Abstract
Psoriasis (PsO) has been associated with obesity, and its severity increases in obese subjects. The link between psoriatic condition and obesity is based on shared pathophysiological pathways where local and systemic inflammation promote each other; PsO is an inflammatory, immune-mediated disease, and the adipose tissue is the source of proinflammatory adipokines. Moreover, psoriatic arthritis (PsA) is an important comorbidity of PsO that reduces quality of life and makes difficult the patient's management. Treatment of obese subjects with moderate to severe PsO, even more if PsA is present, is challenging because of reduced efficacy of several systemic drugs and increased risk of adverse events. Secukinumab, a monoclonal antibody that selectively binds to and neutralizes interleukin 17A, shows efficacy on PsO in all body weight groups, even in the highest, whose response has a slight downward trend. Clinical features of two obese subjects, affected by PsO and PsA, successfully treated with secukinumab, are described.
Keywords: Psoriasis, Psoriatic arthritis, Obesity, Secukinumab
Psoriasis Comorbidities
Psoriasis is a complex, chronic, immune-mediated inflammatory skin disorder. Psoriatic arthritis and metabolic syndrome are common comorbidities in patients with psoriasis; their frequency presents a wide variation depending on population groups observed. In a recent publication by Souza et al. [1], the authors stated that among comorbidities associated with psoriasis and strictly related to the patient's quality of life, the prevalence of metabolic syndrome and psoriatic arthritis was 50 and 41.8%, respectively. Dyslipidemia was the most prevalent secondary comorbidity, followed by hypertension, obesity, and type 2 diabetes mellitus. A diminished health-related quality of life is observed among psoriatic patients with these comorbidities, highlighting the importance of diagnosis and successful treatment beyond the skin lesion resolution [1].
The Association between Psoriasis and Obesity
Epidemiological studies suggested that patients with psoriasis, compared with the general population, have higher prevalence and incidence of obesity [2]. A meta-analysis of 16 observational studies found that the pooled odds ratio (OR) for obesity among patients with psoriasis was 1.66 (95% confidence interval [CI] 1.46–1.89) compared with those without psoriasis [2]. From the studies that reported psoriasis severity, the pooled OR for obesity among patients with mild psoriasis was 1.46 (95% CI 1.17–1.82) and the pooled OR for patients with severe psoriasis was 2.23 (95% CI 1.63–3.05). An incidence study found that psoriasis patients have a hazard ratio of 1.18 (95% CI 1.14–1.23) for new-onset obesity [2]. Psoriasis was found to be associated with abdominal obesity, which is a proxy measure of visceral adipose tissue, and is a well-recognized risk factor for type 2 diabetes mellitus, hypertension, coronary artery disease, and decreased life expectancy [3]. The prospective Nurses' Health Study II observed that psoriasis was more common in subjects with an increased waist circumference, a surrogate marker for abdominal visceral fat [4].
Pathophysiological Basis Linking Psoriasis and Obesity
Although the mechanism underlying the epidemiological association between psoriasis and obesity is not completely understood, inflammation is considered the pathophysiological basis between the two conditions [2]. Obesity is a further source of inflammation in psoriatic subjects. White adipose tissue, which is the primary component of visceral fat, is composed of both adipocytes and other immunologically active cells such as macrophages [5]. Adipocytes secrete adipokines with pro-inflammatory properties, such as leptin, visfatin, and resistin [5]; their expression was found to exacerbate psoriasis [2]. Besides that, a common genetic link between psoriasis and obesity was demonstrated in a cross-sectional, population-based twin study in 33,588 Danish subjects (OR, 1.81; 95% CI, 1.28–2.55; p = 0.001 in subjects with a BMI >35) [6]. On the contrary, body weight reduction has been shown to attenuate the severity of plaque psoriasis. A randomized controlled study investigated the impact of hypocaloric dietary intervention and physical activity over 20 weeks in 303 overweight/obese patients with moderate to severe plaque psoriasis. Psoriasis area and severity index score was reduced by 48% in the interventional arm (where the weight loss target of ≥5% was achieved in 29.8% of subjects), compared to only 25.5% in the information only arm (weight loss target achieved in 14.5% of subjects) (p = 0.02) [7].
Systemic Antipsoriatic Treatments in Obese Patients
The use of systemic antipsoriatic treatments was observed to have poorer benefit and a higher incidence of adverse events in obese subjects compared to patients with normal body weight. With regard to this, methotrexate carries a higher risk of fatty liver and hepatic fibrosis in obese patients and therefore should be avoided [8]. In addition, obese patients require higher doses of acitretin and cyclosporine, which leads to increased risk of side effects [9]. Furthermore, several studies have shown that therapeutic response to anti-tumor necrosis factor (TNF)-Α agent is better in patients with normal BMI, versus those with higher BMI, and that reduction in body weight leads to a better response [9]. Indeed, one of the variables most related to increased clearance of the drug is weight. Individuals weighing more than 100 kg clear the drug 55% faster and have 35% greater volume of distribution, which both lead to lower trough levels at the time of re-dosing [10]. Obesity-related drug excretion may be compounded by the presence of diabetes mellitus, and individuals with both a weight greater than 100 kg and diabetes were found to have very high clearance rates and approximately 50% lower trough levels during therapy. This issue has been demonstrated for ustekinumab, a monoclonal antibody targeting interleukin (IL)-12 and IL-23, in the PHOENIX trials, at the recommended doses of 45/90 mg, where the nonresponders were found to have the highest rates of apparent drug clearance that may be associated with ustekinumab efficacy [10].
Secukinumab and Obesity
Secukinumab is a monoclonal antibody that selectively binds to and neutralizes IL-17A. It has shown efficacy in the treatment of psoriasis, ankylosing spondylitis, and psoriatic arthritis [11]. In subjects with psoriasis, expression of IL-17 mRNA is higher in lesional compared with nonlesional skin [12]. Moreover, IL-17A levels were found to be significantly correlated to disease severity [13, 14]. Blockade of IL-17 reduced keratinocyte hyperproliferation, T-cell infiltration into the dermis, and mRNA expression of disease-propagating genes [15]. Thus, there is a considerable amount of data supporting the central role of IL-17 in the pathogenesis of psoriasis and the value of IL-17-targeted biologic therapy. Finally, some data seem to suggest that adipocytokines could also interfere with the IL-17 pathway [16, 17, 18].
Secukinumab is a highly efficacious drug irrespective of body weight. In a pooled analysis of phase III trials, ERASURE and FIXTURE, responses were analyzed by body weight quartiles (42–69.9, 70–82, 82.1–97, and 97.1–219.1 kg): pooled PASI 75, PASI 90, and PASI 100 response rates were high across weight quartiles and were maintained at week 52. Even though responses remained clinically meaningful in all weight groups, there was a trend for a lower response by increasing body weight. Moreover, secukinumab 300 mg dose demonstrated consistently greater benefit than the 150 mg dose across weight quartiles [19]. Also, data from the CLEAR phase III study showed that secukinumab 300 mg had a clinically and statistically significantly higher efficacy (PASI 90 at week 16) than ustekinumab 90 mg in patients with a body weight over 100 kg. This result was fully sustained at week 52 [20]. Unlike TNF-Α inhibitors, IL-17 inhibitors have not been observed to cause weight gain in clinical trials [9].
Case Reports
Clinical experiences with two obese subjects, with very high BMI, affected by moderate to severe psoriasis and by psoriatic arthritis, successfully treated with secukinumab are described. These experiences represent typical examples of difficult patients that need to be managed in agreement with the previous literature.
Patient 1
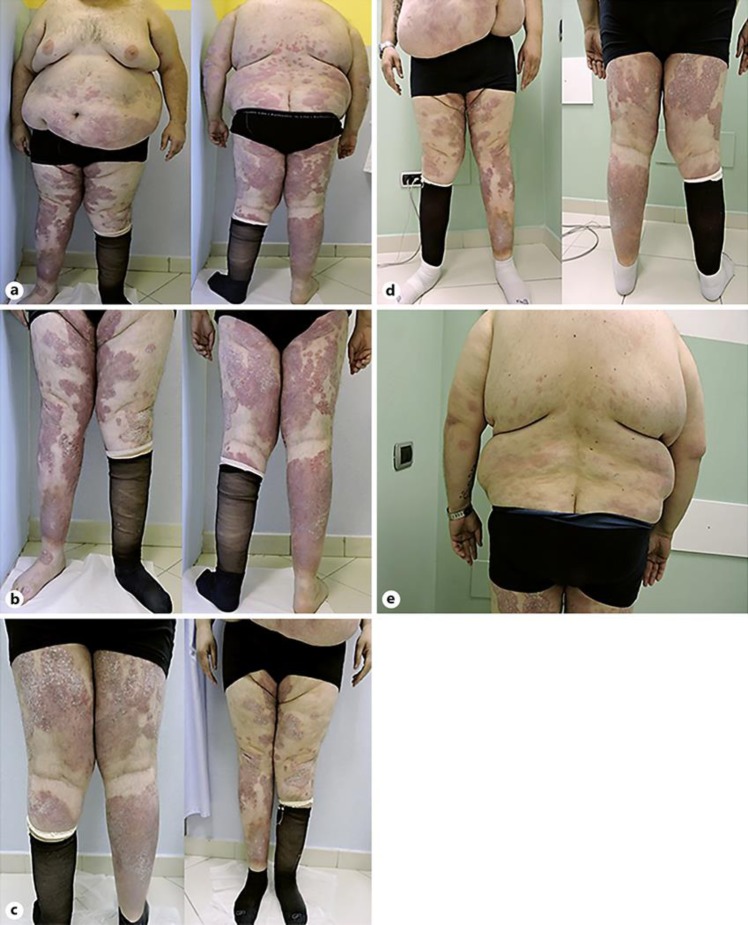
A male patient with a family history of psoriasis had been diagnosed with plaque psoriasis when he was 27 years old and with psoriatic arthritis when he was 29. He had received repeated cycles of acitretin without benefit and had been treated for 3 years with sulfasalazine and an oral corticosteroid. Methotrexate had been then administered without improvement of skin lesions. At first presentation, in December 2016, the patient was 36 years old, had BMI 45.38, and plaque psoriasis with a PASI 24 (Fig. 1a, b). The patient reported articular pain and itching. Methotrexate had been discontinued 1 month before. Secukinumab 300 mg subcutaneous was administered at weeks 0, 1, 2, 3, 4, and subsequently every 4 weeks.
Fig. 1.
Patient No. 1, at presentation (a, b), after 1 month (c), and 4 months (d, e) of secukinumab treatment.
After 1 month of treatment, an unsatisfactory improvement was observed, with PASI 20 (Fig. 1c). After 4 months of therapy, PASI was 7, the trunk was cleared and limb lesions were not infiltrated (Fig. 1d, e). Itching and articular pain were not reported.
Currently, after 2 years of therapy, the PASI index is 3 and we observe only few nummular erythematous lesions with little infiltration at the bilateral pre-tibia regions. The patient also denies the appearance of joint pain.
Patient 2
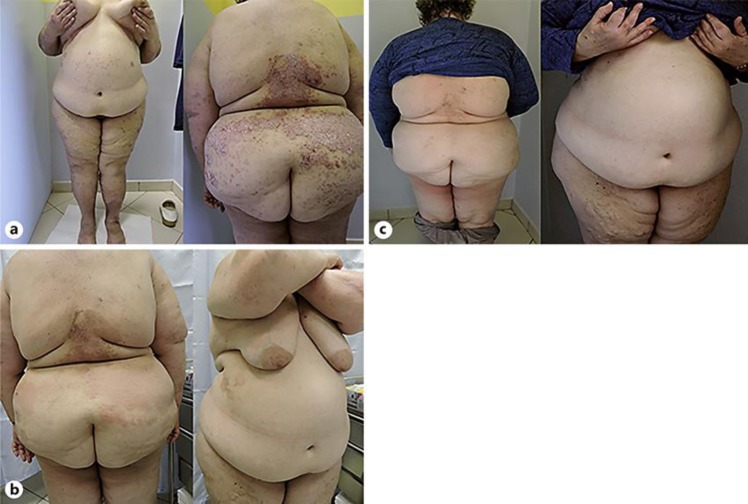
A 58-year-old female presented with itching disseminated psoriatic lesions, with a PASI 14 (Fig. 2a). She was obese, with BMI 49.59. The woman had been diagnosed with psoriatic arthritis 3 years before because of joint pain and had received nonsteroidal anti-inflammatory drugs and later methotrexate 15 mg/week for 1 year. This therapy had not been effective. Secukinumab 300 mg subcutaneous was administered at weeks 0, 1, 2, 3, 4, and subsequently every 4 weeks. Skin lesion improvement was observed 3 months later, but psoriasis persisted on the back, with a PASI 6 (Fig. 2b). Psoriasis was completely cleared (PASI 0) after 6 months of treatment (Fig. 2c).
Fig. 2.
Patient No. 2, at presentation (a), after 3 months (b), and 6 months (c) of secukinumab treatment.
Currently, the patient has almost reached 2 years of treatment and her PASI index has always remained at 0. The patient reported that joint pain was absent.
Discussion and Conclusion
Obese subjects have a high prevalence of psoriasis and are less efficiently treated with systemic agents, due to both reduced efficacy and increased risk of adverse events. Psoriatic arthritis is a common comorbidity of psoriasis. Secukinumab is effective on both skin psoriasis and psoriatic arthritis, and efficacy in psoriasis was demonstrated by clinical trials in all body weight groups. In this article, two exemplary complex cases are reported: two obese patients, with comorbid psoriatic arthritis, were successfully treated with secukinumab, for both arthritis and skin psoriasis.
Key Message
An association between psoriasis and obesity was found. Secukinumab is effective in obese patients with psoriasis and psoriatic arthritis.
Statement of Ethics
The authors declare that the research was conducted in accordance with the World Medical Association Declaration of Helsinki. The patients have given their written informed consent to publish their case, including publication of images.
Disclosure Statement
The authors have no conflicts of interest to declare.
Author Contributions
All the authors contributed to study design, data collection, and study execution, as well as to manuscript preparation.
Funding Sources
Editorial assistance was funded by Novartis Farma Italy.
Acknowledgement
Laura Brogelli, PhD, on behalf of Content Ed Net, provided editorial assistance for the preparation of the manuscript.
References
- 1.Souza CS, de Castro CC, Carneiro FR, Pinto JM, Fabricio LH, Azulay-Abulafia L, et al. Metabolic syndrome and psoriatic arthritis among patients with psoriasis vulgaris: quality of life and prevalence. J Dermatol. 2019 Jan;46((1)):3–10. doi: 10.1111/1346-8138.14706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Armstrong AW, Harskamp CT, Armstrong EJ. The association between psoriasis and obesity: a systematic review and meta-analysis of observational studies. Nutr Diabetes. 2012 Dec 3;2:e54. doi: 10.1038/nutd.2012.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Janssen I, Katzmarzyk PT, Ross R. Body mass index, waist circumference, and health risk: evidence in support of current National Institutes of Health guidelines. Arch Intern Med. 2002 Oct;162((18)):2074–9. doi: 10.1001/archinte.162.18.2074. [DOI] [PubMed] [Google Scholar]
- 4.Setty AR, Curhan G, Choi HK. Obesity, waist circumference, weight change, and the risk of psoriasis in women: Nurses' Health Study II. Arch Intern Med. 2007 Aug;167((15)):1670–5. doi: 10.1001/archinte.167.15.1670. [DOI] [PubMed] [Google Scholar]
- 5.Ahima RS, Flier JS. Adipose tissue as an endocrine organ. Trends Endocrinol Metab. 2000 Oct;11((8)):327–32. doi: 10.1016/s1043-2760(00)00301-5. [DOI] [PubMed] [Google Scholar]
- 6.Lønnberg AS, Skov L, Skytthe A, Kyvik KO, Pedersen OB, Thomsen SF. Association of psoriasis with the risk for type 2 diabetes mellitus and obesity. JAMA Dermatol. 2016 Jul;152((7)):761–7. doi: 10.1001/jamadermatol.2015.6262. [DOI] [PubMed] [Google Scholar]
- 7.Naldi L, Conti A, Cazzaniga S, Patrizi A, Pazzaglia M, Lanzoni A, et al. Psoriasis Emilia Romagna Study Group Diet and physical exercise in psoriasis: a randomized controlled trial. Br J Dermatol. 2014 Mar;170((3)):634–42. doi: 10.1111/bjd.12735. [DOI] [PubMed] [Google Scholar]
- 8.Rosenberg P, Urwitz H, Johannesson A, Ros AM, Lindholm J, Kinnman N, et al. Psoriasis patients with diabetes type 2 are at high risk of developing liver fibrosis during methotrexate treatment. J Hepatol. 2007 Jun;46((6)):1111–8. doi: 10.1016/j.jhep.2007.01.024. [DOI] [PubMed] [Google Scholar]
- 9.Kaushik SB, Lebwohl MG. Psoriasis: Which therapy for which patient: Psoriasis comorbidities and preferred systemic agents. J Am Acad Dermatol. 2019 Jan;80((1)):27–40. doi: 10.1016/j.jaad.2018.06.057. [DOI] [PubMed] [Google Scholar]
- 10.Thibodaux RJ, Triche MW, Espinoza LR. Ustekinumab for the treatment of psoriasis and psoriatic arthritis: a drug evaluation and literature review. Expert Opin Biol Ther. 2018 Jul;18((7)):821–7. doi: 10.1080/14712598.2018.1492545. [DOI] [PubMed] [Google Scholar]
- 11. Secukinumab, Summary of Products Characteristic.
- 12.Li J, Chen X, Liu Z, Yue Q, Liu H. Expression of Th17 cytokines in skin lesions of patients with psoriasis. J Huazhong Univ Sci Technolog Med Sci. 2007 Jun;27((3)):330–2. doi: 10.1007/s11596-007-0329-1. [DOI] [PubMed] [Google Scholar]
- 13.Arican O, Aral M, Sasmaz S, Ciragil P. Serum levels of TNF-alpha, IFN-gamma, IL-6, IL-8, IL-12, IL-17, and IL-18 in patients with active psoriasis and correlation with disease severity. Mediators Inflamm. 2005 Oct;2005((5)):273–9. doi: 10.1155/MI.2005.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Takahashi H, Tsuji H, Hashimoto Y, Ishida-Yamamoto A, Iizuka H. Serum cytokines and growth factor levels in Japanese patients with psoriasis. Clin Exp Dermatol. 2010 Aug;35((6)):645–9. doi: 10.1111/j.1365-2230.2009.03704.x. [DOI] [PubMed] [Google Scholar]
- 15.Krueger JG, Fretzin S, Suarez-Farinas M, et al. IL-17A is essential for cell activation and inflammatory gene circuits in subjects with psoriasis. J Allergy Clin Immunol. 2012;130:145–154. doi: 10.1016/j.jaci.2012.04.024. e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shibata S, Tada Y, Hau CS, Mitsui A, Kamata M, Asano Y, et al. Adiponectin regulates psoriasiform skin inflammation by suppressing IL-17 production from Γδ-T cells. Nat Commun. 2015 Jul;6((1)):7687. doi: 10.1038/ncomms8687. [DOI] [PubMed] [Google Scholar]
- 17.Piccio L, Cantoni C, Henderson JG, Hawiger D, Ramsbottom M, Mikesell R, et al. Lack of adiponectin leads to increased lymphocyte activation and increased disease severity in a mouse model of multiple sclerosis. Eur J Immunol. 2013 Aug;43((8)):2089–100. doi: 10.1002/eji.201242836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kasahara DI, Kim HY, Williams AS, Verbout NG, Tran J, Si H, et al. Pulmonary inflammation induced by subacute ozone is augmented in adiponectin-deficient mice: role of IL-17A. J Immunol. 2012 May;188((9)):4558–67. doi: 10.4049/jimmunol.1102363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Szepietowski J. Secukinumab 300 mg shows superior efficacy across subject body weight groups: pooled analysis of phase 3 ERASURE and FIXTURE trials. J Am Acad Dermatol. 2015;72((5 Supplement 1)):AB248. [Google Scholar]
- 20.Blauvelt A, Reich K, Tsai TF, Tyring S, Vanaclocha F, Kingo K, et al. Secukinumab is superior to ustekinumab in clearing skin of subjects with moderate-to-severe plaque psoriasis up to 1 year: results from the CLEAR study. J Am Acad Dermatol. 2017 Jan;76((1)):60–69.e9. doi: 10.1016/j.jaad.2016.08.008. [DOI] [PubMed] [Google Scholar]