Highlights
-
•
Hydroxychloroquine overdose can cause hypokalemia.
-
•
Hydroxychloroquine overdose can result in electrocardiographic abnormalities.
-
•
Hydroxychloroquine can interfere with urine chemistry and drug screening assays.
-
•
Urine concentrations of hydroxychloroquine can exceed 500 mg/L in acute overdose.
Keywords: Arrhythmias, Drug overdose complications, Electrocardiography, Hydroxychloroquine adverse effects, Hypokalemia, Norepinephrine therapeutic use
Abstract
Hydroxychloroquine is a medication used to treat autoimmune conditions. Overdoses of hydroxychloroquine are uncommon, with most recommendations on monitoring drawing from experience with more common overdoses of the related drug chloroquine. We present a case of an adolescent with intentional overdose of approximately 12 g of hydroxychloroquine. The prominent clinical features were hypokalemia and widened QRS and QT intervals on the electrocardiogram. Therapy included epinephrine by intravenous drip and bicarbonate infusions along with supportive care and cardiac monitoring. The patient recovered without sequelae. Urine drug testing showed an absorbance alarm for one of the components of the institution drug of abuse screening panel, an oxycodone screen using an enzyme immunoassay. Analysis of two urine specimens collected during the hospitalization revealed hydroxychloroquine concentrations of greater than 500 mg/L (approximately 7.5 h after ingestion) and 130 mg/L (approximately 14 h after ingestion). Only the urine with greater than 500 mg/L hydroxychloroquine produced absorbance alarms on the drug of abuse testing. We separately analyzed the impact on 24 urine assays of varying concentrations of hydroxychloroquine spiked into de-identified pooled urine samples. For 6 of the assays (buprenorphine, cotinine, oxycodone, and tetrahydrocannabinol qualitative drug screens; microalbumin and urine myoglobin quantitative assays), hydroxychloroquine produced significant bias and/or instrument alarms. Overall, our study demonstrates that urine concentrations of hydroxychloroquine can reach very high concentrations (exceeding 500 mg/L) following overdose, with the potential to interfere with a range of urine assays including drug of abuse screening and microalbumin. Similar to previous reports, hydroxychloroquine overdose can produce hypokalemia and electrocardiographic abnormalities.
1. Introduction
Hydroxychloroquine is a medication used to treat autoimmune conditions such as rheumatoid arthritis and systemic lupus erythematosus [[1], [2], [3], [4]]. Hydroxychloroquine and the related quinine derivatives chloroquine and amodiaquine have also been used for malaria treatment and prophylaxis, though this is becoming less common as rates of resistance to these drugs increase [[4], [5], [6], [7]]. Overdoses of hydroxychloroquine are rare, and most recommendations on monitoring and treatment of these patients reflect experiences with chloroquine overdoses, which are more commonly seen and usually more toxic [8]. Hydroxychloroquine was initially synthesized from chloroquine in 1946 to decrease the incidence of associated toxicities [9]. Chloroquine is estimated to be several times more toxic [10]; however, deaths have been reported from hydroxychloroquine overdose [11,12]. Published case reports of hydroxychloroquine toxicity are found as early as 1960 [13].
Hydroxychloroquine toxicity is largely due to its effects on the heart [4]. Sodium and potassium channel blockade result in QRS and QT prolongation, respectively, on the electrocardiogram (ECG). These prolonged intervals put patients at significant risk for dysrhythmias, included torsade de pointes and ventricular fibrillation. Other cardiac effects include delayed conduction as well as decreased contractility due to a negative inotropic effect [8]. These effects can lead to profound hypotension, dysrhythmias, and cardiovascular collapse [14].
Hypokalemia is usually seen with significant ingestions and is a result of decreased potassium efflux secondary to blockade of potassium membrane channels [15]. While these patients are often hypokalemic, the benefit from potassium supplementation is unclear. The low potassium likely does not represent a shortage of overall body stores but rather a shifting of the potassium to the intracellular compartment [8].
Ophthalmologic complications, specifically irreversible retinal injury, have been reported with chronic exposure to both chloroquine and hydroxychloroquine, but there are no reports of ophthalmologic complications after acute overdose as the toxicity is related to the overall total dosing and duration of these medications [16]. Some of the pathologic differences between chloroquine and hydroxychloroquine may be due to differences in binding to melanin [17].
Interference with some laboratory testing has also been reported with hydroxychloroquine including drug of abuse immunoassays [18] and urine protein dipstick measurements [19,20]. We report a patient with acute overdose of hydroxychloroquine (approximately 12 g) who presented with cardiac symptoms, hypokalemia, electrocardiographic abnormalities, and interference with urine drug of abuse screening testing. We also investigated the impact of high concentrations of hydroxychloroquine on a variety of urine laboratory tests.
2. Case history
A 16 year old girl was transferred to our hospital after an intentional overdose of hydroxychloroquine. She ingested an estimated 60 hydroxychloroquine 200 mg tablets (12 g) at 20.00 in a suicide attempt. The medication belonged to her mother, and the patient had a similar presentation approximately 2 months prior after ingesting 20 hydroxychloroquine 200 mg tablets (4 g). The patient was also prescribed fluoxetine, risperidone, lamotrigine, and lisdexamfetamine dimesylate, but she denied any other ingestions.
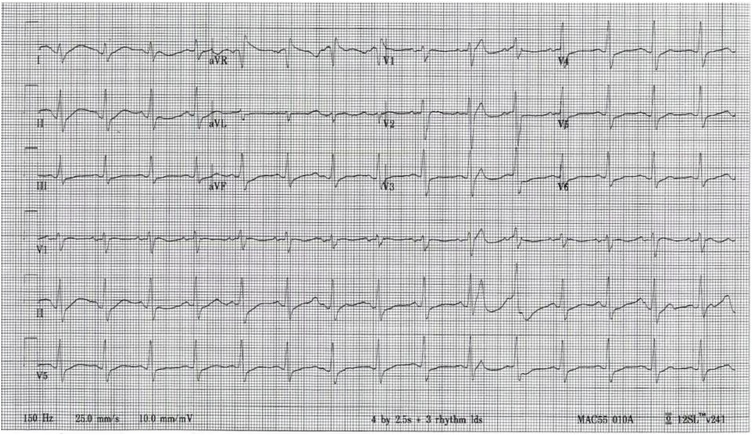
She initially presented to another hospital and was drowsy but arousable with Glasgow Coma Scale score of 15 (out of maximum 15) and an initial heart rate (HR) of 88 beats per minute (bpm), respiratory rate of 24 per minute, and a blood pressure (BP) of 85/51 mm Hg. Her ECG taken approximately 2 h after ingestion showed sinus rhythm with HR of 91 bpm, QRS duration of 132 msec (normal range: 120 msec or less), and a corrected QT interval (QTc) of 728 msec (normal range: 440 msec or less; see Fig. 1). Her initial laboratory studies were notable for a plasma potassium of 3.0 mmol/L (normal range: 3.5–5.0 mmol/L) and a whole blood lactate of 2.5 mmol/L (normal range: 0.5–2.0 mmol/L). The anion gap was 11 (normal range: < 16). Urine potassium was 11.1 mmol/L (normal range: 25–126 mmol/L). Venous blood gas analysis revealed the following: pH 7.32 (normal range: 7.30–7.40) with a pCO2 of 49 mm Hg (normal range: 32–45 mm Hg) and HCO3 of 25 mmol/L (normal range: 22–26). The patient was given 3 L of normal saline.
Fig. 1.
Electrocardiogram (ECG) taken approximately 2 h after ingestion of hydroxychloroquine. The ECG shows sinus rhythm with a rate of 91 bpm, a QRS duration of 132 msec, and a corrected QT interval (QTc) of 728 msec.
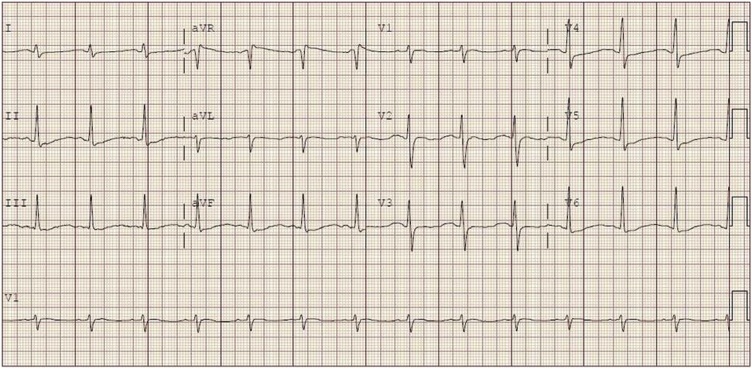
The regional Poison Center was contacted and recommended bicarbonate and epinephrine infusions. These were started, and she was transferred to our hospital for further management. On arrival to our hospital, her mental status and BP had improved, and she no longer required the epinephrine infusion. An ECG obtained shortly after arrival showed sinus rhythm with a rate of 82 bpm, and a QRS of 112 msec (see Fig. 2). The QTc was recorded by the machine as 320 msec but is closer to 600 msec by manual measurement. This error was likely the result of diffuse T wave flattening seen on her early ECGs. The bicarbonate infusion was continued, and she was admitted to the pediatric intensive care unit (PICU).
Fig. 2.
Electrocardiogram showing sinus rhythm with a rate of 82 bpm and a QRS of 112 msec. The QTc was recorded by the machine as 320 msec but is closer to 600 msec by manual measurement.
Laboratory studies in the PICU on arrival were notable for a potassium of 1.8 mmol/L and a white blood count of 15.5 k/mm3 (normal range: 3.7–10.5 k/mm3). The remainder of her laboratory studies, including blood gas analysis and routine toxicology tests, were unremarkable. Screening drug of abuse immunoassays were performed and was presumptive positive for amphetamines (explainable by known lisdexamfetamine prescription), but there was an absorbance alarm on the oxycodone immunoassay screen (this type of alarm occurs when substances in the specimen absorb light at the measured wavelength for the assay and produce a reading outside the analyzer photometer range). After it was hypothesized that the hydroxychloroquine may be interfering with the drug screens, her blood and urine was sent for quantification of hydroxychloroquine to a reference laboratory. Two separate urine samples and one blood sample were sent to specialized reference laboratory (NMS Labs, Willow Grove, PA, USA) for confirmatory testing by high performance liquid chromatography with tandem mass spectrometry (LC/MS/MS). The first urine, taken approximately 7.5 h after ingestion, had a hydroxychloroquine level of >500 mg/L (no reference range available). The second urine, taken about 14 h after ingestion, had a level of 130 mg/L. This sample tested negative for the urine drug screen panel, with no absorbance alarms. A blood sample was taken approximately 6 h after ingestion and showed a level of 10 mg/L (no clearly defined reference range, but reference laboratory result cited data that peak plasma concentrations of 0.41 +/- 0.13 mg/L were achieved approximately 2 h after a 400 mg hydroxychloroquine dose). The patient had a relatively uneventful hospital course. Her plasma potassium returned to normal range, her QRS and QTc prolongations on the ECG quickly resolved, and she was transferred to inpatient psychiatric care.
3. Effects of hydroxychloroquine on laboratory urine assays
We investigated the effect on urine assays of hydroxychloroquine (Sigma-Aldrich) spiked into pools of de-identified urine specimens from the clinical laboratory. The 24 specific urine assays tested are summarized in Table 1. Four different urine specimen pools were tested in triplicate. Absorbance alarms and/or interference bias were clearly evident in 6 of the assays (Fig. 3, Fig. 4show representative data for each of these 6 drugs).
Table 1.
Urine assays analyzed in the present study.
| Assay | Analyzer | Vendor | Assay version | Methodology | Effect of hydroxycholorquine? |
|---|---|---|---|---|---|
| Amphetamines | c502 | Roche | Amphetamines II (AMPS) 2015-10 V9 | KIMS | No |
| Amylase | c701 | Roche | α-Amylase EPS ver. 2 2015-08 V4 | Enzymatic, colorimetric | No |
| Benzodiazepines | c502 | Roche | Benzodiazepines Plus 2016-08 V10 | KIMS | No |
| Buprenorphine | c502 | Lin-Zhi | Buprenorphine Enzyme Immunoassay 2018-04 | EIA | Yes, absorbance errors |
| Calcium | c701 | Roche | Calcium Gen. 2 2014-02 V3 | Photometric | No |
| Chloride | c701 | Roche | ISE indirect Na, K, Cl Gen. 2 2016-01 V8 | Ion-selective electrode | No |
| Cocaine metabolite | c502 | Roche | Cocaine II 2014-03 V7 | KIMS | No |
| Cotinine | c502 | Thermo-Fisher | DRI Cotinine Assay 2017-09 | EIA | Yes, positive bias and absorbance errors |
| Creatinine | c701 | Roche | Creatinine plus ver. 2 2016-12 V9 | Enzymatic | No |
| Glucose | c701 | Roche | Glucose HK Gen. 3 2016-06 V5 | Hexokinase/UV | No |
| hCG | c602 | Roche | Human chorionic gonadotropin, STAT 2013-06 V16 | ECLIA | No |
| Magnesium | c701 | Roche | Magnesium Gen. 2 2017-06 V13 | Colorimetric endpoint | No |
| Microalbumin | c502 | Roche | Tina-quant Albumin 2015-08 V10 | Immunoturbidometric | Yes, biphasic biases |
| Myoglobin | c602 | Roche | Tina-quant myoglobin Gen. 2 2015-08 V6 | ECLIA | Yes, negative bias |
| NGAL | c501 | Bioporto | NGAL 2015-09-RUO | Particle-enhanced turbidimetric immunoassay | No |
| Opiates | c502 | Roche | Opiate II 2014-07 V11 | KIMS | No |
| Oxycodone | c502 | Roche | Oxycodone 2014-12 V7 | EIA | Yes, absorbance errors |
| pH | c501 | Roche | Roche SVTpH 190.2015-06 V2.0 | Spectrophotometric | No |
| Phosphorus | c701 | Roche | Phospate (inorganic) ver. 2 2016-05 V8 | Molybdate/UV | No |
| Potassium | c701 | Roche | ISE indirect Na, K, Cl Gen. 2 2016-01 V8 | Ion-selective electrode | No |
| Protein | c501 | Roche | Total Protein Gen. 2 2017-08 V10 | Colorimetric | No |
| Sodium | c701 | Roche | ISE indirect Na, K, Cl Gen. 2 2016-01 V8 | Ion-selective electrode | No |
| THC | c502 | Roche | Cannabinoids II 2014-03 V9 | KIMS | Yes, positive bias |
| Urea nitrogen | c701 | Roche | Urea/BUN 2016-01 V6 | Kinetic test, urease and glutamate dehydrogenase | No |
Abbreviations: ECLIA, electrochemiluminescence immunoassay; EIA, enzyme immunoassay; hCG, human chorionic gonadotropin; KIMS, kinetic interaction of microparticles in solution; NGAL, neutrophil gelatinase-associated lipocalin; THC, tetrahydrocannabinol; UV, ultraviolet.
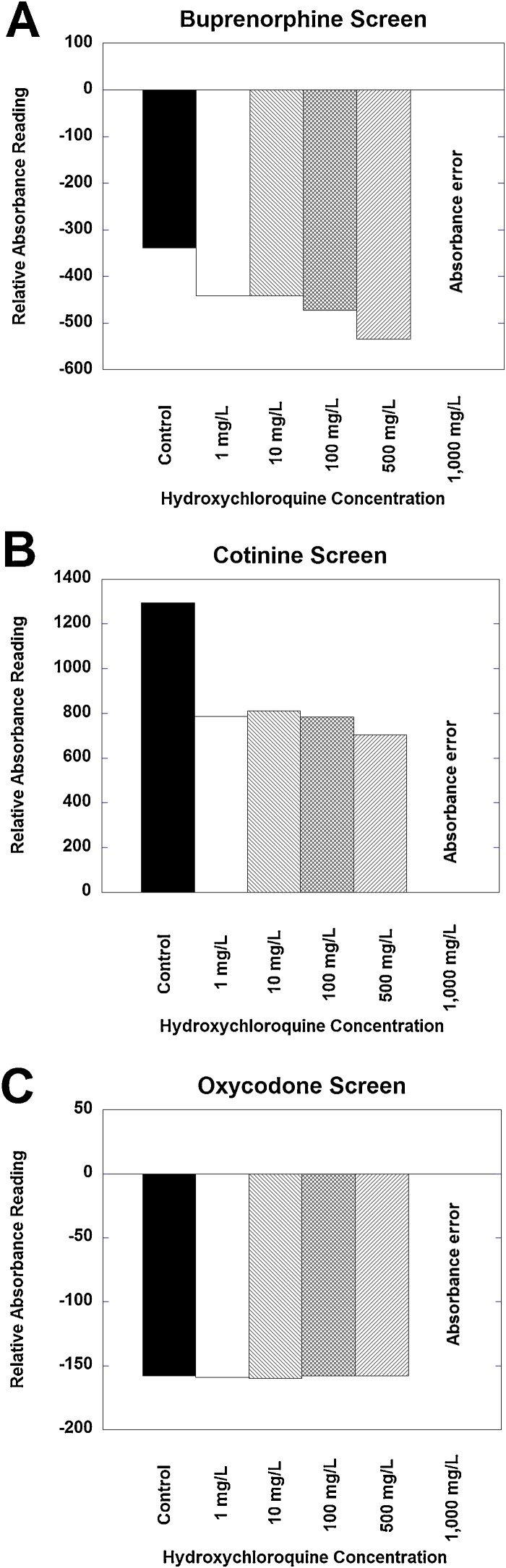
Fig. 3.
Representative examples of interference or errors produced by varying concentrations of hydroxychloroquine spiked in urine. (A) Buprenorphine and (B) cotinine screens shows decrease in relative absorbance relative to baseline from hydroxychloroquine concentrations ranging from 1 to 500 mg/L. For both assays, a hydroxychloroquine concentration of 1000 mg/L produces an absorbance error on the instrument. (C) Oxycodone screen shows no effect of hydroxychloroquine concentrations from 1 to 500 mg/L but does have an absorbance error at 1000 mg/L. See Table 1 for details on assay versions.
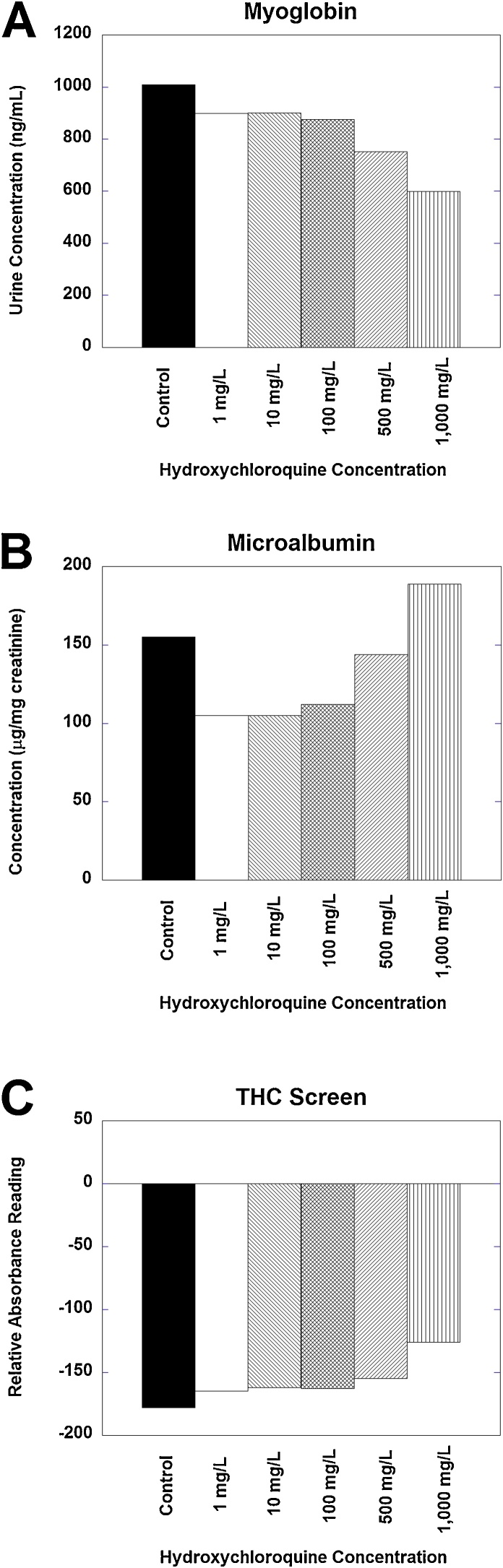
Fig. 4.
Representative examples of interference produced by hydroxychloroquine spiked in urine. (A) Urine myoglobin (quantitative assay) shows negative bias from hydroxychloroquine, especially evident at hydroxychloroquine concentrations of 500 and 1000 mg/L. (B) Urine microalbumin (quantitative assay) shows biphasic interference, with negative bias evident at 1–100 mg/L and a positive bias at 1000 mg/L. (C) THC screen shows slight positive bias from hydroxychloroquine, especially at hydroxychloroquine concentrations of 500 and 1000 mg/L. See Table 1 for details on assay versions.
Buprenorphine, cotinine, and oxycodone qualitative screens all showed absorbance alarm at 1000 mg/L; buprenorphine and cotinine additionally showed negative bias related to control (not spiked with hydroxychloroquine) at all concentrations starting at 1 mg/L (Fig. 3). Oxycodone screen did not show any evident bias at hydroxychloroquine concentrations up to 500 mg/L but registered an absorbance alarm at 1000 mg/L.
Myoglobin, microalbumin, and THC screen were examples of assays that showed evident bias but did not register any instrument alarm or error even up to 1000 mg/L (Fig. 4). A urine specimen pool with myoglobin concentration of 1008 ng/mL showed progressive negative bias with an apparent myoglobin concentration of 599 ng/mL when the sample was spiked with hydroxychloroquine concentration of 1000 mg/L (Fig. 4A). Microalbumin showed biphasic effects of hydroxychloroquine with negative bias at lower concentrations and a positive bias at higher concentrations (Fig. 4B); this was consistent across 4 urine specimen pools with varying baseline microalbumin concentrations. The THC screen showed slight positive bias (Fig. 4C); this would have potential to impact positive/negative results in specimens with baseline reactivity just below positive cutoff in the absence of hydroxychloroquine.
4. Discussion
Toxicity from chloroquine or hydroxychloroquine is often apparent shortly after overdose, and cardiac arrest can be the first sign [21]. These medications are rapidly absorbed from the gastrointestinal tract which leads to early onset of symptoms, usually within the first 1–3 h, and as soon as 30 min after ingestion [8,14]. Despite the long half-life of hydroxychloroquine of around 50 days [22], the duration of effect is short and often less than 24 h due to redistribution of the drug into other tissues [14]. This time course was observed in our patient, who developed toxicity within 1–2 h, which resolved within 24 h.
While seizures are well documented with chloroquine overdoses, there have been no reports in the literature of seizures after an overdose of hydroxychloroquine [23]. There have been reports of other neurologic symptoms, including generalized weakness, blurred vision, and vertigo [9,23]. A proximal myopathy has also been reported with therapeutic dosing, as well as overdose [9].
Hypokalemia has been previously reported with significant ingestions and is thought to be a result of decreased potassium efflux secondary to blockade of potassium membrane channels [15]. One retrospective study showed that the severity of toxicity was directly related to the degree of hypokalemia [24]. Some animal data, however, has shown that the hypokalemia may be protective against QRS widening and dysrhythmias [8]. While some amount of potassium replacement will likely be needed, this should be done with caution as rebound hyperkalemia is possible once toxicity is resolving and potassium redistributes [25].
Treatment of both chloroquine and hydroxychloroquine focuses on good supportive care, including early mechanical ventilation and vasopressor support [9]. Other treatment options include potassium repletion, sodium bicarbonate, lipid emulsion, and high dose diazepam. No studies have looked specifically at which vasoactive agent is the most effective for these overdoses. Most of the cases described in the literature recommend using epinephrine. This seems reasonable given the improvements in both contractility and peripheral vascular resistance seen with epinephrine [26]. Norepinephrine may be a reasonable alternative, though it does not provide the same degree of inotropic support [26].
Sodium bicarbonate is frequently used in treatment of medications that cause QRS prolongation via sodium channel blockade. Its evidence for efficacy for chloroquine and hydroxychloroquine is limited. Animal studies show mild improvement in QRS duration and human case reports are limited by confounders such as co-ingestions and other therapies [27]. If sodium bicarbonate is used, caution should be taken as it may cause worsening of QT prolongation due to further intracellular shifting of potassium [8]. Hydroxychloroquine is highly lipophilic, so theoretically intravenous lipid emulsion would be beneficial. Data on efficacy, however, is lacking and limited to case reports [28]. While case reports describe the use of enhanced elimination techniques, such as hemodialysis, these are not thought to be beneficial due to high volumes of distribution and high protein binding for hydroxychloroquine [4,29].
One therapy that is unique to these overdoses is high dose diazepam. The initial data that this was derived from includes animal models and case reports of patients that were found to have less severe toxicity if they had overdosed on both chloroquine and diazepam [30]. Diazepam will help with sedation, as well as seizure treatment and prophylaxis, but otherwise the exact mechanism is unclear [8]. Hypotheses include a direct anti-dysrhythmia effect, a pharmacokinetic interaction between diazepam and chloroquine, and a central antagonistic effect [4]. While the benefit is unclear, in a case of severe life-threatening toxicity, it seems prudent to use a medication with minimal side effects such as diazepam [8].
Our study demonstrates the potential for hydroxychloroquine to interfere with the performance of urine assays, either by causing interference bias or an instrument absorbance alarm. The ability to produce an absorbance alarm depends on the extent of light absorption by hydroxychloroquine at the measured wavelength(s) of the assay such as the 340 nm wavelength for the oxycodone screen in the present study. An alarm will register if the specimen yields a reading outside the measuring range of the photometer. Our subsequent in vitro analysis of urine specimens spiked with varying concentrations of hydroxychloroquine showed clear evidence of interference bias and/or absorbance alarm on 6 of 24 urine assays examined (buprenorphine, cotinine, oxycodone, and THC qualitative drug screens; microalbumin and urine myoglobin quantitative assays). The buprenorphine, cotinine, and oxycodone screens are all enzyme immunoassays. Buprenorphine and cotinine showed both negative bias at concentrations of 1–500 mg/L and also an absorbance alarm at 1000 mg/L. Oxycodone did not show bias at hydroxychloroquine concentrations up to 500 mg/L but registered an absorbance alarm at 1000 mg/L. This is consistent with the patient in the present case, where a urine specimen with hydroxychloroquine concentration exceeding 500 mg/L flagged an absorbance error for the oxycodone screen on urine drug screening ordered in the hospital. A urine specimen collected later, with a hydroxychloroquine concentration of 130 mg/L, did not flag any absorbance errors. Note that some categories of urine assays, such as those using ion-selective electrodes (chloride, potassium sodium), did not show any interference.
A previous study did not show positive cross-reactivity (positive bias) of hydroxychloroquine up to 455 mg/L for the same buprenorphine enzyme immunoassay used in the present study but did show cross-reactivity for a buprenorphine CEDIA (cloned enzyme donor immunoassay) assay [18]. Our study showed inhibited signal (negative bias) and then an absorbance alarm at 1000 mg/L hydroxychloroquine. Two previous studies that examined cross-reactivity of various compounds with drug of abuse and therapeutic drug monitoring assays found that hydroxychloroquine has low structural similarity with common targets of these assays using computational chemistry methods, consistent with minimal ability to produce positive cross-reactivity (false positives) in assay package insert data [31,32]. This type of analysis suggests that interference produced by hydroxychloroquine is not due to structural similarity but instead other mechanisms such as absorption interference or chemical reaction with assay reagents. Given how commonly drug of abuse screening in used in the emergency department setting [33], understanding of potential interferences are important to avoid diagnostic confusion [34]. Overdoses such as hydroxychloroquine can cause additional challenges in producing cardiovascular symptoms (e.g., arrhythmias) that may overlap with common street drugs of abuse [[35], [36], [37]] or other ingestions such as caffeine [38] or tricyclic antidepressants [39].
Myoglobin, microalbumin, and THC assays showed varying degrees of bias by hydroxychloroquine without instrument alarms. The interference of hydroxychloroquine on the microalbumin assay was biphasic across hydroxychloroquine concentrations. We did not see any interference with the Roche Total Protein assay; in contrast, two prior studies found hydroxychloroquine falsely elevated urine protein dipstick methods using tetrabromophenol blue [20] and pyrogallol red-molybdate methods [19]. The Roche protein assay used in the current operates using a colorimetric assay based on the biuret method [40].
5. Conclusions
The present study shows that large overdoses of hydroxychloroquine have the potential to cause both electrocardiographic abnormalities and interference with urine laboratory assays. Urine concentrations of hydroxychloroquine can reach very high concentrations (exceeding 500 mg/L), with the potential to interfere with a range of urine assays including drug of abuse screening and microalbumin. As a future direction, it would be of interest to study hydroxychloroquine effects on more subtle effects such as metabolomics [36].
Funding
The study was funded by internal funding from the University of Iowa Hospitals and Clinics Department of Pathology.
Declaration of Competing Interest
None of the authors have any conflict to report.
Acknowledgements
None
References
- 1.Hu C., Lu L., Wan J.P., Wen C. The pharmacological mechanisms and therapeutic activities of hydroxychloroquine in rheumatic and related diseases. Curr. Med. Chem. 2017;24(20):2241–2249. doi: 10.2174/0929867324666170316115938. [DOI] [PubMed] [Google Scholar]
- 2.Plantone D., Koudriavtseva T. Current and future use of chloroquine and hydroxychloroquine in infectious, immune, neoplastic, and neurological diseases: a mini-review. Clin. Drug Investig. 2018;38(8):653–671. doi: 10.1007/s40261-018-0656-y. [DOI] [PubMed] [Google Scholar]
- 3.Rainsford K.D., Parke A.L., Clifford-Rashotte M., Kean W.F. Therapy and pharmacological properties of hydroxychloroquine and chloroquine in treatment of systemic lupus erythematosus, rheumatoid arthritis and related diseases. Inflammopharmacology. 2015;23(5):231–269. doi: 10.1007/s10787-015-0239-y. [DOI] [PubMed] [Google Scholar]
- 4.Barry J.D. Chapter 59: antimalarials. In: Hoffman R.S., Howland M.A., Lewin N.A., Nelson L.S., Goldfrank L.R., editors. Goldfrank’s Toxicologic Emergencies. McGraw-Hill Education; New York City, NY: 2014. [Google Scholar]
- 5.Ashley E.A., Pyae Phyo A., Woodrow C.J. Malaria. Lancet. 2018;391(10130):1608–1621. doi: 10.1016/S0140-6736(18)30324-6. [DOI] [PubMed] [Google Scholar]
- 6.Hanboonkunupakarn B., White N.J. The threat of antimalarial drug resistance. Trop. Dis. Travel Med. Vaccines. 2016;2:10. doi: 10.1186/s40794-016-0027-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Noedl H. The need for new antimalarial drugs less prone to resistance. Curr. Pharm. Des. 2013;19(2):266–269. [PubMed] [Google Scholar]
- 8.Marquardt K., Albertson T.E. Treatment of hydroxychloroquine overdose. Am. J. Emerg. Med. 2001;19(5):420–424. doi: 10.1053/ajem.2001.25774. [DOI] [PubMed] [Google Scholar]
- 9.Jordan P., Brookes J.G., Nikolic G., Le Couteur D.G. Hydroxychloroquine overdose: toxicokinetics and management. J. Toxicol. Clin. Toxicol. 1999;37(7):861–864. doi: 10.1081/clt-100102466. [DOI] [PubMed] [Google Scholar]
- 10.McChesney E.W. Animal toxicity and pharmacokinetics of hydroxychloroquine sulfate. Am. J. Med. 1983;75(1A):11–18. doi: 10.1016/0002-9343(83)91265-2. [DOI] [PubMed] [Google Scholar]
- 11.Isbister G.K., Dawson A., Whyte I.M. Hydroxychloroquine overdose: a prospective case series. Am. J. Emerg. Med. 2002;20(4):377–378. doi: 10.1053/ajem.2002.33775. [DOI] [PubMed] [Google Scholar]
- 12.Kemmenoe A.V. An infant fatality due to hydroxychloroquine poisoning. J. Anal. Toxicol. 1990;14(3):186–188. doi: 10.1093/jat/14.3.186. [DOI] [PubMed] [Google Scholar]
- 13.Graham J.D. An overdose of “plaquenil”. Br. Med. J. 1960;1(5181):1256. doi: 10.1136/bmj.1.5181.1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smith E.R., Klein-Schwartz W. Are 1-2 dangerous? Chloroquine and hydroxychloroquine exposure in toddlers. J. Emerg. Med. 2005;28(4):437–443. doi: 10.1016/j.jemermed.2004.12.011. [DOI] [PubMed] [Google Scholar]
- 15.Gunja N., Roberts D., McCoubrie D., Lamberth P., Jan A., Simes D.C., Hackett P., Buckley N.A. Survival after massive hydroxychloroquine overdose. Anaesth. Intensive Care. 2009;37(1):130–133. doi: 10.1177/0310057X0903700112. [DOI] [PubMed] [Google Scholar]
- 16.Melles R.B., Marmor M.F. The risk of toxic retinopathy in patients on long-term hydroxychloroquine therapy. JAMA Ophthalmol. 2014;132(12):1453–1460. doi: 10.1001/jamaophthalmol.2014.3459. [DOI] [PubMed] [Google Scholar]
- 17.Schroeder R.L., Gerber J.P. Chloroquine and hydroxychloroquine binding to melanin: some possible consequences for pathologies. Toxicol. Rep. 2014;1:963–968. doi: 10.1016/j.toxrep.2014.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Melanson S.E., Snyder M.L., Jarolim P., Flood J.G. A new highly specific buprenorphine immunoassay for monitoring buprenorphine compliance and abuse. J. Anal. Toxicol. 2012;36(3):201–206. doi: 10.1093/jat/bks003. [DOI] [PubMed] [Google Scholar]
- 19.da Silva A.S., Falkenberg M. Analytical interference of quinolone antibiotics and quinine derived drugs on urinary protein determined by reagent strips and the pyrogallol red-molybdate protein assay. Clin. Biochem. 2011;44(12):1000–1004. doi: 10.1016/j.clinbiochem.2011.05.018. [DOI] [PubMed] [Google Scholar]
- 20.Wang J.M., Li J.Y., Huang W.C., Wen C.Y., Lee C.H., Yang C.Y., Wu M.F. Confirmed false positive proteinuria in patients with systemic lupus erythematosus taking hydroxychloroquine: a spot sample measurement. Clin. Lab. 2015;61(5–6):581–586. doi: 10.7754/clin.lab.2014.140706. [DOI] [PubMed] [Google Scholar]
- 21.Jaeger A., Sauder P., Kopferschmitt J., Flesch F. Clinical features and management of poisoning due to antimalarial drugs. Med. Toxicol. Adverse Drug Exp. 1987;2(4):242–273. doi: 10.1007/BF03259868. [DOI] [PubMed] [Google Scholar]
- 22.Tett S.E., Cutler D.J., Day R.O., Brown K.F. Bioavailability of hydroxychloroquine tablets in healthy volunteers. Br. J. Clin. Pharmacol. 1989;27(6):771–779. doi: 10.1111/j.1365-2125.1989.tb03439.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chansky P.B., Werth V.P. Accidental hydroxychloroquine overdose resulting in neurotoxic vestibulopathy. BMJ Case Rep. 2017;2017 doi: 10.1136/bcr-2016-218786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Clemessy J.L., Favier C., Borron S.W., Hantson P.E., Vicaut E., Baud F.J. Hypokalaemia related to acute chloroquine ingestion. Lancet. 1995;346(8979):877–880. doi: 10.1016/s0140-6736(95)92711-5. [DOI] [PubMed] [Google Scholar]
- 25.Clemessy J.L., Taboulet P., Hoffman J.R., Hantson P., Barriot P., Bismuth C., Baud F.J. Treatment of acute chloroquine poisoning: a 5-year experience. Crit. Care Med. 1996;24(7):1189–1195. doi: 10.1097/00003246-199607000-00021. [DOI] [PubMed] [Google Scholar]
- 26.Hollenberg S.M. Vasoactive drugs in circulatory shock. Am. J. Respir. Crit. Care Med. 2011;183(7):847–855. doi: 10.1164/rccm.201006-0972CI. [DOI] [PubMed] [Google Scholar]
- 27.Bruccoleri R.E., Burns M.M. A literature review of the use of sodium bicarbonate for the treatment of QRS widening. J. Med. Toxicol. 2016;12(1):121–129. doi: 10.1007/s13181-015-0483-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ten Broeke R., Mestrom E., Woo L., Kreeftenberg H. Early treatment with intravenous lipid emulsion in a potentially lethal hydroxychloroquine intoxication. Neth. J. Med. 2016;74(5):210–214. [PubMed] [Google Scholar]
- 29.McBeth P.B., Missirlis P.I., Brar H., Dhingra V. Novel therapies for myocardial irritability following extreme hydroxychloroquine toxicity. Case Rep. Emerg. Med. 2015;2015 doi: 10.1155/2015/692948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yanturali S., Aksay E., Demir O.F., Atilla R. Massive hydroxychloroquine overdose. Acta Anaesthesiol. Scand. 2004;48(3):379–381. doi: 10.1111/j.0001-5172.2004.0302.x. [DOI] [PubMed] [Google Scholar]
- 31.Krasowski M.D., Siam M.G., Iyer M., Ekins S. Molecular similarity methods for predicting cross-reactivity with therapeutic drug monitoring immunoassays. Ther. Drug Monit. 2009;31(3):337–344. doi: 10.1097/FTD.0b013e31819c1b83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Krasowski M.D., Siam M.G., Iyer M., Pizon A.F., Giannoutsos S., Ekins S. Chemoinformatic methods for predicting interference in drug of abuse/toxicology immunoassays. Clin. Chem. 2009;55(6):1203–1213. doi: 10.1373/clinchem.2008.118638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lager P.S., Attema-de Jonge M.E., Gorzeman M.P., Kerkvliet L.E., Franssen E.J.F. Clinical value of drugs of abuse point of care testing in an emergency department setting. Toxicol. Rep. 2018;5:12–17. doi: 10.1016/j.toxrep.2017.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Krasowski M.D., Pizon A.F., Siam M.G., Giannoutsos S., Iyer M., Ekins S. Using molecular similarity to highlight the challenges of routine immunoassay-based drug of abuse/toxicology screening in emergency medicine. BMC Emerg. Med. 2009;9:5. doi: 10.1186/1471-227X-9-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tsatsakis A., Docea A.O., Calina D., Tsarouhas K., Zamfira L.M., Mitrut R., Sharifi-Rad J., Kovatsi L., Siokas V., Dardiotis E., Drakoulis N., Lazopoulos G., Tsitsimpikou C., Mitsias P., Neagu M. A mechanistic and pathophysiological approach for stroke associated with drugs of abuse. J. Clin. Med. 2019;8(9) doi: 10.3390/jcm8091295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tsoukalas D., Alegakis A., Fragkiadaki P., Papakonstantinou E., Nikitovic D., Karataraki A., Nosyrev A.E., Papadakis E.G., Spandidos D.A., Drakoulis N., Tsatsakis A.M. Application of metabolomics: focus on the quantification of organic acids in healthy adults. Int. J. Mol. Med. 2017;40(1):112–120. doi: 10.3892/ijmm.2017.2983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wood K.E., McCarthy P.J., Krasowski M.D. A case series involving young children presenting with accidental ingestion of amphetamine based stimulants. Toxicol. Rep. 2018;5:1129–1133. doi: 10.1016/j.toxrep.2018.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Willson C. The clinical toxicology of caffeine: a review and case study. Toxicol. Rep. 2018;5:1140–1152. doi: 10.1016/j.toxrep.2018.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Giwa A., Oey E. The return of an old nemesis: survival after severe tricyclic antidepressant toxicity, a case report. Toxicol. Rep. 2018;5:357–362. doi: 10.1016/j.toxrep.2018.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weichselbaum T.E. An accurate and rapid method for the determination of proteins in small amounts of blood serum and plasma. Am. J. Clin. Pathol. 1946;10:40–49. [PubMed] [Google Scholar]