Abstract
Aims
This study aims to determine if traditional markers of disadvantage [female sex, low socio‐economic status (SES), and remoteness] are associated with lower prescription of evidence‐based therapy and higher mortality among patients with moderate–severe heart failure with reduced ejection fraction.
Methods and results
We recruited 452 consecutive class II–III heart failure with reduced ejection fraction patients. Baseline clinical data were recorded prospectively. The primary outcome was the association of female sex on overall survival. Secondary outcomes included association between evidence‐based therapy delivery and sex and association of SES and remoteness on heart failure therapy and survival. The Australian Bureau of Statistics generated all indices. Median follow‐up was 37.9 months. One hundred and nine patients (24.3%) were women. There was no difference in overall survival based on sex (hazard ratio = 1.19, 95% confidence interval: 0.74–1.92, 0.48). There was no difference in prescription of beta‐blockers [χ 2(1) = 0.91, 0.66], angiotensin‐converting enzyme inhibitors [χ 2(1) = 0.001, 0.97], nor aldosterone antagonists [χ 2(1) = 2.71, 0.10]. There was no difference in rates of primary prevention implantable cardioverter‐defibrillator implantation in men compared with women [χ 2(1) = 0.35, 0.56]. Neither higher SES nor inner city residence conferred an overall survival benefit.
Conclusions
In this Australian cohort of heart failure patients, delivery of care and likelihood of death are comparable between the sexes, SES groups, and rural vs. city residents.
Keywords: Heart failure with reduced ejection fraction, Non‐ischaemic cardiomyopathy, Sex, Socio‐economic status, Remoteness
Introduction
Disparity in health status and the delivery of evidence‐based treatment for cardiovascular disease based on sex and socio‐economic status (SES) is extremely concerning.1 Gender inequality in the treatment and outcomes post‐acute coronary syndrome has been extensively reported in the medical literature2, 3 as has a marked female predominance in the incidence of heart failure with preserved ejection fraction.4, 5 The same cannot be said for heart failure with reduced ejection fraction (HFrEF). While older trials have not shown a higher mortality among women with HFrEF,6 there is a lack of recent data on outcomes in women with HFrEF, especially non‐ischaemic cardiomyopathy (NICM) alone. This may be due to the fact that only 24% of those enrolled in pure HFrEF trials are women,7 despite accounting for 35–50% of HFrEF diagnoses.8 As a result, there are no sex‐specific heart failure guidelines9 even though there are known differences in response to cardiovascular drugs.10
Socio‐economic status describes a person's access to material and social resources as well as their ability to participate in society. It is often measured as a combination of education, income, and occupation. SES is a key factor in determining quality of life for women11 and a known determinant of poor outcomes12 and a lack of access to evidence‐based treatment13 in heart failure patients. Similarly, living in a rural setting has been associated with poorer health and a decrease in uptake of heart failure therapies.14
We sought to investigate whether prescription of evidence‐based therapies and defibrillator implantation was equal regardless of gender, SES, and remoteness in a contemporary Australian cohort of patients with moderate–severe HFrEF and to determine if this has an effect on survival.
Methods
This was a post hoc analysis of data collected for a recently published trial that evaluated the reduction in mortality from implantable cardioverter‐defibrillators (ICDs) in NICM patients.15 Methods have been described previously, but in brief, New York Heart Association (NYHA) class II–III patients with non‐ischaemic systolic heart failure and a left ventricular ejection fraction (LVEF) ≤35% were recruited from The Alfred Hospital, Victoria, Australia, between 2005 and 2016. All patients underwent cardiac magnetic resonance imaging (CMR), and only those with NICM were eligible. Exclusion criteria included an ischaemic pattern of scar on CMR, complex congenital heart disease, prior ventricular fibrillation or sustained ventricular tachycardia, and severe valvular disease requiring intervention in the follow‐up period. Patients with ischaemic cardiomyopathy were defined as having segmental wall motion abnormalities or wall thinning in a particular coronary territory with subendocardial or transmural late gadolinium enhancement. Patients with severe left ventricular dysfunction and a small amount of ischaemic late gadolinium enhancement (i.e. an infarct out of keeping with the amount of left ventricular dysfunction) were included only if a recent coronary angiogram without obstructive coronary disease (defined as ≥70% stenosis in any major epicardial vessel or sub‐branch) or a negative functional study was performed. There were 6788 CMR studies performed during the study period from which we identified 888 patients with an LVEF ≤35%. Four hundred and fifty‐two patients were eligible for inclusion. The Alfred ethics committee and The Baker Heart and Diabetes Institute Clinical Governance Unit approved the study.
Measures of socio‐economic status
Each patient's residential address was used to determine SES. We used the Index of Relative Socio‐economic Advantage and Disadvantage (IRSAD) generated by the Australia Bureau of Statistics.16 The IRSAD ranks areas in terms of relative socio‐economic advantage and disadvantage on a continuum from most disadvantaged (1) to most advantaged (5). A multidimensional framework to guide the variable selection process is used. The dimensions included were income variables, education variables, employment variables, occupation variables, housing variables, and other miscellaneous indicators of relative advantage or disadvantage (see Appendix 1 for a full list of variables and their weighting).
We also investigated how living remotely may influence the outcomes of interest. The Accessibility/Remoteness Index of Australia (ARIA) is an unambiguously geographical approach to defining remoteness.17 Geographic remoteness is essentially a measure of a physical location's level of access to goods and services. Large population centres tend to have a greater range of goods and services available than small centres. The lower the ARIA score for a location, the better its level of access to goods and services. Socio‐economic, urban/rural, and population size factors are not considered for incorporation into the measure. There are four categories (major cities, inner regional, outer regional, and remote and/or very remote area). Victoria is the most densely populated state in Australia with a lower rate of residence in outer regional, remote, and very remote areas. Given the majority of our cohort resided in Victoria, we classified our patients into two categories:
ARIA index value 0–2.4 (major cities of Australia and inner regional Australia).
ARIA index value >2.4 (outer regional Australia, remote Australia, and very remote Australia).
Baseline clinical and demographic data
Baseline demographic and clinical data, including sex and address, were recorded in all patients at the time of CMR. The presence and date of implantation of ICD devices were recorded for the entire follow‐up period.
Medical and device therapy
Evidence‐based medical therapies included in the analysis were drugs and ICD. The decision to prescribe medications and implant an ICD was according to the institutional guidelines of the treating centre based on the guidelines for the prevention, detection, and management of people with chronic heart failure in Australia.18 All patients who received an ICD in the follow‐up period were analysed as part of the ICD group.
Definition of endpoints
The primary outcome was all‐cause mortality based on sex, and the secondary outcomes were all‐cause mortality based on SES and remoteness, cardiovascular mortality based on sex, SES, and remoteness, and prescription of beta‐blockers, angiotensin‐converting enzyme (ACE) inhibitors or angiotensin receptor blockers (ARBs), and aldosterone antagonists based on sex, SES, and remoteness. All‐cause mortality was defined as any cause of death. Cardiovascular cause of death was defined as death attributable to myocardial ischaemia and infarction, heart failure, or cardiac arrest because of other or unknown cause. Cause of death was obtained from the death certificate. The medical records were reviewed, and if the requisite information was not available, the Department of Births, Deaths and Marriages was contacted. Duration of follow‐up was calculated from the baseline scan until last patient contact or occurrence of an endpoint.
Statistical analysis
Patients were censored either at the date of the last follow‐up, or if transplantation occurred, at the date of transplantation. Continuous data are reported as median ± interquartile range or mean ± standard deviation, and comparisons between groups were performed using the Mann–Whitney U test or a Kruskal–Wallis test as appropriate. Categorical variables are presented as frequencies (percentages) and compared with the χ 2 test. Kaplan–Meier curves and the log‐rank test assessed cumulative survival. Cox proportional hazard models assessed the significance of hazard ratios (HRs). Multiple imputation (by fully conditional specification) was used for missing variables (Little's missing completely at random test, 0.33). Statistical analyses were performed using SPSS version 25.
Results
From January 2005 to December 2016, 452 patients were enrolled. Baseline characteristics of the entire cohort are shown in Table 1. The median [interquartile range] LVEF was 25.2% [19.0–30.9], 70.6% were NYHA class II, and 29.4% were NYHA class III. The median follow‐up period was 37.9 [19.3–67.3] months. There was a high rate of optimal medical therapy, with 78.1% of patients taking ACE inhibitors/ARBs, 74.6% taking beta‐blockers, and 36.5% taking an aldosterone antagonist (Table 1). There were 77 (17%) deaths. In total, 246 (54%) patients received an ICD. The median time between CMR and insertion of ICD was 54 days [11–160].
Table 1.
Baseline characteristics—men and women
| All (N = 452) | Men (N = 343) | Women (N = 109) | P value | |
|---|---|---|---|---|
| Age (years) | 53.4 (42.4–65.3) | 52.02 (42.4–65.2) | 52.02 (36.93–65.78) | 0.27 |
| BMI (kg/m2) | 26.7 (23.0–30.1) | 27.12 (23.35–30.37) | 25.65 (21.37–29.28) | 0.033 |
| NYHA functional class | 0.017 | |||
| II | 319 (70.6) | 252 (73.47) | 67 (61.48) | |
| III | 133 (29.4) | 91 (26.53) | 42 (38.53) | |
| Presence of diabetes | 66 (14.6) | 54 (15.7) | 12 (11.0) | 0.22 |
| LVEF (%) | 25.2 (19.0–30.9) | 24.75 (18.76–30.52) | 27.16 (20.17–31.56) | 0.11 |
| LVEDVI (mL/m2) | 151.2 (121.8–184.3) | 155.23 (125.69–187.69) | 137.30 (114.49–170.90) | 0.001 |
| LV mass index (g/m2) | 75.5 (63.7–93.3) | 76.32 (64.13–94.04) | 73.06 (61.72–91.11) | 0.30 |
BMI, body mass index; LV, left ventricular; LVEDVI, left ventricular end diastolic volume indexed for body surface area; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association.
Values are median (interquartile range) or n (%) men vs. women, comparison for total cohort unless otherwise specified.
There were 109 (24%) women and 353 (76%) men in the final cohort. There were some differences in baseline characteristics between men and women specifically a lower body mass index in women [25.65 (21.37–29.28) vs. 27.12 (23.35–30.37), 0.033], a higher proportion of women with NYHA class III symptoms [39% vs. 27%, 0.017], and a lower left ventricular diastolic volume indexed for body surface area [137.30 (114.49–170.90) vs. 155.23 (125.69–187.69), 0.001] (Table 1).
Thirty‐six (8%) patients had an IRSAD score of 1 (most disadvantaged quintile relative to the Australia population), 64 (14.2%) patients had an IRSAD score of 2 (2nd most disadvantaged quintile relative to the Australia population), 110 (24.3%) patients had an IRSAD score of 3 (3rd most disadvantaged quintile relative to the Australia population), 127 (28.1%) patients had an IRSAD score of 4 (2nd most advantaged quintile relative to the Australian population), and 115 (25.4%) patients had an IRSAD score of 5 (most advantaged quintile relative to the Australian population). The same baseline characteristics shown in Table 1 were compared over the IRSAD groups. There were no significant differences in baseline characteristics between the IRSAD groups.
Three hundred and eighty‐four (85%) patients had an ARIA index value 0–2.4. Sixty‐eight (15%) patients had an ARIA score of >2.4. The same baseline characteristics shown in Table 1 were compared over the ARIA groups. The more remote group (higher ARIA index value) had a lower body mass index (kg/m2) [25.0 (21.0–29.0) vs. 27.0 (23.4–30.4), 0.015], a lower left ventricular end diastolic volume indexed for body surface area (mL/m2) [128.5 (113.1–166.7) vs. 153.6 (124.6–186.7), 0.001], and a lower proportion of NYHA class II patients (60.3 vs. 72.4, 0.044). All other variables were the same across the groups.
Outcomes according to sex
Fifty‐one (53%) women underwent ICD insertion, which was not significantly different, compared with the 195 (57%) men that underwent ICD insertion [χ 2(1) = 0.35, 0.56] [Figure 1 A]. Similarly, there was no difference in rate of beta‐blocker therapy; 80 (73%) women received beta‐blockers compared with 257 (75%) men [χ 2(1) = 0.91, 0.66] [Figure 1 B]. Eighty‐five (78%) women received ACE inhibitors, which was identical to the rate in the 268 (78%) men [χ 2(1) = 0.001, 0.97] [Figure 1 C]. In addition, the rate of aldosterone antagonists was similar with 39 (36%) women receiving aldosterone antagonists compared with 126 (37%) men [χ 2(1) = 2.71, 0.10] [Figure 1 D].
Figure 1.
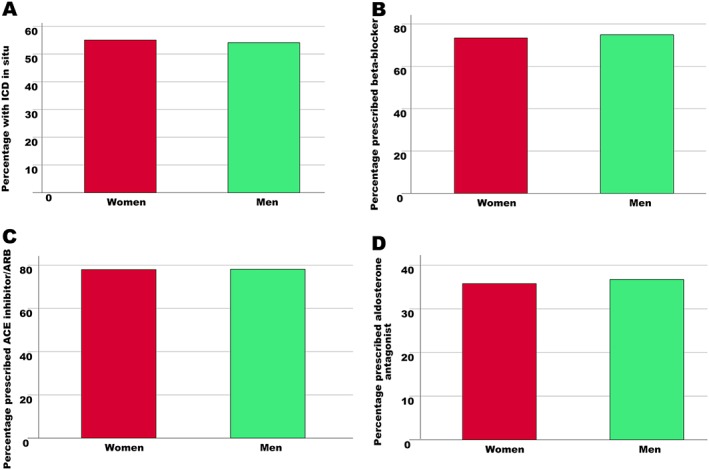
Association between sex and evidence‐based therapy. There was no difference rates in implantable cardioverter‐defibrillator (ICD) implantation (A), nor prescription of beta‐blockers (B), angiotensin‐converting enzyme (ACE) inhibitors/angiotensin receptor blockers (ARBs) (C), and angiotensin receptor antagonists (D) between men and women.
Twenty‐five (23%) women and 52 (15%) men died during the study. There was no difference in overall survival based on sex [HR = 1.19, 95% confidence interval (CI): 0.74–1.92, 0.48] [Figure 2 A]. Sixteen (64%) of the deaths in women were attributed to a cardiovascular cause, which was no difference compared with the 33 (63%) cardiovascular deaths in men (HR = 0.88, 95% CI: 0.49–1.61, 0.63) [Figure 2 B].
Figure 2.
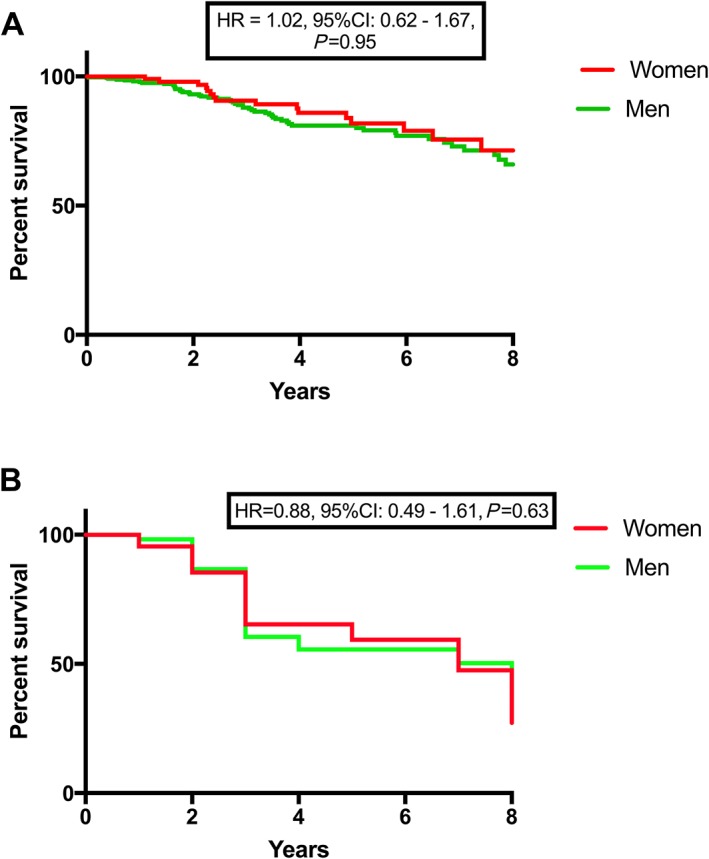
The effect of sex on survival in advanced heart failure. There was no difference in all‐cause (A) nor cardiovascular (B) mortality between men and women. CI, confidence interval; HR, hazard ratio.
Outcomes according to socio‐economic status
Twenty‐four (66.7%) patients with an IRSAD score of 1 received an ICD compared with 38 (59.3%) patients with an IRSAD score of 2, 54 (49%) patients with an IRSAD score of 3, 75 (59%) patients with an IRSAD score of 4, and 54 (49.0%) patients with an IRSAD score of 5. There was no difference between the rate of ICD insertion based on SES [χ 2(4) = 7.53, 0.11] (Supporting Information, Figure S1 A ). In addition, there was no difference between rates of beta‐blockers [χ 2(4) = 3.43, 0.49] (Supporting Information, Figure S1 B ), ACE inhibitor/ARB [χ 2(4) = 1.77, 0.78] (Supporting Information, Figure S1 C ), and aldosterone antagonist [χ 2(4) = 3.91, 0.42] (Supporting Information, Figure S1 D ) prescription based on SES. There were no differences in overall [Figure 3 A] nor cardiovascular mortality [Figure 3 B] based on SES.
Figure 3.
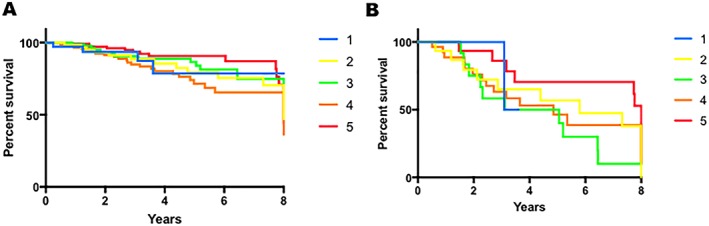
The effect of socio‐economic status on survival in advanced heart failure. There was no difference in all‐cause (A) nor cardiovascular (B) mortality based on socio‐economic status.
Outcomes according to remoteness
Two hundred and seven (53.9%) patients living in a major city or inner regional area underwent ICD implantation compared with 38 (55.9%) in outer regional and rural areas. There was no difference between rates of ICD implantation according to ARIA [χ 2(1) = 0.078, 0.78] [Figure 4 A]. Two hundred and ninety‐nine (77.8%) patients in major cities/inner regional areas received beta‐blockers compared with 38 (55.9%) in outer regional and remote areas. Three hundred and nine (80.5%) patients in major cities/inner regional areas received ACE inhibitors/ARBs compared with 44 (64.7%) living remotely. Patients living more remotely were less likely to receive beta‐blockers [χ 2(1) = 14.72, P < 0.001] [Figure 4 B] and ACE inhibitors/ARBs [χ 2(1) = 8.39, 0.004] [Figure 4 C] compared with their city counterparts. There was no difference in prescription of aldosterone antagonists based on place of residence [χ 2(1) = 0.60, 0.44] [Figure 4 D].
Figure 4.
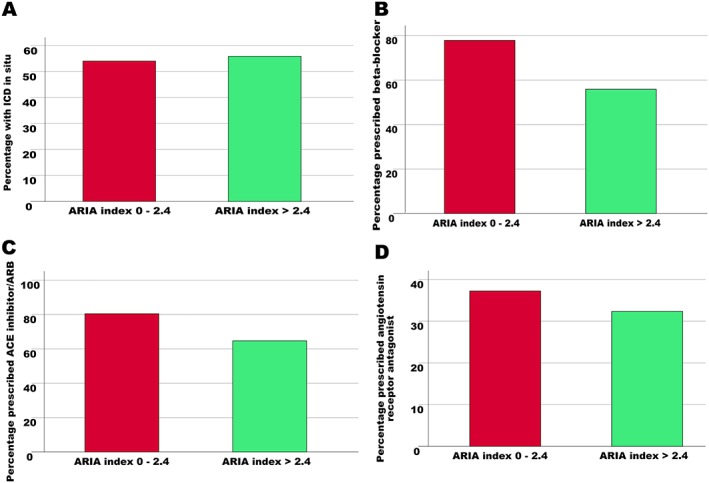
Association between remoteness and evidence‐based therapy. There was no difference in rates of implantable cardioverter‐defibrillator (ICD) implantation (A), nor prescription angiotensin receptor antagonists (D) based on remoteness. Patients living more remotely were less likely to receive beta‐blockers (B) and angiotensin‐converting enzyme (ACE) inhibitors/angiotensin receptor blockers (ARBs) (C). ARIA, Accessibility/Remoteness Index of Australia.
There were 63 (16%) deaths in the group with an ARIA score of 0–2.4, 43 (68%) of which were due to a cardiovascular cause. There were 14 (21%) deaths in those with an ARIA score >2.4, six (42%) of which were due to a cardiovascular cause. There was no difference in overall (HR = 1.15, 95% CI: 0.64–2.05, 0.64) [Figure 5 A] nor cardiovascular mortality (HR = 0.71, 95% CI: 0.30–1.68, 0.44) [Figure 5 B] based on place of residence.
Figure 5.
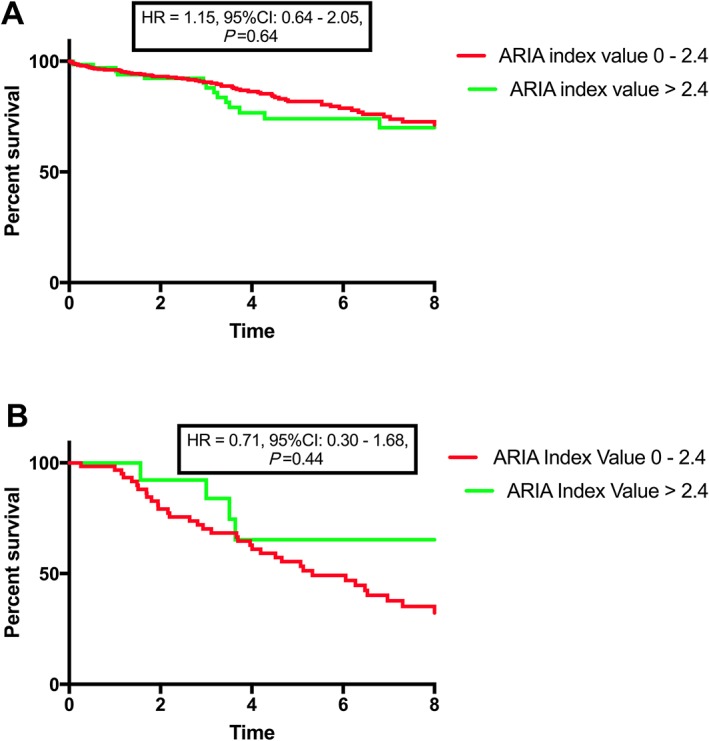
The effect of remote residence on survival in advanced heart failure. There was no difference in all‐cause (A) nor cardiovascular (B) mortality based on remoteness. ARIA, Accessibility/Remoteness Index of Australia; CI, confidence interval; HR, hazard ratio.
The interaction between gender, remoteness, and SES was tested, and there was no evidence of an interaction.
Discussion
The findings from our study are the following. First, in HFrEF, due to NICM, there is no effect of sex on either all‐cause or cardiovascular mortality. Second, rates of prescription of evidence‐based therapies are equal in this cohort of patients with moderate–severely reduced EF. We observed the same trend when patients were analysed according to SES and place of residence. Taken together, we believe that when treatment is equal, many of the factors leading to worse outcomes in disadvantaged patients are mitigated.
Data from the Framingham study suggest that all‐cause mortality from heart failure is decreasing.19 Some studies have shown a parallel decrease in mortality in both sexes20 or even improved outcomes in women, especially in NICM.6, 21, 22 Unfortunately, it has also been reported that this positive trend exists in men but not in women.23 Although parity in the prescription of heart failure therapies in the sexes has been documented,24 under prescription of evidence‐based medications also exists25 and has been postulated as a reason for worse outcomes in women. Our data suggest that when tangible evidence of significant cardiac dysfunction exists (i.e. severely reduced ejection fraction), delivery of evidence‐based therapy is equal. This resulted in near identical survival between groups based on factors traditionally thought to worsen outcomes in cardiovascular conditions. A similar phenomenon has been reported in a systematic review of cardiac resynchronization therapy trials26—although women are markedly under‐represented in cardiac resynchronization therapy trials, when a cardiac resynchronization therapy device is implanted, women have a therapeutic response that is equivalent to or better than in men. Similarly, despite lower rates of ICD implantation in women in the USA compared with men,27 it has been shown that when access to treatment and counselling around ICD implantation is equal between the sexes, rates of ICD implantation are also equal.28
The intersectionality of sex and low SES drives poor outcomes in many disease states. Low SES has consistently been associated with worse outcomes in heart failure; however, this has tended to occur in tandem with lower rates of prescription of evidence‐based therapy.29 Our data suggest that when guidelines are followed equally stringently among socio‐economic groups, outcomes are equal.
Remote residence is arguably the most problematic barrier in delivery of health services. Even in countries where other markers of disadvantage are overcome, longer distances to heath care centres and decreased physician density seem to be insurmountable obstacles.30 Our study demonstrated lower rates of beta‐blocker and ACE inhibitor/ARB therapy among those living more remotely. Although there was no difference in overall nor cardiovascular mortality based on place of residence, due to the population distribution in Victoria, only 68 patients were classified as living in outer regional and remote areas, and survival differences may have been borne out in a larger cohort.
Limitations
All patients in our cohort had NICM. While this narrows theapplicability of the data, the inclusion of patients with ischaemic cardiomyopathy adds potentially complicating factors into the analysis. Women are known to have worse outcomes for women post‐acute coronary syndrome and reduced rates of revascularization and appropriate medical therapy3; in addition, the role of revascularization in ischaemic cardiomyopathy has previously been controversial.31 NICM, conversely, has a discrete amount of evidence‐based treatments that can be delivered, allowing for a clearer dataset to examine equity in health. Although most trials examining the outcomes according to sex in HFrEF have included pre‐specified analyses on the NICM group,6 we believe that this is the only trial to exclude ischaemic cardiomyopathy using CMR, and therefore, we present findings in a unique cohort.
Only 24% of our patients were women; however, this is reflective of the number of women enrolled in other heart failure trials.32 Finally, although data were collected prospectively, this was a post hoc analysis of an observational trial and is therefore subject to confounders.
In conclusion, equality in delivery of evidence‐based therapy in women and the underprivileged may be the answer to improving mortality in these traditionally disadvantaged groups. Those living in the most remote areas still have lower rates of prescription‐based therapy; however, this did not effect outcomes in this study.
Conflict of interest
None declared.
Funding
S.J.G. is supported by a dual funded National Health and Medical Research Council (Australia) and National Heart Foundation Postgraduate Scholarship (Australia) and a Baker Brightsparks Top‐up Scholarship (Melbourne, Australia).
Supporting information
Figure S1. Association between SES and evidence based therapy. There was no difference in rates of ICD implantation (A), nor prescription of beta‐blockers (B), ACE inhibitors/ARBs (C) and angiotensin receptor antagonists (D) based on SES status.
Acknowledgements
The authors wish to acknowledge Dr Eldho Paul and Dr Quan Huynh for assistance with statistical analysis.
Variables used in the IRSAD16
Each variable has a loading that indicates the correlation of that variable with the index. A positive loading indicates an advantaging variable, whereas a negative loading indicates a disadvantaging variable.
| Variable loading | Variable description |
|---|---|
| −0.89 | % People with stated annual household equivalized income between $1 and $20 799 |
| −0.82 | % Occupied private dwellings with no Internet connection |
| −0.82 | % People aged 15 years and over whose highest level of education is Year 11 or lower |
| −0.80 | % Families with children under 15 years of age who live with jobless parents |
| −0.78 | % Employed people classified as ‘labourers’ |
| −0.69 | % One parent families with dependent offspring only |
| −0.69 | % People (in the labour force) unemployed |
| −0.67 | % People aged under 70 who have a long‐term health condition or disability and need assistance with core activities |
| −0.67 | % Occupied private dwellings paying rent less than $166 per week (excluding $0 per week) |
| −0.57 | % People aged 15 and over who are separated or divorced |
| −0.57 | % Employed people classified as machinery operators and drivers |
| −0.51 | % Employed people classified as low skill community and personal service workers |
| −0.49 | % Occupied private dwellings with no cars |
| −0.45 | % Occupied private dwellings requiring one or more extra bedrooms (based on Canadian National Occupancy Standard) |
| −0.37 | % People aged 15 years and over who have no educational attainment |
| 0.35 | % Occupied private dwellings with three or more cars |
| 0.36 | % People aged 15 years and over at university or other tertiary institution |
| 0.37 | % Occupied private dwellings with one or more bedrooms spare |
| 0.40 | % Occupied private dwellings paying rent greater than $370 per week |
| 0.42 | % Employed people classified as managers |
| 0.52 | % Occupied private dwellings with four or more bedrooms |
| 0.62 | % Employed people classified as professionals |
| 0.63 | % People aged 15 years and over whose highest level of education attainment is a diploma qualification |
| 0.70 | % Occupied private dwellings paying mortgage greater than $2800 per month |
| 0.84 | % People with stated annual household equivalized income greater than $52 000 |
Gutman, S. J. , Costello, B. T. , Papapostolou, S. , Iles, L. , Ja, J. , Hare, J. L. , Ellims, A. , Marwick, T. H. , and Taylor, A. J. (2019) Impact of sex, socio‐economic status, and remoteness on therapy and survival in heart failure. ESC Heart Failure, 6, 944–952. 10.1002/ehf2.12481.
References
- 1. Breathett K. Health status equity: a right not a privilege. JACC Heart Fail 2018; 6: 474–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Berger JS, Elliott L, Gallup D, Roe M, Granger CB, Armstrong PW, Simes RJ, White HD, Van de Werf F, Topol EJ, Hochman JS, Newby LK, Harrington RA, Califf RM, Becker RC, Douglas PS. Sex differences in mortality following acute coronary syndromes. JAMA 2009; 302: 874–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Khan E, Brieger D, Amerena J, Atherton JJ, Chew DP, Farshid A, Ilton M, Juergens CP, Kangaharan N, Rajaratnam R, Sweeny A, Walters DL, Chow CK. Differences in management and outcomes for men and women with ST‐elevation myocardial infarction. Med J Aust 2018; 209: 118–123. [DOI] [PubMed] [Google Scholar]
- 4. Beale AL, Meyer P, Marwick TH, Lam CSP, Kaye DM. Sex differences in cardiovascular pathophysiology: why women are overrepresented in heart failure with preserved ejection fraction. Circulation 2018; 138: 198–205. [DOI] [PubMed] [Google Scholar]
- 5. Gori M, Lam CS, Gupta DK, Santos AB, Cheng S, Shah AM, Claggett B, Zile MR, Kraigher‐Krainer E, Pieske B, Voors AA, Packer M, Bransford T, Lefkowitz M, McMurray JJ, Solomon SD, Investigators P. Sex‐specific cardiovascular structure and function in heart failure with preserved ejection fraction. Eur J Heart Fail 2014; 16: 535–542. [DOI] [PubMed] [Google Scholar]
- 6. Adams KF Jr, Sueta CA, Gheorghiade M, O'Connor CM, Schwartz TA, Koch GG, Uretsky B, Swedberg K, McKenna W, Soler‐Soler J, Califf RM. Gender differences in survival in advanced heart failure. Insights from the FIRST study. Circulation 1999; 99: 1816–1821. [DOI] [PubMed] [Google Scholar]
- 7. Tahhan AS, Vaduganathan M, Greene SJ, Fonarow GC, Fiuzat M, Jessup M, Lindenfeld J, O'Connor CM, Butler J. Enrollment of older patients, women, and racial and ethnic minorities in contemporary heart failure clinical trials: a systematic review. JAMA Cardiol 2018; 3: 1011–1019. [DOI] [PubMed] [Google Scholar]
- 8. Gerber Y, Weston SA, Redfield MM, Chamberlain AM, Manemann SM, Jiang R, Killian JM, Roger VL. A contemporary appraisal of the heart failure epidemic in Olmsted County, Minnesota, 2000 to 2010. JAMA Intern Med 2015; 175: 996–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mentzer G, Hsich EM. Heart failure with reduced ejection fraction in women: epidemiology, outcomes, and treatment. Heart Fail Clin 2019; 15: 19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tamargo J, Rosano G, Walther T, Duarte J, Niessner A, Kaski JC, Ceconi C, Drexel H, Kjeldsen K, Savarese G, Torp‐Pedersen C, Atar D, Lewis BS, Agewall S. Gender differences in the effects of cardiovascular drugs. Eur Heart J Cardiovasc Pharmacother 2017; 3: 163–182. [DOI] [PubMed] [Google Scholar]
- 11. Association AP . Fact sheet: women & socioeconomic status.
- 12. Hawkins NM, Jhund PS, McMurray JJ, Capewell S. Heart failure and socioeconomic status: accumulating evidence of inequality. Eur J Heart Fail 2012; 14: 138–146. [DOI] [PubMed] [Google Scholar]
- 13. Wilson D, Harding SA, Melton I, Lever NA, Stiles MK, Boddington D, Heald S, Larsen PD. Geographic, ethnic and socioeconomic factors influencing access to implantable cardioverter defibrillators (ICDs) in New Zealand. Heart Lung Circ 2012; 21: 576–581. [DOI] [PubMed] [Google Scholar]
- 14. Verdejo HE, Ferreccio C, Castro PF. Heart failure in rural communities. Heart Fail Clin 2015; 11: 515–522. [DOI] [PubMed] [Google Scholar]
- 15. Gutman SJ, Costello BT, Papapostolou S, Voskoboinik A, Iles L, Ja J, Hare JL, Ellims A, Kistler PM, Marwick TH, Taylor AJ. Reduction in mortality from implantable cardioverter‐defibrillators in non‐ischaemic cardiomyopathy patients is dependent on the presence of left ventricular scar. Eur Heart J 2018; 39. [DOI] [PubMed] [Google Scholar]
- 16. Pink B. Socio‐Economic Indexes for Areas (SEIFA). http://www.ausstats.abs.gov.au/ausstats/subscriber.nsf/0/22CEDA8038AF7A0DCA257B3B00116E34/$File/2033.0.55.001 seifa 2011 technical paper.pdf.
- 17. Health TAGDo . Measuring remoteness: Accessibility/Remoteness Index of Australia (ARIA). 2001.
- 18. Krum H, Jelinek MV, Stewart S, Sindone A, Atherton JJ, Hawkes AL, Writers CHFGC. Guidelines for the prevention, detection and management of people with chronic heart failure in Australia 2006. Med J Aust 2006; 185: 549–557. [DOI] [PubMed] [Google Scholar]
- 19. Levy D, Kenchaiah S, Larson MG, Benjamin EJ, Kupka MJ, Ho KK, Murabito JM, Vasan RS. Long‐term trends in the incidence of and survival with heart failure. N Engl J Med 2002; 347: 1397–1402. [DOI] [PubMed] [Google Scholar]
- 20. De Maria R, Gavazzi A, Recalcati F, Baroldi G, De Vita C, Camerini F. Comparison of clinical findings in idiopathic dilated cardiomyopathy in women versus men. The Italian Multicenter Cardiomyopathy Study Group (SPIC). Am J Cardiol 1993; 72: 580–585. [DOI] [PubMed] [Google Scholar]
- 21. O'Meara E, Clayton T, McEntegart MB, McMurray JJ, Pina IL, Granger CB, Ostergren J, Michelson EL, Solomon SD, Pocock S, Yusuf S, Swedberg K, Pfeffer MA, Investigators C. Sex differences in clinical characteristics and prognosis in a broad spectrum of patients with heart failure: results of the Candesartan in Heart failure: Assessment of Reduction in Mortality and morbidity (CHARM) program. Circulation 2007; 115: 3111–3120. [DOI] [PubMed] [Google Scholar]
- 22. Dewan P, Rorth R, Jhund PS, Shen L, Raparelli V, Petrie MC, Abraham WT, Desai AS, Dickstein K, Kober L, Mogensen UM, Packer M, Rouleau JL, Solomon SD, Swedberg K, Zile MR, McMurray JJV. Differential impact of heart failure with reduced ejection fraction on men and women. J Am Coll Cardiol 2019; 73: 29–40. [DOI] [PubMed] [Google Scholar]
- 23. Roger VL, Weston SA, Redfield MM, Hellermann‐Homan JP, Killian J, Yawn BP, Jacobsen SJ. Trends in heart failure incidence and survival in a community‐based population. JAMA 2004; 292: 344–350. [DOI] [PubMed] [Google Scholar]
- 24. Yancy CW, Fonarow GC, Albert NM, Curtis AB, Stough WG, Gheorghiade M, Heywood JT, McBride ML, Mehra MR, O'Connor CM, Reynolds D, Walsh MN. Influence of patient age and sex on delivery of guideline‐recommended heart failure care in the outpatient cardiology practice setting: findings from IMPROVE HF. Am Heart J 2009; 157: 754–62 e2. [DOI] [PubMed] [Google Scholar]
- 25. Komajda M, Follath F, Swedberg K, Cleland J, Aguilar JC, Cohen‐Solal A, Dietz R, Gavazzi A, Van Gilst WH, Hobbs R, Korewicki J, Madeira HC, Moiseyev VS, Preda I, Widimsky J, Freemantle N, Eastaugh J, Mason J, Study Group on Diagnosis of the Working Group on Heart Failure of the European Society of C . The EuroHeart Failure Survey programme—a survey on the quality of care among patients with heart failure in Europe. Part 2: treatment. Eur Heart J 2003; 24: 464–474. [DOI] [PubMed] [Google Scholar]
- 26. Herz ND, Engeda J, Zusterzeel R, Sanders WE, O'Callaghan KM, Strauss DG, Jacobs SB, Selzman KA, Pina IL, Canos DA. Sex differences in device therapy for heart failure: utilization, outcomes, and adverse events. J Womens Health (Larchmt) 2015; 24: 261–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hernandez AF, Fonarow GC, Liang L, Al‐Khatib SM, Curtis LH, LaBresh KA, Yancy CW, Albert NM, Peterson ED. Sex and racial differences in the use of implantable cardioverter‐defibrillators among patients hospitalized with heart failure. JAMA 2007; 298: 1525–1532. [DOI] [PubMed] [Google Scholar]
- 28. Hess PL, Hernandez AF, Bhatt DL, Hellkamp AS, Yancy CW, Schwamm LH, Peterson ED, Schulte PJ, Fonarow GC, Al‐Khatib SM. Sex and race/ethnicity differences in implantable cardioverter‐defibrillator counseling and use among patients hospitalized with heart failure: findings from the Get With The Guidelines‐Heart Failure Program. Circulation 2016; 134: 517–526. [DOI] [PubMed] [Google Scholar]
- 29. Khariton Y, Nassif ME, Thomas L, Fonarow GC, Mi X, DeVore AD, Duffy C, Sharma PP, Albert NM, Patterson JH, Butler J, Hernandez AF, Williams FB, McCague K, Spertus JA. Health status disparities by sex, race/ethnicity, and socioeconomic status in outpatients with heart failure. JACC Heart Fail 2018; 6: 465–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Murphy GK, McAlister FA, Eurich DT. Cardiovascular medication utilization and adherence among heart failure patients in rural and urban areas: a retrospective cohort study. Can J Cardiol 2015; 31: 341–347. [DOI] [PubMed] [Google Scholar]
- 31. Velazquez EJ, Lee KL, Deja MA, Jain A, Sopko G, Marchenko A, Ali IS, Pohost G, Gradinac S, Abraham WT, Yii M, Prabhakaran D, Szwed H, Ferrazzi P, Petrie MC, O'Connor CM, Panchavinnin P, She L, Bonow RO, Rankin GR, Jones RH, Rouleau JL, Investigators S . Coronary‐artery bypass surgery in patients with left ventricular dysfunction. N Engl J Med 2011; 364: 1607–1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Frazier CG, Alexander KP, Newby LK, Anderson S, Iverson E, Packer M, Cohn J, Goldstein S, Douglas PS. Associations of gender and etiology with outcomes in heart failure with systolic dysfunction: a pooled analysis of 5 randomized control trials. J Am Coll Cardiol 2007; 49: 1450–1458. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Association between SES and evidence based therapy. There was no difference in rates of ICD implantation (A), nor prescription of beta‐blockers (B), ACE inhibitors/ARBs (C) and angiotensin receptor antagonists (D) based on SES status.


