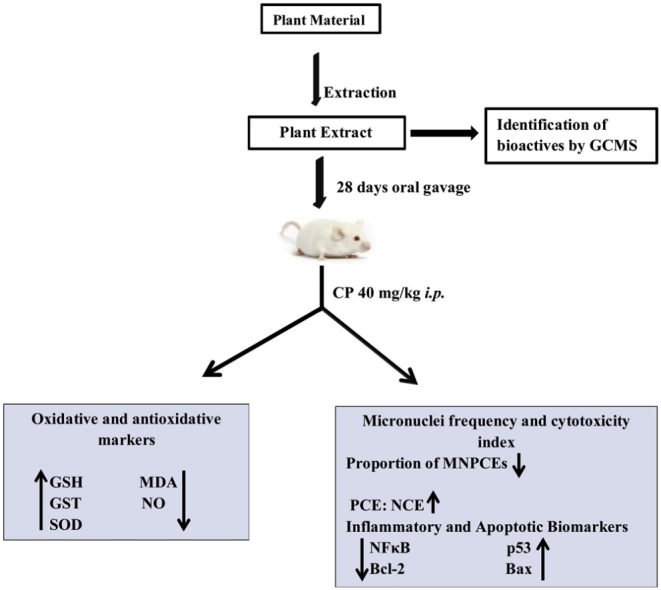
Graphical abstract
Keywords: Antioxidant enzymes, Apoptotic biomarkers, Micronuclei, N. vogelii
Highlights
-
•
N. vogelii extract protects against micronuclei induction, oxidative stress and lipid peroxidation in mice.
-
•
It upregulates the expression of cytoplasmic pro-apoptotic Bax and p53 proteins.
-
•
Downregulates the expression of cytoplasmic anti-apoptotic NFκB and Bcl-2 proteins.
-
•
It protects bone marrow cells against the cytotoxic effects of cyclophosphamide.
Abstract
Napoleona vogelii is used in traditional medicine for the management of pain, inflammatory conditions and cancer.
This study was conducted to investigate the modulatory mechanisms of methanol stem bark extract of N. vogelii on induction of micronuclei, apoptotic biomarkers and in vivo antioxidant enzymes in mice.
Forty male albino mice were randomly divided into eight groups (n = 5) and were administered distilled water (DW, 5 mL/kg) as negative control, 100, 200 or 400 mg/kg of the extract respectively for 28 days before the injection of cyclophosphamide (CP, 40 mg/kg) i.p. on the 28th day. The remaining groups were administered 100, 200 or 400 mg/kg of the extract only for 28 days. Twenty four hours after injection of CP or administration of the last dose of extract, animals were euthanized by cervical dislocation and blood samples collected for determination of in vivo antioxidants, the spleen harvested for immunohistochemical expression of NFκB, Bcl-2, Bax and p53. Bone marrow smears were also made for the micronucleus assay.
Treatment with the extract resulted in a significant (p < 0.0001) reduction in frequency of micronucleated polychromatic erythrocytes (MNPCEs) compared to CP exposed control conferring protection of 75.09, 94.74 and 96.84% at 100, 200 or 400 mg/kg respectively. In extract and CP exposed animals, there were significant (p < 0.05) increases in GSH, GST and SOD with a corresponding significant (p < 0.05) reduction in MDA. In addition, the extract significantly downregulated cytoplasmic levels of NFκB and Bcl-2 and upregulated Bax and p53.
These findings demonstrate that N. vogelli may serve as an interesting lead for chemo-preventive drug development.
1. Introduction
Bioactive compounds and medicinal plants have been reputed to elicit adverse toxicological effects on biological systems which may include mutagenicity and carcinogenicity. These effects therefore, necessitates due caution in their use as therapeutic agents [[1], [2], [3], [4]]. Conversely, it is important to note that these compounds have also been reported to possess antigenotoxic or anticarcinogenic benefits [[5], [6], [7]]. Hence, the scientific investigation of medicinal plants and their products is important because they may serve as potential chemotherapeutic agents or adjuncts to ameliorate the toxic effects of these classes of drugs. It is also imperative to subject these plants to these investigations in order to elucidate their toxicities [8,9]. The in vivo bone marrow micronucleus assay was developed in mouse bone marrow cells by Schmid [10] and has been repeatedly used to assess the genotoxicity of several physical and chemical agents [[11], [12], [13], [14]] in the search for novel chemoprevention drug candidates [15,16].
The ability of plant extracts to induce increase in detoxifying enzymes, scavenge reactive oxygen species (ROS) and induce apoptosis represents an important technique in the prevention of carcinogenesis [17]. According to IARC, 57% of new cancer cases and 65% of cancer deaths are prevalent in less developed regions of the world which includes Central America and some parts of Africa and Asia in 2012. This report also stated that the projected number of new cancer cases will rise to 23.6 million per year by 2030. Since a significant proportion of all cancers are linked to lifestyle and faulty genes [18], exposure to and /or consumption of the right phytochemicals provided by medicinal food and plants could result in the avoidance of certain cancers and prevent a number of cancer deaths [19,20].
Nuclear factor-kappaB (NF-κB) can be activated by carcinogens, tumor promoters and chemotherapeutic agents and can suppress apoptosis, promote chemoresistance and tumorigenesis. However, natural products can downregulate NFκB in vitro and in vivo as a mechanism of chemoprevention [21,22]. The chemopreventive effect of Hibiscus sabdariffa is mediated through the suppression of the Akt/NF-κB signaling pathway, which results in reduced invasiveness of cancer cells [22]. Celastrol, a natural chemopreventive agent, elicits its therapeutic benefits by suppression of constitutively active NF-κB [23]. Therefore, the downregulation or inactivation of NFκB and upregulation of pro-apoptotic factors by medicinal plants may abolish the risk for development of carcinogenic outcomes and secondary neoplasms secondary to chemotherapy.
The spleen plays a prevalent role in several disorders of the hematological system in mice and humans because of its ability to sustain hematologic malignancies [24]. Hematologic stromal cells including those of splenic origin in mice and humans have been reported to demonstrate anti-apoptotic effect in cells of various hematological malignancies by the upregulation of NFκB thereby allowing pathologic relapse [[25], [26], [27], [28]].
Napoleona vogelii is an evergreen shrub or a low branching tree with a dense crown growing up to 15 m tall. It is found mostly in the rain forest regions of Sierra Leone and Nigeria [29]. It is used in traditional medicine for cough, pain, inflammatory conditions [[30], [31], [32]] and cancer [33]. The stem bark is used locally to flavor rice and also chewed with kolanuts as a stimulant [34]. It is used in making wooden poles, chewing sticks and mats and the stem bark decoction is applied topically for the treatment of dermatosis [35,36]. Some researchers have also documented the antidiabetic [37], antiasthma and in vitro cytotoxic [36,38] activities of extracts of the plant. Based on its reported therapeutic benefits and the need to discover novel drug candidates with the potential for chemoprevention, this study was conducted to determine the safety profile of the methanol stem bark extract of N. vogelii on bone marrow cells using the micronucleus assay and to establish its effect on antioxidant responses and apoptotic biomarkers in mice.
2. Materials and methods
2.1. Drugs and chemicals
Cyclophosphamide (Endoxan™ Baxter Oncology GmbH, Frankfurt am Main, Germany), Phosphate buffered saline (PBS), Methanol (Sigma-Aldrich Chemie GmbH, Germany), Normal saline (Unique Pharmaceuticals Ltd, Ogun State, Nigeria), Giemsa stain (Sigma- Aldrich Chemie GmbH, Germany), May-Grunwald Stain (Sigma-Aldrich Chemie GmbH, Germany) Fetal Bovine Serum (Sigma-Aldrich, Brazil), rabbit polyclonal anti-NFκB/p65, Bax (Abcam, Cambridge MA, USA), Bcl-2, p53 (Santa Cruz Biotechnology, Delaware Ave, Santa Cruz, USA).
2.2. Plant extraction
The stem bark of N. vogelii was collected from a secondary forest in Abatadu village, Ikire township of Osun state in the South Western part of Nigeria and duly authenticated by Prof. J.D. Olowokudejo in the Department of Botany and Microbiology, University of Lagos, Nigeria where a voucher specimen (LUH 6524) was deposited in the herbarium. It was then prepared, extracted and reconstituted as described by Ikumawoyi et al. [39].
2.3. Experimental animals
Male albino mice (Mus musculus) of about 7–8 weeks weighing between 17–30 g used were obtained and maintained as described in another study [39]. The animals were acclimatized for two weeks before commencement of the study and the procedures employed were in conformity with The Guide for the Care and Use of Laboratory Animals published by the U.S. National Institutes of Health (NIH Publication No 8023, revised 1978) for studies involving experimental animals. Ethical approval was granted by the Health Research Ethics Committee, College of Medicine University of Lagos (CMUL/HREC/02/18/334).
2.4. Identification of bioactive constituents
The bioactive constituents in the extract were identified by Gas chromatography mass spectrometry [40].
2.5. Acute toxicity
A previous study by Ikumawoyi et al. [39] had reported that the LD50 oral of the extract is greater than 4000 mg/kg. The Miller and Tainter method [41] was employed in this study. Five (5) groups of 5 mice each were fasted for 12 h and were then treated with the extract at doses of 0.5, 1, 2, 3, 4 or 5 g/kg p.o. and 0.25, 0.5, 0.8, 1 or 2 g/kg i.p. Animals were observed for 2 h after extract administration for behavioural symptoms of toxicity and mortality and then after 24 h for mortality. They were further observed for 14 days for signs of delayed toxicity.
2.6. Micronuclei and prophylactic study
Micronuclei were induced with CP, 10, 20 or 40 mg/kg. However, CP 40 mg/kg was used as the positive control. The protocols of Schmid [10] and Alabi and Bakare [42] were adopted. Animals were divided into eight groups of five mice each comprising four groups for the prophylactic study and four groups for extract treatment only. For the prophylactic study, animals were administered 100, 200 or 400 mg/kg of the extract respectively for 28 days before the injection of CP, 40 mg/kg on the 28th day. The groups administered only the extract proceeded with DW, 5 mL/kg as control, 100, 200 or 400 mg/kg of extract respectively for 28 days. Twenty four hours after injection of CP or administration of the last dose of extract, animals were euthanized by cervical dislocation and both femurs surgically removed and cleaned.
2.7. Bone marrow smears
The epiphyses were cut off and the bone marrow flushed with 1 ml fetal bovine serum (FBS) into different eppendorf tubes (1.5 ml). The bottom of the tubes was tapped gently to allow for proper dispersion of the cells and then centrifuged at 2000 rpm for 5 min. The supernatant was removed with the pellet re-suspended in another 1 ml of FBS in the eppendorf tube and then mixed properly using a micropipette before being centrifuged again at the same rate.
The supernatant was removed and 0.5 ml of FBS added to the pellet, mixed properly and then drops of the viscous suspension added to a clean, grease-free slide. A smear of this drop was made and allowed to air-dry. It was then fixed in 70% methanol for 3 min and air dried.
2.8. Staining
The slides were stained in 0.4% May-Grunwald in absolute methanol for 3–4 min and were immediately transferred into a coplin jar containing May-Grunwald and distilled water in a ratio of 1:1 and allowed to stain for another 3–4 minutes. Following this, they were rinsed in distilled water and allowed to air dry completely. Slides were then stained in 5% Giemsa for 5 min, rinsed and allowed to air dry completely before dipping in xylene. They were then finally covered with a cover slip after adding 2–3 drops of dibutyl pthalate xylene.
2.9. Slide scoring
Slides were scored at 1000X (oil immersion) for the frequency of the number of micronucleated polychromatic erythrocytes (MNPCEs) out of 1000 PCEs scored per animal. The number of PCE to NCE ratio out of 1000 cells scored per animal served as the cytotoxicity index. The differential staining of PCEs (bluish-purple) and NCEs (pink), and the relative size of the erythrocytes are indices for differentiating them [43]. The cytotoxicity index (PCE : NCE ratio) was estimated using the formula:
While percentage protection was derived using the formula:
2.10. Antioxidant study
Superoxide dismutase activity was determined as described by Sun and Zigma [44], catalase activity by the method of Beers and Sizer as described by Usoh et al. [45], reduced glutathione by the method of Sedlak and Lindsay [46]. Glutathione-S-transferase was determined according to Habig et al. [47], GPx was determined using the method of Hafemann et al. [48]. Malondialdehyde (MDA) was determined using the method of Buege and Aust [49] while nitric oxide was determined by the Griess assay [50].
2.11. Immunohistochemical detection of apoptotic factors
2.11.1. Procedure
After preparation of tissue blocks, immunohistochemical detection of NFκB and apoptosis regulatory proteins; Bcl-2, Bax and p53 were conducted. Paraffin sections (5μ) were used. Paraformaldehyde-fixed paraffin sections were de-waxed and rehydrated by immersion in descending grade alcohol and brought down to distilled water (reversal of parafinization). Antigen retrieval was done by incubation in 0.01 M sodium citrate (pH 6.0) at 98⁰C for 20 min, after which the slides were washed in PBS. Nonspecific binding was blocked by incubation with 1% BSA (Bovine Serum Albumin) for 1 h. The sections were then incubated with the primary antibodies for 90 min. at room temperature with dilutions as follows: NFκB/p65 – 1: 700, Bax – 1: 400, p53 – 1: 75, Bcl-2 – 1: 50. It was thereafter washed and incubated with secondary antibodies for 30 min at room temperature. Thereafter, it was incubated with fresh DAB solution for 8 min, rinsed in distilled water and counterstained with Haematoxylin before being dehydrated with alcohol and cleared in xylene. The sections were mounted in DPX and photographed under light microscopy (Model CX41RF Olympus Corporation, Tokyo, Japan). The percentage of positive immunolabeled cells over the total cells in each selected area was then counted and recorded [[51], [52], [53]].
2.12. Splenic histopathological assessment
Tissues fixed in 10% formol-saline were dehydrated in graded alcohol, embedded in paraffin, and cut into 4- to 5- μ m-thick sections. The sections were stained with hematoxylin-eosin for photomicroscopic assessment using a Model N-400ME photomicroscope (CEL-TECH Diagnostics, Hamburg, Germany).
3. Statistical analysis
Statistical analysis was done using One-way Analysis of Variance (ANOVA) followed by Tukey’s post-hoc multiple comparison test using Graphpad Prism 6.0 (GraphPad Software, CA, USA). Results were considered significant at p < 0.05.
4. Result
The oral LD50 from a previous study [39] was greater than 4000 mg/kg. However, in this study, there was no mortality recorded on administration of methanol stem bark extract of N. vogelii up to 5000 mg/kg p.o. Hence, the oral LD50 is greater than 5 g/kg while the LD50 i.p. was found to be 668 mg/kg.
4.1. Phytochemical analysis
4.1.1. Qualitative phytochemical analysis
From a previous study [39], the methanol stem bark extract of N. vogelii was found to contain flavonoids, phenols, saponins, tannins, phlobatanin and cardiac glycoside. Quantitatively, the extract consists of 87.88 ± 0.32 mg flavonoid gallic acid equivalent (GAE)/100 g extract, 24.88 ± 0.47 mg phenol GAE/100 g extract, 35.55 ± 0.19 mg saponin GAE/100 g extract and 13.01 ± 0.84 mg tannin GAE/100 g extract.
4.1.2. Structures of bioactives identified
The extract was found to contain the following compounds; Tridecanoic acid (0.38%), Pentadecanoic acid (0.97%), n- Hexadecanoic acid (29.81%), 9, 12 – Octadecadienoic acid (0.23%), 9 – Octadecenoic acid (2.64%), 7- Hexadecenoic acid (0.41%), phytol (0.34%), methyl stearate (0.67%), Oleic acid (48.57%), Octadecanoic acid (14.66%) and cis–10–Nonadecenoic acid (1.33%) [40]. The structures of these compounds are as shown (see supplementary file).
4.2. Micronuclei, prophylactic and anti – oxidant study
4.2.1. Micronuclei study with extract
4.2.1.1. Body weight variation
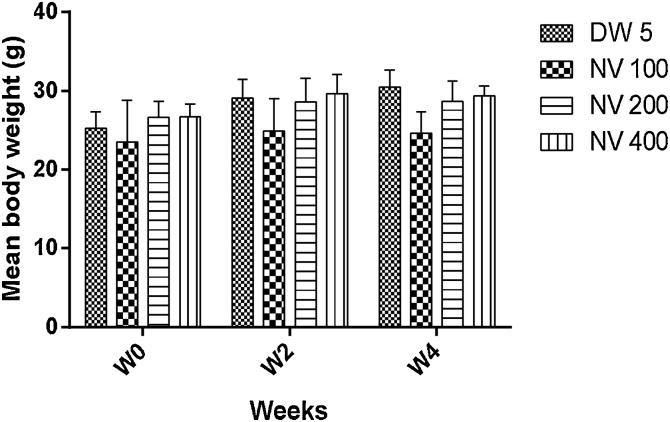
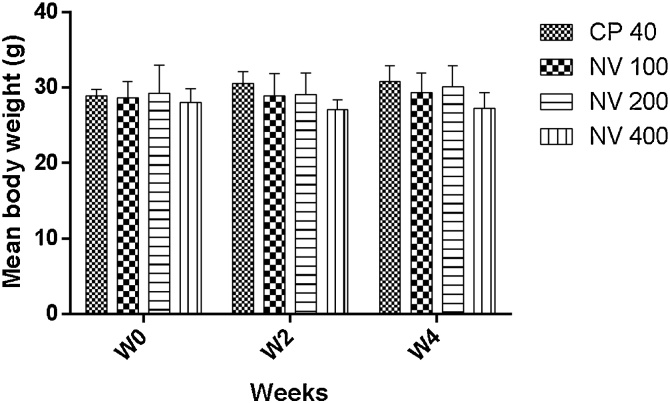
There was no significant difference in body weight variation compared to distilled water treated control (Fig. 1).
Fig. 1.
Effect of N. vogelii on body weight variation in the micronuclei study.
Results are mean ± SEM (n = 5) No significant difference between means (F (6, 36) = 0.39, p > 0.05). Two Way ANOVA followed Tukey’s posthoc multiple comparison test. W; weeks, DW; distilled water, CP; cyclophosphamide, NV; Napoleona vogelii.
4.3. Frequency of MNPCEs
4.3.1. Frequency of micronucleated polychromatic erythrocytes (MNPCEs)
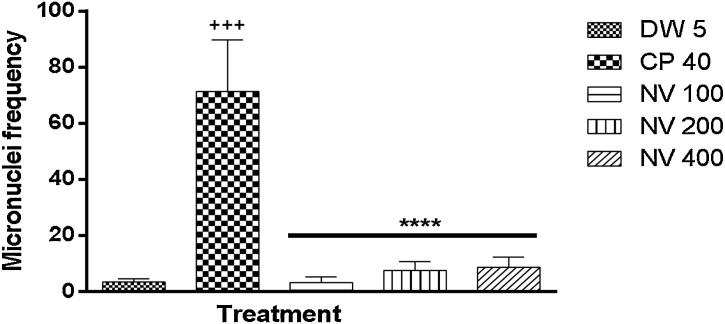
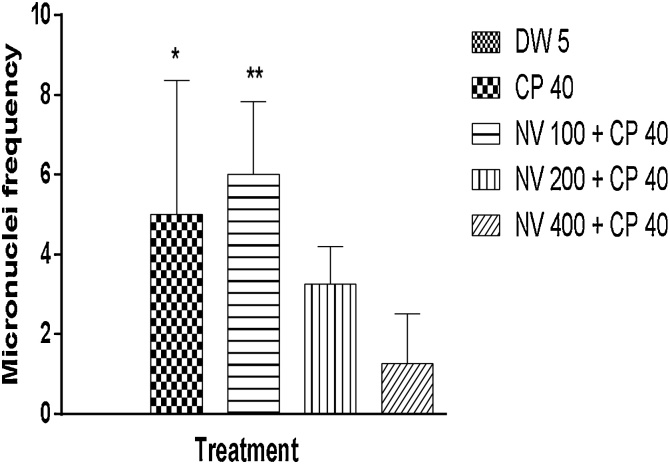
There was significant (p < 0.0001) reduction in frequency of micronucleated polychromatic erythrocytes (MNPCEs) at 100 (3.00 ± 1.08), 200 (7.50 ± 1.56) and 400 mg/kg (8.50 ± 1.85) compared to cyclophosphamide (CP) treated control (71.25 ± 9.18). No significant difference in frequency of MNPCE compared to distilled water treated control (Fig. 2).
Fig. 2.
Effect of N. vogelii on micronuclei frequency in PCEs in the micronuclei study.
Results are mean ± SEM (n = 5) +++ p < 0.0001 versus DW 5 mL/kg, ****p < 0.0001 versus CP 40 mg/kg (F (4, 15) = 47.42). One Way ANOVA followed by Tukey’s posthoc multiple comparison test. CP; Cyclophosphamide, DW; Distilled water, NV; Napoleona vogelii.
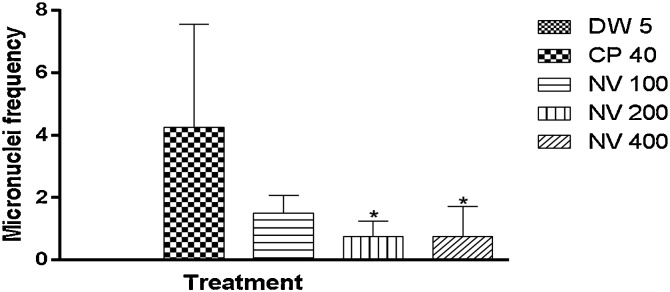
There was significant increase (p < 0.05) in normochromatic erythrocytes at CP 40 mg/kg (4.25 ± 1.65) compared to DW 5 mL/kg treated control (0.00 ± 0.00). However, normochromatic erythrocytes (NCEs) were significantly (p < 0.05) reduced at 200 (0.75 ± 0.25) and 400 mg/kg (0.75 ± 0.47) compared to CP treated control (4.25 ± 1.65) (Fig. 3).
Fig. 3.
Effect of N. vogelii on micronuclei frequency in NCEs in the micronuclei study.
Results are mean ± SEM (n = 5) * p < 0.05 versus DW 5 mL/kg, CP 40 mg/kg (F (4, 15) = 4.39). One Way ANOVA followed by Tukey’s posthoc multiple comparison test. CP; Cyclophosphamide, DW; Distilled water, NV; Napoleona vogelii.
4.4. Cytotoxicity index
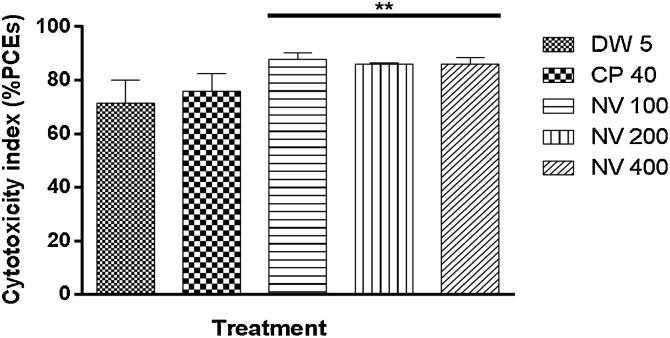
There was significant (p < 0.01) increase in PCE/NCE ratio at 100 (87.73 ± 1.29), 200 (85.97 ± 0.22) and 400 mg/kg (86.01 ± 1.21) compared to CP 40 mg/kg (75.97 ± 3.20) and DW 5 mL/kg (71.40 ± 4.31) treated controls (Fig. 4).
Fig. 4.
Effect of N. vogelii on cytotoxicity index in the micronuclei study.
Results are mean ± SEM (n = 5) ** p < 0.01 versus CP 40 mg/kg (F (4, 15) = 8.27). One Way ANOVA followed by Tukey’s posthoc multiple comparison test. CP; Cyclophosphamide, DW; Distilled water, NV; Napoleona vogelii.
4.5. Antioxidant enzymes
4.5.1. Effect of N. vogelii on antioxidant enzymes in the micronuclei study
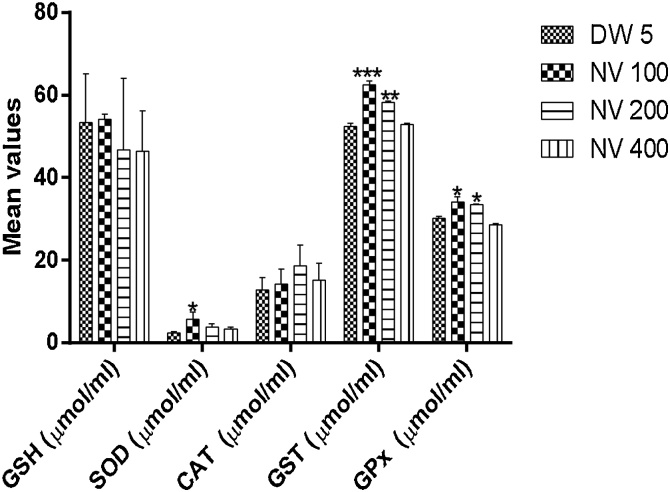
Glutathione-S-transferase (GST) was significantly (p < 0.001) increased at 100 (62.58 ± 0.57 μmol/ml/min) and 200 mg/kg (p < 0.01, 58.27 ± 0.18 μmol/ml/min) compared to DW 5 mL/kg (52.40 ± 0.52 μmol/ml/min).
Glutathione peroxidase (GPx) was significantly (p < 0.05) increased at 100 (34.08 ± 0.90 μmol/ml/min) and 200 mg/kg (33.52 ± 0.11 μmol/ml/min) compared to DW 5 mL/kg (30.20 ± 0.26 μmol/ml/min).
Superoxide dismutase (SOD) was significantly (p < 0.05) increased at 100 mg/kg (5.67 ± 0.97 μmol/ml/min) compared to DW 5 mL/kg (2.39 ± 0.16 μmol/ml/min).
Catalase (CAT) was non-significantly increased compared to distilled water treated control.
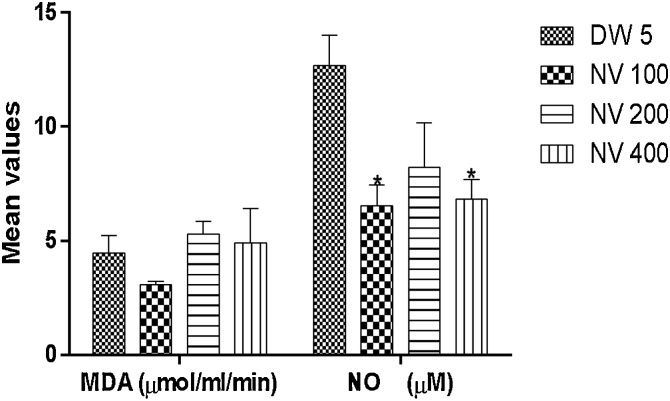
Nitric oxide (NO) was significantly (p < 0.05) reduced at 100 (6.53 ± 0.65 μM) and 400 mg/kg (6.84 ± 0.61 μM) compared to DW 5 mL/kg (12.69 ± 0.93 μM).
Malondialdehyde (MDA) was non-significantly different from control (Fig. 5, Fig. 6).
Fig. 5.
Effect of N. vogelii on antioxidant enzymes in the micronuclei study.
Results are mean ± SEM (n = 5) * p < 0.05, ** p < 0.01, *** p < 0.001 versus DW 5 mL/kg (F (3, 24) = 1.98). One Way ANOVA followed Tukey’s posthoc multiple comparison test. GSH; reduced glutathione, SOD; superoxide dismutase, CAT; catalase, GST; glutathione-S-transferase, GPx; glutathione peroxidase, DW; distilled water, CP; cyclophosphamide, NV; Napoleona vogelii.
Fig. 6.
Effect of N. vogelii on malondialdehyde and nitric oxide in the micronuclei study. Results are mean ± SEM (n = 5) MDA; No significant difference versus DW 5 mL/kg, NO.
* p < 0.05 versus DW 5 mL/kg (F (3, 8) = 7.87). One Way ANOVA followed by Tukey’s posthoc multiple comparison test. MDA; malondialdehyde, NO; Nitric oxide, DW; Distilled water, NV; Napoleona vogelii.
4.6. Prophylactic study
4.6.1. Body weight variation
There was no significant difference in body weight variations in extract treated compared to distilled water treated and cyclophosphamide treated controls (Fig. 7).
Fig. 7.
Effect of N. vogelii on body weight variation in the prophylactic study.
Results are mean ± SEM (n = 5) No significant difference between means (F (3, 6) = 2.74, p > 0.05). Two Way ANOVA followed Tukey’s posthoc multiple comparison test. W; weeks, DW; distilled water, CP; cyclophosphamide, NV; Napoleona vogelii.
4.7. Frequency of MNPCEs
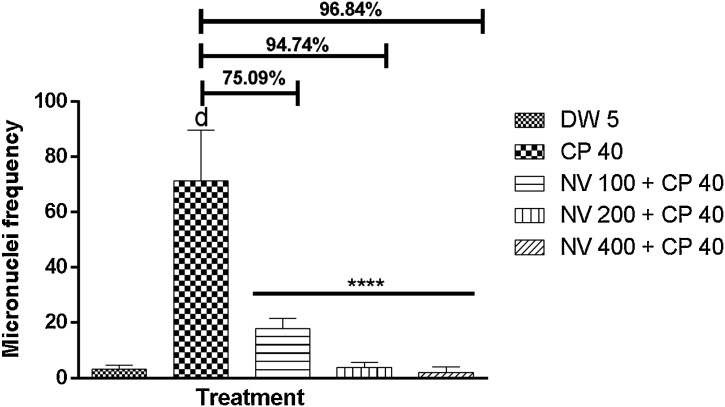
There was significant (p < 0001) reduction in frequency of micronucleated polychromatic erythrocytes (MNPCEs) and dose dependent increase in percentage protection at 100 (17.75 ± 1.89, 75.09%), 200 (3.75 ± 0.95, 94.74%) and 400 mg/kg (2.00 ± 1.08, 96.84%) in mouse bone marrow compared to CP 40 mg/kg (71.25 ± 9.18) treated control. Normochromatic erythrocytes (NCEs) also exhibited a dose dependent decrease compared to CP treated control (Fig. 8, Fig. 9).
Fig. 8.
Effect of N. vogelii on micronuclei frequency in PCEs in the prophylactic study. Results are mean ± SEM (n = 5) d p < 0.0001 versus DW 5 mL/kg, **** p < 0.0001 versus CP 40 mg/kg. (F (4, 15) = 48.47). One Way ANOVA followed by Tukey’s posthoc multiple comparison test. DW; Distilled water, CP; Cyclophosphamide, NV; Napoleona vogelii.
Fig. 9.
Effect of N. vogelii on micronuclei frequency in NCEs in the prophylactic study. Results are mean ± SEM (n = 5) * p < 0.05, ** p < 0.01 versus DW 5 mL/kg (F (4, 15) = 7.30). One Way ANOVA followed by Tukey’s posthoc multiple comparison test. DW; Distilled water, CP; Cyclophosphamide, NV; Napoleona vogelii.
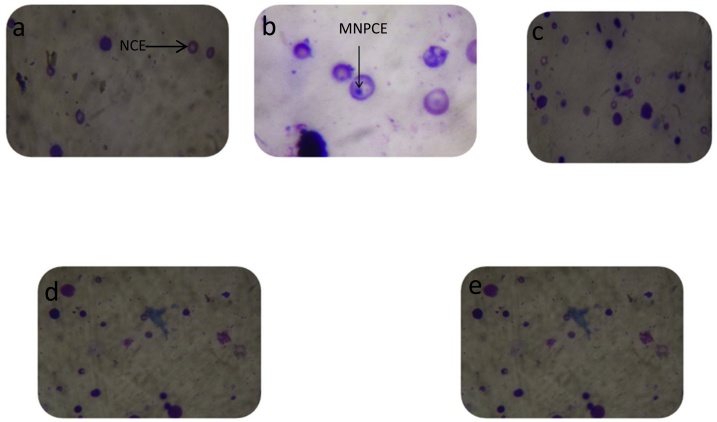
4.8. Bone marrow cytology
The bone marrow cell smears of extract, DW and CP treated mice are represented as showing intact cells, normochromatic erythrocytes and micronucleated polychromatic erythrocytes (Fig. 10).
Fig. 10.
Representative cytology of bone marrow cell smears in extract and CP treated mice.
a. DW treated control showing intact cells b. MNPCE in CP treated mice c. 100 mg/kg extract treated mice d. 200 mg/kg extract treated mice e. 400 mg/kg extract treated mice.
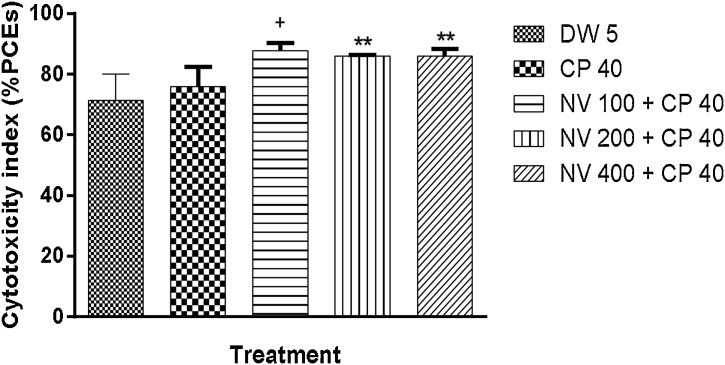
4.9. Cytotoxicity index
There was significant (p < 0.05) increase in PCE/NCE ratio at 100 mg/kg (87.73 ± 1.29) compared to CP treated control and p < 0.01 at 100 mg/kg (87.73 ± 1.29), 200 mg/kg (85.97 ± 0.22) and 400 mg/kg (86.01 ± 1.21) compared to 5 mL/kg DW (71.40 ± 4.31) (Fig. 11).
Fig. 11.
Effect of N. vogelii on cytotoxicity index in the prophylactic study.
Results are mean ± SEM (n = 5) + p < 0.05 versus CP 40 mg/kg, DW 5 mL/kg ** p < 0.01 versus DW 5 mL/kg (F (4, 15) = 8.27). One Way ANOVA followed by Tukey’s posthoc multiple comparison test. DW; Distilled water, CP; Cyclophosphamide, NV; Napoleona vogelii.
4.10. Antioxidant enzymes
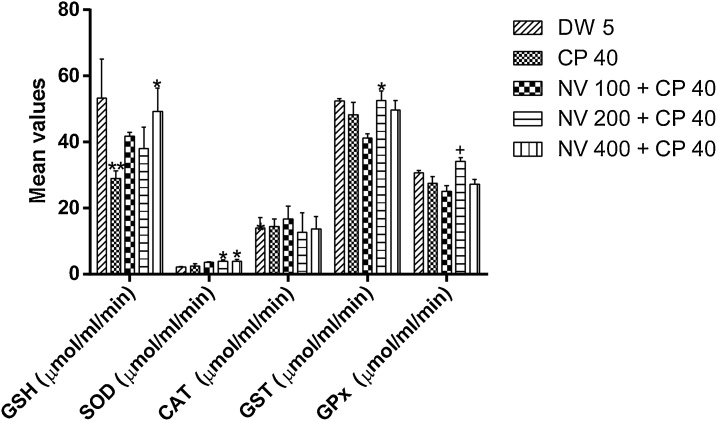
4.10.1. Effect of N. vogelii on antioxidant enzymes in the prophylactic study
Reduced glutathione (GSH) was significantly (p < 0.01) reduced at CP 40 mg/kg (29.03 ± 1.58 μmol/ml/min) compared to DW 5 mL/kg (53.40 ± 8.29 μmol/ml/min) and significantly (p < 0.05) increased at 400 mg/kg (49.29 ± 4.98 μmol/ml/min) compared to CP 40 mg/kg (29.03 ± 1.58 μmol/ml/min).
Glutathione-S-transferase (GST) was significantly (p < 0.05) increased at 200 mg/kg (52.61 ± 2.03 μmol/ml/min) compared to CP 40 mg/kg (48.21 ± 2.65 μmol/ml/min). Glutathione peroxidase (GPx) was significantly increased at 200 mg/kg (34.16 ± 0.59 μmol/ml/min) compared to CP 40 mg/kg (27.55 ± 1.02 μmol/ml/min) and DW 5 mL/kg (30.73 ± 0.33 μmol/ml/min) while superoxide dismutase (SOD) was significantly (p < 0.05) increased at 200 (3.91 ± 0.26 μmol/ml/min) and 400 mg/kg (3.97 ± 0.38 μmol/ml/min) compared to CP 40 mg/kg (2.41 ± 0.57 μmol/ml/min) and DW 5 mL/kg (2.23 ± 0.06 μmol/ml/min) treated controls. Catalase (CAT) was non-significantly increased compared to the controls.
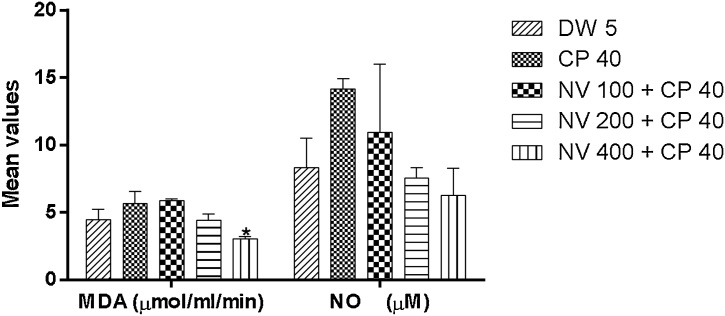
Nitric oxide (NO) was non-significantly decreased, while malondialdehyde (MDA) was significantly (p < 0.05) reduced at 400 mg/kg (3.03 ± 0.13 μmol/ml/min) compared to CP 40 mg/kg (5.65 ± 0.65 μmol/ml/min) and DW 5 mL/kg (4.46 ± 0.54 μmol/ml/min) (Fig. 12, Fig. 13).
Fig. 12.
Effect of N. vogelii on antioxidant enzymes in the prophylactic study.
Results are mean ± SEM (n = 5) * p < 0.05, + p < 0.001 versus CP 40 mg/kg (F (4, 40) = 6.52). One Way ANOVA followed Tukey’s posthoc multiple comparison test. GSH; reduced glutathione, SOD; superoxide dismutase, CAT; catalase, GST; glutathione-S-transferase, GPx; glutathione peroxidase, DW; distilled water, CP; cyclophosphamide, NV; Napoleona vogelii.
Fig. 13.
Effect of N. vogelii on malondialdehyde and nitric oxide in the prophylactic study. Results are mean ± SEM (n = 5) MDA; * p < 0.05 versus CP 40 mg/kg. NO; No significant difference versus controls (F (4, 10) = 4.64, p > 0.05). One Way ANOVA followed by Tukey’s posthoc multiple comparison test. MDA; malondialdehyde, NO; Nitric oxide, CP; Cyclophosphamide, NV; Napoleona vogelii.
4.11. Effect of N. vogelii on apoptotic factors
In the micronuclei induction study, the extract significantly (p < 0.0001) increased NFκB expression at 100 (48.80 ± 2.44%) and 200 mg/kg (42.20 ± 1.24%) compared to DW 5 mL/kg (21.80 ± 1.39%). NFκB was significantly (p < 0.01) reduced at 400 mg/kg (13.00 ± 0.71%) compared to DW 5 mL/kg (21.80 ± 1.39%) (Fig. 14).
Fig. 14.
Effect of N. vogelii on splenic level of NFκB expression in the micronuclei study. Results are mean ± SEM. ** p < 0.01, **** p < 0.0001 versus DW 5 mL/kg (F (3, 16) = 114.3). One Way ANOVA followed by Tukey’s posthoc multiple comparison test. NFκB; Nuclear factor κappa B, Napoleona vogelii.
p53 was significantly (p < 0.001) reduced at 100 (12.60 ± 1.60%) but significantly (p < 0.0001) increased at 200 (67.80 ± 2.67%) and 400 mg/kg (51.20 ± 2.76%) respectively compared to 5 DW mL/kg (28.60 ± 1.44%) (Fig. 15).
Fig. 15.
Effect of N. vogelii on splenic level of p53 expression in the micronuclei study. Results are mean ± SEM. *** p < 0.001, **** p < 0.0001 versus DW 5 mL/kg (F (3, 16) = 122.3). One Way ANOVA followed by Tukey’s posthoc multiple comparison test. p53; protein 53, NV; Napoleona vogelii.
Bax was significantly (p < 0.05) reduced at 100 (9.80 ± 1.66%) but significantly increased at 200 (32.8 ± 02.13%) and non-significantly (p > 0.05) increased at 400 mg/kg (22.40 ± 1.50%) respectively compared to DW 5 mL/kg (18.80 ± 1.77%) (Fig. 16).
Fig. 16.
Effect of N. vogelii on splenic level of Bax expression in the micronuclei study. Results are mean ± SEM. * p < 0.05, *** p < 0.001 versus DW 5 mL/kg (F (3, 16) = 28.55). One Way ANOVA followed by Tukey’s posthoc multiple comparison test. Bax; B-cell lymphoma associated protein X, NV; Napoleona vogelii.
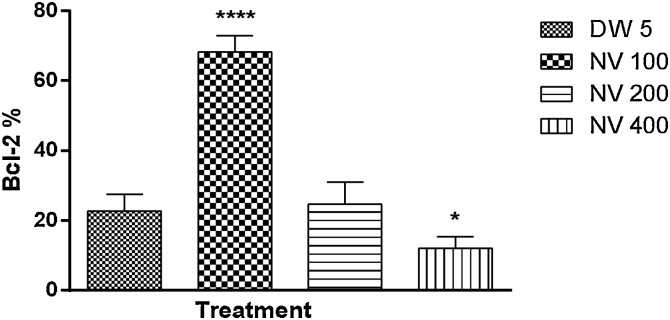
Bcl-2 was significantly (p < 0.0001) increased at 100 (68.20 ± 2.08%) and significantly (p < 0.05) reduced at 400 mg/kg (12.00 ± 1.52%) respectively compared to DW 5 mL/kg (22.80 ± 2.13%) (Fig. 17).
Fig. 17.
Effect of N. vogelii on splenic level of Bcl-2 expression in the micronuclei study. Results are mean ± SEM. * p < 0.05, **** p < 0.0001 versus DW 5 mL/kg (F (3, 16) = 128.2). One Way ANOVA followed by Tukey’s posthoc multiple comparison test. Bcl-2; B-cell lymphoma-2, NV; Napoleona vogelii.
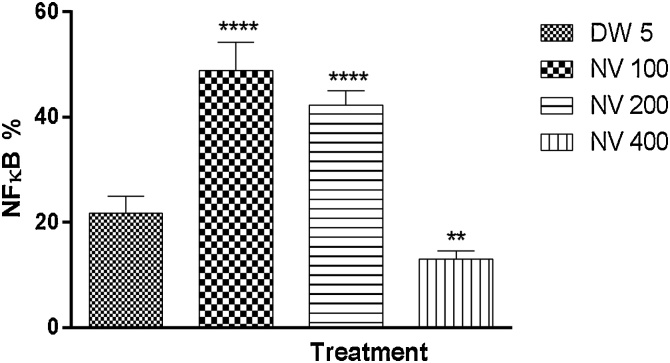
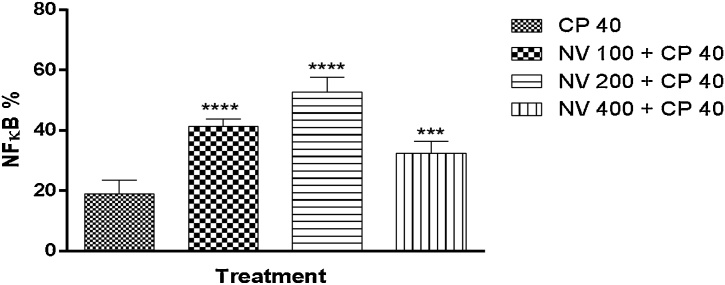
For the prophylactic study, NFκB was significantly (p < 0.0001) increased at 100 (41.20 ± 1.16%), 200 (52.80 ± 2.13%) and p < 0.001 at 400 mg/kg (32.40 ± 1.81%) respectively compared to CP 40 mg/kg (19.00 ± 2.00%) Fig. 18.
Fig. 18.
Effect of N. vogelii on splenic level of NFκB expression in the prophylactic study. Results are mean ± SEM. *** p < 0.001, **** p < 0.0001 versus CP 40 mg/kg (F (3, 16) = 61.97). One Way ANOVA followed by Tukey’s posthoc multiple comparison test. NFκB; Nuclear factor κappa B, CP; Cyclophosphamide, NV; Napoleona vogelii.
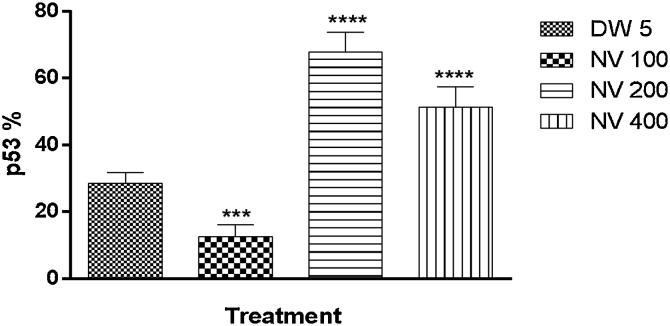
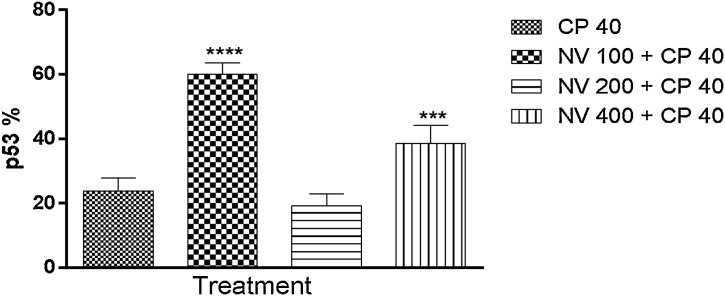
p53 was significantly (p < 0.0001) increased at 100 (60.00 ± 1.61%) and p < 0.001 at 400 mg/kg (38.60 ± 2.50%) respectively compared to CP 40 mg/kg (23.80 ± 1.77%) (Fig. 19).
Fig. 19.
Effect of N. vogelii on splenic level of p53 expression in the prophylactic study. Results are mean ± SEM. *** p < 0.001, **** p < 0.0001 versus CP 40 mg/kg (F (3, 16) = 90.96). One Way ANOVA followed by Tukey’s posthoc multiple comparison test. p53; protein 53, CP; Cyclophosphamide, NV; Napoleona vogelii.
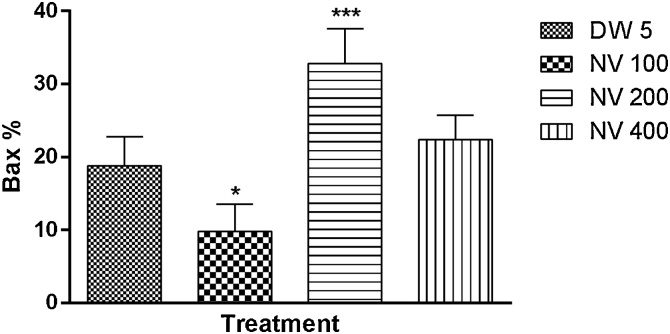
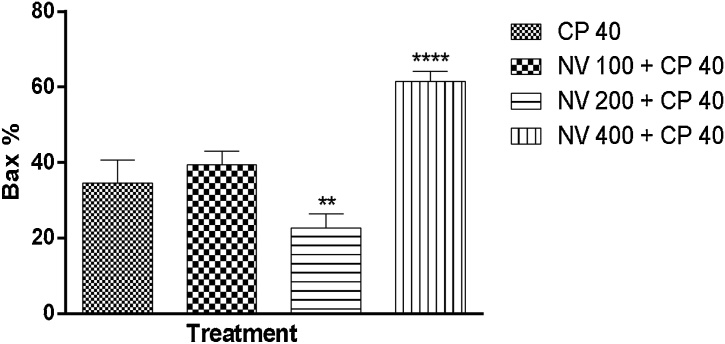
Bax was non significantly (p > 0.05) increased at 100 (39.40 ± 1.63%) and significantly (p < 0.0001) increased at 400 mg/kg (61.40 ± 1.21%) respectively compared to CP 40 mg/kg (34.60 ± 2.73%) (Fig. 20).
Fig. 20.
Effect of N. vogelii on splenic level of Bax expression in the prophylactic study. Results are mean ± SEM. ** p < 0.01, **** p < 0.0001 versus CP 40 mg/kg CP (F (3, 16) = 72.37). One Way ANOVA followed by Tukey’s posthoc multiple comparison test. Bax; B-cell lymphoma associated protein X, CP; Cyclophosphamide, NV; Napoleona vogelii.
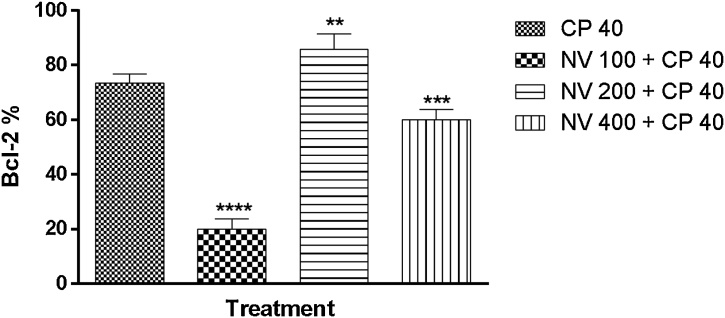
Bcl-2 was significantly (p < 0.0001%) reduced at 100 (20.00 ± 1.70%) and 400 mg/kg (p < 0.001, 60.00 ± 1.70%) respectively compared to CP 40 mg/kg (73.40 ± 1.50%) (Fig. 21).
Fig. 21.
Effect of N. vogelii on splenic level of Bcl-2 expression in the prophylactic study. Results are mean ± SEM. ** p < 0.01, *** p < 0.001, **** p < 0.0001 versus CP 40 mg/kg (F (3, 16) = 226.4). One Way ANOVA followed by Tukey’s posthoc multiple comparison test. Bcl-2; B-cell lymphoma-2, CP; Cyclophosphamide, NV; Napoleona vogelii.
4.11.1. Micronuclei induction
4.11.2. Prophylactic study
4.12. Splenic histology
There was no visible pathology in the spleen of mice in the micronuclei induction and prophylactic study respectively.
5. Discussion
This study was conducted to investigate the safety profile of the methanol stem bark extract of Napoleona vogelii on bone marrow cells in addition to its modulatory effect on expression of apoptotic markers and anti-oxidant responses as an aid to the discovery of novel candidates that will protect against micronuclei induction, NFκB-upregulation and modulate apoptotic transcription factors in the hematological system.
From the results obtained, there was no mortality observed on acute oral administration up to 5 g/kg of the extract in mice. Therefore, the LD50 oral of the extract is greater than 5 g/kg and suggests that the extract is safe on acute oral intake. However, the LD50 i.p. was found to be 0.668 g/kg. This could be attributed to the bypassing of some factors influencing drug absorption. The intraperitoneal route bypasses the gastro intestinal tract and hepatic first pass metabolism hence drugs may elicit toxic effects at low doses when delivered into the systemic circulation via this route.
The assessment of hematological indices as a determinant of cytotoxicity to hematologic cells is important in safety assessment in drug development. The degree of cytotoxicity is determined by the proportion of polychromatic erythrocytes (PCEs) relative to normochromatic erythrocytes (NCEs). Hence, the PCE/NCE ratio is determined as part of the micronucleus assessment to elucidate the cytotoxicity of test agents [54]. In this study, the extract was not toxic to bone marrow cells but was able to abate the cytotoxic effect of CP; it enhanced proliferation of PCEs evident in the significant increase in PCE/NCE ratio observed in extract treated animals compared to CP and DW treated controls.
Micronuclei induction is secondary to genotoxic insult on mammalian cells. This credible and worthwhile technique is often used to determine cytogenetic disturbances and chromosomal lesions or aberrations [55,56]. There was no significant difference in frequency of MNPCE observed in extract treated mice compared to distilled water treated control. This suggests that the extract is not genotoxic but enhanced proliferation of erythroblasts in mouse bone marrow cells [57]. Genotoxic chemicals including chemotherapeutic drugs produce their deleterious effects on the genome by triggering genotoxic stress which produces free radicals that perturbs the genetic material and promotes carcinogenesis [58]. There was significantly increased level of MNPCEs induced by treatment with CP suggesting that it damaged the chromosome [59,60] and reduced proliferation in erythropoietic stem cells. This is similar to a study conducted by Ikumawoyi et al. [39] in which CP, 5 mg/kg induced micronuclei in rat bone marrow cells.
The extract was able to significantly inhibit the induction of micronuclei and protected mouse bone marrow cells from the deleterious effect of CP. It conferred percentage protection of 75.09, 94.74 and 96.84% on mouse bone marrow cells at 100, 200 and 400 mg/kg respectively compared to CP treated control. This presents a significant anti-genotoxic effect of the extract and suggests that the induction of genotoxic stress by CP was significantly abated. Micronuclei may present as predictive factors for carcinogenesis [61]. The deleterious effect of accumulation of damage in hematopoietic stem cells can be observed as dysfunctional hematopoiesis, bone marrow failure or leukemic malignancy [62]. Hence, these results suggest that the extract can lower the risk for the development of cyclophosphamide-induced bone marrow depression triggered by genotoxic insults.
The assessment of enzymatic and non-enzymatic indices of oxidative stress is cardinal to the effectiveness of a therapeutic regimen in CP-induced micronuclei induction. Treatment with CP has been reported to promote the induction of oxidative stress by its mechanism of production of free radicals and ROS which results in lipid peroxidation in cell membranes [63]. Free radicals can induce DNA damage, which can lead to genetic mutations and ultimately result in cancer [64]. In this study, CP resulted in significant elevation of MDA and nitric oxide level with significant reduction in the level of antioxidant enzymes demonstrating the induction of oxidative stress [65] which is consistent with high ROS levels crucial to cancer initiation and promotion [66]. The extract however, significantly increased the level of GSH, GST and SOD with a non-significant increase in CAT compared to CP and distilled water treated controls. SOD and CAT are primary components of antioxidant defense mechanisms and plays very significant roles in the antioxidant defense system. SOD converts the superoxide radical (∗O2) or singlet oxygen radical (O2−) generated in tissues through metabolism or reactions in cells to hydrogen peroxide (H2O2) and molecular oxygen (O2) [67]. Because the accumulation of H2O2 is toxic to body tissues and cells, catalase acts by breaking down H2O2 into water and molecular oxygen, consequently preventing or abolishing the damage induced by free radicals. The observed increase in GSH portends that the extract may inhibit the lipid peroxidation process and confers protection on cells against oxidative stress [68]. Eggler et al. [69] had reported that natural products with the potential for chemoprevention can serve as activators for the increased expression of GST and other antioxidant enzymes through the antioxidant response element (ARE). Reduction in the level of the endocoid, NO and MDA with corresponding significant increases in antioxidant enzymes is a pointer to the antioxidant and anti-inflammatory activity of the extract hence, it acts through antioxidant and anti-inflammatory mechanisms to elicit the reduction in micronuclei.
The spleen plays a prevalent role in several disorders of the hematological system in mice and humans because of its ability to sustain hematologic malignancies [24]. Hematologic stromal cells including those of splenic origin in mice and humans have been reported to protect cells of various hematological malignancies against apoptosis through the upregulation of NFκB and anti-apoptotic factors thereby allowing pathologic relapse [27,28]. In this study, extract of N. vogelii decreased the expression of NFκB/p65 and Bcl-2 while it upregulated the expression of p53 and Bax respectively compared to DW and CP exposed controls. The efficacy of plant extracts in chemoprevention can result from their ability to inhibit and downplay certain upstream signals that results to genotoxic damage, redox imbalances and other forms of cellular stress. The mechanistic role of phytochemicals in chemoprevention is elicited by the inhibition of genotoxic effects including increased antioxidant and anti-inflammatory activity and modulation of apoptosis [70,71]. These findings have therefore indicated the prophylactic potential of the extract through the upregulation of the expression of Bax and p53 in addition to downregulating the expression of NFκB and Bcl-2 and could provide novel avenues for chemoprevention.
The subunits of NFκB are localized in the cytoplasm and on activation; they translocate into the nucleus and bind to promotor regions on the DNA, modifying gene expression [72]. Its activation can result in increased resistance to anti-cancer therapies, and upregulation of anti-apoptotic genes consequently increasing resistance to apoptosis [73]. Elevated levels of NFκB and its activation have been reported to suppress apoptosis and hence induce cellular transformation, proliferation, chemo-resistance and inflammation [74]. Downregulation of NFκB expression on the other hand results in a strong induction of the intrinsic apoptotic pathway by increasing Bax expression [75]. Results obtained from this study hence suggests that the extract would be expected to disrupt or inhibit the ability of cancer cells to thrive as correlated with its suppression of the level of these transcription factors.
Reports have established that plant extracts can induce apoptosis through a number of mechanisms in particular by decreasing Bcl-2, increasing pro-apoptotic protein Bax and by the activation of MAPK that results in increased p53 protein levels [76]. On exposure to genotoxic stresses, p53 trans-activates several target genes involved in the inhibition of cell cycle progression and promotion of apoptosis hence preventing genomic instability [77]. p53 has been reported to play a very important role in apoptosis [78]. As a tumor suppressor, it is responsible for protecting cells from tumorigenic alterations [79] and malignant transformations.
The presence of the bioactives identified in the extract may be supportive of the results obtained in this study. Oleic acid has been reported to increase apoptotic cells by downregulating NFκB and Bcl-2 while upregulating p53 expressions [[80], [81], [82], [83]]. Feldstom et al. [84] and Shen et al. [85] had documented the ability of n- Hexadecanoic and Octadecanoic acids to induce Bax activation and expression while phytol induces apoptosis by reducing the level of Bcl-2 and increasing Bax [86,87].
Correlating the observed effects of the extract on the level of these transcription factors in this study and the findings of Somade et al suggest that the extract could inhibit inflammation, attenuate NFκB level [88] and promote apoptosis. According to Lenzi et al. [89], plant extracts with chemopreventive potentials are expected to have the ability to induce one or more hallmarks of apoptosis, interact with targets involved in cancer development and act as cytoprotective agents against toxicity induced by genotoxins.
Increased level and activation of NFκB together with Bc1-2 plays a significant role in the inhibition of apoptosis. Bcl-2 family is the main regulatory gene protein that prevents the apoptotic process. Its anti-apoptotic activity may be effective in increased resistance to chemotherapy [90]. However, the effect of the extract observed as downregulation of Bcl-2 portends that it may abolish chemoresistance.
6. Conclusion
This study has shown that the methanol stem bark extract of N. vogelii possess significant bioactivity against micronuclei induction and antioxidant activity which may be associated with the presence of flavonoids, phytol, oleic acid and other unsaturated fatty acids. It up regulates expression of pro-apoptotic Bax and p53 and down regulates expression of anti-apoptotic NFκB and Bcl-2. Hence, it may serve as an interesting lead for chemo-preventive drug development.
Declaration of Competing Interest
The authors declare no conflict of interest.
Acknowledgements
The authors wish to acknowledge the technical efforts of Mr. Olajide Okedere of the Institute for Advanced Medical Research and Training, University College Hospital, Ibadan, Dr. Ajani Mustapha Akanji of the Department of Pathology, Babcock University Teaching Hospital, Ilishan-Remo, Mr. Sunday Adenekan and Mr. Daniel Osuagwu of the Departments of Biochemistry and Anatomic and Molecular Pathology, College of Medicine, University of Lagos, Nigeria.
Contributor Information
Victor Olabowale Ikumawoyi, Email: walevt@gmail.com.
Oluyemi Akinloye, Email: oluyemiakinloye@hotmail.com.
References
- 1.Agbaje E.O., Onabanjo A.O. Toxicological study of the extracts of antimalarial medicinal plant Enantia chlorantha. Cent. Afr. J. Med. 1994;40(3) [PubMed] [Google Scholar]
- 2.Marques R.C.P., Medeiros S.R.B., Dias C.S., Barbosa-Fil ho J.M., Agnez-Lima L.F. Evaluation of the mutagenic potential of yangambin and of the hydroalcoholic extract of Ocotea duckei by the Ames test. Mutat. Res. 2003;536 doi: 10.1016/s1383-5718(03)00040-8. [DOI] [PubMed] [Google Scholar]
- 3.Déciga-Campos M., Rivero-Cruz I., Arriaga-Alba M., Castaneda-Corral G., Angeles-López G.E., Navarrete A., Mata R. Acute toxicity and mutagenic activity of mexican plants used in traditional medicine. J. Ethnopharmacol. 2007;110 doi: 10.1016/j.jep.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 4.Akintonwa A., Awodele O., Olofinnade A.T., Anyakora C., Afolayan G.O., Coker H.A.B. Assessment of the mutagenicity of some pharmaceutical effluents. Am. J. Pharmacol. Toxicol. 2009;4 [Google Scholar]
- 5.Waters M.D., Stack H.F., Jackson M.A., Brockman H.E., De Flora S. Activity profiles of antimutagens: in vitro and in vivo data. Mutat. Res. 1996;350 doi: 10.1016/0027-5107(95)00097-6. [DOI] [PubMed] [Google Scholar]
- 6.Gupta C.P., Dubey R.C., Kang S.C., Maheswari D.K. Antibiosis mediated necrotrophic effect of Pseudomonas GRC2 against two fungal plant pathogens. Curr. Sci. 2001;81 [Google Scholar]
- 7.Aruoma O. Methodological considerations for characterizing potential antioxidant actions of bioactive components in plant foods. Mutat. Res. 2003:523–524. doi: 10.1016/s0027-5107(02)00317-2. [DOI] [PubMed] [Google Scholar]
- 8.Verschaeve L., Kestens V., Taylor J.L.S., Elgorashi E.E., Maes A., Van Puyelde L. Investigation of the antimutagenic effects of selected South African medicinal plant extracts. Toxicol. In Vitro. 2004;18 doi: 10.1016/s0887-2333(03)00131-0. [DOI] [PubMed] [Google Scholar]
- 9.Borges F.V.F., Machado T.C., Cunha K.S., Pereira K.C., Costa E.A., De Paula J.R. Assessment of the cytotoxic, genotoxic, and antigenotoxic activities of Celtis iguanaea (Jacq.) in mice. Anais Da Academia Brasileira de Ciências. 2013;85(3) doi: 10.1590/S0001-37652013005000054. [DOI] [PubMed] [Google Scholar]
- 10.Schmid W. The micronucleus test. Mutat. Res. 1975;31 doi: 10.1016/0165-1161(75)90058-8. [DOI] [PubMed] [Google Scholar]
- 11.Majer B.J., Laky B., Knasmuller S., Kassie F. Use of the micronucleus assay with exfoliated epithelial cells as a biomarker for monitoring individuals at elevated risk of genetic damage and in chemoprevention trials. Mutat. Res. 2001;489 doi: 10.1016/s1383-5742(01)00068-0. [DOI] [PubMed] [Google Scholar]
- 12.Chung H.W., Kang S.J., Kim S.Y. A combination of the micronucleus assay and a FISH technique for evaluation of the genotoxicity of 1,2,4-benzenetriol. Mutat. Res. 2002;516 doi: 10.1016/s1383-5718(02)00018-9. [DOI] [PubMed] [Google Scholar]
- 13.Ding G.R., Nakahara T., Miyakoshi J. Induction of kinetochore-positive and kinetochore-negative micronuclei in CHO cells by ELF magnetic fields and/or X-rays. Mutagenesis. 2003;18 doi: 10.1093/mutage/geg019. [DOI] [PubMed] [Google Scholar]
- 14.Bolognesi C., Landini E., Perrone E., Roggieri P. Cytogenetic biomonitoring of a floriculturist population in Italy: micronucleus analysis by fluorescence in situ hybridization (FISH) with an all-chromosome centromeric probe. Mutat. Res. 2004;557 doi: 10.1016/j.mrgentox.2003.09.013. [DOI] [PubMed] [Google Scholar]
- 15.Izzotti A., Balansky R.M., Dagostini F., Bennicelli C., Myers S.R., Grubbs C.J. Modulation of biomarkers by chemopreventive agents in smoke-exposed rats. Cancer Res. 2001;61 PMID: 11289117. [PubMed] [Google Scholar]
- 16.Roy M., Chakrabarty S., Sinha D., Bhattacharya R.K., Siddiqi M. Anticlastogenic, antigenotoxic and apoptotic activity of epigallocatechin gallate: a green tea polyphenol. Mutat. Res. 2003:523–524. doi: 10.1016/s0027-5107(02)00319-6. [DOI] [PubMed] [Google Scholar]
- 17.Neergheen V.S., Bahorun T., Taylor E.W., Jen L.S., Aruoma O.I. Targeting specific cell signaling transduction pathways by dietary and medicinal phytochemicals in cancer chemoprevention. Toxicol. 2010;278(2) doi: 10.1016/j.tox.2009.10.010. [DOI] [PubMed] [Google Scholar]
- 18.Aggarwal B.B., Van Kuiken M.E., Iyer L.H., Harikumar K.B., Sung B. Molecular targets of nutraceuticals derived from dietary spices: potential role in suppression of inflammation and tumorigenesis. Exp. Biol. Med. 2009;234 doi: 10.3181/0902-MR-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Doll R., Peto R. The causes of cancer: quantitative estimates of avoidable risks of cancer in the United States today. J. Natl. Cancer Inst. 1981;66 [PubMed] [Google Scholar]
- 20.Hardy G., Hardy I., Ball P. Nutraceuticals - A pharmaceutical viewpoint, part II. Curr. Opin. Clin. Nutr. Metab. Care. 2003;6(6) doi: 10.1097/00075197-200311000-00010. [DOI] [PubMed] [Google Scholar]
- 21.Shishodia S., Majumdar S., Banerjee S., Aggarwal B.B. Ursolic acid inhibits nuclear factor-κappaB activation induced by carcinogenic agents through suppression of Ikappa-Balpha kinase and p65 phosphorylation: correlation with downregulation of cyclooxygenase 2, matrix metalloproteinase 9 and cyclin D1. Cancer Res. 2003;63 [PubMed] [Google Scholar]
- 22.C.T Chiu, Chen J.H., Chou F.P., Lin H.H. Hibiscus sabdariffa Leaf Extract Inhibits Human Prostate Cancer Cell Invasion via Down-Regulation of Akt/NF-κB/MMP-9 Pathway. Nutrients. 2015;7(7) doi: 10.3390/nu7075065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kannaiyan R., Hay H.S., Rajendran P., Li F., Shanmugam M.K., Vali S. Celastrol inhibits proliferation and induces chemosensitization through down-regulation of NF-κB and STAT3 regulated gene products in multiple myeloma cells. Brit. J. Pharmacol. 2011;164(5) doi: 10.1111/j.1476-5381.2011.01449.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shaked Y., Cervi D., Neuman M., Chen L., Klement G., Michaud C.R. The splenic microenvironment is a source of proangiogenesis/inflammatory mediators accelerating the expansion of murine erythroleukemic cells. Blood. 2005;105(11) doi: 10.1182/blood-2004-08-3210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mudry R.E., Fortney J.E., York T., Hall B.M., Gibson L.F. Stromal cells regulate survival of B-lineage leukemic cells during chemotherapy. Blood. 2000;96 [PubMed] [Google Scholar]
- 26.Konopleva M., Konoplev S., Hu W., Zaritskey A.Y., Afanasiev B.V., Andreeff M. Stromal cells prevent apoptosis of AML cells by up-regulation of anti-apoptotic proteins. Leukemia. 2002;16 doi: 10.1038/sj.leu.2402608. [DOI] [PubMed] [Google Scholar]
- 27.Nefedova Y., Landowski T.H., Dalton W.S. Bone marrow stromal-derived soluble factors and direct cell contact contribute to de novo drug resistance of myeloma cells by distinct mechanisms. Leukemia. 2003;17 doi: 10.1038/sj.leu.2402924. [DOI] [PubMed] [Google Scholar]
- 28.Sison E.A.R., Brown P. The bone marrow microenvironment and leukemia: biology and therapeutic targeting. Exp. Rev. Hematol. 2011;4:271–283. doi: 10.1586/ehm.11.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Keay R.W.J., Onochie C.F.A., Standfield D.P. Nigerian trees. Department of Forest Research. 1964;1 [Google Scholar]
- 30.Iwu M. CRC Press; 1993. Handbook of African Medicinal Plants. [Google Scholar]
- 31.Akah P.A., Nnaeto O., Nworu S., Ezike A.C. Medicinal plants used in the traditional treatment of peptic ulcer diseases: a case study of Napoleona vogelii hook & planch Lecythidaceae. Res. J. Pharmacol. 2007;1 [Google Scholar]
- 32.Odugbemi T. University of Lagos Press; 2008. Outlines and Pictures of Medicinal Plants From Nigeria. [Google Scholar]
- 33.Soladoye M.O., Amusa N.A., Raji-Esan S.O., Chukwuma E.C., Taiwo A.A. Ethnobotanical survey of anti-cancer plants in Ogun State, Nigeria. Ann. Biol. Res. 2010;1(4) [Google Scholar]
- 34.Burkill H.M. Vol 3. 1985. (The Useful Plants of West Tropical Africa). [Google Scholar]
- 35.Jhansi M., Rani S., Mohana Lakshmi A., Kumar S. Review on herbal drugs for anti-ulcer property. Int. J. Biol. Pharm. Res. 2010;1(1) [Google Scholar]
- 36.Muganzaa D.M., Fruth B.I., Lamia J.N., Mesiaa G.K., Kambua O.K., Tonaa G.L. In vitro antiprotozoal and cytotoxic activity of 33 ethonopharmacologically selected medicinal plants from Democratic Republic of Congo. J. Ethnopharmacol. 2012;141 doi: 10.1016/j.jep.2012.02.035. [DOI] [PubMed] [Google Scholar]
- 37.Adejayan A.A., Ozolua R.I., Uwaya D.O., Eze G.I., Ezike A.C. Evaluation of the anti-asthmatic and antitussive potentials of methanol leaf extract of Napoleona vogelii in rodents. Biomed. Pharmacother. 2019;109 doi: 10.1016/j.biopha.2018.10.058. [DOI] [PubMed] [Google Scholar]
- 38.Owolabi O.J., Inninh S.O., Anaka O.N., Iyamu O.A. Antidiabetic and hypolipidemic effects of methanol leaf extract of Napoleona vogelii (Lecythidaceae) hook & Planch on alloxan–induced diabetes mellitus in rats. Trop. J. Pharm. Res. 2014;13 [Google Scholar]
- 39.Ikumawoyi V.O., Agbaje E.O., Awodele O., Akinyede A.A. Biochemical, hematological, and hormonal profile of rats orally administered methanol stem bark extract of Napoleona vogeliiHook and Planch (Lecythidaceae) Drug Chem. Toxicol. 2018 doi: 10.1080/01480545.2018.1454460. [DOI] [PubMed] [Google Scholar]
- 40.Ikumawoyi V., Agbaje E., Awodele O. Antigenotoxic and antioxidant activity of methanol stem bark extract of Napoleona vogelii hook & planch (Lecythidaceae) in cyclophosphamide-induced genotoxicity. Open Access Maced. J. Med. Sci. 2017;5(7) doi: 10.3889/oamjms.2017.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Miller L.C., Tainter M.L. Estimation of LD50 and its error by means of log-probit graph paper. Proc. Soc. Exp. Biol. Med. 1944;57 [Google Scholar]
- 42.Alabi O.A., Bakare A.A. Genotoxicity and mutagenicity of electronic waste leachates using animal bioassays. Toxicol. Environ. Chem. Rev. 2011;93(5) [Google Scholar]
- 43.Bakare A.A., Okunola A.A., Adetunji O.A., Jenmi H.B. Genotoxicity assessment of a pharmaceutical effluent using four bioassays. Genet. Mol. Biol. 2009;32(2) doi: 10.1590/S1415-47572009000200026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sun M., Zigman S. An improved spectrophotometer assay of superoxide dismutase based on epinephrine autoxidation. Anal. Biochem. 1978;90 doi: 10.1016/0003-2697(78)90010-6. [DOI] [PubMed] [Google Scholar]
- 45.Usoh I.F., Akpan E.J., Etim E.O., Farombi E.O. Antioxidant actions of dried flower extract of Hibiscus sabdariffa L. On sodium arsenite-induced oxidative stress in rats. Pak. J. Nutr. 2005;4 [Google Scholar]
- 46.Sedlak J., Lindsay R.H. Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman’s reagent. Anal. Biochem. 1968;25 doi: 10.1016/0003-2697(68)90092-4. [DOI] [PubMed] [Google Scholar]
- 47.Habig W.A., Pabst M.J., Jacoby W.B. Glutathione transferases. The first enzymatic step in mercapturic acid formation. J. Biol. Chem. 1974;249 PMID: 4436300. [PubMed] [Google Scholar]
- 48.Hafemann D.G., Sunde R.A., Houestra W.G. Effect of dietary selenium on erythrocyte and liver glutathione peroxidase in the rat. J. Nutr. 1974;104 doi: 10.1093/jn/104.5.580. [DOI] [PubMed] [Google Scholar]
- 49.Buege J.A., Aust S.D. Microsomal lipid peroxidation. Methods Enzymol. 1978;52 doi: 10.1016/s0076-6879(78)52032-6. [DOI] [PubMed] [Google Scholar]
- 50.Sun J., Zhang X., Broderick M., Harry F. Measurement of nitric oxide production in biological systems by using griess reaction assay. Sensors. 2003;3 [Google Scholar]
- 51.Moon Y., Park G., Han K., Kang K.S., Lee W. Mouse spleen tissue as a staining intensity reference for immunohistochemistry. Ann. Clin. Lab. Sci. 2008;38(3) PMID: 18715848. [PubMed] [Google Scholar]
- 52.Wittenburg G., Volkel C., Mai R., Lauer G. Immunohistochemical comparison of differentiation markers on paraffin and plastic embedded human bone samples. J. Physiol. Pharmacol. 2009;60(Suppl 8) PMID: 20400791. [PubMed] [Google Scholar]
- 53.Wehrhan F., Amann K., Molenberg A., Lutz R., Neukam F.W., Schlegel K.A. Critical size defect regeneration using PEG-mediated BMP-2 gene delivery and the use of cell occlusive barrier membranes - the osteopromotive principle revisited. Clin. Oral Implants Res. 2013;24 doi: 10.1111/j.1600-0501.2012.02489.x. [DOI] [PubMed] [Google Scholar]
- 54.Jain R., Jain S.K. Effect of Buchanania lanzan Spreng. Bark extract on cyclophosphamide-induced genotoxicity and oxidative stress in mice. Asian Pac. J. Trop. Med. 2012 doi: 10.1016/S1995-7645(12)60022-4. [DOI] [PubMed] [Google Scholar]
- 55.Ilyushina N., Goumenou M., Stivaktakis P.D., Vardavas A.I., Masaltsev G., Averianova N., Dmitricheva O., Revazova Y., Tsatsakis A.M., Rakitskii V. Maximum tolerated doses and erythropoiesis effects in the mouse bone marrow by 79 pesticides’ technical materials assessed with the micronucleus assay. Toxicol. Rep. 2019;6 doi: 10.1016/j.toxrep.2018.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vijayalaxmi V., Reiter R.J., Herman T.S., Meltz M.L. Melatonin and radioprotection from genetic damage: in vivo/in vitro studies with human volunteers. Mutat. Res. 1996;371(3–4) doi: 10.1016/s0165-1218(96)90110-x. [DOI] [PubMed] [Google Scholar]
- 57.Madhyastha S., Prabhu L.V., Saralaya V., Rai R. A comparison of vitamin a and leucovorin for the prevention of methotrexate-induced micronuclei production in rat bone marrow. Clinics. 2008;63(6) doi: 10.1590/S1807-59322008000600019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tiwari A.K. Imbalance in antioxidant defence and human diseases: multiple approach of natural antioxidants therapy. Curr. Sci. 2001;81(9) [Google Scholar]
- 59.Moore F.R., Urda G.A., Krishna G., Theiss J.C. An in vivo/in vitro method for assessing micronucleus and chromosome aberration induction in rat bone marrow and spleen. 1. Studies with cyclophosphamide. Mutat. Res. 1995;335(2) doi: 10.1016/0165-1161(95)90055-1. [DOI] [PubMed] [Google Scholar]
- 60.Murata M., Suzuki T., Midorikawa K., Oikawa S., Kawanishi S. Oxidative DNA damage induced by a hydroperoxide derivative of cyclophosphamide. Free Rad. Biol. Med. 2004;37(6) doi: 10.1016/j.freeradbiomed.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 61.Stich H.F., Stich W., Rosin M.P., Vallejera M.O. Use of the micronucleus test to monitor the effect of vitamin A, beta-carotene and canthaxanthin on the buccal mucosa of betel nut/tobacco chewers. Int. J. Cancer. 1984;15(34) doi: 10.1002/ijc.2910340602. [DOI] [PubMed] [Google Scholar]
- 62.Lane S.W., Gilliland D.G. Leukemia stem cells. Semin. Cancer Biol. 2010;20 doi: 10.1016/j.semcancer.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 63.Abarikwu S.O., Otuechere C.A., Ekor M., Monwuba K., Osobu D. Rutin ameliorates cyclophosphamide-induced reproductive toxicity in male rats. Toxicol. Int. 2012;19(2) doi: 10.4103/0971-6580.97224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ames B.N., Shigenaga M.K. Oxidants are a major contributor to aging. Ann. New York Acad. Sci. 1992;663 doi: 10.1111/j.1749-6632.1992.tb38652.x. [DOI] [PubMed] [Google Scholar]
- 65.Oyagbemi A.A., Omobowale O.T., Asenuga E.R., Akinleye A.S., Ogunsanwo R.O., Saba A.B. Cyclophosphamide-induced hepatotoxicity in wistar rats: the modulatory role of gallic acid as a hepatoprotective and chemopreventive phytochemical. Int. J. Prev. Med. 2016;7 doi: 10.4103/2008-7802.177898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yeh C.C., Hou M.F., Tsai S.M., Lin S.K., Hsiao J.K., Huang J.C. Superoxide anion radical, lipid peroxides and antioxidant status in the blood of patients with breast cancer. Clin. Chim. Acta. 2005;361(1-2) doi: 10.1016/j.cccn.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 67.Ighodaro O.M., Akinloye O. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): their fundamental role in the entire antioxidant defence grid. Alexandria J. Med. 2017 [Google Scholar]
- 68.Deshmukh N.S., Stohs S.J., Magar C.C., Kale A., Sowmya B. Bitter orange (Citrus aurantium L.) extract subchronic 90-day safety study in rats. Toxicol. Rep. 2017;4 doi: 10.1016/j.toxrep.2017.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Eggler A.L., Gay K.A., Mesecar A.D. Molecular mechanisms of natural products in chemoprevention: induction of cytoprotective enzymes by Nrf2. Mol. Nutr. Food Res. 2008;52 doi: 10.1002/mnfr.200700249. [DOI] [PubMed] [Google Scholar]
- 70.Chen C., Kong A.N. Dietary chemopreventive compounds and ARE/EpRE signalling. Free Rad. Biol. Med. 2004;15(12) doi: 10.1016/j.freeradbiomed.2004.03.015. [DOI] [PubMed] [Google Scholar]
- 71.Soobrattee M.A., Bahorun T., Aruoma O.I. Chemopreventive actions of polyphenolic compounds in cancer. Biofactors. 2006;27 doi: 10.1002/biof.5520270103. [DOI] [PubMed] [Google Scholar]
- 72.Aggarwal B.B. Nuclear factor-κB: the enemy within. Cancer Cell. 2004;6(3) doi: 10.1016/j.ccr.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 73.Hertlein E., Byrd J.C. Signalling to drug resistance in CLL. Best Pract. Res. Clin. Haematol. 2010;23(1) doi: 10.1016/j.beha.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 74.Aggarwal B.B., Shishodia S. Molecular targets of dietary agents for prevention and therapy of cancer. Biochem. Pharmacol. 2006;71 doi: 10.1016/j.bcp.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 75.Simon-Gabriel C.P., Foerster K., Saleem S., Bleckmann D., Benkisser-Petersen M., Thornton N., Umezawa K., Decker S., Burger M., Veelken H., Claus R., Dierks C., Duyster J., Zirlik K. The microenvironmental stromal cells abrogate NF-κB inhibitor induced apoptosis in chronic lymphocytic leukemia. Haematologica. 2017 doi: 10.3324/haematol.2017.165381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sung M.H., Kwon O.K., Oh S.R., Lee J., Park S.H., Han S.B. Azorella compacta methanolic extract induces apoptosis via activation of mitogen-activated protein kinase. Mol. Med. Rep. 2015;12 doi: 10.3892/mmr.2015.4317. [DOI] [PubMed] [Google Scholar]
- 77.Batchelor E., Loewer A., Lahav G. The ups and downs of p53: understanding protein dynamics in single cells. Nat. Rev. Cancer. 2009;9(5) doi: 10.1038/nrc2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Aggarwal M.L., Taylor W.R., Chernov M.V., Chernova O.B., Stark G.R. The p53 network. J. Biol. Chem. 1998;273 doi: 10.1074/jbc.273.1.1. [DOI] [PubMed] [Google Scholar]
- 79.Levine A.J. p53: the cellular gatekeeper for growth and cell division. Cell. 1997;88 doi: 10.1016/s0092-8674(00)81871-1. [DOI] [PubMed] [Google Scholar]
- 80.Ramakers J.D., Mensink R.P., Schaart G., Plat J. Arachidonic acid but not eicosapentaenoic acid (EPA) and oleic acid activates NF-κB and elevates ICAM-1 expression in Caco-2 cells. Lipids. 2007;42(8) doi: 10.1007/s11745-007-3071-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fontana A., Spolaore B., Polverino de Laureto P. The biological activities of Protein/Oleic acid complexes reside in the fatty acid. Biochim. Biophys. Acta. 2013;1834 doi: 10.1016/j.bbapap.2013.02.041. [DOI] [PubMed] [Google Scholar]
- 82.Moon H.S., Batirel S., Mantzoros C.S. Alpha linolenic acid and oleic acid additively down-regulate malignant potential and positively cross-regulate AMPK/S6 Axis in OE19 and OE33 esophageal Cancer cells. Metabolism. 2014;63 doi: 10.1016/j.metabol.2014.07.009. [DOI] [PubMed] [Google Scholar]
- 83.Jiang L., Wang W., He Q., Wu Y., Lu Z., Sun J. Oleic acid induces apoptosis and autophagy in the treatment of Tongue Squamous cell carcinomas. Sci. Rep. 2017;7 doi: 10.1038/s41598-017-11842-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Feldstein A.E., Werneburg N.W., Li Z., Bronk S.F., Gores G.J. Bax inhibition protects against free fatty acid-induced lysosomal permeabilization. Am. J. Physiol-Gastrointest. Liver Physiol. 2006;290(6) doi: 10.1152/ajpgi.00509.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Shen M.C., Zhao X., Siegal G.P., Desmond R., Hardy R.W. Dietary stearic acid leads to a reduction of visceral adipose tissue in athymic nude mice. PLoS One. 2014;9(9) doi: 10.1371/journal.pone.0104083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Song Y., Cho S.K. Phytol induces apoptosis and ROS-Mediated protective autophagy in human gastric adenocarcinoma AGS cells. Biochem. Anal. Biochem. 2015;4 [Google Scholar]
- 87.Sakthivel R., Malar D.S., Devi K.P. Phytol shows anti-angiogenic activity and induces apoptosis in A549 cells by depolarizing the mitochondrial membrane potential. Biomed. Pharmacother. 2018;105 doi: 10.1016/j.biopha.2018.06.035. [DOI] [PubMed] [Google Scholar]
- 88.Lenzi M., Malaguti M., Cocchi V., Hrelia S., Hrelia P. Castanea sativa Mill. Bark extract exhibits chemopreventive properties triggering extrinsic apoptotic pathway in Jurkat cells. BMC Comp. Alt. Med. 2017;17 doi: 10.1186/s12906-017-1756-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Somade O.T., Ajayi B.O., Safiriyu O.A., Oyabunmi O.S., Akamo A.J. Renal and testicular up-regulation of pro-inflammatory chemokines (RANTES and CCL2) and cytokines (TNF-α, IL-1β, IL-6) following acute edible camphor administration is through activation of NF-kB in rats. Toxicol. Rep. 2019;6 doi: 10.1016/j.toxrep.2019.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Phuoc B., Ehara H., Gotoh T., Nakano M., Yokoi S., Deguchi T., Hirose Y. Immunohistochemical analysis with multiple antibodies in search of prognostic markers for clear cell renal cell carcinoma. Urology. 2007;69 doi: 10.1016/j.urology.2007.01.069. [DOI] [PubMed] [Google Scholar]