Abstract
In this study, the protective effect of curcumin on sodium nitrite (NaNO2) induced hepatotoxicity was assessed in male Wistar rats. Wistar rats were administered orally daily with 20 mg/kg of curcumin for 28 days and NaNO2 was administered as a single dose of 60 mg/kg on day 28. Lipid profile, liver function biomarkers and C-reactive protein were assessed in the serum; lipid peroxidation, non-enzymatic and enzymatic antioxidants were assessed in the liver. Alanine amino transferases (94.67 U/L), aspartate amino transferases (194.33 U/L), alkaline phosphatases, C-reactive proteins (19.56 ng/L) and lipid peroxidation (8.03 × 10−6 μmol/mg protein) were significantly elevated (P < 0.05), while a significant decrease in lipid profiles (total cholesterol, HDL,LDL, and triglycerides): (0.61,0.37, 0.4 and 0.47 mg/dl respectively), reduced glutathione level (4.16 μmol/mg protein), and decreased catalase, superoxide dismutase and glutathione peroxidase activities with severe histological alterations were observed in the livers of rats exposed to NaNO2. Pre-treatment with curcumin significantly (P < 0.05) prevented these alterations by adjusting the lipid profile, liver function markers, and C-reactive proteins and abrogating the elevated markers of oxidative stress as supported by the liver histology. This suggests that dietary consumption of curcumin is beneficial against NaNO2 induced oxidative stress of the liver via its antioxidant potential.
Keywords: Sodium nitrite, Hepatotoxicity, Curcumin, Hepatoprotection, Oxidative stress, Wistar rats
1. Introduction
Sodium nitrite (NaNO2) is a widely employed color fixative and preservative in the food industry and in the processing of fish and meat products. It is also employed in the industry for other purposes including flavour-enhancing, microbial growth inhibition and prevention of fat oxidation, thereby delaying rancidity. In addition, it also is used in the manufacturing of dyes, nitrous compounds and chemicals used in production of rubber [1]. Apart from the benefits offered by NaNO2 in the food and other industries, other associated beneficial activities include its medicinal use for bronchodilation and vasodilation, its use as an antidote for poisonous chemicals, and other health roles at low physiological concentrations [2]. However, there have been reports of induced toxicities in several organs following acute exposure to high concentrations or chronic exposure to low doses of NaNO2. A single acute exposure to NaNO2 at doses ranging between 20–75 mg/kg body weight resulted in organ damage in rats [1]. Several anthropogenic activities, such as the use of nitrogenous fertilizers to improve yield of crops and improper treatment of industrial and sewage wastes, have contributed greatly to the increased concentration of nitrite in the environment [3]. Levels of nitrite greater than the acceptable limit also have been reported in drinking water across the globe [1,4,5]. Exposure to NaNO2 has been associated with several adverse health effects ranging from respiratory tract impairment, birth defects, neurological damage, and oxidative stress [1], to carcinogenicity and mutagenicity [6,7]. The various factors mentioned earlier have made exposure of humans to NaNO2 less avoidable; hence, the resulting health risks associated with NaNO2 may be inevitable. The liver is one of the most susceptible organs to toxic exogenous substances and consequently, hepatotoxicity induced by NaNO2 has been reported to be associated with mitochondrial injury and oxidative stress in rat isolated hepatocytes [8]. However, any phytochemical with established antioxidant properties may be an agent in the prevention of the induced hepatotoxicity [9].
One of such phytochemicals is curcumin, a natural bioactive constituent found in the turmeric (Curcuma longa) rhizome and a member of the ginger (Zingiberaceae) family. Curcumin has been reported for its myriad medicinal properties, established for its ability to suppress oxidative damage, and possesses anti-inflammatory, anticancer, antiangiogenesis, chemopreventive, and chemotherapeutic properties [[10], [11], [12], [13], [14], [15]]. Curcumin possesses no toxic effects based on animal and human studies [16,17] and has been reported to alleviate several xenobiotic-induced toxicities, including those induced by cypermethrin, Lindane, lead acetate and cadmium [[18], [19], [20], [21]]. However, there is a dearth of information regarding the preventive effect of curcumin in NaNO2-induced hepatotoxicity. Hence, this study was carried out to assess the preventive capacity of curcumin against hepatotoxicity induced by acute dose of NaNO2.
2. Materials and methods
2.1. Chemicals
NaNO2 was purchased from Aldrich Chemical Co. Inc. (St. Paul Avenue Wisconsin USA). Curcumin, Glutathione (GSH), 5′,5′-dithiobis-2-nitrobenzene (DTNB), 2-thiobarbituric acid (TBA), Biuret and 1 chloro-2, 4-dinitrobenzene (CDNB) and hydrogen peroxide (H2O2) were purchased from Sigma-Aldrich (St Louis, MO, USA). All other reagents and chemicals used in this study were of analytical grade and water was glass distilled.
2.2. Experimental animals
Twenty-four male rats of the Wistar strain, (160–210 g) used in this study were obtained from the Central Animal House, College of Health Sciences, Osun State University, Osogbo. They were acclimatized for a week in plastic cages at an ambient temperature of 25 °C and a relative humidity of 45–55%, with 12 h each of dark and light cycles. The animals were maintained on a normal laboratory chow and water ad libitum. The handling of experimental animals was in accordance with the guidelines for the use of laboratory animals [22] and as adopted at the Osun State University, Nigeria. All procedures were approved by the Ethics Committee of the College of Health Sciences, Osun State University, Osogbo, Nigeria.
2.3. Experimental design and sample collection
Animals were distributed into four experimental groups (?? = 6). The negative control group received oral administration of 2 mL/kg olive oil daily. The positive control group received oral administration of 2 ml/kg b.wt olive oil and a single dose of NaNO2 at 60 mg/kg body weight equivalent to one of the highest single doses initially reported to produce organ damage [1,23]. The curcumin group received daily oral administration of 2 ml/kg b.wt of olive oil containing 20 mg/kg b.wt of curcumin. This dose is within the acceptable range reported for daily intake by Clinical development plan: curcumin 1996 [24]. In addition, this oral dose has been reported earlier by Tajik et al. [25], in rats, against the acetic acid induced visceral nociception. The duration of the experiment (28 days) was chosen based on the literature, which is 7 days to 12 weeks for the oral curcumin treatment [26,27]. The combination group received both a daily dose of curcumin and a single oral administration of NaNO2. A single dose of sodium nitrite was administered on day 28. After 24 h of NaNO2 exposure, rats were sacrificed by cervical dislocation. Blood samples were collected from the hepatic portal vein into non-heparinized bottles and processed to obtain serum. Serum samples were used for biochemical analysis. The liver tissues were excised and rinsed in ice cold 1.15% KCl and stored at −20 °C for biochemical estimations. A portion was fixed in 10% neutral buffered formalin solution for histological examination.
2.4. Tissue homogenate preparation
The portion of excised liver tissues were homogenized in 50 mmol/l Tris–HCl buffer (pH 7.4) and then centrifuged at 10,000 × g for 15 min at 4 °C. The resulting pellet was discarded and the supernatants were immediately kept frozen and later used for biochemical estimations.
2.5. Determination of serum hepatic function biomarkers
The hepatic function biomarkers Alanine amino transferases (ALT) and Aspartate amino transferases (AST) activities were determined following the principle reported [[28], [29], [30]]. Alkaline phosphatases (ALP) activity was determined following the method described by Goodla et al., [30]. Total protein concentrations of the serum were determined according to Biuret method as described by Gornall et al., [31]. Albumin concentration was determined following the principle reported by Grant [32].
2.6. Determination of lipid profile
Triglyceride (TGs) concentrations were determined by following the principle reported by Bucolo and David [33], HDL (high density lipoprotein) was determined by following the principle of quantitative precipitation of low density lipoproteins (LDL and VLDL) and chylomicron fractions by addition of phosphotungstic acid in the presence of magnesium ions. LDL (low density lipoprotein) was determined by following the principle of quantitative elimination of chylomicron, VLDL-Cholesterol and HDL-Cholesterol by cholesterol esterase, cholesterol oxidase and subsequently catalase.
2.7. Estimation of antioxidant status
Lipid peroxidation was determined as the formation of malondialdehyde (MDA) during an acid-heating reaction according to Ohkawa et al., [34]. Catalase (CAT) activity was measured using hydrogen peroxide as the substrate according to the method previously described Manubolu et al., [35]. Superoxide dismutase (SOD) activity was determined by measuring the inhibition of autoxidation of epinephrine at pH 10.2 and 30 °C according to Misra and Fridovich [36]. Glutathione (GSH) was determined according to the method of Jollow et al., [37]. Activity of Glutathione peroxidase was estimated following the method reported by Rotruck et al., [38] while Total thiol (TSH) content in the liver homogenate was determined as described by Ellman [39].
2.8. Assay of serum C-reactive protein
An enzyme-linked immunosorbent assay (ELISA) (rat C-reactive protein (CRP) ELISA Kit, e-Biosceince, Inc), was used to measure the concentration of high sensitive C reactive protein by following the instructional manual.
2.9. Histopathological analysis
The liver tissues were excised from the animals after sacrifice and stored in 10% formalin solution, for tissue sections and subsequent histopathological examination. The tissues were then embedded in paraffin. A rotary microtome was used to collect five micrometer-thick paraffin sections, and tissues were thereafter stained by Hematoxylin and Eosin (H&E). The specimens were examined and photographed under a light microscope.
2.10. Statistical analysis
The data were expressed as mean ± standard deviation (SD) after analysis by one-way analysis of variance (ANOVA) with the aid of Graph Pad Prism version 6.02, for windows (GraphPad software, San Diego, CA). Differences between mean values of different groups were considered statistically significant at P < 0.05.
3. Results
3.1. Effect of curcumin on liver function biomarkers and C-reactive proteins in rats exposed to NaNO2
NaNO2 resulted in significant elevations of hepatic transaminases (AST and ALT) and ALP activities, and caused significant reductions in the concentration of total protein, globulin and albumin, with an increase in C-reactive protein in the serum. Pre-treatment with curcumin significantly lowered these enzyme activities and increased the concentrations of the different proteins in the serum (Table 1).
Table 1.
Effect of curcumin on liver function biomarkers in rats exposed to NaNO2.
| Parameters | Group I | Group II | Group III | Group IV |
|---|---|---|---|---|
| AST (U/L) | 121.13 ± 1.52 | 118.32 ± 5.03 | 194.33 ± 6.43* | 146.23 ± 5.77*# |
| ALT (U/L) | 47.21 ± 6.1 | 43.23 ± 8.62 | 94.67 ± 4.93* | 61.33 ± 6.43 *# |
| ALP (U/L) | 21.33 ± 43.21 | 20.67 ± 2.54 | 93.33 ± 5.69* | 54.00 ± 9.52*# |
| Total protein | 91.67 ± 5.69 | 93.33 ± 3.79 | 62.67 ± 4.049* | 83.00 ± 4.36 # |
| Albumin (mg/dL) | 40.33 ± 4.51 | 37.32 ± 4.95 | 25.00 ± 3.0* | 28.33 ± 2.89* |
| Globulin (mg/dL) | 51.33 ± 1.527 | 56.00 ± 3.61 | 37.67 ± 2.08* | 54.67 ± 6.11# |
| C-reactive protein (ng/L) | 12.04 ± 1.42 | 10.88 ± 0.65 | 19.56 ± 2.33* | 14.93 ± 2.39# |
Group I: Control; Group II: 20 mg/kg Curcumin; Group III: 60 mg/kg NaNO2; Group IV: 20 mg/kg Curcumin +60 mg/kg NaNO2.Values are mean ± SD, n = 6.
AST = Aspartate amino transferase; ALT = Alanine amino transferase; ALP = Alkaline phosphatase.
*Significantly different from control group (P < 0.05).
#Significantly different from NaNO2 group (P < 0.05).
3.2. Effect of curcumin on the lipid profile of rats exposed to NaNO2
NaNO2 resulted in significant reductions in the concentration of serum lipids (cholesterol, TGs, LDL and HDL) rats as compared to the control (Table 2), while pre-treatment with curcumin produced no significant change in the serum lipid profile concentrations in the exposed rats as compared to the control (Table 2).
Table 2.
Effect of curcumin on the lipid profile of rats exposed to NaNO2.
| Parameters | Group I | Group II | Group III | Group IV |
|---|---|---|---|---|
| Total cholesterol (mg/dL) | 2.33 ± 0.25 | 2.47 ± 0.40 | 0.61 ± 0.20* | 1.97 ± 0.50# |
| HDL (mg/dL) | 1.90 ± 0.10 | 2.00 ± 0.10 | 0.37 ± 0.58* | 1.50 ± 0.70# |
| LDL (mg/dL) | 1.80 ± 0.10 | 1.56 ± 0.52 | 0.4 ± 0.15* | 1.16 ± 0.18*# |
| Triglycerides (mg/dL) | 1.27 ± 0.57 | 1.2 ± 0.1 | 0.47 ± 0.05* | 0.93 ± 0.15# |
Group I: Control; Group II: 20 mg/kg Curcumin; Group III: 60 mg/kg NaNO2; Group IV: 20 mg/kg Curcumin +60 mg/kg NaNO2. Values are expressed as mean ± (SD), n = 6.
HDL = High density lipoprotein; LDL = Low density lipoprotein.
*Significantly different from control group (P < 0.05).
#Significantly different from NaNO2 group (P < 0.05).
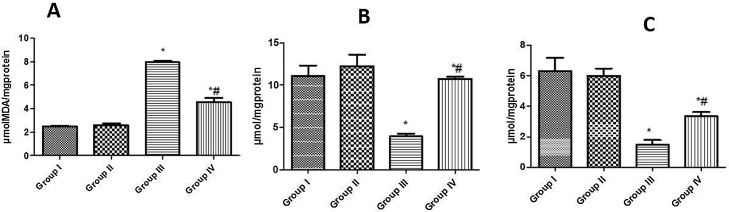
3.3. Effect of curcumin on the lipid peroxidation, GSH and TSH in rat liver exposed to NaNO2
A single dose of the NaNO2 treatment caused a significant elevation of MDA concentration as well as significant decreases in GSH and TSH concentrations in the livers of rats (Fig. 1a–c). Pre-treatment with curcumin prior to NaNO2 exposure significantly reduced MDA concentration and increased GSH concentration in the liver of NaNO2 -induced rats.
Fig. 1.
Effect of curcumin on MDA, GSH and TSH concentrations in the liver of NaNO2 exposed rats. [A] MDA; [B] GSH and [C] TSH. Group I: Control; Group II: 20 mg/kg Curcumin; Group III: 60 mg/kg NaNO2; Group IV: 20 mg/kg Curcumin +60 mg/kg NaNO2. Values are expressed as mean ± SD; n = 6. *Significantly different from the control group (P < 0.05). #Significantly different from NaNO2 group (P < 0.05).
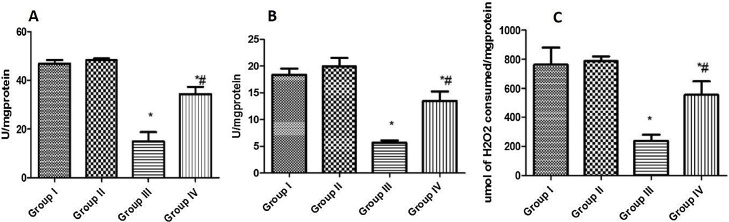
3.4. Effect of curcumin on antioxidant enzyme activities in rat liver exposed to NaNO2
A single dose of the NaNO2 treatment caused a significant reduction in the activities of the antioxidant enzymes in the liver of rats (Fig. 2a–c), while pre-treatment with curcumin prior to NaNO2 exposure significantly abrogated the decrease.
Fig. 2.
Effect of curcumin on antioxidant enzyme activities in the liver of NaNO2 exposed rats. [A] SOD [B] GPx and [C] CAT. Group I: Control; Group II: 20 mg/kg Curcumin; Group III: 60 mg/kg NaNO2; Group IV: 20 mg/kg Curcumin +60 mg/kg NaNO2. Values are expressed as mean ± SD; n = 6. *Significantly different from the control group (P < 0.05). #Significantly different from NaNO2 group (P < 0.05).
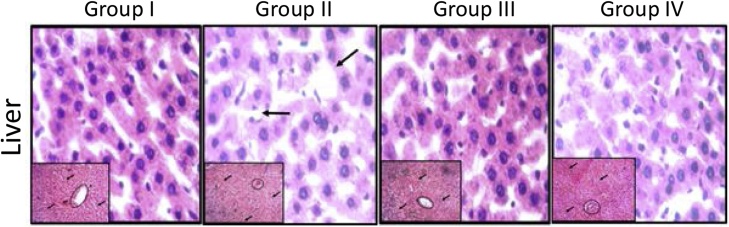
3.5. Histological examination of the liver section
The photomicrographs of the liver sections of rats exposed to NaNO2 after pre-treatment with curcumin are presented in Fig. 3. Control (Group I) and curcumin (Group II) treated groups showed no visible lesions, whereas NaNO2 treated group shows fatty liver with increased deposition of fat droplets with reduced cellular density and cellular distribution. Pretreatment with curcumin significantly protected the liver injury from NaNO2 stress.
Fig. 3.
Representative photomicrographs of liver section viewed under light microscope: Group I control: showing no visible lesion; Group II: 20 mg/kg curcumin, showing no visible lesion and normal morphology of the liver cells; Group III: 60 mg/kg NaNO2 showing Fatty liver with increased deposition of fat droplets with reduced cellular density and cellular distribution; Group IV: curcumin + NaNO2 showing nuclei of hepatocytes distinctively stained and properly disposed within their respective cytoplasm. H&E stain at 100x (embedded) and 400x Magnification.
4. Discussion
Exposure to compounds of nitrite in the environment or through consumption of contaminated water or food constantly predisposes humans to the risk of the induced toxicities [40]. Exposure to a single acute dose other than those used in food preservation or found in water accidentally or intentionally has been associated with wide range of organ toxicities. Sodium nitrite was has been reported to have capacity in inhibiting growth of disease-causing microorganisms, it gives taste and colour to the meat and inhibit lipid oxidation that leads to rancidity [[41], [42], [43]]. Sodium nitrite is well known for its role in inhibiting the growth of Clostridium botulinum spores in refrigerated meats [43]. It has been reported that as little as 2 to 14 ppm (ppm) is needed to induce this desirable effects especially in curing of meat, however, to extend the shelf life span and maintain cured color of meat, or to increase control of microorganisms like Clostridium botulinum, significantly higher levels are needed [44,45] hence, predisposing humans to its toxic effects. Consumption of such foods preserved with high concentration of nitrites by humans is very likely to predispose them to severe nitrite damage. It is possible to find naturally consumed substances to suppress or prevent these risks. In this study, curcumin was used as a pre-treatment against NaNO2 induced hepatotoxicity from a single acute dose. The liver is constantly and adversely affected as a result of its usual direct or indirect contact to various xenobiotics and free radicals. Many markers were considered very useful to regularly assess the proper function of the liver and reports exist on the significant alterations caused by NaNO2 on these markers, suggesting adverse toxic effects on this organ [[46], [47], [48]]. Curcumin has been reported in various studies to act as a scavenger for the oxygen-derived free radicals, [19,49].
In this study, a single acute dose of NaNO2 resulted in significant elevations of the activities of serum aminotransferases (ALT and AST) and ALP, which suggests damage to the liver cells because these hepatic biomarkers have typically been reported to be essential for determination of liver injury [[50], [51], [52], [53]]. Total protein, albumin and globulin concentrations also were significantly reduced following a single dose of NaNO2; this may have been due to loss of hepatocytes as a result of the damage created by NaNO2; as the reduction in these molecules has been reported earlier in liver damage [54]. This reduction by NaNO2 is in agreement with earlier reports [55]. The protein reduction also could indicate a switch in the direction of C-reactive protein synthesis (an inflammatory protein), as this was significantly increased in the serum following NaNO2 exposure. The reduction in albumin synthesis has earlier been linked to C-reactive protein synthesis (Du Clos, 2000). Increase in tissue damage has been reported to induce increased synthesis of C-reactive oxygen species; therefore, its significant increase in this study might be related to the liver damage induced by NaNO2 exposure.
Lipids are part of the important molecules necessary for the control of cellular functions and homeostasis. The liver happens to play a critical role in their metabolism, including their synthesis, degradation and transportation [56]. Thus, it is expected that liver dysfunction will also affect lipid profile. The result of this study revealed significant decreases in all of the lipid profiles assessed (cholesterol, triglycerides, HDL and LDL). To support this standpoint, a significant reduction in plasma cholesterol, HDL, LDL and triglyceride (TG) levels was earlier reported in patients with severe hepatitis and hepatic failure, which has been linked to the reduction of lipoprotein biosynthesis by the liver [56]. This result also is in accordance with the report of Mehboob et al., [57]. In addition, the decrease in the lipid profiles after nitrite or nitrate consumption has earlier been reported - [55,58]. The decrease observed in the lipids was suggested to be probably due to lipolysis, through stimulation of hormone sensitive lipase [55].
It would have been appropriate to conclude that the liver could not synthesize lipids as a result of damage elicited by NaNO2. However, the histology of the livers of rats exposed to NaNO2 revealed Fatty liver with increased expression of fatty droplets and reduced hepatocyte density. Thus, this may mean that the reduction in the liver cell density led to reduction in lipid synthesis and the ones that are synthesized could not be metabolized from the liver through blood circulation, hence, the accumulation of the fat seen in the histology. The biochemical analysis reveals that decrease in lipid profiles in serum and on the other hand the histopathology analysis revealed fatty liver with increased expression of fatty droplets and reduced hepatocyte density in NaNO2 exposed rats. This reveals that the multiple toxicity mechanisms of NaNO2 by damaging the liver cells and also affecting the critical role of liver and their fatty acid metabolism, which includes their synthesis, degradation and transportation.
In contrast, pre-treatment with curcumin significantly reduced the serum levels of AST, ALT, and ALP, prevented reduction in total protein synthesis, and lowered the concentration of C-reactive protein, showing amelioration of the induced liver damage. It may be suggested that the reason for lowered C-reactive protein concentration following pre-treatment with curcumin is a result of lack of damage to the liver, which could have activated the synthesis of C-reactive protein. To further illustrate this amelioration, the liver histopathological analysis in the curcumin pre-treated group showed hepatocytes preserved, with normal morphology with no visible lesions or the presence of fat. The density also was increased compared to the NaNO2 group. It is not surprising therefore that the concentrations of the parameters in the lipid profiles were significantly modulated in the group administered curcumin + NaNO2 when compared with the NaNO2 group. This may be explained by the result of histology; the fats that were accumulated in the NaNO2 group were freely metabolized in the curcumin group, and therefore the effect is seen in the serum with an increase in concentration, then a clear and regular morphology of the liver. Previous studies indicated the efficacy of curcumin in the modulation of the compromised serum lipid profile [59,60].
This study revealed that a significant oxidative stress was induced by NaNO2 as evidenced by the significant (p < 0.05) increase in MDA level, hence lipid peroxidation, with an associated decrease in GSH and TSH levels and CAT, GPx and SOD activities in the liver of rats. Oxidative damage induced by NaNO2 has been widely reported [1,48,55]. Nitrite is reported as a highly reactive compound which is capable of oxidizing, reducing or nitrosylating compounds. It also can be transformed into a myriad of related compounds, such as nitrous acid, nitric oxide and nitrite in biological matter [61]. The significantly induced oxidative stress could be related to these properties. However, there are several reports that have linked the medicinal effects of curcumin to its antioxidative properties and have suggested that antioxidants play an important role in the hepatoprotective mechanisms of liver function [[62], [63], [64], [65]]. Pre-treatment of animals before exposure to NaNO2 revealed a significant decrease in MDA level indicating that the lipid peroxidation rate was decreased by the antioxidant property of curcumin. With regard to the decreased lipid peroxidation level, it may be suggested that dietary consumption of curcumin may be protective against NaNO2-induced membrane damage. Furthermore, the therapeutic ability of curcumin in reducing free radical-induced tissue damage has been previously reported [21,66]. Curcumin has earlier been reported to prevent tissue damage induced by some agents such as paracetamol, cadmium, lead, and lindane [19,21,67]. This ability to prevent lipid peroxidation must have resulted from its reported antioxidative ability as evidenced by increased levels of GSH, TSH and activities of antioxidant enzymes (SOD, GPx and CAT) in the livers of the treated rats.
5. Conclusions
This study reports the protective capacity of curcumin against hepatotoxicity induced by acute dose of NaNO2. The modulation of NaNO2 induced hepatotoxicity (such as increase in C-reactive proteins, liver fat deposition, and decreased serum lipids, reduced antioxidants and increased serum- hepatic transaminases and phosphatase) was ameliorated by pretreatment of curcumin. Curcumin effectively abrogated NaNO2- induced hepatotoxicity via modulation of the antioxidant status and, inhibition of liver lipid peroxidation and accumulation as evidenced by clear liver cell morphology. Disruption in the ability to synthesize proteins caused by NaNO2 also was prevented. Since curcumin is a well-known anti-inflammatory agent, it is likely that these protective effects of curcumin are also mediated by the reduction in liver inflammation; hence, further studies are warranted. Therefore, it may be suggested that prior dietary consumption of curcumin might be able to prevent hepatic damage induced by some toxic food additives.
Author contributions
O.O.A. and E.S.S. designed the experiment, analyzed the data, and wrote the paper. O.O.A. performed the experiments. M.M. and K.P. revised and edited the manuscript. All authors have read and approved the manuscript.
Funding
This research received no external funding.
Declaration of Competing Interest
The authors declare no conflict of interest.
Acknowledgements
The authors are thankful to Dr. Asogwa Nnaemeka Tobechukwu, Director of Research and Innovations for using the facility for analyzing the antioxidant enzymes. The authors are thankful to Dr. Mike Fraker of Ohio State University for his proofreading service.
References
- 1.Ansari F.A., Ali S.N., Arif H., Khan A.A., Mahmood R. Acute oral dose of sodium nitrite induces redox imbalance, DNA damage, metabolic and histological changes in rat intestine. PLoS One. 2017;12(4):e0175196. doi: 10.1371/journal.pone.0175196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McNally B., Griffin J.L., Roberts L.D. Dietary inorganic nitrate: from villain to hero in metabolic disease? Mol. Nutr. Food Res. 2016;60(1):67–78. doi: 10.1002/mnfr.201500153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Galloway J.N., Aber J.D., Erisman J.W., Seitzinger S.P., Howarth R.W., Cowling E.B., Cosby B.J. The nitrogen cascade. Aibs Bull. 2003;53(4):341–356. [Google Scholar]
- 4.Edition F. Guidelines for drinking-water quality. WHO Chron. 2011;38(4):104–108. [PubMed] [Google Scholar]
- 5.Lawniczak A.E., Zbierska J., Nowak B., Achtenberg K., Grześkowiak A., Kanas K. Impact of agriculture and land use on nitrate contamination in groundwater and running waters in central-west Poland. Environ. Monit. Assess. 2016;188(3):172. doi: 10.1007/s10661-016-5167-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gui G., Meng S., Li L., Liu B., Liang H., Huangfu C. Sodium nitrite enhanced the potentials of migration and invasion of human hepatocellular carcinoma SMMC-7721 cells through induction of mitophagy. Yao Xue Xue Bao= Acta Pharm. Sin. 2016;51(1):59–67. [PubMed] [Google Scholar]
- 7.Zhou L., Zahid M., Anwar M.M., Pennington K.L., Cohen S.M., Wisecarver J.L., Shostrom V., Mirvish S.S. Suggestive evidence for the induction of colonic aberrant crypts in mice fed sodium nitrite. Nutr. Cancer. 2016;68(1):105–112. doi: 10.1080/01635581.2016.1102298. [DOI] [PubMed] [Google Scholar]
- 8.Kiani A., Yousefsani B.S., Doroudian P., Seydi E., Pourahmad J. The mechanism of hepatotoxic effects of sodium nitrite on isolated rat hepatocytes. Toxicol. Environ. Health Sci. 2017;9(3):244–250. [Google Scholar]
- 9.Goodla L., Manubolu M., Pathakoti K., Jayakumar T., Sheu J.R., Fraker M., Tchounwou P.B., Poondamalli P.R. Protective effects of ammannia baccifera against CCl4-induced oxidative stress in rats. Int. J. Environ. Res. Public Health. 2019;16(8) doi: 10.3390/ijerph16081440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Venkatesan N., Punithavathi D., Arumugam V. Curcumin prevents adriamycin nephrotoxicity in rats. Br. J. Pharmacol. 2000;129(2):231–234. doi: 10.1038/sj.bjp.0703067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kuhad A., Pilkhwal S., Sharma S., Tirkey N., Chopra K. Effect of curcumin on inflammation and oxidative stress in cisplatin-induced experimental nephrotoxicity. J. Agric. Food Chem. 2007;55(25):10150–10155. doi: 10.1021/jf0723965. [DOI] [PubMed] [Google Scholar]
- 12.Strimpakos A.S., Sharma R.A. Curcumin: preventive and therapeutic properties in laboratory studies and clinical trials. Antioxid. Redox Signal. 2008;10(3):511–546. doi: 10.1089/ars.2007.1769. [DOI] [PubMed] [Google Scholar]
- 13.Bagchi A. Extraction of curcumin. IOSR J. Environ. Sci. Toxicol. Food Technol. 2012;1(3):1–16. [Google Scholar]
- 14.Trujillo J., Chirino Y.I., Molina-Jijón E., Andérica-Romero A.C., Tapia E., Pedraza-Chaverrí J. Renoprotective effect of the antioxidant curcumin: recent findings. Redox Biol. 2013;1(1):448–456. doi: 10.1016/j.redox.2013.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hongsibsong S., Stuetz W., Sus N., Prapamontol T., Grune T., Frank J. Dietary exposure to continuous small doses of alpha-cypermethrin in the presence or absence of dietary curcumin does not induce oxidative stress in male Wistar rats. Toxicol. Rep. 2014;1:1106–1114. doi: 10.1016/j.toxrep.2014.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Qureshi S., Shah A., Ageel A. Toxicity studies on Alpinia galanga and Curcuma longa. Planta Med. 1992;58(02):124–127. doi: 10.1055/s-2006-961412. [DOI] [PubMed] [Google Scholar]
- 17.Anand P., Kunnumakkara A.B., Newman R.A., Aggarwal B.B. Bioavailability of curcumin: problems and promises. Mol. Pharm. 2007;4(6):807–818. doi: 10.1021/mp700113r. [DOI] [PubMed] [Google Scholar]
- 18.Sankar P., Telang A.G., Manimaran A. Protective effect of curcumin on cypermethrin-induced oxidative stress in Wistar rats. Exp. Toxicol. Pathol. 2012;64(5):487–493. doi: 10.1016/j.etp.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 19.Singh P., Deora K., Sankhla V., Mogra P. Curcumin rendered protection against cadmium chloride induced testicular damage in Swiss albino mice. J. Cell Mol. Biol. 2012;10(2) [Google Scholar]
- 20.Akinyemi A.J., Onyebueke N., Faboya O.A., Onikanni S.A., Fadaka A., Olayide I. Curcumin inhibits adenosine deaminase and arginase activities in cadmium-induced renal toxicity in rat kidney. J. Food Drug Anal. 2017;25(2):438–446. doi: 10.1016/j.jfda.2016.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sudjarwo S.A., Giftania Wardani Sudjarwo K. Protective effect of curcumin on lead acetate-induced testicular toxicity in Wistar rats. Res. Pharm. Sci. 2017;12(5):381. doi: 10.4103/1735-5362.213983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.I.o.L.A.R.C.o. Care, U.o.L. Animals, N.I.o.H.D.o.R. Resources . National Academies; 1985. Guide for the Care and Use of Laboratory Animals. [Google Scholar]
- 23.Ansari F.A., Ali S.N., Khan A.A., Mahmood R. Acute oral dose of sodium nitrite causes redox imbalance and DNA damage in rat kidney. J. Cell. Biochem. 2018;119(4):3744–3754. doi: 10.1002/jcb.26611. [DOI] [PubMed] [Google Scholar]
- 24.Clinical development plan: curcumin. J. Cell. Biochem. Supplement. 1996;26:72–85. [PubMed] [Google Scholar]
- 25.Tajik H., Tamaddonfard E., Hamzeh-Gooshchi N. The effect of curcumin (active substance of turmeric) on the acetic acid-induced visceral nociception in rats. Pak. J. Biol. Sci. 2008;11(2):312–314. doi: 10.3923/pjbs.2008.312.314. [DOI] [PubMed] [Google Scholar]
- 26.Tapia E., Zatarain-Barrón Z.L., Hernández-Pando R., Zarco-Márquez G., Molina-Jijón E., Cristóbal-García M., Santamaría J., Pedraza-Chaverri J. Curcumin reverses glomerular hemodynamic alterations and oxidant stress in 5/6 nephrectomized rats. Phytomedicine. 2013;20(3–4):359–366. doi: 10.1016/j.phymed.2012.11.014. [DOI] [PubMed] [Google Scholar]
- 27.Sun L.-N., Yang Z.-Y., Lv S.-S., Liu X.-C., Guan G.-J., Liu G. Curcumin prevents diabetic nephropathy against inflammatory response via reversing caveolin-1 Tyr14 phosphorylation influenced TLR4 activation. Int. Immunopharmacol. 2014;23(1):236–246. doi: 10.1016/j.intimp.2014.08.023. [DOI] [PubMed] [Google Scholar]
- 28.Reitman S., Frankel S. A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am. J. Clin. Pathol. 1957;28(1):56–63. doi: 10.1093/ajcp/28.1.56. [DOI] [PubMed] [Google Scholar]
- 29.Frankel E.N., Meyer A.S. The problems of using one-dimensional methods to evaluate multifunctional food and biological antioxidants. J. Sci. Food Agric. 2000;80(13):1925–1941. [Google Scholar]
- 30.Goodla L., Manubolu M., Pathakoti K., Poondamalli P.R. Preventive and curative effects of Cocculus hirsutus (Linn.) Diels leaves extract on CCl4 provoked hepatic injury in rats. Egypt. J. Basic Appl. Sci. 2017;4(4):264–269. [Google Scholar]
- 31.Gornall A.G., Bardawill C.J., David M.M. Determination of serum proteins by means of the biuret reaction. J. Biol. Chem. 1949;177(2):751–766. [PubMed] [Google Scholar]
- 32.GRANT G.H. 1987. Amino Acids and Proteins, Fundamentals of Clinical Chemistry. [Google Scholar]
- 33.Bucolo G., David H. Quantitative determination of serum triglycerides by the use of enzymes. Clin. Chem. 1973;19(5):476–482. [PubMed] [Google Scholar]
- 34.Ohkawa H., Ohishi N., Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979;95(2):351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 35.Manubolu M., Goodla L., Ravilla S., Thanasekaran J., Dutta P., Malmlöf K., Obulum V.R. Protective effect of Actiniopteris radiata (Sw.) Link. against CCl4 induced oxidative stress in albino rats. J. Ethnopharmacol. 2014;153(3):744–752. doi: 10.1016/j.jep.2014.03.040. [DOI] [PubMed] [Google Scholar]
- 36.Misra H.P., Fridovich I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J. Biol. Chem. 1972;247(10):3170–3175. [PubMed] [Google Scholar]
- 37.Jollow D., Mitchell J., Zampaglione Na., Gillette J. Bromobenzene-induced liver necrosis. Protective role of glutathione and evidence for 3, 4-bromobenzene oxide as the hepatotoxic metabolite. Pharmacology. 1974;11(3):151–169. doi: 10.1159/000136485. [DOI] [PubMed] [Google Scholar]
- 38.Rotruck J.T., Pope A.L., Ganther H.E., Swanson A., Hafeman D.G., Hoekstra W. Selenium: biochemical role as a component of glutathione peroxidase. Science. 1973;179(4073):588–590. doi: 10.1126/science.179.4073.588. [DOI] [PubMed] [Google Scholar]
- 39.Ellman G.L. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959;82(1):70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 40.Ward M.H., DeKok T.M., Levallois P., Brender J., Gulis G., Nolan B.T., VanDerslice J. Workgroup report: drinking-water nitrate and health—recent findings and research needs. Environ. Health Perspect. 2005;113(11):1607–1614. doi: 10.1289/ehp.8043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yun J., Shahidi F., Rubin L., Diosady L. Oxidative stability and flavour acceptability of nitrite-free meat-curing systems. Can. Inst. Food Sci. Technol. J. 1987;20(4):246–251. [Google Scholar]
- 42.Vasavada M.N., Cornforth D.P. Evaluation of milk mineral antioxidant activity in beef meatballs and nitrite‐cured sausage. J. Food Sci. 2005;70(4):C250–C253. [Google Scholar]
- 43.Parthasarathy D.K., Bryan N.S. Sodium nitrite: the “cure” for nitric oxide insufficiency. Meat Sci. 2012;92(3):274–279. doi: 10.1016/j.meatsci.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 44.Sebranek J., Bacus J. Natural and organic cured meat products: regulatory, manufacturing, marketing, quality and safety issues. Am. Meat Sci. Assoc. White Paper Ser. 2007;1:115. [Google Scholar]
- 45.Sofos J.N., Busta F.F., Allen C. Botulism control by nitrite and sorbate in cured meats: a review. J. Food Prot. 1979;42(9):739–770. doi: 10.4315/0362-028X-42.9.739. [DOI] [PubMed] [Google Scholar]
- 46.OE E., EO F., IF U., EJ A. The protective effect of aloe vera juice on lindane induced hepatotoxicity and genotoxicity. Pak. J. Pharm. Sci. 2006;19(4):333–337. [PubMed] [Google Scholar]
- 47.El-Khayat Z., Ezzat A., Arbid M., Rasheed W., Elias T. Potential effects of bee honey and propolis against the toxicity of ochratoxin A in rats. Maced. J. Med. Sci. 2009;2(4):311–318. [Google Scholar]
- 48.Ateya R.H., Taha N.M., Mandour A.E.A., Lebda M.A., El-Morshedy A.M. Effect of monosodium glutamate and sodium nitrite on some biochemical parameters in Japanese quails. Alexandria J. Vet. Sci. 2016;48(1) [Google Scholar]
- 49.Soliman M.M., Baiomy A.A., Yassin M.H. Molecular and histopathological study on the ameliorative effects of curcumin against lead acetate-induced hepatotoxicity and nephrototoxicity in Wistar rats. Biol. Trace Elem. Res. 2015;167(1):91–102. doi: 10.1007/s12011-015-0280-0. [DOI] [PubMed] [Google Scholar]
- 50.Green T.J., Sivilotti M.L., Langmann C., Yarema M., Juurlink D., Burns M.J., Johnson D.W. When do the aminotransferases rise after acute acetaminophen overdose? Clin. Toxicol. 2010;48(8):787–792. doi: 10.3109/15563650.2010.523828. [DOI] [PubMed] [Google Scholar]
- 51.Alkiyumi S.S., Abdullah M.A., Alrashdi A.S., Salama S.M., Abdelwahab S.I., Hadi A.H.A. Ipomoea aquatica extract shows protective action against thioacetamide-induced hepatotoxicity. Molecules. 2012;17(5):6146–6155. doi: 10.3390/molecules17056146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hu Z., Lausted C., Yoo H., Yan X., Brightman A., Chen J., Wang W., Bu X., Hood L. Quantitative liver-specific protein fingerprint in blood: a signature for hepatotoxicity. Theranostics. 2014;4(2):215. doi: 10.7150/thno.7868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Freitag A.F., Cardia G.F.E., da Rocha B.A., Aguiar R.P., Silva-Comar F.M., Spironello R.A., Grespan R., Caparroz-Assef S.M., Bersani-Amado C.A., Cuman R.K.N. Hepatoprotective effect of silymarin (Silybum marianum) on hepatotoxicity induced by acetaminophen in spontaneously hypertensive rats. Evid. Based Complement. Altern. Med. 2015;2015 doi: 10.1155/2015/538317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li S., Tan H.-Y., Wang N., Zhang Z.-J., Lao L., Wong C.-W., Feng Y. The role of oxidative stress and antioxidants in liver diseases. Int. J. Mol. Sci. 2015;16(11):26087–26124. doi: 10.3390/ijms161125942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Helal E., Zahkok S., Soliman G.Z., Al-Kassas M., Abdel Wahed H. Biochemical studies on the effect of sodium nitrite and/or glutathione treatment on male rats. Egypt. J. Hosp. Med. 2008;30 [Google Scholar]
- 56.Ghadir M.R., Riahin A.A., Havaspour A., Nooranipour M., Habibinejad A.A. The relationship between lipid profile and severity of liver damage in cirrhotic patients. Hepat. Mon. 2010;10(4):285. [PMC free article] [PubMed] [Google Scholar]
- 57.Mehboob F., Ranjha F., Masud S. Changes in serum lipid profile among patients suffering from chronic liver disease. Ann. King Edward Med. Univ. 2007;13(3) 209-209. [Google Scholar]
- 58.AL-fatlawi H.G., AL-Zamely H.A. Study the effect of green tea extract on lipid profile in nitrate treated rabbits. Al-Qadisiyah J. Vet. Med. Sci. 2015;14(1):4–9. [Google Scholar]
- 59.Baum L., Cheung S.K., Mok V.C., Lam L.C., Leung V.P., Hui E., Ng C.C., Chow M., Ho P.C., Lam S. Curcumin effects on blood lipid profile in a 6-month human study. Pharmacol. Res. 2007;56(6):509–514. doi: 10.1016/j.phrs.2007.09.013. [DOI] [PubMed] [Google Scholar]
- 60.Qin S., Huang L., Gong J., Shen S., Huang J., Ren H., Hu H. Efficacy and safety of turmeric and curcumin in lowering blood lipid levels in patients with cardiovascular risk factors: a meta-analysis of randomized controlled trials. Nutr. J. 2017;16(1):68. doi: 10.1186/s12937-017-0293-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Honikel K.O. The use and control of nitrate and nitrite for the processing of meat products. Meat Sci. 2008;78(1–2):68–76. doi: 10.1016/j.meatsci.2007.05.030. [DOI] [PubMed] [Google Scholar]
- 62.Ak T., Gülçin İ. Antioxidant and radical scavenging properties of curcumin. Chem. Biol. Interact. 2008;174(1):27–37. doi: 10.1016/j.cbi.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 63.Ghosh S., Bhattacharyya S., Rashid K., Sil P.C. Curcumin protects rat liver from streptozotocin-induced diabetic pathophysiology by counteracting reactive oxygen species and inhibiting the activation of p53 and MAPKs mediated stress response pathways. Toxicol. Rep. 2015;2:365–376. doi: 10.1016/j.toxrep.2014.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Al Jameil N., Tabassum H., Fatima S., Ali M.N., Rizwana H., Khan F.A. Ameliorating effect of vitamin C against potassium dichromate induced oxidative stress and inflammatory response in rats. Int. J. Pharmacol. 2017;13(8):990–999. [Google Scholar]
- 65.El-Desoky G., Abdel-Ghaffar A., Al-Othman Z., Habila M., Al-Sheikh Y., Ghneim H., Giesy J., Aboul-Soud M. Curcumin protects against tartrazine-mediated oxidative stress and hepatotoxicity in male rats. Eur. Rev. Med. Pharmacol. Sci. 2017;21:635–645. [PubMed] [Google Scholar]
- 66.Elsayed A.S.I. The curcumin as an antioxidant natural herb with emphasize on its effects against some diseases. Int. J. Appl. Biol. Pharm. 2016;7:26–40. [Google Scholar]
- 67.Mohajeri M., Rezaee M., Sahebkar A. Cadmium‐induced toxicity is rescued by curcumin: a review. BioFactors. 2017;43(5):645–661. doi: 10.1002/biof.1376. [DOI] [PubMed] [Google Scholar]