for delimitation of “Podospora anserina” and its closely related species.
Phylogenetic identification of the host of Thielavia basicola (Fig. 1)
The ITS and rpb2 sequences generated from the host of Th. basicola in culture CBS 178.82 were added and aligned with the sequences used in Nel et al. (2018). The asexual host in the culture CBS 178.82 resides in the ITS (Fig. 1A) and rpb2 (Fig. 1B) phylograms with three strains identified as Berkeleyomyces rouxiae. The reference strain CBS 341.33 clusters in the sister clade Berkeleyomyces basicola. These two species proved to be separated from the Thielaviopsis lineage as shown in the ITS tree (Fig. 1A).
Phylogeny of morphologically identified Thielavia species and potentially related taxa
The rpb2 and the concatenated ITS, LSU, tub2 and rpb2 sequences data set were both used to resolve the phylogenetic positions of the studied Thielavia species. Thirty-three strains that were previously described or identified in Thielavia and 34 additional strains were included in our study. The latter 34 strains were found to be related to the targeted species based on a preliminary phylogenetic analysis of LSU sequences (data not shown) and the majority of them belonged to the Chaetomiaceae, Lasiosphaeriaceae and Sordariaceae in Sordariales. In the Chaetomiaceae, five representative species of Acrophialophora (nine strains), five Madurella species (eight strains), Boothiella (two strains) and six chaetomium-like species (16 strains) were selected, which were not yet included in our previous studies within this family. Twenty-seven species presumably belonging to the Lasiosphaeriaceae were included which were traditionaly identified in the genera Apiosordaria, Cladorrhinum, Podospora, Triangularia or Zopfiella. The remaining strains were traditionally identified in Stellatospora (considered to be in the Sordariaceae), Microthecium (six isolates) in the Ceratostomataceae (Melanosporales) or Canariomyces notabilis (considered to be in the Microascaceae, Microascales).
Rpb2 phylogeny (Fig. 2)
The rpb2 data set contained sequences of 283 strains. Compared with the four-locus concatenated dataset, 26 additional species of Lasiosphaeriaceae were included, representing the genera Bombardia, Cercophora, Corylomyces, Jugulospora, Lasiosphaeria, Rinaldiella, Strattonia and Schizothecium. Seven species belonging to Gelasinospora, Neurospora and Sordaria in Sordariaceae were included in the analysis, as well as Xylaria hypoxylon, a representative of the order Xylariales. Microascus trigonosporus and the two species of Berkeleyomyces (Microascales) were selected as outgroup species, based on the phylogenetic relationships between orders in the Sordariomycetes (Maharachchikumbura et al. 2016). The ML tree topology confirmed the tree topology obtained from the BI analysis, and therefore, only the ML tree is presented with the PP values indicated at the nodes.
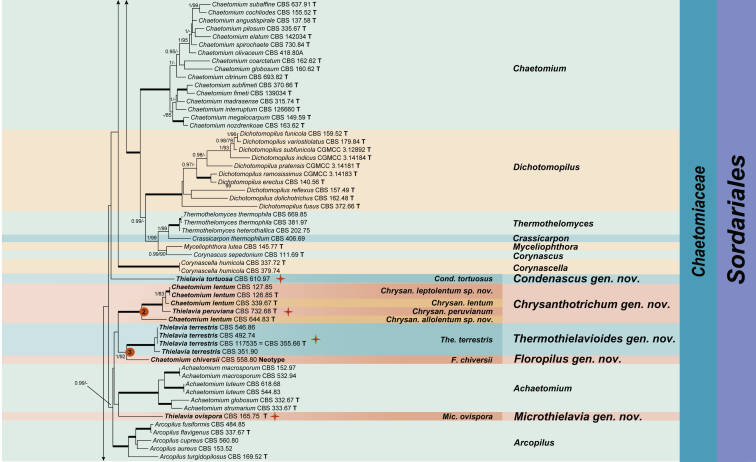
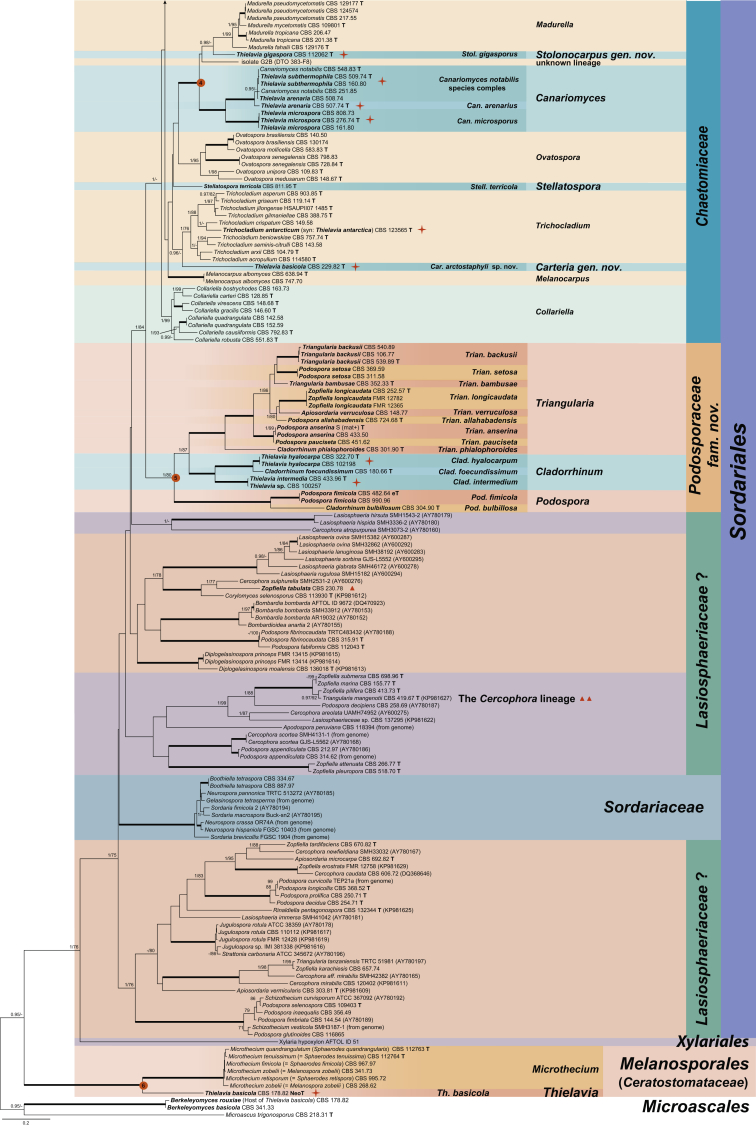
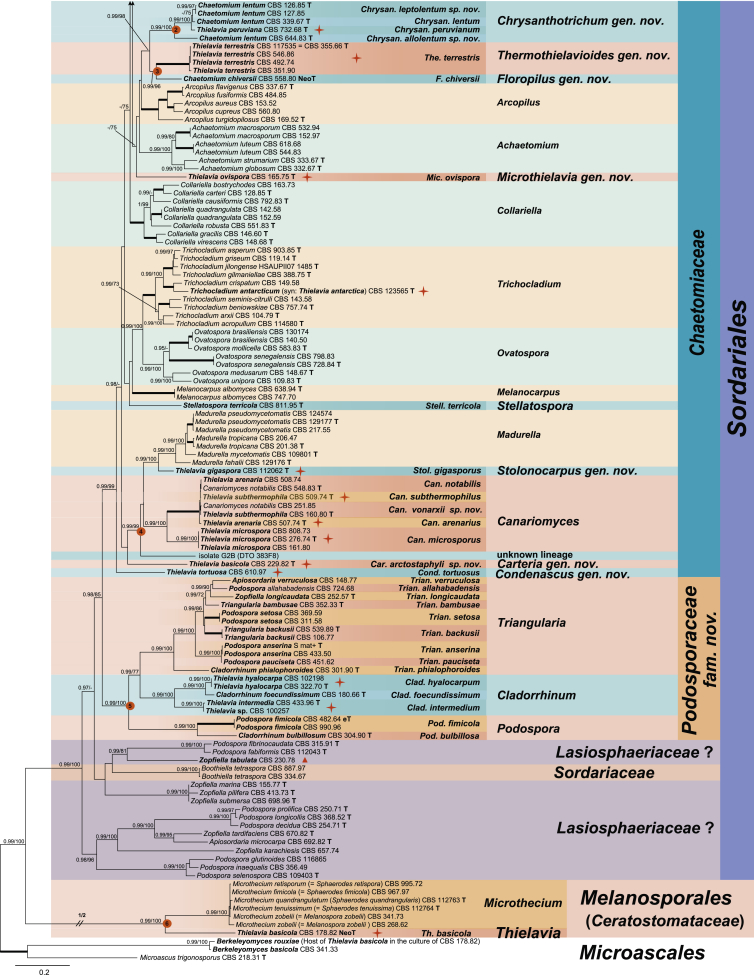
The resulting phylogenetic tree resolved the 33 studied Thielavia strains as 18 species-level clades (marked with an orange star) in 11 generic lineages. Fifteen species-level clades belonged to six well-supported main clades (indicated with 1–6 in red circle boxes at the nodes). The remaining three strains formed single lineages in the Chaetomiaceae: Thielavia tortuosa (CBS 610.97), Th. ovispora (CBS 165.75) and the strain CBS 229.82 which was once used as a representative strain of Th. basicola (Stchigel et al. 2002). Four of the recognised main clades belonged to the Chaetomiaceae (Clades 1–4; PP = 1 and ML-BS ≥ 92 %). Clade 1 (PP = 1; ML-BS = 98 %) grouped into six lineages: three encompassing Thielavia species, two chaetomium-like species (Ch. lucknowense and Ch. variosporum) and the existent genus Acrophialophora. Clade 2 (PP = 1; ML-BS = 100 %) consisted of Th. peruviana (ex-type CBS 732.68) and four isolates of Ch. lentum, which were split into three species lineages. In Clade 3 (PP = 1; ML-BS = 92 %), Ch. chiversii (ex-type CBS 558.80) clustered close but separate from the thermophilic species Th. terrestris. Clade 4 (PP = 1; ML-BS = 100 %) consisted of four generic lineages: Th. gigaspora (ex-type CBS 112062) clustered close but separate from the existent genus Madurella and an unknown lineage (G2B); Th. arenaria and Th. subthermophila grouped with the type species of Canariomyces, Can. notabilis, which formed a sister clade to another species, Th. microspora.
The fifth main clade (Clade 5, PP = 1 and ML-BS = 80 %) was part of the polyphyletic Lasiosphaeriaceae containing three fully-supported generic lineages (PP = 1 and ML-BS = 100 %). Two “Thielavia” species, Th. hyalocarpa and Th. intermedia, grouped with the type species of Cladorrhinum, Clad. foecundissimum. Two isolates of Podospora fimicola, the type species of Podospora (Pod.), grouped with Clad. bulbillosum. The third lineage contained members of five genera: Apiosordaria, Cladorrhinum, Podospora, Triangularia and Zopfiella. The type species of both Apiosordaria (A. verruculosa, 1967) and Triangularia (Trian. bambusae, 1934) were included in this lineage. The type species of Zopfiella, Z. tabulata (ex-type CBS 230.78, marked with a red triangle on the right), was located in another lineage, distant from Clade 5 in the polyphyletic Lasiosphaeriaceae. In Clade 6 (PP = 1 and ML-BS = 100 %), the representative isolate of the type species Th. basicola, CBS 178.82, clustered close but separate from the genus Microthecium in the Ceratostomataceae (Melanosporales). Members of the monophyletic family Sordariaceae form a distinct lineage, while members of the Lasiosphaeriaceae are distributed over at least four unrelated clades (with exception of Clade 5). Stellatospora terricola formed a single lineage in the Chaetomiaceae rather than in the Sordariaceae, while Boothiella tetraspora clustered in the Sordariaceae.
Four-locus phylogeny (Fig. 3)
Fig. 3.
Phylogenetic tree resulting from ML analysis of the concatenated partial rpb2, tub2, ITS and LSU gene region alignment, with the confidence values indicated at the notes same to Fig. 1. Genus/species clades in the Chaetomiaceae and potential family/genus lineages in the other traditional family are discriminated with boxes in different colours. Type strains are marked with “T” after the culture number. “eT” represents the ex-epitype designated in this study. Thielavia-like strains are indicated with a red star on its right side. The scale bar shows the expected number of changes per site. The tree is rooted with three species in the Microascales.
The concatenated alignment included 224 isolates, with the same outgroups as in the rpb2 phylogeny. The sequence dataset consisted of 3 648 characters (including gaps) composed of four partitions: 891 characters for rpb2, 1 373 characters for tub2, 805 characters for ITS and 579 characters for the D1/D2 regions of LSU. All nine thielavia-related clades (Clades 1–6 and three single lineages) recognised in the rpb2 alignment were supported in the four-locus phylogeny with robust supports (PP = 0.99 and ML-BS ≥ 96 %). The phylogenetic relationships of the 11 generic lineages containing “Thielavia” species with their related genera were also confirmed with confident support. At the same time, the four-locus phylogeny confirmed the sister relationship between the Chaetomiaceae and Clade 5 (PP = 0.98, ML-BS = 85 %), and provided further evidence for the polyphyly of the Lasiosphaeriaceae. In addition, the classification of Stellatospora terricola in Chaetomiaceae and Boothiella tetraspora in Sordariaceae was confirmed in this phylogenetic analysis.
Confirmation of the placement of Thielavia basicola in the Ceratostomataceae based on LSU phylogeny (Fig. 4)
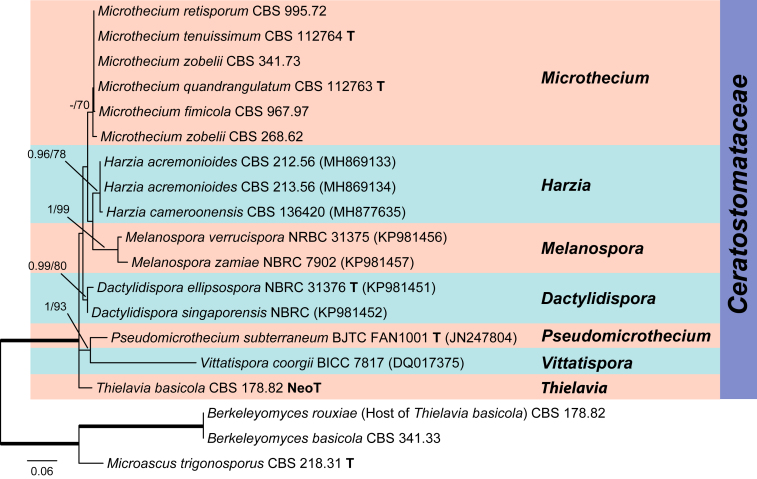
The LSU alignment consisted of 19 isolates, including representatives of six genera in the family Ceratostomataceae, namely Dactylidispora, Harzia, Melanospora, Microthecium, Pseudomicrothecium and Vittatispora. Microascus trigonosporus and the two species of Berkeleyomyces residing in the Microascales were used as outgroup species. This analysis clearly showed that Th. basicola clustered within the Ceratostomataceae (PP = 1; ML-BS = 100 %).
Delimitation of species in the Canariomyces clade (Fig. 5)
The rpb2 phylogeny (Fig. 2) failed to differentiate Th. arenaria, Th. subthermophila and Can. notabilis, even though these species are morphologically different (this study, Mouchacca, 1973, von Arx, 1975, von Arx, 1984, von Arx et al., 1988). Single gene trees based on tub2 and tef1-α data sets were constructed to delimit the species in the Canariomyces clade. The tub2 and tef1-α phylogenies were concordant. Four lineages were recognised and these lineages agreed with the observed or reported morphology.
Re-delimitation of Podospora anserina and its closely related species (Fig. 6)
To better understand the relationship between Podospora anserina and its closely related species, we re-analysed the published sequence data generated by Boucher et al. (2017), including ITS sequences and sequences of three other intergenic loci from different chromosomes (Rchr3, Rchr4 and Rchr6). Single gene trees were constructed based on each locus. ITS failed to distinguish Pod. anserina from Pod. pauciseta, Pod. bellae-mahoneyi and Pod. pseudocomata (Fig. 6A). In contrast, Rchr3 (Fig. 6B), Rchr4 (Fig. 6C) and Rchr6 (Fig. 6D) differentiated all seven species which were accepted by Boucher et al. (2017), and Rchr3 even recognised two subclades within the P. anserina clade.
Taxonomy
Nineteen species are recognised in the 33 studied “Thielavia” strains. Thielavia basicola is transferred to the Ceratostomataceae (Melanosporales) based on its phylogenetic affinity with Microthecium as well as five other genera in the family (Fig. 2, Fig. 3, Fig. 4). Clade 5 is a sister clade to the Chaetomiaceae and is proposed as the new family Podosporaceae. This family accommodates the three re-defined genera Cladorrhinum, Podospora and Triangularia, which were all previously positioned in the polyphyletic Lasiosphaeriaceae. Thielavia hyalocarpa and Th. intermedia are transferred to Cladorrhinum based on their phylogenetic affinities with the type species of this genus. Canariomyces was previously classified in the Microascaceae and is here transferred to Chaetomiaceae. This re-defined genus includes Th. arenaria, Th. microspora and Th. subthermophila. Furthermore, nine new genera in the Chaetomiaceae are introduced to accommodate species with a thielavia-morph. These genera are Carteria, proposed for CBS 229.82 which was previously identified as Th. basicola, but doesn’t have an association with Thielaviopsis basicola; Chrysanthotrichum for Th. peruviana, Ch. lentum and two new species which are morphologically similar but phylogenetically separate from Ch. lentum; Condenascus for Th. tortuosa; Hyalosphaerella for Th. fragilis; Microthielavia for Th. ovispora; Parathielavia for Th. appendiculata, Th. hyrcaniae and Th. kuwaitensis; Pseudothielavia for Th. arxii, Ch. hamadae, Th. terricola and CBS 473.86 representing a new species which was deposited in the CBS collection as Ch. hamadae; Stolonocarpus for Th. gigaspora; Thermothielavioides for the thermophilic species Th. terrestris. To clarify the phylogenetic relationships of thielavia-like taxa in the Chaetomiaceae, three chaetomium-like single lineages are introduced as new genera. These are Chrysocorona to accommodate Ch. lucknowense, Brachychaeta for Ch. variosporum in Clade 1 and Floropilus for Ch. chiversii in clade 3, which is a sister to Thermothielavioides. In addition, the genus Acrophialophora is redefined to include two sexually reproducing chaetomium-like species (Acr. jodhpurensis and Acr. teleoafricana). Our phylogenetic analysis also showed that monotypic Stellatospora, typified with Stell. terricola, is a genus in the Chaetomiaceae and the monotypic genus Boothiella, typified with Booth. tetraspora, belongs to the Sordariaceae. New combinations are provided for those species names where the generic classification changed. Twenty genera and 46 species are (re-)described and illustrated, including the species available that were previously described in Thielavia and those that are related to the studied “Thielavia” species.
Melanosporales, Ceratostomataceae
Thielavia Zopf, Verh. Bot. Vereins. Prov. Brandenburg. 18: 105. 1876.
Micromorphology: Growing in close association with Berkeleyomyces species. Ascomata superficial, usually solitary, non-ostiolate, globose or subglobose, often surrounded by the conidiophores and conidia of the host. Ascomatal wall subhyaline to brown, translucent. Asci subglobose to ellipsoidal or ovate, evanescent before ascospores become mature. Ascospores 1-celled, brown when mature, smooth, ellipsoidal with attenuated ends, or fusiform, with a germ pore.
Type species: Thielavia basicola Zopf.
Notes: Thielavia basicola was originally described to develop on the root of the plant species Senecio elegans (common names: redpurple ragwort, purple groundsel, wild cineraria and purple ragwort) in Germany and is associated with the morphologicaly defined Thielaviopsis basicola (Zopf 1876). The lack of type material of the type species hinders the attempt to properly resolve the taxonomy of Thielavia. In the present study, the type species Th. basicola is neotypified with CBS H-18808 (see the details below). This material originates from CBS 178.82, a strain isolated from the plant species Phaseolus vulgaris (common names: kidney bean, pea bean and French bean) and containing Th. basicola and its host which was previously identified as Thielaviopsis basicola. The taxonomy of Thielaviopsis basicola and allied genera/species was subject of various studies (Mbenoun et al., 2014, de Beer et al., 2014, Nel et al., 2018). Mbenoun et al. (2014) epitypified Thielaviopsis ethacetica which is the type species of Thielaviopsis, and transferred Thielaviopsis to the genus Ceratocystis. De Beer et al. (2014) resurrected and redefined the genus Thielaviopsis. Thielaviopsis was considered to produce both a sexual and asexual state, and the members of this genus have stellate appendages on the globose basal part of the ascomata. Thielaviopsis basicola was not included in their study and this species would not fit in their generic description. More recently, Nel et al. (2018) phylogenetically re-evaluated Thielaviopsis basicola. They introduced the new genus Berkeleyomyces to accommodate Thielaviopsis basicola and described a new, cryptic sister species, B. rouxiae. These two species are morphologically undistinguishable (Nel et al. (2018). In the present study, we obtained ITS, LSU, rpb2 and tub2 sequences of both fungi in the culture CBS 178.82. The hyphomycete in CBS 178.82 is identified as B. rouxiae (Fig. 1). The host fungus in the culture of Zopf (1876) used for the description of Th. basicola could have been B. rouxiae or B. basicola based on its morphology. The association of Th. basicola in CBS 178.82 with Berkeleyomyces (= Thielaviopsis basicola sensu lato) and the substrate (isolated from plant roots) agrees with the original description of Th. basicola (Zopf 1876). Even though CBS 178.82 originates from Canada and the holotype is from Germany, and their plant host species differ, still von Arx et al. (1988) used CBS 178.82 as a representative strain for Th. basicola. Our morphological examination of CBS 178.82 confirmed that the sexually reproducing fungus agrees well with the original description of the holotype of Th. basicola (Fig. 7), and the hyphomycete fits with the description of Berkeleyomyces species as noted above (Fig. 8). Phylogenetic analyses (Fig. 2, Fig. 3, Fig. 4) indicated that the Th. basicola strain in CBS 178.82 clustered close, but separate from Microthecium and five other genera in the family Ceratostomataceae (Melanosporales).
Fig. 8.
Berkeleyomyces rouxiae, host of Thielavia basicola (CBS 178.82). A–B. Part of the colony on TSA, showing thick-walled conidia arising from aerial hyphae. C–G. Dimorphic synanamorphs: conidiophores, phialides and two types of conidia. Scale bars: C–G = 10 μm.
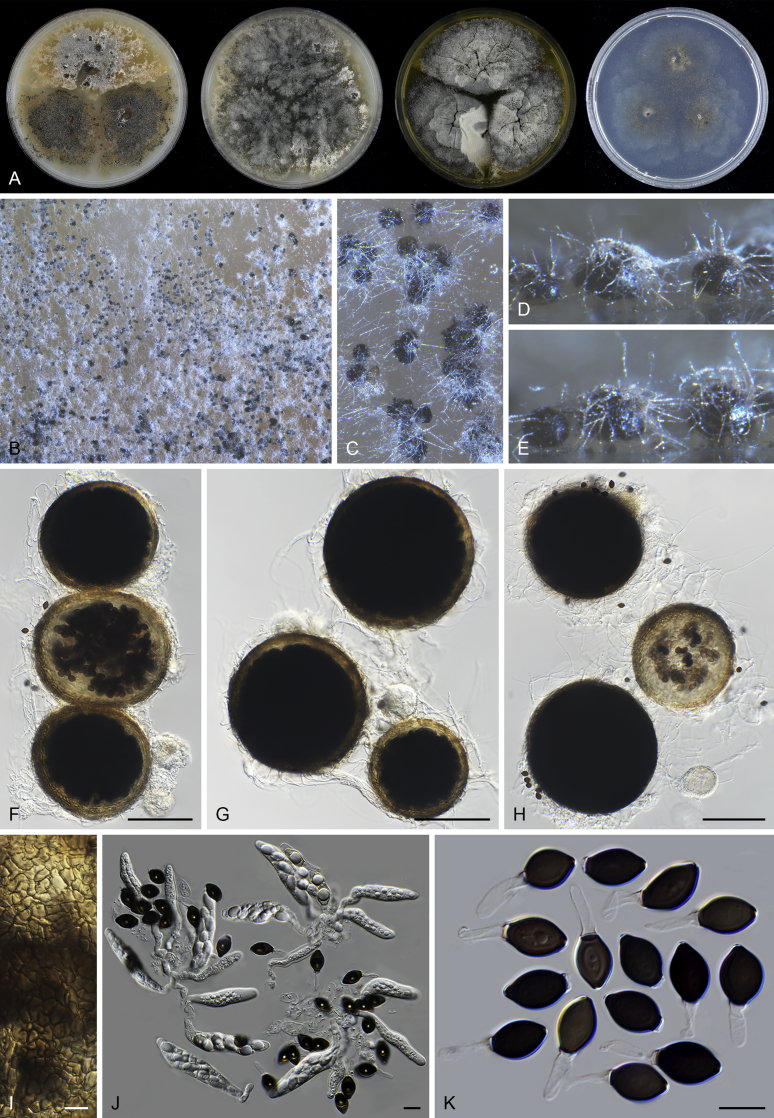
Microthecium, Melanospora and Sphaerodes in the Ceratostomataceae have a tumultuous taxonomic history with conflicts between morphology and phylogeny (Cannon and Hawksworth, 1982, Zhang and Blackwell, 2002, Schultes et al., 2017). In the past, many Microthecium species were transferred to Melanospora or Sphaerodes, including those selected in this study as the representatives of Ceratostomataceae (Fig. 2, Fig. 3). A recent study (Marin-Felix et al. 2018) phylogenetically re-evaluated the taxonomy of Melanospora and related taxa. Melanospora was restricted to species producing ostiolate ascomata whose neck is composed of intermixed hyphae, and having a phialidic asexual morph. Sphaerodes was treated as a synonym of Microthecium. Microthecium was re-established for Melanospora and Sphaerodes species without the typical characters of Melanospora described above. An analysis of a LSU dataset including representative species of six genera in Ceratostomataceae confirmed the placement of Th. basicola in this family (Fig. 4).
Species in the order Melanosporales (comprises the family Ceratostomataceae) are characterised by the production of usually translucent ascomata, unitunicate asci, and unicellular, pigmented ascospores with germ pores or germ slits (Marin-Felix et al. 2018). Thielavia basicola produces translucent ascomata, unitunicate asci, and unicellular, pigmented ascospores with a germ pore, fitting in the family Ceratostomataceae. Moreover, most of the species in the Ceratostomataceae are known to be parasitic on or closely associated with other fungi, including basidiomycetes and sexual and asexual reproducing ascomycetes (Jeffries and Young, 1994, Harveson, 1999, Zhang and Blackwell, 2002, Marin-Felix et al., 2018). These data provide robust support for the phylogenetic placement of Th. basicola in the Ceratostomataceae, rather than Chaetomiaceae. As a consequence, the Thielavia species classified in the latter family should be combined in other or new genera.
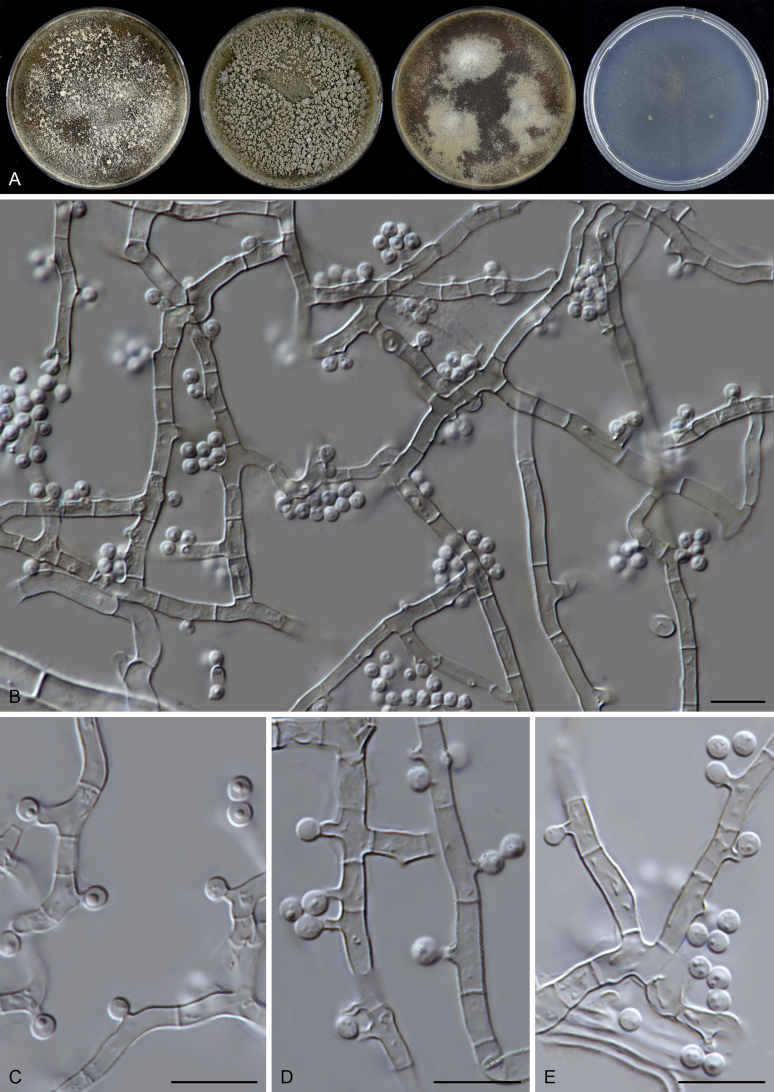
Thielavia basicola Zopf, Verh. Bot. Vereins Prov. Brandenburg 18: 105. 1876. Fig. 7.
Micromorphology: Ascomata superficial, usually solitary, non-ostiolate, globose or subglobose, often surrounded by the conidiophores and conidia of the host (Berkeleyomyces rouxiae), (80–)105–260 μm diam. Ascomatal wall subhyaline to brown, translucent, composed of 4–6 layers of cells. Asci subglobose to ellipsoidal or ovate, 14–19 × 12.5–17 μm, usually without visible stalks, containing eight irregularly-arranged ascospores, evanescent, but often persistent until ascospores mature. Ascospores 1-celled, brown when mature, smooth, fusiform, umbonate at both ends, (8.5–)9.5–11.5(–13.5) × (5–)5.5–7 μm, with an apical germ pore. Asexual morph not observed.
Culture characteristics (mixed with the host): On OA with an entire edge, 40–46 mm diam in 7 d at 25 °C, obverse fawn due to conidia of the host, reverse uncoloured. On CMA similar to those on OA, 35–41 mm diam in 7 d at 25 °C. On MEA with an entire or slightly crenate edge, 37–43 mm diam in 7 d at 25 °C, texture floccose due to the host, obverse vinaceous buff to fawn, reverse fawn. On PCA transparent, with a crenate edge, 22–28 mm diam in 7 d at 25 °C, with sparse aerial mycelium, obverse vinaceous buff, reverse uncoloured.
Typus: Canada, Ontario, Toronto, isolated from diseased root of Phaseolus vulgaris, Mar. 1981, A. Carter (CBS H-18808, neotype designated here, MBT 385801, culture ex-neotype CBS 178.82 = MUCL 40417).
Notes: Our attempts in finding the holotype of Th. basicola in B (Botanischer Garten und Botanisches Museum Berlin-Dahlem, Germany), HAL (Martin-Luther-Universität, Halle-Wittenberg, Halle, Germany) and K (Royal Botanic Gardens, Kew, UK) were unsuccessful. The holotype of this species seems to be lost. There are two strains deposited as Th. basicola in the CBS culture collection: CBS 178.82 and CBS 229.82. The two isolates produce similar ascomata and asci in shape. CBS 229.82 grows independently on the agar media, while CBS 178.82 grows in close association with Berkeleyomyces rouxiae (Fig. 8). This association hampered the use of CBS 178.82 as it was often poorly sporulating and difficult to obtain sequence data from its culture. Probably that is why CBS 229.82 was once used as a representative strain of Th. basicola (Stchigel et al. 2002). After successfully inducing ascomata production of CBS 178.82 in the present study (Fig. 7B, C), morphological examination showed that CBS 178.82 produces fusiform and larger ascospores (9.5–11.5 × 5.5–7 μm vs 8–9 × 4.5–5.5 μm) than the ellipsoidal ones of CBS 229.82. The former strain also produced larger ascomata than those of CBS 229.82 (105–260 μm vs 25–85 μm diam). The holotype of Th. basicola was described with ascomata developing on roots of Senecio elegans in association with Thielaviopsis basicola, measuring 80–170 μm diam, containing fusiform ascospores 9–12 × 5–6.5 μm (fide Booth, 1961, von Arx, 1975). It is clear that the morphology and ecology of CBS 178.82 fits that of the holotype of Th. basicola quite well. To stabilize the use of the species name, we propose the specimen CBS H-18808 from the strain CBS 178.82 as the neotype of Th. basicola.
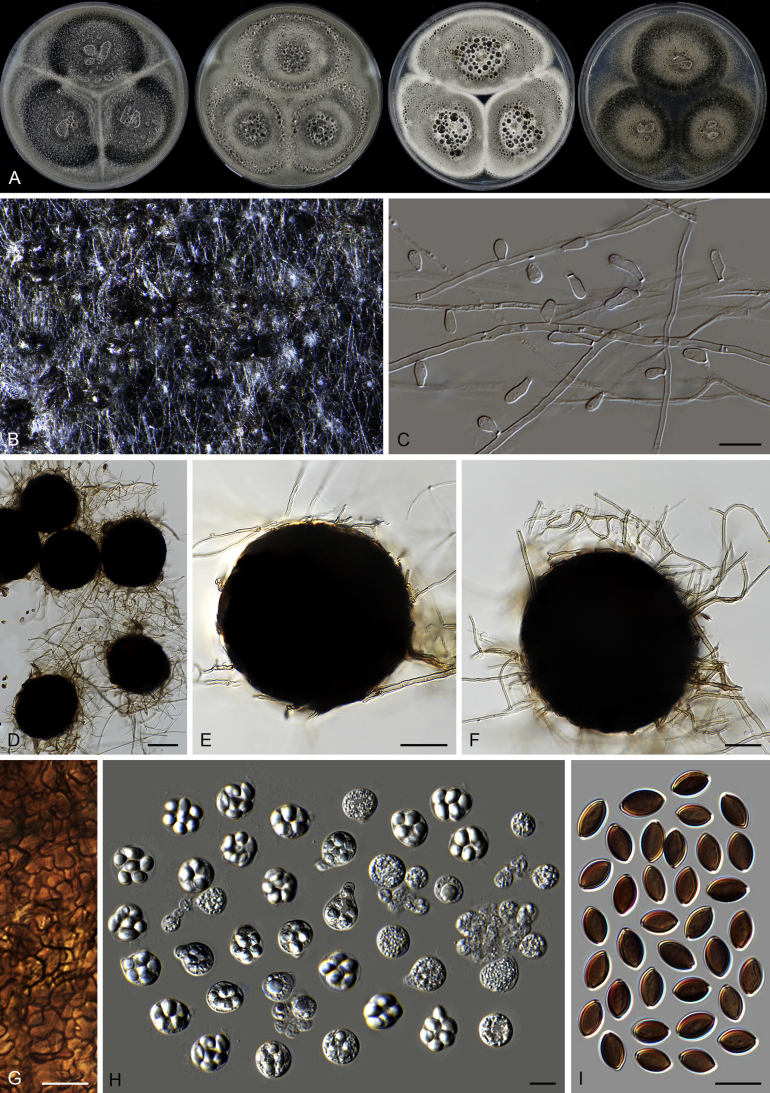
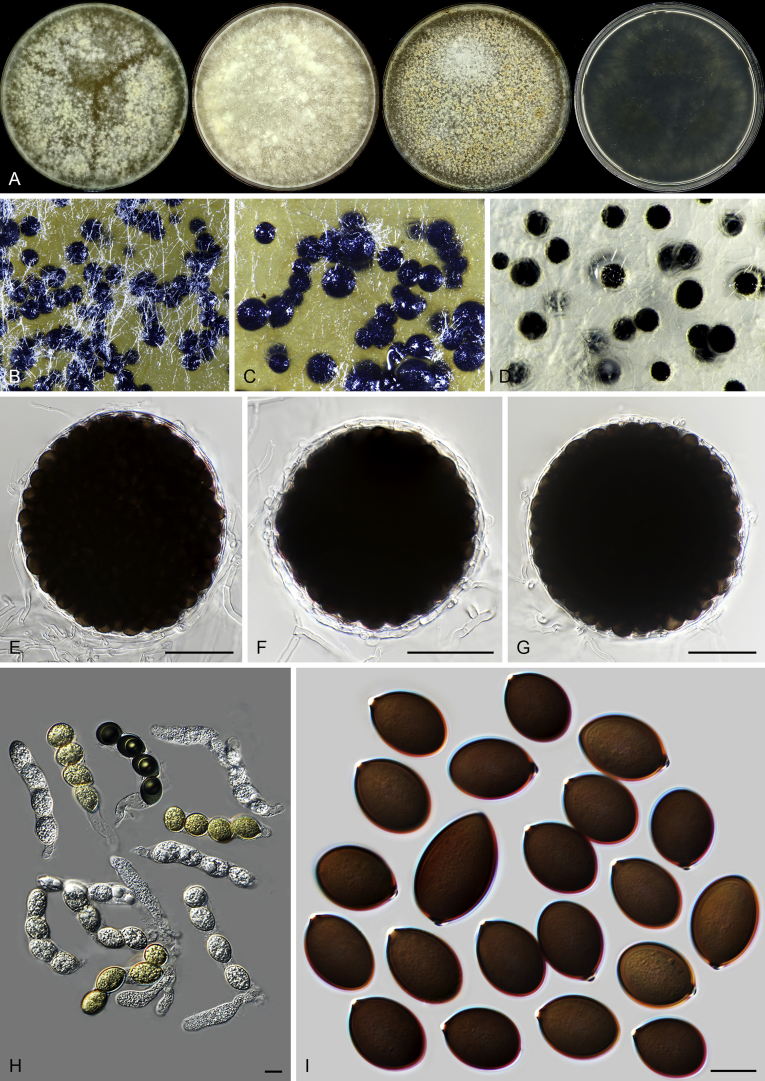
The morphology of Microthecium tenuissimum (syn.: Sphaerodes tenuissima) is also presented in this study as a representative species in the Ceratostomataceae (Fig. 9). This species possesses a similar morphology to that of Th. basicola: non-ostiolate and translucent ascomata, evanescent asci which are often persistent until ascospores mature, and fusiform and pigmented ascospores with two apical germ pores.
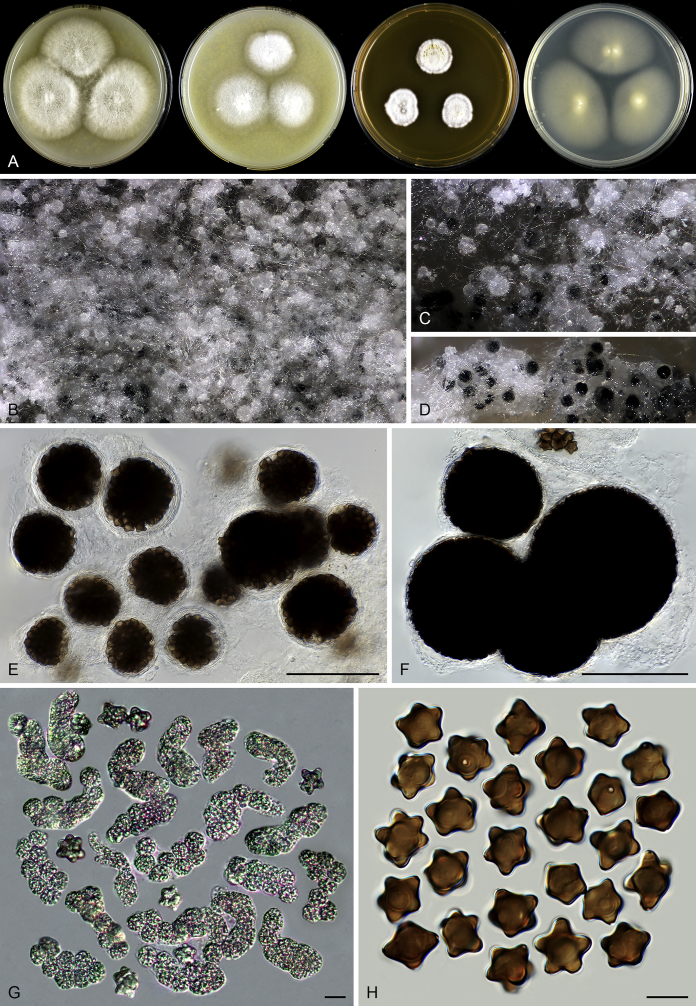
Fig. 9.
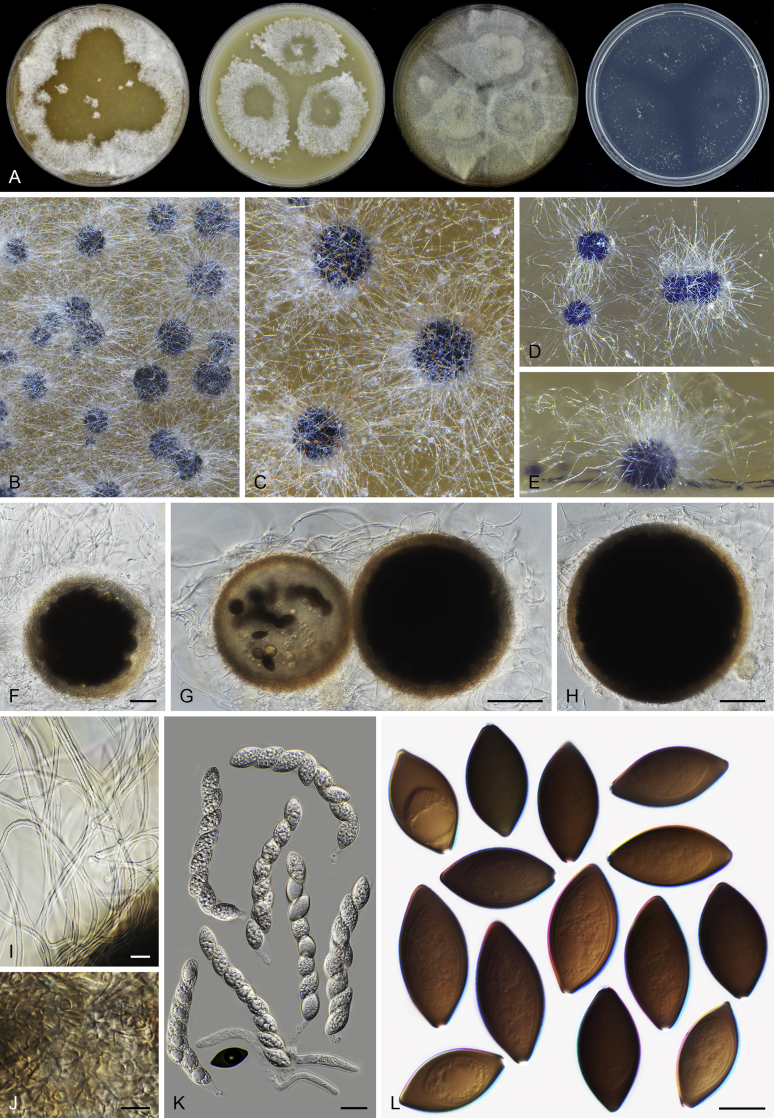
Microthecium tenuissimum (CBS 112764, ex-type culture). A. Colonies from left to right on OA, CMA, MEA and PCA after 3 wk incubation. B–C. Mature ascomata on MEA, top view. D–F. Ascomata mounted in lactic acid. G. Structure of ascomatal wall in surface view. H. Asci. I. Ascospores. Scale bars: D = 50 μm; E–F = 20 μm; G–I = 10 μm.
Sordariales, Chaetomiaceae
Acrophialophora Edward, Mycologia 51: 784. 1961.
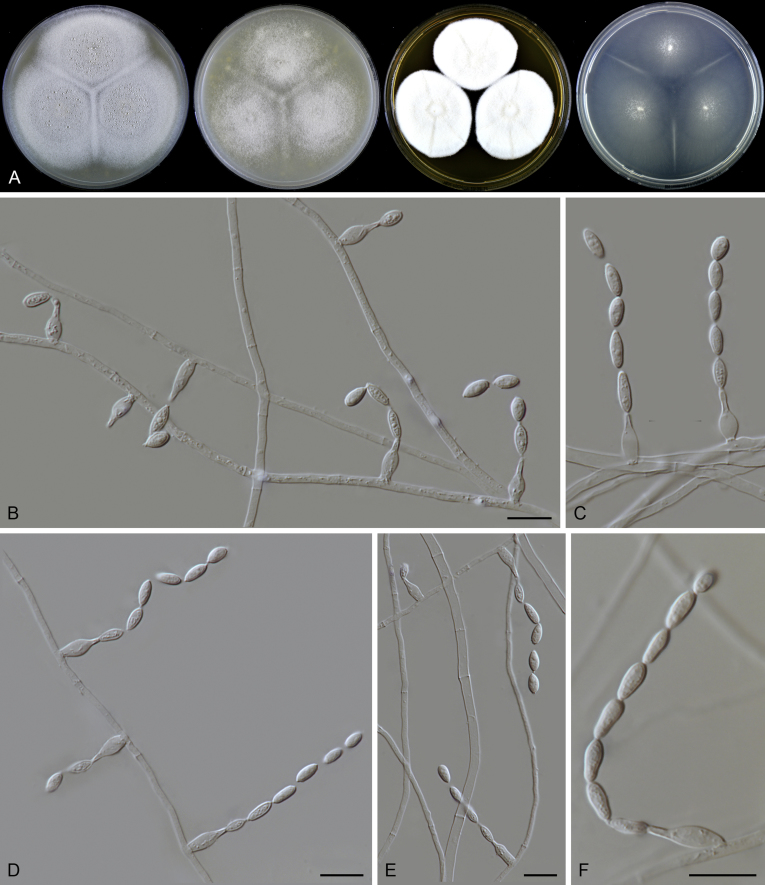
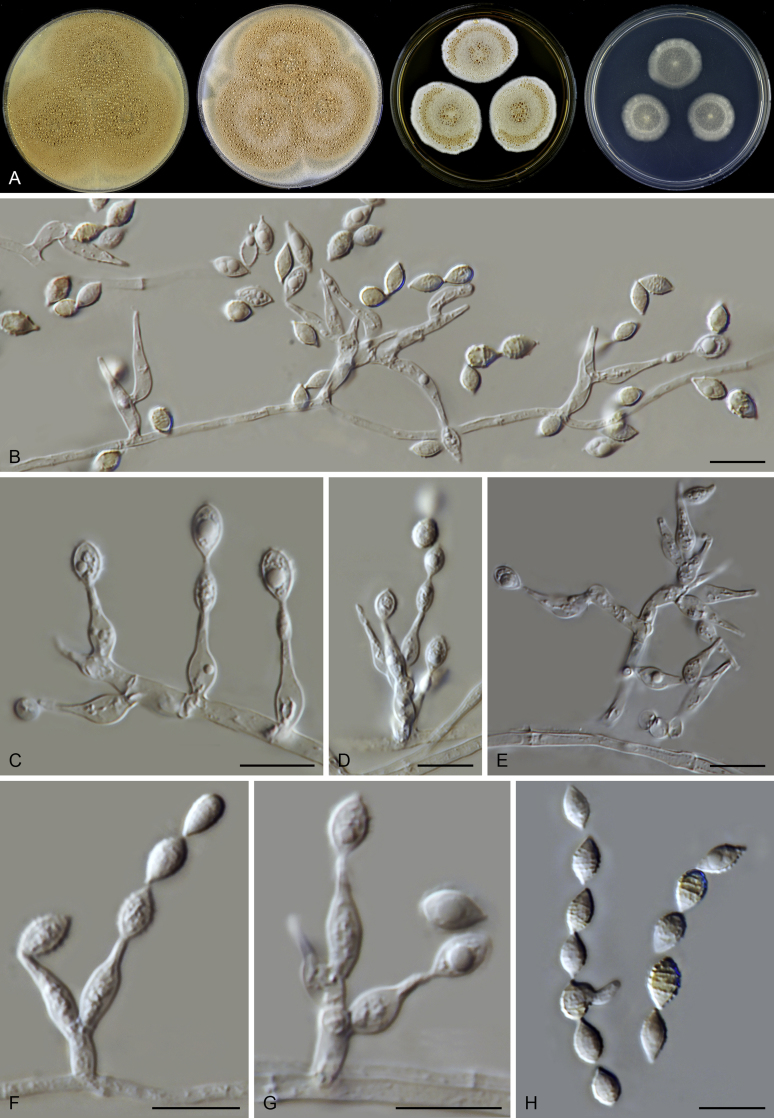
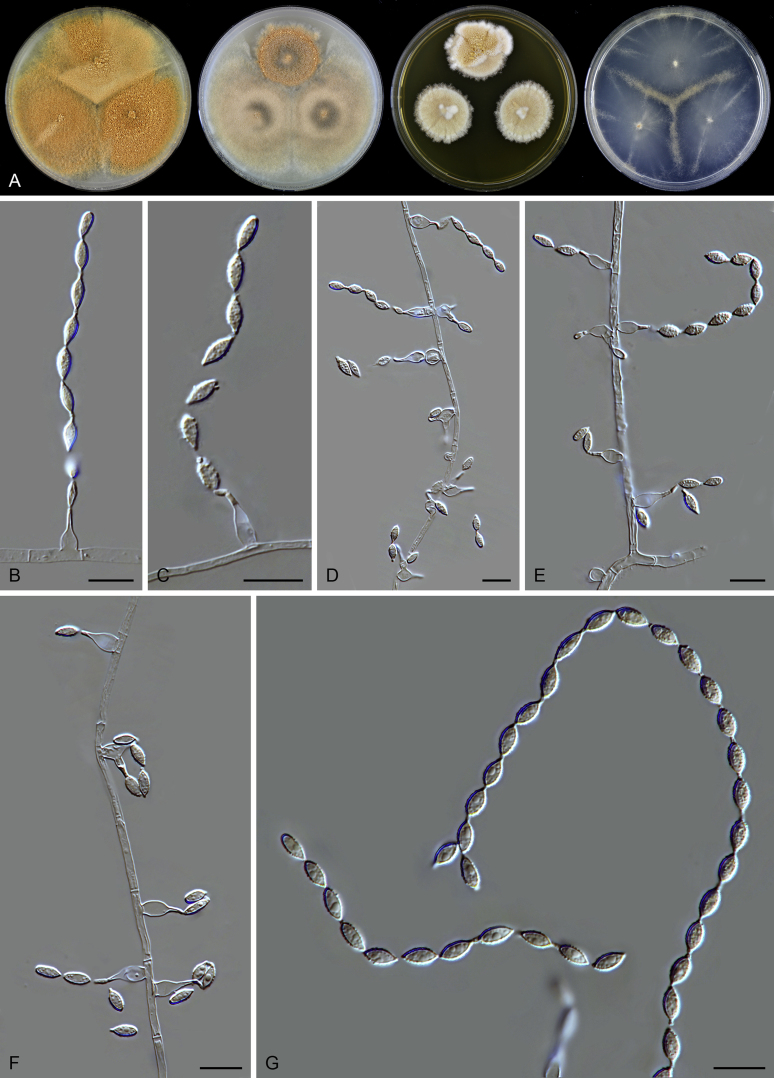
Micromorphology of asexual species: Hyphae hyaline or pigmented, branched, septate. Conidiophores arising laterally from hyphae, differentiated to be thick-walled, warty, unbranched, erect, pigmented, fading towards tips, or reduced to conidiogenous cells. Conidiogenous cells phialidic, flask-shaped, swollen at the base, taping to a narrow neck, either on differentiated conidiophores, arranged in whorls or verticils near or at the apex, or on undifferentiated aerial hyphae, in latter case solitary, sometimes proliferating. Conidia 1-celled. formed in basipetal chains, hyaline to subhyaline, ovoid, ellipsoidal to fusiform, smooth or punctate. Sexual morph not observed in the known asexual species. Micromorphology of sexual species: Ascomata superficial, ostiolate, subglobose to ovate. Ascomatal wall brown, of textura intricata in surface view. Terminal hairs flexuous or undulate, occasionally branched, brown, septate. Lateral hairs flexuous. Asci fasciculate, clavate or fusiform, stalked, containing eight biseriate or irregularly-arranged ascospores, evanescent. Ascospores 1-celled, olivaceous brown when mature, smooth, 1-celled, fusiform or ellipsoidal, with a subapical or lateral germ pore. Asexual morph not observed in the known asexual species.
Type species: Acrophialophora nainiana Edward.
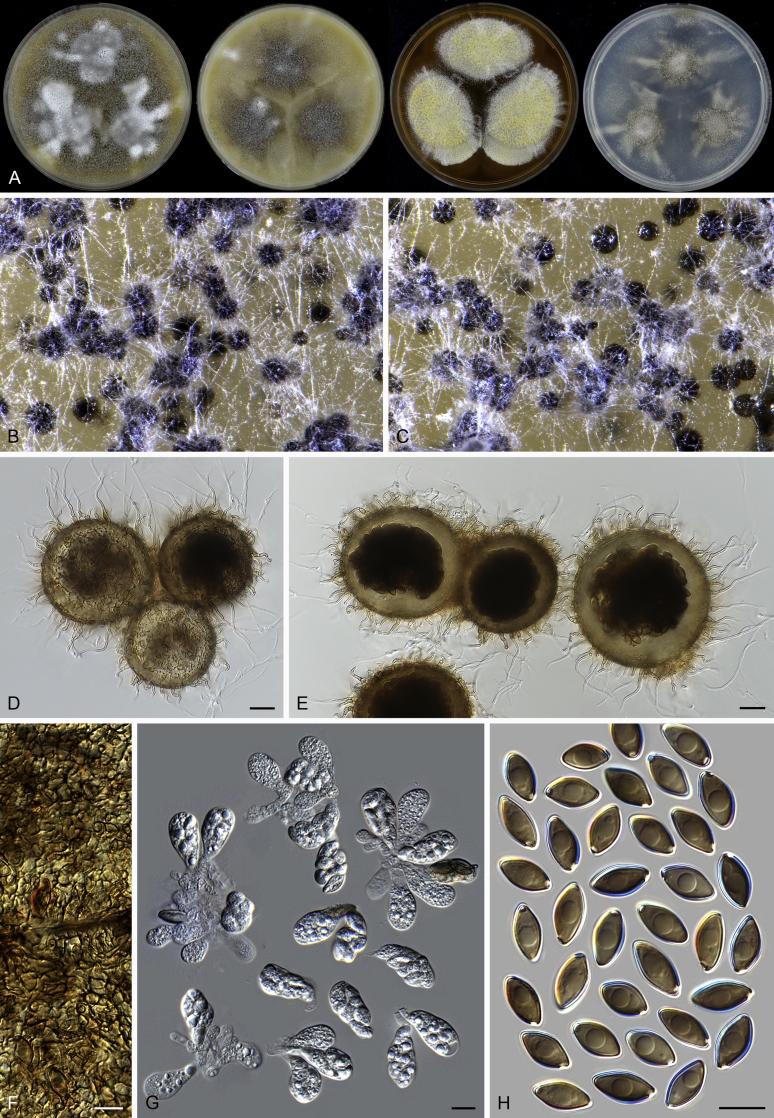
Notes: Both rpb2 and four-locus phylogenies indicated that Acrophialophora is closely related to three genera (Parathielavia, Hyalosphaerella and Pseudothielavia) containing thielavia-like species and two genera (Chrysocorona, Brachychaeta) with former Chaetomium species in Clade 1 (Fig. 2, Fig. 3). The taxonomy of this genus was therefore treated in the present study to better show the phylogenetic position of the related “Thielavia” species in the Chaetomiaceae. Five traditional Acrophialophora species were selected as representatives of the genus (Acr. ellipsoidea, Acr. fusispora, Acr. hechuanensis, Acr. major and Acr. nainiana). Two sexual species (Acr. jodhpurensis and Acr. teleoafricana) formerly classified in Chaetomium, clustered in the monophyletic Acrophialophora clade (PP ≥ 0.99, ML-BS = 100, Fig. 2, Fig. 3). The generic description thus was emended to include these sexually reproducing species.
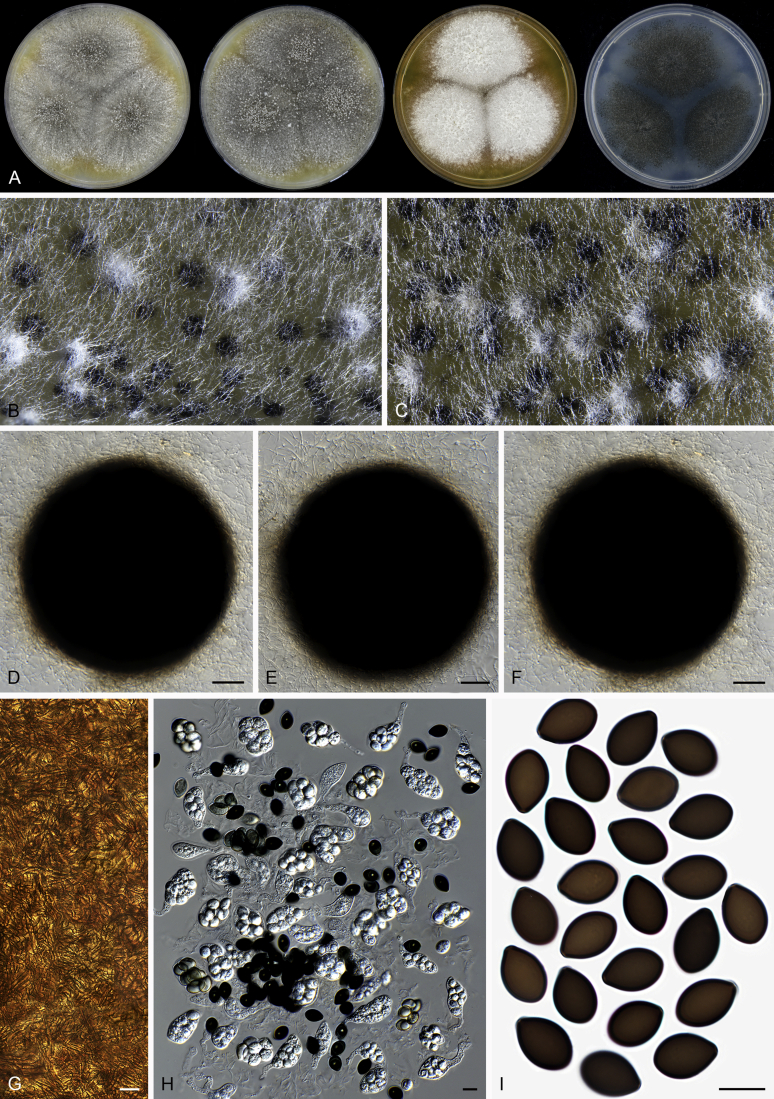
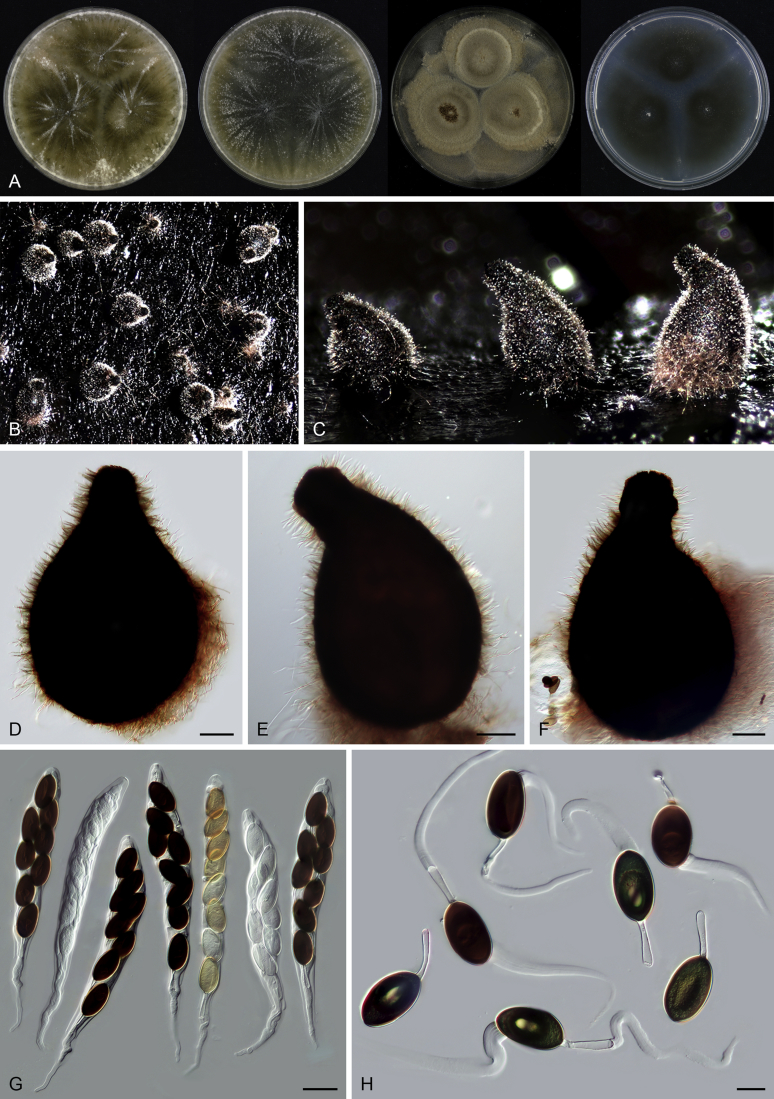
Edward (1961) introduced Acrophialophora based on its distinct conidiophores and phialides and suggested it to be closely related to the genus Paecilomyces. However, Dal Vesco & Peyronel (1968) treated the type species of the genus, Acr. nainiana, as a synonym of Paecilomyces fusisporus and also Barron (1968) did not accept Acrophialophora as separate genus from Paecilomyces. In the subsequent study of Samson & Mahmood (1970), Acrophialophora was reintroduced and they emphasized the differences from Paecilomyces species in the presence of pigmented hyphae and pigmented conidiophores with thick and verrucose walls, and the presence of proliferating phialides. Three Acrophialophora species were accepted in Acrophialophora sensu Samson & Mahmood, Acr. fusispora, Acr. levis and Acr. nainiana, each being thermotolerant and producing more or less differentiated conidiophores. The morphological concept of Paecilomyces was demonstrated to be polyphyletic by the phylogenetic analysis of SSU sequences (Luangsa-ard et al. 2004). The type species Pae. variotii and its thermophilic relatives belong in the order Eurotiales, Paecilomyces farinosus and its related mesophilic species belong to the order Hypocreales, while the monophialidic species Pae. inflatus was moved to Phialemonium as Ph. inflatum in the Cephalothecaceae (Perdomo et al. 2013). On the other hand, many more monophialidic species were described in Paecilomyces (Matushima 1971, Liang et al., 2006a, Liang et al., 2006b, Liang et al., 2007). In 2009, Liang et al. proposed the genus Taifanglania and transferred eight monophialidic Paecilomyces species to Taifanglania. Recent phylogenetic evidence using ITS, SSU and tub2 sequences showed that Acrophialophora and Taifanglania are congeneric, and Taifanglania was therefore treated as a synonym of Acrophialophora (Zhang et al. 2015). Their emended genus Acrophialophora contained 16 thermotolerant species with an optimal growth temperature between 35–40 °C.
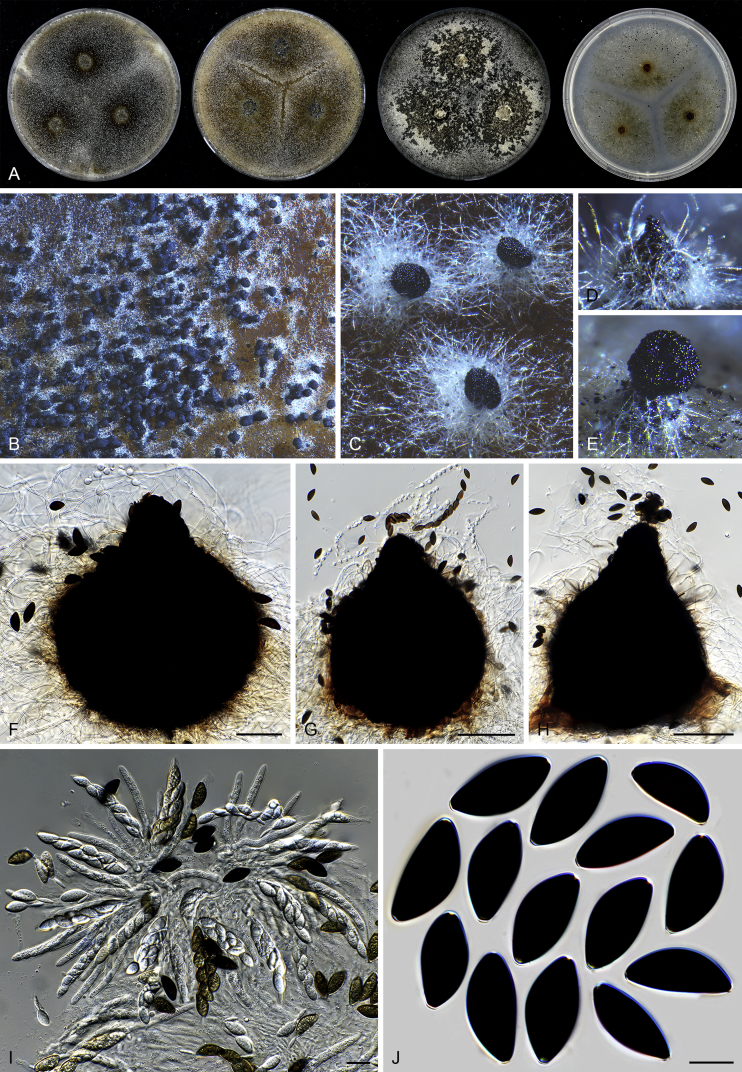
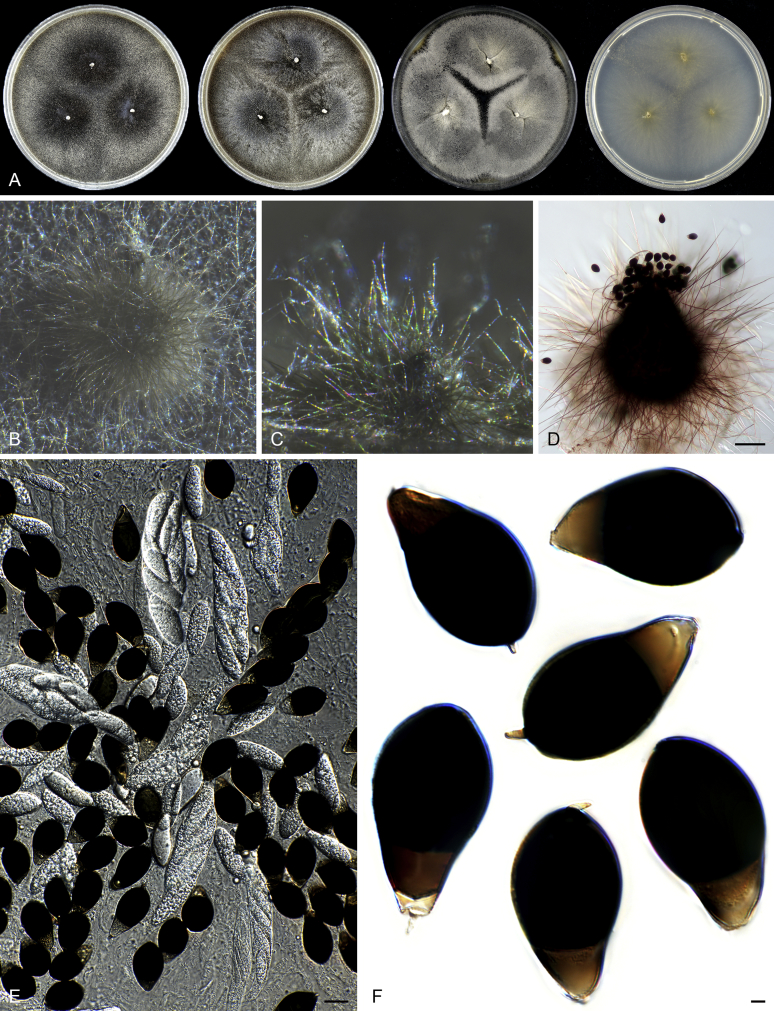
Acrophialophora is commonly known as an asexual genus because the majority of species produce only the asexual state and no species is known to form both asexual and sexual states. Species of the five closely related genera are all known to produce sexual states. Parathielavia and Hyalosphaerella produce non-ostiolate ascomata. The majority of Pseudothielavia species also produce non-ostiolate ascomata with the exception of Pse. hamadae which possesses ascomata with an inconspicuous ostiole and covered by sparse and hyaline hypha-like hairs (Fig. 37), quite different from the sexual Acrophialophora species that produce ascomata with a conspicuous ostiole and covered by well-developed flexuous or undulate ascomatal hairs (Fig. 13, Fig. 16). The monotypic genus Chrysocorona can be distinguished from sexual Acrophialophora species by its ascomata covered by amber to luteous and arcuate ascomatal hairs usually with numerous short and easily-exfoliated branches near the tips (Fig. 28). Brachychaeta can be distinguished by its ascomata covered by its short, yellow-green, arcuate or flexuous and unbranched ascomatal hairs and by its irregularly-shaped ascospores.
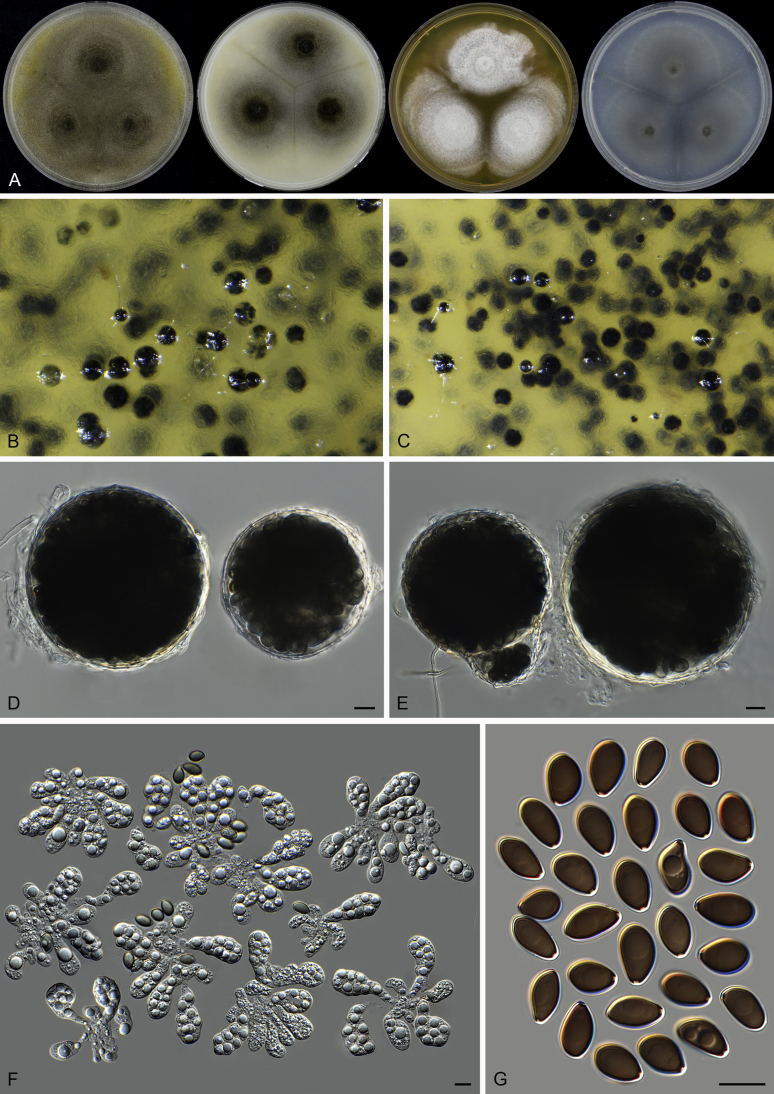
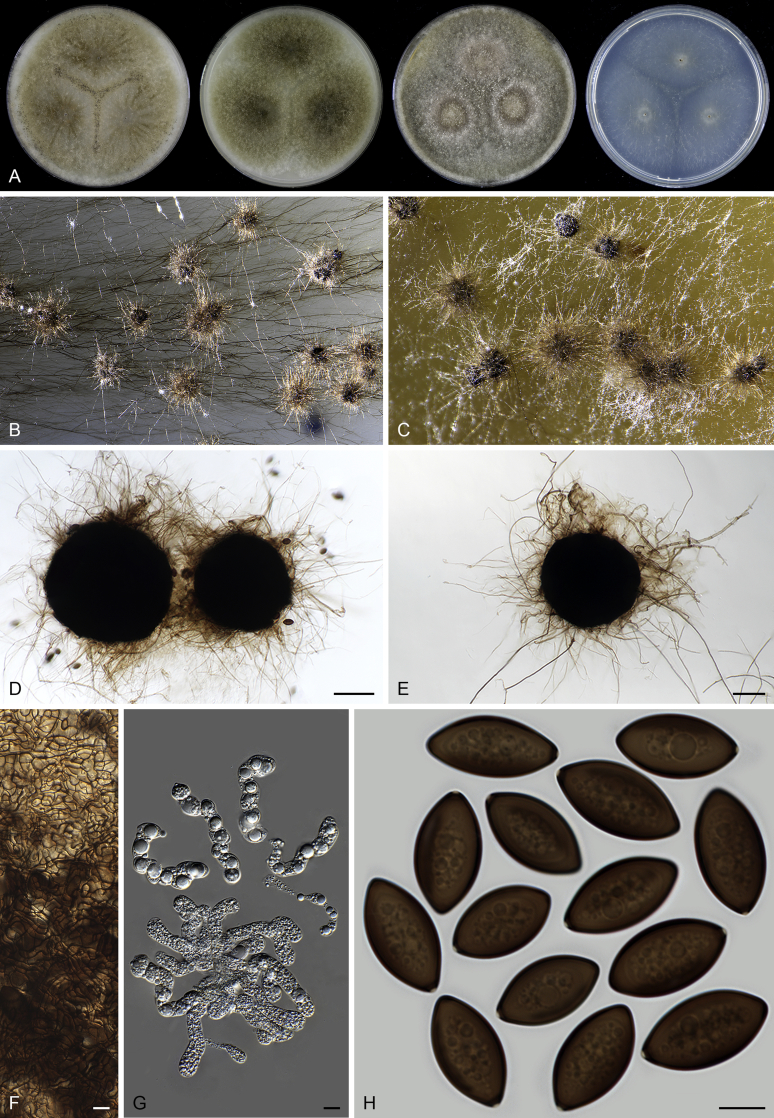
Fig. 37.
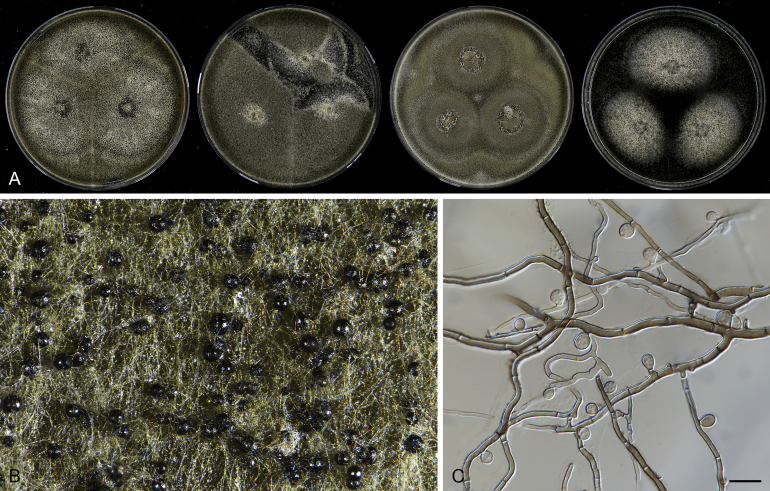
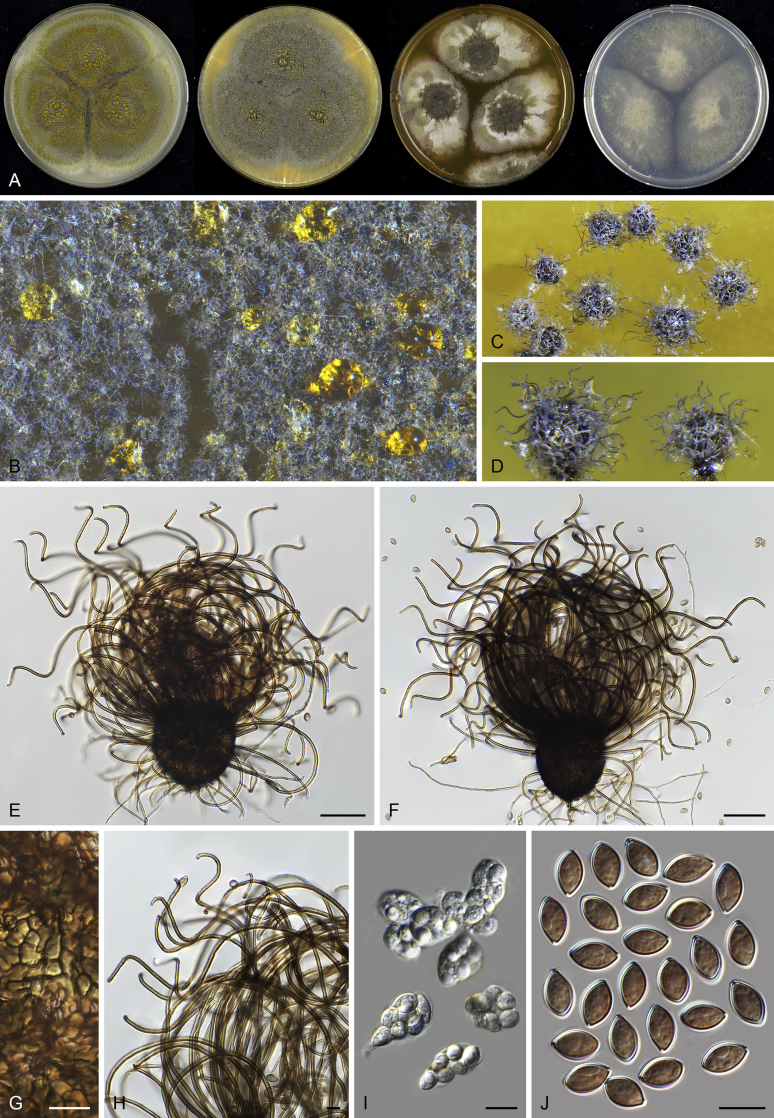
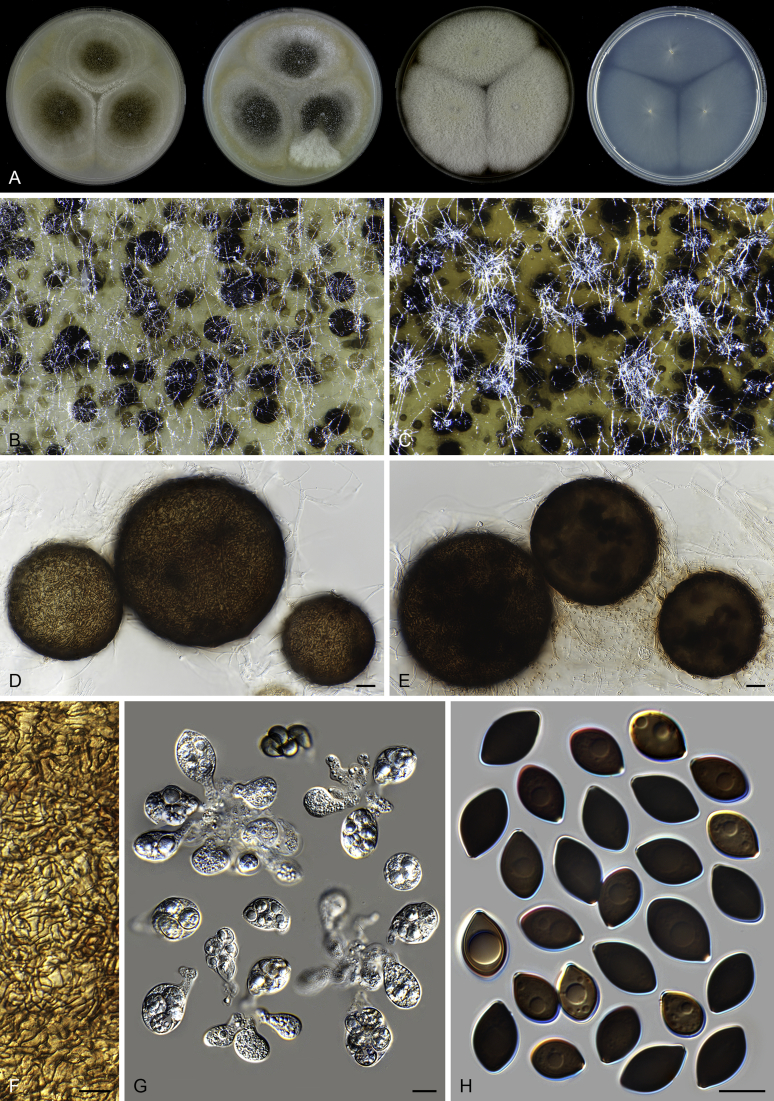
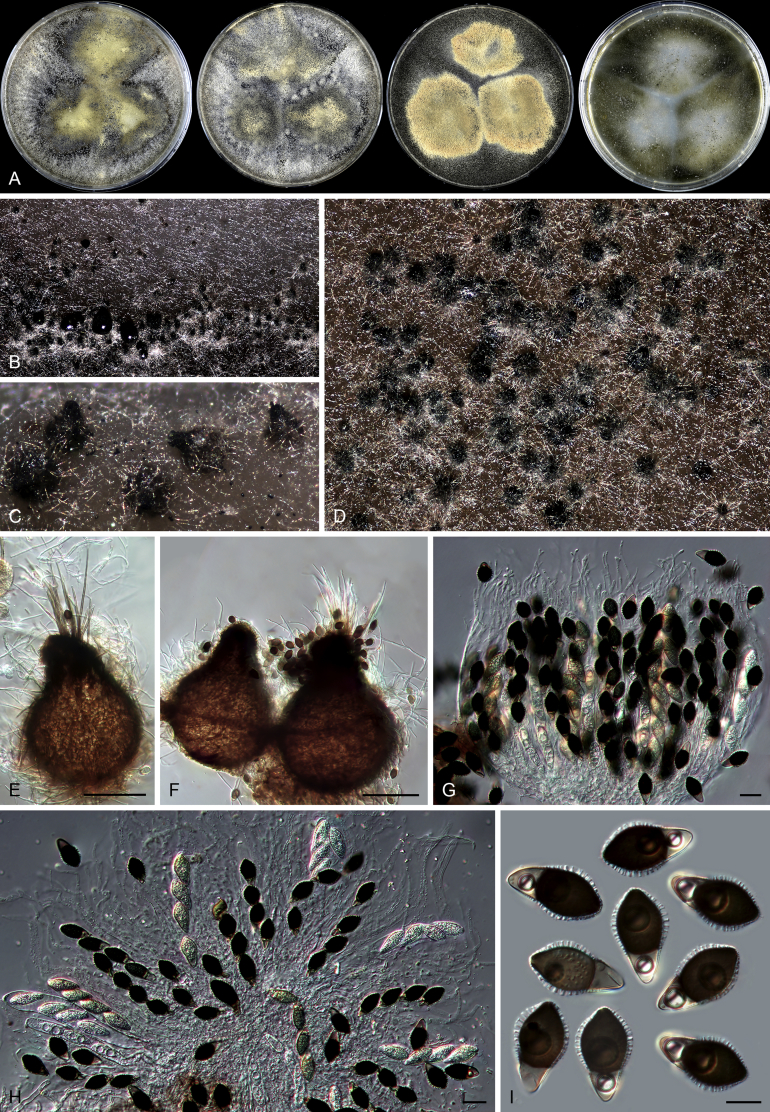
Pseudothielavia hamadae (CBS 499.83, ex-type culture). A. Colonies from left to right on OA, CMA, MEA and PCA after 18 d incubation. B. Young ascomata on OA. C–D. Mature ascomata on OA. E–G. Ascomata mounted in lactic acid. H. Structure of ascomatal wall in surface view. I. Asci. J. Ascospores. Scale bars: E–G = 20 μm; H–J = 10 μm.
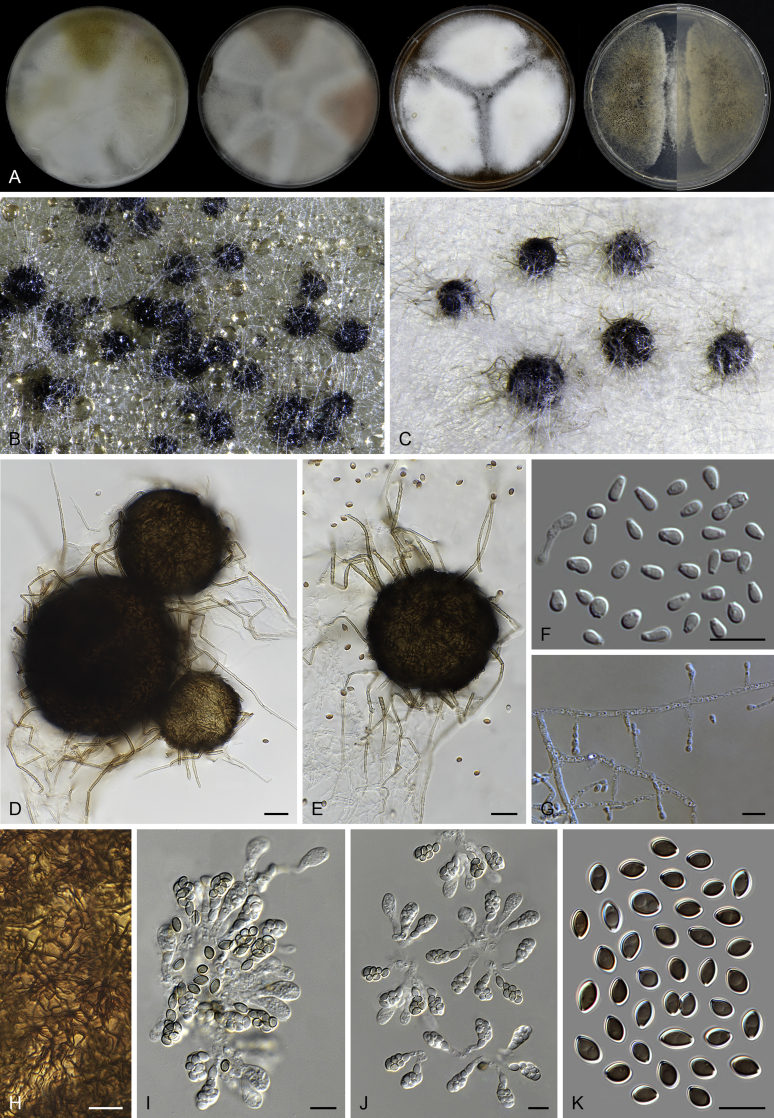
Fig. 13.
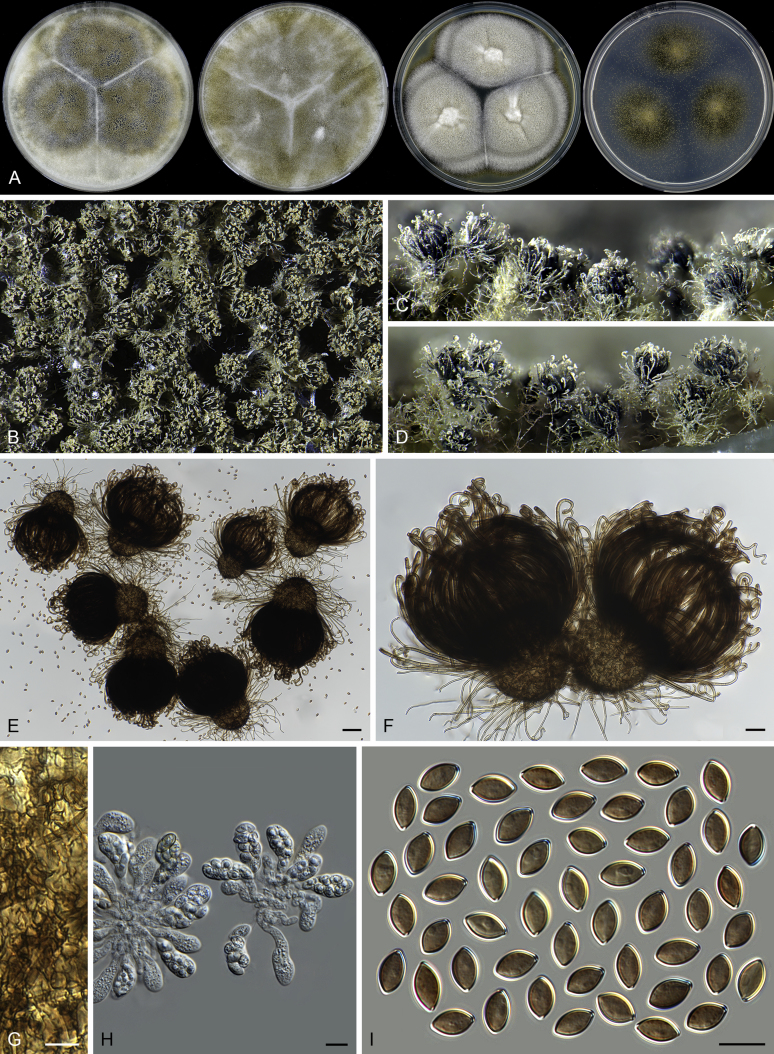
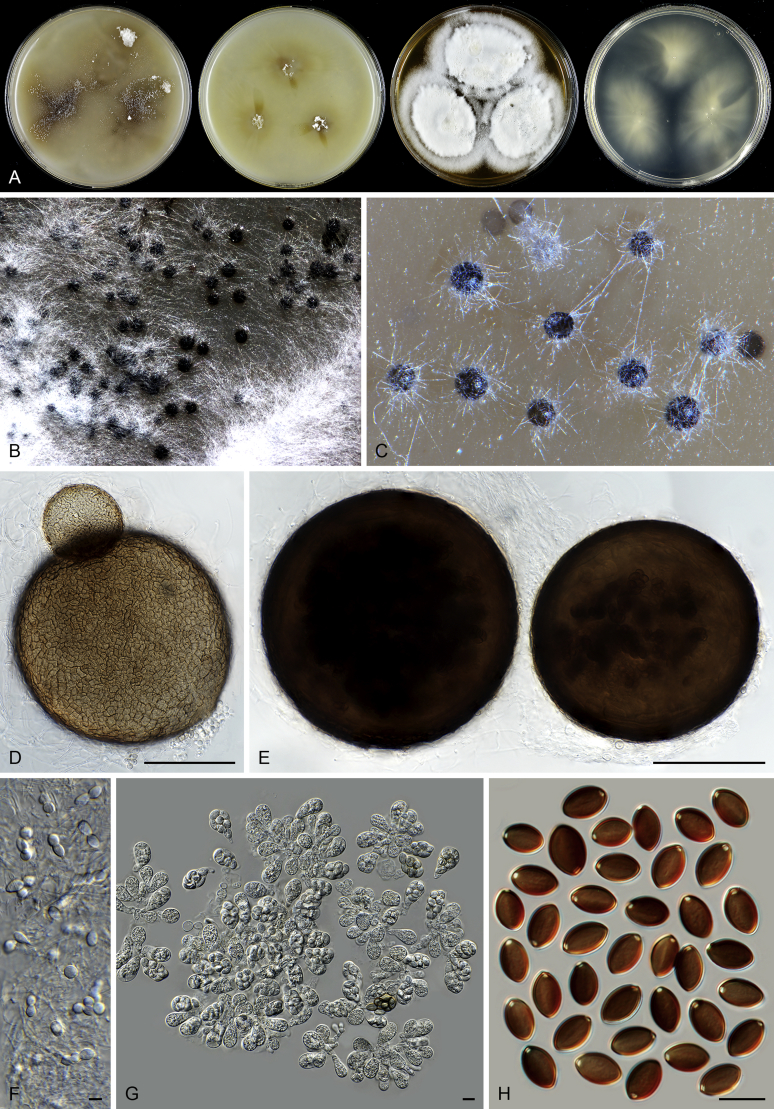
Acrophialophora jodhpurensis (CBS 602.69, ex-epitype culture). A. Colonies from left to right on OA, CMA, MEA and PCA after 4 wk incubation. B. Part of the colony on OA. C. Mature ascomata on OA, top view. D. Mature ascomata on OA, side view. E–G. Ascomata mounted in lactic acid. H. Structure of ascomatal wall in surface view. I. Terminal ascomatal hairs. J. Asci. K. Ascospores. Scale bars: E–G = 50 μm; H–K = 10 μm.
Fig. 16.
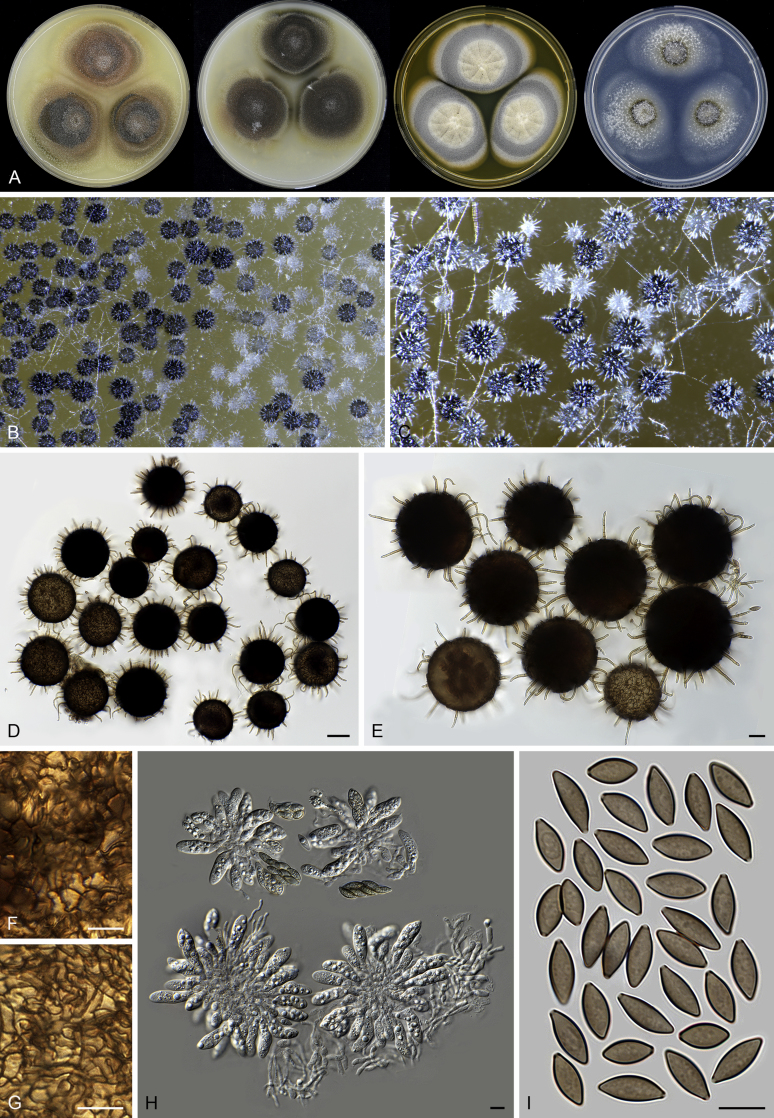
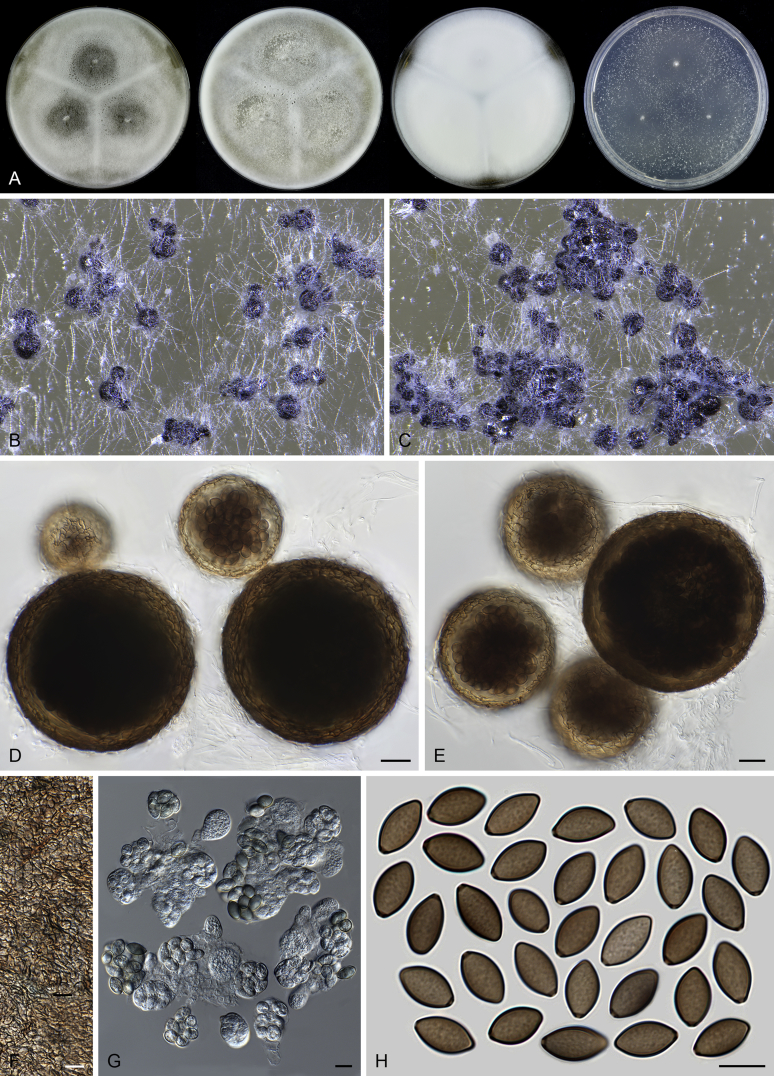
Acrophialophora teleoafricana (CBS 280.79, ex-type culture). A. Colonies from left to right on OA, CMA, MEA and PCA after 3 weeks incubation. B. Part of the colony on OA. C. Mature ascomata on OA, top view. D. Mature ascomata on OA, side view. E–F. Ascomata mounted in lactic acid. G. Structure of ascomatal wall in surface view. H. Ascomatal hairs. I. Asci. J. Ascospores. Scale bars: E–F = 50 μm; G–J = 10 μm.
Fig. 28.
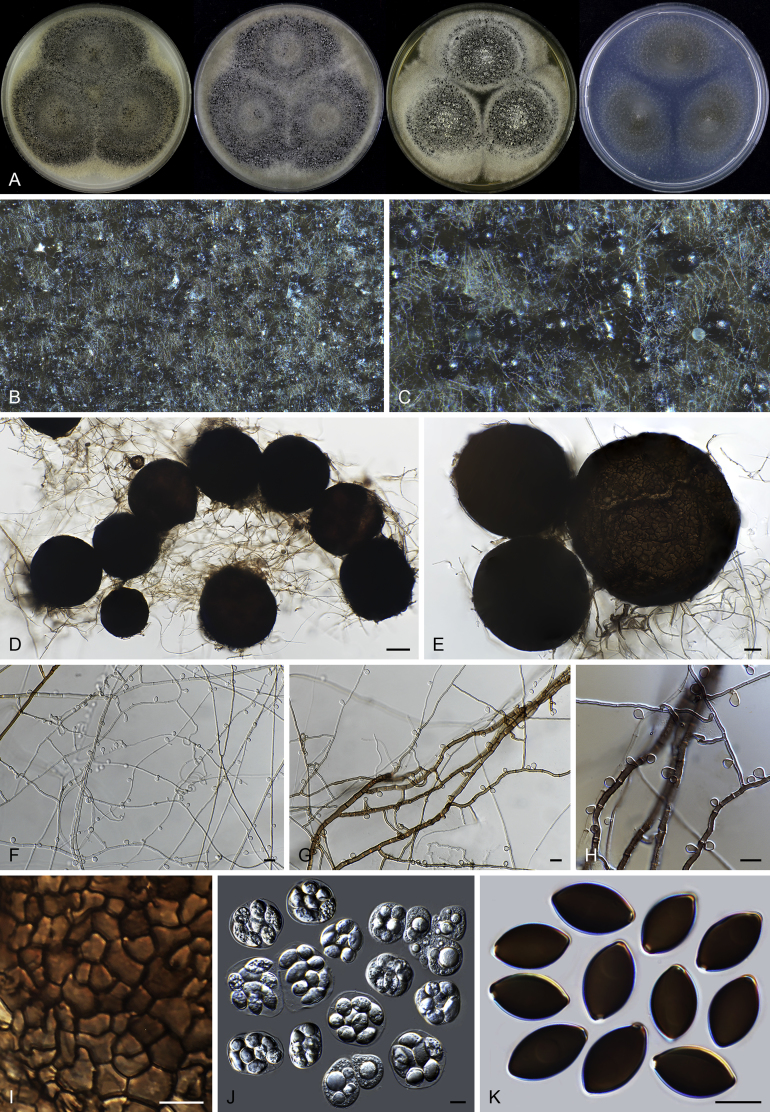
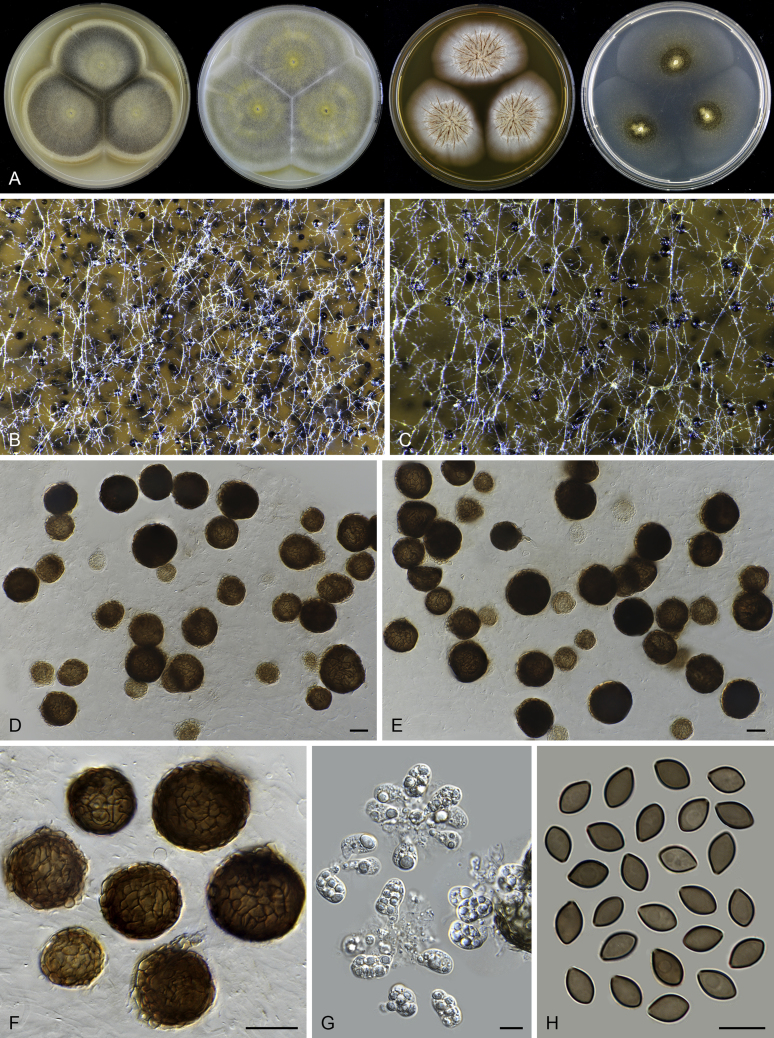
Chrysocorona lucknowensis (CBS 727.71, ex-epitype culture). A. Colonies from left to right on OA, CMA, MEA and PCA after 4 wk incubation. B. Mature ascomata on OA, top view. C. Mature ascomata on OA, side view. D–F. Ascomata mounted in lactic acid. G–H. Terminal ascomatal hairs. I. Asci. J. Ascospores. Scale bars: D–F = 20 μm; G–J = 10 μm.
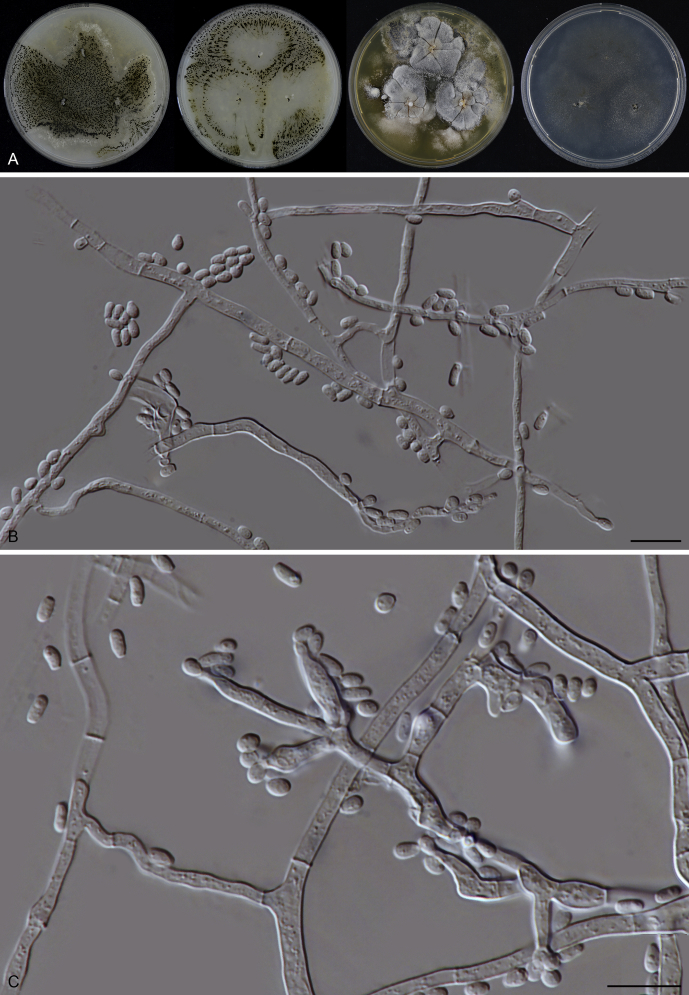
Acrophialophora ellipsoidea Yu Zhang & L. Cai, Mycologia 107: 772. 2015. Fig. 10.
Fig. 10.
Acrophialophora ellipsoidea (CBS 102.61). A. Colonies from left to right on OA, CMA, MEA and PCA after 2 wk incubation. B–F. Hyphae, phialidic conidiogenous cells and conidia. Scale bars: B–F = 10 μm.
Micromorphology: Sexual morph not observed. Somatic hyphae hyaline, 1.0–4 μm wide. Conidiophores reduced to conidiogenous cells. Conidiogenous cells arising laterally from hyphae, phialidic, usually solitary, flask-shaped or obclavate, swollen near base, tapering abruptly to a narrow neck, 8–16 × 2.5–4.5 μm. Conidia 1-celled, formed in basipetal chains, hyaline, ellipsoidal or ovoid, smooth, (4–)5.5–7(–9) × (2–)2.5–3 μm.
Culture characteristics: On OA with an entire edge, 34–40 mm diam after 7 d at 25 °C, obverse white and floccose due to aerial mycelium, reverse buff. On CMA similar to those on OA, with a relative thin layer of aerial mycelium. On MEA with an entire edge, 17–23 mm diam after 7 d at 25 °C, texture floccose, obverse white to buff, reverse luteous to orange. On PCA 17–23 mm diam after 7 d at 25 °C; edge entire; with sparse aerial mycelium, reverse uncoloured.
Material examined: Belgium, Luik, isolated from soil, date unknown, J.L. Ramaut (CBS 102.61). China, Guangxi, isolated from compost, Oct. 2011, W.Q. Ma (CGMCC 3.17487).
Notes: Acrophialophora ellipsoidea can be recognised by its solitary conidiogenous cells (reduced conidiophores) producing smooth, ellipsoidal or ovoid conidia, rather than fusiform conidia as in most of the other studied species. CBS 102.61 was deposited in the CBS culture collection as Acr. levis. Acrophialophora ellipsoidea produces conidiogenous cells and conidia similar to those of Acr. levis, but can be distinguished by the absence of differentiated conidiophores which are pigmented in Acr. levis and have a coarsely warty surface in the lower part. Samson & Mahmood (1970) mentioned that differentiated conidiophores were also often absent in Acr. levis. Based on Zhang et al. (2015), the two species are closely related, but cluster in two distinct clades. Our examined strains CGMCC 3.17487 and CBS 102.61 group with CGMCC 3.15256, the ex-type of Acr. ellipsoidea. Further study is needed to determine the taxonomic value of conidiophore complexity in Acrophialophora.
Acrophialophora fusispora (S.B. Saksena) Samson, Acta Bot. Neerl. 19: 805. 1970. Fig. 11.
Fig. 11.
Acrophialophora fusispora (CBS 380.55, ex-type culture). A. Colonies from left to right on OA, CMA, MEA and PCA after 18 d incubation. B–G. Hyphae, phialidic conidiogenous cells and conidia. H. Conidia. Scale bars = 10 μm.
Basionym: Paecilomyces fusisporus S.B. Saksena, J. Indian Bot. Soc. 32: 188. 1953.
Micromorphology: Sexual morph not observed. Somatic hyphae hyaline, 1–4 μm wide. Conidiophores simple, hyaline, smooth, cylindrical or slightly clavate, 3.5–13 × 3–4 μm, or reduced to conidiogenous cells. Conidiogenous cells phialidic, generating apically on conidiophores in verticils, or arising laterally from hyphae and solitary, often proliferating, obclavate or flask-shaped, swollen near base, tapering gradually or abruptly to a narrow neck, (4–)9–22 × 2.5–4(–4.5) μm. Conidia 1-celled, formed in basipetal chains, hyaline to subhyaline, ellipsoidal, fusiform or ovoid, with warts which are usually striated or spirally arranged, (5–)6–8 (–11) × 2.5–4(–5) μm.
Culture characteristics: On OA 30–36 mm diam after 7 d at 25 °C; edge entire; obverse ochraceous, reverse buff to saffron. On CMA 29–35 mm diam after 7 d at 25 °C; edge entire; obverse rosy buff, reverse buff. On MEA 24–30 mm diam after 7 d at 25 °C, edge slightly crenate; obverse buff, reverse saffron to orange. On PCA 16–22 mm diam after 7 d at 25 °C; edge entire or slightly crenate; with sparse aerial mycelium, reverse uncoloured.
Typus: India, Sagar, Patharia Forest, isolated from forest soil, date unknown, S.B. Saksena (culture ex-type CBS 380.55 = ATCC 22556 = IMI 057442 = LSHB Pa64 = UAMH 10771).
Notes: According to Samson & Mahmood (1970), Acr. fusispora produces darkly pigmented conidiophores measuring up to 1.2 mm long. In our examination of the ex-type, conidiophores were absent, or when present, hyaline and less than 15 μm in length. This is another example to show that the presence of pigmented conidiophores may not be a stable character. Also, the spiral ornamentation reported on the surface of the conidia was not as prominently present as the previous description (Samson & Mahmood 1970). These morphological variations might be caused by the long-term preservation of CBS 380.55. Acrophialophora fusispora is closely related to Acr. hechuanensis and Acr. nainiana. It can be distinguished from Acr. hechuanensis by ornamented and larger conidia (6–8 × 2.5–4 μm vs 5–6 × 2–3.5 μm). Acrophialophora nainiana differs from both Acr. fusispora and Acr. hechuanensis by its larger conidia (6–8.5 × 3.5–4.5 μm) and by the persistent presence of well-developed conidiophores which are pigmented, warty and up to 1300(–1500) μm long with conidiogenous cells borne apically in verticils.
Acrophialophora hechuanensis (Z.Q. Liang, Y.F. Han, H.L. Chu & R.T.V. Fox) Yu Zhang & L. Cai, Mycologia 107: 775. 2015. Fig. 12.
Fig. 12.
Acrophialophora hechuanensis (GZUIFR-H08-1, ex-type culture). A. Colonies from left to right on OA, CMA, MEA and PCA after 18 d incubation. B–F. Hyphae, phialidic conidiogenous cells and conidia. G. Conidia. Scale bars = 10 μm.
Basionym: Taifanglania hechuanensis Z.Q. Liang, Y.F. Han, H.L. Chu & R.T.V. Fox, Fungal Diversity 34: 72. 2009.
Micromorphology: Sexual morph not observed. Somatic hyphae hyaline, 1.5–4 μm wide. Conidiophores usually reduced to conidiogenous cells. Conidiogenous cells arising laterally from hyphae, phialidic, often proliferating or solitary, obclavate or flask-shaped, swollen near base, tapering abruptly to a narrow neck, (4–)8–19 × 2.5–4 μm. Conidia 1-celled, formed in basipetal chains, sometimes formed in heads at apex of the conidiogenous cells, hyaline, smooth, fusiform or ovoid, usually with a spine-like extension at the base, (4–)5–6 (–8) × 2–3.5 (–4) μm.
Culture characteristics: On OA with an entire edge, 32–38 mm diam after 7 d at 25 °C, texture floccose, obverse white or pale white, reverse buff to saffron. On CMA 32–38 mm diam after 7 d at 25 °C, texture floccose, edge entire or slightly crenate; obverse white, reverse pale luteous. On MEA with an entire edge, 32–38 mm diam after 7 d at 25 °C; obverse rosy buff, with a relative thick layer of aerial mycelium, reverse orange. On PCA with an entire edge, 27–33 mm diam after 7 d at 25 °C, with sparse aerial mycelium, reverse uncoloured.
Typus: China, Chongqing, Hechuan, isolated from soil, 2003, Y.F. Han & Z.Q. Liang (culture ex-type GZUIFR-H08-1).
Notes: Acrophialophora hechuanensis produces smooth conidia, like Acr. ellipsoidea, but can be distinguished by usually fusiform conidia with a spine-like extension at base, often proliferating conidiogenous cells, and by the presence of simple conidiophores. Moreover, conidia of Acr. hechuanensis are formed not only in chains, but also in heads (Fig. 12F). This species was designated as the type species of Taifanglania, a genus characterized by lacking conidiophores (or maximally having simple conidiophores), and having hyaline, solitary phialides, with a swollen base and tapering into a thin neck, and hyaline, smooth walled conidia (Liang et al. 2009). This study supports the conclusion of Zhang et al. (2015) that Taifanglania is congeneric with Acrophialophora.
Acrophialophora jodhpurensis (Lodha) X. Wei Wang & Houbraken, comb. nov. MycoBank MB829844. Fig. 13.
Basionym: Chaetomium jodhpurense Lodha, J. Indian Bot. Soc. 43: 132. 1964.
Micromorphology: Ascomata superficial, greyish yellow-green due to ascomatal hairs in reflected light, subglobose to ovate, ostiolate, 130–220 μm high, (100–)120–180 μm diam. Ascomatal wall brown, composed of textura intricata in surface view. Terminal hairs flexuous or undulate, occasionally branched, brown, septate, 1.5–3 μm diam near base. Lateral hairs flexuous. Asci fasciculate, clavate or fusiform, spore-bearing part 30–43 × 12.5–17 μm, with stalks 6–19 μm long, containing eight biseriate or irregularly arranged ascospores, evanescent. Ascospores 1-celled, olivaceous brown when mature, smooth, fusiform or ellipsoidal, (11–)13–14.5(–16) × (5.5–) 6–7(–7.5) μm, with a subapical or lateral germ pore. Asexual morph not observed.
Culture characteristics: On OA with entire edge, 25–31 mm diam in 7 d at 25 °C, often with mouse grey concentric rings due to the formation of ascomata, without aerial mycelium, fuscous black around the colonies due to coloured exudates diffusing into the medium, reverse olivaceous black. On CMA with a slightly crenate edge, 26–32 mm diam in 7 d at 25 °C, obverse mouse grey due to ascomata, without aerial mycelium, with fuscous black exudates diffusing into the medium and a concentric ascomata ring around the pale center, reverse pale mouse grey. On MEA with a crenate edge, 22–28 mm diam in 7 d at 25 °C, obverse pale olivaceous grey to olivaceous grey due to aerial mycelium, without coloured exudates, reverse olivaceous grey. On PCA with a slightly crenate edge, 30–36 mm diam in 7 d at 25 °C, with sparse ascomata, without aerial mycelium, with olivaceous exudates diffusing into the medium, reverse buff to olivaceous.
Typus: Lectotype of Chaetomium jodhpurense designated here: fig. 8 in Lodha, J. Indian Bot. Soc. 43: 132, 1964 (based on the original culture from rabbit dung collected at Jodhpur, Rajasthan, India), MBT385817. Pakistan, Peshawar, substrate and date unknown, T. Mahmood (CBS H-10019, epitype designated here, MBT385818, culture ex-epitype CBS 602.69).
Notes: Four strains maintained as Ch. jodhpurense in the CBS collection were studied. These strains cluster with other strictly asexual Acrophialophora species with statistical support in both the rpb2 (PP = 1, ML-BS = 100 %; Fig. 2) and the four-locus phylogeny (PP = 0.99, ML-BS = 100 %; Fig. 3). As a result, they are transferred to the genus Acrophialophora. Phylogenetically, the four studied Ch. jodhpurense strains split into two species lineages and the other one is described here as a novel species, Acr. teleoafricana (Fig. 16, see below). The thermotolerant nature of the sexual species (Zhang et al. 2017) also matches with the genus Acrophialophora. Since the type specimen seemed to be lost (von Arx et al. 1986), an illustration in the protologue is designated here as the lectotype of Ch. jodhpurense and CBS 602.69 is selected as the ex-epitype culture in order to fix the application of the species name. This strain was collected in Pakistan, adjacent to the country (India) of the holotype location. Furthermore, the morphology of the species lineage represented by CBS 602.69 matches with the protologue (Lodha 1964).
Acrophialophora major (Z.Q. Liang, H.L. Chu & Y.F. Han) Yu Zhang & L. Cai, Mycologia 107: 775. 2015. Fig. 14.
Fig. 14.
Acrophialophora major (GZUIFR- H57-2, ex-type culture). A. Colonies from left to right on OA, CMA, MEA and PCA after 18 d incubation. B–F. Hyphae, phialidic conidiogenous cells and conidia. G. Conidia. Scale bars = 10 μm.
Basionym: Paecilomyces inflatus var. major Z.Q. Liang, H.L. Chu & Y.F. Han, J. Fungal Res. 2: 43. 2004.
Synonyms: Paecilomyces major (Z.Q. Liang, H.L. Chu & Y.F. Han) Z.Q. Liang, H.L. Chu & Y.F. Han, J. Fungal Res. 4: 47. 2006.
Taifanglania major (Z.Q. Liang, H.L. Chu & Y.F. Han) Z.Q. Liang, Y.F. Han & H.L. Chu, Fungal Diversity 34: 74. 2009.
Micromorphology: Sexual morph not observed. Somatic hyphae hyaline, 1–3.5 μm wide. Conidiophores reduced to conidiogenous cells. Conidiogenous cells arising laterally from hyphae, phialidic, solitary, sometimes proliferating, flask-shaped or obclavate, swollen near base, tapering abruptly to a narrow neck, (4–)7.5–12 × 2–4.5(–5) μm. Conidia 1-celled, formed in basipetal chains, hyaline, ellipsoidal, fusiform or ovoid, smooth to ornamented with sparse warts which are irregularly or slightly spirally arranged, (6–)6.5–7.5(–8.5) × 2.5–3.5 μm.
Culture characteristics: On OA with an entire edge, 27–33 mm diam after 7 d at 25 °C, obverse saffron, reverse buff to pale luteous. On CMA with an entire or crenate edge 27–33 mm diam after 7 d at 25 °C, obverse rosy buff or saffron, reverse rosy buff. On MEA with a crenate or fimbriate edge, 20–26 mm diam after 7 d at 25 °C, obverse rosy buff to saffron, reverse orange with ochraceous margin. On PCA with an entire edge, 28–34 mm diam after 7 d at 25 °C, without aerial mycelium, reverse uncoloured.
Typus: China, Tangshan, Hebei Province, isolated from soil, 2003, H.L. Chu, Y.F. Han & Z.Q. Liang (culture ex-type GZUIFR- H57-2).
Additional material examined: China, Tengchong, Yunnan, isolated from soil, 2003, Y.F. Han & Z.Q. Liang (GZUIFR- H52-1).
Notes: Based on our examination of Acr. major, the majority of conidia are ornamented with sparse warts which are irregularly or slightly spirally arranged and this slightly differs from the observation reported in Liang et al. (2009). Acrophialophora major is morphologically similar to Acr. hechuanensis, but can be distinguished by ornamented and larger conidia (6.5–7.5 × 2.5–3.5 μm vs 5–6 × 2–3.5 μm) and by less proliferating conidiogenous cells.
Acrophialophora nainiana Edward, Mycologia 51: 784. 1961. Fig. 15.
Fig. 15.
Acrophialophora nainiana (CBS 100.60, ex-type culture). A. Colonies from left to right on OA, CMA, MEA and PCA after 2 wk incubation. B. Hyphae, conidiophores and conidia. C–E. Conidiophores, conidiogenous cells and macronematous conidia. F–G. Phialidic conidiogenous cells and micronematous conidia arising from hyphae. H. Conidia. Scale bars: B = 20 μm; C–H = 10 μm.
Micromorphology: Sexual morph not observed. Somatic hyphae hyaline or pale brown often where conidiophores bearing, 1–4 μm wide. Conidiophores arising laterally from hyphae, septate, unbranched, erect, warty, pigmented, fading towards tips, 2–5.5 μm wide, (300–)600–1300(–1500) μm long. Conidiogenous cells born apically in whorls or in verticils on conidiophores or arising directly from hyphae, phialidic, flask-shaped, swollen at the base, tapering abruptly to a narrow neck, sometimes proliferating, 7.5–15 × 2.5–5 μm. Conidia 1-celled, formed in basipetal chains, hyaline to subhyaline, ovoid, ellipsoidal to fusiform, smooth to sparsely punctate, (5–)6–8.5(–10.5) × (2.5–)3.5–4.5(–5) μm.
Culture characteristics: On OA with an entire edge, 32–38 mm diam after 7 d at 25 °C, obverse hazel to olivaceous, reverse olivaceous. On CMA with an entire edge, 24–30 mm diam after 7 d at 25 °C, obverse olivaceous buff to olivaceous, reverse olivaceous. On MEA with an entire edge, 17–23 mm diam after 7 d at 25 °C, obverse white and floccose, reverse pale luteous to luteous at the edge. On PCA with an entire edge, 17–23 mm diam after 7 d at 25 °C, obverse grey white to vinaceous buff due to aerial mycelium, reverse buff.
Typus: India, Allahabad, isolated from farm soil, 1957, J.C. Edward (culture ex-type CBS 100.60 = ATCC 22555 = IMI 076567 = LSHB BB399 = UAMH 10774).
Additional material examined: India, Allahabad, isolated from soil, collection date and collector unknown, (CBS 417.67 = IARI 1316).
Notes: In our study of the five representatives, asexually reproducing Acrophialophora species, Acr. nainiana, the type species of the genus, is the only one where typically differentiated pigmented conidiophores were observed. Conidia of this species are produced on phialides on differentiated conidiophores or on solitary phialides on hyphae. The conidia of Acr. nainiana are larger than those of the other studied Acrophialophora species. Conidiophores of the four other examined species were often absent, or simple and hyaline. The observed conidia of the ex-type culture were less distinctly ornamented as reported by Samson & Mahmood (1970). In their description, the conidia were ornamented with a fine echinulation which is sometimes slightly spirally arranged, while the conidia we observed were smooth to sparsely punctate. It seems evident that differentiated pigmented conidiophores and conidial ornamentation are variable characters in the genus and its species.
Acrophialophora teleoafricana X. Wei Wang & Houbraken, sp. nov. MycoBank MB829843. Fig. 16.
Etymology: Name refers to a sexual species of Acrophialophora isolated from Africa.
Micromorphology: Ascomata superficial, greyish yellow-green due to ascomatal hairs in reflected light, subglobose to ovate, ostiolate, 90–185 μm high, 90–150 μm diam. Ascomatal wall brown, of textura epidermoides or intricata in surface view. Terminal hairs flexuous or slightly undulate, brown, septate, 1.5–3 μm diam near base. Lateral hairs flexuous. Asci fasciculate, clavate or fusiform, spore-bearing part 20–30 × 11.5–17 μm, with stalks 3–7.5 μm long, containing eight biseriate or irregularly-arranged ascospores, evanescent. Ascospores 1-celled, olivaceous brown when mature, smooth, ellipsoidal to fusiform, 11–12.5(–13.5) × (5.5–)6–7 μm, with a subapical or lateral germ pore. Asexual morph not observed.
Culture characteristics: On OA with an entire edge, 22–28 mm diam in 7 d at 25 °C, obverse buff due to mycelium and mouse grey in the central part due to ascomata, with olivaceous grey exudates diffusing into the medium, reverse smoke grey to olivaceous grey or mouse grey. On CMA with an entire edge, 26–32 mm diam in 7 d at 25 °C, obverse buff due to mycelium mixed with ascomata, with olivaceous grey exudates diffusing into the medium, reverse pale mouse grey to mouse grey. On MEA with a slightly crenate edge, 21–27 mm diam in 7 d at 25 °C, obverse floccose, buff to smoke grey due to aerial mycelium, with coloured exudates diffusing into the medium, reverse dark mouse grey with a pale luteous edge. On PCA with an entire edge, 27–33 mm diam in 7 d at 25 °C, obverse buff due to mycelium, reverse olivaceous to dark mouse grey due to exudates diffusing into the medium.
Typus: Sudan, isolated from soil, collection date unknown, B.P.R. Vittal (holotype CBS H-23631, culture ex-type CBS 280.79).
Additional material examined: Sudan, isolated from soil, collection date unknown, B.P.R. Vittal (CBS 281.79).
Notes: Acrophialophora teleoafricana is a sister to Acr. jodhpurensis in both the rpb2 (PP = 1, ML-BS = 98 %; Fig. 2) and the four-locus phylogram (PP = 1, ML-BS = 100 %; Fig. 3). The two species are morphologically similar, but Acr. teleoafricana can be distinguished from Acr. jodhpurensis by shorter ascospores (11–12.5 × 6–7 μm vs 13–14.5 × 6–7 μm) and shorter asci (20–30 × 11.5–17 μm vs 30–43 × 12.5–17 μm).
Brachychaeta X. Wei Wang & Houbraken, gen. nov. MycoBank MB829842.
Etymology: Name refers to the short ascomatal hairs on the ascomata of the type species of this genus.
Micromorphology: Ascomata superficial, subglobose, ostiolate. Ascomatal wall brown, of textura epidermoidea in surface view. Terminal hairs arcuate or flexuous, brown, septate, less than 180 μm long. Lateral hairs flexuous. Asci fasciculate, clavate or fusiform, stalked, containing eight biseriate ascospores, evanescent. Ascospores 1-celled, brown when mature, smooth, ellipsoidal, ovate or reniform, often irregular, with two apical germ pores. Asexual morph not observed.
Type species: Brachychaeta variospora (Udagawa & Y. Horie) X. Wei Wang & Houbraken.
Notes: Brachychaeta is a new chaetomium-like genus proposed for a single lineage in Clade 1 (Fig. 2, Fig. 3). This lineage was basal in Clade 1, however, statistical support is lacking and the relationship of this monotypic genus with other genera in Clade 1 remains unknown. Brachychaeta can be easily distinguished from the other five related genera in Clade 1 (Fig. 2, Fig. 3) by producing ostiolate ascomata covered by short and arcuate or flexuous ascomatal hairs and by irregularly-shaped ascospores with two apical germ pores.
Brachychaeta variospora (Udagawa & Y. Horie) X. Wei Wang & Houbraken, comb. nov. MycoBank MB829845. Fig. 17.
Fig. 17.
Brachychaeta variospora (CBS 414.73, ex-type culture). A. Colonies from left to right on OA, CMA, MEA and PCA after 3 wk incubation. B. Mature ascomata on OA, top view. C–D. Mature ascomata on OA, side view. E–F. Ascomata mounted in lactic acid. G. Structure of ascomatal wall in surface view. H. Ascomatal hairs. I. Asci. J. Ascospores. Scale bars: E–F = 100 μm; G–J = 10 μm.
Basionym: Chaetomium variosporum Udagawa & Y. Horie, Rep. Tottori Mycol. Inst. 10: 430. 1973.
Micromorphology: Ascomata superficial, greyish yellow-green due to ascomatal hairs in reflected light, subglobose, ostiolate, 140–200 μm high, 120–180 μm diam. Ascomatal wall brown, of textura epidermoidea in surface view. Terminal hairs arcuate or flexuous, brown, septate, 2–3.5 μm diam near base, less than 180 μm long. Lateral hairs flexuous. Asci fasciculate, clavate or fusiform, spore-bearing part 35–47 × 14.5–18 μm, with stalks 8–16 μm long, containing eight biseriate ascospores, evanescent. Ascospores 1-celled, brown when mature, smooth, ellipsoidal, ovate or reniform, often irregular, (13–)14.5–17(–18) × (9–)10–12(–14) μm, with two apical germ pores. Asexual morph not observed.
Culture characteristics: On OA with an entire edge, 20–26 mm diam in 7 d at 25 °C, without aerial mycelium, obverse honey to greenish olivaceous, citrine or sometimes luteous due to coloured exudates diffusing into the medium, reverse citrine. On CMA with an entire edge, 18–24 mm diam in 7 d at 25 °C, without aerial mycelium, obverse orange due to coloured exudates diffusing into the medium, covered with pale mouse grey to mouse grey ascomata, reverse saffron or citrine. On MEA with a slightly crenate edge, 11–17 mm diam in 7 d at 25 °C, obverse floccose, grey white or pale olivaceous grey, without coloured exudates, reverse olivaceous grey. On PCA with a slightly crenate edge, 17–23 mm diam in 7 d at 25 °C, without aerial mycelium and coloured exudates, reverse greenish olivaceous to iron grey.
Typus: Thailand, Lodpure Muang, isolated from soil, date unknown, S. Udagawa (culture ex-type CBS 414.73 = IMI 172986 = NHL 2698).
Notes: Brachychaeta variospora forms a single lineage in Clade 1. The ascomata of Bra. variospora morphologically resemble those of Collariella gracilis (see fig. 25 in Wang et al. 2016a). These two species are phylogenetically distant. This species can also be easily distinguished from Col. gracilis by its larger and often irregular ascospores (14.5–17 × 10–12 μm vs 10–12.5 × 6–7.5 μm) with two germ pores. In contrast, the ascospores of Col. gracilis are regularly ellipsoidal or fusiform with one germ pore.
Canariomyces Arx, Persoonia 12: 185. 1984.
Micromorphology: Ascomata superficial, solitary to aggregated, non-ostiolate, often covered by subhyaline to brown aerial hyphae, black, globose or subglobose. Ascomatal wall brown, non-translucent, textura angularis in surface view. Asci obvoid, ellipsoidal or subglobose, usually without visible stalks, containing eight irregularly-arranged ascospores, evanescent. Ascospores 1-celled, dark brown when mature, smooth, ellipsoidal, with attenuated ends, with a subapical or apical germ pore. Conidiogenous cells reduced to a hyphal cell, monoblastic, laterally producing conidia. Conidia solitary or in basipetal chains, subhyaline to pigmented, obovoid, pyriform or clavate.
Type species: Canariomyces notabilis Arx.
Notes: According to the original description, two types of conidia were observed in the type species of Canariomyces: “type I conidia” are formed terminally on hyphae or on short branches of hyphae in a basipetal chain; “type II conidia” are produced laterally from hyphae, solitary and appressonium-like. Canariomyces was assigned in the family Microascaceae (Microascales) mainly based on its sessile asci, ascomatal wall and type I conidia, which are similar to those of Scedosporium desertorum and several other species in Microascaceae (von Arx 1984). Based on our phylogenetic analyses of the rpb2 and the combined four-locus datasets, Canariomyces belongs in Chaetomiaceae, and is closely related to Madurella and Stolonocarpus (Fig. 2, Fig. 3). Canariomyces is morphologically different from Madurella and Stolonocarpus. Madurella gained attention as the fungal etiologic agent of mycetoma, a chronic subcutaneous inflammatory disease often occurring on extremities. Madurella species usually produce only sterile (non-sporulating) hyphae and sparse aerial mycelium, growing restrictedly in culture and often producing buff, cinnamon, sienna or orange exudates diffusing into the agar (Fig. 18). Stolonocarpus also produces non-ostiolate ascomata, but can be distinguished from Canariomyces by cylindrical (rather than obvoid to subglobose) asci, larger ascospores (25.5–28.5 × 14–15.5 μm vs less than 20 × 12 μm) and by the absence of a conidial state.
Fig. 18.
Colony morphology of Madurella. A.Madurella fahalii (ex-type CBS 129176). Colonies from left to right on OA, CMA, MEA and PCA after 3 wk incubation. B.Madurella mycetomatis (ex-neotype CBS 109801). Colonies from left to right on OA, CMA, MEA and PCA after 3 wk incubation. C.Madurella pseudomycetomatis (ex-type CBS 129177). Colonies from left to right on OA, CMA, MEA and PCA after 3 wk incubation. D.Madurella tropicana (ex-type CBS 201.38). Colonies from left to right on OA, CMA, MEA and PCA after 3 wk incubation.
The ex-type strains of Th. arenaria (CBS 507.74), Th. microspora (CBS 276.74) and Th. subthermophila (CBS 509.74) clustered within the Canariomyces lineage represented by the type species Can. notabilis (ex-type CBS 548.83). These four species also have similar culture characteristics, ascomata and asci shapes. We therefore transfer Th. arenaria, Th. microspora and Th. subthermophila to Canariomyces.
The Canariomyces strains seem to degenerate easily. In this study, we only observed type II conidia in CBS 548.83 (ex-type of Can. notabilis) using the inclined coverslip method (Wang et al. 2019), but its type I conidia and sexual structures were not observed. The ex-type strains of Th. arenaria and Th. subthermophila also degenerated. Strain CBS 507.74 507.74 produced asexual structures together with empty ascomata with no asci and ascospores inside (Fig. 19). This makes the use of morphological characters for classification of Can. notabilis and related species challenging. Several studies (Mouchacca, 1973, von Arx, 1975, von Arx, 1984, von Arx et al., 1988) accepted Can. arenarius, Can. notabilis and Can. subthermophilus as separate species mainly based on the difference in their ascospore sizes (Table 3). As both the rpb2 and the combined four-locus phylogenies were unable to differentiate these three species well (Fig. 2, Fig. 3), further phylogenetic analyses were made based on partial tub2 (which has proved to be a good marker to delimit species in the Chaetomiaceae, Wang et al. 2016b) and tef1-α sequences. In the resulting trees (Fig. 5), five lineages were recognised in Canariomyces. In the tub2 phylogeny (Fig. 5A), Can. notabilis clustered closely but separate from the Th. subthermophila clade, which differ in their ascospore size (11–14 × 7–8.5 μm vs 14–19 × 8–10 μm, Tab. 3). Also an unnamed clade clustered close, but separate from the Th. arenaria clade, both also differing in their ascospore size (15–18.5 × 9.5–11 μm vs 8–12.5 × 5–7.5 μm, Tab. 3). As a result, five species are accepted, including a novel one. Further morphological and phylogenetic work is required to confirm the recognition of these species.
Fig. 19.
Canariomyces arenarius (CBS 507.74, ex-type culture). A. Colonies from left to right on OA, CMA, MEA and PCA after 4 wk incubation. B. Part of the colony on OA, showing sterile ascomata, top view. C. Hyphae, conidiogenous cells and conidia. Scale bar: C = 10 μm.
Table 3.
Comparison of asci, ascospores and conidia between the five Canariomyces species.
| Species names | Asci | Ascospores | Conidia |
|---|---|---|---|
| Can. arenarius | 17–26 × 15–20 μm (M) | 9–12.5 × 6–7.5 μm (M) 8–12 × 5–6.5 μm (A1) |
3.5–8 × 2–6 μm (M) 4–8 × 3–5 μm (A1) |
| Can. microsporus | 15–22 × 11–15 μm (M) 15–25 × 10–15 μm (A) 15–24 × 11–17 μm (W) |
7.5–11 × 5–6 μm (M) 8–10 × 5.5–6.5 μm (A1) 7.5–9 × 4.5–6 μm (W) |
4–10 × 2.5–4 μm (M) 4–10 × 3–5 μm (A1) 4–10.5 × 2–3.5 μm (W) |
| Can. notabilis | 20–26 μm (A2) | 11–14 × 7–8.5 μm (A2) | Type I: 9–16 × 5–7 μm (A2) Type II: 3–5 μm (A2) |
| Can. subthermophilus | 23–32 × 20–25 ìm (M) | 13–17 × 7.5–9.5 μm (M) 14–19 × 8–10 μm (A1) |
4–18 × 3–5 μm (M) 4–7 × 3–4 μm (A1) |
| Can. vonarxii | 26–45 × 22–38 μm (W) | 15–18.5 ×9.5–11 μm (W) | 3–7.5 × 2.5–5.5 μm (W) |
(M): from Mouchacca (1973); (A1): from von Arx (1975); (A2): from von Arx (1984); (W): from the present study.
Canariomyces arenarius (Mouch.) X. Wei Wang & Houbraken, comb. nov. MycoBank MB829846. Fig. 19.
Basionym: Thielavia arenaria Mouch., Bull. Trimestriel Soc. Mycol. France 89: 308. 1973.
Micromorphology: Description fide von Arx (1975) and von Arx et al. (1988): Ascomata non-ostiolate, globose, black, 60–120 μm diam. Ascomatal wall composed of textura epidermoidea and covered by dark hyphae. Ascospores 1-celled, brown when mature, smooth, fusiform or ellipsoidal, 8–12 × 5–6.5 μm. Conidiogenous cells reduced to a hyphal cell, monoblastic, laterally producing conidia. Conidia borne laterally, terminally or intercalary on the aerial hyphae, spherical or broadly clavate, hyaline or light brown, 4–8 × 3–5 μm.
Culture characteristics: On OA with an entire edge, 32–38 mm diam in 7 d at 25 °C, texture floccose, obverse mouse grey to dark mouse grey due to ascomata and aerial mycelium, reverse leaden black. On CMA similar to those on OA. On MEA with an entire edge, 29–35 mm diam in 7 d at 25 °C, texture floccose, obverse smoke grey to olivaceous grey, reverse black. On PCA with an entire edge, 28–34 mm diam in 7 d at 25 °C, obverse black, with smoke grey, reverse pale olivaceous grey with black margin.
Typus: Egypt, Kharga, isolated from desert soil, date unknown, J. Mouchacca (culture ex-type CBS 507.74).
Notes: Only sterile ascomata and the asexual morph were observed during the morphological examination of the ex-type strain (Fig. 19). According to literature (Mouchacca, 1973, von Arx, 1984, Tab. 3), this species has ascospores (8–12 × 5–6.5 μm) smaller than those of Can. notabilis (11–14 × 7–8.5 μm), Can. subthermophilus (13–17 × 7.5–9.5 μm) and Can. vonarxii (15–18.5 × 9.5–11), but larger than those of Can. microsporus (7.5–9 × 4.5–6 μm).
Canariomyces microsporus (Mouch.) X. Wei Wang & Houbraken, comb. nov. MycoBank MB829847. Fig. 20.
Fig. 20.
Canariomyces microsporus (CBS 161.80). A. Colonies from left to right on OA, CMA, MEA and PCA after 4 wk incubation. B. Part of the colony on OA, showing mature ascomata, top view. C. Hyphae, conidiogenous cells and conidia. D–F. Ascomata mounted in lactic acid. G. Structure of ascomatal wall in surface view. H. Asci. I. Ascospores. Scale bars: C, G–I = 10 μm; D = 50 μm; E–F = 20 μm.
Basionym: Thielavia microspora Mouch., Bull. Trimestriel Soc. Mycol. France 89: 300. 1973.
Micromorphology: Ascomata superficial, solitary to aggregated, non-ostiolate, often covered by subhyaline to brown aerial hyphae in 1.5–3.5 μm diam, black, globose or subglobose, 85–120 μm diam. Ascomatal wall brown, non-translucent, composed of irregular or angular cells. Asci ellipsoidal to ovoid, spore-bearing part 13–19 × 11–17 μm, with short stalks 2–5 μm long, containing eight irregularly arranged ascospores, evanescent. Ascospores 1-celled, dark brown when mature, smooth, ellipsoidal, with attenuated ends, (7.5–)8–8.5(–9) × 4.5–5.5(–6) μm, with an apical or slightly subapical germ pore. Conidiogenous cells reduced to a hyphal cell, monoblastic, laterally producing conidia. Conidia laterally produced on aerial hyphae subhyaline, obovoid to clavate, 4–10.5 × 2–3.5 μm.
Culture characteristics: On OA with an entire edge, 31–37 mm diam in 7 d at 25 °C, texture floccose, obverse pale mouse grey to black due to ascomata and aerial mycelium, reverse pale olivaceous. On CMA similar to those on OA, 30–36 mm diam in 7 d at 25 °C, obverse pale mouse grey to olivaceous grey. On MEA with an entire edge, 35–41 mm diam in 7 d at 25 °C, texture floccose, obverse grey white to smoke grey, reverse black. On PCA with an entire edge, 35–41 mm diam in 7 d at 25 °C, with sparse aerial mycelium, obverse pale olivaceous grey to dark mouse grey, reverse dark mouse grey.
Typus: Egypt, Kharga, isolated from desert soil, date unknown, J. Mouchacca (culture ex-type CBS 276.74).
Additional material examined: Japan, isolated from leaf of imported Thymus sp., date and collector unknown (CBS 161.80).
Notes: The ex-type culture of Can. microsporus, CBS 276.74, is degenerated and does not produce a sexual state. The above description is based on CBS 161.80. Canariomyces microsporus exhibits the culture characteristics and asexual morph that match Canariomyces. Phylogenetic analyses unambiguously place this species in Canariomyces, distant from the other species in the genus (Fig. 2, Fig. 3, Fig. 5). This species can be distinguished from the other known species in the genus by having the smallest ascospores (8–8.5 × 4.5–5.5 μm) and elongated conidia (4–10.5 × 2–3.5 μm).
Canariomyces notabilis Arx, Persoonia 12: 185. 1984.
Micromorphology: Description fide von Arx (1984): Hyphae at first hyaline, partly becoming brown especially in advancing regions, often closely septate, 2–5 μm. Ascomata superficial, non-ostiolate, globose, glabrous, dark brown or black, 120–180 μm diam. Ascomatal wall dark brown, non-translucent, composed of angular or irregular cells. Asci spherical or broadly obovate, sessile, 8-spored, evanescent, 20–26 μm diam. Ascospores 1-celled, brown and often with 2 or 3 darker, longitudinal bands when mature, smooth, ellipsoidal or broadly fusiform, with attenuated ends, 11–14 × 7–8.5 μm. Conidiogenous cells reduced to a hyphal cell, monoblastic, laterally producing conidia. Conidia spherical, ellipsoidal or clavate, formed terminally from hyphae or short branches of hyphae in basipetal chains, 1-celled or 2-celled, 9–16 × 5–7 μm (type I); or formed laterally from hyphae, solitary, 1-celled, appressonium-like, 3–5 μm long (type II).
Culture characteristics (fide von Arx 1984): On corn meal agar with a daily growth rate of 2.5–3.5 mm at 28 °C, texture floccose or fasciculate, exudate present, orange or ochraceous.
Typus: Spain, Canary Islands, Gran Canaria, Maspalomas, isolated from litter of Phoenix canariensis, Oct. 1982, J.A. von Arx (culture ex-type CBS 548.83).
Notes: We failed to observe the sexual state and type I conidia of this species because of the degeneration of the ex-type culture. The genome of CBS 508.74 is sequenced by the US Department of Energy Joint Genome Institute (JGI, http://genome.jgi.doe.gov/) and this strain is identified as Can. notabilis. Within the genus, Can. notabilis produces ascospores in intermediate size (Tab. 3). It is closely related to Can. subthermophilus (Fig. 5A), but can be distinguished by smaller ascospores (11–14 × 7–8.5 μm vs 14–19 × 8–10 μm, Tab. 3).
Canariomyces subthermophilus (Mouch.) X. Wei Wang & Houbraken, comb. nov. MycoBank MB829848.
Basionym: Thielavia subthermophila Mouch., Bull. Trimestriel Soc. Mycol. France 89: 297. 1973.
Micromorphology: Description fide Mouchacca (1973): Ascomata non-ostiolate, globose, black, 60–120 μm diam. Ascomatal wall composed of textura epidermoidea and covered by dark hyphae. Ascospores 1-celled, brown when mature, smooth, fusiform or ellipsoidal, 13–17 × 7.5–9.5 μm. Conidiogenous cells reduced to a hyphal cell, monoblastic, laterally producing conidia. Conidia borne laterally, terminally or intercalarary on the aerial hyphae, spherical or broadly clavate, hyaline or light brown, 4–8 × 3–5 μm.
Typus: Egypt, Kharga, isolated from desert soil, date unknown, J. Mouchacca (culture ex-type CBS 509.74).
Notes: We generated molecular data from the ex-type stain, but failed to obtain morphological data. More work is needed to confirm the morphology and phylogeny of this species.
Canariomyces vonarxii X. Wei Wang & Houbraken, sp. nov. MycoBank MB829849. Fig. 21, Fig. 22.
Fig. 21.
Canariomyces vonarxii (CBS 160.80, ex-type culture). A. Colonies from left to right on OA, CMA, MEA and PCA after 4 wk incubation. B. Mature ascomata on PCA, top view. C. Mature ascomata on OA, top view. D. Hyphae, conidiogenous cells and conidia. E–F. Ascomata mounted in lactic acid. G. Asci. H. Ascospores. Scale bars: D, G–H = 10 μm; E–F = 20 μm.
Fig. 22.
Canariomyces vonarxii (CBS 251.85). A. Colonies from left to right on OA, CMA, MEA and PCA after 4 wk incubation. B–C. Part of the colony on OA, showing mature ascomata, top view. D–E. Ascomata mounted in lactic acid. F–H. Hyphae and conidia. I. Structure of ascomatal wall in surface view. J. Asci. K. Ascospores. Scale bars: D = 50 μm; E = 20 μm; F–K = 10 μm.
Etymology: Named after J.A. von Arx, who introduced the genus Canariomyces.
Micromorphology: Ascomata superficial, solitary to aggregated, non-ostiolate, often covered by subhyaline to brown aerial hyphae in 1–4.5 μm diam, black, globose or subglobose, 80–200 μm diam. Ascomatal wall brown, non-translucent, textura angularis in surface view. Asci subglobose to ellipsoidal, 26–45(–47) × 22–38 μm, usually without visible stalks, containing eight irregularly-arranged ascospores, evanescent. Ascospores 1-celled, dark brown when mature, smooth, ellipsoidal, with attenuated ends, (13.5–)15–18.5(–19.5) × (8.5–)9.5–11(–12) μm, with a subapical, or occasionally apical germ pore. Conidiogenous cells reduced to a hyphal cell, monoblastic, laterally producing conidia. Conidia laterally produced on aerial hyphae, subhyaline to pigmented, obovoid or pyriform, (2.5–)3–7.5 × 2.5–5.5 μm.
Culture characteristics: On OA with an entire edge, 27–33 mm diam in 7 d at 25 °C, texture floccose, obverse buff to olivaceous grey due to ascomata and aerial mycelium, reverse pale mouse grey to mouse grey. On CMA similar to those on OA. On MEA with an entire or slightly crenate edge, 26–32 mm diam in 7 d at 25 °C, texture floccose, obverse olivaceous buff to mouse grey or dark mouse grey due to ascomata and aerial mycelium, reverse hazel. On PCA with an entire edge, 27–33 mm diam in 7 d at 25 °C, with sparse aerial mycelium, obverse olivaceous grey, reverse pale olivaceous grey to olivaceous grey.
Typus: Sudan, isolated from a dried flower of Hibiscus sp., date unknown, S. Udagawa (holotype CBS H-18817, culture ex-type CBS 160.80 = NHL 2831).
Additional material examined: Nigeria, substrate unknown, date and collector unknown (CBS 251.85).
Notes: Strain CBS 160.80 was deposited in the CBS collection as Th. subthermophila and CBS 251.85 was deposited as Can. notabilis. Phylogenetic analyses of tub2 and tef1-α showed that these two strains belong to the same species, separate from the other species in the genus (Fig. 5). In the tub2 phylogeny, Can. vonarxii is closely related to Can. arenarius (Fig. 5A), but can be distinguished by its larger ascospores (15–18.5 × 9.5–11 μm vs 8–12.5 × 5–7.5 μm, Tab. 3). This species also differs from Can. notabilis by having larger ascospores (15–18.5 × 9.5–11 μm vs 11–14 × 7–8.5 μm) and from Can. subthermophilus by wider ascospores (9.5–11 μm vs 7.5–9.5 μm).
Carteria X. Wei Wang & Houbraken, gen. nov. MycoBank MB829850.
Etymology: Named after Dr Adrian Carter, who collected the ex-neotype culture of Th. basicola that made the re-evaluation of Thielavia possible.
Micromorphology: Ascomata superficial or immersed in medium, solitary to aggregated, non-ostiolate, globose or subglobose. Ascomatal wall brown, semi-translucent, composed of textura epidermoidea or angularis in surface view. Asci subglobose, ellipsoidal or obovate, without visible stalks, containing eight irregularly-arranged ascospores, evanescent. Ascospores 1-celled, olivaceous brown when mature, smooth, ellipsoidal, attenuated at both ends, with an apical or slightly subapical germ pore. Asexual morph not observed.
Type species: Carteria arctostaphyli X. Wei Wang & Houbraken.
Notes: Carteria is a monotypic genus represented by Car. arctostaphyli. This genus forms a unique lineage in the Chaetomiaceae and no close relatives were found. Carteria produces asci and ascospores similar to those of Thielavia sensu stricto in shape; however, its ascomatal wall is not translucent, which is different from Thielavia and other genera in the Ceratostomataceae. Moreover, Thielavia sensu stricto is not able to grow on agar media without a fungal host, while Carteria can. A new genus is proposed to accommodate this distinct fungus.
Carteria arctostaphyli X. Wei Wang & Houbraken, sp. nov. MycoBank MB829851. Fig. 23.
Fig. 23.
Carteria arctostaphyli (CBS 229.82, ex-type culture). A. Colonies from left to right on OA, CMA, MEA and PCA after 4 wk incubation. B–C. Part of the colony on OA, showing mature ascomata, top view. D–E. Ascomata mounted in lactic acid. F. Structure of ascomatal wall in surface view. G. Asci. H. Ascospores. Scale bars: D–E = 20 μm; F–H = 10 μm.
Etymology: Name refers to Arctostaphylos, the original substrate of the type strain.
Micromorphology: Ascomata superficial, occasionally immersed in medium, solitary to aggregated, often covered by aerial mycelium, non-ostiolate, olivaceous black in reflected light, spherical or subspherical, 25–85 μm diam. Ascomatal wall brown, semi-translucent, composed of textura epidermoidea or angularis in surface view. Asci subglobose, ellipsoidal or obovate, 14–18.5 × 11.5–16 μm, without visiable stalks, containing eight irregularly-arranged ascospores, evanescent. Ascospores 1-celled, olivaceous brown when mature, smooth, ellipsoidal, attenuated at both ends, (7–)8–9(–9.5) × 4.5–5.5 μm, with an apical or slightly subapical germ pore. Asexual morph not observed.
Culture characteristics: On OA with an entire or slightly crenate edge, 7–13 mm diam in 7 d at 25 °C, obverse white to pale smoke grey due to masses of ascomata mixed with aerial mycelium, with smoke grey margin, usually without coloured exudates, reverse fawn. On CMA similar to those on OA, obverse white to pale mouse grey, with hazel margin due to dark immersed hyphae, reverse fawn to hazel. On MEA with a crenate edge, 9–15 mm diam in 7 d at 25 °C, texture floccose, obverse white due to aerial mycelium, with radiating furrows on the margins, reverse fuscous black cinnamon with a thin margin in sienna. On PCA transparent, with an entire edge, 9–15 mm diam in 7 d at 25 °C, with sparse aerial mycelium, obverse smoke grey, without coloured exudates, reverse dark mouse grey.
Typus: Switzerland, Graubünden, Davos, Parsenn, isolated from Arctostaphylos uva-ursi, date unknown, B. Widler (holotype CBS H-23640, culture ex-type CBS 229.82).
Notes: Strain CBS 229.82 was deposited as one of the two isolates of Th. basicola in the CBS culture collection. Phylogenetic data shows that CBS 229.82 and the ex-neotype strain of Th. basicola CBS 178.82 belong to two different orders. Carteria arctostaphyli produces smaller asci (25–85 μm vs 105–260 μm diam) and smaller ascospores (8–9 × 4.5–5.5 μm vs 9.5–11.5 × 5.5–7 μm) than those of Th. basicola. Furthermore, the pure culture of Car. arctostaphyli grows well on the agar media.
Chrysanthotrichum X. Wei Wang & Houbraken, gen. nov. MycoBank MB829852.
Etymology: Name refers to the ascomatal hairs looking like the flower of the plant genus Chrysanthemum.
Micromorphology: Ascomata superficial, globose, subglobose or ovoid, ostiolate or non-ostiolate. Ascomatal wall brown, composed of irregular cells. Ascomatal hairs smooth to verrucose, brown, septate, arcuate around the ostiole of ostiolate ascomata, apically circinate or coiled, with flexuous lateral hairs; or short and flexuous around non-ostiolate ascomata, sometimes slightly undulate or apically circinate. Asci fasciculate, clavate or fusiform, stalked, containing eight biseriate or irregularly-arranged ascospores, evanescent, occasionally persistent until ascospores mature. Ascospores 1-celled, olivaceous brown when mature, smooth, elongated ellipsoidal with attenuated ends to fusiform or elongated fusiform, with an apical germ pore. Asexual morph not observed.
Type species: Chrysanthotrichum lentum (Van Warmelo) X. Wei Wang & Houbraken.
Notes: This genus is closely related to the monotypic genera Thermothielavioides and Floropilus (in Clade 3), two new genera introduced in this study (Fig. 3; PP = 0.99; BS = 98 %). Chrysanthotrichum includes mesophilic species, while Thermothielavioides is introduced for the thermophilic species The. terrestris (syn.: Th. terrestris). Floropilus differs from Chrysanthotrichum by its arcuate ascomatal hairs with undulate to loosely coiled upper parts. Furthermore, colonies of Floropilus often have amber or luteous exudates on OA and CMA (Fig. 30). The ex-type strain of Th. peruviana clustered with three strains in Clade 2, which were deposited as Ch. lentum in the CBS culture collection. Phylogenetic analysis showed that these strains fell into four different lineages (Fig. 2, Fig. 3). Therefore, they are treated as different species in the genus Chrysanthotrichum.
Fig. 30.
Floropilus chiversii (CBS 558.80, ex-type culture). A. Colonies from left to right on OA, CMA, MEA and PCA after 3 wk incubation. B. Part of the colony on OA. C. Mature ascomata on OA, top view. D. Mature ascomata on OA, side view. E–F. Ascomata mounted in lactic acid. G. Structure of ascomatal wall in surface view. H. Terminal ascomatal hairs. I. Asci. J. Ascospores. Scale bars: E–F = 50 μm; G–J = 10 μm.
Chrysanthotrichum allolentum X. Wei Wang & Houbraken, sp. nov. MycoBank MB829853. Fig. 24.
Fig. 24.
Chrysanthotrichum allolentum (CBS 644.83, ex-type culture). A. Colonies from left to right on OA, CMA, MEA and PCA after 3 wk incubation. B. Part of the colony on OA. C. Mature ascomata on OA, top view. D. Mature ascomata on OA, side view. E–F. Ascomata mounted in lactic acid. G. Structure of ascomatal wall in surface view. H. Asci. I. Ascospores. Scale bars: E = 50 μm; F = 20 μm; G–I = 10 μm.
Etymology: Name refers to a fungus morphologically similar but separate from Chrysanthotrichum lentum.
Micromorphology: Ascomata superficial, olivaceous buff to greenish olivaceous in reflected light due to ascomatal hairs, subglobose, ostiolate, 65–110 μm high, 70–115 μm diam. Ascomatal wall brown, composed of textura epidermoidea in surface view. Terminal hairs arcuate, apically coiled or circinate and tapering towards tips, finely verrucose, brown, septate, (3.5–)4–5.5 μm diam near base. Lateral hairs flexuous. Asci clavate or fusiform, spore-bearing part 18.5–26.5 × 9–12.5 μm, with stalks 7.5–14 μm long, containing eight biseriate or irregularly-arranged ascospores, evanescent. Ascospores 1-celled, olivaceous when mature, smooth, elongated ellipsoidal with attenuated ends to fusiform, (8.5–)9–10(–10.5) × 5–6.5(–7) μm, with an apical germ pore. Asexual morph not observed.
Culture characteristics: On OA with an entire edge, 28–34 mm diam in 7 d at 25 °C, with a thin layer of aerial mycelium, obverse greenish olivaceous due to ascomatal hairs, reverse mouse grey due to coloured exudates diffusing into the medium. On CMA similar to those on OA, reverse olivaceous buff. On MEA with an entire edge, 22–28 mm diam in 7 d at 25 °C, texture floccose, obverse pale olivaceous grey, reverse leaden black due to coloured exudates diffusing into the medium. On PCA with a slightly crenate edge, 29–35 mm diam in 7 d at 25 °C, obverse uncoloured, without aerial mycelium, reverse uncoloured.
Typus: USA, California, Cachuma Lake, isolated from soil, date unknown, M. Dreyfuss (holotype CBS H-23634, culture ex-type CBS 644.83).
Notes: Chrysanthotrichum allolentum is phylogenetically distant from the three other species in the genus (Fig. 2, Fig. 3). Morphologically, this species can be distinguished by its terminal hairs which usually have longer coiled apices than Chrysan. lentum (Fig. 25) and Chrysan. leptolentum (Fig. 26), while Chrysan. peruvianum can be easily distinguished by its non-ostiolate ascomata and elongated fusiform ascospores (Fig. 27)
Fig. 25.
Chrysanthotrichum lentum (CBS 339.67, ex-type culture). A. Colonies from left to right on OA, CMA, MEA and PCA after 5 wk incubation. B. Part of the colony on OA. C. Mature ascomata on OA, top view. D. Mature ascomata on OA, side view. E–G. Ascomata mounted in lactic acid. H. Structure of ascomatal wall in surface view. I. Terminal ascomatal hairs. J. Asci. K. Ascospores. Scale bars: E–G = 20 μm; H–K = 10 μm.
Fig. 26.
Chrysanthotrichum leptolentum (CBS 126.85, ex-type culture). A. Colonies from left to right on OA, CMA, MEA and PCA after 3 wk incubation. B. Part of the colony on OA. C. Mature ascomata on OA, top view. D. Mature ascomata on OA, side view. E–G. Ascomata mounted in lactic acid. H. Structure of ascomatal wall in surface view. I. Terminal ascomatal hairs. J. Asci. K. Ascospores. Scale bars: E–G = 20 μm; H–K = 10 μm.
Fig. 27.
Chrysanthotrichum peruvianum (CBS 732.68, ex-type culture). A. Colonies from left to right on OA, CMA, MEA and PCA after 3 wk incubation. B–C. Mature ascomata on OA, top view. D–E. Ascomata mounted in lactic acid. F–G. Structure of ascomatal wall in surface view. H. Asci. I. Ascospores. Scale bars: D = 50 μm; E = 20 μm; F–I = 10 μm.
Chrysanthotrichum lentum (Van Warmelo) X. Wei Wang & Houbraken, comb. nov. MycoBank MB829855. Fig. 25.
Basionym: Chaetomium lentum Van Warmelo, Mycologia 58: 850. 1967.
Micromorphology: Ascomata superficial, olivaceous black in reflected light due to ascomatal hairs, subglobose or ovoid, ostiolate, 55–85 μm high, 55–100 μm diam. Ascomatal wall brown, composed of irregular cells. Terminal hairs arcuate, apically circinate or slightly coiled, verrucose, brown, septate, (3.5–)4–7 μm diam near base. Lateral hairs flexuous. Asci fasciculate, clavate or fusiform, spore-bearing part 20–28 × 9.5–12.5 μm, with stalks 5.5–9.5 μm long, containing eight biseriate or irregularly-arranged ascospores, evanescent, occasionally persistent until mature. Ascospores 1-celled, olivaceous brown when mature, smooth, elongated ellipsoidal with attenuated ends to fusiform, (7.5–)9–10.5 × (5–)5.5–6(–6.5) μm, with an apical germ pore. Asexual morph not observed.
Culture characteristics: On OA with an entire edge, 27–33 mm diam in 7 d at 25 °C, without aerial mycelium, obverse leaden black due to ascomatal hairs, reverse olivaceous grey to iron grey due to coloured exudates diffusing into the medium. On CMA similar to those on OA, reverse dark mouse grey. On MEA with an entire edge, 27–33 mm diam in 7 d at 25 °C, obverse floccose, smoke grey with leaden black ascomata scattering on the surface, reverse fuscous black due to coloured exudates diffusing into the medium. On PCA with an entire edge, 28–34 mm diam in 7 d at 25 °C, obverse olivaceous grey to dark mouse grey due to coloured exudates diffusing into the medium, with sparse white aerial mycelium, reverse olivaceous grey to dark mouse grey.
Typus: South Africa, Johannesburg, isolated from soil, K.T. van Warmelo, date unknown (culture ex-type CBS 339.67).
Note: This species can be distinguished from Chrysan. allolentum by producing terminal hairs that are circinate or with short coiled apices. Chrysanthotrichum lentum is closely related to Chrysan. leptolentum and Chrysan. peruvianum in a well-supported lineage (PP ≥ 0.99, BS = 100 %; Fig. 2, Fig. 3). The latter deviates from Chrysan. lentum by its non-ostiolate ascomata and elongated fusiform ascospores (Fig. 27), and Chrysan. leptolentum differs from Chrysan. lentum by thinner ascomatal hairs (2.5–5 μm vs 4–7 μm diam near base) and thinner ascospores (9–10 × 4.5–5.5 μm vs 9–10.5 × 5–6.5 μm).
Chrysanthotrichum leptolentum X. Wei Wang & Houbraken, sp. nov. MycoBank MB829856. Fig. 26.
Etymology: Name refers to Chrysan. lentum with relatively slender ascomatal hairs.
Micromorphology: Ascomata superficial, grey olivaceous in reflected light due to ascomatal hairs, subglobose or ovate, ostiolate, 75–125 μm high, 70–125 μm diam. Ascomatal wall brown, composed of irregular cells. Terminal hairs arcuate, apically circinate or coiled, smooth or finely punctulate, brown, septate, 2.5–5 μm diam near base. Lateral hairs flexuous. Asci fasciculate, clavate or fusiform, spore-bearing part 18–29 × 8–10.5 μm, with stalks 6–12 μm long, containing eight biseriate ascospores, evanescent. Ascospores 1-celled, brown when mature, smooth, elongated ellipsoidal with attenuated ends to fusiform, (8.5–)9–10 × 4.5–5.5 μm, with an apical germ pore. Asexual morph not observed.
Culture characteristics: On OA with an entire edge, 27–33 mm diam in 7 d at 25 °C, without aerial mycelium, or occasionally with sparse white aerial mycelium, obverse olivaceous grey due to ascomatal hairs, reverse fuscous black due to coloured exudates diffusing into the medium. On CMA similar to those on OA. On MEA with an entire edge, 28–34 mm diam in 7 d at 25 °C, obverse floccose, smoke grey, reverse greenish black due to coloured exudates diffusing into the medium. On PCA with a slightly crenate edge, 24–30 mm diam in 7 d at 25 °C, obverse greenish black due to coloured exudates diffusing into the medium, with sparse white aerial mycelium, reverse olivaceous grey to iron grey.
Typus: Kenya, Mt. Kenya, Naro Moro Track, isolated from dung of elephant, 13 Jul. 1966, R.F. Cain, H.D. Griffin & J.C. Krug (holotype CBS H-23633, culture ex-type CBS 126.85).
Additional material examined: Canada, Ontario, Haliburton Co., Dorset, isolated from dung of moose or deer, 21 Sep. 1980, D.G. Lahaie (CBS 127.85).
Notes: Chrysanthotrichum leptolentum and Chrysan. lentum are closely related. For the morphological comparison, see notes of Chrysan. lentum.
Chrysanthotrichum peruvianum (Goch.) X. Wei Wang & Houbraken, comb. nov. MycoBank MB829857. Fig. 27.
Basionym: Chaetomidium peruvianum Goch., Mycologia 60: 1118. 1968.
Synonym: Thielavia peruviana (Goch.) Malloch & Cain, Mycologia 65: 1067. 1973.
Micromorphology: Ascomata superficial, solitary to aggregated, non-ostiolate, leaden black when mature in reflected light due to the dark ascomatal wall, spherical, pilose, 70–120 μm diam. Ascomatal wall brown, semi-translucent, composed of textura epidermoidea in surface view. Ascomatal hairs brown, short, flexuous, sometimes slightly undulate or apically circinate, smooth, septate, 2.5–4.5 μm diam near base, less than 100 μm long. Asci fusiform or clavate, spore-bearing part 25–38 × 8.5–13 μm, with stalks 6–12 μm long, containing eight biseriate or irregularly-arranged ascospores, evanescent, occasionally persistent until mature. Ascospores 1-celled, olivaceous when mature, smooth, elongated fusiform, often inequilateral, (9–)11–13.5(–14.5) ×5–6 μm, with an apical germ. Asexual morph not observed.
Culture characteristics: On OA with an entire edge, 12–18 mm diam in 7 d at 25 °C, without or with sparse aerial mycelium, obverse mouse grey or olivaceous grey due to the masses of ascomata, or ochraceous to cinnamon or fulvous due to coloured exudates diffusing into the medium, reverse olivaceous grey, with margin fulvous. On CMA similar to those on OA. On MEA with an entire edge, 15–21 mm diam in 7 d at 25 °C, texture slightly floccose, obverse pale smoke grey, with a mouse grey ring near margin, reverse greenish black. On PCA with an entire edge, 13–19 mm diam in 7 d at 25 °C, without white aerial mycelium mainly in the central part, obverse pale olivaceous grey, without coloured exudates, reverse dark mouse grey in the central part.
Typus: Peru, near Manazo, isolated from high mountain tundra soil, 1 Jan. 1963, S.E. Gochenaur (culture ex-type CBS 732.68).
Notes: Chrysanthotrichum peruvianum is morphologically distinctly different from the three other species in the genus in producing non-ostiolate ascomata. Interestingly, this species is phylogenetically closer to Chrysan. lentum and Chrysan. leptolentum than to Chrysan. allolentum, while the latter three species are morphologically similar. The presence of ostiolate and non-ostiolate ascomata in this genus makes these species good examples to study divergent and convergent evolution of this character.
Chrysocorona X. Wei Wang & Houbraken, gen. nov. MycoBank MB829858.
Etymology: Name refers to ascomata covered by orange crowns composed of ascomatal hairs.
Micromorphology: Ascomata superficial, amber to luteous due to ascomatal hairs in reflected light, subglobose or ellipsoidal, ostiolate. Ascomatal wall brown, composed of textura angularis in surface view. Terminal hairs arcuate, verrucose, with numerous short, flexuous and easily-exfoliated branches near the apical part, brown, septate, usually constricted at the septa, verrucose. Lateral hairs flexuous. Asci fasciculate, clavate, fusiform or pyriform, stalked, containing eight irregularly-arranged ascospores, evanescent. Ascospores 1-celled, olivaceous brown when mature, smooth, 1-celled, ellipsoidal, with a slightly subapical germ pore. Asexual morph not observed.
Type species: Chrysocorona lucknowensis (J.N. Rai & J.P. Tewari) X. Wei Wang & Houbraken.
Notes: Chrysocorona is a newly proposed monotypic chaetomium-like genus closely related to three thielavia-like genera (Hyalosphaerella, Parathielavia, Pseudothielavia), Acrophialophora and another chaetomium-like genus (Brachychaeta) in Clade 1. In the combined tree (Fig. 3), Bayesian analysis indicates that Chrysocorona is basal to the three thielavia-like genera (PP = 0.99); however, this is not supported in the ML analysis (BS <70 %). The genus can be distinguished from Brachychaeta and the two sexual species of Acrophialophora by its arcuate and brightly coloured terminal ascomatal hairs having numerous short branches near the apical part.
Chrysocorona lucknowensis (J.N. Rai & J.P. Tewari) X. Wei Wang & Houbraken, comb. nov. MycoBank MB829859. Fig. 28.
Basionym: Chaetomium lucknowense J.N. Rai & J.P. Tewari, Canad. J. Bot. 40: 1380. 1962.
Synonym: Chaetomium venezuelense L.M. Ames, Monograph of the Chaetomiaceae: 42. 1963.
Micromorphology: Ascomata superficial, amber to luteous due to ascomatal hairs in reflected light, subglobose or ellipsoidal, ostiolate, 115–175 μm high, 90–120 μm diam. Ascomatal wall brown, composed of textura angularis in surface view. Terminal hairs arcuate, 3–4.5 μm diam near base, with numerous short, flexuous and easily-exfoliated branches near the apical part, brown, septate, usually constricted at the septa, verrucose. Lateral hairs flexuous. Asci fasciculate, clavate, fusiform or pyriform, spore-bearing part 23–31.5 × 11.5–15.5 μm, with stalks 9–19.5 μm long, containing eight irregularly arranged ascospores, evanescent. Ascospores 1-celled, olivaceous brown when mature, smooth, ellipsoidal, (9.5–)10–11.5(–12) × 6–7 μm, with a slightly subapical germ pore. Asexual morph not observed.
Culture characteristics: On OA with an entire edge, 27–33 mm diam in 7 d at 25 °C, without aerial mycelium, olivaceous grey to violaceous black because of pigmented exudates diffusing into the medium, with luteous or orange ascomata mainly distributing near the edges, reverse mouse grey. On CMA similar to those on OA, obverse presenting orange due to the dense formation of ascomata. On MEA with an fimbriate edge, 24–30 mm diam in 7 d at 25 °C, texture floccose, obverse buff to pale luteous, or rosy buff at the edge due to the formation of young ascomata, reverse mouse grey due to exudates diffusing into the medium. On PCA with an entire edge, 27–33 mm diam in 7 d at 25 °C, without aerial mycelium, sparsely forming ascomata mainly in the central point, without coloured exudates, reverse uncoloured or olivaceous grey under ascomata in the central point.
Typus: Lectotype of Chaetomium lucknowense designated here: figs 16–28 illustrated by J.N. Rai and J.P. Tewari based on original culture from soil in Uttar Pradesh, India, in Canadian Journal of Botany 40: 1380, 1962, MBT385833. India, Bharatpur, isolated from dung of deer, Jan. 1971, B.C. Lodha (CBS H-10081, epitype designated here, MBT385834, culture ex-epitype CBS 727.71).
Additional material examined: Germany, isolated from dung of rabbit, date unknown, H.K. Seth (CBS 562.67). Venezuela, State Sucre, isolated from dung of donkey, 14 Jul. 1972, J.C. Krug (CBS 124.85); isolated from soil and vegetable detritus, date unknown, L.M. Ames (CBS 385.66, ex-type of Ch. venezuelense L.M. Ames).
Note: In Clade 1, both Chrysocorona and Brachychaeta are monotypic genera and produce arcuate terminal ascomatal hairs. The type species Chrysoc. lucknowensis can be distinguished from Bra. variospora by amber to luteous ascomatal hairs with short branches near the apical part which easily fall off, and by ellipsoidal (rather than irregular) and smaller ascospores (10–11.5 × 6–7 μm vs 14.5–17 × 10–12 μm) with one (rather than two) germ pore.
Condenascus X. Wei Wang & Houbraken, gen. nov. MycoBank MB829860.
Etymology: Name refers to the aggregated ascomata and densely congregated asci.
Micromorphology: Ascomata superficial, usually aggregated and mixed with aerial mycelium, globose or subglobose, non-ostiolate, glabrous. Ascomatal wall brown, semi-translucent, composed of angular or irregular cells. Asci cylindrical, twisted, stalked, containing eight uniseriate ascospores, evanescent. Ascospores 1-celled, olivaceous brown when mature, smooth, fusiform, with a subapical or oblique germ pore. Asexual morph not observed.
Type species: Condenascus tortuosus (Udagawa & Y. Sugiy.) X. Wei Wang & Houbraken.
Note: The four-locus concatenated tree showed that this is a monotypic genus forming a single, basal lineage in the Chaetomiaceae and no closely related genera were found in the present study. Condenascus produces dense conglomerations of asci in the ascomata, similar to Trichocladium antarcticum (syn.: Th. antarctica, figs 16–22 in Stchigel et al. 2003; fig. 46H in Wang et al. 2019). However, Condenascus can be easily distinguished from Tri. antarcticum by its twisted asci and large, fusiform ascospores, sparse mycelium and by the lack of an asexual state.
Condenascus tortuosus (Udagawa & Y. Sugiy.) X. Wei Wang & Houbraken, comb. nov. MycoBank MB829861. Fig. 29.
Fig. 29.
Condenascustortuosus (CBS 610.97). A. Colonies from left to right on OA, CMA, MEA and PCA after 3 wk incubation. B–C. Mature ascomata on OA, top view. D–E. Ascomata mounted in lactic acid. F–G. Asci. H. Ascospores. Scale bars: D–E = 50 μm; F = 20 μm; G–H = 10 μm.
Basionym: Thielavia tortuosa Udagawa & Y. Sugiy., Trans. Mycol. Soc. Japan 22: 197. 1981.
Micromorphology: Ascomata superficial, usually aggregated, often covered by or mixed with aerial mycelium, dark slate blue in reflected light, globose or subglobose, non-ostiolate, glabrous,130–400 μm diam. Ascomatal wall brown, semi-translucent, composed of angular or irregular cells. Asci fasciculate, densely congregated in the ascomata, cylindrical, twisted, spore-bearing part 108–146 × 13–21.5 μm, with stalks 6–12 μm long, containing eight uniseriate ascospores, evanescent. Ascospores 1-celled, olivaceous brown when mature, smooth, fusiform, (25–)26–29(–30.5) × (13.5–)14–16(–16.5) μm, with a subapical or oblique germ pore. Asexual morph not observed.
Culture characteristics: On OA with a crenate edge, 18–24 mm diam in 7 d at 25 °C, obverse pale mouse grey due to masses of ascomata mixed with aerial mycelium, with white margin because of aerial mycelium, without coloured exudates, reverse uncoloured or buff. On CMA similar to those on OA. On MEA with a crenate edge, 37–43 mm diam in 7 d at 25 °C, obverse pale olivaceous grey due to aggregated ascomata mixed with white aerial mycelium, or white to buff due to aerial mycelium that covers the ascomata, reverse ochraceous or cinnamon. On PCA transparent, with an entire edge, 18–24 mm diam in 7 d at 25 °C, without aerial mycelium, obverse smoke grey in the centre with a ring around the centre due to ascomata, without coloured exudates, reverse uncoloured.
Material examined: India, Jaipur, isolated from soil, 30 Oct. 1995, J. Guarro (representative culture CBS 610.97).
Notes: The holotype was isolated from imported spices (thyme plant) in Japan (Udagawa & Sugiyama 1981) and the original location of the holotype is unknown. The ex-type culture CBS 691.82 deposited in the CBS culture collection has died. The examined isolate CBS 610.97 morphologically matches with the protologue (Udagawa & Sugiyama 1981) and was used as the representative of this species in this study.
Floropilus X. Wei Wang & Houbraken, gen. nov. MycoBank MB829862.
Etymology: Name refers to the arcuate terminal hairs with undulate or loosely coiled upper parts hairs which look like a flower covering the ascomata of the fungus.
Micromorphology: Ascomata superficial, subglobose, ostiolate. Ascomatal wall brown, composed of irregular or angular cells. Terminal hairs arcuate, with undulate to circinate or loosely coiled upper parts, brown, septate, verrucose. Lateral hairs flexuous, undulate or circinate near the apical part. Asci fasciculate, pyriform, obovate or clavate, stalked, containing eight irregularly-arranged ascospores, evanescent. Ascospores 1-celled, olivaceous brown when mature, smooth, fusiform or ovate, with an apical or slightly subapical germ pore. Asexual morph not observed.
Type species: Floropilus chiversii (J.C. Cooke) X. Wei Wang & Houbraken.
Notes: Floropilus is a chaetomium-like monotypic genus which forms a sister lineage of the newly introduced genus Thermothielavioides in Clade 2 (Fig. 2, Fig. 3). It can be distinguished from Thermothielavioides by ostiolate ascomata and by the absence of a conidial state. Moreover, Thermothielavioides is thermophilic, while Floropilus is mesophilic. The type species F. chiversii produces arcuate terminal ascomatal hairs and coloured exudates, similar to members of Arcopilus, but can be distinguished by its terminal ascomatal hairs with undulate apices. Arcopilus species produce terminal hairs with incurved, circinate to coiled apices (Wang et al. 2016a). Phylogenetic analyses indicated that this species is not closely related to the genus Arcopilus (Fig. 2, Fig. 3). Floropilus chiversii will be a good reference species to study how thermophilic and mesophilic species diverged from each other in the evolutionary history of the Chaetomiaceae.
Floropilus chiversii (J.C. Cooke) X. Wei Wang & Houbraken, comb. nov. MycoBank MB829863. Fig. 30.
Basionym: Chaetomium trilaterale var. chiversii J.C. Cooke, Mycologia 65: 1218. 1973.
Synonym: Chaetomium chiversii (J.C. Cooke) A. Carter, Beih. Nova Hedwigia 84: 19. 1986.
Micromorphology: Ascomata superficial, pale mouse grey due to ascomatal hairs in reflected light, subglobose, ostiolate, 60–125 μm high, 50–110 μm diam. Ascomatal wall brown, composed of irregular or angular cells. Terminal hairs arcuate, apically undulate, brown, septate, verrucose, 2–3.5 μm diam near base. Lateral hairs flexuous, undulate or circinate near the apical part. Asci fasciculate, pyriform, obovate or clavate, spore-bearing part 16–24.5 × 10–15.5 μm, with stalks 5–6 μm long, containing eight irregularly arranged ascospores, evanescent. Ascospores 1-celled, olivaceous brown when mature, smooth, fusiform or ovate, (9.5–)10–11.5(–12) × 6–7 μm, with an apical or slightly subapical germ pore. Asexual morph not observed.
Culture characteristics: On OA with an entire or slightly crenate edge, 21–27 mm diam in 7 d at 25 °C, texture floccose, obverse honey, often with amber or luteous drops of exudates on the surface, reverse honey to ochraceous due to coloured exudates diffusing into the medium. On CMA similar to those on OA, 16–22 mm diam in 7 d at 25 °C, obverse pale olivaceous grey, reverse saffron or citrine. On MEA with a crenate edge, 21–27 mm diam in 7 d at 25 °C, irregularly wrinkled, obverse floccose, honey or hazel, reverse orange. On PCA with an entire edge, 25–31 mm diam in 7 d at 25 °C, without aerial mycelium, without coloured exudates, reverse uncoloured or pale luteous.
Typus: Canada, Ontario, Timiskaming District, Burt Lake, isolated from dung of moose, date unknown, A. Carter (CBS H-10077, neotype of Chaetomium trilaterale var. chiversii designated here MBT386363, culture ex-neotype CBS 558.80 = IMI 250966 = MUCL 40052 = TRTC 48533).
Notes: This species was originally described as a variety of Ch. trilaterale based on a strain from the USA (Cooke 1973). Carter (1986) raised this variety to species level based on CBS 558.80, because the ex-type culture of Ch. trilaterale var. chiversii was unavailable (von Arx et al. 1986). Therefore, the herbarium specimen CBS H-10077, a dried culture of CBS 558.80, is designated here as the neotype of this species.
Hyalosphaerella X. Wei Wang & Houbraken, gen. nov. MycoBank MB829864.
Etymology: Name refers to the small spherical non-ostiolate ascomata with translucent walls.
Micromorphology: Ascomata immersed in the medium, solitary to aggregated, non-ostiolate, spherical, glabrous, usually less than 120 μm diam. Ascomatal wall subhyaline, translucent, thin, composed of textura epidermoidea in surface view. Asci clavate to pyriform, stalked, containing eight irregularly-arranged ascospores, evanescent. Ascospores 1-celled, olivaceous brown when mature, smooth, 1-celled, ovoid, ellipsoidal or reniform, often inequilateral, with an apical germ pore at the most attenuated end. Asexual morph not observed.
Type species: Hyalosphaerella fragilis (Natarajan) X. Wei Wang & Houbraken.
Notes: The monotypic genus Hyalosphaerella forms a single lineage which is a sister to another thielavia-like lineage Parathielavia in Clade 1 (Fig. 2, Fig. 3). Hyalosphaerella can be distinguished from Parathielavia by the production of immersed ascomata with a subhyaline and translucent ascomatal wall. The subhyaline and translucent ascomatal wall of the type species of Hyalosphaerella is reminiscent of that of Boothiella in the Sordariaceae. However, Boothiella differs from Hyalosphaerella by the production of cylindrical and 4-spored asci, larger ascomata and larger ascospores. For detailed comparison see notes of Hya. fragilis.
Hyalosphaerella fragilis (Natarajan) X. Wei Wang & Houbraken, comb. nov. MycoBank MB829865. Fig. 31.
Fig. 31.
Hyalosphaerella fragilis (CBS 456.73, ex-type culture). A. Colonies from left to right on OA, CMA, MEA and PCA after 3 wk incubation. B–C. Mature ascomata on OA, top view. D–E. Ascomata mounted in lactic acid. F. Asci. G. Ascospores. Scale bars: D–E = 20 μm; F–G = 10 μm.
Basionym: Chaetomidium fragile Natarajan, Proc. Indian Natl. Sci. Acad., B. 37: 124. 1972.
Synonym: Thielavia fragilis (Natarajan) Arx, Stud. Mycol. 8: 8. 1975.
Micromorphology: Ascomata immersed in the medium, solitary to aggregated, non-ostiolate, leaden black when mature in reflected light due to the dark ascospores inside, spherical, glabrous, 50–115 μm diam. Ascomatal wall subhyaline, translucent, thin, composed of textura epidermoidea in surface view. Asci clavate to pyriform, spore-bearing part 23.5–36.5 × 13.5–20 μm, with stalks 5–13 μm long, containing eight irregularly arranged ascospores, evanescent. Ascospores 1-celled, olivaceous brown when mature, smooth, ovoid, ellipsoidal or reniform, often inequilateral, (10–)11–13 (–14.5) × (6–)6.5–7.5(–8.5) μm, with an apical germ pore at the most attenuated end. Asexual morph not observed.
Culture characteristics: On OA with an entire edge, 49–55 mm diam in 7 d at 25 °C, without aerial mycelium, obverse olivaceous grey due to masses of ascomata, with margin olivaceous buff to honey due to coloured exudates diffusing into the medium, reverse isabelline. On CMA with an entire edge, 37–43 mm diam in 7 d at 25 °C, without aerial mycelium, obverse olivaceous grey due to masses of ascomata, without coloured exudates, reverse uncoloured. On MEA with an entire or slightly crenate edge, 41–47 mm diam in 7 d at 25 °C, with white aerial mycelium, texture floccose, obverse white, reverse ochraceous to fulvous. On PCA with an entire edge, 43–49 mm diam in 7 d at 25 °C, without aerial mycelium, obverse pale olivaceous grey, without coloured exudates, reverse pale olivaceous grey.
Typus: India, Tamil Nadu, isolated from rhizosphere of Pennisetum typhoideum in garden soil, 27 Oct. 1966, K. Natarajan (culture ex-type CBS 456.73).
Notes: Hyalosphaera fragilis is morphologically similar to Pse. subhyaloderma in ascomata, asci and ascospores, but can be distinguished by immersed ascomata and by lacking aerial mycelium on OA and CMA. Its ascomata with subhyaline and translucent ascomatal wall are also similar to those of Boothiella tetraspora in the Sordariaceae (Fig. 58). However, Hya. fragilis differs from B. tetraspora by smaller ascomata (50–115 μm vs 115–390 μm diam), 8-spored, clavate to pyriform asci and distinct ascospores (often inequilateral, 11–13 × 6.5–7.5 μm vs ellipsoidal to broad ovoid, 17.5–22 ×13–15 μm).
Fig. 58.
Boothiella tetraspora (CBS 887.97). A. Colonies from left to right on OA, CMA, MEA and PCA after 4 wk incubation. B. Part of the colony on OA. C. Mature ascomata on OA, top view. D. Mature ascomata on PCA, top view. E–G. Ascomata mounted in lactic acid. H. Asci. I. Ascospores. Scale bars: E–G = 50 μm; H–I = 10 μm.
Microthielavia X. Wei Wang & Houbraken, gen. nov. MycoBank MB829866.
Etymology: Name refers to small and thielavia-like ascomata.
Micromorphology: Ascomata immersed or sub-immersed in the medium, solitary to loosely aggregated, small, non-ostiolate, spherical or subspherical, glabrous, usually less than 60 μm diam. Ascomatal wall brown, semi-translucent or non- translucent, composed of textura angularis in surface view. Asci fasciculate, clavate to pyriform, stalked, containing eight irregularly-arranged ascospores, evanescent. Ascospores 1-celled, olivaceous when mature, smooth, 1-celled, fusiform or ovoid, attenuated at both ends, with an apical germ pore. Asexual morph not observed.
Type species: Microthielavia ovispora (Pidopl., Kiril. & Zakharch.) X. Wei Wang & Houbraken.
Notes: Microthielavia is one of the three thielavia-like single lineages (Carteria, Condenascus and Microthielavia) in the Chaetomiaceae. All three are monotypic and known only from their type species. No close relatives of these three genera have been found. Microthielavia can be distinguished from the other genera by its quite small and semi-translucent or non- translucent ascomata which are immersed or sub-immersed in the medium. Hyalosphaerella also produces small and immersed ascomata, but can be distinguished by its subhyaline and translucent ascomatal wall. Moreover, the type species of Hyalosphaerella has larger ascomata than those of Mic. ovispora (50–115 μm vs 25–56 μm diam). Carteria is also similar to Microthielavia in having small and non-ostiolate ascomata, but the type species Car. arctostaphyli differs from Mic. ovispora by having superficial and slightly larger ascomata (25–85 μm vs 25–56 μm diam), subglobose, ellipsoidal or obovate asci without visiable stalks and ellipsoidal ascospores (Fig. 23).
Microthielavia ovispora (Pidopl., Kiril. & Zakharch.) X. Wei Wang & Houbraken, comb. nov. MycoBank MB829867. Fig. 32.
Fig. 32.
Microthielavia ovispora (CBS 165.75, ex-type culture). A. Colonies from left to right on OA, CMA, MEA and PCA after 2 wk incubation. B–C. Part of the colony on OA, showing mature ascomata, top view. D–E. Ascomata mounted in lactic acid. F. Ascomata mounted in lactic acid, showing structure of ascomatal wall in surface view. G. Asci. H. Ascospores. Scale bars: D–F = 20 μm; G–H = 10 μm.
Basionym: Thielavia ovispora Pidopl., Kiril. & Zakharch., Mikrobiol. Zhurn. 35: 724. 1973.
Synonym: Thielavia kirilenkoae Beliakova, Mikol. Fitopatol. 8: 73. 1974.
Micromorphology: Ascomata immersed or sub-immersed in the medium, solitary to loosely aggregated, non-ostiolate, leaden black when mature in reflected light, spherical or subspherical, glabrous, 25–56 μm diam. Ascomatal wall brown, semi-translucent, composed of textura angularis in surface view. Asci clavate to pyriform, spore-bearing part 13.5–20.5 ×9–13.5 μm, with stalks 3–7 μm long, containing eight irregularly arranged ascospores, evanescent. Ascospores 1-celled, olivaceous when mature, smooth, ovoid to fusiform, attenuated at both ends, (7–)8–9(–9.5) × 5–6 μm, with an apical germ pore. Asexual morph not observed.
Culture characteristics: On OA with an entire edge, 18–24 mm diam in 7 d at 25 °C, with sparse aerial mycelium, obverse buff to mouse grey, with margin buff, reverse ochraceous or mouse grey. On CMA with an entire edge, 18–24 mm diam in 7 d at 25 °C, with aerial mycelium, obverse smoke grey, slightly pale luteous due to ascomata mixed with aerial mycelium, reverse isabelline. On MEA with an entire or slightly crenate edge, 13–19 mm diam in 7 d at 25 °C, without aerial mycelium, wrinkled to form radiating furrows, obverse vinaceous buff with fawn margin, reverse ochraceous to orange. On PCA transparent, with an entire edge, 13–19 mm diam in 7 d at 25 °C, obverse near the centre olivaceous grey due to ascomata, with sparse and pale luteous aerial mycelium, without coloured exudates, reverse pale mouse grey, or olivaceous grey near the centre.
Typus: Ukraine, Zhitomir region, isolated from the root of Avena sativa, 26 Jun. 1962, T.S. Kirilenko (culture ex-type CBS 165.75 = IMI 196525 = VKM F-1596).
Notes: Strain CBS 165.75 was deposited as the ex-type of Th. kirilenkoae. The type strains of both Th. ovispora and Th. kirilenkoae were isolated by T.S. Kirilenko in 1962 originating from the same location and source (Index of Fungi 4: 291. 1971–1980). There is a note written by L.A. Beliakova (the author of Th. kirilenkoae) linked to CBS 165.75 and saved in the CBS collection, which indicated that Th. kirilenkoae and Th. ovispora are based on the same specimen. Microthielavia ovispora has priority and Th. kirilenkoae is thus reduced to a synonym of Th. ovispora. For the morphological comparison, see notes of the genus.
Parathielavia X. Wei Wang & Houbraken, gen. nov. MycoBank MB829868.
Etymology: Name refers to the morphologically similarity but phylogenetically distance from Thielavia sensu stricto.
Micromorphology: Ascomata superficial to immersed in the medium, solitary to aggregated, non-ostiolate, spherical to oblate, pilose or glabrous. Ascomatal wall brown, semi-translucent, composed of textura epidermoidea in surface view. Asci clavate, obovoid or pyriform, with stalks, containing eight irregularly arranged ascospores, evanescent, sometimes persistent until ascospores mature. Ascospores 1-celled, olivaceous when mature, smooth, fusiform or ellipsoidal and attenuated at both ends, with a subapical germ pore. Asexual morph absent or producing conidia directly on hyphae.
Type species: Parathielavia hyrcaniae (Nicot) X. Wei Wang & Houbraken.
Notes: Several thielavia-like species were previously used as the representatives of the genus Thielavia (Wang et al., 2016a, Wang et al., 2019). Phylogenetic analyses in this study included a large sampling of Chaetomiaceae members. In the resulting trees (Fig. 2, Fig. 3), those representative species were split into three different lineages and mixed with several non-thielavia-like lineages in Clade 1 in the Chaetomiaceae. Hyalosphaerella, Parathielavia and Pseudothielavia were proposed to accommodate those three thielavia-like lineages. These three genera are different from Thielavia sensu stricto in having no association with Berkeleyomyces species. Parathielavia and Pseudothielavia also differ in possessing a semi-translucent (rather than translucent) ascomatal wall. Parathielavia is most closely related to Hyalosphaerella, but differs by its ascomata that are superficial and larger (60–300 μm vs 50–115 μm diam) with pigmented and semi-translucent ascomatal walls. It can also be distinguished from Pseudothielavia by the ascospores which have a subapical germ pore. Pseudothielavia species usually have ascospores with an apical germ pore with the exception of Pse. arxii which has ascospores with an oblique to lateral germ pore.
Parathielavia appendiculata (M.P. Srivast., Tandon, Bhargava & A.K. Ghosh) X. Wei Wang & Houbraken, comb. nov. MycoBank MB829869. Fig. 33.
Fig. 33.
Parathielavia appendiculata (CBS 723.68, ex-type culture). A. Colonies from left to right on OA, CMA, MEA and PCA after 3 wk incubation. B–C. Mature ascomata on OA, top view. D–F. Ascomata mounted in lactic acid. G. Asci. H. Ascospores. Scale bars: D–F = 20 μm; G–H = 10 μm.
Basionym: Thielavia appendiculata M.P. Srivast., Tandon, Bhargava & A.K. Ghosh, Mycopath. Mycol. Appl. 30: 205. 1966.
Micromorphology: Ascomata superficial, solitary to loosely aggregated, non-ostiolate, leaden black when mature in reflected light due to the dark ascomatal wall, spherical, pilose, (90–)110–235(–280) μm diam. Ascomatal wall brown, semi-translucent, composed of textura epidermoidea in surface view. Ascomatal hairs brown, short, finger-like or tapering towards tips, erect or flexuous, smooth, septate, 2–3.5 μm diam near base, usually less than 20 μm long. Asci clavate, spore-bearing part 24–36 × 12.5–17 μm, with stalks 5–14 μm long, containing eight irregularly arranged ascospores, evanescent, sometimes persistent until ascospores mature. Ascospores 1-celled, olivaceous when mature, smooth, ellipsoidal to fusiform, attenuated at both ends, (11–)12–14.5(–17) × (6–)6.5–8.5(–9.5) μm, with a subapical germ pore. Asexual morph not observed.
Culture characteristics: On OA with an entire edge, 20–26 mm diam in 7 d at 25 °C, with sparse aerial mycelium, obverse usually pale luteous to luteous due to coloured exudates diffusing into the medium, reverse pale luteous to luteous. On CMA similar to those on OA. On MEA with an entire edge, 20–26 mm diam in 7 d at 25 °C, texture floccose, obverse white or pale luteous, reverse orange or sienna. On PCA with an entire edge, 18–24 mm diam in 7 d at 25 °C, without aerial mycelium, obverse uncoloured, without coloured exudates, reverse uncoloured.
Typus: India, Jodhpur, isolated from leaf of Punica granatum, Nov. 1963, collector unknown (culture ex-type CBS 723.68 = IMI 104944).
Additional material examined: UK, Wales, near Aberystwyth, isolated from dung of rabbit, date unknown, H.K. Seth (CBS 731.68). Unknown, substrate unknown, date and collector unknown, J.N. Kapoor (CBS 417.73).
Note: This species can be distinguished from the other known thielavia-like species by short, usually finger-like ascomatal hairs and ellipsoidal to fusiform ascospores with a subapical germ pore. Among the three known species in the genus, Par. hyrcaniae can be distinguished from Par. appendiculata by longer (up to almost 60 μm vs less than 20 μm long) and geniculate, flexuous or undulate ascomatal hairs and slightly smaller fusiform ascospores (11–13 × 6–7 μm vs 12–14.5 × 6.5–8.5 μm), and Par. kuwaitensis differs from Par. appendiculata by glabrous ascomata, smaller ascospores (9.5–10.5 × 6–7 μm) and the presence of conidia.
Parathielavia hyrcaniae (Nicot) X. Wei Wang & Houbraken, comb. nov. MycoBank MB829870. Fig. 34.
Fig. 34.
Parathielavia hyrcaniae (CBS 945.72, ex-type culture). A. Colonies from left to right on OA, CMA, MEA and PCA after 3 wk incubation. B–C. Part of the colony on OA, showing mature ascomata, top view. D–E. Ascomata mounted in lactic acid. F. Structure of ascomatal wall in surface view. G. Asci. H. Ascospores. Scale bars: D–E = 20 μm; F–H = 10 μm.
Basionym: Thielavia hyrcaniae Nicot, Compt. Rend. Hebd. Séances Acad. Sci., Paris 253: 304. 1961.
Micromorphology: Ascomata superficial to immersed in the medium, solitary to aggregated, non-ostiolate, fuscous black when mature in reflected light due to dark ascospores inside, spherical to oblate, pilose, 60–130 μm diam. Ascomatal wall brown, semi-translucent, composed of textura epidermoidea in surface view. Ascomatal hairs olivaceous at the base, tapering and fading towards tips, geniculate, flexuous or undulate, with a swollen basal cell, smooth, septate, 3–6 μm diam near base, usually less than 60 μm long. Asci clavate, spore-bearing part 23–28 × 10.5–14.5 μm, with stalks 4–9.5 μm long, containing eight irregularly arranged ascospores, evanescent. Ascospores 1-celled, olivaceous or olivaceous brown when mature, smooth, fusiform, sometimes inequilateral, (10–)11–13(–15) × (5.5–)6–7 μm, with a subapical or oblique germ pore. Asexual morph not observed.
Culture characteristics: On OA with an entire edge, 22–28 mm diam in 7 d at 25 °C, with sparse aerial mycelium, obverse mouse grey due to ascomata, reverse hazel. On CMA similar to those on OA, 27–33 mm diam in 7 d at 25 °C. On MEA with an entire or slightly crenate edge, 21–27 mm diam in 7 d at 25 °C, texture floccose, obverse mouse grey or buff, reverse apricot to scarlet due to coloured exudates diffusing into the medium. On PCA with an entire edge, 24–30 mm diam in 7 d at 25 °C, without aerial mycelium, obverse pale olivaceous grey, without coloured exudates, reverse fawn.
Typus: Iran, southern shore of Caspian Lake, near Nochahr, isolated from sand dune soil, date unknown, J. Nicot (culture ex-type CBS 353.62).
Notes: Parathielavia hyrcaniae produces pilose ascomata similar to Par. appendiculata. They differ in ascomatal hairs and ascospores. For their morphological comparison, see notes of Par. appendiculata.
Parathielavia kuwaitensis (Moustafa) X. Wei Wang & Houbraken, comb. nov. MycoBank MB829871. Fig. 35.
Fig. 35.
Parathielavia kuwaitensis (CBS 353.62, ex-type culture). A. Colonies from left to right on OA, CMA, MEA and PCA after 3 wk incubation. B–C. Part of the colony on OA, showing mature ascomata, top view. D–E. Ascomata mounted in lactic acid, showing structure of ascomatal wall in surface view in D. F. Conidia arising from hyphae. G. Asci. H. Ascospores. Scale bars: D–E = 100 μm; F–H = 10 μm.
Basionym: Thielavia kuwaitensis Moustafa, Trans. Brit. Mycol. Soc. 66: 336. 1976.
Micromorphology: Ascomata superficial to immersed, or covered by mycelium, solitary to loosely aggregated, non-ostiolate, olivaceous grey to leaden black when mature in reflected light due to the dark ascomatal wall, spherical, glabrous, often covered by more or less mycelium, 130–300 μm diam. Ascomatal wall brown, semi-translucent, composed of textura epidermoidea in surface view. Asci obovoid, pyriform or clavate, spore-bearing part 21–28.5 × 12–17.5 μm, with stalks 3–6.5 μm long, containing eight irregularly arranged ascospores, evanescent, occasionally persistent until ascospores mature. Ascospores 1-celled, fulvous to olivaceous brown when mature, smooth, ellipsoidal, attenuated at both ends, (9–)9.5–10.5(–12) × 6–7(–8) μm, with a subapical germ pore. Conidiogenous cells reduced to a hyphal cell. Conidia 1-celled, hyaline, ellipsoidal, ovoid or subglobose, intercalary, or terminal on hyphae, solitary or two in chains, (7–)8–12(–14) × (6–)7–8.5(–9) μm.
Culture characteristics: On OA with an entire edge, 21–27 mm diam in 7 d at 25 °C, without or with sparse aerial mycelium, obverse irregularly fawn to olivaceous due to coloured exudates diffusing into the medium, reverse partly olivaceous. On CMA with an entire edge, 20–26 mm diam in 7 d at 25 °C, without or with sparse aerial mycelium, obverse occasionally partly honey to cinnamon due to coloured exudates diffusing into the medium, reverse uncoloured or honey. On MEA with an entire edge, 18–24 mm diam in 7 d at 25 °C, texture floccose, obverse white due to mycelium, reverse ochraceous to fulvous. On PCA with an entire edge, 22–28 mm diam in 7 d at 25 °C, without aerial mycelium, obverse uncoloured, without coloured exudates, reverse uncoloured.
Typus: Kuwait, isolated from desert soil, unknown date, A.F. Moustafa (culture ex-type CBS 945.72).
Additional material examined: China, Xinjiang, Hetian, Mingfeng county, isolated from desert soil, Aug. 2003, X.W. Wang (CBS 119771 = AS 3.9412).
Notes: Parathielavia kuwaitensis differs from the other two species of Parathielavia in forming conidia and glabrous ascomata. The ascospores of this species are similar to those of Canariomyces arenarius (syn.: Th. arenaria). However, Can. arenarius can be distinguished from Par. kuwaitensis by the production of dark coloured mycelium and by its thermotolerant nature (von Arx, 1975, von Arx et al., 1988, van den Brink et al., 2015). Parathielavia kuwaitensis was classified as a mesophilic species (van den Brink et al. 2015).
Pseudothielavia X. Wei Wang & Houbraken, gen. nov. MycoBank MB829872.
Etymology: Name refers to a genus similar to, but different from Thielavia sensu stricto.
Micromorphology: Aerial mycelium white, usually covering ascomata on OA and CMA. Ascomata superficial, occasionally immersed in medium, non-ostiolate, or ostiolate when mature in some species, solitary to aggregated, globose or subglobose, glabrous, or with sparse hypha-like hairs on the ostiolate ascomata. Ascomatal wall brown, semi-translucent or translucent, composed of textura epidermoidea in surface view. Asci clavate to pyriform, with stalks containing eight irregularly arranged ascospores, evanescent. Ascospores 1-celled, olivaceous brown when mature, smooth, fusiform, with an apical, oblique or lateral germ pore. Asexual morph not observed.
Type species: Pseudothielavia terricola (J.C. Gilman & E.V. Abbott) X. Wei Wang & Houbraken.
Notes: Species in this genus present similar culture characteristics. Most of the species produce non-ostiolate ascomata only with the exception of Pse. hamadae. The ascomata of Pse. hamadae are non-ostiolate at the beginning, but develop an inconspicuous ostiole in time. This genus could be an interesting candidate for studying the genetic relationships between the species with ostiolate and non-ostiolate ascomata. For morphological comparison with the closely related genera, see notes of Parathielavia.
Pseudothielavia arxii (Stchigel & Guarro) X. Wei Wang & Houbraken, comb. nov. MycoBank MB829873. Fig. 36.
Fig. 36.
Pseudothielavia arxii (CBS 603.97, ex-type culture). A. Colonies from left to right on OA, CMA, MEA and PCA after 2 wk incubation. B–C. Mature ascomata on OA, top view. D–E. Ascomata mounted in lactic acid. F. Structure of ascomatal wall in surface view. G. Asci. H. Ascospores. Scale bars: D–E = 50 μm; F–H = 10 μm.
Basionym: Thielavia arxii Stchigel & Guarro, Mycol. Res. 106: 979. 2002.
Micromorphology: Ascomata superficial, solitary to aggregated, non-ostiolate, leaden black when mature in reflected light due to the dark ascomatal wall and asospores inside, spherical, glabrous, 60–250 μm diam. Ascomatal wall brown, semi-translucent, composed of textura epidermoidea in surface view. Asci clavate to pyriform, spore-bearing part 24–31 × 15–19.5 μm, with stalks 8–18.5 μm long, containing eight irregularly arranged ascospores, evanescent. Ascospores 1-celled, olivaceous brown when mature, smooth, fusiform, (11–)12–14.5(–17) ×(6–)6.5–8.5(–9.5) μm, with an oblique to lateral germ pore. Asexual morph not observed.
Culture characteristics: On OA with an entire edge, 41–47 mm diam in 7 d at 25 °C, with sparse aerial mycelium, obverse pale olivaceous grey due to masses of ascomata mixed with mycelium, without coloured exudates, reverse buff. On CMA similar to those on OA, 38–44 mm diam in 7 d at 25 °C. On MEA with an entire or slightly crenate edge, 33–39 mm diam in 7 d at 25 °C, texture floccose, obverse white or pale mouse grey, reverse ochraceous. On PCA with an entire edge, 34–40 mm diam in 7 d at 25 °C, without aerial mycelium, obverse smoke grey, without coloured exudates, reverse uncoloured.
Typus: Chile, Pascua Island, Hanga-Roa, isolated from soil, date unknown, L. Zaror (culture ex-type CBS 603.97 = FMR 5875).
Additional material examined: India, Ajmer, isolated from soil, 2 Nov. 1995, A.M. Stchigel (CBS 102199 = FMR 5765).
Notes: Phylogenetic analyses (Fig. 2, Fig. 3) failed to differentiate Pse. arxii from Pse. terricola. Pseudothielavia arxii is similar to Pse. terricola in culture characteristics, ascomata and asci, but differs in producing ascospores with an oblique to lateral germ pore (Fig. 36H), while those of Pse. terricola have an apical germ pore (Fig. 39H). Because of their distinctly different ascospores, we practically accept them as two separate species. Further study is required to deeply re-evaluate the relationship of the two morphological species.
Fig. 39.
Pseudothielavia terricola (CBS 165.88, ex-epitype culture). A. Colonies from left to right on OA, CMA, MEA and PCA after 3 wk incubation. B–C. Mature ascomata on OA, top view. D–E. Ascomata mounted in lactic acid. F. Structure of ascomatal wall in surface view. G. Asci. H. Ascospores. Scale bars: D–E = 20 μm; F–H = 10 μm.
Pseudothielavia hamadae (Udagawa) X. Wei Wang & Houbraken, comb. nov. MycoBank MB829874. Fig. 37.
Basionym: Achaetomium hamadae Udagawa, Trans. Mycol. Soc. Japan 23: 287. 1982.
Synonym: Chaetomium hamadae (Udagawa) Arx, Proc. Indian Acad. Sci., Pl. Sci. 94: 343. 1985.
Micromorphology: Ascomata superficial, non-ostiolate when young, forming an inconspicuous ostiole when mature, leaden black in reflected light due to the dark ascomatal wall and masses of ascospores, spherical, or broad ovoid, with a short papillate beak, 160–245 μm high, 140–205 μm diam. Ascomatal wall brown, semi-translucent, composed of textura intricata in surface view. Ascomatal hairs sparse, hypha-like, hyaline or subhyaline, erect or flexuous, smooth, septate, 1.5–3.5 μm diam near base. Asci clavate, spore-bearing part 28–41.5 × 10–14.5 μm, with stalks 8–19 μm long, containing eight irregularly arranged or biseriate ascospores, evanescent. Ascospores 1-celled, olivaceous when mature, smooth, ovoid to irregularly, often inaequilateral, (8–)9.5–11.5(–12) × (6–)6.5–8(–8.5) μm, with an indistinct apical germ at the most attenuated end. Asexual morph not observed.
Culture characteristics: On OA with an entire edge, 30–36 mm diam in 7 d at 25 °C, with white aerial mycelium, texture floccose, obverse white or pale olivaceous grey due to mycelium mixed with ascomata, without coloured exudates, reverse buff to honey. On CMA similar to those on OA. On MEA with an entire edge, 26–31 mm diam in 7 d at 25 °C, texture thick floccose, obverse buff, often with two pale luteous concentric rings, reverse saffron. On PCA with an entire edge, 28–34 mm diam in 7 d at 25 °C, without aerial mycelium, obverse smoke grey due to ascomata, without coloured exudates, reverse uncoloured.
Typus: Japan, Tosayamada-cho, Kami-gun, isolated from soil, date unknown, S. Udagawa (culture ex-type CBS 499.83 = IMI 288714ii = NHL 2910).
Notes: Pseudothielavia hamadae presents an intermediate form between species with ostiolate and non-ostiolate ascomata. This species is mainly characterized by subglobose to broad ovoid ascomata covered by sparse, hyaline hypha-like hairs, with an inconspicuous ostiole and by ovoid to irregularly ascospores with an indistinct apical germ pore.
Pseudothielavia subhyaloderma X. Wei Wang & Houbraken, sp. nov. MycoBank MB829875. Fig. 38.
Fig. 38.
Pseudothielavia subhyaloderma (CBS 473.86, ex-type culture). A. Colonies from left to right on OA, CMA, MEA and PCA after 7 d incubation. B–C. Mature ascomata on OA, top view. D–F. Ascomata mounted in lactic acid. G. Structure of ascomatal wall in surface view. H. Asci. I. Ascospores. Scale bars: D–E = 50 μm; F = 20 μm; G–I = 10 μm.
Etymology: Name refers to ascomata with translucent walls.
Micromorphology: Ascomata superficial, often covered by white aerial mycelium, occasionally immersed in the medium, solitary to aggregated, non-ostiolate, leaden black when mature in reflected light due to the dark ascomatal wall, spherical, glabrous, 60–185 μm diam. Ascomatal wall hyaline, subhyaline to pale mouse grey, translucent, composed of textura epidermoidea in surface view. Asci clavate to pyriform, spore-bearing part 21–31 × 13–18 μm, with stalks 6–16.5 μm long, containing eight irregularly arranged or biseriate ascospores, evanescent. Ascospores 1-celled, olivaceous brown when mature, smooth, ovoid or irregular, often inequilateral, (8.4–)10.5–13(–14) × (6.5–)7–8 μm, with an apical or slightly subapical germ pore at the most attenuated end. Asexual morph not observed.
Culture characteristics: On OA with an entire edge, 42–48 mm diam in 7 d at 25 °C, with white aerial mycelium, texture floccose, obverse white or pale olivaceous grey due to masses of ascomata mixed with mycelium, without coloured exudates, reverse uncoloured. On CMA similar to those on OA, 51–57 mm diam in 7 d at 25 °C, with thin aerial mycelium. On MEA with an entire edge, 36–42 mm diam in 7 d at 25 °C, with thick white aerial mycelium, texture floccose, obverse white, reverse cinnamon. On PCA with an entire edge, 34–40 mm diam in 7 d at 25 °C, with white aerial mycelium, obverse white, without coloured exudates, reverse uncoloured.
Typus: Papua New Guinea, near Madang, isolated from forest soil, date unknown, R.S. Khan (holotype CBS H-6866, culture ex-type CBS 473.86 = TRTC 36863).
Notes: The ex-type culture of Pse. subhyaloderma was deposited as Pse. hamadae in the CBS culture collection, probably because both species produce irregular ascospores; however, it can be easily distinguished from the latter by the formation of non-ostiolate ascomata. Pseudothielavia subhyaloderma and Hya. fragilis are not closely related to each other, but morphologically similar in having ascomata with subhyaline and translucent ascomatal wall and ovoid or irregular ascospores. Cultures of Pse. subhyaloderma produce aerial mycelium on OA and CMA, and the ascomata of this species are superficial and larger (60–185 μm diam). In contrast, cultures of Hya. fragilis lack aerial mycelium on OA and CMA, and their ascomata are immersed in the medium and smaller (50–115 μm diam).
Pseudothielavia terricola (J.C. Gilman & E.V. Abbott) X. Wei Wang & Houbraken, comb. nov. MycoBank MB829876. Fig. 39.
Basionym: Coniothyrium terricola J.C. Gilman & E.V. Abbott, Iowa State Coll. J. Sci. 1: 267. 1927.
Synonym: Thielavia terricola (J.C. Gilman & E.V. Abbott) C.W. Emmons, Bull. Torrey Bot. Club 57: 124. 1930.
Micromorphology: Ascomata superficial, solitary to aggregated, non-ostiolate, leaden black when mature in reflected light due to the dark ascomatal wall, spherical, glabrous, 70–200 μm diam. Ascomatal wall brown, semi-translucent, composed of textura epidermoidea in surface view. Asci clavate to pyriform, spore-bearing part 18.5–28.5 × 15.5–22 μm, with stalks 4–9 μm long, containing eight irregularly arranged ascospores, evanescent. Ascospores 1-celled, olivaceous brown when mature, smooth, fusiform, (11–)12–14(–14.5) × (6–)7–8.5 μm, with an apical germ pore. Asexual morph not observed.
Culture characteristics: On OA with an entire edge, 42–48 mm diam in 7 d at 25 °C, with white aerial mycelium, texture floccose, obverse white or pale olivaceous grey due to masses of ascomata mixed with mycelium, without coloured exudates, reverse buff. On CMA similar to those on OA, 40–46 mm diam in 7 d at 25 °C. On MEA with an entire edge, 45–51 mm diam in 7 d at 25 °C, with thick white aerial mycelium, texture floccose, obverse white, reverse ochraceous to fulvous. On PCA with an entire edge, 38–44 mm diam in 7 d at 25 °C, without aerial mycelium, obverse uncoloured, without coloured exudates, reverse uncoloured.
Typus: Lectotype of Coniothyrium terricola designated here: Fig. 17 based on the original culture of Coniothyrium terricola, isolated from soil in Iowa, USA, in Gilman & Abbott, Iowa State Coll. J. Sci. 1(3): 267, 1927, MBT385836. USA, North Carolina, isolated from barren soil, 5 Feb. 1986, J. Shaw (CBS H-24049, epitype designated here, MBT387689, culture ex-epitype CBS 165.88 = TRTC 50997).
Additional material examined: USA, Gaudsia, isolated from kernel of Arachis hypogaea, date unknown, G.A. Gilman (CBS 487.74 = IMI 124876).
Notes: Pseudothielavia terricola is widely distributed in the world. The holotype of Coniothyrium terricola, the basionym of Pse. terricola, was isolated from soil in Iowa, USA. This holotype is neither preserved in FH (Harvard University Herbaria) nor in the ATCC culture collection and seems to be lost. In order to fix the application of the species name, an illustration in the protologue is designated here as the lectotype of this species. CBS 165.88 is selected as the ex-type culture. It is from the same substrate and country, and its morphology agrees well with the protologue (Gilman & Abbott 1927, von Arx 1975). This species is related to Pse. arxii. For morphological comparison, see notes of Pse. arxii.
Stellatospora Tad. Ito & Nakagiri, Mycoscience 35: 413. 1994.
Micromorphology: Ascomata usually immersed in the mycelium, solitary to aggregated, non-ostiolate, globose to subglobose. Ascomatal wall subhyaline, translucent, composed of depressed cells. Asci without visible stalks, 3–8 spored based on the original description, often irregularly-shaped, evanescent. Ascospores 1-celled, olivaceous brown when mature, smooth, 1-celled, stellate, with apical germ pores. Asexual morph not observed.
Type species: Stellatospora terricola Tad. Ito & Nakagiri.
Note: This is a monotypic genus, which was originally regarded to reside in the Sordariaceae (Ito & Nakagiri 1994). This classification was followed by Maharachchikumbura et al., 2015, Maharachchikumbura et al., 2016, even though Kirk et al. (2008) suggested the genus to belong to the Chaetomiaceae. The sequences of ITS and the D1/D2 domain of LSU from Vu et al. (2019) indicated that the type species, Stell. terricola belongs to the Chaetomiaceae. Our phylogenetic analyses based on the rpb2 and the four-locus datasets confirmed that this genus is a single lineage in the Chaetomiaceae (Fig. 2, Fig. 3). No genera or species have been found to be closely related to this genus.
Stellatospora terricola Tad. Ito & Nakagiri, Mycoscience 35: 413. 1994. Fig. 40.
Fig. 40.
Stellatospora terricola (CBS 811.95, ex-type culture). A. Colonies from left to right on OA, CMA, MEA and PCA after 4 wk incubation. B–C. Mature ascomata on OA, top view. D. Mature ascomata on OA, side view. E–F. Ascomata mounted in lactic acid. G. Asci. H. Ascospores. Scale bars: E–F = 100 μm; G–H = 10 μm.
Micromorphology: Ascomata usually immersed in the mycelium, solitary to aggregated, non-ostiolate, globose to subglobose, leaden black when mature in reflected light due to the dark ascospores inside, glabrous, 50–150 μm diam. Ascomatal wall subhyaline, translucent, composed of textura epidermoidea in surface view. Asci elongated clavate, elongated pyriform, dumbbell-shaped or irregularly-shaped, often geniculate or twisted, spore-bearing part 34.5–48.5 × 12–19.5 μm, without visible stalks, containing eight biseriate, irregularly-arranged ascospores, soon evanescent. Ascospores 1-celled, olivaceous brown when mature, smooth, stellate, with up to 7–8 papillate protuberances, (8.5–)10.5–13(–16) μm diam, with at least two visible apical germ pores at the ends of protuberances. Asexual morph not observed.
Culture characteristics: On OA with an entire edge, 3–9 mm diam in 7 d at 25 °C, with, obverse white due to white aerial mycelium, without coloured exudates, reverse uncoloured or buff. On CMA similar to those on OA. On MEA with an entire or slightly crenate edge, 2–8 mm diam in 7 d at 25 °C, texture floccose, obverse white due to white aerial mycelium, without coloured exudates, reverse ochraceous. On PCA with an entire edge, 1–6 mm diam in 7 d at 25 °C, without aerial mycelium, without coloured exudates, reverse uncoloured.
Typus: Japan, Ikeda, Osaka Pref., isolated from paddy soil, May 1966, T. Ito & A. Nakagiri (culture ex-type CBS 811.95 = IFO 32597).
Notes: The unique stellate shape of the ascospores is a diagnostic character to identify Stell. terricola. In the original description, the asci were described as “3–8-spored, pyriform to ovate” (Ito & Nakagiri 1994). In our examination, however, no ovate asci containing less than eight spores were observed, but most asci observed were irregularly-shaped (Fig. 40G).
Stolonocarpus X. Wei Wang & Houbraken, gen. nov. MycoBank MB829877.
Etymology: Name refers to ascomata arising from stolon-like mycelium creeping on or immersed in medium.
Micromorphology: Mycelium sparse, composed of olivaceous to brown hyphae, branched, septate, creeping along medium like stolon or immersed in the medium. Ascomata superficial, usually arising from aerial mycelium along the edge, subglobose, non-ostiolate. Ascomatal wall brown, non-translucent, composed of irregular, angular or elongated cells. Ascomatal hairs hypha-like, flexuous, brown, septate, some thicker than the others. Asci fasciculate, cylindrical, geniculate or twisted, stalked, containing eight uniseriate or occasionally biseriate ascospores, evanescent. Ascospores 1-celled, brown when mature, smooth, ellipsoidal, with attenuated ends, usually over 20 μm long, with an apical germ pore. Asexual morph not observed.
Type species: Stolonocarpus gigasporus (Moustafa & Abdel-Azeem) X. Wei Wang & Houbraken.
Notes: Stolonocarpus is a monotypic genus which is most closely related (PP = 0.99, BS = 100 %; Fig. 3) to Madurella, a group of etiologic fungi that can cause human mycetoma. Madurella strains often do not sporulate and do not produce a sexual state.
Stolonocarpus gigasporus (Moustafa & Abdel-Azeem) X. Wei Wang & Houbraken, comb. nov. MycoBank MB829878. Fig. 41.
Fig. 41.
Stolonocarpus gigasporus (CBS 112062, ex-type culture). A. Colonies from left to right on OA, CMA, MEA and PCA after 2 wk incubation. B. Mature ascomata on PCA, top view. C. Mature ascomata on OA, top view. D–E. Ascomata mounted in lactic acid. F. Structure of ascomatal wall in surface view. G. Asci. H. Ascospores. Scale bars: D–E = 100 μm; F–H = 10 μm.
Basionym: Thielavia gigaspora Moustafa & Abdel-Azeem, Microbiol. Res. 163: 442. 2008.
Micromorphology: Mycelium sparse, composed of pigmented hyphae, branched, septate, often creeping radially along medium like stolon, vinaceous buff to olivaceous in reflected light. Ascomata superficial, usually arising from stolon-like mycelium and easily forming along the edge of colonies, fawn to olivaceous due to ascomatal hairs in reflected light, subglobose, non-ostiolate, 160–410 μm diam. Ascomatal wall brown, non-translucent, composed of irregular, angular or elongated cells. Ascomatal hairs hypha-like, flexuous, partly brown, septate, 1.5–3 μm diam near base, partly dark brown, 3.5–5.5 μm diam near base. Asci fasciculate, cylindrical, often geniculate or twisted, spore-bearing part 50–75 × 7.5–13 μm, with stalks 5.5–10 μm long, containing eight uniseriate or occasionally biseriate ascospores, evanescent. Ascospores 1-celled, brown when mature, smooth, ellipsoidal with attenuated ends or fusiform, (22.5–)25.5–28.5(–29.5) × (13–)14–15.5 μm, with an apical germ pore. Asexual morph not observed.
Culture characteristics: On OA with an entire edge, 38–44 mm diam in 7 d at 25 °C, obverse vinaceous buff to olivaceous due to aerial mycelium with ascomata forming along edge, reverse hazel. On CMA with an entire edge, 29–35 mm diam in 7 d at 25 °C, texture floccose, obverse hazel to isabelline due to aerial mycelium mixed with ascomata, reverse isabelline. On MEA with an entire edge, 44–50 mm diam in 7 d at 25 °C, obverse floccose, vinaceous buff, reverse fawn to olivaceous due to immered hyphae. On PCA transparent, with an entire edge, 37–43 mm diam in 7 d at 25 °C, with extremely sparse aerial mycelium, without coloured exudates, reverse uncoloured.
Typus: Egypt, El-Sheikh Zweid, North Sinai, isolated from dung of Camelus dromedarius, 2002, A.F. Moustafa (culture ex-type CBS 112062).
Notes: The type species, Stol. gigasporus is distinct from the other known related species in the Chaetomiaceae in its sparse, pigmented and stolon-like mycelium which creep radially along medium, ascomata mostly forming along the edge of the colony and large ascospores (25.5–28.5 × 14–15.5 μm). This genus is related to Madurella and further study is needed to determine its potential in animal or human infection.
Thermothielavioides X. Wei Wang & Houbraken, gen. nov. MycoBank MB829879.
Etymology: Name refers to its thermophilic nature and its morphological similarity to Thielavia sensu stricto.
Micromorphology: Ascomata superficial or covered by aerial mycelium, solitary to aggregated, non-ostiolate, globose or subglobose. Ascomatal wall brown, semi-translucent, composed of irregular, angular or elongated cells. Ascomatal hairs brown, septate, flexuous, verrucose, tapering and fading to hyaline towards tips. Asci fasciculate, ellipsoidal to ovoid, stalked, containing eight biseriate or irregularly-arranged ascospores, evanescent. Ascospores 1-celled, olivaceous brown when mature, smooth, ellipsoidal or ovoid, with an apical germ pore at the most attenuated end. Conidiogenous cells arising laterally from aerial hyphae, hyaline, phialidic. Conidia in basipetal chains, hyaline, aseptate, smooth, obovoid to clavate, usually with a truncated base and a rounded apex. Thermophilic.
Type species: Thermothielavioides terrestris (Apinis) X. Wei Wang & Houbraken.
Notes: The monotypic genus Thermothielavioides is closely related to Floropilus in Clade 3. Floropilus is mesophilic and produces chaetomium-like ascomata, while Thermothielavioides is thermorphilic with an optimal growth temperature ≥45 °C (van den Brink et al. 2015) and has a thielavia-morph.
Thermothielavioides terrestris (Apinis) X. Wei Wang & Houbraken, comb. nov. MycoBank MB829880. Fig. 42.
Fig. 42.
Thermothielavioides terrestris (CBS 492.74). A. Colonies from left to right on OA, CMA, MEA and PCA after 4 wk incubation at 37 °C (left half showing obverse colony and right half showing the reverse on PCA). B–C. Mature ascomata on PCA, top view. D–E. Ascomata mounted in lactic acid. F. Conidia. G. Hyphae, conidiophores and conidia. H. Structure of ascomatal wall in surface view. I–J. Asci. K. Ascospores. Scale bars: D–E = 20 μm; F–K = 10 μm.
Basionym: Allescheria terrestris Apinis, Nova Hedwigia 5: 68. 1963.
Synonym: Thielavia terrestris (Apinis) Malloch & Cain, Canad. J. Bot. 50: 66. 1972.
Micromorphology: Ascomata superficial or covered by aerial mycelium, solitary to aggregated, non-ostiolate, lead black in reflected light, globose or subglobose, 80–270 μm diam. Ascomatal wall brown, non- translucent or semi-translucent, composed of irregular, angular or elongated cells. Ascomatal hairs brown, septate, flexuous, verrucose, tapering and fading to hyaline towards tips, 2–3.5 μm diam near base. Asci ellipsoidal to ovoid, spore-bearing part 13.5–20.5 × 6–8 μm, with stalks 4.5–11.5 μm long, containing eight biseriate or irregularly arranged ascospores, evanescent. Ascospores 1-celled, olivaceous brown when mature, smooth, ellipsoidal or ovoid, 4–6.5(–7) × (3–)3.5–4.5(–5) μm, with an apical germ pore at the most attenuated end. Conidiogenous cells arising laterally from aerial hyphae, hyaline, phialidic, occasionally branched, (6.5–)9–24(–33) × (1–)1.5–3.5 μm. Conidia in basipetal chains, hyaline, aseptate, smooth, obovoid to clavate, usually with a truncated base and a rounded apex, (3–)3.5–5(–5.5) × 1.5–2.5(–3) μm.
Culture characteristics: On OA with an entire edge, 53–59 mm diam in 7 d at 37 °C, texture thick floccose, obverse white to pale smoke grey, or rosy buff to hazel, reverse cinnamon to hazel. On CMA similar to those on OA. On MEA with an entire edge, 39–45 mm diam in 7 d at 37 °C, texture thick floccose, obverse white, reverse fawn. On PCA with an entire edge, 41–47 mm diam in 7 d at 37 °C, with sparse aerial mycelium, producing ascomata when growing on cellophane membrane covering the surface of the medium, obverse white to smoke grey, reverse smoke grey.
Typus: UK, isolated from dry pasture soil, date unknown, T. Funahashi (culture ex-type CBS 117535 = CBS 355.66).
Additional material examined: Japan, Hiroshima, isolated from soil, 11 Jul. 1972, K. Minoura (CBS 492.74 = ATCC 26917 = HUT 4081). Malaysia, Pahang, isolated from cellulose in soil from palm oil estate, date unknown, S.C. Cheah (CBS 351.90). Unknown, substrate and date unknown, K.F. Gregory (CBS 546.86). USA, Indiana, Monroe Co., Kent Farm, isolated from sun-heated soil, 1971, M.R. Tansey (CBS 455.75); Florida, Everglades Nat. Park, 5 km N of Flamingo, isolated from sun-heated dung of rabbit, 1973, M.R. Tansey (CBS 454.75 = IAM 14666).
Notes: This species easily degenerates and produces only conidial morph. Cross cultivation induced the formation of ascomata (Samson et al. 1977). Although the culture CBS 117535 = CBS 355.66 was not marked as the ex-type of the species in the CBS database, von Arx (1975) described this culture as the type with the following text: “culture CBS 355.66, type strain, isolated from pasture soil, sent by A.E. Apinis”.
Podosporaceae X. Wei Wang & Houbraken, fam. nov. MycoBank MB829841.
Etymology: Named after Podospora, the oldest genus in this family, but differentiating from the existing but invalid name “Podosporaceae Hochb. 1930” (Art. 32.1(c), Melbourne).
Micromorphology: Sexual morph: Ascomata superficial to immersed in medium, solitary or loosely aggregated, ostiolate and ovoid to obpyriform, or non-ostiolate and globose to subglobose, glabrous or possessing hypha-like to seta-like hairs. Ascomatal wall membranaceous to coriaceous, usually opaque, in some species semi-translucent. Asci cylindrical to elongated clavate or fusiform, stipitate, with or without a thickened ring at apex, (2–)4- or 8- or multi-spored, evanescent or persistent until ascospores mature. Ascospores 1-celled and pigmented, or 2-celled and composed of a larger, pigmented upper cell and a smaller, pale or hyaline cell, with or without appendage, usually smooth, in a few species ornamented. Asexual morph not observed or cladorrhinum-like: Conidiophores micronematous, reduced to conidiogenous cells. Conidiogenous cells intercalary or occasionally terminal, originating lateral or terminal peg-like structure with a flaring collarette, producing blastic conidia. Conidia single-celled, hyaline, smooth, usually with a truncated base and a rounded apex.
Type genus: Podospora (Corda) Ces.; other included genera: Cladorrhinum Sacc. & Marchal; Triangularia Boedijn.
Notes: Previous studies have showed phylogenetic evidence for the polyphyly of the family Lasiosphaeriaceae, and noted that the relationships at family and genus level in the Sordariales are complicated (Huhndorf et al., 2004, Cai et al., 2005, Miller and Huhndorf, 2005, Cai et al., 2006, Zhang et al., 2006, Kruys et al., 2015). Based on our phylogenetic analyses, a monophyletic lineage, containing species of several different genera, is sister to the Chaetomiaceae. The new family name Podosporaceae is proposed here to accommodate this sister lineage of the Chaetomiaceae (Fig. 2, Fig. 3). Aside from the molecular data in the present study, a previous study also showed that several Cercophora species belonged to this lineage, however, the type species of Cercophora, Cer. mirabilis grouped with Triangularia mangenotii and Podospora decipiens, distant from this lineage (Clade A in Cai et al. 2006). Although there were not many sequences of Cercophora species available for this study, our data enable us to mark the Cercophora sensu stricto lineage in the rpb2 phylogram (marked with double red triangles in Fig. 2).
Podosporaceae Hochb. was invalidly introduced (Art. 32.1(c), Melbourne). Our newly proposed Podosporaceae is based on the same type (Podospora), but they are differently delimited (phenotype-based vs sequence-based). Future studies are required to show the diversity within this family.
Cladorrhinum Sacc. & Marchal, Bull. Soc. Roy. Bot. Belgique 24: 64. 1885.
Micromorphology: Sexual morph: Ascomata superficial to immersed in medium, solitary or loosely aggregated, non-ostiolate, globose to subglobose, with hypha-like ascomatal hairs or covered by aerial mycelium. Ascomatal wall membranaceous, semi-translucent or opaque, composed of textura intricata or epidermoidea in surface view. Asci cylindrical, pyriform, obovoid or fusiform, stalked, without a thickened ring at apex, 8-spored, evanescent. Ascospores 1-celled, pigmented, smooth, with an apical germ pore, without appendage. Asexual morph: Conidiophores micronematous, reduced to conidiogenous cells. Conidiogenous cells intercalary or occasionally terminal, originating lateral or terminal peg-like structure with a flaring collarette, producing blastic conidia. Conidia single-celled, hyaline, smooth, broad obovoid, ellipsoidal or subglobose, usually with a truncated base and a rounded apex.
Type species: Cladorrhinum foecundissimum Sacc. & Marchal.
Notes: Cladorrhinum was originally introduced for asexually reproducing species. Our phylogenetic analyses show that two former Thielavia species (Th. hyalocarpa and Th. intermedia) group with the type species of Cladorrhinum in the Podosporaceae lineage. This genus is therefore redefined to also accommodate sexually reproducing species. Phylogenetic evidence indicated that morphologically-defined traditional Cladorrhinum is polyphyletic (Carmarán et al. 2015). On the other hand, several sexual genera also produce a cladorrhinum-like state (Mouchacca and Gams, 1993, Cai et al., 2006). Based on our phylogenetic analyses, only the type species of the traditional Cladorrhinum is maintained in the modified genus. The two other studied Cladorrhinum species, Clad. bulbillosum and Clad. phialophoroides, belong to the redefined genera Podospora and Triangularia, respectively, in the family Podosporaceae. More work is required to determine the phylogenetic position of the remaining “Cladorrhinum” species.
Cladorrhinum foecundissimum Sacc. & Marchal, Bull. Soc. Roy. Bot. Belgique 24: 64. 1885. Fig. 43.
Fig. 43.
Cladorrhinum foecundissimum (CBS 180.66, ex-type culture). A. Colonies from left to right on OA, CMA, MEA and PCA after 3 wk incubation. B–E. Hyphae, conidiogenous cells and conidia. Scale bars: B–E = 10 μm.
Micromorphology: Sexual morph not observed. Somatic hyphae hyaline, 1.0–2.5 μm wide. Conidiophores micronematous, reduced to conidiogenous cells. Conidiogenous cells intercalary, originating lateral peg-like structure with a flaring collarette, producing lateral blastic conidia. Conidia single-celled, hyaline, smooth, broad obovoid, ellipsoidal or subglobose, usually with a truncated base and a rounded apex, (2.5–)3–4(–4.5) × 2.5–3(–3.5) μm.
Culture characteristics: On OA with an entire edge, 26–32 mm diam after 7 d at 25 °C; obverse pale luteous due to coloured exudates diffusing into the medium, with aerial mycelium, reverse honey. On CMA similar to those on OA. On MEA with an entire edge, 18–24 mm diam after 7 d at 25 °C, obverse honey due to aerial mycelium and conidia, reverse olivaceous grey due to coloured exudates diffusing into the medium. On PCA translucent, with an entire edge, 23–29 mm diam after 7 d at 25 °C, without aerial mycelium, obverse and reverse honey.
Typus: Netherlands, Wageningen, isolated from soil, 22 Jul. 1965, J.H. van Emden (culture ex-neotype CBS 180.66).
Notes: The type species Clad. foecundissimum is currently the only asexual species in this genus. This species was originally isolated from dung in Belgium (Marchal 1885). The holotype seems to be lost, and von Arx & Gams (1967) designated a herbarium specimen from CBS 180.66 as the neotype of this species. The ex-neotype strain keeps a strictly asexual reproduction. In contrast, no asexual morph was observed in the closely related species Clad. hyalocarpum and Clad. intermedium. The latter two species only produced sexual morphs.
Cladorrhinum hyalocarpum (Arx) X. Wei Wang & Houbraken, comb. nov. MycoBank MB829881. Fig. 44.
Fig. 44.
Cladorrhinum hyalocarpum (CBS 322.70, ex-type culture). A. Colonies from left to right on OA, CMA, MEA and PCA after 4 wk incubation. B. Part of the colony on OA. C–D. Mature ascomata on OA, top view. E. Mature ascomata on OA, side view. F–H. Ascomata mounted in lactic acid. I. Ascomatal hairs. J. Structure of ascomatal wall in surface view. K. Asci. L. Ascospores. Scale bars: F = 50 μm; G–H = 100 μm; I, J, L = 10 μm; K = 20 μm.
Basionym: Thielavia hyalocarpa Arx, Stud. Mycol. 8: 6. 1975.
Micromorphology: Ascomata superficial or slightly sub-immersed, occationally immersed in the medium, solitary, often covered by aerial mycelium, non-ostiolate, black, globose, (160–)265–500(–605) μm diam. Ascomatal wall brown, semi-translucent, translucent, composed of textura epidermoidea or intricata in surface view. Ascomatal hairs hypha-like, hyaline or subhyaline, 2–4 μm diam near base. Asci cylindrical, spore-bearing part 81.5–148 × 11.5–19.5 μm, with stalks 7–20.5 μm long, containing eight uniseriate ascospores, evanescent. Ascospores 1-celled, dark brown when mature, smooth, fusiform, (22.5–)24.5–31(–36) × (11.5–)13–15.5(–16) μm, with an apical germ. Asexual morph not observed.
Culture characteristics: On OA with an entire edge, over 70 mm diam in 7 d at 25 °C, with floccose aerial mycelium near the edge, obverse buff or honey due to coloured exudates diffusing into the medium, or white due to aerial mycelium, reverse buff. On CMA similar to those on OA. On MEA with an entire edge, over 70 mm diam in 7 d at 25 °C, texture floccose, obverse grey white, reverse fulvous. On PCA with an entire edge, over 70 mm diam in 7 d at 25 °C, without aerial mycelium, without coloured exudates, reverse uncoloured.
Typus: Netherlands, Zuidelijk Flevoland, isolated from soil, 1969, C.V. Subramanian (culture ex-type CBS 322.70).
Additional material examined: Spain, Beseit, isolated from forest soil, 14 Mar. 1999, A.M. Stchige (CBS 102198).
Notes: Cladorrhinum hyalocarpum is most closely related to Clad. foecundissimum, but differ in its ability to produce a sexual state and lacking a conidial state. This species can also be easily distinguished from Clad. intermedium by usually superficial or slightly sub-immersed ascomata, cylindrical asci, and larger fusiform ascospores (24.5–31 × 13–15.5 μm vs 12.5–14.5 × 9–10 μm).
Cladorrhinum intermedium (Stchigel & Guarro) X. Wei Wang & Houbraken, comb. nov. MycoBank MB829882. Fig. 45, Fig. 46.
Fig. 45.
Cladorrhinum intermedium (CBS 433.96, ex-type culture). A. Colonies from left to right on OA, CMA, MEA and PCA after 3 wk incubation. B–C. Part of the colony showing mature ascomata on OA, top view. D–F. Ascomata mounted in lactic acid. G. Structure of ascomatal wall in surface view. H. Asci. I. Ascospores. Scale bars: D–F = 50 μm; G–I = 10 μm.
Fig. 46.
Cladorrhinum intermedium (CBS 100257). A. Colonies from left to right on OA, CMA, MEA and PCA after 10 d incubation. B–C. Mature ascomata on OA, top view. D–E. Ascomata mounted in lactic acid. F. Structure of ascomatal wall in surface view. G. Asci. H. Ascospores. Scale bars: D = 50 μm; E = 20 μm; F–H = 10 μm.
Basionym: Thielavia intermedia Stchigel & Guarro, Mycol. Res. 106: 976. 2002.
Micromorphology: Ascomata immersed or sub-immersed in the medium, solitary, non-ostiolate, fuscous black, globose, 245–460 μm diam. Ascomatal wall brown, opaque or semi-translucent, composed of textura intricata in surface view. Ascomatal hairs hypha-like, hyaline or subhyaline, 0.6–1.5 μm diam near base. Asci pyriform, obovoid or fusiform, spore-bearing part 20–35 × 13–21.5 μm, with stalks 14–25 μm long, containing eight irregularly arranged ascospores, evanescent. Ascospores 1-celled, olivaceous brown when mature, smooth, ovoid, (10–)12.5–14.5(–15) × (8–)9–10 μm, with an apical germ at the attenuated end. Asexual morph not observed.
Culture characteristics: On OA with an entire edge, 55–61 mm diam in 7 d at 25 °C, obverse pale grey to pale mouse grey due to aerial mycelium mixed with ascomata, reverse olivaceous grey due to coloured exudates diffusing into the medium. On CMA similar to those on OA, 52–58 mm diam in 7 d at 25 °C . On MEA with a slightly crenate edge, 55–61 mm diam in 7 d at 25 °C, texture floccose, obverse white, reverse fulvous. On PCA with a crenate edge, 56–62 mm diam in 7 d at 25 °C, without aerial mycelium, obverse mouse grey due to ascomata, without coloured exudates, reverse mouse grey.
Typus: India, isolated from soil, 29 Oct. 1995, A.M Stchigel (culture ex-type CBS 433.96 = FMR 5594).
Additional material examined: Tunisia, 5 km NW of Ksar Haddada, NW from Tatahouine, 470 m asl, isolated from soil attached to rhizoids of Grimmia orbicularis, 11 Apr. 1981, J.P. Frahm (CBS 100257 = ATCC 201454 = TRTC 52049).
Notes: The above description is based on the ex-type culture CBS 433.96 (Fig. 45). The isolate CBS 100257 was deposited as Th. dacrydioides J.C. Krug in the CBS culture collection, but no publication was found and the name was probably never validly published. Although this strain differs slightly from CBS 433.96 in colony morphology due to the formation of denser ascomata covered by less aerial mycelium (Fig. 46), our phylogenetic analyses and microscopic examination identified it as Clad. intermedium. Cladorrhinum intermedium can be easily distinguished from Clad. hyalocarpum by immersed or occasionaly sub-immersed ascomata, pyriform, obovoid or fusiform but never cylindrical asci and smaller ovoid ascospores (12.5–14.5 × 9–10 μm vs 24.5–31 × 13–15.5 μm).
Podospora Ces., Hedwigia 1: 103. 1856.
Micromorphology: Sexual morph: Ascomata usually superficial, solitary, ostiolate, ampulliform or papillate. Ascomatal wall membranaceous to coriaceous. Asci with an apical ring, usually persistent until ascospore mature. Ascospores composed of a pigmented, swollen upper cell and a lower hyaline pedicel (or referred to as primary appendage), often with apical and/or basal gelatinous appendages (also referred to as secondary appendages). Asexual morph: cladorrhinum-like or not observed. Conidiophores micronematous, reduced to conidiogenous cells. Conidiogenous cells usually intercalary, originating lateral peg-like structure with a flaring collarette, producing blastic conidia. Conidia single-celled, hyaline, smooth, usually with a truncated base and a rounded apex.
Type species: Podospora fimicola (Corda) Ces.
Notes: The genus Podospora was validly published in Hedwigia 1(15): 103, Tab. XIV, A, 1–11 (Cesati 1856) with a description later added in Rabenhorst’s Klotzschii Herb. Viv. Mycol., Ed. Nova, no. 259 (see Braun 2018, fig. 22). It is a large genus containing over 100 species. Among the species included in this study, only the type species is maintained in Podospora sensu stricto based on our phylogenetic analyses. More work is required to determine the additional taxa in the genus.
Podospora bulbillosa (W. Gams & Mouch.) X. Wei Wang & Houbraken, comb. nov. MycoBank MB829883. Fig. 47.
Fig. 47.
Podospora bulbillosa (CBS 304.90). A. Colonies from left to right on OA, CMA, MEA and PCA after 3 wk incubation. B–E. Hyphae, conidiogenous cells and conidia. Scale bars: B–E = 10 μm.
Basionym: Cladorrhinum bulbillosum W. Gams & Mouch., Mycotaxon 48: 425. 1993.
Micromorphology: Sexual morph not observed. Fertile hyphae hyaline, 1.5–4(–6) μm wide. Conidiophores micronematous, reduced to intercalary hyphal conidiogenous cells. Conidiogenous hyphal cells 5.5–14 × 2–4 μm, usually producing a single (rarely two) conidiogenous protrusion laterally. Conidiogenous protrusion papillate to cylindrical, up to 2.5 μm long, 1–2 μm wide, apically with a flaring collarette opening that produces blastic conidia continuously. Conidia single-celled, hyaline, smooth, subglose or broad obovoid, usually with a truncated base and a rounded apex, 2.5–3.5 × 2–3 μm.
Culture characteristics: On OA with an entire edge, over 70 mm diam after 7 d at 25 °C, texture floccose, obverse buff to honey due to aerial mycelium mixed with masses of young ascomata, later becoming dark due to the mature ascomata, reverse buff to cinnamon. On CMA similar to those on OA. On MEA with an entire edge, over 70 mm diam after 7 d at 25 °C, with buff to vinaceous buff aerial mycelium, obverse greyish sepia, reverse fawn. On PCA translucent, with an entire edge, over 70 mm diam after 7 d at 25 °C, without or with sparse aerial mycelium, reverse uncoloured.
Typus: Egypt, New Valley region, Western Desert, isolated from desert soil, 15 Jan. 1993, J. Mouchacca (culture ex-type CBS 304.90 = CBS 979.72K).
Note: Phylogenetic analyses place this species in Podospora sensu stricto, sister to Pod. fimicola. No sexual state is observed in Podospora bulbillosa, while in Pod. fimicola, only the sexual morph is present.
Podospora fimicola (Corda) Ces., Hedwigia 1: 103. 1856. Fig. 48.
Fig. 48.
Podospora fimicola (CBS 482.64, ex-epitype culture). A. Colonies from left to right on OA, CMA, MEA and PCA after 2 wk incubation. B. Mature ascomata on OA, top view. C. Mature ascomata on OA, side view. D–F. Ascomata mounted in lactic acid. G. Asci. H. Ascospores. Scale bars: D–F = 100 μm; G = 50 μm; H = 20 μm.
Basionym: Schizothecium fimicola Corda, Icon. Fung. 2: 29. 1838.
Synonyms: Pleurage fimicola (Corda) Kuntze, Revis. Gen. Pl. 3: 504. 1898.
Sordaria fimiseda Ces. & De Not., Comment. Soc. Crittog. Ital. 1: 226. 1863.
Micromorphology: Ascomata superficial, purplish grey or iron grey in reflected light, solitary, ampulliform with a short and black beak, ostiolate, 630–1250 μm high, 330–710 μm diam. Ascomatal wall brown, opaque, of textura intricata or epidermoidea in surface view. Ascomatal hairs covering the whole ascoma, erect or flexuous, brown, 2.5–5 μm diam near base, less than 75 μm long. Asci fasciculate, elongated fusiform, occasionally cylindrical, spore-bearing part 245–320 × 35–60 μm, with stalks (90–)120–150 μm long, containing eight biseriate (occasionally uniseriate) ascospores, persistent until ascospore mature. Ascospores composed of a olivaceous brown cell and a hyaline and clavate or cylindrical pedicel (primary appendage) when mature, of which the pigmented cell ellipsoidal, 44.5–55 × 27.5–46 μm, with an apical germ pore and often with a hyaline and gelatinous apical secondary appendage; the primary appendage at the opposite end, 25–37 × 4.5–6.5(–8) μm, often with hyaline and gelatinous basal secondary appendage. Asexual morph not observed.
Culture characteristics: On OA with an entire edge, 31–37 mm diam in 7 d at 25 °C, with sparse aerial mycelium, obverse olivaceous to iron grey due to pigmented hyphae and coloured exudates diffusing into the medium, reverse purplish grey. On CMA similar to those on OA, obverse dark mouse grey. On MEA with a fimbriate edge, 17–23 mm diam in 7 d at 25 °C, obverse hazel due to aerial mycelium, reverse dark slate blue. On PCA with a slightly crenate edge, 25–31 mm diam in 7 d at 25 °C, without aerial mycelium; obverse dark mouse grey, reverse mouse grey to dark mouse grey.
Typus: Lectotype of Schizothecium fimicola designated here: Tab. XIII, fig. 105, illustrated by Corda based on the holotype collected from dried cow dung in Czech Republic, near Prague, in Corda, Icon. Fung. 2, 1831, MBT 385838. Switzerland, Kt. Aargau, Ober-Erlinsbach, Barmelweid, isolated from dung of cow, 21 Aug. 1958, W. Schaffner (CBS H-24048, epitype designated here, MBT387690, culture ex-epitype CBS 482.64 = ETH 2812).
Additional material examined: New Zealand, Cape Foulwind, South Island, isolated from dung of horse, Aug. 1990, D.P. Mahoney (CBS 990.96).
Notes: The genus Podospora is typified by Pod. fimicola, which is based on Corda's name Schizothecium fimicola. Type material of S. fimicola could neither by traced in PRC (Charles University in Prague) nor PRM (National Museum, Prague) and seems to be lost. In order to fix the application of the name, an illustration in the protologue is designated here as the lectotype of Schizothecium fimicola, and we selected CBS 482.64 as ex-epitype culture. CBS 482.64 matches with the protologue of the fungus described by Corda (1838) and it was collected on the same substrate and continent as the original material.
Triangularia Boedijn, Ann. Mycol. 32: 302. 1934.
Synonym: Apiosordaria Arx & W. Gams, Nova Hedwigia 13: 201. 1967.
Micromorphology: Sexual morph: Ascomata often superficial, sometimes semi-immersed to immersed in medium, solitary or loosely aggregated, ostiolate and ovoid, obpyriform to ampulliform and with a papilla-like beak, or non-ostiolate and globose to subglobose, glabrous or possessing hypha-like or seta-like hairs. Ascomatal wall membranaceous to coriaceous, opaque or semi-translucent, in some species translucent. Asci cylindrical to elongated clavate or fusiform, stipitate, without or with a usually inconspicuous apical ring, (2–)4– or 8– or multi-spored, evanescent or persistent until ascospores mature. Ascospores often 2-celled, composed of a larger, pigmented upper cell with an apical germ pore, and a smaller, pale or hyaline cell (also referred to as the primary appendage or pedicel when elongated and narrow), in some species without gelatinous appendages (secondary appendages), usually smooth, in a few species ornamented. Asexual morph usually cladorrhinum-like or not observed. Conidiophores micronematous, reduced to conidiogenous cells. Conidiogenous cells intercalary or occasionally terminal, originating lateral or terminal peg-like structure with a flaring collarette, producing blastic conidia. Conidia single-celled, hyaline, smooth, usually with a truncated base and a rounded apex.
Type species: Triangularia bambusae (J.F.H. Beyma) Boedijn.
Notes: Triangularia was morphologically defined by producing cylindrical or clavate asci with a thickened apical ring, 2-celled smooth ascospores, with a larger, pigmented and conical to triangular upper cell and a smaller, paler or hyaline and triangular to hemispherical lower cell, without gelatinous appendages (Guarro & Cano 1988). Our phylogenetic analyses did not support the morphologically defined genus Triangularia (Fig. 2, Fig. 3). Therefore, this genus is redefined which includes morphologically diverse species.
Triangularia pauciseta and related species (“Podospora anserina/pauciseta/comata species complex”, Boucher et al. 2017) needs further attention. Podospora pauciseta and Pod. anserina are morphologically indistinguishable and the former species was once treated as a synonym of Pod. pauciseta (Traversog 1907). At the molecular level, Pod. anserina strains differ by one base pair in their ITS sequence in comparison with Pod. pauciseta, six base pairs in rpb2 (total 852 bp) and two base pairs in tub2 (total 689 bp). Following a polyphasic approach and the taxonomic criteria used in our previous work in the Chaetomiaceae (Wang et al., 2016a, Wang et al., 2016b, Wang et al., 2019), we would have treated Pod. anserina as a synonym of Pod. pauciseta, due to the lack of morphological differences and minor sequence differences. A recent study (Boucher et al. 2017) employed ITS sequences and three new intergenic loci to delimit cryptic species in the Podospora anserina/pauciseta/comata species complex (Rchr3, on chromosome 3; Rchr4 on chromosome 4, and Rchr6 on chromosome 6). Unfortunately, the phylogenetic markers rpb2 and tub2, generally used in the Chaetomiaceae, were not included in their study. Based on their phylogenetic analysis, they recognised not only Pod. anserina, Pod. pauciseta and Pod. comata, but also four new species. In addition to the phylogenetic analyses, Boucher et al. (2017) performed crosses and pointed out that interspecific crosses were nearly sterile and most F1 progeny is female sterile, demonstrating congruence between their phylogenetic and biologic defined species. Based on the results (Fig. 6) of the re-analysed datasets of Boucher et al. (2017), we decided to follow them, and accept all seven species in the “Podospora anserina/pauciseta/comata species complex”: Trian. anserina, Trian. bellae-mahoneyi, Trian. comata, Trian. pauciseta, Trian. pseudoanserina, Trian. pseudocomata and Trian. pseudopauciseta. These species are morphologically identical (Boucher et al. (2017) and only the species description of Trian. pauciseta is given here.
Triangularia allahabadensis (M.P. Srivast., Tandon, Bhargava & A.K. Ghosh.) X. Wei Wang & Houbraken, comb. nov. MycoBank MB829884. Fig. 49.
Fig. 49.
Triangularia allahabadensis (CBS 724.68, ex-type culture). A. Colonies from left to right on OA, CMA, MEA and PCA after 4 wk incubation. B. Part of the colony on OA. C. Mature ascomata on OA, top view. D–E. Mature ascomata on OA, side view. F–H. Ascomata mounted in lactic acid. I. Asci and young ascospores with a primary appendage. J. Ascospores. Scale bars: F = 50 μm; G–H = 100 μm; I = 20 μm; J = 10 μm.
Basionym: Sordaria allahabadensis M.P. Srivast., Tandon, Bhargava & A.K. Ghosh, Mycopathol. Mycol. Appl. 30: 203. 1966.
Micromorphology: Ascomata superficial, greenish black due to the mass of released ascospores in reflected light, solitary to aggregated, ampulliform with a short beak, ostiolate, 188–350 μm high, 144–325 μm diam. Ascomatal wall dark brown, opaque. Ascomatal hairs covering the whole ascoma, hypha-like, hyaline to subhyaline, 0.5–1 μm diam near base. Asci fasciculate, cylindrical to elongated fusiform, without conspicuous apical ring, spore-bearing part 70–125 × 12–20 μm, with stalks 13–35 μm long, containing eight biseriate or irregularly arranged (occasionally uniseriate) ascospores, evanescent after ascospores become mature. Ascospores 1-celled, hyaline when young and continuous with a hyaline and elongated fusiform appendage (9–13.5 × 2.5–3 μm in size and then falling off from mature ascospores), dark brown when mature, smooth, fusiform or navicular (19.5–)21.5–26(–28.5) × (8.5–)10–12 μm, with an apical germ pore. Asexual morph not observed.
Culture characteristics: On OA with an entire edge, 31–37 mm diam in 7 d at 25 °C, without aerial mycelium, obverse violaceous black due to ascomata and coloured exudates diffusing into the medium, reverse mouse grey. On CMA similar to those on OA, reverse olivaceous grey. On MEA with an entire edge, 34–40 mm diam in 7 d at 25 °C, with a thin layer of grey white aerial mycelium, sporulating well, obverse olivaceous grey due to coloured exudates diffusing into the medium, reverse dark slate blue. On PCA with an entire edge, 27–33 mm diam in 7 d at 25 °C, without aerial mycelium; obverse pale olivaceous grey or olivaceous grey, reverse mouse grey.
Typus: India, Allahabad, isolated from flower of Carica papaya, 1964, collector unknown (culture ex-type CBS 724.68 = IMI 104947).
Notes: The ex-type culture CBS 724.68 was deposited as Podospora austroamericana in the CBS culture collection. Previous studies showed that these two species are morphologically similar (Srivastava et al. 1966, Mirza & Cain 1969). Guarro et al. (1991) treated Sordaria allahabadensis as a synonym of Pod. austroamericana (basionym: Hypocopra austroamericana Speg. 1880); however, they did not study the holotype of the latter species. Based on literature (Srivastava et al. 1966, Mirza & Cain 1969), Podospora austroamericana can be distinguished from Tri. allahabadensis by the production of a phialophora-like morph and ascospores with clavate, persistent and broader primary appendages (3.5–4 μm vs 2.5–3 μm wide). More work is required to determine the relationship between these two species.
Triangularia anserina (Rabenh.) X. Wei Wang & Houbraken, comb. nov. MycoBank MB829885.
Basionym: Malinvernia anserina Rabenh., Hedwigia 1: 116. 1857.
Synonym: Sordaria anserina (Rabenh.) G. Winter, Abh. Naturf. Ges. Halle 13: 99. 1873.
Pleurage anserina (Rabenh.) Kuntze, Revis. Gen. Pl. 3: 504. 1898.
Podospora anserina (Rabenh.) Niessl, Hedwigia 22: 156. 1883.
Description and illustrations: See Boucher et al. (2017) and Triangularia pauciseta (present study).
Material examined: Canada, Ontario, Glenn Morris, isolated from dung of cow, 11 May 1944, R.F. Cain (CBS 433.50 = TRTC 12114). France, Normandy, isolated from dung, around 1940, collector unknown, culture ex-epitype of Malinvernia anserina, strain S deposited at Museum National d’Histoire Naturelle, Paris n° 6597. Unknown, substrate unknown, date and collector unknown, deposited by K. McCluskey (CBS 141519 = ATCC MYA-4624 = DSM 980 = FGSC 10383; CBS 141520 = ATCC MYA-4625 = FGSC 10384).
Notes: The rpb2 and tub2 sequences of the ex-epitype strain S (deposited at the Museum National d’Histoire Naturelle, Paris n° 6597) were retrieved from the released genomic data, but we didn't examine the morphology of this strain. Triangularia anserina is an important model ascomycete, being used for over a century to study various biological processes such as cell fusion, aging, sexual reproduction, differentiation and development and plant biomass degradation (Silar 2013). Triangularia anserina, Trian. pauciseta and the other species in this complex are morphologically indistinguishable. For further details, see notes of Triangularia.
Triangularia backusii L.H. Huang, Canad. J. Bot. 53: 560. 1975. Fig. 50.
Fig. 50.
Triangularia backusii (CBS 539.89, ex-type culture). A. Colonies from left to right on OA, CMA, MEA and PCA after 4 wk incubation. B. Mature ascomata on OA, top view. C. Mature ascomata on OA, side view. D. Ascomata mounted in lactic acid. E. Asci. F. Ascospores. Scale bars: D = 100 μm; E = 20 μm; F = 10 μm.
Synonyms: Zopfiella backusii (L.H. Huang) Guarro, Int. J. Mycol. Lichenol. 2: 253. 1986.
Apiosordaria backusii (L.H. Huang) Guarro, Trans. Brit. Mycol. Soc. 91: 589. 1988.
Micromorphology: Ascomata superficial, ostiolate, obpyriform to ampulliform with a conical or papilla-like dark-brown to black beak, 350–480 μm high, 290–360 μm wide, covered with hypha-like grey olivaceous to isabelline hairs. Ascomatal wall coriaceous, opaque, brown. Asci elongated clavate or fusiform, without an apical ring, spore-bearing part 120–210 × 29–39 μm, with stalks 21–40.5 μm long, containing eight biseriate or irregularly-arranged ascospores, evanescent, often persistent till ascospores mature. Ascospores 2-celled, smooth under light microscope, obovoid to pyriform, (39–)40–50(–56.5) × (21.5–)23.5–26(–27) μm, of which the upper cell is violaceous black when mature and usually possessing a pale apical stab-like appendage, (28–)30–35.5(–37.5) μm long, with an inconspicuous apical germ pore; and the lower cell straw to cinnamon, (10.5–)12–16(–18.5) × (13–)14.5–17(–18) μm, often collapsed at the base. Asexual morph not observed.
Culture characteristics: On OA with an entire edge, over 70 mm diam after 7 d at 25 °C, obverse greyish sepia, with a thin layer of smoke grey aerial mycelium, reverse mouse grey. On CMA similar to those on OA, obverse olivaceous, reverse mouse grey. On MEA with an entire edge, over 70 mm diam after 7 d at 25 °C, texture floccose, obverse smoke grey, reverse isabelline. On PCA translucent, with an entire edge, over 70 mm diam after 7 d at 25 °C, without or with sparse aerial mycelium, obverse pale olivaceous grey to pale luteous, reverse pale olivaceous grey or partly pale luteous.
Typus: USA, Montgomery Co. in Ohio, isolated from soil, L.H. Huang, date unknown (culture ex-type CBS 539.89 = ATCC 28796 = CBS 273.75).
Additional material examined: Japan, Okinawa Pref., isolated from sandy soil by J. Horie, 28 Sep. 1973 (CBS 106.77 = ATCC 34568 = IFM 4533 = IMI 210877 = NHL 2739). Spain, Castillejos, Tarragona, isolated from dung of rabbit, J. Guarro, date unknown (CBS 540.89 = ATCC 62830 = FMR 842).
Notes: The name changes of this species illustrate the complication of delimiting of Apiosordaria, Triangularia and Zopfiella morphologically. These three genera share the production of unequally 2-celled ascospores, with one larger, pigmented upper cell (which is occasionally septated) and a smaller, paler or hyaline lower cell, but lacking gelatinous appendages (Guarro and Cano, 1988, Guarro et al., 1991). Apiosordaria species were regarded to produce ornamented ascospores, while the ascospores of Triangularia and Zopfiella were considered to be smooth walled (Guarro and Cano, 1988, Wu et al., 2016). Zopfiella could be differentiated by the production of non-ostiolate ascomata (Guarro and Cano, 1988, Guarro et al., 1991). SEM micrographs showed that there were shallow pits on the ascospores of Trian. backusii, which were hardly visible under light microscopy (Guarro & Cano 1988). For this reason, the species was transferred to Apiosordaria (Guarro & Cano 1988). Our phylogenetic data (Fig. 2, Fig. 3) do not support the classification of Trian. backusii in Apiosordaria. Our data show that the morphological characters used for the delimitation of Apiosordaria needs to be re-evaluated.
Triangularia bambusae (J.F.H. Beyma) Boedijn, Ann. Mycol. 32: 302. 1934. Fig. 51, Fig. 52.
Fig. 51.
Triangularia bambusae (CBS 352.33, ex-type culture). A. Colonies from left to right on OA, CMA, MEA and PCA after 4 wk incubation. B. Part of the colony on OA. C. Mature ascomata on OA, top view. D–F. Ascomata mounted in lactic acid. G. Asci. H. Ascospores. Scale bars: D–F = 100 μm; G = 20 μm; H = 10 μm.
Fig. 52.
Triangularia bambusae (CBS 352.33, ex-type culture). A–B. Asexual structures covering ascomata on OA, top view. C. Asexual structures covering an ascoma mounted in lactic acid. D–E. Hyphae, conidiogenous cells and conidia. F. Conidia. Scale bars: C = 100 μm; D–F = 10 μm.
Basionym: Trigonia bambusae J.F.H. Beyma, Zentralbl. Bakteriol., 2. Abt. 89: 236. 1933.
Micromorphology: Ascomata superficial, sometimes semi-immersed, ostiolate, obpyriform with a conical or papilla-like dark-brown to black beak, 200–370 μm high, 150–275 μm wide, usually covered by hyaline hypha-like hair, sometimes with brown setae near the ostiole. Ascomatal wall coriaceous, opaque or slightly semi-translucent, brown. Asci cylindrical, with a thickened ring at apex, spore-bearing part 90–125 × 9.5–17 μm, with stalks 16.5–55 μm long, containing eight uniseriate (occasionally biseriate) ascospores, evanescent, sometimes persistent till ascospores mature. Ascospores 2-celled, (15.5–)16–18.5(–20) μm long, composed of an upper triangular cell and a lower vinaceous buff cell, of which, the upper triangular cell olivaceous brown when mature, (13–)13.5–15.5(–17) × (9–)9.5–11(–13) μm, with a subapical germ pore; and the lower paler cell,(2–)3–4(–4.5) μm high, (10–)11.5–13.5(–14) μm wide. Asexual morph cladorrhinum-like, abundantly present, covering the ascomata. Conidiogenous cells terminally or laterally arising from aerial hyphae, hyaline, short cylindrical, slightly tapering, up to 2.5 μm long, 1–1.5 μm wide, apically with a flaring collarette opening in 1.5–2.5 μm wide, which produces blastic conidia continuously. Conidia single-celled, hyaline, smooth, subglobose, ellipsoidal or broad obovoid, often with a truncated base and a rounded apex, (2.5–)3–3.5(–4) × (2–)2.5–3(–4) μm.
Culture characteristics: On OA with an entire edge, over 70 mm diam after 7 d at 25 °C, with a layer of white aerial mycelium, obverse salmon to peach due to coloured exudates diffusing into the medium, distributing black dot-like ascomata, reverse peach. On CMA similar to those on OA. On MEA with an entire edge, over 70 mm diam after 7 d at 25 °C, with a thick layer of white aerial mycelium, obverse fulvous due to coloured exudates diffusing into the medium, distributing black dot-like ascomata, reverse apricot. On PCA with an entire edge, over 70 mm diam after 7 d at 25 °C, with a thin layer of white aerial mycelium, obverse and reverse uncoloured, without coloured exudates.
Typus: Unknown, isolated from shoot of Bambusa sp., Kol. Instituut, date unknown (ex-type culture CBS 352.33).
Notes: The position of Tri. bambusae, the type species of Triangularia, determined the phylogenetic placement of this genus in the Podosporaceae fam. nov. Triangularia bambusae is most closely related to Trian. allahabadensis, Trian. backusii, Trian. longicaudata, Trian. setosa and Trian. verruculosa (Fig. 2, Fig. 3), but can be easily distinguished by its ascospores consisting of a dark and triangular shaped upper cell and a pale, broad and very short lower cell. Triangularia backusii and Trian. verruculosa also produce 2-celled ascospores, but ascospores of Trian. backusii are obovoid to pyriform, and those of Trian. verruculosa are unequally fusiform, never having a triangular upper cell. Triangularia allahabadensis produces 1-celled fusiform or navicular ascospores with a hyaline, elongated fusiform and easily falling-off appendage. Triangularia longicaudata and Trian. setosa produce 2-celled ascospores with a persistent hyaline primary appendage.
Triangularia bellae-mahoneyi (C. Boucher, T.S. Nguyen & Silar) X. Wei Wang & Houbraken, comb. nov. MycoBank MB829886.
Basionym: Podospora bellae-mahoneyi C. Boucher, T.S. Nguyen & Silar, Cryptog. Mycol. 38: 497. 2017.
Description and illustrations: See Boucher et al. (2017) and Triangularia pauciseta (present study).
Notes: This species is accepted based on the phylogenetic analysis using sequence data of the loci Rchr3, Rchr4 and Rchr6 (Fig. 6) from Boucher et al. (2017). This species cannot be recognised by ITS sequences. Chr3, Chr4 and Chr5 sequences should be generated for identification (Boucher et al. 2017). Tub2 sequencing is recommended for identification of Chaetomiaceae (Wang et al. 2016b); however, no tub2 references sequences of this species are available. Future studies including tub2 sequences should provide information whether this species (and other closely related Podosporaceae species) could be identified using this secondary barcode.
Triangularia comata (Milovtz.) X. Wei Wang & Houbraken, comb. nov. MycoBank MB829887.
Basionym: Podospora comata Milovtz., Trav. Inst. Bot. Charkov 2: 20. 1937.
Description and illustrations: See Boucher et al. (2017) and Triangularia pauciseta (present study).
Notes: This species is accepted and transferred to the genus Triangularia. For additional information, see notes of Trian. bellae-mahoneyi.
Triangularia longicaudata (Cain) X. Wei Wang & Houbraken, comb. nov. MycoBank MB829888. Fig. 53.
Fig. 53.
Triangularia longicaudata (CBS 252.57, ex-type culture). A. Colonies from left to right on OA, CMA, MEA and PCA after 3 wk incubation. B. Part of the colony on OA. C. Mature ascomata on OA, top view. D–E. Mature ascomata on OA, side view. F–H. Ascomata mounted in lactic acid. I. Structure of ascomatal wall in surface view. J. Asci. K. Ascospores. Scale bars: F–H = 100 μm; I–K = 10 μm.
Basionym: Tripterospora longicaudata Cain, Canad. J. Bot. 34: 702. 1956.
Synonym: Zopfiella longicaudata (Cain) Arx, Proc. Kon. Ned. Akad. Wetensch. C 76: 291. 1973.
Micromorphology: Ascomata superficial to immersed in the medium, fuscous black in reflected light, solitary to aggregated, subglobose, non-ostiolate, 110–245 μm diam. Ascomatal wall brown, semi-translucent, composed of irregular or angular cells. Ascomatal hairs sparse, hyaline, hypha-like, 2–3.5 μm diam near base. Asci fasciculate, cylindrical or elongated fusiform, without an apical ring, spore-bearing part 46–63 × 11–13 μm, with stalks 14–26 μm long, containing eight biseriate or irregularly arranged ascospores, evanescent. Ascospores composed of a olivaceous brown cell and a hyaline, cylindrical primary appendage when mature, of which the pigmented cell is ellipsoidal, attenuated at both ends, (11–)12–14.5(–15) × (7.5–)8–9(–9.5) μm, with an apical germ pore; the primary appendage at the opposite end, 9.5–13.5 × 2–3.5(–4) μm. Asexual morph not observed.
Culture characteristics: On OA with a lobate edge, 22–28 mm diam in 7 d at 25 °C, with white or buff sparse aerial mycelium or without aerial mycelium, obverse vinaceous buff or ochraceous due to pigmented exudates diffusing into the medium, or mouse grey due to the formation of ascomata, reverse mouse grey. On CMA similar to those on OA, obverse mouse grey to dark mouse grey. On MEA with a crenate or slightly fimbriate edge, 24–30 mm diam in 7 d at 25 °C, irregularly wrinkled, obverse white to mouse grey, with white to grey white aerial mycelium, reverse olivaceous grey. On PCA with a lobate edge, 19–25 mm diam in 7 d at 25 °C, without aerial mycelium; obverse pale olivaceous grey due to the formation of ascomata, reverse uncoloured.
Typus: Canada, Ontario, Peel Co., N of Palgrave, isolated from dung of horse, 10 Oct. 1955, R.F. Cain (culture ex-type CBS 252.57 = TRTC 31528).
Notes: Triangularia longicaudata was once placed in the genus Zopfiella (von Arx 1973). Our phylogenetic analyses indicated that this species is distant from Zopfiella tabulata, the type species of the genus Zopfiella (authentic strain CBS 230.78, marked with a red triangle in Fig. 2, Fig. 3). Traditionally, Zopfiella is differentiated from Triangularia by the production of non-ostiolate ascomata, clavate to cylindrical asci lacking an apical ring, and small ascospores with short hyaline cells (primary appendages), but without gelatinous appendages (Guarro et al. 1991). Cai et al. (2006) suggested restricting Zopfiella to species having ascospores with a septum in the dark upper cell. The taxonomic value of this character and the possible presence of other species than Zopfiella tabulata, Pod. didyma and Cercophora sulphurella in Zopfiella sensu stricto needs to be evaluated. Triangularia longicaudata produces similar ascospores to those of Trian. setosa in shape, but can be distinguished from the latter species by 8-spored asci and smaller ascospores (12–14.5 × 8–9 μm vs 17–20.5 × 10.5–12 μm). It can be distinghuised from the other closely related species by the production of non-ostiolate ascomata. For more morphological comparisons, see notes of Trian. bambusae.
Triangularia pauciseta (Ces.) X. Wei Wang & Houbraken, comb. nov. MycoBank MB829889. Fig. 54.
Fig. 54.
Triangularia pauciseta (CBS 451.62). A. Colonies from left to right on OA, CMA, MEA and PCA after 3 wk incubation. B. Mature ascomata on OA, top view. C. Mature ascomata on OA, side view. D–E. Ascomata mounted in lactic acid. F–G. Asci. H. Ascospores. Scale bars: D–E = 100 μm; F = 50 μm; G = 20 μm; H = 10 μm.
Basionym: Sphaeria pauciseta Ces., Rabenhorst, Klotzschii Herb. Viv. Mycol., Cent. 17: no. 1642, 1852.
Synonym: Podospora pauciseta (Ces.) Traverso, Fl. ital. crypt., Fungi 2: 431. 1907.
Micromorphology: Ascomata superficial, iron grey to fuscous black in reflected light, solitary to aggregated, ampulliform with a short and black beak, ostiolate, 310–685 μm high, 220–440 μm diam. Ascomatal wall brown, semi-translucent, composed of irregular or angular cells. Ascomatal hairs arising from the lower half of ascomata, erect or flexuous, subhyaline to pale brown, 1.5–3 μm diam near base, up to 200 μm long. Asci fasciculate, cylindrical, apically attenuated, without conspicuous apical ring, spore-bearing part 124–164 × 18.5–24.5 μm, stalks 30–55 μm long, containing four uniseriate ascospores, persistent. Ascospores composed of a olivaceous brown cell and a hyaline and cylindrical primary appendage when mature, of which the pigmented cell is ellipsoidal, (30–)31–36(–38) × (14.5–)16–19 μm, with an apical or slightly subapical germ pore and often geminating at or near apex to form a hyaline secondary appendage; the primary appendage at the opposite end, 25–37 × 4.5–6.5(–8) μm. Asexual morph cladorrhinum-like (fide Mirza & Cain 1969 and Boucher et al. 2017): Conidiophores micronematous, reduced to conidiogenous cells. Conidiogenous cells intercalary, originating lateral peg-like structure with a flaring collarette, producing blastic conidia. Conidia single-celled, hyaline, smooth, globose to ovoid, usually with a truncated base and a rounded apex, 1.5–2.5 μm diam.
Culture characteristics: On OA with a crenate edge, 29–35 mm diam in 7 d at 25 °C, without aerial mycelium, obverse vinaceous buff to olivaceous grey due to pigmented hyphae and coloured exudates diffusing into the medium, reverse pale mouse grey to mouse grey. On CMA similar to those on OA, but less sporulating, obverse olivaceous to fuscous black. On MEA with a lobate edge, 23–29 mm diam in 7 d at 25 °C, obverse presenting smoke grey aerial mycelium and olivaceous black ascomata, reverse greenish grey. On PCA with a slightly crenate edge, 25–31 mm diam in 7 d at 25 °C, without aerial mycelium; obverse pale olivaceous grey, reverse uncoloured.
Material examined: Argentina, Buenos Aires, Km 38, isolated from dung of cow, date unknown, J.E. Wright (CBS 451.62).
Note: This species has been typified by Boucher et al. (2017). We did not study the ex-epitype CBS 237.71, but the phylogenetic analyses indicated that our examined strain (CBS 451.62) clusters with the ex-type of Trian. pauciseta in the same species lineage (Fig. 6). Triangularia pauciseta and the other species in the “Podospora anserina/pauciseta/comata species complex” (Boucher et al. 2017) are mainly characterised by 4-spored cylindrical asci, 1-celled ascospores with a hyaline primary appendage at base and a secondary appendage at or near apex. These characters can be used to differentiate the species in this species complex from the other known species in the genus.
Triangularia phialophoroides (Mouch. & W. Gams) X. Wei Wang & Houbraken, comb. nov. MycoBank MB829891. Fig. 55.
Fig. 55.
Triangularia phialophoroides (CBS 301.91, ex-type culture). A. Colonies from left to right on OA, CMA, MEA and PCA after 3 wk incubation. B–C. Hyphae, conidiogenous cells and conidia. Scale bars: B–C = 10 μm.
Basionym: Cladorrhinum phialophoroides Mouch. & W. Gams, Mycotaxon 48: 428. 1993.
Micromorphology: Sexual morph not observed. Somatic hyphae hyaline, 1.5–5 μm wide. Conidiophores phialidic, terminally or laterally arising from aerial hyphae, 7–20 × 2–3 μm, or reduced to intercalary conidiogenous cells that produce lateral blastic conidia. Conidia single-celled, hyaline, smooth, broad obovoid, ellipsoidal, elongated ellipsoidal or cylindrical, usually with a truncated base and a rounded apex, 2.5–3.5(–4) × 1.5–2.5 μm.
Culture characteristics: On OA with a slightly crenate edge, 25–31 mm diam after 7 d at 25 °C, obverse dark mouse grey due to pigmented sclerotial masses immerged in the medium, with sparse aerial mycelium, reverse mouse. On CMA similar to those on OA. On MEA with a crenate edge, 18–24 mm diam after 7 d at 25 °C, obverse pale mouse grey due to aerial mycelium and conidia, reverse greenish black. On PCA translucent, with a crenate edge, 22–28 mm diam after 7 d at 25 °C, without sparse aerial mycelium, obverse pale mouse grey, reverse uncoloured.
Typus: Egypt, New Valley region, Western Desert, isolated from desert sand soil, 24 Aug. 1990, J. Mouchacca (culture ex-type CBS 301.90).
Notes: This species clustered in the Triangularia lineage in both rpb2 and combined four-locus phylogenetic analyses (rpb2: PP = 1, ML-BS = 100 %; Fig. 2 and the four-locus tree: PP = 0.99, ML-BS = 100 %; Fig. 3). No sexual morph was observed in our analysis. On the other hand, we have observed that several sexual species in the re-defined genus Triangularia produce cladorrhinum-like asexual structures.
Triangularia pseudoanserina (C. Boucher, T.S. Nguyen & Silar) X. Wei Wang & Houbraken, comb. nov. MycoBank MB829890.
Basionym: Podospora pseudoanserina C. Boucher, T.S. Nguyen & Silar, Cryptog. Mycol. 38: 497. 2017.
Description and illustrations: See Boucher et al. (2017) and Triangularia pauciseta (present study).
Notes: This species is accepted and transferred to the genus Triangularia. For additional information, see notes Trian. bellae-mahoneyi.
Triangularia pseudocomata (C. Boucher, T.S. Nguyen & Silar) X. Wei Wang & Houbraken, comb. nov. MycoBank MB829892.
Basionym: Podospora pseudocomata C. Boucher, T.S. Nguyen & Silar, Cryptog. Mycol. 38: 498. 2017.
Description and illustrations: See Boucher et al. (2017) and Triangularia pauciseta (present study).
Notes: This species is accepted and transferred to the genus Triangularia. For additional information, see notes Trian. bellae-mahoneyi.
Triangularia pseudopauciseta (C. Boucher, T.S. Nguyen & Silar) X. Wei Wang & Houbraken, comb. nov. MycoBank MB829893.
Basionym: Podospora pseudopauciseta C. Boucher, T.S. Nguyen & Silar, Cryptog. Mycol. 38: 498. 2017.
Description and illustrations: See Boucher et al. (2017) and Triangularia pauciseta (present study).
Notes: Triangularia pseudopauciseta is accepted and transferred to the genus Triangularia. For additional information, see notes Trian. bellae-mahoneyi.
Triangularia setosa (G. Winter) X. Wei Wang & Houbraken, comb. nov. MycoBank MB829894. Fig. 56.
Fig. 56.
Triangularia setosa (CBS 311.58). A. Colonies from left to right on OA, CMA, MEA and PCA after 3 wk incubation. B. Mature ascomata on OA, top view. C. Mature ascomata on OA, side view. D–E. Ascomata mounted in lactic acid. F, G, I. Asci. H. Structure of ascomatal wall in surface view. J. Ascospores. Scale bars: D–E = 100 μm; F–G = 50 μm; H, J = 10 μm; I = 20 μm.
Basionym: Sordaria setosa G. Winter, Abh. Naturf. Ges. Halle 13: 97. 1873.
Synonym: Philocopra setosa (G. Winter) Sacc., Syll. Fung. 1: 249. 1882.
Podospora setosa (G. Winter) Niessl, Hedwigia 22: 156. 1883.
Pleurage setosa (G. Winter) Kuntze, Revis. Gen. Pl. 3: 505. 1898.
Cladochaete setosa (G. Winter) Sacc., Ann. Mycol. 10: 318. 1912.
Micromorphology: Ascomata superficial, mouse grey in reflected light, solitary, ovoid to ampulliform with a short and black beak, ostiolate, 230–590 μm high, 185–410 μm diam. Ascomatal wall brown, opaque, of textura intricata or epidermoidea in surface view. Ascomatal hairs arising mainly around the lower half part, hypha-like, erect or flexuous, brown, 1.5–3 μm diam near base. Asci fasciculate, fusiform or elongated fusiform, spore-bearing part 145–238 × 25–49(–57) μm, without a conspicuous apical ring, with stalks 21.5–62 μm long, containing numerous irregularly- and densely-arranged ascospores, evanescent or persistent until ascospores mature. Ascospores composed of a olivaceous brown cell and a hyaline, cylindrical primary appendage when mature, of which the pigmented cell ellipsoidal, usually with attenuated ends, (15–)17–20.5(–22) × (9–) 10.5–12(–12.5) μm, with an apical germ pore, the primary appendage at the opposite end, 8–12 × 2–3 μm. Asexual morph not observed.
Culture characteristics: On OA with an entire edge, 24–30 mm diam in 7 d at 25 °C, without aerial mycelium, obverse vinaceous buff with sparse ascomata mainly along the edge, reverse hazel. On CMA similar to those on OA. On MEA with a fimbriate edge, 18–24 mm diam in 7 d at 25 °C, obverse grey white to vinaceous buff due to aerial mycelium, with numerous ascomata on the surface, reverse saffron to orange. On PCA with a slightly crenate edge, 21–27 mm diam in 7 d at 25 °C, without aerial mycelium, reverse uncoloured.
Material examined: UK, Lakenheath Warren, isolated from soil, 1949, J.H. Warcup (CBS 311.58 = LSHTM BB244); isolated from dung of horse, Dec. 1957, C.T. Ingold (CBS 369.59).
Notes: Triangularia setosa produces ascospores similar to those of Trian. longicaudata in shape, but can be distinguished from the latter species by multi-spored asci (containing more than 64 ascospores) and larger ascospores (17–20.5 × 10.5–12 μm vs 12–14.5 × 8–9 μm). For more characters that can be used for morphological comparisons, see notes of Trian. bambusae. In the morphologically-defined Podospora, species seem to be highly diverse in the number of ascospores in an ascus, including those with 4-spored, 8-spored, 16-spored, 32-spored, 64-spored, 128-spored, 156-spored, 256-spored, 512-spored and 1024-spored asci (Cain, 1962, Mirza and Cain, 1969). More work is needed to assess the relationships of those species with asci containing different numbers of ascospores. The holotype of the basionym Sordaria setosa was collected from goose dung in Leipzig, Germany, and needs to be examined.
Triangularia verruculosa (C.N. Jensen) X. Wei Wang & Houbraken, comb. nov. MycoBank MB829895. Fig. 57.
Fig. 57.
Triangularia verruculosa (CBS 148.77). A. Colonies from left to right on OA, CMA, MEA and PCA after 4 wk incubation. B. Part of the colony on OA. C. Mature ascomata on OA, side view. D. Mature ascomata on OA, top view. E–F. Ascomata mounted in lactic acid. G–H. Asci. I. Ascospores. Scale bars: E–F = 100 μm; G–H = 20 μm; I = 10 μm.
Basionym: Pleurage verruculosa C.N. Jensen, Bull. Cornell Univ. Agric. Exp. Sta 315: 472. 1912.
Synonym: Apiosordaria verruculosa (C.N. Jensen) Arx & W. Gams, Nova Hedwigia 13: 201. 1967.
Micromorphology: Ascomata superficial to semi-immersed, ostiolate, obpyriform to ampulliform with a papilla-like dark-brown to black beak, 230–340 μm high, 160–260 μm wide, usually with brown setae near the ostiole. Ascomatal wall coriaceous, opaque, brown. Asci cylindrical, with an apical ring, spore-bearing part 75–115 × 12–18.5 μm, with stalks 19–53 μm long, containing (two to) four uniseriate ascospores, evanescent, often persistent until ascospores mature. Ascospores 2-celled, unequally fusiform, umbonate at both ends, (23–)25.5–28.5(–29.5) μm long, composed of an upper olivaceous brown cell and a lower vinaceous buff cell when mature, of which the upper cell is ornamented with spines up to 2 μm long, (15.5–)18–20 × (12.5–)13–14 μm, with an apical germ pore; and the lower cell smooth, (7–)8–9.5(–10) × (7–)7.5–8.5(–9) μm. Asexual morph cladorrhinum-like (fide Mouchacca & Gams 1993): Conidiophores micronematous, reduced to conidiogenous cells. Conidiogenous cells intercalary or occasionally terminal, originating lateral or terminal peg-like structure with a flaring collarette, producing blastic conidia. Conidia single-celled, hyaline, smooth, ovoid to ellipsoid, usually with a truncated base and a rounded apex, 3–3.5 × 2–2.5 μm.
Culture characteristics: On OA with an entire edge, over 70 mm diam after 7 d at 25 °C, with white aerial mycelium, obverse buff to olivaceous grey, reverse pale greenish grey to pale mouse grey. On CMA similar to those on OA. On MEA with an entire edge, over 70 mm diam after 7 d at 25 °C, obverse buff to straw due to aerial mycelium and conidia, with olivaceous grey margin due to ascomata, reverse greenish grey. On PCA an entire edge, over 70 mm diam after 7 d at 25 °C, with sparse aerial mycelium, obverse grey olivaceous to olivaceous grey, reverse olivaceous grey.
Material examined: Japan, Niyako-shi, isolated from soil, 2 Aug. 1974, K. Furuya (CBS 148.77 = NHL 2736 = SANK 10374).
Notes: Apiosordaria verruculosa is the type species of the genus Apiosordaria. This genus was also defined to possess 2-celled ascospores, and was considered to be different from Triangularia in the production of ellipsoidal to subglobose ascospores with an upper dark cell which is often ornamented with striate, pitted or verrucose walls, or covered by spines (Arx & Gams 1967, Guarro & Cano 1988). Our rpb2 and four-locus phylogenetic analyses clearly indicated that Apio. verruculosa belongs to the Triangularia lineage. Triangularia (1934) has priority over Apiosordaria (1967), and this species is therefore transferred to the re-defined genus Triangularia here, and Apiosordaria is synonymized with Triangularia. The species remains to be typified.
Triangularia verruculosa is most closedly related to Trian. allahabadensis, Trian. backusii, Trian.bambusae, Trian. longicaudata, Trian. setosa and Trian. verruculosa (Fig. 2, Fig. 3), but can be easily distinguished by its 2-celled ascospores with the upper cell ornamented with conspicuous spines. For more characters that can be used for morphological comparison, see notes of Trian. bambusae.
Sordariaceae
Boothiella Lodhi & J.H. Mirza, Mycologia 54: 217. 1962.
Micromorphology: Ascomata superficial to immersed in the medium, solitary to aggregated, non-ostiolate, globose to subglobose. Ascomatal wall hyaline, translucent. Asci cylindrical, containing four uniseriate ascospores, evanescent. Ascospores olivaceous brown when mature, smooth, 1-celled, with apical germ pores. Asexual morph not observed.
Type species: Boothiella tetraspora Lodhi & J.H. Mirza.
Note: Boothiella is a monotypic genus which was thought to be related to Thielavia, but separated from this genus based on its colourless ascomatal wall (Lodhi & Mirza 1962). Later, Eriksson et al. (2004) and Kirk et al. (2008) classified this genus in the Sordariaceae; however, more recently Maharachchikumbura et al., 2015, Maharachchikumbura et al., 2016 placed this genus in the Chaetomiaceae. Comparison of ITS and LSU (D1/D2 domain) sequences obtained from CBS 334.67 (Vu et al. 2019) showed that Boothiella tetraspora belongs to the Sordariaceae. Our phylogenetic analyses based on rpb2 and four-locus datasets robustly confirmed the result of Vu et al. (2019).
Boothiella tetraspora Lodhi & J.H. Mirza, Mycologia 54: 217. 1962. Fig. 58.
Synonyms: Thielaviella humicola Arx & T. Mahmood, Trans. Brit. Mycol. Soc. 51: 611. 1968.
Thielavia tetraspora (Lodhi & Mirza) Arx, The genera of fungi sporulating in pure culture. 115. 1974.
Micromorphology: Ascomata superficial to immersed in the medium, solitary to aggregated, non-ostiolate, globose to subglobose, leaden black when mature in reflected light due to the dark ascospores inside, glabrous, 115–390 μm diam. Ascomatal wall subhyaline, translucent, composed of textura epidermoidea in surface view. Asci cylindrical, spore-bearing part 44–70 × 11.5–17 μm, with stalks 8–26 μm long, without apical structure, containing four uniseriate ascospores, evanescent. Ascospores 1-celled, olivaceous brown when mature, smooth, ellipsoidal to broad ovoid, (16.5–)17.5–22(–26.5) × 13–15(–17.5) μm, with an apical germ pore. Asexual morph not observed.
Culture characteristics: On OA with an entire edge, over 70 mm diam after 7 d at 25 °C, texture floccose, obverse buff to honey due to aerial mycelium mixed with masses of young ascomata, later becoming umber due to the mature ascomata, reverse buff to ochraceous. On CMA similar to those on OA. On MEA with an entire edge, over 70 mm diam after 7 d at 25 °C, texture floccose, obverse buff to ochraceous due to aerial mycelium mixed with masses of ascomata, reverse umber. On PCA translucent, with an entire edge, over 70 mm diam after 7 d at 25 °C, without or with sparse aerial mycelium, reverse uncoloured.
Material examined: India, Kanpur, isolated from soil, 5 Nov. 1995, J. Guarro (CBS 887.97 = FMR 6192). Pakistan, Lahore area, isolated from barley field soil, date unknown, S.H. Iqbal (CBS 334.67 = IMI 291723 = MUCL 11462, culture ex-isotype of Thielaviella humicola).
Notes: Boothiella tetraspora possesses glabrous and subglobose ascomata with subhyaline and translucent ascomatal wall, resembling Hya. fragilis and Pse. subhyaloderma in the Chaetomiaceae, but differs mainly in cylindrical and 4-spored asci. Hyalosphaerella fragilis can also be distinguished by smaller ascomata (50–115 μm vs 115–390 μm diam), clavate to pyriform asci, smaller and inequilaterally ovoid to reniform ascospores (11–13 × 6.5–7.5 μm vs 17.5–22 × 13–15 μm). Pseudothielavia subhyaloderma differs by smaller ascomata (60–185 μm vs 115–390 μm diam), clavate to pyriform asci and smaller and often irregular ascospores (10.5–13 × 7–8 μm vs 17.5–22 × 13–15 μm).
Discussion
Thielavia basicola is neotypified and this species (and genus) is classified in the Ceratostomataceae (Melanosporales). The remaining 18 studied “Thielavia” species fall into two related family-level lineages in the Sordariales: the Chaetomiaceae and Podosporaceae fam. nov. (Fig. 2, Fig. 3). Seventeen species that were previously classified in Thielavia are recognised in the Chaetomiaceae, including our previously combined Trichocladium antarcticum (Wang et al. 2019). These species belong to eleven genera, of which nine are newly described in the present study. The genera containing thielavia-like species are not all closely related to each other and they are mostly intermingled with other genera in the Chaetomiaceae. Six of the newly described genera are monotypic: three of them (Carteria, Condenascus, Microthielavia) are lone lineages without known close relatives; Hyalosphaerella clusters closely but separate from Parathielavia and Pseudothielavia within Clade 1; Thermothielavioides is closely related to the chaetomium-like ostiolate genus Floropilus; and Stolonocarpus is basal to Madurella. Furthermore, several species cluster within Canariomyces; Chrysan. peruvianum (syn. Th. peruviana) groups with three chaetomium-like ostiolate species in Chrysanthotrichum, and Trich. antarcticum (syn: Th. antarctica) falls in the morphologically diverse genus Trichocladium. These results show that the thielavia-like (non-ostiolate, glabrous, setose or tomentose ascomata with a thin wall composed of textura epidermoidea) is a homoplastic structure that originated from several separate evolutionary events, similar to what happened in the chaetomidium-like ascomata (Greif et al., 2009, Wang et al., 2016b). According to Index Fungorum and MycoBank, there are 26 more species which were described in Thielavia, but no strains of those species are available in the present study. They are likely to be distant from Thielavia sensu stricto because none of them are known to grow in association with any other fungus. Their exact position in the current classification of Chaetomiaceae (or other families) remains to be studied in future. As mentioned in the introduction, the morphologically defined Thielavia exhibits a diverse ecology. It is required to further study the influence of ecological factors on the divergence of thielavia-like species.
Three re-defined genera are currently classified in the newly proposed family Podosporaceae. Based on some previous studies and our phylogenetic analyses, several morphological defined genera in the Lasiosphaeriaceae appear to be polyphyletic. For example, the genus Cladorrhinum is phylogenetically restricted to the type species (Clad. foecundissimum), and also includes two thielavia-like species in which no asexual state was observed. On the other hand, the cladorrhinum-form asexual state has also been reported in several sexually reproducing species, such as Apiosordaria verruculosa (von Arx & Gams 1967), Cercophora samala (Udagawa & Muroi 1979), Cercophora striata (Miller & Huhndorf 2001) and Podospora fimicola (Bell & Mahoney 1997). The family Podosporaceae corresponds to clade A of Cai et al. where species with a cladorrhinum-like asexual state grouped in their Clade A (Cai et al. 2006). Our observation supported the study of Cai et al. (2006), although we did not include Cercophora species in this study, and did not carefully examine the cladorrhinum-like state in all the studied species. The morphologically defined genus Podospora is one of the largest genera in the Lasiosphaeriaceae and is mainly characterised by producing ascospores with an apical germ pore, a basal hyaline cell, and gelatinous appendages (Mirza & Cain 1969). Nearly 200 species have been described in this genus according to Index Fungorum and MycoBank. Numerous previous studies have provided phylogenetic evidence that the morphologically defined Podospora is polyphyletic and taxonomic confusion between this genus and several other genera in the Lasiosphaeriaceae exists (Huhndorf et al., 2004, Cai et al., 2005, Miller and Huhndorf, 2005, Cai et al., 2006, Zhang et al., 2006, Kruys et al., 2015). Based on our rpb2 phylogeny (Fig. 2), the morphologically defined Podospora species are distributed over at least seven generic- or even higher-level clades and this confirms that a revision of Podospora is necessary. We restrict Podospora to a small clade containing the type species Pod. fimicola and Pod. bulbillosa, an asexually reproducing species which was originally described as Cladorrhinum bulbillosum.
The genera Triangularia and Apiosordaria were defined mainly based on their differences in ascospores morphology. Triangularia was characterised by having ascospores composed of two cells: the upper cell is dark and conical or triangular shaped and has a germ pore, and the lower cell is hyaline and triangular or hemisphaerical shaped (Guarro & Cano 1988). The upper cells of the 2-celled ascospores of Apiosordaria are also dark pigmented, but ellipsoidal to subglobose, and often ornamented with striate, pitted or verrucose walls (Arx & Gams 1967, Guarro & Cano 1988). In this study, the genus Triangularia is redefined, and includes nine species which were previously classified in Apiosordaria, Cladorrhinum, Podospora, Triangularia or Zopfiella. Our results indicate not only a highly diverse morphology in Triangularia (especially in the morphology of their ascospores), but also the polyphyly of the four other traditional genera. Aside from Cladorrhinum and Podospora (discussed above), the two Apiosordaria species included in our rpb2 phylogram fall in two distant clades: the type species (Apio. verruculosa) is combined in Triangularia and belongs to the Podosporaceae, while Apio. microcarpa is related to Zopfiella tardifaciens and is classified in a different, maybe new family lineage. Similarly, Zopfiella species are scattered throughout the polyphyletic Lasiosphaeriaceae, and probably belong to four different family lineages. More work is required to re-evaluate the species of Apiosordaria, Cercophora, Podospora, and Zopfiella, as well as additional Triangularia species that were not involved in the present study.
Species delimitation is the basic work of taxonomy. Triangularia anserina (syn.: Pod. anserina) is a model species that has been widely used for over a century to study many biological and genetic phenomena (Silar 2013). Following the taxonomic criteria in our previous work in the Chaetomiaceae (Wang et al., 2016a, Wang et al., 2016b, Wang et al., 2019), we would have accepted Trian. anserina as a synonym of Trian. pauciseta, because there are no morphological differences or phylogenetic evidence based on ITS, LSU, rpb2 or tub2 sequences to distinguish both species. Boucher et al. (2017) recognised seven species in the Podospora anserina/pauciseta/comata species complex on the basis of their phylogenetic analysis of ITS and three new intergenic loci. At the same time they stated that their recognised seven species appeared to poorly mate in culture, pointing towards biological species. ITS has poor resolution in differentiating species (Fig. 6A) and three uncommonly used intergenic loci (Rchr3, Rchr4 and Rchr6) were able to differentiate the seven species in the species complex (Fig. 6B–D). Their data challenged to some extent our current phylogenetic delimitation of Chaetomiaceae species. It remains to study whether the markers commonly used in Chaetomiaceae for phylogeny (LSU, ITS, tub2 and rpb2) and identification (tub2) have sufficient resolution in this species complex. There is an opposite situation in the Chaetomiaceae where species are phylogenetically indistinguishable but morphologically distinct. One example is the two species in Pseudothielavia: Pse. terricola and Pse. arxii, which are phylogenetically indistinguishable but produce morphologically distinct ascospores. A similar case occurs in Ch. globosum and Ch. cruentum. Wang et al. (2016b) treated Ch. cruentum as Ch. globosum with the morphological form ‘cruentum’; however, we now prefer to accepted them as separate species as well. Phylogenetic species recognition (PSR) is based on the consensus that “once progeny evolutionary species have formed from an ancestor, changes in gene sequences occur and can be recognized before changes have occurred in mating behavior or morphology” (Taylor et al. 2000). We prefer to use a polyphasic approach, combining phenotypic and molecular data, for the delimitation of species in Chaetomiaceae. More studies (e.g. other loci, genome data) are needed for sufficient recognition of species in the Chaetomiaceae.
Acknowledgements
This study was financially supported by the National Natural Science Foundation of China (NSFC, Project No. 31770021), partly supported by the National Key R&D Program of China “Research on the key technology of supplying high-quality forage and recycling of cultivation and husbandry” (Project No. 2017YFD0502100) and NSFC’s project No. 31460010. The authors are grateful to the CBS culture collection at the Westerdijk Fungal Biodiversity Institute, the curator Gerard Verkleij and the technical staff Bart Kraak, Trix Merkx, Diana Vos, Yda Vlug and Arien van Iperen for providing strains from the culture collection, for their help in the loan of herbarium specimens and for the deposit of herbarium material. We thank Wen-Tao Li in Utrecht University for his kind help in cloning work. We would also like to thank Marc Stadler at Department Microbial Drugs, Helmholtz Centre for Infection Research for his kind providing with their unpublished strain G2B. We acknowledge Markéta Šandová in the PRM Herbarium, Ondrei Kokoul in the PRC Herbarium, Genevieve E. Tocci in FH (Harvard University Herbaria), Robert Vogt in B (Botanischer Garten und Botanisches Museum Berlin-Dahlem), Uwe Braun in HAL (Martin-Luther-Universität, Halle-Wittenberg, Halle, Germany) and the Kew Garden for their kind help in searching the type specimens we needed for this study.
Footnotes
Peer review under responsibility of Westerdijk Fungal Biodiversity Institute.
Contributor Information
X.W. Wang, Email: wangxw@im.ac.cn.
J. Houbraken, Email: j.houbraken@wi.knaw.nl.
References
- Alexopoulos C.J., Mims C.W., Blackwell M. 4th Edition. John Wiley & Sons, Inc.; New York: 1996. Introductory Mycology. [Google Scholar]
- Apinis A.E. Occurrence of thermophilous microfungi in certain alluvial soils near Nottingham. Nova Hedwigia. 1963;5:57–78. [Google Scholar]
- Badali H., Chander J., Gupta A. Fatal cerebral phaeohyphomycosis in an immunocompetent individual due to Thielavia subthermophila. Journal of Clinical Microbiology. 2011;49:2336–2341. doi: 10.1128/JCM.02648-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr M.E. Prodromus to nonlichenized pyrenomycetous member of class Hymenoascomycetes. Mycotaxon. 1990;39:43–184. [Google Scholar]
- Barron G.L. Williams & Wilkins Co.; Baltimore: 1968. The genera of Hyphomycetes from soil. [Google Scholar]
- Bell A., Mahoney D.P. Coprophilous fungi in New Zealand. II. Podospora species with coriaceous perithecia. Mycologia. 1997;89:908–915. [Google Scholar]
- Berka R.M., Grigoriev I.V., Otillar R. Comparative genomic analysis of the thermophilic biomass-degrading fungi Myceliophthora thermophila and Thielavia terrestris. Nature Biotechnology. 2011;29:922–927. doi: 10.1038/nbt.1976. [DOI] [PubMed] [Google Scholar]
- Booth C. Studies of Pyrenomyectes: VI. Thielavia, with notes on some allied genera. Mycological Papers. 1961;83:1–15. [Google Scholar]
- Boucher C., Nguyen T.-S., Silar P. Species delimitation in the Podospora anserina / P. pauciseta / P. comata species complex (Sordariales) Cryptogamie, Mycologie. 2017;38:485–506. [Google Scholar]
- Braun U. Annotated list of taxonomic novelties published in "Klotzschii Herbarium Vivum Mycologicum, Editio Nova" issued by G. L. Rabenhorst between 1855 and 1858. Schlechtendalia. 2018;35:1–43. [Google Scholar]
- Cai L., Jeewon R., Hyde K.D. Phylogenetic evaluation and taxonomic revision of Schizothecium based on ribosomal DNA and protein coding genes. Fungal Diversity. 2005;19:1–21. [Google Scholar]
- Cai L., Jeewon R., Hyde K.D. Molecular systematics of Zopfiella and allied genera: evidence from multi-gene sequence analyses. Mycological Research. 2006;110:359–368. doi: 10.1016/j.mycres.2006.01.007. [DOI] [PubMed] [Google Scholar]
- Cain R.F. Studies of coprophilous ascomycetes VIII. new species of Podospora. Canadian Journal of Botany. 1962;40:447–490. [Google Scholar]
- Cannon P.F., Hawksworth D.L. A re-evaluation of Melanospora Corda and similar Pyrenomycetes, with a revision of the British species. Botanical Journal of the Linnean Society. 1982;84:115–160. [Google Scholar]
- Carmarán C.C., Berretta M., Martínez S. Species diversity of Cladorrhinum in Argentina and description of a new species, Cladorrhinum australe. Mycological progress. 2015;14:94. [Google Scholar]
- Cesati V. Explicatio Iconum. Hedwigia. 1856;1:103–104. [Google Scholar]
- Cooke J.C. Variation in isolates of Chaetomium trilaterale. Mycologia. 1973;65:1212–1220. [PubMed] [Google Scholar]
- Corda A.C.J. Vol. 2. 1838. pp. 1–43. (Icones fungorum hucusque cognitorum). [Google Scholar]
- Dal Vesco G., Peyronel B. Funghi isolati dal suolo di due isole del Pacifico meridionale. Allionia. 1968;14:31–49. [Google Scholar]
- de Beer Z.W., Duong T.A., Barnes I. Redefining Ceratocystis and allied genera. Studies in Mycology. 2014;79:187–219. doi: 10.1016/j.simyco.2014.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vries R.P., Benoit I., Doehlemann G. Post-genomic approaches to understanding interactions between fungi and their environment. IMA Fungus. 2011;2:81–86. doi: 10.5598/imafungus.2011.02.01.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edward J.C. A new genus of the Moniliaceae. Mycologia. 1961;51:781–786. [Google Scholar]
- Eriksson O.E., Hawksworth D.L. Outline of the ascomycetes. Systema Ascomycetum. 1993;12:51–257. [Google Scholar]
- Eriksson O.E., Baral H.O., Currah R.S. Outline of Ascomycota - 2004. Myconet. 2004;10:1–99. [Google Scholar]
- Carbone I., Kohn L.M. A method for designing primer sets for speciation studies in filamentous ascomycetes. Mycologia. 1999;91:553–556. [Google Scholar]
- Gao J., Huang J.W., Qian L.Q. Characterization and crystal structure of a thermostable glycosidehydrolase family 45 1,4-β-endoglucanase from Thielavia terrestris. Enzyme and Microbial Technology. 2017;99:32–37. doi: 10.1016/j.enzmictec.2017.01.005. [DOI] [PubMed] [Google Scholar]
- García-Huante Y., Cayetano-Cruz M., Santiago-Hernández A. The thermophilic biomass-degrading fungus Thielavia terrestris Co3Bag1 produces a hyperthermophilic and thermostable β-1,4-xylanase with exo- and endo-activity. Extremophiles. 2017;21:175–186. doi: 10.1007/s00792-016-0893-z. [DOI] [PubMed] [Google Scholar]
- Gilman J.C., Abbott E.V. A summary of the soil fungi. Iowa State College Journal of Science. 1927;1:225–343. [Google Scholar]
- Greif M.D., Stchigel A.M., Huhndorf S.M. A re-evaluation of genus Chaetomidium based on molecular and morphological characters. Mycologia. 2009;101:554–564. doi: 10.3852/08-200. [DOI] [PubMed] [Google Scholar]
- Grum-Grzhimaylo A.A., Georgieva M.L., Bondarenko S.A. On the diversity of fungi from soda soils. Fungal Diversity. 2016;76:27–74. [Google Scholar]
- Guarro J., Cano J. The genus Triangularia. Transactions of the British Mycological Society. 1988;91:587–591. [Google Scholar]
- Guarro J., Gene J., Stchigel A.M. CBS Biodiversity Series 10. CBS-KNAW Fungal Biodiversity Centre; Utrecht, The Netherlands: 2012. Atlas of Soil Ascomycetes. [Google Scholar]
- Guarro J., Cannon P.F., van der AA H.A. A synopsis of the genus Zopfiella (ascomycetes, Lasiosphaeriaceae) Systema Ascomycetum. 1991;10:79–112. [Google Scholar]
- Han Z., Li Y.X., Liu L.L. Thielavins W–Z7, new antifouling thielavins from the marine-derived fungus Thielavia sp. UST030930-004. Marine Drugs. 2017;15:128. doi: 10.3390/md15050128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harveson R.M. University of Florida; Gainesville: 1999. Evaluation of the parasitic relationship of Melanospora and other allied genera with Fusarium oxysporum. PhD thesis. [Google Scholar]
- Hawksworth D.L., Sutton B.C., Ainsworth G.C. 7th ed. CAB International; Wallingford: 1983. Ainsworth & Bisby’s Dictionary of the Fungi. [Google Scholar]
- Huhndorf S.M., Miller A.M., Fernández F.A. Molecular systematics of the Sordariales: the order and the family Lasiosphaeriaceae redefined. Mycologia. 2004;96:368–387. [PubMed] [Google Scholar]
- Ito T., Nakagiri A. Stellatospora, a new genus of the Sordariaceae. Mycoscience. 1994;35:413–415. [Google Scholar]
- Jeffries P., Young T.W.K. CAB International; Wallingford: 1994. Interfungal Parasitic Relationships. [Google Scholar]
- Kallio J.P., Gasparetti C., Andberg M. Crystal structure of an ascomycete fungal laccase from Thielavia arenaria – common structural features of asco-laccases. FEBS Journal. 2011;278:2283–2295. doi: 10.1111/j.1742-4658.2011.08146.x. [DOI] [PubMed] [Google Scholar]
- Katoh K., Standley D.M. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Molecular Biology and Evolution. 2013;30:772–780. doi: 10.1093/molbev/mst010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitahara N., Endo A., Furuya K. Thielavin A and B, New inhibitors of prostaglandin biosynthesis produced by Thielavia terricola. The Journal of Antibiotics. 1981;34:1562–1568. doi: 10.7164/antibiotics.34.1562. [DOI] [PubMed] [Google Scholar]
- Kirk P.M., Cannon P.F., Minter D.W. 10th ed. CABI; UK: 2008. Ainsworth & Bisby's Dictionary of the Fungi. [Google Scholar]
- Kruys Å., Huhndorf S.M., Miller A.N. Coprophilous contributions to the phylogeny of Lasiosphaeriaceae and allied taxa within Sordariales (Ascomycota, Fungi) Fungal Diversity. 2015;70:101–113. [Google Scholar]
- Liang Z.Q., Han Y.F., Chu H.L. Three prospectively applicable species of Paecilomyces from soils in China. Journal of Fungal Research. 2006;4:45–48. [Google Scholar]
- Liang Z.Q., Han Y.F., Chu H.L. Studies on the genus Paecilomyces in China IV. Two new species of Paecilomyces with monophialides. Mycotaxon. 2006;97:13–20. [Google Scholar]
- Liang Z.Q., Han Y.F., Chu H.L. A new thermotolerant Paecilomyces species that produces laccase and a biform sporogenous structure. Fungal Diversity. 2007;27:95–102. [Google Scholar]
- Liang Z.Q., Han Y.F., Chu H.L., Fox R.T.V. Studies on the genus Paecilomyces in China V. Taifanglaniagen. nov. for some monophialidic species. Fungal Diversity. 2009;34:69–77. [Google Scholar]
- Lodha B.C. Studies on coprophilous fungi. The Journal of the Indian Botanical Society. 1964;43:121–140. [Google Scholar]
- Lodhi S.A., Mirza J.H. A new genus of the Eurotiales. Mycologia. 1962;54:217–219. [Google Scholar]
- Lu H., Zhang H.T., Shi P.J. A family 5 β-mannanase from the thermophilic fungus Thielavia arenaria XZ7 with typical thermophilic enzyme features. Applied Microbiology and Biotechnology. 2013;97:8121–8128. doi: 10.1007/s00253-012-4656-1. [DOI] [PubMed] [Google Scholar]
- Luangsa-ard J.J., Hywel-Jones N.L., Samson R.A. The polyphyletic nature of Paecilomyces sensu lato based on 18S-generated rDNA phylogeny. Mycologia. 2004;96:773–780. doi: 10.1080/15572536.2005.11832925. [DOI] [PubMed] [Google Scholar]
- Luttrell E.S. Taxonomy of the pyrenomycetes. University of Missouri Studies. 1951;24:1–120. [Google Scholar]
- Malloch D., Cain R.F. New cleistothecial Sordariaceae and a new family, Coniochaetaceae. Canadian Journal of Botany. 1971;49:869–880. [Google Scholar]
- Maharachchikumbura S.N., Hyde K.D., Jones E.B.G. Towards a natural classification and backbone tree for Sordariomycetes. Fungal Diversity. 2015;72:199–301. [Google Scholar]
- Maharachchikumbura S.N., Hyde K.D., Jones E.B.G. Families of Sordariomycetes. Fungal Diversity. 2016;79:1–317. [Google Scholar]
- Malloch D., Cain R.F. The genus Thielavia. Mycologia. 1973;65:1055–1077. [Google Scholar]
- Marchal E. Champignons coprophiles de Belgique. Bulletin de la Société Royale de Botanique de Belgique. 1885;24:57–77. [Google Scholar]
- Marin-Felix Y., Guarro J., Cano-Lira J.F. Melanospora (Sordariomycetes, Ascomycota) and its relatives. MycoKeys. 2018;44:81–122. doi: 10.3897/mycokeys.44.29742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matushima T. Published by the Author, Kobe; 1971. Microfungi of the Solomon Islands and Papua-New Guinea; p. 42. [Google Scholar]
- Mbenoun M., de Beer Z.W., Wingfield M.J. Reconsidering species boundaries in the Ceratocystis paradoxa complex, including a new species from oil palm and cacao in Cameroon. Mycologia. 2014;106:757–784. doi: 10.3852/13-298. [DOI] [PubMed] [Google Scholar]
- McCormick F.A. Perithecia of Thielavia basicola Zopf in culture and the stimulation of their production by extracts from other fungi. Bulletin of Connecticut Agricultural Experiment Station. 1925;269:539–554. [Google Scholar]
- Miller A.N., Huhndorf S.M. Neotropical ascomycetes 10. New and interesting Cercophora species. Sydowia. 2001;53:211–226. [Google Scholar]
- Miller A.N., Huhndorf S.M. Multi-gene phylogenies indicate ascomal wall morphology is a better predictor of phylogenetic relationships than ascospore morphology in the Sordariales (Ascomycota, Fungi) Molecular Phylogenetics and Evolution. 2005;35:60–75. doi: 10.1016/j.ympev.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Miller M.A., Pfeiffer W., Schwartz T. Proceedings of the Gateway Computing Environments Workshop (GCE), November 14, 2010, New Orleans, Louisiana. 2010. Creating the CIPRES Science Gateway for inference of large phylogenetic trees; pp. 1–8. [Google Scholar]
- Mirza J.H., Cain R.F. Revision of the genus Podospora. Canadian Journal of Botany. 1969;47:1999–2048. [Google Scholar]
- Mouchacca J. Les Thielavia des sols arides: epsèces nouvelles et analyse générique. Bulletin trimestriel de la Société mycologique de France. 1973;89:295–311. [Google Scholar]
- Mouchacca J., Gams W. The hyphomycete genus Cladorrhinum and its teleomorph connections. Mycotaxon. 1993;48:415–440. [Google Scholar]
- Moustafa A.F., Abdel-Azeem A.M. Thielavia gigaspora, a new thermotolerant ascomycete from Egypt. Microbiological Research. 2008;163:441–444. doi: 10.1016/j.micres.2006.07.008. [DOI] [PubMed] [Google Scholar]
- Mtibaà R., Olicón-Hernández D.R., Pozo C. Degradation of bisphenol A and acute toxicity reduction by different thermo-tolerant ascomycete strains isolated from arid soils. Ecotoxicology and Environmental Safety. 2018;156:87–96. doi: 10.1016/j.ecoenv.2018.02.077. [DOI] [PubMed] [Google Scholar]
- Nel W.J., Duong T.A., Wingfield B.D. A new genus and species for the globally important, multihost root pathogen Thielaviopsis basicola. Plant Pathology. 2018;67:871–882. [Google Scholar]
- Nylander J.A.A. Uppsala University; 2004. MrModeltest v. 2. Programme distributed by the author. Evolutionary Biology Centre. [Google Scholar]
- Perdomo H., García D., Gené J. Phialemoniopsis, a new genus of Sordariomycetes, and new species of Phialemonium and Lecythophora. Mycologia. 2013;105:398–421. doi: 10.3852/12-137. [DOI] [PubMed] [Google Scholar]
- Qadri M., Rajput R., Abdin M.Z. Diversity, molecular phylogeny, and bioactive potential of fungal endophytes associated with the Himalayan blue pine (Pinus wallichiana) Microbial Ecology. 2014;67:877–887. doi: 10.1007/s00248-014-0379-4. [DOI] [PubMed] [Google Scholar]
- Rambaut A. 2009. FigTree v. 1.3.1.http://tree.bio.ed.ac.uk/software/ Computer program and documentation distributed by the author at. [Google Scholar]
- Ronquist F., Teslenko M., van der Mark P. MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Systematic Biology. 2012;61:539–542. doi: 10.1093/sysbio/sys029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saccardo P.A. Vols.1–26. 1882-1931. (Sylloge Fungorum). Pavia: Saccardo PA. Johnson. New York. [Google Scholar]
- Sambrook J., Fritsch E.F., Maniatis T. 2nd ed. Cold Spring Harbor, Cold Spring Laboratory Press; New York: 1989. Molecular cloning. A laboratory manual. [Google Scholar]
- Samson R.A., Mahmood T. The genus Acrophialophora (Fungi, Moniliales) Acta Botanica Neerlandica. 1970;19:804–808. [Google Scholar]
- Samson R.A., Crisman J.M., Tansey M.R. Observations on the thermophilous ascomycete Thielavia terrestris. Transactions of the British Mycological Society. 1977;69:417–423. [Google Scholar]
- Schultes N.P., Murtishi B., Li D.W. Phylogenetic relationships of Chlamydomyces, Harzia, Olpitrichum, and their sexual allies, Melanospora and Sphaerodes. Fungal Biology. 2017;121:890–904. doi: 10.1016/j.funbio.2017.07.004. [DOI] [PubMed] [Google Scholar]
- Silar P. Podospora anserina: from laboratory to biotechnology. In: Horwitz B.A., Mala Mukherjee P.K.M., Kubicek C.P., editors. Genomics of Soil – and Plant-Associated Fungi. Springer; Heidelberg: 2013. pp. 283–309. [Google Scholar]
- Srivastava M.P., Tandon R.N., Bhargava S.N., Ghosh A.K. Studies on fungal diseases of some tropical fruits. IV. Some new fungi. Mycopathologia et Mycologia Applicata. 1966;30:203–208. [Google Scholar]
- Stamatakis A. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics. 2014;30:1312–1313. doi: 10.1093/bioinformatics/btu033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stchigel A.M., Figuera L., Cano J. New species of Thielavia, with a molecular study of representative species of the genus. Mycological Research. 2002;106:975–983. [Google Scholar]
- Stchigel A.M., Guarro J., Cormack W.M. Apiosordaria antarctica and Thielavia antarctica, two new ascomycetes from Antarctica. Mycologia. 2003;95:1218–1226. doi: 10.1080/15572536.2004.11833030. [DOI] [PubMed] [Google Scholar]
- Syed K., Shale K., Nazir K.H.M.N.H. Genome-wide identification, annotation and characterization of novel thermostable cytochrome P450 monooxygenases from the thermophilic biomass-degrading fungi Thielavia terrestris and Myceliophthora thermophila. Genes & Genomics. 2014;36:321–333. [Google Scholar]
- Tamura K., Stecher G., Peterson D. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Molecular Biology and Evolution. 2013;30:2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor J.W., Jacobson D.J., Kroken S. Phylogenetic species recognition and species concepts in fungi. Fungal Genetics and Biology. 2000;31:21–32. doi: 10.1006/fgbi.2000.1228. [DOI] [PubMed] [Google Scholar]
- Theoulakis P., Goldblum D., Zimmerli S. Keratitis resulting from Thielavia subthermophila Mouchacca. Cornea. 2009;28:1067–1069. doi: 10.1097/ICO.0b013e31819717f4. [DOI] [PubMed] [Google Scholar]
- Traversog B. Flora italica Cryptogama. Pyrenomycetinae. 1907;2:420–470. [Google Scholar]
- Udagawa S., Muroi T. Coprophilous pyrenomycetes from Japan V. Transactions of the Mycological Society of Japan. 1979;20:453–468. [Google Scholar]
- Udagawa S., Sugiyama Y. Additions to the interesting species of Ascomycetes from imported spices. Transactions of the Mycological Society of Japan. 1981;22:197–212. [Google Scholar]
- van den Brink J., Facun K., de Vries M. Thermophilic growth and enzymatic thermostability are polyphyletic traits within Chaetomiaceae. Fungal Biology. 2015;119:1255–1266. doi: 10.1016/j.funbio.2015.09.011. [DOI] [PubMed] [Google Scholar]
- van den Brink J., Samson R.A., Hagen F. Phylogeny of the industrial relevant, thermophilic genera Myceliophthora and Corynascus. Fungal Diversity. 2012;52:197–207. [Google Scholar]
- von Arx J.A. Cramer; Lehre, Germany: 1967. Pilzkunde. 1–356. [Google Scholar]
- von Arx J.A., Gams W. Űber Pleurage verruculosa und die zugehörige Cladorrhinum-Konidienform. Nova Hedwigia. 1967;13:199–208. [Google Scholar]
- von Arx J.A., Mahmood T. Thielaviella humicolagen. et sp. nov. from Pakistan. Transactions of the British Mycological Society. 1968;51:611–613. [Google Scholar]
- von Arx J.A. Ostiolate and nonostiolate pyrenomycetes. Proceedings of the Koninklijke Nederlandse Akademie van Wetenschappen, ser. C. 1973;76:289–296. [Google Scholar]
- von Arx J.A. On Thielavia and some similar genera of ascomycetes. Studies in Mycology. 1975;8:1–32. [Google Scholar]
- von Arx J.A. Canariomyces notabilis, a peculiar ascomycete from the Canary Isolands. Persoonia. 1984;12:185–187. [Google Scholar]
- von Arx J.A., Guarro J., Figueras M.J. The ascomycete genus Chaetomium. Beihefte zur Nova Hedwigia. 1986;84:1–162. [Google Scholar]
- von Arx J.A., Figueras M.J., Guarro J. Sordariaceous ascomycetes without ascospore ejaculation. Beiheft zur Nova Hedwigia. 1988;94:1–104. [Google Scholar]
- Vu D., Groenewald M., de Vries M. Large-scale generation and analysis of filamentous fungal DNA barcodes boosts coverage for kingdom fungi and reveals thresholds for fungal species and higher taxon delimitation. Studies in Mycology. 2019;92:135–154. doi: 10.1016/j.simyco.2018.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X.W., Houbraken J., Groenewald J.Z. Diversity and taxonomy of Chaetomium and chaetomium-like fungi from indoor environments. Studies in Mycology. 2016;84:145–224. doi: 10.1016/j.simyco.2016.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X.W., Lombard L., Groenewald J.Z. Phylogenetic reassessment of the Chaetomium globosum species complex. Persoonia. 2016;36:83–133. doi: 10.3767/003158516X689657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X.W., Yang F.Y., Meijer M. Redefining Humicola sensu stricto and related genera in the Chaetomiaceae. Studies in Mycology. 2019;93:65–153. doi: 10.1016/j.simyco.2018.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteside W.C. Morphological studies in the Chaetomiaceae. II. Ascotricha and Chaetomidium. Mycologia. 1962;54:152–159. [Google Scholar]
- Woon J.S.K., Mackeen M.M., Sudin A.H. Production of an oligosaccharide-specific cellobiohydrolase from the thermophilic fungus Thielavia terrestris. Biotechnology Letters. 2016;38:825–832. doi: 10.1007/s10529-016-2045-z. [DOI] [PubMed] [Google Scholar]
- Wu B., Tian J.Q., Wang L. Apiosordaria hamatasp. nov. from lake sediment in China. Mycotaxon. 2016;131:847–857. [Google Scholar]
- Xu H.B., Yan Q.J., Duan X.J. Characterization of an acidic cold-adapted cutinase from Thielavia terrestris and its application in flavor ester synthesis. Food Chemistry. 2015;188:439–445. doi: 10.1016/j.foodchem.2015.05.026. [DOI] [PubMed] [Google Scholar]
- Zhang N., Blackwell M. Molecular phylogeny of Melanospora and similar pyrenomycetous fungi. Mycological Research. 2002;106:148–155. [Google Scholar]
- Zhang N., Castlebury L.A., Miller A.N. An overview of the systematics of the Sordariomycetes based on four-gene phylogeny. Mycologia. 2006;98:1077–1088. doi: 10.3852/mycologia.98.6.1076. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Liu F., Wu W. A phylogenetic assessment and taxonomic revision of the thermotolerant hyphomycete genera Acrophialophora and Taifanglania. Mycologia. 2015;107:768–779. doi: 10.3852/14-173. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Wu W., Cai L. Polyphasic characterisation of Chaetomium species from soil and compost revealed high number of undescribed species. Fungal Biology. 2017;121:21–43. doi: 10.1016/j.funbio.2016.08.012. [DOI] [PubMed] [Google Scholar]
- Zopf W. Thielavia basicola Zopf. Genus novum Perisporiacearum. Verhandlungen des Botanischen Vereins für die Provinz Brandenburg. 1876;18:101–105. [Google Scholar]
- Zopf W. Zur Entwicklungsgeschichte der Ascomyceten: Chaetomium. Nova Acta der Kaiserlich Leopoldinisch-Carolinisch Deutschen Akademie der Naturforscher. 1881;42:199–292. [Google Scholar]