Abstract
Objectives
Under representation of black subjects in trials of hepatitis C virus (HCV) direct-acting antivirals (DAAs) complicates assessment of differential outcomes for black individuals vs non-black individuals. HCV trials submitted to the Food and Drug Administration (2013–2017) to support approval or to expand an indication of 12-week interferon-free DAA regimens with or without ribavirin to treat HCV genotype 1 (GT1) infection were pooled to explore efficacy comparisons by ethnicity.
Methods
Twenty-six trials were pooled and included 2869 individuals with HCV GT1 alone and 742 individuals with both HCV GT1 and HIV.
Results
Of the 2869 HCV GT1-mono-infected subjects, 408 (14.2%) were black. Sustained virological response assessed 12 weeks following cessation of treatment (SVR12) was 92%–100% in black individuals and 87.5%–100.0% in non-black individuals. In pooled analyses, SVR12 was numerically similar between black and non-black subjects (97.1% vs 97.3%). Baseline characteristics did not affect SVR12 for the two groups. Of the 742 subjects with both HCV GT1 and HIV, 243 (32.7%) were black: SVR12 was 89.5%–100% in black individuals and 94.4%–100% in non-black individuals. In pooled analyses for HCV GT1/HIV co-infection, black individuals had a 4% (95% confidence interval −7.7% to 0.3%) lower SVR12 than non-black individuals (93.4% vs 97.0%). This difference was driven by ION-4 in which study SVR12 was approximately 10% lower for black than for non-black individuals (89.5% vs 99.1%). Baseline characteristics did not affect SVR12 for the two groups.
Conclusion
No notable SVR12 differences were seen in between black and non-black individuals with HCV GT1 alone. Although a numerical difference was observed between black and non-black individuals with both HCV GT1 and HIV, this finding was driven by results from a single trial and may be due to reasons other than ethnicity: 19 subgroup analyses showed baseline characteristics did not affect SVR12 for black and non-black individuals with both HCV GT1 and HIV.
Keywords: hepatitis C, clinical trial, Food and Drug Administration
Introduction
Chronic hepatitis C virus (HCV) infection is a serious and life-threatening condition that can lead to cirrhosis and hepatocellular carcinoma, and affects approximately 71 million people worldwide and approximately 3.5 million people in the USA [1–4]. Black subjects are disproportionately affected by HCV [2]. In the USA, approximately 23% of the population with HCV infection are black [2,3]. Additionally, co-infection with HCV and HIV is common in the USA. Of the estimated 1.2 million individuals living with HIV in the USA, about a quarter (approximately 300,000) are also infected with HCV [3].
Recent studies have shown that achievement of sustained virological response (SVR) is associated with halting the progression of liver disease and decreasing chronic hepatitis C-related complications, including cirrhosis, hepatic decompensation, hepatocellular carcinoma and liver-related mortality [5,6].
Black subjects are often under represented in clinical trials of HCV direct-acting antivirals (DAAs), resulting in small sample sizes for the subset of black subjects [7]. This makes the assessment of differential outcomes for black vs non-black subjects challenging.
In the interferon (IFN) era, SVR rates were lower among black subjects and likely to be due to the high prevalence of interleukin (IL)-28B CT/TT genotype in black individuals. This genotype is associated with a reduced response to IFN [8,9]. Also IFN-based regimens, including those using the DAA protease inhibitors telaprevir and boceprevir, resulted in lower SVR rates among black subjects even when controlling for IL-28B status [10,11]. DAAs are safer, better tolerated and more effective than IFN-based regimens. If IL-28B genotype status was the main predictor of decreased SVR rates among black individuals, then ethnicity differences with non-IFN DAA regimens may not be apparent. However, the Food and Drug Administration (FDA) noted differences in SVR rates during the review of the ION-4 trial in which SVR rates were lower in black than in non-black individuals with both HCV genotype 1 (GT1) and HIV receiving ledipasvir/sofosbuvir (LDV/SOF) for 12 weeks. Some cohort analyses have found comparable SVR rates between black and non-black subjects, whereas other cohort analyses have found lower SVR rates in black compared with non-black subjects [12–27]. These observations focused on specific regimens, and not all FDA-approved 12-week products for the treatment of HCV GT1 were included. This has prompted the US FDA to pool data from clinical trials approved for 12-week IFN-free DAA regimens for the treatment of HCV GT1 infection to assess if SVR rates differed between black and non-black individuals with HCV GT1 alone and with both HCV GT1 and HIV [27–34].
Methods
Definitions
Treatment outcomes were defined as described in current FDA guidance for HCV DAA development [35]. Antiviral treatment efficacy referred to sustained virological response assessed 12 weeks following cessation of treatment (SVR12). On-treatment virological failure was defined as HCV RNA ≥ lower limit of quantification (LLOQ) at the end of treatment (e.g. virological breakthrough or non-response). Virological relapse was defined as HCV RNA <LLOQ at the end of treatment but HCV RNA quantifiable (≥LLOQ) during follow-up. Non-virological failure referred to subjects who did not achieve SVR and did not meet any virological failure criteria (e.g. discontinued due to adverse event, lost to follow-up or subject withdrawal). The assays used to assess SVR included COBAS TaqMan HCV test (version 2.0, Roche, Branchburg, USA) for use with the High Pure System, COBAS AmpliPrep/COBAS TaqMan HCV test (version 2.0, Roche, Branchburg, USA).
Clinical trials
HCV trials to support approval or to expand an indication of 12-week IFN-free DAA regimens with or without ribavirin (RBV) in the treatment of HCV GT1 infection submitted to the FDA between 2013 and 2017 were pooled (Supplemental Table S1). The intent of these analyses was to explore efficacy comparisons by ethnicity, not to compare SVR across different regimens.
Supplemental Table S1.
Data included in analysis for 12-week regimens
| Drugs | Study | Study population/subgroups | Number of subjects (n) | Approved 12-week regimen |
|---|---|---|---|---|
| DCV | ALLY-2 | GT1, TN/TE, ±cirrhosis, HCV/HIV co-infection | 127 | DCV + SOF |
| EBR and GZR | C-EDGE co-infection | GT1a without baseline NS5A polymorphisms/GT1b, TN, ±cirrhosis, HCV/HIV co-infection | 179 | EBR/GZR |
| C-EDGE TE | GT1a without baseline NS5A polymorphisms/GT1b, TE, ±cirrhosis, HCV mono-infection or HCV/HIV co-infection | 86 (80 mono-infections, 6 co-infections) | EBR/GZR | |
| C-EDGE TN | GT1a without baseline NS5A polymorphisms/GT1b, TN, ±cirrhosis | 267 | EBR/GZR | |
| C-SALVAGE | GT1a without baseline NS5A polymorphisms/GT1b, TE, ±cirrhosis | 76 | EBR/GZR + ribavirin (RBV) | |
| C-SURFER | GT1a without baseline NS5A polymorphisms/GT1b, TN/TE, ±cirrhosis, severe renal impairment | 115 | EBR/GZR | |
| LDV and SOF | ION-1 | GT1, TN, ±cirrhosis | 213 | LDV/SOF |
| ION-2 | GT1, TE, without cirrhosis | 87 | LDV/SOF + RBV | |
| ION-3 | GT1, TN, without cirrhosis | 216 | LDV/SOF | |
| ION-4 | GT1, TN/TE, ±cirrhosis, HIV/HCV co-infection | 327 | Same as mono-infection | |
| GLE and PIB | Expedition-1 | GT1, TN/TE (PRS), with cirrhosis | 90 | GLE/PIB |
| Expedition-4 | GT1, TN/TE (PRS), ±cirrhosis, severe renal impairment | 55 | GLE/PIB | |
| Magellon-1 | GT1, TE (NS3/4A PI), ±cirrhosis | 25 | GLE/PIB | |
| OBV and PTV-r + DAS | PEARL-II | GT1b, TE, without cirrhosis | 91 | OBV/PTV-r + DAS |
| PEARL-III | GT1b, TN, without cirrhosis | 209 | OBV/PTV-r + DAS | |
| PEARL-IV | GT1a, TN, without cirrhosis | 100 | OBV/PTV-r + DAS + RBV | |
| SAPPHIRE-I | GT1a, TN, without cirrhosis | 322 | OBV/PTV-r + DAS + RBV | |
| SAPPHIRE-II | GT1a, TE, without cirrhosis | 173 | OBV/PTV-r + DAS + RBV | |
| TURQUOISE I | GT1, TN/TE, ±cirrhosis, HCV/HIV co-infection | 25 | Same as mono-infection | |
| TURQUOISE III | GT1b, TN/TE, with cirrhosis | 60 | OBV/PTV-r + DAS | |
| SMV | COSMOS | GT1, TN/TE, without cirrhosis | 21 | SMV + SOF |
| OPTIMIST-1 | GT1, TN/TE, without cirrhosis | 155 | SMV + SOF | |
| SOF and VEL | ASTRAL-1 | GT1, TN/TE, ±cirrhosis | 328 | SOF/VEL |
| ASTRAL-5 | GT1, TN/TE, ±cirrhosis, HIV co-infection | 78 | SOF/VEL | |
| SOF, VEL and VOX | Polaris-1 | GT1, TE, ±cirrhosis | 150 | SOF/VEL/VOX |
| Polaris-4 | GT1a, TE, ±cirrhosis | 36 | SOF/VEL/VOX |
DAS: dasabuvir; DCV: daclatasvir; EBR: elbasvir; GLE: glecaprevir; GT1: genotype 1; GZR: grazoprevir; HCV: hepatitis C virus; LDV: ledipasvir; NS3/4A PI: HCV NS3/4A protease inhibitor; NS: HCV nonstructural protein; OBV: ombitasvir; PIB: pibrentasvir; PRS: Prior treatment experience with regimens containing (peg) interferon, ribavirin, and/or sofosbuvir, but no prior treatment experience with an HCV NS3/4A PI or NS5A inhibitor; PTV-r: paritaprevir/ritonavir; RBV: ribavirin; SMV: simeprevir; SOF: sofosbuvir; TE: treatment experienced; TN: treatment naive; VEL: velpatasvir; VOX: voxilaprevir.
Data analyses included trials of daclatasvir (DCV), elbasvir (EBR)/grazoprevir (GZR), LDV/SOF, glecaprevir (GLE)/pibrentasvir (PIB), ombitasvir/paritaprevir/ritonavir plus dasabuvir (OBV/PTV-r+DSV, OPrD), simeprevir (SMV), SOF/velpatasvir (VEL) and SOF/VEL/voxilaprevir (VOX).
Study determinants
The following determinants were evaluated: age, gender, ethnicity (black vs non-black ethnicity), body mass index (BMI), country (USA vs non-USA), cirrhosis, treatment experience, baseline HCV RNA, IL-28B genotype and HIV-1 status. In our analyses, we do not make the assumption that black subjects in non-US sites are genetically similar to black subjects in the USA.
Statistical analysis
Descriptive statistics including the point estimate and corresponding exact confidence intervals (CIs) based on inverting a two-sided test for difference in SVR12 rates between black and non-black subjects are presented in each study; the overall subjects with HCV GT1 alone and both HCV GT1 and HIV and subgroups defined by baseline characteristics include age, gender, country, BMI, cirrhotic status, GT1 subtype, HCV treatment history, baseline HCV RNA and IL-28B status.
There were 19 subgroup analyses conducted in those with HCV GT1 alone and those with both HCV GT1 and HIV, respectively. Although the analyses were exploratory, Bonferroni's method was used to address multiple comparisons.
Results
Demographics and baseline characteristics
Overall, 26 clinical trials (20 trials with subjects with HCV GT1 alone, five trials with subjects with both HCV GT1 and HIV and one trial with both mono-infected and co-infected subjects) were pooled and included 2869 indivudals with HCV GT1 alone and 742 individuals with both HCV GT1 and HIV. Eleven trials enrolled both treatment-naive (TN) and treatment-experienced (TE) subjects; the remaining 15 trials were conducted in either TN subjects only (n = 7) or TE subjects only (n = 8). Only five trials (19%) included the use of RBV. All FDA-approved 12-week IFN-free DAA regimens were represented in this analysis, and most subjects received an OPrD-based regimen (27%), an LDV/SOF-based regimen (25%) or an EBR/GZR-based regimen (20%).
Table 1 summarises the overall baseline characteristics by ethnicity for the 12-week regimens for both individuals with HCV GT1alone and both HCV GT1 and HIV. Most subjects included in our analyses were male (65%), and 18% were black. A higher proportion of black subjects with HCV GT1 alone or both HCV GT1 and HIV were from US sites. Additionally, fewer black subjects with both HCV GT1 and HIV compared with non-black subjects had HCV GT1a. A higher proportion of IL-28B CT/TT genotype was seen among black subjects with HCV GT1 alone and those with both HCV GT1 and HIV compared with non-black subjects. However, there were similar proportions of black and non-black subjects with cirrhosis at baseline among those with HCV GT1 alone and those with both HCV GT1 and HIV. Cirrhosis is generally considered to be the most clinically relevant baseline covariate to predict SVR12 rate even for DAA regimens.
Table 1.
Baseline characteristics by ethnicity (black vs non-black subjects) for 12-week regimens
| Mono-infection | Co-infection | |||||
|---|---|---|---|---|---|---|
| Overall (N = 2869) | Black (n = 408) | Non-black (n = 2461) | Overall (N = 742) | Black (n = 243) | Non-black (n = 499) | |
| Age (years) | ||||||
| Mean (SD) | 53.6 (10.7) | 57.5 (7.8) | 53.0 (11.0) | 51.5 (8.8) | 54.8 (7.9) | 49.9 (8.7) |
| Median (Q1, Q3) | 56.0 (48.0, 61.0) | 59.0 (54.0, 62.0) | 55.0 (47.0, 60.0) | 52.0 (47, 58) | 56.0 (51.0, 60.0) | 51.0 (45.0, 56.0) |
| Male | 60.5% (1737) | 67.6% (408) | 59.4% (1461) | 84.8% (629) | 75.7% (184) | 89.2% (445) |
| USA | 56.2% (1612) | 94.6% (386) | 49.8% (1226) | 80.1% (594) | 97.9% (238) | 71.3% (356) |
| BMI (kg/m2) | ||||||
| Mean (SD) | 27.1 (5.0) | 29.5 (4.8) | 26.7 (4.9) | 26.6 (4.8) | 28.7 (6.0) | 25.6 (3.8) |
| Median (Q1, Q3) | 26.5 (23.6, 30.0) | 28.7 (26.1, 32.6) | 26.1 (23.3, 29.4) | 25.9 (23.5, 28.7) | 27.7 (24.6, 31.3) | 25.3 (23.1, 27.7) |
| Cirrhosis | ||||||
| Yes | 16.6% (475) | 15.4% (63) | 16.7% (412) | 18.1% (134) | 17.7% (43) | 18.2% (91) |
| No | 83.3% (2391) | 84.6% (345) | 83.1% (2046) | 81.3% (603) | 80.7% (196) | 81.6% (407) |
| Missing | 0.1% (3) | 0% | 0.1% (3) | 0.7% (5) | 1.6% (4) | 0.2% (1) |
| Subtype | ||||||
| GT1a | 63.4% (1820) | 68.6% (280) | 62.6% (1540) | 77.9% (578) | 70.0% (170) | 81.8% (408) |
| GT1b | 36.1% (1036) | 29.9% (122) | 37.1% (914) | 21.8% (162) | 29.6% (72) | 18.0% (90) |
| Other* | 0.5% (13) | 1.5% (6) | 0.3% (7) | 0.3% (2) | 0.4% (1) | 0.2% (1) |
| HCV treatment experienced | 34.1% (979) | 37.3% (152) | 33.6% (827) | 59.3% (440) | 54.3% (132) | 61.7% (308) |
| IL-28B | ||||||
| CC | 23.3% (668) | 12.3% (50) | 25.1% (618) | 25.9% (192) | 11.1% (27) | 33.1% (165) |
| CT | 57.5% (1650) | 49.3% (201) | 58.9% (1449) | 53.5% (397) | 50.2% (122) | 55.1% (275) |
| TT | 19.0% (544) | 37.5% (153) | 15.9% (391) | 20.6% (153) | 38.7% (94) | 11.8% (59) |
| Missing | 0.2% (7) | 1.0% (4) | 0.1% (3) | 0% | 0% | 0% |
| Baseline HCV RNA ≥ 800,000 IU/mL | 77.6% (2227) | 78.7% (321) | 77.4% (1906) | 79.2% (588) | 81.5% (198) | 78.2% (390) |
BMI: body mass index; HCV: hepatitis C virus; IL: interleukin.
*Including subjects with other subtypes, undetermined subtype and missing information on subtype.
SVR12 rates in HCV GT1-mono-infected black vs non-black subjects
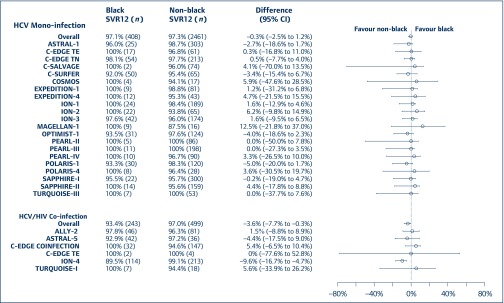
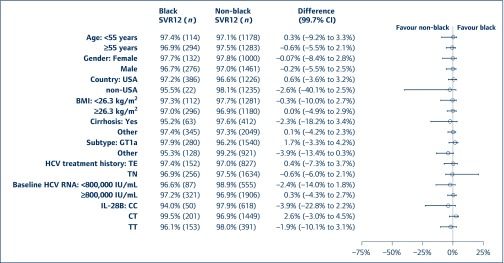
Of the 2869 HCV GT1-mono-infected subjects, 408 (14.2%) were black. Across 21 clinical trials, SVR12 rates were between 92% and 100% in black individuals and between 87.5% and 100.0% in non-black individuals. In pooled analyses of each DAA regimen, the difference in SVR12 rates was numerically similar for black and non-black subjects (97.1% vs 97.3%, Figure 1). Baseline characteristics did not appear to affect SVR12 rates for the two groups (Figure 2). SVR12 rates were also numerically similar for black and non-black subjects with cirrhosis (95.2% vs 97.6%, Figure 2). As shown in Table 2, for mono-infected subjects, the proportions of on-treatment virological failure and relapse were similar between black and non-black subjects.
Figure 1.
Difference in SVR12 rate between black and non-black subjects for those with HCV GT1 alone and those with both HCV GT1 and HIV for 12-week regimens. CI: confidence interval; GT1: genotype 1; HCV: hepatitis C virus; SVR12: sustained virological response assessed 12 weeks following cessation of treatment; TE: treatment experienced; TN: treatment naive
Figure 2.
Difference in SVR12 rates between black and non-black subjects by subgroups for those with HCV GT1 alone in 12-week regimens. *: Including non-cirrhotic subjects and subjects with missing cirrhosis status; **: including GT1b subjects and subjects with other or undetermined subtype; ***: excluding subjects with missing IL-28B status. BMI: body mass index; CI: confidence interval; HCV: hepatitis C virus; SVR12: sustained virological response assessed 12 weeks following cessation of treatment; TE: treatment experienced; TN: treatment naive
Table 2.
Virological outcome at post-treatment week 12 for 12-week regimens
| Study | Black | Non-black |
|---|---|---|
| Mono-infection | ||
| n | 408 | 2461 |
| SVR12 rate | 97.1% (396) | 97.3% (2395) |
| Not achieving SVR12 | ||
| On-treatment virological failure | 0.2% (1) | 0.1% (2) |
| Relapse | 1.0% (4) | 1.4% (34) |
| Other | 1.7% (7) | 1.2% (30) |
| Co-infection (including ION-4) | ||
| n | 243 | 499 |
| SVR12 rate | 93.4% (227) | 97.0% (484) |
| Not achieving SVR12 | ||
| On-treatment virological failure | 0.8% (2) | 0.2% (1) |
| Relapse | 5.3% (13) | 1.0% (5) |
| Other | 0.4% (1) | 1.8% (9) |
| Co-infection (excluding ION-4) | ||
| n | 129 | 286 |
| SVR12 rate | 96.9% (125) | 95.5% (273) |
| Not achieving SVR12 | ||
| On-treatment virological failure | 0% (0) | 0.3% (1) |
| Relapse | 2.3% (3) | 1.7% (5) |
| Other | 0.8% (1) | 2.4% (7) |
| ION-4 | ||
| n | 114 | 213 |
| SVR12 rate | 89.5% (102) | 99.1% (211) |
| Not achieving SVR12 | ||
| On-treatment virological failure | 1.8% (2) | 0% (0) |
| Relapse | 8.8% (10) | 0% (0) |
| Other | 0% (0) | 0.9% (2) |
SVR12: sustained virological response assessed 12 weeks following cessation of treatment.
SVR12 rates in black and non-black subjects with both HCV GT1 and HIV
Of the 742 subjects with HCV GT1 and HIV, 243 (32.7%) were black. Across six clinical trials, SVR12 rates were between 89.5% and 100% in black individuals and between 94.4% and 100.0% in non-black subjects. In pooled analyses for HCV GT1/HIV co-infection, black subjects had a 4% lower SVR12 rate than non-black subjects (93.4% vs 97.0%, 95% CI −7.7% to −0.3%).
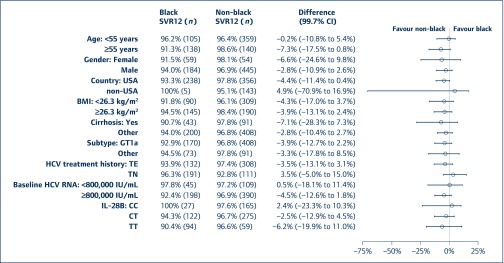
As illustrated in Figure 1, this difference was driven by ION-4 in which the SVR12 rate for black subjects was approximately 10% lower than that for non-blacks (89.5% vs 99.1%). Baseline characteristics did not appear to affect SVR12 rates for the two ethnicity groups (Figure 3). Of note, SVR12 rates were numerically higher for non-black subjects with cirrhosis (n = 91) than for black subjects (n = 43) (97.8% vs 90.7%, Figure 3). This finding is limited by the number of individuals in this subgroup. For individuals with both HCV GT1 and HIV who did not take part in ION-4, SVR12 rates for black subjects (96.9%) were numerically comparable with that of non-black subjects (95.5%). Additionally, the proportion of on-treatment virological failure was similar between black and non-black subjects (Table 2). There was a numerical difference in the proportion of black and non-black subjects who experienced virological relapse (5.3% vs 1.0%). This result was driven by ION-4, where there was relapse in 8.8% of black subjects but 0% in non-black subjects.
Figure 3.
Difference in SVR12 rates between black and non-black subjects by subgroups for those with both HCV GT1 and HIV in 12-week regimens. *: Including non-cirrhotic subjects and subjects with missing cirrhosis status; **: including GT1b subjects and subjects with other or undetermined subtype. BMI: body mass index; CI: confidence interval; HCV: hepatitis C virus; SVR12: sustained virological response assessed 12 weeks following cessation of treatment; TE: treatment experienced; TN: treatment naïve
Of the 43 black subjects with HCV GT1, HIV and cirrhosis, the SVR12 rate was 90.7%. Of 200 black subjects without cirrhosis and both HCV GT1 and HIV, SVR12 rates were 94%. These differences were not statistically significant.
Discussion
Prior to the advent of IFN-free DAA regimens, HCV GT1 was considered difficult-to-treat [36]. Since 2013, several IFN-free DAA regimens have been approved with SVR12 rates that exceed 90% for the overall GT1 population and 95% for certain GT1 subpopulations [27–34]. Additional data to further characterise SVR12 rates in various subgroups are helpful in establishing outcomes. The pooled analysis population comprises a substantially larger dataset compared with individual clinical development programmes for black subjects with HCV GT1 infection and allows for several observations, including among a multitude of baseline characteristics (age, gender, country, BMI, cirrhosis, treatment experience, HCV RNA and IL-28B genotype), as well as for HIV co-infection. A sizeable proportion of the overall study population is from the USA, including a high proportion of black subjects from the USA (386 out of 408 subjects with HCV GT1 alone and 238 out of 243 subjects with both HCV GT1 and HIV). As there were few black subjects who were not from the USA (22 [5.4%] out of 408 of those with HCV GT1 alone and five [2.1%] of 243 with both HCV GT1 and HIV), we grouped them together for simplicity although we do not make the assumption that these subgroups are genetically similar, and acknowledge that the overall number of black non-US subjects is limited and separate analyses of this subgroup would not generate meaningful data.
To our knowledge, our analyses represent the most systematic review of 12-week IFN-free DAA SVR12 clinical trial data in black subjects to date and provide another data source regarding SVR12 rates among black and non-black subjects with HCV GT1 alone and with both HCV GT1 and HIV [27–34].
SVR12 rates in black and non-black individuals with HCV GT1 alone
Despite varying SVR12 rates among black and non-black individualss reported from real-world observational cohorts, our pooled analysis showed consistently high SVR12 rates of at least 94% (range 94%–99.5%) in all 19 subgroups evaluated. Notably, ethnicity did not affect SVR12 rates. Our results provide data on all the approved 12-week DAAs for the treatment of HCV GT1 infection, and our SVR 12 findings are comparable with those findings reported in observational cohorts, retrospective subgroup analyses for a given DAA and meta-analyses of published literature [12–17].
SVR12 rates in black and non-black individuals with both HCV GT1 and HIV
Individuals with both HCV GT1 and HIV have increased liver-related morbidity and mortality, non-hepatic organ dysfunction, and overall mortality than those with HCV alone [36–40]. Efforts are ongoing to ensure that treatment of HCV infection in individuals living with HIV is a priority. Additional advances related to IFN-free DAA regimens include improved HCV treatment uptake and high SVR rates that are independent of HIV co-infection [36,40]. Although a numerical difference in SVR12 rates between black and non-black subjects with both HCV GT1 and HIV was seen in our analyses, it is noteworthy that this difference was driven by a single trial (ION-4) with its associated uncertainty. In ION-4, relapse was observed only in black subjects (relapse rate 9%), all of whom were IL-28B non-CC genotype. This difference in relapse rate between ION-4 black and non-black subjects is not explained by differences in LDV/SOF exposure, concomitant antiretroviral regimen or adherence, or pharmacogenomic markers; thus, there remains a degree of uncertainty in understanding why this difference in relapse rate was observed [18,29]. Given the overall sample size in ION-4 and the overall high SVR in this study, it is unclear if clinically meaningful differences exist. In the ION-1, ION-2 and ION-3 HCV mono-infection trials submitted with the original LDV/SOF new drug application (NDA), relapse rates were 3% (10/305) in black subjects and 2% (26/1637) in non-black subjects [29]. Overall, our pooled findings of high SVR12 rates between black and non-black individuals differ from the single ION-4 trial and from the Veterans Affairs observational cohort data, which lower SVR12 rates in black compared with non-black individuals [15,18].
Aside from the ION-4 trial, our extensive subgroup analyses of baseline characteristics (age, gender, country, BMI, cirrhosis, treatment experience, HCV RNA and IL-28B status) showed no differences between SVR12 rates for black and non-black individuals with both HCV GT1 and HIV.
Given the degree of uncertainty regarding the relapse rate difference in ION-4, we believe our findings overall support the treatment guidelines as well as the published literature (primarily derived from observational cohorts or from retrospective subgroup analysis of a given individual DAA regimen) that DAAs have similar SVR12 rates among those with both HCV GT1 and HIV, and those with HCV alone [19–26].
Limitations of the FDA's HCV GT1 database
We do acknowledge that treatment durations could impact SVR and have differential outcomes for black vs non-black subjects; however, the small numbers of GT1 subjects from clinical trials with other treatment durations (i.e. 8, 16 and 24 weeks) precluded the ability to conduct the analyses described earlier. Only 24 (4.7%) black subjects with HCV alone were enrolled in clinical trials with other treatment durations. No black subjects with both HCV GT1 and HIV were enrolled in clinical trials with other treatment durations [27–34]. As a result, the assessment of differential outcomes for black vs non-black subjects in clinical trials with other treatment durations is not a part of this work. Additionally, we acknowledge that other factors may affect SVR rates. Real-world cohorts such as the Veterans Cohort study may differ from clinical trial participants and may include those with more advanced disease and other factors not otherwise represented in clinical trials that could affect SVR rates.
Conclusion
The balance of clinical trial data and real-world observational data can help inform treatment guideline decisions regarding regimen and duration for various subgroups such as ethnicity. Our pooled analyses included all 12-week approved DAA regimens; however, some regimens had a limited number of HCV GT1 black subjects. Although the small numbers of black subjects with HCV GT1from clinical trials precluded the ability to make definitive conclusions when evaluating efficacy by ethnicity the individual SVR12 rates for the trials included in our analyses ranged from 89.5% to 100% and did not substantially differ between HCV GT1-mono-infection and HCV GT1/HIV-co-infection.
Further evaluation from clinical trials and real-world observational cohorts will help inform possible underlying reasons that affect SVR12 rates based on ethnicity or other factors. At the time of initial approval, it is not possible to address all factors for varying response rates among subgroups in clinical trials. Post-approval trials and other sources of data, such as real-world observational cohorts, can help identify factors associated with treatment success or failure. More representation of minority subgroups is needed in clinical trials to better assess possible efficacy or safety differences at the time of initial approval.
FDA has a Drug Trials Snapshot database to provide consumers with information about who participated in clinical trials that supported the FDA approval of new drugs. The information provided in these snapshots also highlights whether there were any differences in the benefits and side effects among gender, ethnicity and age groups. Drug Trials Snapshots is part of an overall FDA effort to make demographic data more available and transparent (www.fda.gov/Drugs/InformationOnDrugs/ucm412998.htm).
We hope these data help raise awareness of the need for widespread participation in clinical trials, including among black subjects in general, as well as those with both HCV and HIV, with the potential to address the issue of ethnicity differences throughout drug development and to have data available at the time of initial approval regarding any potential subgroup differences.
Acknowledgements
The data analysed for this manuscript were submitted in the NDAs for DCV, EBR/GZR, GLE/PIB, LDV/SOF, OBV/PTV-r and DSV, SMV, SOF/VEL and SOF/VEL/VOX. The authors acknowledge the study sponsors, investigators and study participants as the source of these data. The authors acknowledge John Farley for his assistance with the manuscript.
Part of this work was presented at The Liver Meeting 2017, Washington, DC, October 2017; Poster 1140: SVR12 Outcomes for approved HCV DAAs in black subjects with HCV genotype (GT) 1 infection. The authors acknowledge Wen Zeng, Fraser Smith, Guoxing Soon, Sarita Boyd, Adam Sherwat, Wendy Carter, Russell Fleischer, Prabha Viswanathan, Takashi Komatsu, Patrick Harrington, Lisa Naeger and Julian O’Rear.
Disclaimer
This article reflects the views of the authors and should not be construed to represent FDA's views or policies.
Conflicts of interest
All authors have no potential conflicts of interest related to this article.
Funding
There was no funding source for this study. The corresponding authors had full access to all data and were responsible for the decision to submit for publication.
References
- 1. World Health Organization (WHO) Global hepatitis report, 2017. Geneva 2017. Available at: apps.who.int/iris/bitstream/handle/10665/255017/WHO-HIV-2017. 06-eng.pdf;jsessionid=0B90A84BC8751B26238BE6001D464B99?sequence=1 ( accessed May 2019).
- 2. Centers for Disease Control and Prevention Epidemiology and prevention of HIV and viral hepatitis co-infections. CDC, 2018. Available at: www.cdc.gov/hepatitis/populations/hiv.htm ( accessed May 2019).
- 3. Edlin BR, Eckhardt BJ, Shu MA et al. Toward a more accurate estimate of the prevalence of hepatitis C in the United States. Hepatology 2015; 62: 1353– 1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Platt L, Easterbrook P, Gower E et al. Prevalence and burden of HCV co-infection in people living with HIV: a global systematic review and meta-analysis. Lancet Infect Dis 2016; 16: 797– 808. [DOI] [PubMed] [Google Scholar]
- 5. Backus LI, Boothroyd DB, Phillips BR et al. A sustained virologic response reduces risk of all-cause mortality in patients with hepatitis C. Clin Gastroenterol Hepatol 2011; 9: 509– 516. [DOI] [PubMed] [Google Scholar]
- 6. Meer AJ, Veldt BJ, Feld JJ et al. Association between sustained virological response and all-cause mortality among patients with chronic hepatitis C and advanced hepatic fibrosis. JAMA 2012; 308: 2584– 2593. [DOI] [PubMed] [Google Scholar]
- 7. Wilder J, Saraswathula A, Hasselblad V et al. A systematic review of race and ethnicity in hepatitis C clinical trial enrollment. J Natl Med Assoc 2016; 108: 24– 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Reddy KR, Hoofnagel JH, Tong MJ et al. Racial differences in responses to therapy with interferon in chronic hepatitis C. Consensus Interferon Study Group. Hepatology 1999; 30: 787– 793. [DOI] [PubMed] [Google Scholar]
- 9. Ge D, Fellay J, Thompson AJ et al. Genetic variation in IL28B predicts hepatitis C treatment-induced viral clearance. Nature 2009; 461: 399– 401. [DOI] [PubMed] [Google Scholar]
- 10. Victrelis® (package insert) Whitehouse Station, NJ: Schering Corporation, 2017. Available at: www.accessdata.fda.gov/drugsatfda_docs/label/2017/202258s016lbl.pdf ( accessed May 2019).
- 11. Incivek® (package insert) Cambridge, MA: Vertex Pharmaceuticals, Inc., 2013. www.accessdata.fda.gov/drugsatfda_docs/label/2013/201917s012lbl.pdf ( accessed May 2019).
- 12. Su F, Green PK, Berry K et al. The association between race/ethnicity and the effectiveness of direct antiviral agents for hepatitis C virus infection. Hepatology 2017; 65: 426– 438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. O’Brien TR, Kottilil S, Feld JJ et al. Race or genetic makeup for hepatitis C virus treatment decisions? Hepatology 2017; 65: 2124– 2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Backus LI, Belperio PS, Shahoumian TA et al. Real-world effectiveness of ledipasvir/sofosbuvir in 4,365 treatment-naive, genotype 1 hepatitis C-infected patients. Hepatology 2016; 64: 405– 414. [DOI] [PubMed] [Google Scholar]
- 15. Backus LI, Belperio PS, Shahoumian TA et al. Real-world effectiveness and predictors of sustained virological response with all-oral therapy in 21,242 hepatitis C genotype-1 patients. Antivir Ther 2017; 22: 481– 493. [DOI] [PubMed] [Google Scholar]
- 16. Wilder JM, Jeffers LJ, Ravendhran N et al. Safety and efficacy of ledipasvir-sofosbuvir in black patients with hepatitis C virus infection: a retrospective analysis of phase 3 data. Hepatology 2016; 63: 437– 444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Falade-Nwulia O, Suarez-Cuervo C, Nelson DR et al. Oral direct-acting agent therapy for hepatitis C virus infection: a systematic review. Ann Intern Med 2017; 166: 637– 648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Naggie S, Cooper C, Saag M et al. Ledipasvir and sofosbuvir for HCV in patients coinfected with HIV-1. N Engl J Med 2015; 373: 705– 713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sulkowski MS, Eron JJ, Wyles DL et al. Ombitasvir, paritaprevir co-dosed with ritonavir, dasabuvir, and ribavirin for hepatitis C in patients co-infected with HIV-1: a randomized trial. JAMA 2015; 313: 1223– 1231. [DOI] [PubMed] [Google Scholar]
- 20. Wyles DL, Ruane PJ, Sulkowski MS et al. Daclatasvir plus sofosbuvir for HCV in patients coinfected with HIV-1. N Engl J Med 2015; 373: 714– 725. [DOI] [PubMed] [Google Scholar]
- 21. Wyles D, Bräu N, Kottilil S et al. Sofosbuvir and velpatasvir for the treatment of HCV in patients coinfected with HIV-1: an open-label, phase 3 study. Clin Infect Dis 2017; 65: 6– 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bhattacharya D, Belperio PS, Shahoumian TA et al. Effectiveness of all-oral antiviral regimens in 996 human immunodeficiency virus/hepatitis C virus genotype 1-coinfected patients treated in routine practice. Clin Infect Dis 2017; 64: 1711– 1720. [DOI] [PubMed] [Google Scholar]
- 23. Burton MJ, Naggie S. Real-world effectiveness of DAA therapies in human immunodeficiency virus/hepatitis C virus coinfection: 996 veterans can't be wrong. Clin Infect Dis 2017; 64: 1721– 1723. [DOI] [PubMed] [Google Scholar]
- 24. Falade-Nwulia O, Sutcliffe C, Moon J et al. High hepatitis C cure rates among black and non-blacks human immunodeficiency virus-infected adults in an urban center. Hepatology 2017; 66: 1402– 1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Patel M, Rab S, Kalapila AG et al. Highly successful hepatitis C virus (HCV) treatment outcomes in human immunodeficiency virus/HCV-coinfected patients at a large, urban, Ryan White clinic. Open Forum Infect Dis 2017; 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sikavi C, Chen PH, Lee AD et al. Hepatitis C and human immunodeficiency virus co-infection in the era of direct-acting antiviral agents: no longer a difficult to treat population. Hepatology 2018; 67: 847– 857. [DOI] [PubMed] [Google Scholar]
- 27. Daklinza (package insert) Princeton, NJ: Bristol-Myers Squibb Company, 2017. Available at: www.accessdata.fda.gov/drugsatfda_docs/label/2017/206843s006lbl.pdf ( accessed May 2019).
- 28. Zepatier (package insert) Whitehouse Station, NJ: Merck & Co., Inc., 2017. Available at: www.accessdata.fda.gov/drugsatfda_docs/label/2017/208261s002lbl.pdf ( accessed May 2019).
- 29. Harvoni (package insert) Foster City, CA: Gilead Sciences, Inc., 2017. Available at: www.accessdata.fda.gov/drugsatfda_docs/label/2017/205834s017lbl.pdf ( accessed May 2019).
- 30. Mavyret (package insert) North Chicago, IL: AbbVie Inc., 2017. Available at: www.accessdata.fda.gov/drugsatfda_docs/label/2017/209394s003lbl.pdf ( accessed May 2019).
- 31. Viekira Pak (package insert) North Chicago, IL: AbbVie Inc., 2017. Available at: www.accessdata.fda.gov/drugsatfda_docs/label/2017/206619s013s015lbl.pdf ( accessed May 2019).
- 32. Olysio (package insert) Titusville, NJ: Janssen Therapeutics, 2017. Available at: www.accessdata.fda.gov/drugsatfda_docs/label/2017/205123s013lbl.pdf ( Accessed May 2019).
- 33. Epclusa (package insert) Foster City, CA: Gilead Sciences, Inc., 2017. Available at: www.accessdata.fda.gov/drugsatfda_docs/label/2017/208341s002lbl.pdf ( accessed May 2019).
- 34. Vosevi (package insert) Foster City, CA: Gilead Sciences, Inc., 2017. Available at: www.accessdata.fda.gov/drugsatfda_docs/label/2017/209195s000lbl.pdf ( accessed May 2019).
- 35. Struble K, Murray J, Sherwat A et al. Guidance for industry chronic hepatitis C virus infection: developing direct-acting antiviral drugs for treatment guidance for industry. 2017. Available at: www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm225333.pdf ( accessed May 2019).
- 36. Chung RT, Ghany MG, Kim AY et al. Hepatitis C guidance: AASLD-IDSA recommendations for testing, managing, and treating hepatitis C. 2018. Available at: www.hcvguidelines.org/ ( accessed May 2019). [DOI] [PMC free article] [PubMed]
- 37. Lo Re V, Kallan MJ, Tate JP et al. Hepatic decompensation in antiretroviral-treated patients co-infected with HIV and hepatitis C virus compared with hepatitis C virus-mono-infected patients: a cohort study. Ann Intern Med 2014; 160: 369– 379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kirk GD, Mehta SH, Astemborski J et al. HIV, age, and the severity of hepatitis C virus-related liver disease: a cohort study. Ann Intern Med 2013; 158: 658– 666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Chen TY, Ding EL, Seage-Iii GR et al. Meta-analysis: increased mortality associated with hepatitis C in HIV-infected persons is unrelated to HIV disease progression. Clin Infect Dis 2009; 49: 1605– 1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Collins LF, Chan A, Zheng J et al. Direct-acting antivirals improve access to care and cure for patients with HIV and chronic HCV infection. Open Forum Infect Dis 2018; 5. [DOI] [PMC free article] [PubMed] [Google Scholar]