Abstract
Objectives
Enrolling people living with HIV with undetectable viral load into HIV cure-related clinical trials (HCRCT) is challenging. Few data are currently available about the individual factors that influence willingness to participate in HCRCT (WPHCRCT). We hypothesised that WPHCRCT would be more frequent among people living with HIV considering themselves HIV activists. The objective of this study was to investigate the individual characteristics associated with both WPHCRCT and self-identification as an HIV activist.
Methods
The study enrolled 195 long-term ART-treated and virologically suppressed people living with HIV, followed-up in 19 French HIV services, 2016–2017. A Bayesian model averaging approach was used to assess correlates of both outcomes i.e. WPHCRCT and self-identified HIV activism.
Results
WPHCRCT was reported by 43% of participants and was positively associated with self-identification as an HIV activist (adjusted odds ratio [aOR] 2.90 95% confidence interval [CI] 2.17–3.63], P<0.05) and self-confidence as an HIV positive person (aOR 1.17, 95% CI 0.99–1.35, P<0.1). Self-identified HIV activists (56% of participants) were more likely to have a higher ‘relationship with others’ score using the post-traumatic growth inventory (aOR 1.10, 95% CI 0.99–1.20, P<0.1), to obtain information about HIV from a greater number of sources (aOR 1.35 [95% CI 1.00–1.68], P<0.1), and to feel greatly affected by mandatory daily treatment (aOR 2.15, 95% CI 1.27–3.03, P<0.1). All associations had relative importance weight>0.75, indicating strong evidence.
Conclusions
WPHCRCT is strongly related to HIV activism, and also to positive psychosocial characteristics as a person living with HIV, especially regarding relationships with others. The desire to contribute to the fight against HIV for the sake of the HIV community and society should be taken into account to improve participation in upcoming HCRCT.
Keywords: willingness to participate in clinical trials, cure trial, HIV, activism, cure research, France
Introduction
Ever since the emergence of the epidemic, people living with HIV have had an important influence on shaping the HIV research landscape [1], by staunchly advocating for more ethical, community-based and participant-centred clinical trials [2,3] and care. Thanks to major advances in diagnosis, treatment efficacy, the reduction of mortality and management of side effects, the health and quality of life (QOL) of people living with HIV have significantly improved, with HIV becoming a manageable chronic condition in high-income countries [4,5]. Nevertheless, people living with HIV still have to face stigma, comorbidities and the challenges that lifelong daily adherence to antiretroviral therapy (ART) brings. Despite being indispensable to limit HIV transmission, ART adherence nonetheless serves as a continual reminder of the condition, and negatively affects QOL [6,7]. Recent medical progress has raised the possibility of an HIV cure, either through remission or eradication [8–14]. HIV-cure related clinical trials (HCRCT) have already started in various countries. They are characterised by the recruitment of people living with HIV with sustained suppressed viral load, for whom little or no direct benefit is expected, and who must agree to ART interruption (ATI) and its associated risks of viral rebound and HIV transmission [15,16]. Identifying and characterising people living with HIV willing to participate in HCRCT provides valuable knowledge in terms of trial design and recruitment trials [17]. In 2012, the International AIDS Society launched the ‘Towards an HIV cure’ initiative to promote multidisciplinary research for a safe, scalable and affordable cure [18,19]. Since then, various social sciences researchers have studied the attitudes, beliefs and perceptions regarding participation in HIV cure research [15,20–22] of people living with HIV. However, as no specific criteria – other than clinical criteria – were set out in these studies, the people living with HIV they recruited were not necessarily representative of people living with HIV recruited in present and future HCRCTs [23]. Results from these studies suggest that individual factors including age, socioeconomic status, perceived health status, HIV clinical outcomes, history of ART and time living with HIV, all have an effect on WPHCRCT. As suggested by previous qualitative data from the ANRS-APSEC study (‘Acceptability, expectations and preferences for HCRCT among patients with undetectable viral load and caregivers’) [17,24,25] and other studies [20,26,27], WPHCRCT is strongly dependent on the altruistic desire to help scientific research and on the feeling that one is an HIV activist, which does not necessarily mean being active in an association in the fight against HIV-AIDS. Despite these studies, few quantitative data are available about the effect of psychosocial characteristics, especially self-identified HIV activism, on WPHCRCT.
The multicentre ANRS-APSEC study was implemented in France in order to develop guidelines for ethical and suitable recruitment in HCRCT. It collected data about characteristics of people living with HIV meeting the clinical criteria for inclusion in HCRCT, as well as their viewpoints regarding these trials using complementary mixed methods [17,24].
Using data from ANRS-APSEC, the objective of the present study was to evaluate a) the effect of HIV activism and other psychosocial people living with HIV characteristics on WPHCRCT and b) the factors associated with HIV activism among people living with HIV who meet the clinical criteria for inclusion in HCRCT. When exploring the factors associated with an outcome among a large amount of potentially explanatory variables, one of the main issues in standard regression procedures is the uncertainty linked to the process of selecting a final model, especially regarding research questions, lacking empirical guidance [28]. In order to circumvent this issue, we used an approach which combined the estimates of all possible models given the explanatory variables.
Methods
Setting, design, and data collection
ANRS-APSEC was a cross-sectional study conducted throughout France between October 2016 and March 2017, which collected information about the acceptability, expectations and preferences regarding HCRCT of people living with HIV. The target population were people living with HIV followed up in HIV care centres participating in clinical research, who met the clinical inclusion criteria for eligibility in planned HCRCT (i.e. on a stable ART regimen for at least 6 months, having an undetectable viral load for at least 3 years and having a CD4 cell count >500 cells/mm3). In total 260 eligible people living with HIV were invited to participate in the study by their care provider during a follow-up visit. Of these, 195 patients (75%) from 19 HIV centres were enrolled after providing informed written consent. Clinical data were obtained from medical files. Sociodemographic and psychosocial data were collected using a self-administered questionnaire, including questions about experience with HIV, the search for information on HIV, assessment of ART and perception of HIV cure research.
Outcomes
WPHCRCT was defined as answering ‘Yes, definitely’ to the question ‘If a clinical trial were based on your preferred HIV cure strategy, would you wish to participate in it?’ (versus ‘Not at all’, ‘Not really’ or ‘Yes perhaps’). The preferred strategy was defined by ranking the different attributes of HIV cure strategies (study duration, follow-up visit frequency, moderate and severe side effects, duration, and chance of success of ATI [29–31]), according to the Discrete Choice Experiment method [32,33]. These five attributes and their levels were defined through consensus with the physicians of the scientific committee of the ANRS-APSEC study. Self-identification as an HIV activist was defined as answering ‘Yes, definitely’ or ‘Yes, rather’ (versus ‘Not at all’ or ‘Not really’) to the question ‘Do you define yourself as an activist in the fight against HIV?’.
Explanatory variables
Sociodemographic variables
Demographic and socioeconomic variables included age (continuous), sex, full-time permanent work contract (yes/no), perceived financial situation (difficult: ‘I find it hard to get by’ or ‘I cannot get by without going into debt’/average: ‘I just get by, I have to be careful’/good: ‘I get by without struggling’/very good: ‘I am financially comfortable’), educational level (no high-school diploma/ high-school diploma/post-secondary diploma), having a partner/partners (yes/no), living with one's steady partner (yes/no), having dependents (yes/no) and HIV status of one's steady partner (HIV-positive/HIV-negative/no partner).
Clinical characteristic variables
Clinical characteristic variables included: diagnosed with HIV before 1996 (yes/no), delay between HIV diagnosis and first ART (continuous), time on current ART (continuous), time since the most recent detectable viral load (continuous), on integrase inhibitors (yes/no), on protease inhibitors (yes/no), on combination ART (yes/no), nadir CD4 cell count (<200 cells/mm3; ≥200 cells/mm3 ), hepatitis C co-infection (yes/no), comorbidities (none/one/≥2), lifetime experience of depression and evolution of morale in the previous 5 years (improved or unchanged morale and no depression/deteriorated morale and depression/deteriorated morale and no depression/improved or unchanged morale and depression).
Psychosocial variables
Five visual analogue scales were used as continuous variables for self-description as a person with HIV from 0 to 10 (scale endpoints: ashamed/proud; self-confident/not self-confident, sick/in good health, excluded/socially integrated, and vulnerable/strong, respectively).
We analysed the degree to which participants were affected by the following disadvantages of living with HIV (with answers dichotomised into ‘feeling greatly affected’ versus ‘slightly or not at all affected’): risk of transmitting HIV; condom use during sex; living with a secret; HIV-related discrimination; feeling the future will be uncertain because of HIV; a shorter life expectancy; difficulties in building a steady relationship; side effects of current ART; excessive fatigue; not living a normal life; negative effects of HIV on health, professional life and sexual life; mandatory daily treatment; the cost for the ‘collectivity’ (i.e. the French general population); out-of-pocket expenses related to HIV. Other psychosocial variables and variables related to living with HIV included: a sense of belonging to a community (people living with HIV, LGBT, heterosexuals, drug users: yes/no); moral support (not having someone to talk to about issues related to HIV/having someone or not concerned); perception of health evolution since HIV acquisition (deteriorated/unchanged or improved); ease of daily treatment (easy or very easy/difficult or very difficult); discomfort related to ART-related side effects (slightly or not troublesome side effects or no side effects/quite troublesome/very troublesome). Post-traumatic growth following HIV diagnosis was measured using the 21-item post-traumatic growth inventory (PTGI) scale, which is considered a reliable tool for patients with HIV (34) and evaluated five domains: spiritual change (2 items), new possibilities (5 items), appreciation of life (3 items), relating to others (7 items), and personal strength (4 items). PTGI global score and sub-scores were used as continuous variables.
Information about health and HIV
The six-item questionnaire from the European Health Literacy Survey project (HLS-EU 6) was used to evaluate health literacy. The mean scores were used to create a three-category variable (inadequate, problematic and sufficient health literacy) (35). The number of HIV information sources was used as a continuous explanatory variable with each of the following sources counting for one point: HIV medical staff; attending physician; media (reading, TV, radio, internet, social networks); scientific articles or journals; associations for people living with HIV; friends or relatives; and other people with HIV. Participants’ perceptions of information received from their physician about HIV scientific breakthroughs was defined as a dichotomous variable (not sufficiently or not at all informed/sufficiently or very well informed).
Perception of ART efficacy and HIV cure
Variables related to participants’ perception of ART and HIV cure included: duration of ART efficacy (short-term/mid-term/long-term/do not know); arrival of an HIV cure during one's lifetime (yes/no/do not know); having heard about HCRCT (yes/no); previous participation experience in a clinical trial (positive/ negative/no experience).
Statistical analysis
One of the main issues surrounding our analysis of the factors associated with both WPHCRCT and self-identification as an HIV activist was the lack of empirical guidance. In order to circumvent the risk of erroneous conclusions, arising from the fact that inference was based on a single model, two Bayesian model averaging (BMA) were implemented to estimate the factors associated with each outcome [36]. Briefly, this approach combines the estimates of all possible models according to the explanatory variables. This allows both the uncertainty linked to the process of selecting a final model with standard regression procedures and the ranking of explanatory variables by their relative importance to be incorporated [28,37]. We used relative importance weights (RIW, values between 0 and 1) to classify the explanatory factors according to the weight of the evidence supporting the presence of an actual relationship with the dependent variable [38] using the following classification: [0–0.5]=no evidence; [0.5–0.75]=weak evidence; [0.75–0.90]=positive evidence; [0.95–0.99]=strong evidence; [0.99–1]=very strong evidence [39].
Results
All participants enrolled in the ANRS-APSEC study were included in the present analysis (n=195), with no missing data for the studied outcomes. Participants’ median [IQR] age was 53 [45–61] years, 76% were male, 38% had a full-time permanent work contract, 21% reported financial difficulties, and 39% had no high school diploma (Table 1). Most participants declared to have at least one partner (79%). Approximately half of the participants had a steady partner (54%). Sixty-nine percent of steady partners were seronegative. Most of the study sample (70%) felt they belonged to the community of people living with HIV. Participants were living with HIV for a median of 17 (interquartile range [IQR] 11–25) years and were virally suppressed for a median of 7 (IQR 5–11) years. Two-thirds were diagnosed with HIV in 1996 or later or had a CD4 cell count nadir ≥200 cells/mm3. Almost half reported at least one comorbidity or had experienced depression during their life. Thirty-one percent declared they had personal issues related to HIV but no one to talk to about them. Approximately half felt greatly affected by the risk of transmitting the virus to someone, the cost for the collectivity and mandatory daily medication. Seventeen percent felt that their health had deteriorated since HIV acquisition. Only 16% had sufficient health literacy and only 9% declared/felt not being informed or sufficiently informed about HIV scientific breakthroughs by their doctor. Fifty-six percent self-identified as HIV activists. Fifty-seven percent believed that ART will continue to be effective over the long term. Two-thirds believed that an HIV cure treatment will be available in their lifetime and 41% had already heard about HCRCT.
Table 1.
Characteristics of ART-treated and virally suppressed participants (ANRS-APSEC study, n=195)
| n | % or median (IQR) | |
|---|---|---|
| Sociodemographic characteristics | ||
| Age (years) | 195 | 53 [45–61] |
| Sex | ||
| Male | 148 | 76% |
| Female | 47a | 24% |
| Full-time permanent work contract | ||
| Yes | 75 | 38% |
| No | 120 | 62% |
| Financial situation | ||
| Difficult | 40 | 21% |
| Average | 65 | 33% |
| Comfortable | 50 | 26% |
| Very comfortable | 40 | 21% |
| Educational level | ||
| No high-school diploma | 75 | 39% |
| High-school or post-secondary diploma | 120 | 61% |
| Having a partner or partners | ||
| Yes | 154 | 79% |
| No | 41 | 21% |
| Living with one's steady partner | ||
| Yes | 85 | 44% |
| No | 110b | 56% |
| Having dependents | ||
| Yes | 39 | 20% |
| No | 156 | 80% |
| HIV status of one's steady partner | ||
| No steady partner | 90 | 46% |
| Partner living with HIV | 32 | 16% |
| Partner living without HIV | 70 | 69% |
| Clinical characteristics | ||
| HIV diagnosis | ||
| Time since HIV diagnosis (years) | 195 | 17 [11–25] |
| HIV diagnosis in 1996 or later | 128 | 66% |
| HIV diagnosis before 1996 | 67 | 34% |
| Engagement in HIV care | ||
| Time between HIV diagnosis and first ART (years) | 195 | 1 [0–4] |
| Time on current ART (years) | 195 | 3 [2–5] |
| Time since the most recent detectable viral load (years) | 195 | 7 [5–11] |
| CD4 nadir (cells/mm3) | ||
| <200 | 66 | 34% |
| ≥200 | 122 | 63% |
| Comorbidities | ||
| 1 comorbidity | 63 | 32% |
| ≥2 comorbidities | 31 | 16% |
| Hepatitis C | 22 | 11% |
| Lifetime experience of depression and evolution of morale in the previous 5 years | ||
| Unchanged/improved morale and never experienced depression | 105 | 54% |
| Deteriorated morale and experienced depression | 15 | 8% |
| Deteriorated morale but never experienced depression | 6 | 3% |
| Unchanged/improved morale but had experienced depression | 69 | 35% |
| Psychosocial characteristics and living with HIV | ||
| Sense of belonging to a community | ||
| People living with HIV | 136 | 70% |
| LGBT | 81 | 42% |
| Heterosexuals | 51 | 26% |
| Drug users | 10 | 5% |
| Self-description as a person living with (on a scale from 0 to 10) | ||
| Ashamed (0) to proud (10) | 195 | 5 [5–8] |
| Not confident (0) to confident (10) | 195 | 8 [5–10] |
| Sick (0) to in good health (10) | 195 | 8 [6–10] |
| Excluded (0) to socially integrated (10) | 195 | 9 [6–10] |
| Vulnerable (0) to strong (10) | 195 | 8 [6–10] |
| Post-traumatic growth inventory scalec | ||
| Global score (range 21–84) | 195 | 43 [34–55] |
| ‘New possibilities’ sub-score (range 5–20) | 195 | 10 [7–14] |
| ‘Spiritual change’ sub-score (range 2–8) | 195 | 3 [2–5] |
| ‘Personal strength’ sub-score (range 4–16) | 195 | 9 [7–12] |
| ‘Relating to others’ sub-score (range 7–28) | 195 | 13 [9–17] |
| ‘Appreciation of life’ sub-score (range 3–12) | 195 | 8 [6–9] |
| Self-identification as an activist in the fight against HIV | ||
| Yes, definitely | 58 | 30% |
| Yes, rather | 51 | 26% |
| Not really | 57 | 29% |
| Not at all | 29 | 15% |
| Moral support | ||
| Not having someone to talk to about personal issues related to HIV | 60 | 31% |
| Having someone to talk to about personal issues related to HIV | 112 | 57% |
| Not concerned with personal issues related to HIV | 23 | 12% |
| Feeling greatly affected by | ||
| The risk of transmitting the virus to someone | 94 | 48% |
| The cost for the collectivity (French general population) | 94 | 48% |
| Having to use condoms during sex | 89 | 46% |
| Mandatory daily medication | 88 | 45% |
| Having to live with a secret | 84 | 43% |
| The negative effect of HIV on sexual life | 75 | 39% |
| HIV-related discrimination | 72 | 37% |
| An uncertain future because of HIV | 71 | 36% |
| A shorter life expectancy | 63 | 32% |
| Difficulties to build a stable relationship | 56 | 29% |
| Side effects of current ART | 55 | 28% |
| Excessive fatigue | 48 | 25% |
| Not living a normal life | 47 | 24% |
| A negative effect of HIV on one's professional life | 44 | 23% |
| Out-of-pocket expenses related to HIV | 42 | 22% |
| Perception of overall health evolution since HIV acquisition | ||
| Deteriorated | 34 | 17% |
| Unchanged or improved | 161 | 83% |
| Ease of taking daily treatment | ||
| Difficult or very difficult | 28 | 14% |
| Easy or very easy | 167 | 86% |
| Discomfort related to side effects of ART | ||
| Slightly or not troublesome side effects or no side effects | 120 | 62% |
| Quite troublesome | 50 | 26% |
| Very troublesome | 25 | 13% |
| Information about health and HIV | ||
| Health literacy | ||
| Health literacy score | 195 | 2.8 [2.7–3.0] |
| Inadequate health literacy | 8 | 4% |
| Problematic health literacy | 155 | 80% |
| Sufficient health literacy | 32 | 16% |
| Number of information sources about HIVd | 3 [2–4] | |
| Perceived information about HIV scientific breakthroughs from one's doctor | ||
| Not at all or not sufficiently informed | 18 | 9% |
| Sufficiently or very well informed | 177 | 91% |
| Perception of ART efficacy and HIV cure | ||
| Perception of ART efficacy | ||
| Belief in short-term efficacy of ART | 6 | 3% |
| Belief in mid-term efficacy of ART | 21 | 11% |
| Belief in long-term efficacy of ART | 112 | 57% |
| Did not know | 56 | 29% |
| Important to be cured of HIV | ||
| No | 5 | 3% |
| Yes | 190 | 97% |
| Perception of availability of HIV cure treatment | ||
| Believed that a HIV cure treatment would be available in lifetime | 119 | 61% |
| Did not believe that a HIV cure treatment would be available in lifetime | 25 | 13% |
| Did not know | 51 | 26% |
| Had heard about HIV cure-related clinical trials | ||
| No | 116 | 60% |
| Yes | 79 | 41% |
| Previous clinical trial experience | ||
| Negative experience | 5 | 3% |
| Positive experience | 78 | 40% |
| No experience | 106 | 54% |
| Willingness to participate in HIV-cure related clinical trialse | ||
| Yes, definitely | 83 | 43% |
| Yes, perhaps | 74 | 38% |
| Not really | 21 | 11% |
| Not at all | 17 | 9% |
Includes one transgender person.
20 of whom had a steady partner but did not live with him/her. In total, 105 (54%) declared having a steady partner.
Following HIV diagnosis.
Each of following information sources counts for one point: the HIV medical staff, attending physician, media (reading, TV, radio, internet, social networks), scientific articles or journals, people living with HIV associations, friends or relatives, other people living with HIV.
Outcome ‘willingness to participate in HCRCT’ studied in the present paper was defined as replying ‘Yes, definitely’ versus all other answers (i.e. ‘Yes, perhaps’, ‘Not really’, ‘Yes, perhaps’). ART: antiretroviral therapy; LGBT: lesbian, gay, bisexual, and transgender.
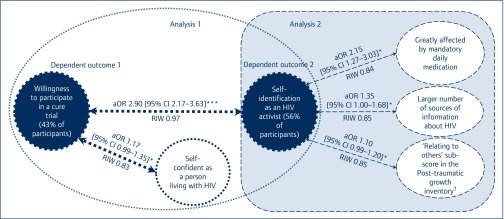
Forty-three percent stated they would definitely participate in an HCRCT based on their preferred HIV cure strategy (Table 1). Results from the first BMA showed that WPHCRCT was more frequent in participants who self-identified as HIV activists (aOR 2.90 (95% confidence interval [CI] 2.17–3.63), P<0.01 RIW 0.97) and in participants with higher scores of self-confidence as persons with (aOR 1.17, 95% CI 0.99–1.35, P<0.1, RIW 0.83) (Figure 1). No effect on WPHCRCT of other variables included in the analysis was found. Three individual factors were independently significantly associated with self-identification as an HIV activist (Figure 1): feeling greatly affected by mandatory daily treatment (aOR 2.15, 95% CI 1.27–3.03, P<0.1, RIW 0.84); obtaining information about HIV from a larger number of sources (aOR 1.35, 95% CI 1.00–1.68, P<0.1, RIW 0.85), and having a higher ‘relating to others’ PTGI sub-score (aOR 1.10, 95% CI 0.99–1.20, P<0.1, RIW 0.85). No effect on self-identification as an HIV activist of other variables included in the analysis was found. Detailed results of the analysis about factors associated with WPHCRCT and self-identification as an HIV-activist are available in supplementary files (S1 and S2, respectively).
Figure 1.
Significant associations with willingness to participate in a HIV-cure related trial (analysis 1, n=195, multimodel averaging method) and with self-identification as an HIV activist (analysis 2, n=195, multimodel averaging method)
Significant associations *P<0.1, **P<0.05, ***P<0.01 and RIW indicating positive evidence [0.75–0.90] and strong evidence ≥0.90. The exhaustive results of analyses 1 and 2 are available in the supplementary tables.
aOR: adjusted odds ratio; RIW: relative importance weights. †Following HIV diagnosis.
Supplementary table S1.
Factors associated with willingness to participate in a HIV-cure related trial (results from multivariate analysis, multi-model averaging method)
| Adjusted OR [95% CI]a | |
|---|---|
| Sociodemographic characteristics | |
| Age (years) | 1.00 [0.99–1.01] |
| Sex | |
| Male | 1.01 [0.87–1.14] |
| Female | Ref |
| Full-time permanent work contract | |
| Yes | 1.00 [0.93–1.07] |
| No | Ref |
| Financial situation | |
| Difficult | 1.00 [0.99–1.01] |
| Average | 1.00 [0.99–1.01] |
| Comfortable | 1.00 [0.98–1.02] |
| Very comfortable | Ref |
| Living with one's steady partner | |
| Yes | 1.01 [0.9–1.11] |
| No | Ref |
| Having dependents | |
| Yes | 1.00 [0.91–1.09] |
| No | Ref |
| HIV status of one's steady partner | |
| No steady partner | Ref |
| Partner living with HIV | 1.00 [0.97–1.03] |
| Partner living without HIV | 1.00 [0.95–1.05] |
| Clinical characteristics | |
| HIV diagnosis | |
| HIV diagnosis after 1996 | Ref |
| HIV diagnosis before 1996 | 0.99 [0.79–1.18] |
| Engagement in HIV care | |
| Time between HIV diagnosis and first ART (years) | 1.00 [0.99–1.01] |
| Time on current ART (years) | 0.98 [0.88–1.08] |
| Time since most recent detectable viral load (years) | 1.00 [0.99–1.01] |
| CD4 nadir (cells/mm3) | |
| <200 | 1.00 [0.92–1.09] |
| ≥200 | Ref |
| Comorbidities | |
| 1 comorbidityb | 1.00 [0.98–1.02] |
| ≥2 comorbiditiesb | 1.00 [0.97–1.03] |
| Hepatitis Cc | 1.00 [0.9–1.1] |
| Lifetime experience of depression and evolution of morale in the previous 5 years | |
| Unchanged/improved morale and no depression | Ref |
| Deteriorated morale and experienced depression | 1.00 [0.81–1.19] |
| Deteriorated morale but no depression | 1.00 [0.77–1.24] |
| Unchanged/improved morale but experienced depression | 1.00 [0.87–1.14] |
| Psychosocial characteristics and living with HIV | |
| Sense of belonging to the people living with HIV community | |
| No | Ref |
| Yes | 1.04 [0.71–1.37] |
| Self-description as a HIV-positive person (on a scale from 0 to 10) | |
| Ashamed (0) to proud (10) | 1.00 [0.98–1.02] |
| Not confident (0) to confident (10) | 1.17 [0.99–1.35]* |
| Sick (0) to in good health (10) | 1.01 [0.94–1.07] |
| Excluded (0) to socially integrated (10) | 1.00 [0.98–1.02] |
| Vulnerable (0) to strong (10) | 1.00 [0.98–1.02] |
| Self-identification as an activist in the fight against HIV | |
| No | Ref |
| Yes | 2.9 [2.17–3.63]*** |
| Moral support | |
| Not having someone to talk to about personal issues related to HIV | 1.00 [0.96–1.04] |
| Having someone to talk to about personal issues related to HIV | 1.00 [0.95–1.05] |
| Not concerned with personal issues related to HIV | Ref |
| Feeling greatly affected byd | |
| The risk of contaminating someone | 1.00 [0.93–1.07] |
| The cost for the collectivity (i.e. French general population) | 1.00 [0.92–1.09] |
| Having to use condoms during sex | 0.95 [0.57–1.33] |
| Mandatory daily medication | 1.00 [0.91–1.10] |
| Having to live with a secret | 1.00 [0.93–1.07] |
| A negative effect of HIV on one's sexual life | 1.00 [0.91–1.08] |
| HIV-related discrimination | 1.00 [0.93–1.07] |
| An uncertain future because of HIV | 1.00 [0.92–1.08] |
| A shorter life expectancy | 1.00 [0.93–1.07] |
| Difficulties to build up a stable relationship | 1.00 [0.93–1.07] |
| Excessive fatigue | 1.00 [0.92–1.08] |
| Not living a normal life | 1.00 [0.89–1.12] |
| A negative effect of HIV on professional life | 1.01 [0.81–1.22] |
| Out-of-pocket expenses related to HIV | 1.00 [0.92–1.08] |
| Perception of overall health evolution since HIV acquisition | |
| Deteriorated | 1.03 [0.73–1.33] |
| Unchanged or improved | Ref |
| Ease of taking daily treatment | |
| Difficult or very difficult | Ref |
| Easy or very easy | 1.01 [0.83–1.19] |
| Discomfort related to side effects of ART | |
| Slightly or not troublesome side effects or no side effects | Ref |
| Quite troublesome | 1.00 [0.95–1.05] |
| Very troublesome | 1.00 [0.94–1.06] |
| Information about health and HIV | |
| Health literacy score | 0.99 [0.86–1.13] |
| Number of information sources about HIVe | 1.00 [0.86–1.14] |
| Perceived information about HIV scientific breakthroughs from one's doctor | |
| Not sufficiently or not at all informed | 0.99 [0.82–1.17] |
| Sufficiently or very well informed | Ref |
| Perception of ART efficacy and HIV cure | |
| Perception of ART efficacy | |
| Belief in short-term efficacy of ART | 1.00 [0.88–1.12] |
| Belief in mid-term efficacy of ART | 1.00 [0.87–1.14] |
| Belief in long-term efficacy of ART | 1.00 [0.89–1.11] |
| Did not know | Ref |
| Important to be cured of HIV | |
| No | Ref |
| Yes | 1.00 [0.81–1.2] |
| Perception of availability of HIV cure treatment | |
| Belief that an HIV cure treatment would be available during lifetime | 1.00 [0.93–1.06] |
| Did not believe that an HIV cure treatment would be available during lifetime | 1.00 [0.94–1.06] |
| Did not know | Ref |
| Had heard about HIV cure-related clinical trials | |
| No | Ref |
| Yes | 1.06 [0.65–1.47] |
| Previous clinical trial experience | |
| Negative experience | 1.00 [0.88–1.12] |
| Positive experience | 1.00 [0.96–1.05] |
| No experience | Ref |
Significant associations *P<0.1, **P<0.05, ***P<0.01 and RIW ≥0.75, indicating positive or strong evidence.
Adjusted OR presented here were different from 1 at minima at the 3th or 4th decimal places. Other explanatory variables were included in the model but not presented in the table because they had no relationship with the outcome (educational level, having a partner or loving relationships; sense of belonging to the LGBT, heterosexual, and/or drug user communities, PTGI global scores and sub-scores; health literacy).
Reference ‘no comorbidity’.
Reference ‘no comorbid hepatitis C’.
Reference ‘not affected by’ or ‘slightly affected by’.
Each of following information sources counts for one point: the HIV medical staff, attending physician, media (reading, TV, radio, Internet, social networks), scientific articles or journals, people living with HIV associations, friends or relatives, other HIV-positive people
ART: antiretroviral therapy; LGBT: lesbian, gay, bisexual, and transgender; Ref: reference; OR: odds ratio; PTGI: Post-traumatic growth inventory
Supplementary table S2.
Factors associated with self-identification as an HIV activist (results from the multivariate analysis, multi-model averaging method)
| Adjusted OR [95% CI]a | |
|---|---|
| Sociodemographic characteristics | |
| Age (years) | 1.00 [1.00–1.00] |
| Financial situation | |
| Difficult | 1.00 [0.98–1.02] |
| Average | 1.00 [0.98–1.02] |
| Comfortable | 1.00 [0.98–1.02] |
| Very comfortable | Ref |
| Having dependents | |
| Yes | 1.01 [0.8–1.22] |
| No | Ref |
| HIV status of steady partner | |
| No partner | Ref |
| HIV-positive | 1.00 [0.90–1.11] |
| HIV-negative | 1.00 [0.91–1.08] |
| Clinical characteristics | |
| Time between HIV diagnosis and first ART (years) | 0.99 [0.92–1.05] |
| Psychosocial characteristics and living with HIV | |
| Sense of belonging to the people living with HIV community | |
| No | Ref |
| Yes | 0.93 [0.47–1.38] |
| Self-description as a HIV-positive person (on a scale from 0 to 10) | |
| Ashamed (0) to proud (10) | 1.00 [0.98–1.02] |
| Sick (0) to in good health (10) | 1.01 [0.95–1.06] |
| Post-traumaticb growth inventory scale | |
| Global score (range 21–84) | 1.00 [1.00–1.00] |
| ‘New possibilities’ sub-score (range 5–20) | 1.01 [0.96–1.05] |
| ‘Spiritual change’ sub-score (range 2–8) | 1.02 [0.88–1.17] |
| ‘Relating to others’ sub-score (range 7–28) | 1.10 [1.00–1.20]* |
| Feeling greatly affected byc | |
| Mandatory daily medication | 2.15 [1.27–3.03]* |
| The negative effect of HIV on sexual life | 1.03 [0.78–1.27] |
| Out-of-pocket expenses related to HIV | 1.14 [0.47–1.81] |
| Number of information sources about HIVd | 1.35 [1.01–1.68]* |
Significant associations *P<0.1 and RIW ≥0.75, indicating positive or strong evidence.
Adjusted OR presented here were different from 1 at minima at the 3th and 4th decimal places. Other explanatory variables were included in the model but not presented in the table because they had no relationship with the outcome (sex; stable professional situation; educational level; having a partner or partners; living with one's steady partner; time of HIV diagnosis; time on current ART; time since last detectable viral load; CD4 nadir; comorbidities; hepatitis C; lifetime experience of depression and evolution of morale in the previous 5 years; sense of belonging to the heterosexual, LGBT or drug user communities; describing oneself as a confident, socially integrated or strong person living with HIV; ‘personal strength’ PTGI sub-score; ‘appreciation of life’ PTGI sub-score; moral support; feeling greatly affected by the following: the risk of contaminating someone, the cost for the collectivity (i.e. the French general population), having to use condoms, having to live with a secret, HIV-related discrimination, an uncertain future because of HIV, a shorter life expectancy, difficulties to build a stable relationship, side effects of current ART, excessive fatigue, not living a normal life, and the negative effect of HIV on professional life; perception of overall health evolution since HIV acquisition; ease of taking daily treatment; discomfort related to side effects of ART; health literacy; perception of information received from one's doctor about HIV scientific breakthroughs; perception of ART efficacy; importance of being cured of HIV; perception of availability of HIV cure treatment).
Following HIV diagnosis.
Reference ‘not affected by’ or ‘slightly affected by’.
Each of following information sources counts for one point: the HIV medical staff, participant's attending physician, media (reading, TV, radio, internet, social networks), scientific articles or journals, people living with HIV associations, friends or relatives, other HIV-positive people. ART: antiretroviral therapy; Ref: reference; OR: odds-ratio; RIW: relative importance weights; LGBT: lesbian, gay, bisexual, and transgender; PTGI: Post-traumatic growth inventory
Discussion
Factors associated with willingness to participate in HIV cure research
Self-identification as an HIV activist was strongly associated with WPHCRCT in the present analysis. This reflects previous qualitative ANRS-APSEC results [17,24,25] and other qualitative researches [20,26,27] highlighting the importance of scientific altruism and HIV activism in willingness to participate in HIV cure research. Indeed, in the qualitative first phase of the ANRS-APSEC project [17], advancing science and contributing to the HIV community and society were frequently quoted as incentives to participate, given that little or no individual benefit was expected, at least for the first clinical trials This involved a strong sense of the community and the desire to be part of the collaboration between people living with HIV and researchers, with HCRCT seen as a continuum of the historical HIV culture embracing innovation and activism. HIV activism was also identified as a main motivation to participate in HCRCT [25].
In our study, HIV activism was measured only through self-identification and not through an analysis of concrete activism actions. However, some research suggests that individuals may in fact be active without self-identifying as such [40]. Accordingly, it is possible that we underestimated the actual level of activism. In the last three decades, activists have been playing a growing role in clinical research in general [41]. Being part of the process of knowledge construction is a goal for HIV/AIDS activists, so that they can build credibility with regard to their participation in biomedical research[24,42]. Previously published research showed that activism was a motivator for both people living with HIV and researchers to participate in clinical trials about ART [43], and for people without HIV at high risk to participate in HIV vaccine trials [44]. Some activist groups, through the ‘science-wise patient’ approach, have launched appeals for people living with HIV to participate in cutting edge research [3]. Beyond collaboration with trial stakeholders, HIV activism also consists in vigilance. Indeed, a number of HIV clinical trials whose designs include little or no people living with HIV consultation and questionable ethical considerations have been interrupted under the pressure of HIV activists [43]. The French context lends itself particularly well to participation in HCRCT, as HIV activism in the country is still one of the most dynamic and politicised in Europe, despite recent difficulties related to decreased state funding [45].
Our results show that the more self-confident persons with HIV are, the more likely they are to be willing to participate in HCRCT. It may be that higher self-confidence as a person with HIV implies a greater sense of control over the condition, which helps them overcome their concerns about HCRCT-related risks [27]. Their high self-confidence might translate into a sense that they may also be useful to society. Accordingly, they would be more willing to lend their body to science for the sake of HIV cure research and society and to play a role in the history of the fight against HIV [46].
We found no effect of demographic or socioeconomic characteristics on WPHCRCT. This suggests that contrary to what was observed in clinical research about other pathologies, participants’ representations of HIV treatment and a HIV cure – and therefore their willingness to participate in HIV cure research – do not differ according to social group in terms of age, sex, or socioeconomic status [47,48]. No effect of clinical characteristics, such as perceived deteriorated health since HIV diagnosis, comorbidities, or time since HIV diagnosis was observed on WPHCRCT. A previously published study showed a positive association of WPHCRCT with more recent diagnosis or being unhealthy in univariate analysis [15], but people living with HIV enrolled in that study were recruited irrespective of ART stability and immune or virological success.
Factors associated with self-identification as an HIV activist
In the present study, people living with HIV self-identifying as HIV activists were more likely to feel affected by mandatory daily treatment and were able to obtain information about HIV from a larger number of sources. Similarly, Brashers et al. showed that HIV/AIDS activists had greater knowledge of HIV than non-activists [49]. Although HIV activists’ attitudes towards medical and scientific knowledge differ [3], appropriation of knowledge by people living with HIV is one of the strategies that all activists use to negotiate with biomedical professionals on more equal terms, both in the USA [1] and in France [3]. In the present analysis, WPHCRCT seemed to be indirectly associated with obtaining information about HIV from a larger number of sources.
Other researchers have shown that people living with HIV with a history of two or more ART regimens were more willing to participate in HCRCT requiring treatment interruption [22]. Previous research also shows that increased knowledge about HIV is associated with increased acceptability of risks related to ATI in HCRCT [21]. This suggests that despite viral rebound being a major concern regarding HCRCT involving ATI [20], people living with HIV who find it difficult to cope with the exigencies of mandatory daily ART may see participation in HCRCT as an opportunity to interrupt their treatment under medical supervision [46]. The potential individual clinical benefit is not substantial. However, it may be overestimated by eligible people living with HIV when they evaluate the risk–benefit balance of participating in HCRCT [50].
HIV diagnosis constitutes a traumatic event. Intrapersonal and interpersonal factors contribute to post-traumatic growth [51]. This growth includes acquiring a sense of control over the condition, which in turn comes about by improving one's biomedical and psychosocial knowledge about HIV/AIDS, and seeking support from health providers or peers [51]. In the present analysis, self-identification as an HIV activist was significantly associated with a greater PTGI ‘relating to others’ sub-score. This score gauges post-traumatic growth in relationships [52], including feelings of greater connection with other people and increased empathy and altruism [53]. In general, social and political activism helps those people who fare better after a trauma than others to find meaning to their lives [54] although in our study, we are not sure that those who self-identified as HIV activists are actually engaged in activist actions. Long-term positive effects of HIV/AIDS activism on post-traumatic growth, specifically greater self-esteem and wellbeing, were found in former members of the association ACT UP New York [55], thanks to the support of the group. HIV activists have a greater HIV social network than non-activist people living with HIV and use problem-focused coping more than emotion-focused coping [49], something which might mediate the positive effects of activism on post-traumatic growth [55]. In the literature, the direction of the causal relationship between HIV activism and individual characteristics is unclear. Some researchers have suggested that behavioural and psychological predisposing factors may lead to participation in an activist group, and that educational and motivational environments together with social support of activist groups may lead to changes in individuals [49]. Furthermore, HIV activism has been historically stimulated by other forms of community activism, given that most people living with HIV, especially LGBT, already belong to discriminated and stigmatised communities [56].
Post-traumatic growth, especially the ‘relating to others’ sub-score, has been associated with social support in people affected by [57] or living with HIV [53,58]. Social support facilitates post-traumatic growth by mobilising cognitive processing and stimulating the search for meaning after a trauma [53]. People living with HIV mobilise social capital from family and community networks as a buffer against the adverse effects of living with HIV, and serving others might be a mechanism for finding optimism and purpose in life [59]. In terms of adapting to life with HIV, post-traumatic growth is associated with more positive personal beliefs regarding the benevolence of people [60], which might be a stimulus for seeking social support [61] and perhaps encourage activism. The PTGI ‘relating to others’ sub-score reflects increased altruism [61], which is an important motivator of HIV activism and therefore of WPHCRCT. Post-traumatic growth following HIV infection is still under researched [34]. According to the present study, it might lead to increased participation in HIV research. It should also be noted that post-traumatic growth is also associated with higher CD4 counts and undetectable viral load among people living with HIV [34], two criteria for recruitment in HCRCT.
Strengths and limitations
Declared WPHCRCT might not exactly reflect future consent to be enrolled in HCRCT. In order to limit overestimation of WPHCRCT, we only used the ‘yes, definitely’ answer to the question on willingness to participate to define WPHCRCT. Furthermore, significant correlation was observed between actual enrolment by persons without HIV at high risk of seroconversion in a vaccine trial, subsequent to an initial study examining their willingness to enrol [62]. More specifically, two-thirds of those who previously stated they would be willing to participate actually signed up [30,62]. The present analysis did not investigate the effect of the doctor–patient relationship, which is known to influence participation in clinical trials [63]. However, our objective was to independently explore the effect of individual factors on WPHCRCT of people living with HIV. Most importantly, participants are long-term ART-treated and virologically suppressed for many years. We assume that these people living with HIV in relatively good health are likely to maintain a long-term good and satisfactory relationship with their HIV doctor. Therefore, it would have been less relevant to evaluate the effect of the patient–doctor relationship on WPHCRCT in the present sample. People living with HIV who agreed to participate in the ANRS-APSEC study probably constitute a population motivated to participate in HIV cure research. Guidelines for future recruitment in HIV cure research should also consider how to reach people living with HIV with profiles other than those in ANRS-APEC who meet the clinical criteria for HCRCT. The present study only explored five attributes of HCRCT which are study duration, visit frequency, side effects, chance of success and duration of ATI. Other parameters such as methods of molecule administration (tablets, injection, perfusions), duration of visits, or scientific objectives could influence WPHCRCT. However, these attributes were selected as the most relevant for the study by the members of the scientific committee of APSEC, which are clinicians involved in HIV clinical research.
The BMA method used here offers the advantage of avoiding issues related to uncertainty linked to the process of selecting a final model, and possible erroneous conclusions arising from the fact that inference was based on a single model. Our results are consistent with, and therefore reinforce, previous results from the ANRS-APSEC study, which suggested the important role of HIV activism in WPHCRCT. The similarity of the characteristics of the source population of the present study to participants in HCRCT constitutes another strength of our study. Indeed, the participants of the present study were already followed up in HIV centres participating in clinical research and were recruited using the same criteria as for HCRCT enrolment (i.e. stable ART, and viral and immunological success). Attributes of the different HIV cure trial strategies offered to participants included practical modalities of trials (study duration, side effects, ATI duration and chance of success).
Conclusion
HIV activism and positive psychosocial characteristics as a person living with HIV, especially regarding relationships with others, were drivers of WPHCRCT in our study. In order to better understand factors that underlie participation in HIV cure research and HIV activism, especially personal experience with the condition, this result must be compared with upcoming ‘real-life’ data collected among patients enrolled in HCRCT. We recommend, as do our peers [22], that the effect of factors such as social organisation and community norms should be investigated, rather than on concentrating on identity-focused characterisation of HCRCT enrolees. As part of the ‘deliberative’ doctor–patient exchange model regarding enrolment in HCRCT [64], the desire to help advance science and to contribute to the fight against HIV for the sake of the HIV community and for society should be emphasised as a main motivator for participation, considering the very little potential individual benefit expected from ATI under medical control [50] and the currently unlikely prospect of being cured.
A successor to the pre-and post- ART eras, and the advent of TASP and PrEP, the ‘Towards an HIV cure’ period might constitute the fourth revolutionary period in HIV care, where activist organisations and the HIV-specific culture of mobilisation for the community and scientific altruism come together to play an important role alongside researchers [24] in the field of HIV.
Acknowledgements
We are grateful to all the people living with HIV and all the members of the medical staff of the following hospitals who agreed to participate in the ANRS-APSEC survey: Aix en Provence (Centre Hospitalier Général, AP Blanc); Avignon (Centre Hospitalier Henri Duffaut, G Lepeu); Bordeaux (Hôpital Pellegrin, JM Ragnaud; Hôpital Saint André, P Morlat); Créteil (Hôpital Intercommunal, I De Lacroix; Hôpital Henri Mondor JD Lelièvre); Dijon (Hôpital Du Bocage, L Piroth); Kremlin-Bicêtre (Hôpital Bicêtre, C Goujard); Lyon (Hôpital Edouard Herriot, JL Touraine; Hôpital De La Croix Rousse, D Peyramond); Marseille (Hôpital Ste-Marguerite, I. Poizot-Martin); Nantes (Hôpital Hôtel-Dieu, F Raffi); Paris (Hôpital Saint Antoine, PM Girard ; Hôpital Pompidou, L Weiss; Hôpital Saint Louis, JM Molina ; Hôpital Bichat, Y Yazdanpanh; G H Pitié Salpêtrière, C Katlama; Hôpital Lariboisière, JF Bergmann; Hôtel Dieu, J Blacher), Rennes (CHRU Pontchaillou, C Michelet); Suresnes (Hôpital Foch, D Zucman); Toulon (Hôpital Sainte Musse, A Lafeuillade); Toulouse (Hôpital Purpan, P Delobel); Tourcoing (Hôpital Gustave Dron, E Senneville).
We also thank the members of the APSEC scientific committee for their helpful advice: J Barbot, F Barré-Sinoussi, A Chéret, P Delobel, V Doré, S Fainzang, C Gasiglia, C Gauzente, J Ghosn, C Goujard, O Lambotte, JD Lelièvre, A Mennecier, L Meyer, M Mora, M.Préau, C Protière, A L Ross, C Rouzioux, B Spire, M Suzan-Monti. Finally, our thanks to Jude Sweeney for revising and editing the English version of the manuscript.
References
- 1. Woods S, McCormack P.. Disputing the ethics of research: the challenge from bioethics and patient activism to the interpretation of the Declaration of Helsinki in clinical trials. Bioethics 2013; 27: 243– 250. [DOI] [PubMed] [Google Scholar]
- 2. Cox LE, Rouff JR, Svendsen KH et al. Community advisory boards: their role in AIDS clinical trials. Health Soc Work 1998; 23: 290– 297. [DOI] [PubMed] [Google Scholar]
- 3. Barbot J. How to build an ‘active’ patient? The work of AIDS associations in France. Soc Sci Med 2006; 62: 538– 551. [DOI] [PubMed] [Google Scholar]
- 4. Barré-Sinoussi F, Ross AL, Delfraissy JF.. Past, present and future: 30 years of HIV research. Nat Rev Microbiol 2013; 11: 877– 883. [DOI] [PubMed] [Google Scholar]
- 5. Antiretrovial Therapy Cohort Collaboration Life expectancy of individuals on combination antiretroviral therapy in high-income countries: a collaborative analysis of 14 cohort studies. Lancet 2008; 372: 293– 299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. McGrath JW, Winchester MS, Kaawa-Mafigiri D et al. Challenging the paradigm: anthropological perspectives on HIV as a chronic disease. Med Anthropol 2014; 33: 303– 317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Degroote S, Vogelaers D, Vandijck DM.. What determines health-related quality of life among people living with HIV: an updated review of the literature. Arch Public Health 2014; 72: 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Douek DC. HIV Infection: Advances toward a cure. Top Antivir Med 2018; 25: 121– 125. [PMC free article] [PubMed] [Google Scholar]
- 9. Autran B, Descours B, Avettand-Fenoel V, Rouzioux C.. Elite controllers as a model of functional cure. Curr Opin HIV AIDS 2011; 6: 181– 187. [DOI] [PubMed] [Google Scholar]
- 10. Okulicz JF, Lambotte O.. Epidemiology and clinical characteristics of elite controllers. Curr Opin HIV AIDS 2011; 6: 163– 168. [DOI] [PubMed] [Google Scholar]
- 11. Hütter G, Nowak D, Mossner M et al. Long-term control of HIV by CCR5 Delta32/Delta32 stem-cell transplantation. N Engl J Med 2009; 360: 692– 698. [DOI] [PubMed] [Google Scholar]
- 12. Goujard C, Girault I, Rouzioux C et al. HIV-1 control after transient antiretroviral treatment initiated in primary infection: role of patient characteristics and effect of therapy. Antivir Ther 2012; 17: 1001– 1009. [DOI] [PubMed] [Google Scholar]
- 13. Sáez-Cirión A, Bacchus C, Hocqueloux L et al. Post-treatment HIV-1 controllers with a long-term virological remission after the interruption of early initiated antiretroviral therapy ANRS VISCONTI Study. PLoS Pathog 2013; 9: e1003211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Shand LK, Cowlishaw S, Brooker JE, Burney S et al. Correlates of post-traumatic stress symptoms and growth in cancer patients: A systematic review and meta-analysis. Psychooncology 2015; 24: 624– 634. [DOI] [PubMed] [Google Scholar]
- 15. Dubé K, Evans D, Sylla L et al. Willingness to participate and take risks in HIV cure research: survey results from 400 people living with HIV in the US. J Virus Erad 2017; 3: 40– 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Johnston R, Barré-Sinoussi F.. Controversies in HIV cure research. J Int AIDS Soc 2012; 15: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Preau M, Doumergue M, Protiere C et al. Acceptability of HIV cure-related trials: the challenges for physicians and people living with HIV (ANRS-APSEC). AIDS Care 2018; 30: 914– 920. [DOI] [PubMed] [Google Scholar]
- 18. Grossman CI, Ross AL, Auerbach JD et al. Towards multidisciplinary HIV-cure research: integrating social science with biomedical research. Trends Microbiol 2016; 24: 5– 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Deeks SG, Lewin SR, Ross AL et al. International AIDS Society global scientific strategy: towards an HIV cure 2016. Nat Med 2016; 22: 839– 850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dubé K, Evans D, Dee L et al. ‘ We Need to Deploy Them Very Thoughtfully and Carefully’: Perceptions of Analytical Treatment Interruptions in HIV Cure Research in the United States—A Qualitative Inquiry. AIDS Res Hum Retroviruses 2018; 34: 67– 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Simmons R, Kall M, Collins S et al. A global survey of HIV-positive people's attitudes towards cure research. HIV Med 2017; 18: 73– 79. [DOI] [PubMed] [Google Scholar]
- 22. Arnold MP, Evans D, Vergel N.. Recruitment and ethical considerations in HIV cure trials requiring treatment interruption. J Virus Erad 2015; 1: 43– 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Protière C, Préau M, Carrieri P et al. Importance of the patient-physician interaction in assessing acceptability of HIV cure trials. HIV Med 2017; 19: e56– e57. [DOI] [PubMed] [Google Scholar]
- 24. Protière C, Préau M, Doumergue M et al. Will CURE trials introduce an uncomfortable revolution in the field of HIV research. HIV Clin Trials 2017; 18: 174– 175. [DOI] [PubMed] [Google Scholar]
- 25. Protière C, Spire B, Mora M et al. Patterns of patient and healthcare provider viewpoints regarding participation in HIV cure-related clinical trials. Findings from a multicentre French survey using Q methodology (ANRS-APSEC). PloS One 2017; 12: e0187489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. US Food and Drug Administration The Voice of the Patient: Human Immunodeficiency Virus (HIV) Patient-Focused Drug Development and HIV Cure Research. 2014. Available at: www.fda.gov/downloads/ForIndustry/UserFees/PrescriptionDrug UserFee/UCM389379.pdf ( accessed July 2019).
- 27. Power J, Westle A, Dowsett GW et al. Perceptions of HIV cure research among people living with HIV in Australia. PloS One 2018; 13: e0202647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Draper D. Assessment and propagation of model uncertainty. J R Stat Soc 1995; 57: 45– 97. [Google Scholar]
- 29. Protière C, Fressard Lisa, Arnold MP, Mora Marion, Préau Marie, Meyer Laurence, et al. Towards the end of ARVs? Which strategy for which population? Results of the ANRS-APSEC study. 9th International Francophone Conference on HIV Hepatitis. Bordeaux, France, 4–7 April 2018.
- 30. Protière C. Investigating patient and physician preferences for different HIV cure trials: a discrete choice experiment In: Aids Impact 2017. Capetown, South Africa, 13– 15 November 2017. [Google Scholar]
- 31. Protière C, Fressard L, Sagaon-Teyssier L et al. Does burden associated with HIV explain preferences for several HIV Cure strategies? A French DCE survey among patients and physicians (ANRS-APSEC). 22nd International AIDS Conference, 23– 27 July 2018. Amsterdam, the Netherlands. Abstract 3275. [Google Scholar]
- 32. Sculpher M, Bryan S, Fry P et al. Patients’ preferences for the management of non-metastatic prostate cancer: discrete choice experiment. BMJ 2004; 328: 382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ryan M, McIntosh E, Shackley P.. Methodological issues in the application of conjoint analysis in health care. Health Econ 1998; 7: 373– 378. [DOI] [PubMed] [Google Scholar]
- 34. Sherr L, Nagra N, Kulubya G et al. HIV infection associated post-traumatic stress disorder and post-traumatic growth–a systematic review. Psychol Health Med 2011; 16: 612– 629. [DOI] [PubMed] [Google Scholar]
- 35. Pelikan JM, Röthlin F, Ganahl K, Peer S.. Measuring comprehensive health literacy in general populations—le HLS-EU instruments. 6th Annual Health Literacy Research Conference. Bethesda, USA, 3–4 November, 2014.
- 36. Turkheimer FE, Hinz R, Cunningham VJ.. On the undecidability among kinetic models: from model selection to model averaging. J Cereb Blood Flow Metab 2003; 23: 490– 498. [DOI] [PubMed] [Google Scholar]
- 37. Hoeting JA, Madigan D, Raftery AE, Volinsky CT.. Bayesian model averaging: a tutorial. Stat Sci 1999; 14: 382– 401. [Google Scholar]
- 38. Kass RE, Raftery AE.. Bayes Factors. J Am Stat Assoc 1995; 90: 773– 795. [Google Scholar]
- 39. Viallefont V, Raftery AE, Richardson S.. Variable selection and Bayesian model averaging in case-control studies. Stat Med 2001; 20: 3215– 3230. [DOI] [PubMed] [Google Scholar]
- 40. Bobel C. ‘I'm not an activist, though I've done a lot of it’: doing activism, being activist and the ‘perfect standard’in a contemporary movement. Soc Mov Stud 2007; 6: 147– 159. [Google Scholar]
- 41. Gross D, Fogg L.. Clinical trials in the 21st century: the case for participant-centered research. Res Nurs Health 2001; 24: 530– 539. [DOI] [PubMed] [Google Scholar]
- 42. Epstein S. The construction of lay expertise: AIDS activism and the forging of credibility in the reform of clinical trials. Sci Technol Hum Values 1995; 20: 408– 437. [DOI] [PubMed] [Google Scholar]
- 43. Mills EJ, Singh S, Singh JA et al. Designing research in vulnerable populations: lessons from HIV prevention trials that stopped early. BMJ 2005; 331: 1403– 1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Newman PA, Duan N, Roberts KJ et al. HIV vaccine trial participation among ethnic minority communities: barriers, motivators, and implications for recruitment. J Acquir Immune Defic Syndr 2006; 41: 210– 217. [DOI] [PubMed] [Google Scholar]
- 45. Girard G. Sida: un monde associatif en crise? The life of ideas, 14 October, 2014 Available at: laviedesidees.fr/Sida-un-monde-associatif-en-crise.html ( accessed July 2019).
- 46. Henderson GE, Peay HL, Kroon E et al. Ethics of treatment interruption trials in HIV cure research: addressing the conundrum of risk/benefit assessment. J Med Ethics 2018; 44: 270– 276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Murthy VH, Krumholz HM, Gross CP.. Participation in cancer clinical trials: race-, sex-, and age-based disparities. JAMA 2004; 291: 2720– 2726. [DOI] [PubMed] [Google Scholar]
- 48. Ford JG, Howerton MW, Lai GY et al. Barriers to recruiting underrepresented populations to cancer clinical trials: a systematic review. Cancer 2008; 112: 228– 242. [DOI] [PubMed] [Google Scholar]
- 49. Brashers DE, Haas SM, Neidig JL, Rintamaki LS.. Social activism, self-advocacy, and coping with HIV illness. J Soc Pers Relatsh 2002; 19: 113– 133. [Google Scholar]
- 50. Eyal N. What can the lived experience of participating in risky HIV cure-related studies establish? J Med Ethics 2018; 44: 277– 278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Harper GW, Bruce D, Hosek SG et al. Resilience processes demonstrated by young gay and bisexual men living with HIV: Implications for intervention. AIDS Patient Care STDs. 2014; 28: 666– 676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Cadell S. Trauma and growth in Canadian carers. AIDS Care 2003; 15: 639– 648. [DOI] [PubMed] [Google Scholar]
- 53. Rzeszutek M. Social support and posttraumatic growth in a longitudinal study of people living with HIV: the mediating role of positive affect. Eur J Psychotraumatology 2017; 8: 1412225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Norman J. Constructive narrative in arresting the impact of post-traumatic stress disorder. Clin Soc Work J 2000; 28: 303– 319. [Google Scholar]
- 55. Rabkin JG, McElhiney MC, Harrington M, Horn T.. Trauma and Growth: impact of AIDS activism. AIDS Res Treat 2018; 2018: 9696725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Horat S. Homosexual activism in the fight against AIDS. EspacesTemps. net Available at: www.espacestemps.net/articles/le-militantisme-homosexuel-a-preuve-du-sida/ ( accessed July 2018). [Google Scholar]
- 57. Cadell S, Regehr C, Hemsworth D.. Factors contributing to posttraumatic growth: a proposed structural equation model. Am J Orthopsychiatry 2003; 73: 279– 287. [DOI] [PubMed] [Google Scholar]
- 58. Rzeszutek M, Oniszczenko W, Firląg-Burkacka E. Social support, stress coping strategies, resilience and posttraumatic growth in a Polish sample of HIV-infected individuals: results of a 1 year longitudinal study. J Behav Med 2017; 40: 942– 954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Hussen SA, Tsegaye M, Argaw MG et al. Spirituality, social capital and service: factors promoting resilience among expert patients living with HIV in Ethiopia. Glob Public Health 2014; 9: 286– 298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Farber EW, Schwartz JA, Schaper PE et al. Resilience factors associated with adaptation to HIV disease. Psychosomatics 2000; 41: 140– 146. [DOI] [PubMed] [Google Scholar]
- 61. Rzeszutek M. A longitudinal analysis of posttraumatic growth and affective well-being among people living with HIV: The moderating role of received and provided social support. PloS One 2018; 13: e0201641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Buchbinder SP, Metch B, Holte SE et al. Determinants of enrollment in a preventive HIV vaccine trial: hypothetical versus actual willingness and barriers to participation. J Acquir Immune Defic Syndr 2004; 36: 604– 612. [DOI] [PubMed] [Google Scholar]
- 63. Grand MM, O’Brien PC.. Obstacles to participation in randomised cancer clinical trials: a systematic review of the literature. J Med Imaging Radiat Oncol 2012; 56: 31– 39. [DOI] [PubMed] [Google Scholar]
- 64. Emanuel EJ, Emanuel LL.. Four models of the physician-patient relationship. JAMA 1992; 267: 2221– 2226. [PubMed] [Google Scholar]