Highlights
-
•
Datura stramonium seed extracts is toxic.
-
•
Its toxicity affects the liver, kidney, heart and brain.
-
•
Lipid peroxidation is one of the mechanisms of causing oxidative stress in target organs.
-
•
Toxicity of Datura stramonium seed largely depends on solvent of extraction.
-
•
Polarity and/or non-polarity of extraction solvent is linked to toxicity of Datura stramonium.
Keywords: Datura stramonium, Biomarkers, Antioxidants, Drugs, Toxicity, Solvent
Abstract
Datura stramonium seed ranks top among major plants commonly abused as drug in Nigeria. The present study therefore sought to unravel the target organs of toxicity as well as underscore the role of extraction solvent in the toxicity of Datura stramonium seed. Twenty male Wistar rats were randomly placed into four groups (I—IV) of five animals per group. Group I served as the control and was administered with distilled water only, while groups II, III and IV animals received 50 mg/kg body weight of aqueous, methanolic and diethylether extracts of Datura stramonium seeds by oral gavage for 14 days. Specific biomarkers of toxicity such as aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP) and lipid peroxidation (MDA) were estimated in the liver, brain, kidney and heart homogenates as well as serum of experimental animals. Lipid profile and activity of antioxidant enzymes such as superoxide (SOD) and catalase were determined in selected tissues while creatinine and urea were estimated in the kidney and serum. Results indicated that Datura stramonium seed extract generally caused a significant decrease in ALT, AST, ALP and MDA in selected tissue homogenates while these parameters increased significantly in the serum relative to the control group. Lipid profile was significantly deranged across selected tissues while SOD and catalase activity were significantly decreased relative to control. Put together, toxicity of Datura stramonium seed extract is diverse depending on the organ involved and solvent used for its extraction. Therefore, illicit use of Datura stramonium seeds as drugs among young people should be discouraged.
1. Introduction
Several reports have identified free radicals as the major culprit in the onset, progression and complications of almost all pathological conditions [1,2]. Antioxidants also exist in the biological system to combat the deleterious effects of free radicals [3]. However, any event that triggers a surge in free radicals generation to a level that overwhelms the body’s antioxidant capacity to scavenge them would inevitably lead to oxidative stress [4]. Natural compounds (phytochemicals) from plants parts that are synthesized as part of defense mechanism of the host plant against predators (and/or other stressors) often act as antioxidants [1,4]. In contrast, some phytochemicals are toxic to humans and other animals alike [2,[5], [6], [7], [8], [9]]. One of such plants is Datura stramonium L. commonly called thorn apple or Jimson weed. It contains alkaloids that are toxic to humans causing hallucination and deaths [10,11]. Botanically, it has whitish- tubular flowers with fruits containing seeds in an encapsulated form. Datura stramonium L has been widely reported to be very toxic [[12], [13], [14]]. The plant also possesses anti-inflammatory potential, stimulatory effects on the central nervous system (CNS), active clearing effect on the respiratory tract thereby aiding the respiratory system as well as keeping the teeth and skin healthy [[15], [16], [17]]. Datura stramonium L is rich in compounds with anticholinergic potential which can be exploited in treating symptoms of organophosphate toxicity [18].
Although, all parts of D. stramonium are toxic, its ripened seeds contain the highest concentration of its active principles [11,12,[19], [20], [21]]. Its frequent poisoning (intentional or accidental) may be intricately linked to its ubiquitous nature, ease of contaminating foodstuffs and portable water and its high toxicity [13,14,[22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32]]. Regardless of the part ingested, D. stramonium may cause complications whose diagnosis may be difficult to unravel [20,33,34]. Several cases of suicide and murder in India and Europe have been traced to Datura stramonium, hence, strict legislation prohibiting its cultivation has been implemented in several places [20,21,35,36]. Despite the widely reported toxicity of the plant, literature is scanty on the role played by extraction solvent in its toxicity as well as the susceptibility of different organs to the toxic effects of the plant. Hence, the present study is aimed at underscoring the effect of different solvents used for extraction on the toxicity of D. stramonium seeds in selected organs of albino rat.
2. Materials and methods
2.1. Collection of plant materials
Matured fruits of Datura stramonium were harvested from Ido-Ekiti, Ekiti State, Nigeria. The fruits were identified botanically and authenticated at the Department of Plant Science, Ekiti State University, Ado Ekiti, Nigeria. Each fruit was cut opened and the seeds collected, air-dried and pulverized in a warring blender. The powdered seed was kept in an airtight container at room temperature prior to extraction.
2.1.1. Chemicals and reagents
Potassium sulphate buffer, Tris-KCl buffer, carbonate buffer and trichloroacetic acid (TCA) were obtained from Sigma Aldrich chemicals. Other chemicals and reagents of high analytical grade were purchased from standard commercial suppliers.
2.1.2. Animals
Male Wistar rats (200–250 g) were obtained from the Animal House, College of Medicine, Ekiti State University, Ado Ekiti. The rats were kept in different animal cages, on a 12-h light:12-h dark cycle, at room temperature of 22–24 ○C. Animals were allowed free access to food and water. Animals were handled in accordance with the National Institutes of Health guide for the care and use of Laboratory animals (NIH Publications No. 8023, revised 1978). Animals were randomly placed into four groups of five rats each: Group I animals were the control that were administered distilled water only while groups II, III and IV animals were administered 50 mg/kg body weight of aqueous, methanolic and diethyl ether extracts respectively for two weeks by oral gavage. This dosage was chosen considering earlier toxicological works done on the plant and reports from traditional parlance. Animals were fasted 24 h before sacrifice.
2.2. Methods
2.2.1. Extraction of active principles
Three hundred (300 g) of dried seeds of Datura stramonium was weighed and extracted with 250 ml each of distilled water, methanol and diethyl ether respectively for 72 h. The resulting mixture was filtered using a muslin cloth and Whatmann’s filter paper number 1.0 to obtain the residue which was discarded and the filtrates which were left opened for 72 h to allow for evaporation of the solvents of extraction. The reason for this step was to guide against toxicity due to residual solvents specifically diethyl ether and methanol. After establishing complete evaporation of the extraction solvents, the crude extract obtained was stored in an airtight container prior to use. Each extract was then reconstituted in distilled water and administered to the animals orally for two weeks.
2.2.2. Preparation of tissue homogenate
Animals were rapidly dissected to excise the liver, brain, heart and kidney. Ten percent homogenate of each tissue was prepared using 6.7 mM potassium phosphate buffer, pH 7.4. Each of the tissue homogenates was centrifuged at 10,000 rpm for 10 min at 4 °C to obtain a clear supernatant which was carefully decanted and kept at 8 °C for biochemical analysis. Whole blood was collected into serum bottles by cardiac puncture and centrifuged at 3000 rpm for 15 min to obtain the serum which was kept on ice.
2.2.3. Determination of alkaline phosphatase (ALP)
Activity of alkaline phosphatase in the serum as well as tissue homogenates was measured by the method of Reitman and Frankel [37]. Fifty microliter (50 μl) of sample was added 500 μl of substrate which had been earlier equilibrated at 37 °C for 3 min. The resulting mixture was then for 10 min at 37 °C. Thereafter, 2.5 ml of a colour reagent containing 0.09 M NaOH and 0.1 M Na2CO3, was added. The mixture was well mixed and absorbance read at 590 nm against the blank.
2.2.4. Determination of aspartate aminotransferase (AST)
Aspartate aminotransferase activity in the serum and tissues homogenates was measured according to the method described by Reitman and Frankel [37]. One hundred microliter of sample was added to 500 μl of AST buffer, the resulting mixture was thoroughly mixed and incubated at 37 °C for 30 min. Five hundred microliter of 2,4-dinitrophenyl hydrazine (2.0 mM) was added. The mixture was mixed thoroughly mixed and incubated at 20 °C for 20 min. Thereafter, five (5) ml of 0.01 M NaOH was then added, the mixture was again mixed and its absorbance was read at 546 nm.
2.2.5. Determination of alanine aminotransferase (ALT)
Alanine aminotransferase was measured using standard Fortress kit from England according to the method of International Federation of Clinical Chemistry [38]. One hundred microliter (100 μl) of homogenates was added to 500 μl of ALT buffer, thoroughly mixed and incubated for 30 min at 37 °C in water bath. Five hundred microliter (500 μl) of dye reagent (2,4 - dinitrophenyl hydrazine, 2.0 mM) was then added. The resulting mixture was thoroughly mixed and allowed to stand for 20 min at 20 °C. Thereafter, 5.0 ml of diluted NaOH was added and mixed thoroughly. Absorbance of the resulting mixture was read at 546 nm against the blank.
2.2.6. Determination of total cholesterol
Total serum cholesterol was measured following the method of Richmond [39]. Ten microliter (10 μl) each of tissue homogenate and serum was thoroughly mixed with 1 ml of working reagent containing (4-aminoantipyrine, cholesterol oxidase, cholesterol esterase and peroxidase) dissolved in 50 ml of pipes buffer and phenol. Thereafter, the mixture was incubated at 25 °C for 10 min. Absorbance of the resulting mixture was read at 546 nm against the blank. Total cholesterol (mg/dl) was then calculated.
2.2.7. Determination of HDL-cholesterol
The method earlier described by Assmann and Schulte [40] was followed in measuring high density lipoproptein-cholesterol (HDL-chol)]. Five microliter (5 μl) each of serum and tissues homogenates was added to 450 μl of Good’s buffer (4-amino antipyrine, POD, ascorbic oxidase and anti-human lipoprotein Ab). The reaction mixture was mixed thoroughly and incubated for five minutes at 37 °C. One hundred and fifty microliter of enzyme reagent (Good’s buffer I, cholesterol esterase and cholesterol oxidase) was then added to the mixture which was incubated at 37 °C for five minutes. Absorbance of the resulting mixture was then read at 600 nm against the blank.
2.2.8. Determination of LDL-cholesterol
Measurement of low density lipoprotein-cholesterol (LDL-chol) was done by the method described by Amstrong and Seidel [41]. Five microliter each of serum and tissue homogenates was added to 450 μl of buffer solution containing (Good’s buffer, cholesterol oxidase, cholesterol esterase, catalase, ascorbate oxidase, and peroxidase). The resulting mixture was incubated at 37 °C for five minutes after thorough mixing. Absorbance I was read at 600 nm. One hundred and fifty microliters (150 μl) of enzyme reagent R2 (Amino antipyrine and POD) was then added to the mixture and incubated at 37 °C for five minutes. Absorbance II of the resulting mixture was read at 600 nm.
2.2.9. Estimation of lipid peroxidation assay (TBARS)
Amount of thiobarbituric acid reactive species (TBARS) in serum and tissue homogenates were determined following the method of Varshney and Kale [42]. Four hundred microliter of serum and tissue homogenates was thoroughly mixed with 1.6 ml of Tris-KCl buffer after the addition of five hundred microliter of 30% trichloroacetic acid (TCA). Five hundred microliter of 0.75% thiobarbituric acid (TBA) was added to the mixture and incubated at 80 °C for 45 min. The reaction mixture was then cooled on ice and centrifuged at 3000 g. The clear supernatant obtained was carefully decanted and its absorbance read at 532 nm against a reference blank. Malondialdehyde (MDA) level was then calculated as earlier described by Adam-vizi and Seregi [43].
2.2.10. Superoxide dismutase (SOD)
Activity of superoxide dismutase (SOD) in serum and tissue homogenates was measured as described by Misra and Fridovich [44]. Aliquots of serum and tissue homogenates mixed with 2.5 ml of 0.05 M carbonate buffer, pH 10.2 and the reaction mixture was equilibrated for two minutes in a spectrophotometer. Three hundred microliter of freshly prepared adrenaline (0.3 mM) was added and mixed by inversion to initiate the reaction. The blank cuvette contained all other assay components except the homogenates (s) and/or serum which were replaced with distilled water. Change in absorbance at 480 nm was monitored every 30 s for 150 s.
2.2.11. Determination of catalase activities
Activity of catalase in serum and tissue homogenates was assayed according to Sinha [45]. Two hundred microliters each of tissue homogenates was mixed with eight hundred microliter of distilled water to obtain five-fold dilution of the sample. Five hundred microliter of diluted homogenates was rapidly mixed with the reaction mixture at room temperature by a gentle swirling. One milliliter (1 ml) of the mixture was withdrawn at one minute interval and blown into 1 ml dichromate/acetic acid reagent. Hydrogen peroxide content of the withdrawn portion of the mixture was estimated at 570 nm as described by Sinha [45].
2.2.12. Statistical analysis
Data were expressed as mean ± SD and analyzed by appropriate analysis of variance (ANOVA), followed by Duncan’s multiple range tests where necessary. This is indicated in the text of results. Statistical significance was considered at p < 0.05.
3. Results
3.1. General observation
Administration of D. stramonium caused a significant decrease in the activity of AST, ALT and ALP. However, the decrease in activity was dependent on the tissue involved and the solvent used for extraction. Meanwhile, serum levels of AST, ALT and ALP were significantly increased relative to the control animals. Serum total cholesterol was decreased but increased in the heart homogenate while LDL-cholesterol and HDL-cholesterol were significantly increased and decreased respectively depending on the tissue involved. Urea and creatinine level was increased in the serum and kidney homogenates relative to the control. Lipid peroxidation was increased in the animals administered with D. stramonium relative to control animals. Superoxide dismutase and catalase were depleted by D. stramonium regardless of the solvent used for extraction.
4. Discussion
Datura poisoning is fast becoming a global phenomenon of vast research attention [[11], [12], [13],30]. Enzyme biomarkers from selected tissues and body fluids have been used routinely to monitor toxicity of plant extracts as well as disease investigation and diagnosis [46]. This approach often helps in identifying tissue-related oxidative assault in experimental animals [47]. Consequently, specific biochemical parameters from the blood are routinely used as indicators to determine the health status of an organism [48,49].
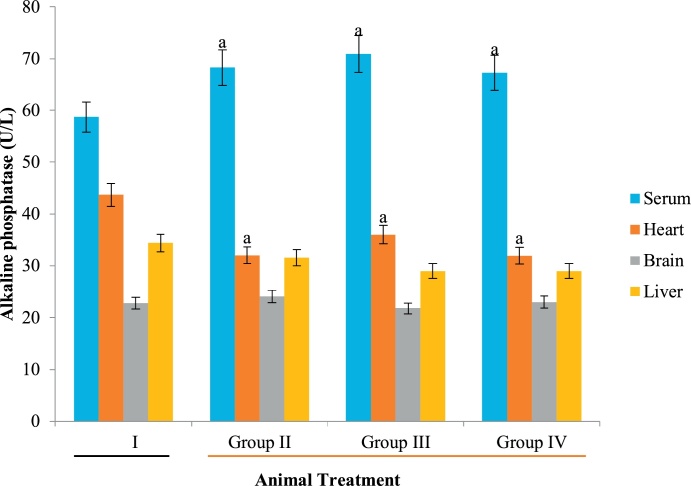
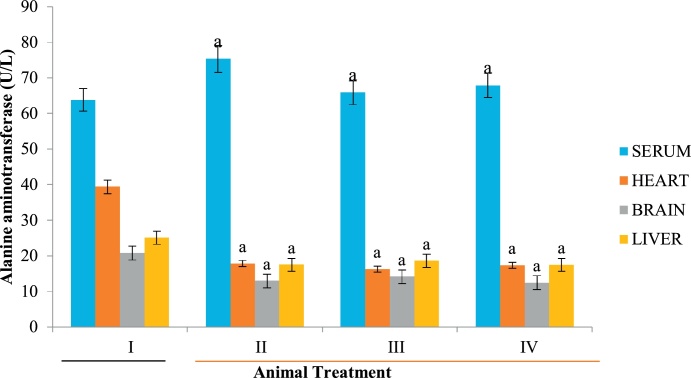
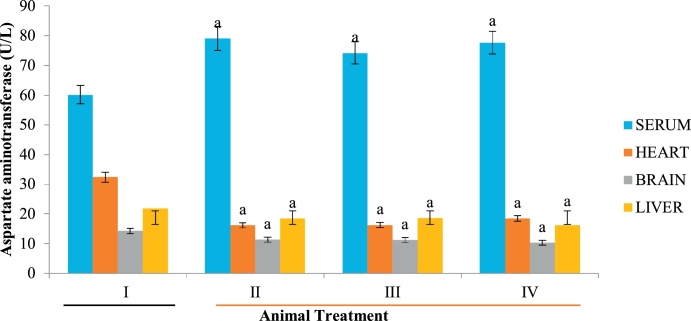
ALT, ALP and AST are specific bio-indicators associated with hepatotoxicity [50]. Hepatic injury is usually detected by significant alteration in the normal serum level of these enzymes [51]. In the present study, increase in serum ALP (Fig. 1) could be attributed to possible biliary obstruction, heart failure, decrease in blood flow to the kidney and dehydration [52,53] caused by Datura stramonium extract. Similarly, high level of serum AST relative to the control animals suggests that extracts of Datura stramonium are hepatotoxic. This elevation is a consequence of hepatic injury, resulting in the leakage of enzymes that are normally localized within the hepatocytes. Although, Clementine and Tar [54] reported that a high level of AST do not always signify liver damage but an elevation in serum ALT is always an indication of a problem with the liver since it is a specific biomarker of liver damage [54]. Since the level of these biomarkers were decreased in the heart and brain homogenates (Fig. 1, Fig. 2, Fig. 3), the plant is both cardiotoxic and neurotoxic. Noteworthy however, is the fact that the aqueous extract of Datura stramonium appeared to contain more toxic principles than methanolic and diethyl ether as reflected in the levels of biomarkers in the groups administered with the respective extracts relative to the control group (Fig. 1, Fig. 2, Fig. 3).
Fig. 1.
Effect of D. stramonium on ALP activity of selected tissues.
Data is expressed as mean ± standard deviation, ‘a’ represents significant difference (p < 0.05) from the control.
Fig. 2.
Effect of D. stramonium on ALT activity of selected tissues.
Data is expressed as mean ± standard deviation, ‘a’ represents significant difference (p < 0.05) from the control.
Fig. 3.
Effect of D. stramonium on AST activity of selected tissues. Data is expressed as mean ± standard deviation, ‘a’ represents significant difference (p < 0.05) from the control.
Derangement in lipid profile is another mechanism of toxicity employed by toxic principles of medicinal plants. Elevated concentration of lipid is a risk factor in cardiovascular disorders [55]. Generally, hyperlipidemia have been identified with certain risk factors including a significant elevation in LDL-cholesterol, total cholesterol coupled with decreased level of HDL-cholesterol in experimental animals [55]. In the present study, total cholesterol was decreased in the serum, brain and liver homogenates of experimental animals relative to the control (Table 1). This observation suggests a possible alteration in membrane stability and fluidity. Cholesterol is among the prominent lipids responsible for membrane integrity. Perhaps, Datura stramonium toxicity involves derangement of lipid profile ultimately resulting in the compromise of membrane integrity and function. Similarly, there was a noticeable increase in total cholesterol in the kidney and heart homogenates of experimental rats relative to the control animals (Table 1). This is suggestive of the fact that the plant is toxic both to the heart and kidney. Although the specific mechanism and active principle involved in both renal and cardiac still remains unclear within the limit of the present study, it is an indication that the plant exhibits selective toxicity to various tissues. Lipids are transported in the blood by lipoproteins. HDL-cholesterol otherwise called ‘good cholesterol’ which returns cholesterol to the liver where it is converted into bile and subsequently removed from the body [55,56]. A noticeable decrease in HDL-cholesterol in the liver, brain and heart homogenates suggested hepatotoxicity, brain toxicity and cardiotoxicity of D. stramonium respectively. This implies that the mechanism involved in the toxicity of D. stramonium is complex and diverse, perhaps depending on the tissue involved (Table 2). On the other hand, an increase in serum LDL cholesterol (although to varying degrees) in the groups administered with aqueous, methanolic and diethylether extracts of D. stramonium relative to the control group further suggests that regardless of the solvent used for extraction, the plant is toxic to the various tissues employed (Table 3).
Table 1.
Effect of D. stramonium on Total cholesterol level of selected tissues.
| GROUP | I | II | III | IV |
|---|---|---|---|---|
| SERUM (mg/dl) | 19.09 ± 2.29 | 15.70 ± 1.89a | 10.28 ± 1.60a | 12.18 ± 1.46a |
| HEART (mg/dl) | 12.14 ± 1.43 | 17.92 ± 2.11 a | 14.31 ± 1.56a | 13.29 ± 1.56 |
| BRAIN (mg/dl) | 6.46 ± 0.54 | 6.10 ± 0.51a | 5.24 ± 0.44a | 6.24 ± 0.52a |
| LIVER (mg/dl) | 12.95 ± 1.08 | 10.51 ± 0.39a | 9.49 ± 0.35a | 11.47 ± 0.42 |
| KIDNEY (mg/dl) | 8.53 ± 0.71 | 10.74 ± 0.90a | 9.65 ± 0.81 | 9.40 ± 0.79 |
Data is expressed as mean ± standard deviation, ‘a’ represents significant difference (p < 0.05) from the control.
Table 2.
Effect of D. stramonium on HDL-cholesterol level of selected tissues.
| GROUP | I | II | III | IV |
|---|---|---|---|---|
| SERUM (mg/dl) | 16.51 ± 7.27 | 9.61 ± 62.00a | 4.36 ± 56.69a | 8.79 ± 58.58a |
| HEART (mg/dl) | 6.01 ± 0.71 | 3.11 ± 0.36a | 3.64 ± 0.43a | 4.25 ± 0.50a |
| BRAIN (mg/dl) | 2.60 ± 0.22 | 1.09 ± 0.09a | 1.62 ± 0.14a | 1.48 ± 0.12a |
| LIVER (mg/dl) | 9.40 ± 0.79 | 5.38 ± 0.45a | 4.92 ± 0.41a | 3.68 ± 0.31a |
| KIDNEY (mg/dl) | 4.38 ± 0.37 | 2.08 ± 0.17a | 0.51 ± 0.04a | 1.47 ± 0.12a |
Data is expressed as mean ± standard deviation, ‘a’ represent significant difference (p < 0.05) from the control.
Table 3.
Effect of D. stramonium on LDL- cholesterol level of selected tissues.
| GROUP | I | II | III | IV |
|---|---|---|---|---|
| SERUM (mg/dl) HEART (mg/dl) BRAIN (mg/dl) LIVER (mg/dl) KIDNEY (mg/dl) |
8.48 ± 1.92 5.15 ± 0.61 2.02 ± 0.34 3.20 ± 0.60 3.15 ± 0.26 |
7.86 ± 2.37 8.67 ± 1.02a 3.18 ± 0.27a 4.28 ± 0.36a 5.38 ± 0.45a |
8.84 ± 2.66 8.87 ± 1.05a 3.88 ± 0.32a 4.92 ± 0.41a 4.92 ± 0.41a |
9.47 ± 2.86a 7.42 ± 0.87a 1.48 ± 0.12 4.77 ± 0.40a 4.78 ± 0.40a |
Data is expressed as mean ± standard deviation, ‘a’ represents significant difference (p < 0.05) from the control.
Urea is made from protein catabolism in the liver but is excreted in the urine. Measurement of amount of urea is an indirect way of determining the amount of excretable nitrogen in the blood [57]. In the present study, serum urea significantly decreased in the group administered with aqueous extract suggesting that the toxic principle was not water-extractible (Table 4). However, in the group administered with methanolic extract, there was an increase in serum urea relative to the control group. This observation suggests that the toxic principle responsible for kidney damage is only extractible in methanol. This suggestion was further supported by the fact that the urea level was least in the kidney homogenate of animals administered with the methanolic extract. Put together, high urea in the serum correlated with a low urea level in the tissue homogenate, which are indication of nephrotoxicity of the methanolic extract of the plant (Table 4). This implies that different toxic principle in the extract got extracted to each of the solvents, perhaps depending on their polarity.
Table 4.
Effect of D. stramonium on urea level of selected tissues.
| GROUP | I | II | III | IV |
|---|---|---|---|---|
| SERUM (mg/dl) | 16.98 ± 2.83 | 9.45 ± 3.2.25a | 17.67 ± 2.95a | 15.75 ± 2.63a |
| KIDNEY (mg/dl) | 1.99 ± 0.17 | 1.24 ± 0.10 | 0.47 ± 0.04a | 1.20 ± 0.10 |
Data is expressed as mean ± standard deviation, ‘a’ represents significant difference (p < 0.05) from the control.
Creatinine is a measure of the level of waste product in the blood and urine. By implication, high level of creatinine suggests a malfunctioning kidney probably resulting from oxidative kidney damage or chronic kidney disease [57]. Extremely low level of creatinine and urea may be suggestive of a severe liver disease [57]. Serum creatinine level was significantly higher in animals administered with the aqueous extract relative to the control (Table 5). Similarly, creatinine level was decreased in the kidney homogenates of experimental animals administered with the extract irrespective of the solvent used for extraction (Table 5). This is an indication that D. stramonium extract is nephrotoxic [58].
Table 5.
Effect of D. stramonium on creatinine level of selected tissues.
| GROUP | I | II | III | IV |
|---|---|---|---|---|
| SERUM (mg/dl) | 3.12 ± 0.76 | 7.39 ± 0.85a | 2.33 ± 0.79 | 2.01 ± 0.81 |
| KIDNEY (mg/dl) | 19.12 ± 1.60 | 15.82 ± 1.32a | 15.27 ± 1.28a | 16.21 ± 1.35a |
Data is expressed as mean ± standard deviation, ‘a’ represents significant difference (p < 0.05) from the control.
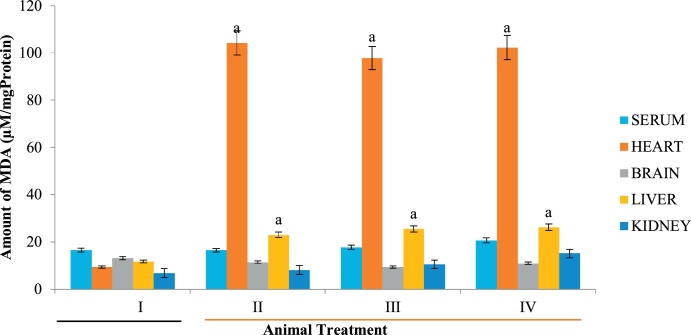
Lipid peroxidation is a free radical-mediated oxidation of polyunsaturated fatty acids involving chain reactions that ultimately cause deleterious effect to critical biological macromolecules. These oxidative reactions often lead to the formation of lipid hydroperoxide which are further degraded into several aldehydic compounds including hydroxy-alkenals [9]. In comparison to free radicals, these aldehydes are more stable and have the tendency to diffuse and escape from the cell thereby attacking targets cells far away. These aldehydes are highly reactive attacking critical macromolecules such as DNA, proteins and membrane phospholipids, producing a wide array of covalent adducts [59]. On interaction, lipoproteins and other lipid-containing compounds can be modified by hydroxy-alkenals thereby deranging membrane configuration and spatial arrangement thereby impairing the function of bound enzymes like Na+ /K + ATPase which plays important role in the functional nerve cells [60,61]. D. stramonium extract caused a significant increase in hepatic and renal lipid peroxidation in all groups administered with the extracts relative to control (Fig. 4). Noteworthy however, is the fact that cardiac lipid peroxidation was far higher than other tissues, suggesting that the mechanism of toxicity of D. stramonium in the heart involves lipid peroxidation. This might imply that cardiovascular disorders and its complications are the perhaps the aftermath of intentional or accidental ingestion of D. stramonium extract.
Fig. 4.
Effect of D. stramonium on MDA level of selected tissues. Data is expressed as mean ± standard deviation, ‘a’ represents significant difference (p < 0.05) from the control.
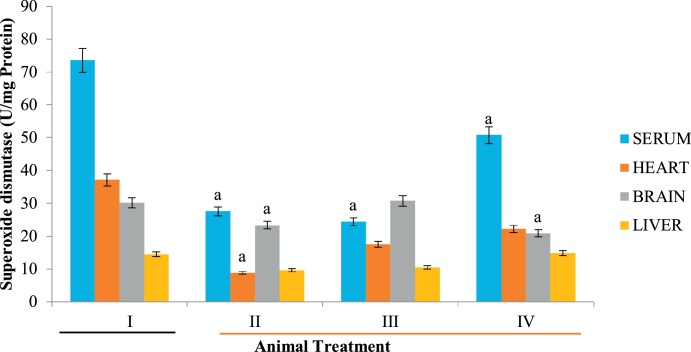
Superoxide dismutase is another antioxidant enzyme responsible for the conversion of singlet oxygen into hydrogen peroxide and subsequently to water and oxygen by catalase. A significant decrease in SOD activity in the various tissues employed in the present study (Fig. 5) suggests that the first line of defense against ROS has been depleted, thereby exposing animals to the deleterious effects of free radicals.
Fig. 5.
Effect of D. stramonium on SOD activity of selected tissues. Data is expressed as mean ± standard deviation, ‘a’ represents significant difference (p < 0.05) from the control.
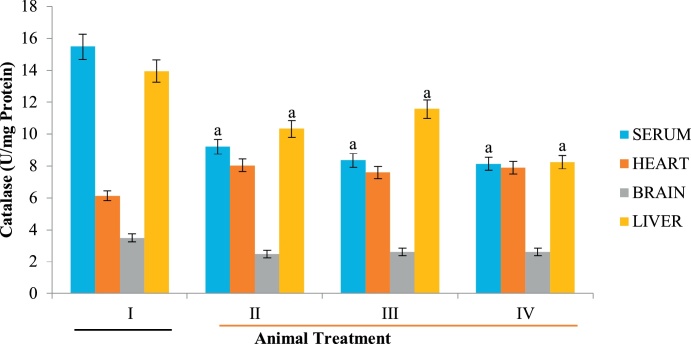
Catalase is a preventive peroxisomal antioxidant enzyme which protects the cell and critical macromolecules against free radicals attack thereby mitigating oxidative stress [62]. Relative to the control group, catalase activity was decreased in animals administered with the extracts regardless of the solvent used for extraction (Fig. 6). This further buttress the claim that D. stramonium extracts are toxic to all tissues investigated. The toxicity appeared to be largely dependent on the tissue involved and solvent used for extraction.
Fig. 6.
Effect of D. stramonium on catalase activity of selected tissues. Data is expressed as mean ± standard deviation, ‘a’ represents significant difference (p < 0.05) from the control.
5. Conclusion
The study concluded that D. stramonium is toxic and attacks various organs including the liver, heart, kidney and brain. Its toxicity to the various organs varies and depends on the solvent used for its extraction.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of Competing Interest
All authors clearly state that there is no conflict of interest of any kind.
Acknowledgements
The authors acknowledged the support of the Department of Medical Biochemistry, Ekiti State University, Ado Ekiti, Nigeria for free usage of the laboratory.
References
- 1.Abdel-Daim M.M., Moustafa Y.M., Umezawa M., Kota V.R., Elena A. Applications of antioxidants in ameliorating drugs and xenobiotics toxicity: mechanistic approach. Oxid. Med. Cell. Longev. 2017;4565127 doi: 10.1155/2017/4565127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yeung A., Nikolay T., El-Tawil O.S., Simona G.B., Mohamed M.A., Atanas G.A. Antioxidants: scientific literature landscape analysis. Oxid. Med. Cell. Longev. 2019 doi: 10.1155/2019/8278454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shaban S., Mostafa W.A., Abdelrahman I.B., Muhammad A.S., Mamdouh M., Abdel-Daim M. Effects of antioxidant supplements on the survival and differentiation of stem cells. Oxid. Med. Cell. Longev. 2017 doi: 10.1155/2017/5032102. 5032102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abdel-Daim M.M., Abo-EL-Sooud K., Aleya L., Bungǎu S.G., Najda A., Saluja R. Alleviation of drugs and chemicals toxicity: biomedical value of antioxidants. Oxid. Med. Cell. Longev. 2018 doi: 10.1155/2018/6276438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.El-Hak H.N.G., Moustafa A.R.A., Mansour S.R. Toxic effect of Moringa peregrine seeds on histological and biochemical analyses of adult male Albino rats. Toxicol. Rep. 2017;12(5):38–45. doi: 10.1016/j.toxrep.2017.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burcia L.M., da Silva C.B., Rondon J.N., da Silva L.M., de Andrade S.F., Miguela O.G., de Fátima Gaspari Diasa J., Miguela M.D. Acute and subacute (28 days) toxicity, hemolytic and cytotoxic effect of Artocarpus heterophyllus seed extracts. Toxicol. Rep. 2018 doi: 10.1016/j.toxrep.2018.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sundarama R., Karuppaiah M., Shanthid P., Sachdanandama P. Acute and sub-acute studies of catechol derivatives from Semecarpus Anacardium. Toxicol. Rep. 2018;5:231–239. doi: 10.1016/j.toxrep.2018.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sureshkumar D., Begum S., Johannah N.M., Maliakel B., Krishnakumar I.M. Toxicological evaluation of a saponin-rich standardized extract of fenugreek seeds (FenuSMARTTM): acute, sub-chronic and genotoxicity studies. Toxicol. Rep. 2018 doi: 10.1016/j.toxrep.2018.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Panigrahia G.K., Vermaa N., Singhb N., Asthanaa S., Guptab S.K., Tripathia A., Dasa M. Interaction of anthraquinones of Cassia occidentalis seeds with DNA and Glutathione. Toxicol. Rep. 2018;5:164–172. doi: 10.1016/j.toxrep.2017.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maibam R.D. Neurotoxic and medicinal properties of Datura stramonium L. – review. Assam Univ. J. Sci. Technol. Biol. Environ. Sci. 2011;7:139–144. [Google Scholar]
- 11.Joshia B.C., Prakashb A., Kalia A.N. Hepatoprotective potential of antioxidant potent fraction from Urticadioica Linn. (whole plant) in CCl4 challenged rats. Toxicol. Rep. 2015;2:1101–1110. doi: 10.1016/j.toxrep.2015.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Benouadah Z., Mahdeb N., Bouzidi A. Evaluation of acute and sub-acute toxicity of alkaloids from Datura stramonium sp. in mice. Int. J. Pharmacogn. Phytochem. Res. 2016;8(11):1759–1766. [Google Scholar]
- 13.Trancă S.D., Szabo R., Cocis M. Acute poisoning due to ingestion of Datura stramonium – a case report. Rom. J. Anaesth. Intensive Care. 2017;24(1):65–68. doi: 10.21454/rjaic.7518.241.szb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Korkmaz M.F., Bostanc M., Onur H., Cagan E. Datura stramonium poisoning: a case report and review of the literature. Eur. Res. J. 2019;5(1):186–188. [Google Scholar]
- 15.De Foe V., Senatore F. Medicinal plants and phytotherapy in the amal fitan cost, Salerno province Campania, Southern Italy. J. Ethnopharm. 1993;39:39–51. doi: 10.1016/0378-8741(93)90049-b. [DOI] [PubMed] [Google Scholar]
- 16.Manandhar N.P. Inventory of some herbal drugs of Myagai district, Nepal. Econ. Bot. 1995;49:371–379. [Google Scholar]
- 17.Abbas D.A. Analgesiac, anti-Inflammatory and antidiarrhoeal effects of Datura stramonium hydroalcoholic leaves extract in mice. IJRRAS. 2013;14(1):22–29. [Google Scholar]
- 18.Theodore C., Bania M.S., Jasan C., Dallas B., Melanie O. Jimson weed extract as a protective agent in severe organophosphate toxicity. Acad. Emerg. Med. 2004;11(4):335–338. doi: 10.1197/j.aem.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 19.Miraldi E., Masti A., Ferri S., Barni C.I. Distribution of hyoscyamine and scopolamine in Datura stramonium. Fitoterap. 2001;72:644–648. doi: 10.1016/s0367-326x(01)00291-x. [DOI] [PubMed] [Google Scholar]
- 20.Jonasson M., Afshari R. Chronicle of Datura toxicity in 18th and 19th century. Asia Pac. J. Med. Toxicol. 2016;5:4–10. [Google Scholar]
- 21.Mishra D.S. Datura stramonium (common name: jimson weed) medicinal uses, side effects and benefits. World J. Pharm. Res. 2018;7(12):1011–1019. [Google Scholar]
- 22.Kurzbaum A., Simsolo C., Kvasha L., Blum A. Toxic delirium due to Datura stramonium. Isr. Med. Assoc. J. 2001;3:538–539. [PubMed] [Google Scholar]
- 23.Steenkamp P.A., Harding N.M., Van Heerden F.R., Van WYk B.E. Fatal Datura poisoning: identification of atropine and scopolamine by high performance liquid chromatography/photodiode array/mass spectrometry. J. For. Sci. Int. 2004;145:31–39. doi: 10.1016/j.forsciint.2004.03.011. [DOI] [PubMed] [Google Scholar]
- 24.Al-Shaikh A.M., Sablay Z.M. Hallucinogenic plant poisoning in children. Saudi Med. J. 2005;26:118–121. [PubMed] [Google Scholar]
- 25.Forrester M.B. Jimsonweed (Datura stramonium) exposures in Texas, 1998–2004. J. Toxicol. Environ. Health. 2006;69:1757–1762. doi: 10.1080/15287390600631284. [DOI] [PubMed] [Google Scholar]
- 26.Monteriol A., Kenane N., Delort G., Asencio Y., Palmier B. Intentional Datura stramonium intoxication: an unknown etiology of mydriasis. Ann. Fr. Anesthés. Réanim. 2007;26:810–813. doi: 10.1016/j.annfar.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 27.Oberndorfer S., Grisold W., Hinterholzer G., Rosner M. Coma with focal neurological signs caused by Datura stramonium intoxication in a young man. J. N. Neuro. Psy. 2002;73:458–459. doi: 10.1136/jnnp.73.4.458-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arouko H., Matray D., Braganca C., Mpaka P., Chinello L., Castaing F., Bartou C., Poisot D. Voluntary poisoning by ingestion of Datura stramonium. Another cause of hospitalisation in youth seeking strong sensations. Annu. Med. Int. 2003;154:46–50. [PubMed] [Google Scholar]
- 29.Boumba A., Mitselou A., Vougiouklakis T. Fatal poisoning from ingestion of Datura stramonium seeds. Vet. Hum. Toxicol. 2005;46:81–82. [PubMed] [Google Scholar]
- 30.Ertekin V., Selimoglu M.A., Altinkaynak S. A combination of unusual presentations of Datura stramonium intoxication in a child: rhabdomyolys and fulminant hepatitius. J. Emergency Med. 2005;28:227–228. doi: 10.1016/j.jemermed.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 31.Dubey P.K., Sanjeev O.P. Acute renal failure: a complication of Datura poisoning. J. Kidney. 2017;3:3–5. [Google Scholar]
- 32.Başaran S., Dündar G., Ogun M.N. Datura stramonium poisoning case with Wernicke aphasia-like symptoms: case report. Kocaeli Med. J. 2018;7(1):83–86. [Google Scholar]
- 33.Diker D., Markovitz D., Rothman M., Sendovski U. Coma as a presenting sign of Datura stramonium seed tea poisoning. Eur. J. Internal Med. 2007;44:336–338. doi: 10.1016/j.ejim.2006.09.035. [DOI] [PubMed] [Google Scholar]
- 34.Uddin F., Hossain A., Das R., Rahman Ahmad M., Akanda R., Islam S. Evaluation of toxic effects of Datura Leaves (Datura stramonium) in rat. Int. J. Agric. Environ. Res. 2017;3(4):3486–3497. [Google Scholar]
- 35.Preissel U., Hans-George P. Firefly Books; New York: 2002. Brugmansia and Datura: Angel’s Trumpets and Thorn Apples Buffalo; pp. 106–129. [Google Scholar]
- 36.Bontoyan W. Jimsonweed poison associated with homemade stew- maryland centers for disease control and prevention. Morbidity Mortality Weekly Rep. 2010;59(4):102–103. 2008. [PubMed] [Google Scholar]
- 37.Reitman S., Frankel S. Glutamic – pyruvate transaminase assay by colorimetric method. Am. J. Clin. Path. 1957;28:56–60. doi: 10.1093/ajcp/28.1.56. [DOI] [PubMed] [Google Scholar]
- 38.International federation of clinical chemistry, methods for the measurement of catalytic concentration of enzymes. Part 3. IFCC method for alanine aminotransferase. J. Clin. Chem. Biochem. 1986;18:521–534. [PubMed] [Google Scholar]
- 39.Richmond W. Preparation and properties of a cholesterol oxidase from Nocardia sp. And its application to the enzymatic assay of total cholesterol in serum. Clin. Chem. 1973;19/12:1350–1356. [PubMed] [Google Scholar]
- 40.Assmann G., Schulte H. Relationship of HDL cholesterol to incidence of atherosclerotic coronary heart disease: the procam experience. Drugs Affecting Lipid Metabol. 1990;2:343–355. [Google Scholar]
- 41.Armstrong V.W., Seidel D. Evaluation of a commercial kit for the determination of LDL cholesterol based on precipitation of LDL with dextran sulfate. Arztliche Laboratorium. 1985;31:325–330. [Google Scholar]
- 42.Varshney R., Kale R.K. Effects of Calmodulin Antagonists on radiation-induced lipid peroxidation in Microsomes. Int. J. Rad. Biol. 1990;58:733–743. doi: 10.1080/09553009014552121. [DOI] [PubMed] [Google Scholar]
- 43.Adam-vizi V., Seregi M. Receptor dependent stimulatory effect of noradrenaline on Na+/K+ ATPase in rat brain homogenate: role of lipid peroxidation. Biochem. Pharmacol. 1982;31:2231–2236. doi: 10.1016/0006-2952(82)90106-x. [DOI] [PubMed] [Google Scholar]
- 44.Misra H.P., Fridovich I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J. Biol. Chem. 1972;247(15):3170–3175. [PubMed] [Google Scholar]
- 45.Sinha A.K. Colorimetric assay of catalase. Anal. Biochem. 1972;47:389–394. doi: 10.1016/0003-2697(72)90132-7. [DOI] [PubMed] [Google Scholar]
- 46.Malomo S.O. Toxicological implication of ceftriaxone administration in rats. Nig. J. Biochem. Mol. Biol. 2000;15(1):33–38. [Google Scholar]
- 47.Yakubu M.T., Salau I.O., Muhammad N.O. Phosphatase activities in selected rat tissues following repeated administration of ranitidine. Nig. J. Biochem. Mol. Biol. 2003;18(1):21–24. [Google Scholar]
- 48.Rehman H., Ali M., Atif F.M., Kaur Bhatia K., Raisuddin S. The modulatory effect of deltamethrin on antioxidants in mice. Clin. Chem. Acta. 2006;369(1):61–65. doi: 10.1016/j.cca.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 49.Saravanan M., Devi K.U., Malarvizhi A. Effects of Ibuprofen on hematological, biochemical and enzymological parameters of blood in an Indian major carp. Cirrhinus Mrigala Environ. Toxicol. Pharmacol. 2012;34(1):14–22. doi: 10.1016/j.etap.2012.02.005. [DOI] [PubMed] [Google Scholar]
- 50.Lavanya V., Viswanathan T., Malar S.A. Prevalence of hepatitis B virus infection among blood donors with antibodies to hepatitis B core antigen. Int. J. Med. Med. Sci. 2012;4(6):128–137. [Google Scholar]
- 51.Whitby L.G., Percy-Robb I.W., Smith A.F. Lecturenotes On Clinical Chemistry. 3rd ed. Blackwell Sci. Publication; London: 1984. Enzymes test in diagnosis; pp. 138–168. [Google Scholar]
- 52.Jaroslaw S., Armand M., Gizowska M., Marcinek M., Sasim E., Szafran E. Ceramic-in-Polymer versus Polymer-In-Ceramic polymeric electrolytes—a novel approach. J. Power Sources. 2009;194:66–72. [Google Scholar]
- 53.Udem S.C., Obidoa O., Asuzu I.U. Acute and chronic toxicity studies of erythrinasen egalensis stem bark extract in mice. Comp. Clin. Pathol. 2009;19:275–282. [Google Scholar]
- 54.Clementine Y.F., Tar C. Liver function tests (LFTs) Lab. Insights. 2010;19(1):1–3. [Google Scholar]
- 55.Ugwu Okechukwu P.C., Nwodo Okwesili F.C., Joshua Parker E., Odo Christian E., Ossai Emmanuel C. Effect Of Ethanol Leaf Extract OfMoringa Oleifera On Lipid Profile Of Mice. Res. J. Pharm. Biol. Chem. Sci. 2013;4(1):1324–1332. [Google Scholar]
- 56.Nwanjo H.U. Efficacy of aqueous leaf extract of Vernonia amygdalina plasma lipoprotein and oxidative status in diabetic rat models. Niger. J. Physiol. Sci. 2005;20:39–42. [PubMed] [Google Scholar]
- 57.Pagana K.D., Pagana T.J. 4th ed. Mosbyelseveir; St. Louis: 2010. Mosby’s Manual Diagnostic and Laboratory Tests. [Google Scholar]
- 58.Gidado A., Zainab A., Hadiza M., Serah D., Anas H., Andmilala M.A. Toxicity studies of ethanol extract of the leaves of Datura stramonium in rats. Afr. J. Biotechnol. 2006;6(8):1012–1015. [Google Scholar]
- 59.Bakan E., Taysi P.M.F., Dalga S., Umudum Z., Bakan N. Nitric oxide levels and lipid peroxidation in plasma of patients with gastric Cancer. Jpn. J. Clin. Oncol. 2002;32:162–166. doi: 10.1093/jjco/hyf035. [DOI] [PubMed] [Google Scholar]
- 60.Kurella M.E., Kukley O., Tyulina D., Dobrota M., Matejovicova V., Mezesova A.B. Kinetic parameters of Na/K‐ATPase modified by free radicals in Vitro and in Vivo. Ann. N Y Acad. Sci. 1997;834:661–665. doi: 10.1111/j.1749-6632.1997.tb52344.x. [DOI] [PubMed] [Google Scholar]
- 61.Stefanello F.M., Emilene B.S., Scherer A.G., Kurek C.B. Effect of hypermethioninemia on some parameters of oxidative stress and on Na+,K+-ATPase activity in hippocampus of rats. Metab. Brain Dis. 2007;22(2):172–182. doi: 10.1007/s11011-007-9052-7. [DOI] [PubMed] [Google Scholar]
- 62.Kirkman H.N., Gaetani G.F. Mammalian catalase: a venerable enzyme with new mysteries. Trends Biochem. Sci. 2007;32:44–50. doi: 10.1016/j.tibs.2006.11.003. [DOI] [PubMed] [Google Scholar]