Abstract
The vault ribonucleoprotein (RNP), comprising vault RNA (vtRNA) and telomerase-associated protein 1 (TEP1), is found in many eukaryotes. However, previous studies of vtRNAs, for example in mammalian cells, have failed to reach a definitive conclusion about their function. vtRNAs are related to Y RNAs, which are complexed with Ro protein and influence Ro's function in noncoding RNA (ncRNA) quality control and processing. In Trypanosoma brucei, the small noncoding TBsRNA-10 was first described in a survey of the ncRNA repertoire in this organism. Here, we report that TBsRNA-10 in T. brucei is a vtRNA, based on its association with TEP1 and sequence similarity to those of other known and predicted vtRNAs. We observed that like vtRNAs in other species, TBsRNA-10 is transcribed by RNA polymerase III, which in trypanosomes also generates the spliceosomal U-rich small nuclear RNAs. In T. brucei, spliced leader (SL)-mediated trans-splicing of pre-mRNAs is an obligatory step in gene expression, and we found here that T. brucei's vtRNA is highly enriched in a non-nucleolar locus in the cell nucleus implicated in SL RNP biogenesis. Using a newly developed permeabilized cell system for the bloodstream form of T. brucei, we show that down-regulated vtRNA levels impair trans-spliced mRNA production, consistent with a role of vtRNA in trypanosome mRNA metabolism. Our results suggest a common theme for the functions of vtRNAs and Y RNAs. We conclude that by complexing with their protein-binding partners TEP1 and Ro, respectively, these two RNA species modulate the metabolism of various RNA classes.
Keywords: RNA metabolism, RNA processing, RNA splicing, RNA, RNA-binding protein, Ro protein, spliced leader (SL), telomerase-associated protein 1 (TEP1), Trypanosoma brucei, vault RNA
Introduction
Vault RNAs (vtRNAs)3 are small noncoding RNAs first identified as components of the large barrel-shaped cytoplasmic vault structures in eukaryotes (1). vtRNAs are transcribed by RNA polymerase III (pol III) (2) and vary in length from 80 to 140 nt (3). Each vtRNA forms a stable RNP particle with the protein TEP1 (4, 5), and only a fraction of this complex is associated in sub-stoichiometric amounts with the barrel-shaped vault assemblies (6). The vaults are formed by multiple copies of the major vault protein (MVP) (7, 8), the building block monomer of the vault particle, which, in addition to the vtRNA/TEP1 RNP, also associates with an ADP-ribose polymerase (9). There appears to be a correlation between the presence of the MVP gene and the occurrence of TEP1 and vtRNA genes in the genomes of eukaryotes, possibly indicating that the vault particles may have an indirect role in sequestering TEP1 and vtRNAs. Notable examples of eukaryotes lacking MVP, vtRNA, and TEP1 are plants, fungi, insects, and nematodes. Like the cellular role of the vaults, the molecular function of vtRNAs has remained enigmatic. Complicating the interrogation of vtRNA function in higher eukaryotes stems from the observations that the vault RNP (vtRNA and TEP1) is not essential for mammals (10, 11), and the levels of vtRNAs are elevated only in mammalian cells that are experiencing suboptimal growth conditions, such as viral infections (12–14), suggesting a possible role for the mammalian vtRNA in cellular stress responses. A multitude of functions has been attributed to mammalian vtRNAs, such as: (i) modulating multidrug resistance of cancer cells (6, 15–17); (ii) protecting cells from undergoing apoptosis (13); (iii) regulating autophagy (18); and (iv) modulating cancer cell growth by interaction with protein kinase R (14, 19, 20). vtRNAs were also proposed to serve as a substrate for microRNA production (21–23) and to be involved in intercellular communication via extracellular vesicles (24–27). Because most of these roles have been attributed to only a single vtRNA out of the four present in human cells, it is unlikely that they represent the general primary molecular function of this noncoding RNA family.
The protein directly contacting vtRNAs is TEP1 (5), which was initially discovered to be associated with telomerase RNP (28). However, it was later demonstrated that TEP1 is not essential for telomerase function, because its knockout in mice does not lead to any noticeable changes in telomere dynamics (10, 11). TEP1 contains a TROVE domain (29), which is a ring-shaped RNA-binding module also found in Ro proteins in eukaryotes and bacteria (30–32). Ro and TEP1 are the only known proteins that contain the TROVE domain, and although very little is known about TEP1's function, data obtained for Ro indicate involvement in RNA metabolism. Ro associates with Y RNAs (33), a separate group of small noncoding RNAs with similarities to vault RNAs. Ro proteins in bacteria and eukaryotes have been shown to participate in RNA processing and quality control (34–37), and current models suggest that Y RNAs regulate the function of the protein and serve as a tether to facilitate larger-order complex assembly (38, 39). The interaction between Ro and Y RNAs depends on a short region in the terminal bulged helix of the RNAs (30, 40). Moreover, similarities in the predicted secondary structures of Y RNAs and vtRNAs hint that the TROVE domain of TEP1 makes contact with a similar region in the bulged terminal stem of vtRNAs, although this has not been demonstrated experimentally.
Here, we identified and characterized the vtRNA in an early diverging branch of the eukaryotic tree, namely trypanosomatids. Trypanosoma brucei is a member of this branch and is the parasite causing African trypanosomiasis (sleeping sickness) in humans and similar diseases in animals (41). Like all trypanosomatids, it relies on spliced leader (SL) trans-splicing to produce mature mRNAs from long polycistronic precursors (42–44). We found that the vtRNA in T. brucei affects the production of trans-spliced mRNAs, and our data suggest a similarity between the function of the vtRNA in trypanosomatids and the functions of Y RNAs in other species in controlling the metabolism of RNA molecules.
Results
TBsRNA-10 is the trypanosome vtRNA
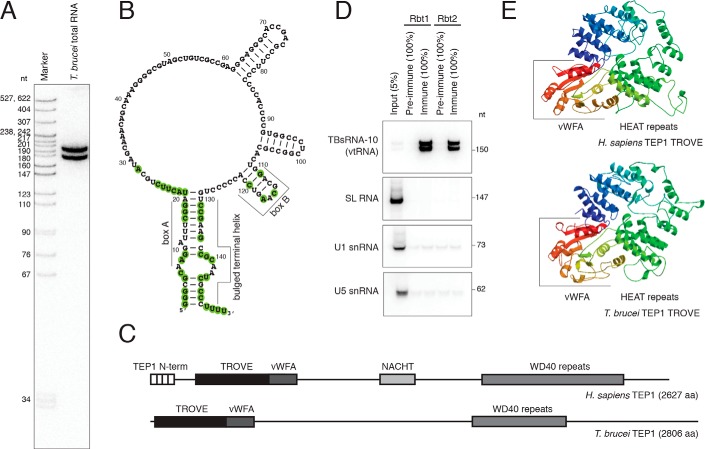
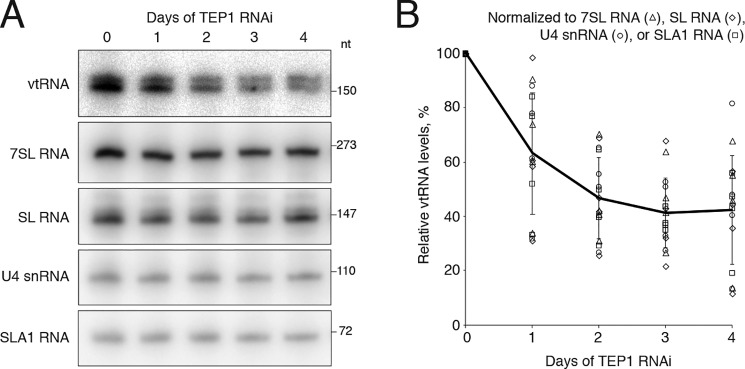
An abundant noncoding RNA, named TBsRNA-10 (Fig. 1, A and B), was discovered in a high-throughput sequencing survey of T. brucei small RNAs (45). It was shown to be more abundant in the bloodstream than the procyclic form trypanosomes, to be highly enriched in a non-nucleolar focus in the cell nucleus, and to distribute during sucrose gradient RNP fractionation in a pattern that is different from that of small RNPs involved in rRNA processing and modification (45). The sequence of TBsRNA-10 (Fig. 1B) has the potential to form a secondary structure that is reminiscent of Y RNAs and vtRNAs from a broad range of species. Because Y RNAs and vtRNAs associate with Ro and TEP1 proteins, respectively, to achieve stability (5, 11, 33), we searched the T. brucei genome (46) for orthologs of both proteins. T. brucei does not appear to contain a gene coding for Ro; however, a gene (Tb927.7.830) encoding a protein that shares homology with TEP1 is present (Fig. 1C). Because TEP1 associates with vtRNAs in mammalian cells and is required for their stability (11), we tested whether the trypanosome protein is complexed with TBsRNA-10. Because attempts to tag the endogenous locus of TEP1 at either the N or C terminus were unsuccessful, we raised antibodies against a C-terminal peptide and performed immunoprecipitations from a whole-cell extract. TBsRNA-10 was pulled down very efficiently by the anti-TEP1 serum, but not by pre-immune serum, in contrast to three snRNAs (SL, U1, and U5) used as controls (Fig. 1D). The efficiency of immunoprecipitation of vtRNA by anti-TEP1 sera varied between different experiments; however, it was often close to 100%, indicating that vtRNA is likely quantitatively bound by TEP1. The apparent lack of the Ro gene in trypanosomes and the ability of anti-TEP1 serum to precipitate TBsRNA-10 provided strong evidence for the identity of this ncRNA as T. brucei vtRNA. Modeling of the TROVE domain of human and T. brucei TEP1 on the available structure of the Xenopus laevis Ro60 protein (30) revealed the characteristic toroidal shape of this RNA-binding module, composed of HEAT repeats (Fig. 1E). Both the trypanosome TEP1 and human TEP1 appear to contain the von Willebrand factor A (vWFA) domain present in Ro proteins (Fig. 1E). However, the highly-conserved metal ion-dependent adhesion site, MIDAS, motif for binding protein ligands (DXSXS … T … D) does not appear to be conserved in either the T. brucei (Fig. S1) or the human protein. T. brucei TEP1 is of similar size (2,806 aa) as human TEP1 (2,627 aa) and, in addition to the TROVE module, contains WD40 repeats like TEP1 from other species (Fig. 1C). The TEP1 N-terminal repeats are absent in the trypanosome TEP1, and the NACHT domain (an NTPase module) is not obvious. The protein is even larger in the related kinetoplastid Leishmania, 4,193 aa in Leishmania braziliensis. Silencing of TEP1 in procyclic cells using a stem–loop RNAi construct resulted in modest inhibition of growth (Fig. S2), accompanied by gradual reduction in the amount of vtRNA present in the cells (Fig. 2, A and B), without a major effect on the levels of SL, U4, SLA1, and 7SL RNAs (Fig. 2A). Similar findings of TEP1 being required for the stability of vtRNA have been reported for mammalian cells (11). This observation provided additional evidence that TBsRNA-10 is indeed vtRNA.
Figure 1.
TBsRNA-10 is the T. brucei vtRNA. A, Northern blot analysis of total T. brucei RNA probed with 32P-labeled antisense TBsRNA-10 oligodeoxyribonucleotide probe. Marker is a 32P-labeled pBR322 DNA MspI digest. B, predicted secondary structure of the T. brucei vtRNA. Shown as double-stranded are only the terminal helix and very stable hairpins emanating from the large internal loop. Evolutionarily conserved residues (see Fig. 3) are highlighted. C, diagram outlining the domain architecture of human and trypanosome TEP1. D, Northern blot analysis of the indicated RNAs after immunoprecipitation with anti-TEP1 sera from two rabbits (Rbt1 and Rbt2). The fractions of the input and precipitated RNA loaded are indicated with percentages. E, three-dimensional structure models for the TROVE domains of human (top) and T. brucei (bottom) TEP1 obtained with SWISS-MODEL (93) based on the crystal structure of X. laevis Ro60 protein (30). The vWFA domains are included in the models.
Figure 2.
TEP1 RNAi silencing affects vtRNA levels. A, reduction of vtRNA levels upon silencing TEP1. Cells carrying the RNAi construct for TEP1 were induced for the indicated number of days, and total RNA was isolated and subjected to Northern blot analysis with the indicated probes. B, graphical representation of the results from four independent TEP1 RNAi experiments showing the levels of vtRNA relative to 7SL, SL, U4, and SLA1 RNA. The individual data points (symbols) for all experiments are presented, and the line connects the average values ±S.D.
The vtRNA of T. brucei invariably migrated as a doublet on denaturing polyacrylamide gels (Fig. 1, A and D), and both bands exhibited changing mobility relative to molecular size markers on gels of different percentage (Fig. S3A). Likewise, a similar doublet was observed upon fractionation of an in vitro T7 RNA polymerase-transcribed vtRNA (Fig. S3B). Taken together, these data strongly suggested that the two bands correspond to two alternative structural forms of the RNA that are not completely denatured, rather than terminal extension of the slower-migrating form. Small amounts of human Y RNAs have been shown to associate with the La protein as a result of the presence of a short oligo(U) tract at the 3′ end of the RNAs (38, 47). Although trypanosomes do possess La (48), examination of publicly available high-throughput sequencing reads of RNA depleted for rRNA (49) showed that the mature form of the vtRNA in T. brucei does not contain the encoded 3′-oligo(U) stretch.
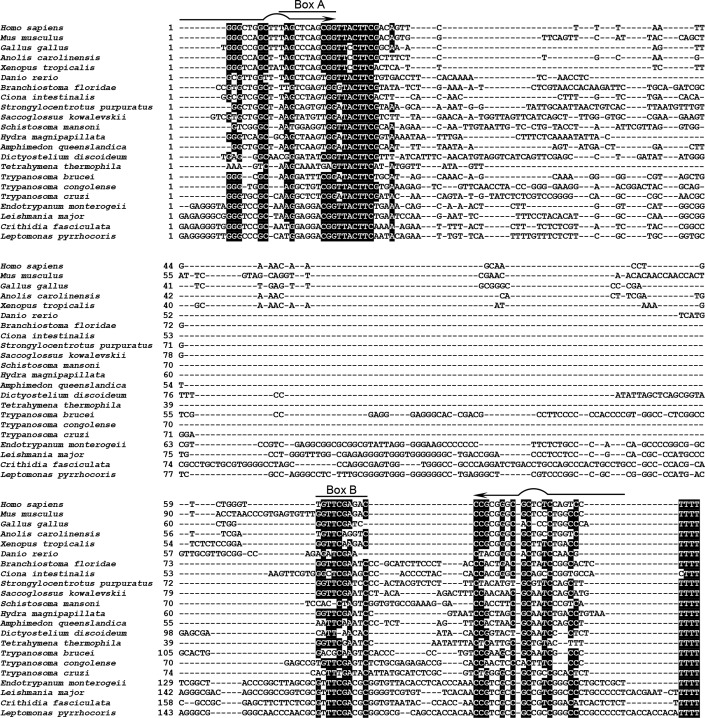
We looked for additional examples of vtRNA genes in trypanosomatid genomes using the Infernal 1.1 tools for sequence/structure bioinformatics searches (50). We identified candidate vtRNA genes in all searched trypanosomatid species (Fig. 3 and data not shown) and confirmed the expression of L. braziliensis vtRNA by Northern blotting (Fig. S4). Being ∼190 nt long, Leishmania vtRNA represents the longest known member of the vtRNA family in eukaryotes so far. Similar to its T. brucei counterpart, the Leishmania ortholog also migrated as a doublet during denaturing PAGE. A very similar property was previously observed for the Leptomonas 7SL RNA, where the migration of a doublet changed as result of changing the denaturation conditions, and this was shown to be due to two distinct structural forms (51).
Figure 3.
Sequence alignment of the newly identified vtRNAs from trypanosomatids with selected vtRNAs from other eukaryotes. Shown are the sequences of the vtRNA genes. Species with more than one vtRNA are represented by a single example. The examples from H. sapiens to S. kowalevskii are from Ref. 3, and the remaining genes are newly identified. The positions of the internal box A and box B transcription elements are indicated, and nucleotides that are at least 60% conserved are highlighted. Arrows indicate the two strands of the bulged terminal helix.
T. brucei vtRNA is transcribed by pol III
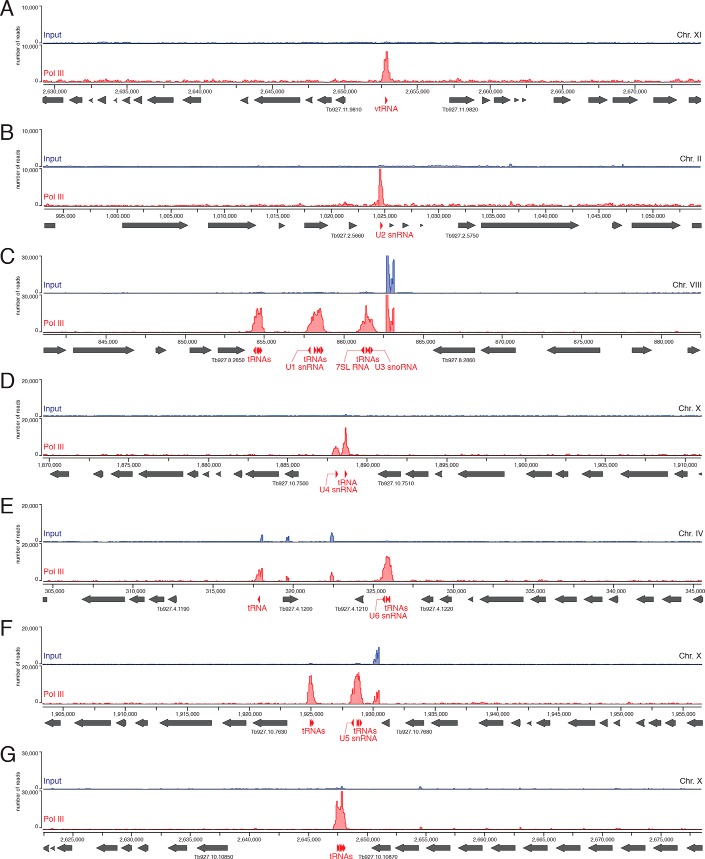
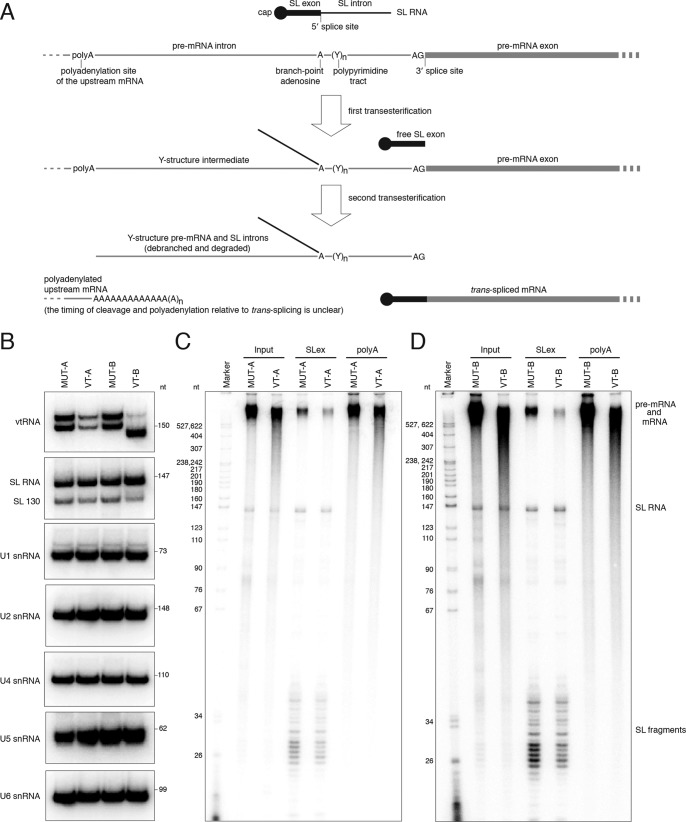
vtRNAs in other eukaryotes are transcribed by pol III, from promoters containing both external and internal sequence elements (2, 52, 53). Proximal element, PSE, and a TATA-box are positioned upstream of the transcription start site, and an A-box and one or two B-boxes are embedded in the vtRNA sequence. In trypanosomatids, pol III has been shown to transcribe not only tRNAs, 5S rRNA, 7SL RNA, U6 snRNA, and U3 snoRNA, but also the rest of the U-rich spliceosomal snRNAs, i.e. U1, U2, U4, and U5 (54, 55). Transcription of U2 snRNA depends on an internal element in close proximity to the start site and two external elements, A-box and B-box, positioned upstream of the gene and in an orientation opposing the direction of U2 transcription (54). The trypanosome vtRNA genes exhibit only limited conservation of the internal A- and B-boxes (Fig. 3). Instead, similar to the U2 snRNA gene, the T. brucei vtRNA gene has well-defined A- and B-boxes upstream of the transcribed region, in the same orientation as in the U2 gene locus, and the L. braziliensis vtRNA gene contains two sets of external upstream A- and B-box, resembling the U2 snRNA gene locus in the same organism (Fig. S5). To confirm that T. brucei vtRNA is transcribed by pol III, we performed ChIP-seq. One of the alleles for the largest subunit of pol III (Tb927.10.2780) was tagged with a Ty1 epitope (EVHTNQDPLD)–mCherry fusion, and the second allele was deleted. After formaldehyde cross-linking and immunoprecipitation of chromatin fragments with mAb against the Ty1 epitope, libraries for high-throughput sequencing were generated and processed on the Illumina platform. There was a clear enrichment of pol III–cross-linked DNA fragments at the vtRNA gene (Fig. 4A), similar to the loci of all tRNAs, snRNAs, U3 snoRNA, and 7SL RNA (Fig. 4, B–G). The height of the pol III ChIP-seq peak at the vtRNA locus was lower than tRNA peaks (Fig. 4, C–G), but similar in scale to the pol III peaks at the spliceosomal snRNAs (Fig. 4, B and D). Based on these ChIP-seq data, the similarities between upstream promoter elements in the vtRNA gene and the U2 snRNA gene and the presence of oligo(T) stretch at the 3′-end of the vtRNA gene, we conclude that T. brucei vtRNA is transcribed by pol III.
Figure 4.
T. brucei vtRNA is transcribed by RNA polymerase III. The plots show the number of ChIP-seq reads aligning to specific genomic locations for either the input (blue) or the anti-pol III-immunoprecipitated (red) material. A, shown are the chromosome regions containing the vtRNA gene; B, U2 snRNA gene; C, U1 snRNA, U3 snoRNA, 7SL RNA, ant tRNAs genes; D, U4 snRNA and a tRNA gene; E, U6 snRNA and tRNAs genes; F, U5 snRNAs and tRNAs genes; G, example with tRNA genes only. The location of the small RNA genes is indicated with red arrowheads according to their orientation. The locations of protein-coding sequences are indicated with gray arrows. Gene accession numbers are shown only for the two genes neighboring the pol III transcription units.
vtRNAs share a highly-conserved sequence element that is accessible for base-pairing interactions
Sequence alignment of vtRNAs from diverse species (Fig. 3) revealed that, as observed previously for higher eukaryotes (3), there is little overall sequence conservation of these noncoding RNAs. Well-conserved are the short sequence stretches in the 5′ and 3′ regions that form the bulged terminal helix, particularly the nucleotides flanking the small internal loop (Fig. 1B). The clustering of conserved nucleotides at the terminal stem of vtRNA suggests that, similarly to Y RNAs, which contain a highly-conserved Ro-binding site in the analogous position in the molecule, vtRNAs are likely utilizing the corresponding region for contacts with the TROVE domain of TEP1. In nontrypanosome species, the A- and B-boxes required for pol III transcription are well-conserved, but the conservation extends to kinetoplastids only for the B-box. vtRNA genes in all species possess a conserved oligo(T) stretch at the 3′ end that is required for pol III transcription termination (56, 57). Adjacent to the end of the box A element, we observed a highly-conserved sequence, 5′-UACUUC-3′, which has not been implicated as a transcriptional element. This element is also present in Deinococcus radiodurans Y RNA, but not in Y RNAs from vertebrates, which in contrast to D. radiodurans also possess vtRNAs. In general, it appears that this highly-conserved element is found in vtRNAs in all species and in Y RNAs in only those species that lack vtRNAs. Because the mode of action of many small noncoding RNAs involves base-pairing interactions with other RNAs, we tested whether this conserved sequence of T. brucei vtRNA is single-stranded and available for hybridization with nucleic acids. We used an RNase H cleavage assay with a series of oligodeoxyribonucleotides (Fig. 5A) and whole-cell T. brucei extracts as a source of native vault ribonucleoprotein particle (vtRNA/TEP1 complex). The bulged terminal helix of vtRNA, which constitutes a putative binding site for TEP1, was targeted by oligos 1 and 10, but was not susceptible to cleavage by RNase H (Fig. 5B). Two regions within the remaining portion of the RNA, targeted by oligos 2/3 and 5/6/7, were susceptible for efficient cleavage (Fig. 5B). Similar results have been previously obtained for the mammalian vtRNP (5) indicating that a couple of segments in the middle portion of the vtRNA in both trypanosomes and mammals have the potential to base pair with nucleic acids, including the highly-conserved 5′-UACUUC-3′ element. Oligo 9 yielded a cleavage pattern similar to the one elicited by oligo 7, possibly reflecting an identical sequence targeted by these two oligos, most likely the 5′-CCACCCC-3′ present twice in T. brucei vtRNA (Fig. 5, A and B).
Figure 5.
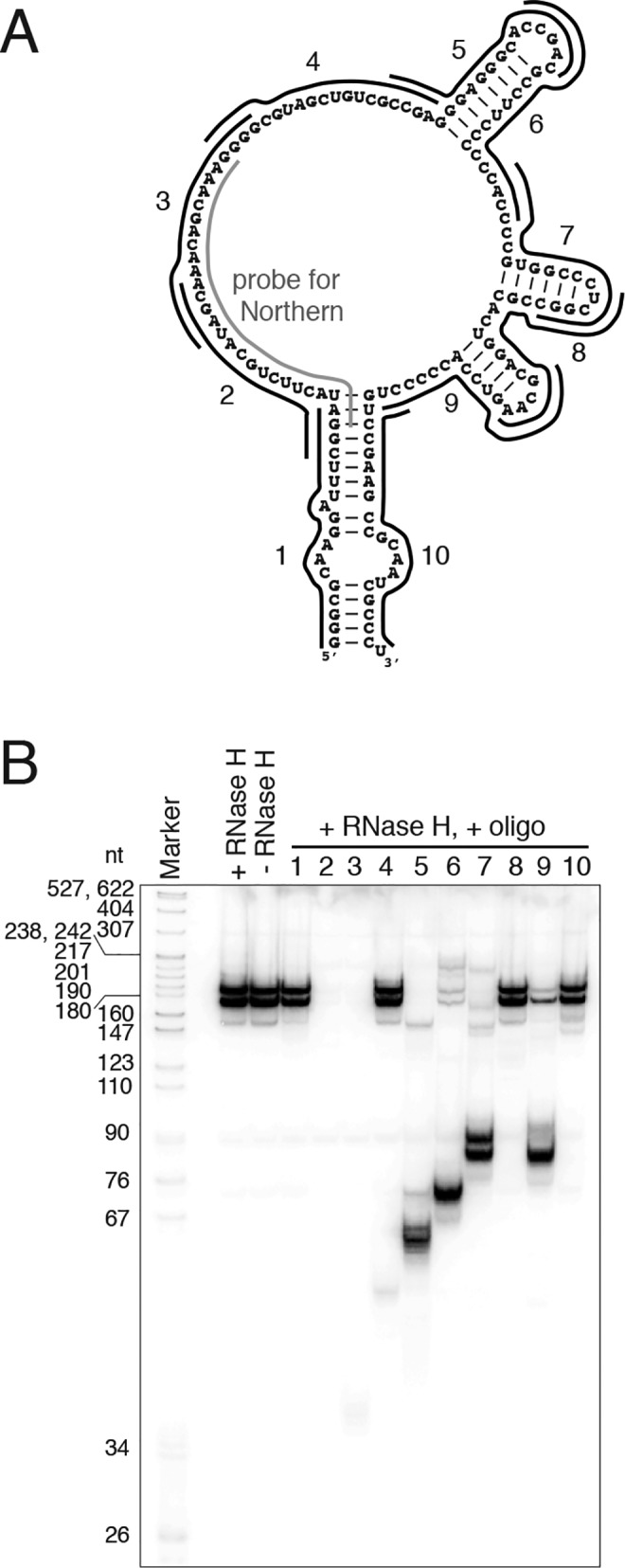
Two regions in T. brucei vtRNA are accessible for base-pairing interactions. A, diagram depicting the antisense oligodeoxyribonucleotides used in the RNase H cleavage assay. Shown is the antisense probe used for Northern blotting. B, Northern blot analysis detecting vtRNA in the samples treated with RNase H and the indicated oligos. Controls without oligo (+RNase H) and without both oligo and RNase H (−RNase H) are loaded at the beginning. Marker is a 32P-labeled pBR322 DNA MspI digest.
vtRNA in T. brucei is highly-enriched in a nuclear domain with a role in SL RNP maturation
vtRNA (TBsRNA-10) was shown previously to be enriched in an extranucleolar body in the cell nucleus (45), in contrast to the described primarily cytoplasmic localization of vtRNAs in mammalian cells (6, 12). In T. brucei, there are two known nuclear subdomains where small noncoding RNAs have been shown to accumulate. One is the nucleolus, where small nucleolar RNPs (snoRNPs) involved in the endonucleolytic processing of rRNA precursors and the 2′-O-methylation and pseudouridylation of rRNA accumulate (45, 58). The second compartment shows an enrichment of a specific subset of box C/D and box H/ACA RNPs, involved in the assembly and processing of the Sm-proteins-containing snRNP particles (59). In particular, the H/ACA RNP containing spliced-leader-associated RNA 1 (SLA1), carrying out the pseudouridylation at position 28 of the SL RNA (60), is a resident of this nuclear subdomain (59). The SLA1 RNP is part of a larger complex containing the methyltransferase TbMTr1 and a methyltransferase-associated protein (MTAP) (61), and it is required for the production of trans-splicing-competent SL RNP. MTAP is the trypanosome protein with the highest degree of homology to the Cajal body marker protein WDR79/WRAP53, a WD40-domain protein, which is required for proper localization of small Cajal body-associated RNAs (62, 63) and for Cajal body maintenance (64). Down-regulation of MTAP by RNAi was previously shown to decrease the levels of SLA1 RNA and telomerase RNA (65).
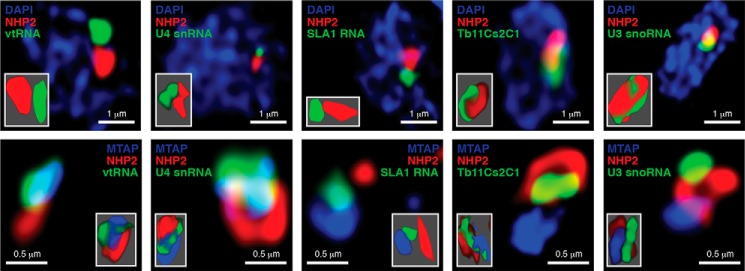
To test whether T. brucei vtRNA is enriched in the MTAP nuclear compartment, we performed fluorescence in situ hybridization (FISH) coupled with immunofluorescence for either NHP2, a box H/ACA RNP core component enriched at the site of rRNA processing and modification in the nucleolus, or for YFP-tagged MTAP. vtRNA, U4 snRNA, and SLA1 RNA did not show appreciable co-localization with NHP2 at the site of pre-rRNA maturation, in contrast to U3 snoRNA and TB11Cs2C1 (a box C/D snoRNA involved in pre-rRNA cleavage), both clearly overlapping the signal for NHP2 (Fig. 6). Partial co-localization with MTAP was detected for vtRNA, U4 snRNA, and SLA1 RNA but not TB11Cs2C1 or U3 snoRNAs (Fig. 6). The overlapping enrichment of vtRNA and MTAP in the nucleus suggested that the vtRNP in T. brucei may affect processes related to the metabolism of the SL RNP particle and thus influence the process of trans-splicing of trypanosome pre-mRNAs, an essential step of protein-coding gene expression in this organism.
Figure 6.
T. brucei vtRNA is highly enriched in a nuclear compartment distinct from the nucleolus. High-resolution fluorescence in situ hybridization coupled with immunofluorescence was performed for the indicated small RNAs (green) and NHP2 (red), top panels. DNA was stained with DAPI (blue). Bottom panels, simultaneous detection by immunofluorescence of NHP2 (red) and MTAP (blue) with in situ hybridization for the indicated RNAs (green). Insets show three-dimensional representation of the detected RNA and proteins.
T. brucei vtRNA is required for the production of trans-spliced mRNA
To assess whether the vtRNA has a role in trans-splicing (Fig. 7A), we wished to use cells that contain the highest amounts of this ncRNA, assuming that higher levels indicate greater need for the vtRNA in a particular trypanosome life-cycle stage. We first tested by semi-quantitative RT-PCR the abundance of this noncoding RNA in three different trypanosome life-cycle stages, namely cultured procyclic cells, purified metacyclic cells derived from procyclics by inducible expression of the RNA-binding protein 6 (RBP6, Tb927.3.2930) (66, 67), and cultured slender bloodstream form cells. Previous results have shown that vtRNA is about 2-fold more abundant in bloodstream form trypanosomes as compared with procyclics (45), and this observation was confirmed in our RT-PCR assay using cDNA from an equivalent number of cells (Fig. S6). An even greater abundance for vtRNA was revealed in metacyclic trypanosomes, at least five times higher than in procyclic cells according to our semi-quantitative RT-PCR results (Fig. S6). However, metacyclic cells are quiescent cells (66) with very limited RNA biosynthetic activity, which makes them unsuitable for studying pre-mRNA processing. Thus, bloodstream form cells were chosen for analyzing a potential role of the vtRNA in trans-splicing.
Figure 7.
vtRNA in T. brucei is required for the production of trans-spliced mRNA. A, diagram of the mechanism of coupled trans-splicing and polyadenylation in T. brucei. Note that the timing of splicing and upstream mRNA polyadenylation is currently unknown. Efficiency of processing events upstream or downstream of any mRNA could result in species that are either not spliced or not polyadenylated. B, Northern blot analysis of the indicated RNAs in permeabilized BF cells treated with the vtRNA antisense oligodeoxynucleotide VT-A or VT-B or their mutant versions, MUT-A or MUT-B, as controls. C, analysis of newly synthesized, 32P-labeled RNA in permeabilized BF cells treated with MUT-A or VT-A oligo. Input is the total cellular RNA; SLex samples represent the RNA enriched on streptavidin beads coupled to biotinylated antisense oligo against the SL exon sequence; poly(A) is the RNA enriched on beads with biotinylated (dT)39 oligonucleotide; D, as in C, but for cells treated with the MUT-B or VT-B oligo. Marker is a 32P-labeled pBR322 DNA MspI digest. Indicated are the positions of the SL RNA and SL RNA fragments. As the conditions for the assay were optimized for pol II transcription, the majority of the high-molecular-weight signal represents pre-mRNA and mRNA.
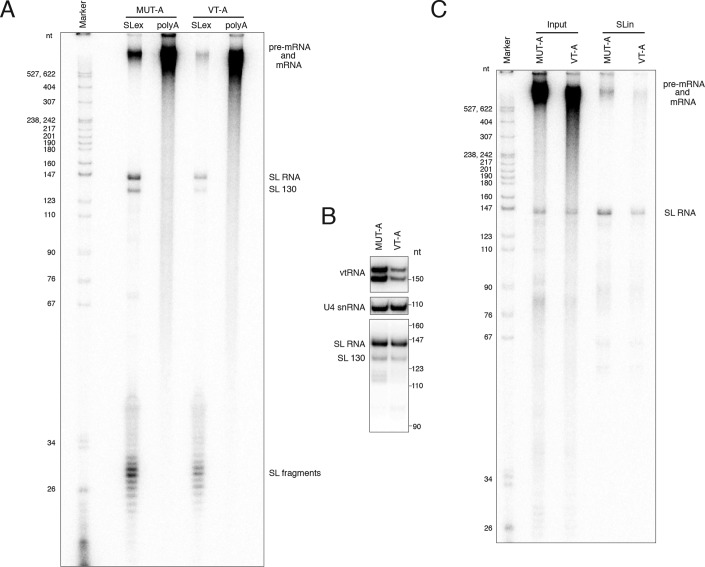
The best option for studying pre-mRNA splicing is an in vitro assay with cell extracts and an in vitro-transcribed RNA substrate. However, repeated attempts to devise a robust in vitro splicing assay for T. brucei that does not require the addition of endogenous SL RNA (68) were unsuccessful. Therefore, we decided that the permeabilized cell system previously developed for procyclic trypanosomes to study pre-mRNA processing (69, 70) was the best-available assay for assessing the potential role of the vtRNA in trans-splicing. The bloodstream form system required optimization (Fig. S7), because we noticed that these cells were more difficult to manipulate compared with permeabilized procyclic trypanosomes (see “Experimental procedures”). One of the most important changes in the protocol was the substitution of sucrose in the buffer with an equimolar concentration of glucose, which allowed the cells to remain alive upon permeabilization and eliminated the need for addition of an ATP-regenerating system (creatine kinase/creatine phosphate), because bloodstream form trypanosomes use primarily glycolysis for their ATP needs. Increasing the Mg2+ concentration to 10 mm and incubating the cells at 30 °C after the addition of ribonucleoside triphosphates produced the largest amounts of newly-synthesized SL RNA (Fig. S7, A and B), a prerequisite for efficient trans-splicing. In addition, these conditions allowed the production of the highest levels of pre-mRNA, mRNA, pre-rRNA, and rRNA (Fig. S7, A–C). To decrease the levels of the vtRNA in the permeabilized cells, we used two different oligodeoxyribonucleotides, VT-A (spanning regions covered by oligos 2 and 3 in Fig. 5) and VT-B (spanning a region of oligo 4, oligo 5, and a region of oligo 6 in Fig. 5), to target the RNA for cleavage by endogenous RNase H (70). As controls, we used mutant versions of the two oligos, MUT-A and MUT-B, in which every second nucleobase was changed to adenine. After permeabilization and incubation with the oligonucleotides, the cells were incubated with a mixture of ribonucleoside triphosphates, including the labeled [α-32P]UTP. Incubation of permeabilized cells at 30 °C after treatment with an anti-vtRNA oligo resulted in decreased amounts of newly-synthesized SL RNA at time points longer than 5 min (Fig. S7D). To eliminate the effects of lower levels of SL RNA synthesis on trans-splicing, we performed our assays for 5 min after the addition of ribonucleoside triphosphates. To test that the oligonucleotides used caused a decrease only in the level of vtRNA and not any of the spliceosomal snRNAs, we performed identically processed reactions in which only cold ribonucleoside triphosphates were used. The total RNA prepared from these cells was subjected to Northern blotting to visualize vtRNA, SL RNA, U1, U2, U4, U5, and U6 snRNAs (Fig. 7B). Full-length vtRNA exhibited a large decrease in abundance, and a previously described 3′-end truncated form of the SL RNA (69), SL RNA 130, showed a modest drop in its levels (Fig. 7B). To monitor the global effects of vtRNA knockdown on trans-splicing and polyadenylation, the radioactively-labeled newly-synthesized RNA was subjected to affinity selection with biotinylated antisense oligonucleotides against the SL exon or oligo(dT) against the poly(A) tail. The input material and the RNA samples highly-enriched for either trans-spliced or polyadenylated molecules were separated by electrophoresis and visualized (Fig. 7, C and D). The specificity of the enrichment protocol was validated by the lack of SL RNA in the poly(A)+-enriched RNA samples (Fig. 7, C and D, and Fig. 8A). The levels of newly-synthesized SL RNA in the anti-vtRNA oligo-treated cells were comparable with the amounts in the cells treated with mutant oligos (Fig. 7, C and D). The largest difference in abundance of labeled RNA species between different samples was observed for the SL exon-enriched long RNA, with a marked decrease in the anti-vtRNA oligo-treated cells, 55% less for VT-A and 70% less for VT-B. We noted that the sizes of these labeled RNAs were comparable with the SL exon-containing RNA in the mutant oligo-treated cells (Fig. 7, C and D). The levels of polyadenylated RNA in the VT-A and VT-B oligo-treated cells were only slightly reduced compared with the cells treated with mutant oligos (by 5 and 25%, respectively); however, there was a difference in the size of the poly(A)+ RNA, which manifested as a smear extending toward smaller-sized molecules (Fig. 7, C and D).
Figure 8.
Effect of reduction of vtRNA levels on the newly synthesized SL RNA after longer RNA synthesis time in permeabilized cells. A, analysis of newly-synthesized, 32P-labeled RNA in permeabilized BF cells treated with the MUT-A or VT-A antisense oligos after a 30-min incubation with ribonucleoside triphosphate mixture containing [α-32P]UTP. SLex, RNA enriched on antisense SL oligonucleotide beads; poly(A), RNA enriched on (dT)39 beads. The positions of pre-mRNA/mRNA, SL RNA, the 3′-end–shortened SL 130 RNA, and the SL RNA fragments are indicated. B, Northern blot analysis of the indicated RNAs in permeabilized cells treated with the MUT-A or VT-A oligos, after incubation for 30 min with only nonradioactive ribonucleoside triphosphates. C, newly synthesized, 32P-labeled RNA in permeabilized cells treated with the MUT-A or VT-A antisense oligos after a 5-min incubation with ribonucleoside triphosphate mixture containing [α-32P]UTP. Input, total RNA in the cells; SLin, RNA enriched on beads coupled to antisense oligonucleotide against the SL RNA intron.
Unexpectedly, we observed very efficient enrichment of fragments corresponding to the SL exon sequence among the RNAs selected by the anti-SL oligo, even though they were barely detectable in the total input RNA (Fig. 7, C and D). The abundance of these SL exon fragments was also different between cells treated with anti-vtRNA oligos VT-A and VT-B and was less prominent in the anti-vtRNA samples by 40% for both oligos (Fig. 7, C and D). Very similar results were obtained with newly-synthesized RNA for 30 min (Fig. 8A), a 75% decrease of SL-containing long RNAs in the anti-vtRNA cells, a 15% decrease in polyadenylated species, which again appeared shorter compared with mutant oligo-treated cell RNAs, and a 45% decrease in the abundance of the SL exon fragments (Fig. 8A). As mentioned above, the longer incubation time caused a decrease in the level of newly synthesized SL RNA by 65% (Fig. 8A) and a more modest decrease in the total (nonradioactive) SL RNA amounts by 20% in the vtRNA-depleted cells (Fig. 8B). We did not observe a major change in the methylation state of the SL RNA cap or the overall abundance of branched or debranched SL intron in the total RNA samples from permeabilized cells upon down-regulation of vtRNA (Fig. S8). However, we detected a shift in the size of high-molecular-weight newly-synthesized RNA containing the branched SL intron toward shorter species (Fig. 8C).
Discussion
Our observation that the T. brucei vtRNA plays a role in the maturation of mRNAs suggests that in addition to structural similarities between vtRNAs and Y RNAs, and between the TEP1 and Ro TROVE domains, the likelihood for similarities in the functions of these RNAs and/or RNPs is higher than previously suspected. In addition, some bacterial Y RNAs appear to have similarities to tRNAs (71–73), suggesting that Y RNAs (and likely vault RNAs) have either originated from tRNA ancestors or evolved to mimic tRNA features. The bacterial systems containing a TROVE-domain protein seem to have evolved to deal with either endonucleolytic cleavages introduced in RNA substrates and/or repair of damaged 5′ or 3′ ends (72). Abundant experimental evidence has shown that bacterial and vertebrate Y RNAs are involved in RNA quality control and processing. In some instances, their role is to tether Ro and its RNA substrate to a specific enzymatic activity (32, 36), and in others they appear to guide Ro to a particular substrate. An example is Y5 RNA guiding Ro via its internal loop to associate with 5S rRNA and its L5 ribosomal protein-binding partner, to presumably facilitate 5S RNP quality control (74). The presence of several different Y RNA and/or vtRNA molecules in many species could be an indication for specialization of these ncRNAs to serve as guide/tethers of the TROVE domain protein factors to different substrates and/or effector RNA-metabolizing complexes. Additionally, Ro and TEP1 likely have functions in RNA metabolism that do not require Y RNA or vtRNA, respectively. Tetrahymena p80 (an ortholog of TEP1) is able to directly interact with telomerase RNA without evidence of the participation of a vtRNA in the complex (75). Additionally, p80 is not a core component of the telomerase complex and exhibits general RNA-binding activity (76). For mammalian TEP1, it was shown that telomerase RNA and vtRNA compete for binding to the protein in vitro (5). Notwithstanding the use of multiple experimental approaches, we were unable to detect an interaction between TEP1 and SL RNA or between vtRNA and SL RNA, or other components of the trypanosome trans-splicing machinery, such as SF1 or U2AF65 (data not shown) (77). Thus, we are inclined to believe that the apparent effect of the vtRNA on trans-splicing, even when tested for a very short 5-min time period, is indirect. Important for the interpretation of our results is the fact that TEP1 is a moderately abundant protein, and we estimate that it is represented by ∼15,000 molecules per cell based on shotgun proteomics (66), and its levels do not change much between procyclic, metacyclic, or bloodstream form trypanosomes (66, 78). The number of vtRNA molecules in a procyclic trypanosome cell probably do not exceed 1,000–2,000 and, in contrast to TEP1, vtRNA abundance varies considerably between life-cycle stages of T. brucei development, and it is conceivable that its copy number per cell can rival the number of TEP1 molecules in quiescent metacyclic-form parasites.
Two possible explanations can account for our observation that the vtRNA in T. brucei appears to be required for the production of SL trans-spliced mRNA. In both scenarios, the vtRNA could either act as a regulator of TEP1 activity or could guide TEP1 to the site of its function. The first possibility is that T. brucei vtRNA guides TEP1 to SL RNA or the SL RNP and stimulates the maturation of trans-splicing–competent SL RNP. It is not clear whether all steps of the complex SL RNP biogenesis pathway have been identified (61). Beyond properly modified SL RNA 5′-end cap structure and assembled Sm-protein core, any additional required characteristics of a splicing-competent SL RNP are unknown. Interestingly, 3′-oligoadenylated SL intron sequences are readily detectable in all RNA-seq data obtained for polyadenylated mRNA samples, whereas sequencing reads spanning the SL exon–intron junction are largely absent. This may indicate that remaining branched SL intron in pre-mRNAs actually contains a short polyadenylate tail. Whether this occurs prior to, during, or after trans-splicing is unknown, although oligoadenylated SL RNA species have been described in T. brucei (79) and Leishmania (80). The highly-conserved and available for base-pairing sequence element in the vtRNA of T. brucei has the potential to anneal to a region of the SL intron that overlaps the Sm-binding site, and one of the genes frequently present in TROVE-domain protein operons in bacteria codes for a nucleotidyltransferase (72). It is particularly tempting to speculate about the possibility of a TEP1-associated nucleotidyltransferase activity that is responsible for the oligoadenylation of SL RNA or the SL intron. Unfortunately, our attempts to demonstrate such enzymatic activity co-purifying with TEP1 have so far been unsuccessful, questioning but not excluding its existence. Such activity could potentially also be involved in degradation of the debranched SL intron and recycling of its Sm protein core. Whereas mammalian Sm snRNPs are known to be extremely stable, and even suggested to not turn over at all (81), the trypanosome SL RNP is consumed during trans-splicing very rapidly (82, 83), and presumably its Sm core-bound intron is recycled with similarly high rates.
The second possibility is that the vtRNA is involved in the stabilization of the SL portion of 5′ ends of newly synthesized mRNAs, if they are targeted for degradation by TEP1. Such a negative regulatory role for the vtRNA in modulating TEP1 function is supported by the observation that vtRNA abundance is highest in quiescent metacyclic cells, which have very limited RNA biosynthetic capacity and presumably minimal RNA metabolism in general. Additionally, mRNAs in these cells need to be stabilized during the few days of quiescence prior to entry into the mammalian host or metacyclic cell death. The SL fragments (∼39 nt and shorter), which we always observed in permeabilized cells, may be the result of nonproductive trans-splicing, where the second step of the trans-splicing reaction is inhibited, and free SL exon accumulates. However, this is unlikely because we did not observe an increase in branched Y-structure splicing intermediates. An intriguing possibility is that the SL fragments could be the result of a new, previously unrecognized mode of (newly synthesized) mRNA degradation, by endonucleolytic removal of the SL exon followed by its exonucleolytic degradation. The net end result would be equivalent to mRNA decapping, which in T. brucei has been shown to be performed by TbALPH1, a protein with a calcineurin-like phosphoesterase domain of the ApaH type (84). If TEP1 is involved in such noncanonical decapping mechanism, the vtRNA could act as an inhibitor and thus promote the accumulation of SL-containing mRNA. The potential roles of vtRNA as a negative regulator of TEP1 function or a guide for TEP1 activity should not be considered mutually exclusive.
In conclusion, we identified vtRNAs in all sequenced trypanosomatids, demonstrated T. brucei vtRNA association with TEP1, and provided evidence that the trypanosome vtRNA functions in regulating mRNA metabolism. This activity is attributed to the TEP1/vtRNA RNP, because the vtRNA is associated with TEP1 and is unstable without its protein-binding partner. It is highly likely that the vtRNP and/or vtRNA-free TEP1 also modulate the metabolism of other classes of RNA molecules. For example, additional potential negative regulatory function for T. brucei vtRNA might be suggested by the inverse correlation between the levels of vtRNA in trypanosome cells and total cellular RNA content. Metacyclic cells contain only one-sixth of the procyclic total RNA and possess five times more vtRNA. If TEP1 is required for normal rRNA production (the bulk of the RNA in the cell) and its activity could be suppressed by the vtRNA, this could ultimately determine the differential RNA content of trypanosomes at various stages of their life cycle. Alternatively, the vtRNA may play a stimulatory effect for a role of TEP1 in rRNA degradation. We propose that the core function of vtRNAs is similar to the general function of Y RNAs and consists of guiding and/or modulating the activity of the TROVE-domain protein module of the complex. Our future studies will focus on elucidating the function of TEP1 as either an enzymatic cofactor or a catalytic entity in RNA metabolism in trypanosomes.
Experimental procedures
T. brucei strains and culture
Procyclic form T. brucei rhodesiense YTat 1.1 and T. brucei Lister strain 427(29-13) (85) were maintained at 28 °C and 5% CO2 in Cunningham's medium supplemented with 10% heat-inactivated FBS (Tet-system approved for Lister 427), 2 mm l-glutamine, 100 units/ml penicillin, 100 μg/ml streptomycin, 10 μg/ml gentamicin. T. brucei Lister 427(29-13) was grown in the presence of 15 μg/ml G418, 50 μg/ml hygromycin B, and additional antibiotics as needed, 5 μg/ml phleomycin, 10 μg/ml blasticidin, and 1 μg/ml puromycin. Bloodstream form single marker T. brucei Lister 427 cells (85) were maintained at 37 °C and 5% CO2 in IMDM supplemented with 10% heat-inactivated FBS, 10 mm hypoxanthine, 50 μm bathocuproine disulfonic acid, 1.5 mm cysteine, 1.25 mm pyruvate, 160 μm thymidine, and 0.2 mm β-mercaptoethanol.
T. brucei whole-cell extract preparation and immunoprecipitation
Whole-cell extract from procyclic T. brucei rhodesiense YTat 1.1 was prepared as described previously (86). Rabbit sera against the peptide VKGQQSLLVEAIRVQRCAVK, located close to the C terminus of T. brucei TEP1, were generated and used for immunoprecipitation, although they were inactive toward the protein in Western blots. Immunoprecipitation followed standard protocols, using protein G–Sepharose (GE Healthcare, catalog no. 17-0618-01) and NET-2 buffer (50 mm Tris-HCl, pH 7.5, 150 mm NaCl, 0.05% Nonidet P-40). Briefly, 50 μl of beads were washed five times with 1 ml of NET-2, and then a mixture of 0.5 ml of NET-2 and 50 μl of rabbit serum was incubated with the beads for 4 h at 4 °C with end-to-end rotation. After that, the beads were washed 5 times with 1 ml of NET-2 and then incubated with rotation for additional 4 h at 4 °C with 0.2 ml of whole-cell extract from procyclic trypanosomes and 0.3 ml of NET-2. The incubation was followed by eight washes with 1 ml of NET-2, and precipitated RNA was purified with the TRIzol reagent (Invitrogen, catalog no. 15596026). The RNA was subjected to electrophoresis in denaturing polyacrylamide gels containing 8 m urea, transferred to Hybond-N+ nylon membrane (Amersham Biosciences), followed by Northern blotting with 5′-end 32P-labeled oligodeoxyribonucleotide probes against the indicated RNAs.
RNAi, immunofluorescence, and FISH
Stem-loop construct targeting TEP1 for RNAi was generated against nucleotides 6,352 to 6,892 of the coding sequence, using previously described protocols (58, 87). N-terminal YFP tagging of the endogenous locus for MTAP (Tb927.11.16490) was performed according to a previously published procedure (59). For high-resolution microscopy, procyclic T. brucei cells were fixed on coverslips using 1.6% formaldehyde and permeabilized using Triton/Tween 20 (1:0.1%) in PBS. In situ hybridization with specific DIG-labeled RNA probes was performed as described (59). The nucleolus was localized using anti-NHP2 antibody (59), and the nucleus was stained with 4′,6-diamidino-2-phenylindole (DAPI). Images were acquired using a Leica SP8 confocal microscope equipped with a white light laser and gating. Cells were oversampled both in the lateral and axial axis. The images were captured using a ×100 HC Plan-Apo 1.4 NA objective at 512 × 512 pixels with Z slices taken every 200 nm (×8 zoom). Excitation wavelengths used in this study were 405 nm for DAPI, 490 nm for detecting anti-DIG conjugated to FITC (hybrid detector at 496–552 nm with emission detection time gated 0.5–9.85 ns, and eight accumulations), and 552 nm for secondary anti-rabbit–conjugated Cy3 antibody. The images were then deconvolved using Huygens Professional software with standard parameters (SVI, Laapersveld 6, 1213 VB Hilversum, The Netherlands).
Bioinformatic search for trypanosomatid vtRNAs
Protozoan and invertebrate genomic RefSeq databases (NCBI, release 65) were queried for vtRNAs using the Infernal 1.1 tools (50). The trypanosomatid genomes not included in the protozoan RefSeq database were downloaded from the TriTrypDB website and searched separately. The vtRNA covariance model was built from the alignment of metazoan sequences (3) supplemented with T. brucei TBsRNA-10 (45). The sequence alignment of vtRNA genes was carried out using the T-Coffee algorithm (88) and refined manually.
ChIP-seq
The parental T. brucei cell line for tagging the largest subunit of pol III, RPC1 (Tb927.10.2780), was the procyclic form Lister 427(29-13) strain for inducible expression of gene products (85), containing a phleomycin-resistance construct for inducible RBP6 expression (67). One of the alleles for RPC1 was tagged by transfecting the cells with a DNA fragment containing the last 500 bp of the RPC1-coding sequence in-frame with sequence encoding the Ty1 tag fused to the mCherry-coding sequence, followed by the β/α-tubulin intergenic sequence, the blasticidin-S deaminase–coding sequence, and the first 500 bp of the RPC1 3′-UTR. After confirming proper integration, the second allele was targeted with transfection of a DNA fragment consisting of the 500 bp upstream of the RPC1-coding sequence, the puromycin N-acetyltransferase–coding sequence, and the first 500 bp of the RPC1 3′-UTR. The tagging of one allele and the knockout of the second allele were verified by PCR with appropriate primers and genomic DNA as template and Western blotting with mouse monoclonal BB2 antibody against the Ty1 tag (89), which was also used for immunoprecipitating RPC1–cross-linked chromatin. ChIP was performed as described previously (90, 91). Gel purification of the precipitated DNA fragments, end-repair, ligation of Illumina adapters for high-throughput sequencing, and amplification by PCR were performed as described previously (92). DNA libraries for the input and immunoprecipitated material were sequenced on the Illumina HiSeq4000 platform at the Yale Center for Genome Analysis. The obtained 75-nt reads were mapped to the T. brucei 11-megabase chromosomes (GeneDB version 5) using the Lasergene 13.1 software package from DNASTAR.
RNase H cleavage assay
Reactions of the 25-μl final volume were assembled by combining 12.5 μl of 2× RNase H buffer (100 mm Tris-HCl, pH 8.0, 150 mm KCl, 6 mm MgCl2, 20 mm DTT) with 10 μl of whole-cell extract from procyclic cells, 40 units of Protector RNase inhibitor (Roche Applied Science, catalog no. 03335399001), and individually, 200 ng of each of the following oligodeoxyribonucleotides: oligo 1, 5′-ATCCGAAATCCTTGCGCCC-3′; oligo 2, 5′-TGCTATGCAGAAGTATCCG-3′; oligo 3, 5′-GCCCCTTTGCTGTTTGCTA-3′; oligo 4, 5′-TCGGCGACAGCTACGCCCC-3′; oligo 5, 5′-GTCGGTGCCCTCCCTCGGC-3′; oligo 6, 5′-GGTGGGGGGAAGGCGTCGG-3′; oligo 7, 5′-GCCGAGGGCCACGGGGTGG-3′; oligo 8, 5′-TTGCGTCCAGTGCGGCCGA-3′; oligo 9, 5′-GACAGGGGGTGGACTTGCG-3′; oligo 10, 5′-GGGCGATTGCGGCTTCGGACAG-3′. After incubation of the mixtures for 10 min at room temperature, 2 units of RNase H (Invitrogen, catalog no. 18021071) were added, and the reactions were incubated at the same temperature for 1 h. TRIzol reagent (Invitrogen, catalog no. 15596026) was used to isolate the RNA from the reactions according to the manufacturer's instructions. RNA was fractionated on denaturing polyacrylamide gels containing 8 m urea, transferred to Hybond-N+ nylon membrane (Amersham Biosciences), and subjected to Northern blotting with 5′-end 32P-labeled probe against the T. brucei vtRNA (5′-CTTTGCTGTTTGCTATGCAGAAGTATC-3′) using standard procedures. The membrane was exposed for analysis with Typhoon FLA 7000 PhosphorImager (GE Healthcare).
Permeabilized bloodstream form cells
For every four reactions/samples, 1 × 108 to 2 × 108 exponentially growing bloodstream form cells were collected by centrifugation. The cells were washed twice at room temperature with 1 ml of plain IMDM, which contained no additions, in a 1.7-ml Eppendorf tube by inverting the tube multiple times and collecting the cells by centrifugation for 30 s at 4,500 rpm. The cells were then washed twice in identical fashion as before with 1 ml of freshly prepared Buffer G+ (20 mm HEPES-KOH, pH 7.9, 150 mm d-glucose, 20 mm KCl, 3 mm MgCl2, 1 mm DTT, 1 tablet per 10 ml of cOmplete, Mini, EDTA-free Protease Inhibitor Mixture (Roche Applied Science, catalog no. 11836170001)). Using glucose instead of sucrose was one of the two critical changes from the original protocol for permeabilized procyclic T. brucei cells (69, 70). The washed cells were resuspended by gentle pipetting in 170 μl of Buffer G+, and 40-μl aliquots were placed into four separate Eppendorf tubes. After incubation on ice for 5 min, a mixture of 38.2 μl of Buffer G+ and 1.8 μl of 5 mg/ml α-lysolecithin (Sigma, catalog no. L1381) was very gently added to each of the 40-μl cell aliquots by dispensing the α-lysolecithin solution and gently swirling the pipette tip to carefully mix the cells with the added new volume. This was the second crucial change in the protocol because after the addition of α-lysolecithin, bloodstream form cells become extremely delicate and cannot be pipetted up and down. From this point, every addition to the cells was performed very gently and without pipetting. The permeabilized cells were incubated on ice for 5 min. When indicated, at this stage 5 μl of 5 mg/ml antisense oligodeoxyribonucleotide solution (VT-A, 5′-CTTTGCTGTTTGCTATGCAGAAGTATC-3′; MUT-A, 5′-CATAGATATATACAAAGAAAAAGAAAC-3′; VT-B, 5′-GCGTCGGTGCCCTCCCTCGGCGACAGC-3′; MUT-B 5′-GAGACAGAGACATACATAGACAAAAAC-3′) were added to the cells, and the mixture was incubated on ice for 30 min. A solution containing 5 μl (50 μCi) of [α-32P]UTP, 3,000 Ci/mmol 10 mCi/ml, 3 μl of 100 mm ATP, 1 μl of 100 mm GTP, 1 μl of 100 mm CTP, and 1 μl of 700 mm MgCl2 (and 5 μl of H2O if the previous step is omitted) was added to the cells, and they were incubated on ice for 2 min and then at 30 °C for up to 30 min. The radioactive UTP was substituted with 1 μl of 100 mm cold UTP and 4 μl of water when labeling of newly-synthesized RNA was not needed. After the incubation at 30 °C, 1 ml of TRIzol reagent (Invitrogen, catalog no. 15596026) was added to each sample, and total RNA was isolated according to the manufacturer's instructions. For visualization of the synthesized RNA, 1/10th of the RNA was fractionated on denaturing polyacrylamide gels containing 8 m urea, and the gels were dried and exposed for analysis with Typhoon FLA 7000 PhosphorImager (GE Healthcare).
Enrichment for trans-spliced and polyadenylated RNAs from total RNA
The isolated total RNA from permeabilized cells was dissolved in 50 μl of H2O; 5 μl were kept as the input sample, and the remaining 45 μl were split equally between two tubes and incubated with an equal volume of (10 mm Tris-HCl, pH 7.4, 200 mm NaCl) solution and either 25 μg of biotinylated anti-SL exon oligo 5′bio-CAATATAGTACAGAAACTGTTCTAATAATAGCGTTAGTT-3′ or 25 μg of biotinylated anti-poly(A) oligo 5′bio-(T)39-3′ at 90 °C for 2 min. The samples were then slowly cooled to room temperature, and an additional 0.2 ml of the same buffer (10 mm Tris-HCl, pH 7.4, 200 mm NaCl) were added to the RNA/oligo mixtures. Dynabeads MyOne Streptavidin C1 (Invitrogen, catalog no. 65001), 0.2 ml of bead slurry per sample, were washed with 0.5 ml of the same buffer, and the RNA/oligo mix was added to the beads and incubated with end–to–end rotation for 2 h at room temperature. The beads were washed four times with 0.5 ml of the same buffer. Selected RNAs were eluted by incubating the beads with 40 μl of 8 m urea-containing gel-loading dye at 70 °C for 5 min, with thorough mixing every minute. RNA (1% of input and 10% of enriched material) was fractionated on denaturing polyacrylamide gels containing 8 m urea, and the gels were dried and exposed for analysis with Typhoon FLA 7000 PhosphorImager (GE Healthcare). RNA was similarly enriched for molecules containing the SL RNA intron, using a biotinylated anti-SL intron oligo, 5′bio-GCGTGTGTTGGCCCAGCTGCTACTGGGAGCTTCTCATAC-3′ against nucleotides 40–78 of the SL RNA.
Author contributions
N. G. K., S. M., and C. T. conceptualization; N. G. K., K. T. T., and C. T. data curation; N. G. K., K. T. T., and C. T. formal analysis; N. G. K., S. M., and C. T. supervision; N. G. K., K. S. R., J. Y. T., H. S., and Y. L. validation; N. G. K., K. S. R., K. T. T., J. Y. T., H. S., Y. L., S. M., and C. T. investigation; N. G. K., K. S. R., K. T. T., J. Y. T., H. S., Y. L., and C. T. visualization; N. G. K., K. S. R., and K. T. T. methodology; N. G. K. writing-original draft; N. G. K., S. M., and C. T. project administration; N. G. K., K. S. R., K. T. T., J. Y. T., S. M., and C. T. writing-review and editing; S. M. and C. T. funding acquisition.
Supplementary Material
Acknowledgment
We thank Joan A. Steitz for critical reading of the manuscript.
This work was supported by National Institutes of Health Grants AI028798 and AI110325 (to C. T.) and by a grant from the Israel–United States Binational Science Foundation (to S. M. and C. T.). The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
This article was selected as one of our Editors' Picks.
This article contains Figs. S1–S8.
ChIP-Seq data from this study have been submitted to the NCBI Sequence Read Archive-SRA under accession number PRJNA526543.
- vtRNA
- vault RNA
- pol
- polymerase
- oligo
- oligonucleotide
- DAPI
- 4′,6-diamidino-2-phenylindole
- MTAP
- methyltransferase-associated protein
- RNP
- ribonucleoprotein
- snRNA
- small nuclear RNA
- snoRNA
- small nucleolar RNA
- FBS
- fetal bovine serum
- ncRNA
- noncoding RNA
- SL
- spliced leader
- MVP
- major vault protein
- vWFA
- von Willebrand factor A
- IMDM
- Iscove's modified Dulbecco's medium
- aa
- amino acid
- nt
- nucleotide
- DIG
- digoxigenin.
References
- 1. Kedersha N. L., and Rome L. H. (1986) Isolation and characterization of a novel ribonucleoprotein particle: large structures contain a single species of small RNA. J. Cell Biol. 103, 699–709 10.1083/jcb.103.3.699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kickhoefer V. A., Searles R. P., Kedersha N. L., Garber M. E., Johnson D. L., and Rome L. H. (1993) Vault ribonucleoprotein particles from rat and bullfrog contain a related small RNA that is transcribed by RNA polymerase III. J. Biol. Chem. 268, 7868–7873 [PubMed] [Google Scholar]
- 3. Stadler P. F., Chen J. J., Hackermüller J., Hoffmann S., Horn F., Khaitovich P., Kretzschmar A. K., Mosig A., Prohaska S. J., Qi X., Schutt K., and Ullmann K. (2009) Evolution of vault RNAs. Mol. Biol. Evol. 26, 1975–1991 10.1093/molbev/msp112 [DOI] [PubMed] [Google Scholar]
- 4. Kickhoefer V. A., Stephen A. G., Harrington L., Robinson M. O., and Rome L. H. (1999) Vaults and telomerase share a common subunit, TEP1. J. Biol. Chem. 274, 32712–32717 10.1074/jbc.274.46.32712 [DOI] [PubMed] [Google Scholar]
- 5. Poderycki M. J., Rome L. H., Harrington L., and Kickhoefer V. A. (2005) The p80 homology region of TEP1 is sufficient for its association with the telomerase and vault RNAs, and the vault particle. Nucleic Acids Res. 33, 893–902 10.1093/nar/gki234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kickhoefer V. A., Rajavel K. S., Scheffer G. L., Dalton W. S., Scheper R. J., and Rome L. H. (1998) Vaults are up-regulated in multidrug-resistant cancer cell lines. J. Biol. Chem. 273, 8971–8974 10.1074/jbc.273.15.8971 [DOI] [PubMed] [Google Scholar]
- 7. Anderson D. H., Kickhoefer V. A., Sievers S. A., Rome L. H., and Eisenberg D. (2007) Draft crystal structure of the vault shell at 9-A resolution. PLoS Biol. 5, e318 10.1371/journal.pbio.0050318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tanaka H., Kato K., Yamashita E., Sumizawa T., Zhou Y., Yao M., Iwasaki K., Yoshimura M., and Tsukihara T. (2009) The structure of rat liver vault at 3.5 angstrom resolution. Science 323, 384–388 10.1126/science.1164975 [DOI] [PubMed] [Google Scholar]
- 9. Kickhoefer V. A., Siva A. C., Kedersha N. L., Inman E. M., Ruland C., Streuli M., and Rome L. H. (1999) The 193-kD vault protein, VPARP, is a novel poly(ADP-ribose) polymerase. J. Cell Biol. 146, 917–928 10.1083/jcb.146.5.917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Liu Y., Snow B. E., Hande M. P., Baerlocher G., Kickhoefer V. A., Yeung D., Wakeham A., Itie A., Siderovski D. P., Lansdorp P. M., Robinson M. O., and Harrington L. (2000) Telomerase-associated protein TEP1 is not essential for telomerase activity or telomere length maintenance in vivo. Mol. Cell. Biol. 20, 8178–8184 10.1128/MCB.20.21.8178-8184.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kickhoefer V. A., Liu Y., Kong L. B., Snow B. E., Stewart P. L., Harrington L., and Rome L. H. (2001) The telomerase/vault-associated protein TEP1 is required for vault RNA stability and its association with the vault particle. J. Cell Biol. 152, 157–164 10.1083/jcb.152.1.157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nandy C., Mrázek J., Stoiber H., Grässer F. A., Hüttenhofer A., and Polacek N. (2009) Epstein-Barr virus-induced expression of a novel human vault RNA. J. Mol. Biol. 388, 776–784 10.1016/j.jmb.2009.03.031 [DOI] [PubMed] [Google Scholar]
- 13. Amort M., Nachbauer B., Tuzlak S., Kieser A., Schepers A., Villunger A., and Polacek N. (2015) Expression of the vault RNA protects cells from undergoing apoptosis. Nat. Commun. 6, 7030 10.1038/ncomms8030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Li F., Chen Y., Zhang Z., Ouyang J., Wang Y., Yan R., Huang S., Gao G. F., Guo G., and Chen J. L. (2015) Robust expression of vault RNAs induced by influenza A virus plays a critical role in suppression of PKR-mediated innate immunity. Nucleic Acids Res. 43, 10321–10337 10.1093/nar/gkv1078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. van Zon A., Mossink M. H., Schoester M., Scheffer G. L., Scheper R. J., Sonneveld P., and Wiemer E. A. (2001) Multiple human vault RNAs. Expression and association with the vault complex. J. Biol. Chem. 276, 37715–37721 10.1074/jbc.M106055200 [DOI] [PubMed] [Google Scholar]
- 16. Gopinath S. C., Wadhwa R., and Kumar P. K. (2010) Expression of noncoding vault RNA in human malignant cells and its importance in mitoxantrone resistance. Mol. Cancer Res. 8, 1536–1546 10.1158/1541-7786.MCR-10-0242 [DOI] [PubMed] [Google Scholar]
- 17. Chen J., OuYang H., An X., and Liu S. (2018) Vault RNA partially induces drug resistance of human tumor cells MCF-7 by binding to the RNA/DNA-binding protein PSF and inducing oncogene GAGE6. PLoS ONE 13, e0191325 10.1371/journal.pone.0191325 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 18. Horos R., Büscher M., Kleinendorst R., Alleaume A. M., Tarafder A. K., Schwarzl T., Dziuba D., Tischer C., Zielonka E. M., Adak A., Castello A., Huber W., Sachse C., and Hentze M. W. (2019) The small non-coding vault RNA1-1 acts as a riboregulator of autophagy. Cell 176, 1054–1067.e12 10.1016/j.cell.2019.01.030 [DOI] [PubMed] [Google Scholar]
- 19. Lee K., Kunkeaw N., Jeon S. H., Lee I., Johnson B. H., Kang G. Y., Bang J. Y., Park H. S., Leelayuwat C., and Lee Y. S. (2011) Precursor miR-886, a novel noncoding RNA repressed in cancer, associates with PKR and modulates its activity. RNA 17, 1076–1089 10.1261/rna.2701111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jeon S. H., Lee K., Lee K. S., Kunkeaw N., Johnson B. H., Holthauzen L. M., Gong B., Leelayuwat C., and Lee Y. S. (2012) Characterization of the direct physical interaction of nc886, a cellular non-coding RNA, and PKR. FEBS Lett. 586, 3477–3484 10.1016/j.febslet.2012.07.076 [DOI] [PubMed] [Google Scholar]
- 21. Persson H., Kvist A., Vallon-Christersson J., Medstrand P., Borg A., and Rovira C. (2009) The non-coding RNA of the multidrug resistance-linked vault particle encodes multiple regulatory small RNAs. Nat. Cell Biol. 11, 1268–1271 10.1038/ncb1972 [DOI] [PubMed] [Google Scholar]
- 22. Hussain S., Sajini A. A., Blanco S., Dietmann S., Lombard P., Sugimoto Y., Paramor M., Gleeson J. G., Odom D. T., Ule J., and Frye M. (2013) NSun2-mediated cytosine-5 methylation of vault noncoding RNA determines its processing into regulatory small RNAs. Cell Rep. 4, 255–261 10.1016/j.celrep.2013.06.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kong L., Hao Q., Wang Y., Zhou P., Zou B., and Zhang Y. X. (2015) Regulation of p53 expression and apoptosis by vault RNA2-1-5p in cervical cancer cells. Oncotarget 6, 28371–28388 10.18632/oncotarget.4948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Li C. C., Eaton S. A., Young P. E., Lee M., Shuttleworth R., Humphreys D. T., Grau G. E., Combes V., Bebawy M., Gong J., Brammah S., Buckland M. E., and Suter C. M. (2013) Glioma microvesicles carry selectively packaged coding and non-coding RNAs which alter gene expression in recipient cells. RNA Biol. 10, 1333–1344 10.4161/rna.25281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lässer C., Shelke G. V., Yeri A., Kim D. K., Crescitelli R., Raimondo S., Sjöstrand M., Gho Y. S., Van Keuren Jensen K., and Lötvall J. (2017) Two distinct extracellular RNA signatures released by a single cell type identified by microarray and next-generation sequencing. RNA Biol. 14, 58–72 10.1080/15476286.2016.1249092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Shurtleff M. J., Yao J., Qin Y., Nottingham R. M., Temoche-Diaz M. M., Schekman R., and Lambowitz A. M. (2017) Broad role for YBX1 in defining the small noncoding RNA composition of exosomes. Proc. Natl. Acad. Sci. U.S.A. 114, E8987–E8995 10.1073/pnas.1712108114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Babatunde K. A., Mbagwu S., Hernández-Castañeda M. A., Adapa S. R., Walch M., Filgueira L., Falquet L., Jiang R. H. Y., Ghiran I., and Mantel P. Y. (2018) Malaria infected red blood cells release small regulatory RNAs through extracellular vesicles. Sci. Rep. 8, 884 10.1038/s41598-018-19149-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Harrington L., McPhail T., Mar V., Zhou W., Oulton R., Bass M. B., Arruda I., and Robinson M. O. (1997) A mammalian telomerase-associated protein. Science 275, 973–977 10.1126/science.275.5302.973 [DOI] [PubMed] [Google Scholar]
- 29. Bateman A., and Kickhoefer V. (2003) The TROVE module: a common element in Telomerase, Ro and Vault ribonucleoproteins. BMC Bioinformatics 4, 49 10.1186/1471-2105-4-49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Stein A. J., Fuchs G., Fu C., Wolin S. L., and Reinisch K. M. (2005) Structural insights into RNA quality control: the Ro autoantigen binds misfolded RNAs via its central cavity. Cell 121, 529–539 10.1016/j.cell.2005.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Fuchs G., Stein A. J., Fu C., Reinisch K. M., and Wolin S. L. (2006) Structural and biochemical basis for misfolded RNA recognition by the Ro autoantigen. Nat. Struct. Mol. Biol. 13, 1002–1009 10.1038/nsmb1156 [DOI] [PubMed] [Google Scholar]
- 32. Chen X., Taylor D. W., Fowler C. C., Galan J. E., Wang H. W., and Wolin S. L. (2013) An RNA degradation machine sculpted by Ro autoantigen and noncoding RNA. Cell 153, 166–177 10.1016/j.cell.2013.02.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wolin S. L., and Steitz J. A. (1984) The Ro small cytoplasmic ribonucleoproteins: identification of the antigenic protein and its binding site on the Ro RNAs. Proc. Natl. Acad. Sci. U.S.A. 81, 1996–2000 10.1073/pnas.81.7.1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. O'Brien C. A., and Wolin S. L. (1994) A possible role for the 60-kD Ro autoantigen in a discard pathway for defective 5S rRNA precursors. Genes Dev. 8, 2891–2903 10.1101/gad.8.23.2891 [DOI] [PubMed] [Google Scholar]
- 35. Chen X., Smith J. D., Shi H., Yang D. D., Flavell R. A., and Wolin S. L. (2003) The Ro autoantigen binds misfolded U2 small nuclear RNAs and assists mammalian cell survival after UV irradiation. Curr. Biol. 13, 2206–2211 10.1016/j.cub.2003.11.028 [DOI] [PubMed] [Google Scholar]
- 36. Chen X., Wurtmann E. J., Van Batavia J., Zybailov B., Washburn M. P., and Wolin S. L. (2007) An ortholog of the Ro autoantigen functions in 23S rRNA maturation in D. radiodurans. Genes Dev. 21, 1328–1339 10.1101/gad.1548207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wurtmann E. J., and Wolin S. L. (2010) A role for a bacterial ortholog of the Ro autoantigen in starvation-induced rRNA degradation. Proc. Natl. Acad. Sci. U.S.A. 107, 4022–4027 10.1073/pnas.1000307107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wolin S. L., Belair C., Boccitto M., Chen X., Sim S., Taylor D. W., and Wang H. W. (2013) Non-coding Y RNAs as tethers and gates: insights from bacteria. RNA Biol. 10, 1602–1608 10.4161/rna.26166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sim S., and Wolin S. L. (2018) Bacterial Y RNAs: gates, tethers, and tRNA mimics. Microbiol. Spectr. 6 10.1128/microbiolspec.RWR-0023-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Green C. D., Long K. S., Shi H., and Wolin S. L. (1998) Binding of the 60-kDa Ro autoantigen to Y RNAs: evidence for recognition in the major groove of a conserved helix. RNA 4, 750–765 10.1017/S1355838298971667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Büscher P., Cecchi G., Jamonneau V., and Priotto G. (2017) Human African trypanosomiasis. Lancet 390, 2397–2409 10.1016/S0140-6736(17)31510-6 [DOI] [PubMed] [Google Scholar]
- 42. Günzl A. (2010) The pre-mRNA splicing machinery of trypanosomes: complex or simplified? Eukaryot. Cell 9, 1159–1170 10.1128/EC.00113-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Michaeli S. (2011) Trans-splicing in trypanosomes: machinery and its impact on the parasite transcriptome. Future Microbiol. 6, 459–474 10.2217/fmb.11.20 [DOI] [PubMed] [Google Scholar]
- 44. Preusser C., Jaé N., and Bindereif A. (2012) mRNA splicing in trypanosomes. Int. J. Med. Microbiol. 302, 221–224 10.1016/j.ijmm.2012.07.004 [DOI] [PubMed] [Google Scholar]
- 45. Michaeli S., Doniger T., Gupta S. K., Wurtzel O., Romano M., Visnovezky D., Sorek R., Unger R., and Ullu E. (2012) RNA-seq analysis of small RNPs in Trypanosoma brucei reveals a rich repertoire of non-coding RNAs. Nucleic Acids Res. 40, 1282–1298 10.1093/nar/gkr786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Berriman M., Ghedin E., Hertz-Fowler C., Blandin G., Renauld H., Bartholomeu D. C., Lennard N. J., Caler E., Hamlin N. E., Haas B., Böhme U., Hannick L., Aslett M. A., Shallom J., Marcello L., et al. (2005) The genome of the African trypanosome Trypanosoma brucei. Science 309, 416–422 10.1126/science.1112642 [DOI] [PubMed] [Google Scholar]
- 47. Hendrick J. P., Wolin S. L., Rinke J., Lerner M. R., and Steitz J. A. (1981) Ro small cytoplasmic ribonucleoproteins are a subclass of La ribonucleoproteins: further characterization of the Ro and La small ribonucleoproteins from uninfected mammalian cells. Mol. Cell. Biol. 1, 1138–1149 10.1128/MCB.1.12.1138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Marchetti M. A., Tschudi C., Kwon H., Wolin S. L., and Ullu E. (2000) Import of proteins into the trypanosome nucleus and their distribution at karyokinesis. J. Cell Sci. 113, 899–906 [DOI] [PubMed] [Google Scholar]
- 49. Fadda A., Ryten M., Droll D., Rojas F., Färber V., Haanstra J. R., Merce C., Bakker B. M., Matthews K., and Clayton C. (2014) Transcriptome-wide analysis of trypanosome mRNA decay reveals complex degradation kinetics and suggests a role for co-transcriptional degradation in determining mRNA levels. Mol. Microbiol. 94, 307–326 10.1111/mmi.12764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Nawrocki E. P., and Eddy S. R. (2013) Infernal 1.1: 100-fold faster RNA homology searches. Bioinformatics 29, 2933–2935 10.1093/bioinformatics/btt509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ben-Shlomo H., Levitan A., Shay N. E., Goncharov I., and Michaeli S. (1999) RNA editing associated with the generation of two distinct conformations of the trypanosomatid Leptomonas collosoma 7SL RNA. J. Biol. Chem. 274, 25642–25650 10.1074/jbc.274.36.25642 [DOI] [PubMed] [Google Scholar]
- 52. Vilalta A., Kickhoefer V. A., Rome L. H., and Johnson D. L. (1994) The rat vault RNA gene contains a unique RNA polymerase III promoter composed of both external and internal elements that function synergistically. J. Biol. Chem. 269, 29752–29759 [PubMed] [Google Scholar]
- 53. Kickhoefer V. A., Emre N., Stephen A. G., Poderycki M. J., and Rome L. H. (2003) Identification of conserved vault RNA expression elements and a non-expressed mouse vault RNA gene. Gene 309, 65–70 10.1016/S0378-1119(03)00507-9 [DOI] [PubMed] [Google Scholar]
- 54. Fantoni A., Dare A. O., and Tschudi C. (1994) RNA polymerase III-mediated transcription of the trypanosome U2 small nuclear RNA gene is controlled by both intragenic and extragenic regulatory elements. Mol. Cell. Biol. 14, 2021–2028 10.1128/MCB.14.3.2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Tschudi C., and Ullut E. (2002) Unconventional rules of small nuclear RNA transcription and cap modification in trypanosomatids. Gene Expr. 10, 3–16 [PMC free article] [PubMed] [Google Scholar]
- 56. Nielsen S., Yuzenkova Y., and Zenkin N. (2013) Mechanism of eukaryotic RNA polymerase III transcription termination. Science 340, 1577–1580 10.1126/science.1237934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Arimbasseri A. G., and Maraia R. J. (2013) Distinguishing core and holoenzyme mechanisms of transcription termination by RNA polymerase III. Mol. Cell. Biol. 33, 1571–1581 10.1128/MCB.01733-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Liang X. H., Uliel S., Hury A., Barth S., Doniger T., Unger R., and Michaeli S. (2005) A genome-wide analysis of C/D and H/ACA-like small nucleolar RNAs in Trypanosoma brucei reveals a trypanosome-specific pattern of rRNA modification. RNA 11, 619–645 10.1261/rna.7174805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Hury A., Goldshmidt H., Tkacz I. D., and Michaeli S. (2009) Trypanosome spliced-leader-associated RNA (SLA1) localization and implications for spliced-leader RNA biogenesis. Eukaryot. Cell 8, 56–68 10.1128/EC.00322-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Liang X. H., Xu Y. X., and Michaeli S. (2002) The spliced leader-associated RNA is a trypanosome-specific sn(o) RNA that has the potential to guide pseudouridine formation on the SL RNA. RNA 8, 237–246 10.1017/S1355838202018290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Zamudio J. R., Mittra B., Chattopadhyay A., Wohlschlegel J. A., Sturm N. R., and Campbell D. A. (2009) Trypanosoma brucei spliced leader RNA maturation by the cap 1 2′-O-ribose methyltransferase and SLA1 H/ACA snoRNA pseudouridine synthase complex. Mol. Cell. Biol. 29, 1202–1211 10.1128/MCB.01496-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Tycowski K. T., Shu M. D., Kukoyi A., and Steitz J. A. (2009) A conserved WD40 protein binds the Cajal body localization signal of scaRNP particles. Mol. Cell 34, 47–57 10.1016/j.molcel.2009.02.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Venteicher A. S., Abreu E. B., Meng Z., McCann K. E., Terns R. M., Veenstra T. D., Terns M. P., and Artandi S. E. (2009) A human telomerase holoenzyme protein required for Cajal body localization and telomere synthesis. Science 323, 644–648 10.1126/science.1165357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Mahmoudi S., Henriksson S., Weibrecht I., Smith S., Söderberg O., Strömblad S., Wiman K. G., and Farnebo M. (2010) WRAP53 is essential for Cajal body formation and for targeting the survival of motor neuron complex to Cajal bodies. PLoS Biol. 8, e1000521 10.1371/journal.pbio.1000521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Gupta S. K., Kolet L., Doniger T., Biswas V. K., Unger R., Tzfati Y., and Michaeli S. (2013) The Trypanosoma brucei telomerase RNA (TER) homologue binds core proteins of the C/D snoRNA family. FEBS Lett. 587, 1399–1404 10.1016/j.febslet.2013.03.017 [DOI] [PubMed] [Google Scholar]
- 66. Christiano R., Kolev N. G., Shi H., Ullu E., Walther T. C., and Tschudi C. (2017) The proteome and transcriptome of the infectious metacyclic form of Trypanosoma brucei define quiescent cells primed for mammalian invasion. Mol. Microbiol 106, 74–92 10.1111/mmi.13754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Kolev N. G., Ramey-Butler K., Cross G. A., Ullu E., and Tschudi C. (2012) Developmental progression to infectivity in Trypanosoma brucei triggered by an RNA-binding protein. Science 338, 1352–1353 10.1126/science.1229641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Shaked H., Wachtel C., Tulinski P., Yahia N. H., Barda O., Darzynkiewicz E., Nilsen T. W., and Michaeli S. (2010) Establishment of an in vitro trans-splicing system in Trypanosoma brucei that requires endogenous spliced leader RNA. Nucleic Acids Res. 38, e114 10.1093/nar/gkq065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Ullu E., and Tschudi C. (1990) Permeable trypanosome cells as a model system for transcription and trans-splicing. Nucleic Acids Res. 18, 3319–3326 10.1093/nar/18.11.3319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Tschudi C., and Ullu E. (1990) Destruction of U2, U4, or U6 small nuclear RNA blocks trans splicing in trypanosome cells. Cell 61, 459–466 10.1016/0092-8674(90)90527-L [DOI] [PubMed] [Google Scholar]
- 71. Chen X., Sim S., Wurtmann E. J., Feke A., and Wolin S. L. (2014) Bacterial noncoding Y RNAs are widespread and mimic tRNAs. RNA 20, 1715–1724 10.1261/rna.047241.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Burroughs A. M., and Aravind L. (2016) RNA damage in biological conflicts and the diversity of responding RNA repair systems. Nucleic Acids Res. 44, 8525–8555 10.1093/nar/gkw722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Wang W., Chen X., Wolin S. L., and Xiong Y. (2018) Structural basis for tRNA mimicry by a bacterial Y RNA. Structure 26, 1635–1644.e3 10.1016/j.str.2018.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Hogg J. R., and Collins K. (2007) Human Y5 RNA specializes a Ro ribonucleoprotein for 5S ribosomal RNA quality control. Genes Dev. 21, 3067–3072 10.1101/gad.1603907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Gandhi L., and Collins K. (1998) Interaction of recombinant Tetrahymena telomerase proteins p80 and p95 with telomerase RNA and telomeric DNA substrates. Genes Dev. 12, 721–733 10.1101/gad.12.5.721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Mason D. X., Autexier C., and Greider C. W. (2001) Tetrahymena proteins p80 and p95 are not core telomerase components. Proc. Natl. Acad. Sci. U.S.A. 98, 12368–12373 10.1073/pnas.221456398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Tkacz I. D., Gupta S. K., Volkov V., Romano M., Haham T., Tulinski P., Lebenthal I., and Michaeli S. (2010) Analysis of spliceosomal proteins in trypanosomatids reveals novel functions in mRNA processing. J. Biol. Chem. 285, 27982–27999 10.1074/jbc.M109.095349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Urbaniak M. D., Guther M. L., and Ferguson M. A. (2012) Comparative SILAC proteomic analysis of Trypanosoma brucei bloodstream and procyclic lifecycle stages. PLoS ONE 7, e36619 10.1371/journal.pone.0036619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Pellé R., and Murphy N. B. (1993) Stage-specific differential polyadenylation of mini-exon derived RNA in African trypanosomes. Mol. Biochem. Parasitol. 59, 277–286 10.1016/0166-6851(93)90225-M [DOI] [PubMed] [Google Scholar]
- 80. Lamontagne J., and Papadopoulou B. (1999) Developmental regulation of spliced leader RNA gene in Leishmania donovani amastigotes is mediated by specific polyadenylation. J. Biol. Chem. 274, 6602–6609 10.1074/jbc.274.10.6602 [DOI] [PubMed] [Google Scholar]
- 81. Yong J., Wan L., and Dreyfuss G. (2004) Why do cells need an assembly machine for RNA–protein complexes? Trends Cell Biol. 14, 226–232 10.1016/j.tcb.2004.03.010 [DOI] [PubMed] [Google Scholar]
- 82. Laird P. W., ten Asbroek A. L., and Borst P. (1987) Controlled turnover and 3′ trimming of the trans splicing precursor of Trypanosoma brucei. Nucleic Acids Res. 15, 10087–10103 10.1093/nar/15.24.10087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Laird P. W., Zomerdijk J. C., de Korte D., and Borst P. (1987) In vivo labelling of intermediates in the discontinuous synthesis of mRNAs in Trypanosoma brucei. EMBO J. 6, 1055–1062 10.1002/j.1460-2075.1987.tb04858.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Kramer S. (2017) The ApaH-like phosphatase TbALPH1 is the major mRNA decapping enzyme of trypanosomes. PLoS Pathog. 13, e1006456 10.1371/journal.ppat.1006456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Wirtz E., Leal S., Ochatt C., and Cross G. A. (1999) A tightly regulated inducible expression system for conditional gene knock-outs and dominant-negative genetics in Trypanosoma brucei. Mol. Biochem. Parasitol. 99, 89–101 10.1016/S0166-6851(99)00002-X [DOI] [PubMed] [Google Scholar]
- 86. Kolev N. G., Günzl A., and Tschudi C. (2017) Metacyclic VSG expression site promoters are recognized by the same general transcription factor that is required for RNA polymerase I transcription of bloodstream expression sites. Mol. Biochem. Parasitol. 216, 52–55 10.1016/j.molbiopara.2017.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Chikne V., Doniger T., Rajan K. S., Bartok O., Eliaz D., Cohen-Chalamish S., Tschudi C., Unger R., Hashem Y., Kadener S., and Michaeli S. (2016) A pseudouridylation switch in rRNA is implicated in ribosome function during the life cycle of Trypanosoma brucei. Sci. Rep. 6, 25296 10.1038/srep25296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Notredame C., Higgins D. G., and Heringa J. (2000) T-Coffee: a novel method for fast and accurate multiple sequence alignment. J. Mol. Biol. 302, 205–217 10.1006/jmbi.2000.4042 [DOI] [PubMed] [Google Scholar]
- 89. Bastin P., Bagherzadeh Z., Matthews K. R., and Gull K. (1996) A novel epitope tag system to study protein targeting and organelle biogenesis in Trypanosoma brucei. Mol. Biochem. Parasitol. 77, 235–239 10.1016/0166-6851(96)02598-4 [DOI] [PubMed] [Google Scholar]
- 90. Siegel T. N., Hekstra D. R., Kemp L. E., Figueiredo L. M., Lowell J. E., Fenyo D., Wang X., Dewell S., and Cross G. A. (2009) Four histone variants mark the boundaries of polycistronic transcription units in Trypanosoma brucei. Genes Dev. 23, 1063–1076 10.1101/gad.1790409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Lee T. I., Johnstone S. E., and Young R. A. (2006) Chromatin immunoprecipitation and microarray-based analysis of protein location. Nat. Protoc. 1, 729–748 10.1038/nprot.2006.98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Kolev N. G., Franklin J. B., Carmi S., Shi H., Michaeli S., and Tschudi C. (2010) The transcriptome of the human pathogen Trypanosoma brucei at single-nucleotide resolution. PLoS Pathog. 6, e1001090 10.1371/journal.ppat.1001090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Waterhouse A., Bertoni M., Bienert S., Studer G., Tauriello G., Gumienny R., Heer F. T., de Beer T. A. P., Rempfer C., Bordoli L., Lepore R., and Schwede T. (2018) SWISS-MODEL: homology modelling of protein structures and complexes. Nucleic Acids Res. 46, W296–W303 10.1093/nar/gky427 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.