Abstract
Aims
Significant mitral regurgitation (MR) is an important predictor for all‐cause mortality and heart failure (HF) hospitalizations independent of left ventricular ejection fraction (LVEF). The aims of this study were to investigate (i) in how many patients referred to a tertiary outpatient HF clinic HF therapy could be optimized, (ii) the effect of optimized treatment on MR severity, and (iii) whether a reduction in MR resulted in improvement of symptoms.
Methods and results
Forty‐seven referred patients with therapy‐resistant symptomatic chronic HF with an LVEF <40% and at least moderate MR were analysed on admission and after optimization of HF treatment after 6–18 months. The patients were classified as a volume responder when LV end‐systolic volume (LVESV) decreased ≥15%, as LVEF responder when LVEF increased by ≥5% points, as clinical responder when New York Heart Association (NYHA) class improved at least one category, and as MR responder when MR severity improved at least one category to maximally moderate. After 14 ± 4 months of treatment optimization, optimal doses of angiotensin‐converting enzyme inhibitors/angiotensin receptor blocker were seen in 18 (38%) patients compared with three (6%) at baseline (P < 0.001), and optimal doses of beta‐blockers were seen in 14 (30%) patients compared with four (9%) at baseline (P < 0.001). In total, 68% of the patients were clinical responders, 57% MR responders, 34% volumetric responders, and 49% LVEF responders. NYHA class improved from 2.9 ± 0.6 to 2.0 ± 0.9 (P < 0.001), MR class from 5.2 ± 0.8 to 3.6 ± 1.5 (P < 0.001), LVEF from 24% ± 9% to 31% ± 12% (P < 0.01), and LVESV non‐significantly improved. The positive predictive value of MR response to NYHA response was 88%; the negative predictive value was 53%, agreement 69%, and kappa 0.39. The positive predictive value of LVEF response to NYHA response was 76%; the negative predictive value was 44%, agreement 60%, and kappa 0.21. The positive predictive value of LVESV volume response to NYHA response was 75%; the negative predictive value was 39%, agreement 51%, and kappa 0.12.
Conclusions
Although this study was limited by a small number of patients, initiation and up‐titration of recommended HF therapy in patients referred to our tertiary HF outpatient clinic resulted in significant MR reduction in over half of the patients, emphasizing the importance of optimal medical treatment in these very sick cardiac patients with otherwise grave prognosis. MR reduction was best correlated to NYHA improvement.
1. Introduction
Both in ischaemic and non‐ischaemic cardiomyopathy, the presence of significant mitral regurgitation (MR) is an important predictor for all‐cause mortality and heart failure (HF) hospitalizations independent of left ventricular ejection fraction (LVEF).1 To date, the most effective therapies for secondary MR are aimed at the underlying LV dysfunction. Given the main pathophysiological mechanism, that is, LV and annular dilatation, these include optimal medical HF therapy and cardiac resynchronization therapy (CRT) when appropriate. In particular, beta‐blockers and angiotensin‐converting enzyme inhibitors (ACE‐Is) are recommended for all patients with LV dysfunction and secondary MR.2 By reversing LV unloading and LV remodelling, optimal HF therapy may reduce MR. Surprisingly, however, few studies have examined the effect of beta‐blockers3, 4, 5, 6 or ACE‐Is7 therapies on secondary MR. Secondary MR may also dramatically improve after optimization of fluid status by diuretics through lowering of the LV filling pressures.8 More robust data are available on the LV remodelling and synchronizing effects of CRT on secondary MR.9, 10, 11, 12, 13, 14
In this study, we report our results in patients with chronic HF and at least moderate MR referred to our tertiary HF outpatient clinic for a second opinion, specific referral for MR intervention, and/or heart transplantation. The aims of this study were to investigate (i) in how many real‐world referred patients HF therapy could be optimized, (ii) the effect of optimized treatment on MR severity, and (iii) whether a reduction in MR resulted in an improvement of symptoms.
2. Methods
2.1. Study patient definition
All patients included in the study fulfilled the following inclusion criteria: (i) referred by a cardiologist to our tertiary HF outpatient clinic between 2005 and 2015 for a second opinion with (ii) therapy‐resistant symptomatic chronic HF New York Heart Association (NYHA) class 2 to 4, (iii) LVEF <40%, and (iv) at least moderate MR. In addition, all included patients were required to have a baseline transthoracic echocardiogram before change in HF treatment at our tertiary HF outpatient clinic and a follow‐up transthoracic echocardiogram between 6 and 18 months. Exclusion criteria were prior valvular surgery and concomitant congenital heart disease.
2.2. Clinical data
The following variables were noted: gender, age, heart rate, systolic blood pressure, aetiology of HF, prior HF hospitalization in the last 12 months, NYHA class, and renal dysfunction [defined as estimated glomerular filtration rate (eGFR) < 45 mL/min/1.73 m2].
2.3. Transthoracic echocardiography
Echocardiography was performed with a Sonos or iE33 system (Philips, Best, The Netherlands), equipped with an S5‐1 transducer according to a standard HF protocol. The following variables were measured both at baseline and follow‐up according to standard guidelines15, 16, 17: LV end‐diastolic diameter and volumes, LV end‐systolic diameter and volumes (LVESV), LVEF, left atrial (LA) diameter and volume, transmitral E‐wave, transmitral deceleration time, diastolic early septal wall velocity as assessed with tissue Doppler imaging (e′), tricuspid valve regurgitation velocity, caval vein diameter, MR severity [according to seven scales (from 0 to 6): none, trivial, mild, mild to moderate, moderate, moderate to severe, and severe],17 and MR jet morphology in the LA (central or eccentric). LV volumes and LVEF were measured with TomTec triplane analysis in Imaging Arena (TomTec Imaging systems, Imaging Arena, version 4.6, Unterschleissheim, Germany). All measurements were performed by blinded observers: MR by M. L. G., LV volumes and ejection fraction by E. S., and all others by L. d. G. d. L.
2.4. Definition of responders
A patient was considered a volume responder when LVESV decreased ≥15%, an LVEF responder when LVEF increased by ≥5% points, a clinical responder when NYHA class improved at least one category, and an MR responder when MR severity improved at least one category to maximally moderate.
2.5. Medication and devices
In all patients, the following drugs (including dosage) were noted at baseline and at the time of follow‐up echocardiography: ACE‐I or angiotensin receptor blocker (ARB), beta‐blocker, loop diuretic, mineralocorticoid receptor antagonist (MRA), and digoxin. Optimal treatment dosages were defined according to the guideline.2 Also, other interventions like thyroid hormone or Vitamin‐D supplementation were noted. Finally, it was noted whether the patient had an implantable cardioverter defibrillator (ICD) or had undergone (upgrade to) CRT.
2.6. Statistical analysis
Statistical analysis was performed using SPSS version 21.0.0.1 (SPSS, IBM, Armonk, NY). Categorical data are presented as numbers and percentages, whereas continuous data are summarized as mean ± standard deviation or median value with range. Comparisons of proportions were performed with a two‐sided Z test. P‐values <0.05 were considered significant.
The agreement between MR response, EF response, and LVESV volume response to the NYHA response was assessed by calculating the kappa coefficient (a value of 0.21 to 0.40 indicating a fair agreement, a value of 0.41 to 0.80 indicating a moderate agreement, and a value of >0.80 indicating an excellent agreement).
3. Results
3.1. Baseline clinical and echocardiographic characteristics
Forty‐seven patients (mean age 52 ± 13 years, 68% male patients) were included in the study, see Figure 1. As seen in Table 1, heart rate was 81 ± 19 b.p.m., and systolic blood pressure 102 ± 15 mmHg. HF aetiologies were ischaemic in 14 (30%) patients, 25 (53%) patients were hospitalized because of HF in the previous 12 months, and NYHA class was 2.9 ± 0.6 [NYHA 2 in 11 (23%), NYHA 3 in 30 (64%), and NYHA 4 in 6 (13%)]. Significant renal dysfunction was present in 12 (26%) patients. Mean volumes were 265 ± 103 mL for LV end‐diastolic volume and 205 ± 97 mL for LVESV, and LVEF was 25% ± 9%. Moderate, moderate‐to‐severe, and severe MR was present in 11 (23%), 15 (32%), and 21 (45%) patients. As seen in Table 2, ACE‐I/ARBs were present in 45 (96%) patients, beta‐blockers in 37 (79%), diuretics in 42 (89%), MRAs in 32 (68%), and digoxin in 12 (26%). However, optimal doses of ACE‐I/ARBs were present in three (6%) patients, and optimal doses of beta‐blockers in four (9%). CRT was present in 10 (21%) patients (CRT‐D in nine and CRT‐P in one), and an isolated ICD was present in 13 (28%) patients.
Figure 1.
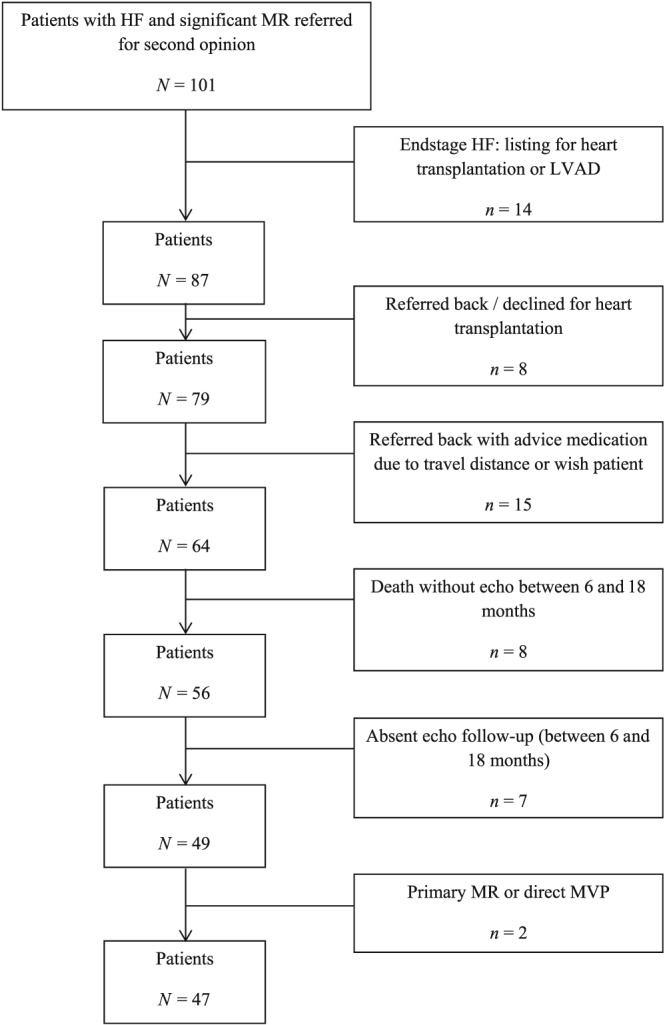
Inclusion patients. HF, heart failure; LVAD, left ventricular assist device; MR, mitral regurgitation; MVP, mitral valve plasty.
Table 1.
Clinical and echocardiographic data
| All patients baseline N = 47 | All patients follow‐up N = 47 | MR responder N = 27 | MR non‐responder N = 20 | |
|---|---|---|---|---|
| Clinical data | ||||
| Male gender | 32 (68%) | 17 (63%) | 15 (75%) | |
| Age (years) | 52 ± 13 | 56 ± 12 | 49 ± 13 | |
| Heart rate (bpm) | 81 ± 19 | 66 ± 12 | 85 ± 20 | 77 ± 16 |
| Systolic blood pressure | 102 ± 15 | 101 ± 13 | 103 ± 18 | |
| Ischaemic aetiology | 14 (30%) | 8 (30%) | 6 (30%) | |
| Prior HF hospitalization | 25 (53%) | 15 (56%) | 10 (50%) | |
| NYHA class III or IV | 36 (77%) | 12 (26%) | 21 (78%) | 15 (75%) |
| Glomerular filtration rate | 61 ± 20 | 58 ± 21 | 64 ± 22 | 56 ± 14 |
| Renal dysfunctiona | 12 (26%) | 10 (21%) | 7 (26%) | 5 (25%) |
| Echocardiographic datab | ||||
| LVEDD | 68.4 ± 13.2 | 66.8 ± 11.9 | 68.3 ± 12.8 | 68.4 ± 13.8 |
| LVEDD delta | −1.6 ± 11.2 | −4.8 ± 13.5 | 1.1 ± 8.2 | |
| LVESD | 61.3 ± 13.8 | 56.4 ± 13.1 | 60.8 ± 13.4 | 61.6 ± 14.5 |
| LVESD delta | −4.9 ± 11.1* | −8.0 ± 13.4* | −2.3 ± 8.1 | |
| LVEDV | 264.6 ± 102.6 | 246.5 ± 100.3 | 272.7 ± 119.6 | 257.8 ± 88.6 |
| LVEDV delta | −18.1 ± 86.6 | −48.0 ± 112.6 | 6.9 ± 46.1 | |
| LVESV | 204.8 ± 97.0 | 179.5 ± 99.1 | 210.4 ± 113.5 | 200.0 ± 83.6 |
| LVESV delta | −25.3 ± 89.9 | −54.2 ± 120.5 | −0.9 ± 42.7 | |
| LVEF | 24.5 ± 9.3 | 30.7 ± 11.7 | 25.3 ± 9.4 | 24.0 ± 9.4 |
| LVEF delta | 6.1 ± 12.2# | 9.0 ± 16.2* | 3.7 ± 7.0* | |
| LA diameter | 49.5 ± 7.8 | 46.4 ± 9.0 | 47.9 ± 7.1 | 50.9 ± 8.3 |
| LA diameter delta | −3.1 ± 8.1# | −6.7 ± 8.3# | −0.1 ± 6.8 | |
| LA volume | 117.1 ± 37.7 | 102.6 ± 49.6 | 115.1 ± 36.0 | 118.8 ± 39.9 |
| LA volume delta | −14.5 ± 52.1 | −41.8 ± 37.4# | 8.5 ± 52.4 | |
| e′ | 4.7 ± 1.7 | 5.2 ± 2.0 | 4.4 ± 1.8 | 4.9 ± 1.7 |
| e′ delta | 0.5 ± 1.9 | 0.5 ± 1.9 | 0.5 ± 1.9 | |
| E/e′ | 22.5 ± 9.1 | 17.6 ± 12.1 | 24.0 ± 10.6 | 21.2 ± 7.7 |
| E/e′ delta | −4.9 ± 13.2* | −8.6 ± 13.9* | −1.8 ± 12.2 | |
| TR velocity | 2.9 ± 0.6 | 2.6 ± 0.8 | 2.9 ± 0.6 | 3.0 ± 0.6 |
| TR velocity delta | −0.3 ± 0.8* | −0.6 ± 0.7# | −0.1 ± 0.7 | |
| IVC diameter | 20.9 ± 5.2 | 16.3 ± 4.9 | 20.1 ± 5.5 | 21.5 ± 5.0 |
| IVC diameter delta | −4.6 ± 5.6# | −5.3 ± 6.1# | −3.9 ± 5.2# | |
| MR central jet | 26 (55%) | 26 (55%) | 16 (59%) | 10 (50%) |
| MR severe | 21 (45%) | 6 (13%) | 13 (48%) | 8 (40%) |
HF, heart failure; IVC, inferior vena cava; LA, left atrium; LVEDD, left ventricular end‐diastolic diameter; LVEDV, LV end‐diastolic volume; LVEF, LV ejection fraction; LVESD, LV end‐systolic diameter; LVESV, LV end‐systolic volume; MR, mitral regurgitation; NYHA, New York Heart Association; TR, tricuspid regurgitation.
Glomerular filtration rate <45 mL/min/1.73 m2.
In the MR responder columns, only baseline and delta values are displayed. Limited to the 35 patients with complete echo data available.
P < 0.05.
P < 0.01.
Table 2.
Baseline heart failure therapy and changes in the study population (n = 47)
| Baseline | Follow‐up | |||||
|---|---|---|---|---|---|---|
| At referral | Optimal dose | Up‐titration | Initiation | Finally | Optimal dose | |
| ACE‐inhibitors/ARBs | 45 (96%) | 3 (6%) | 22 (47%) | 2 (4%) | 47 (100%) | 18 (38%)* |
| Beta‐blocker | 37 (79%) | 4 (9%) | 20 (43%) | 9 (19%) | 46 (98%) | 14 (30%)* |
| Diuretics | 42 (89%) | 22 (47%) | 3 (6%) | 45 (96%) | ||
| MRAs | 32 (68%) | 3 (6%) | 7 (15%) | 39 (83%) | ||
| Digoxin | 12 (26%) | 0 (0%) | 25 (53%) | 37 (76%) | ||
| CRT‐P | 1 (2%) | 0 (0%) | 1 (2%) | |||
| CRT‐D | 9 (19%) | 8 (17%)a | 17 (36%) | |||
| ICD only | 13 (28%) | 5 (11%) | 15 (32%) | |||
ACE, angiotensin‐converting enzyme; ARB, angiotensin receptor blocker; CRT‐D, cardiac resynchronization therapy defibrillator; CRT‐P, cardiac resynchronization therapy pacemaker; ICD, implantable cardioverter defibrillator; MRAs, mineralocorticoid receptor antagonists.
Including three upgrades from ICD only.
P < 0.001 compared with baseline.
3.2. Medical interventions
Ten patients (21%) were immediately after first outpatient assessment hospitalized to optimize HF. As seen in Table 2 and Figure 2, in the total group of patients, ACE‐I/ARBs were initiated in two (4%) patients and up‐titrated in 22 (47%) patients, beta‐blockers were initiated in nine (19%) patients and up‐titrated in 20 (43%) patients, diuretics were initiated in three (6%) patients and up‐titrated in 22 (47%) patients, MRAs were initiated in seven (15%) patients and up‐titrated in three (6%) patients, and digoxin was initiated in 25 (52%) patients and up‐titrated in none. At follow‐up, optimal doses of ACE‐I/ARBs were present in 18 (38%) patients compared with three (6%) at baseline (P < 0.001), and optimal doses of beta‐blockers were present in 14 (30%) patients compared with four (9%) at baseline (P < 0.001). Six (13%) patients were on the evidence‐based dose of both beta‐blockers and ACE‐inhibitors/ARBs at the time of follow‐up echocardiography vs. 0 (0%) at baseline. Heart rate decreased from 81 ± 19 to 66 ± 12 b.p.m. (P < 0.001).
Figure 2.
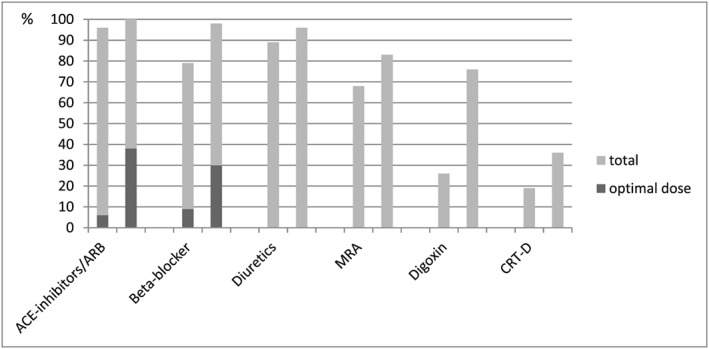
Baseline (left) and change (right) in heart failure therapy. ACE, angiotensin‐converting enzyme; ARB, angiotensin receptor blocker; CRT‐D, cardiac resynchronization therapy defibrillator; MRA, mineralocorticoid receptor antagonist.
3.3. Device interventions
As seen in Table 2, CRT was initiated in eight (17%) patients, of whom in three patients, existing ICD therapy was upgraded to a CRT‐D system. An additional five patients received an isolated ICD.
3.4. Clinical and echocardiographic improvement
After a mean of 14 ± 4 months, NYHA class improved from 2.9 ± 0.6 to 2.0 ± 0.9 (P < 0.001), and 32 patients (68%) were clinical responders. MR class improved from 5.2 ± 0.8 to 3.6 ± 1.5 (P < 0.001), and 27 patients (57%) were MR responders (Figure 3). In these latter patients, vena contracta width improved from 7.0 ± 1.4 to 2.7 ± 1.2 mm (P < 0.001), whereas in the non‐responders, no significant improvement was seen in vena contracta width (7.3 ± 1.5 vs. 6.9 ± 1.6 mm, P = not significant).
Figure 3.
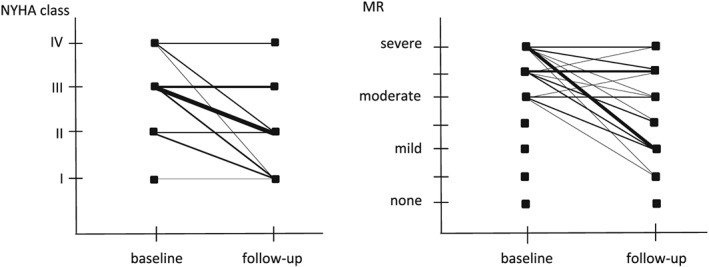
New York Heart Association (NYHA) class (left) and mitral regurgitation (MR) (right) response to optimize treatment. Thickness of the line corresponds to the number of patients.
Left ventricular end‐systolic volume non‐significantly improved from 205 ± 97 to 180 ± 99 mL (P = not significant), and 12 patients (34%) were volumetric responders. LVEF improved from 24% ± 9% to 31% ± 12% (P < 0.01), and 17 patients (49%) were LVEF responders.
3.5. Predictors for mitral regurgitation improvement
As seen in Table 1, none of the baseline variables predicted improvement (responders) in MR. Associated with MR improvement were a decrease in LV end‐systolic diameter, LA diameter and volume, E/e′, tricuspid regurgitation velocity and inferior vena cava dimension, and an increase in LVEF.
3.6. Relation between mitral regurgitation and ejection fraction improvement vs. New York Heart Association response
The positive predictive value of MR response to NYHA response was 88%; the negative predictive value was 53%, agreement 69%, and kappa 0.39. The positive predictive value of LVEF response to NYHA response was 76%; the negative predictive value was 44%, agreement 60%, and kappa 0.21. The positive predictive value of LVESV volume response to NYHA response was 75%; the negative predictive value was 39%, agreement 51%, and kappa 0.12.
3.7. Relation between mitral regurgitation improvement and renal dysfunction
Estimated glomerular filtration rate non‐significantly decreased from 61 ± 20 to 58 ± 21 mL/min/1.73 m2. In MR responders, eGFR remained stable (0 ± 13), whereas in MR non‐responders, eGFR deteriorated with 8 ± 12 mL/min/1.73 m2 (P < 0.05). In both EF responders and non‐responders, eGFR remained stable.
4. Discussion
The main findings of this study in patients referred by cardiologists to our tertiary HF clinic with therapy‐resistant HF and at least moderate MR are (i) although the vast majority of the referred patients received recommended medication, optimal dosages were seen in only a very small minority, (ii) initiation of therapy resulted in the presence of the recommended medication in virtually all patients, (iii) despite up‐titration of recommended medication in almost half of the patients, still approximately only one‐third of patients could tolerate the maximum recommended drug dosages, (iv) MR reduced significantly in over half of the patients, and (v) MR reduction best correlated to NYHA improvement.
4.1. Medical therapy
Medical therapy for HF consists of vasodilators (ACE‐Is), beta‐blockers, MRAs, and diuretics. The main effects of these drugs include reversal or delay of LV remodelling and reduction of MR through lowering filling pressures. The use of afterload‐reducing agents, including ACE‐Is, might reduce MR volume and improve LV forward stroke volume by decreasing the pressure gradient between the LV and the left atrium through systolic unloading. A similar effect of reduction in MR is obtained with preload reduction through the use of diuretics that decrease LV size and tethering with a consequent decrease in MR volume.8 It is well known that ACE‐Is and beta‐blockers reduce mortality and morbidity in symptomatic patients with HF with reduced LVEF18, 19, 20 and are complementary. According to the guideline, these drugs should be gradually up‐titrated to the maximum tolerated dose.2 In this study, it is shown that although referred patients often were on ACE‐Is and beta‐blockers, optimal doses were rarely seen. In a significant number of patients, beta‐blockers could be initialized by the HF specialist, and drugs could be up‐titrated. Still, at the last moment of assessment (between 6 and 18 months), optimal doses of ACE‐Is and beta‐blockers were only seen in one‐third of our patients. Hypotension, bradycardia, and renal failure are well‐known causes of failure to up‐titrate HF drugs, in particular in patients with advanced HF. Patients referred to our outpatient HF clinic represent the sickest of the sick: the majority were hospitalized because of HF in the previous 12 months, and outpatient NYHA class was in the vast majority NYHA class 3 or 4. Further evidence for the severity of HF disease is seen in the haemodynamic characteristics. The mean heart rate of 81 is quite comparable with patients included in the major HF landmark trials,18, 19, 20 but the systolic blood pressure of 102 mmHg is significantly lower than the 120–130 mmHg range reported in the major HF landmark trials18, 19, 20 that included also mainly patients in NYHA class 3 or 4.
Despite these issues, the subscription of ACE‐Is and beta‐blockers in 100% and 98% of patients is a remarkable achievement. For example, in a Spanish prospective cohort of patients hospitalized for HF from 2008 to 2011, beta‐blockers were after 12 months only present in 68% of patients,21 and numbers also seen in other registries like the Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients With Heart Failure (OPTIMIZE‐HF) registry.22 In this latter trial, target doses of metoprolol and carvedilol were seen in only 8% and 18% of patients 3 months after discharge.
In the recently published European Society of Cardiology Heart Failure Long‐Term Registry, patients with chronic HF had 1 year follow‐up ACE‐Is/ARBs, beta‐blockers, and MRAs in 87%, 89%, and 59% of patients, respectively.23 In these trials, baseline values of systolic blood pressure and heart rate were 124 ± 21 mmHg and 73 ± 15 b.p.m., and 25% of patients were in NYHA class III or IV. So our patients referred because of refractory HF were also compared with this contemporary registry clearly the sickest of the sick.
4.2. Device therapy
According to the baseline LVEF, 63% of patients who had a primary prophylactic ICD indication had an ICD (with or without CRT) implanted before referral. At the end of the follow‐up period, this percentage was 94%; in addition, all patients with left bundle branch block had CRT. These numbers seem also much better than the low numbers reported in the European Society of Cardiology Heart Failure Long‐Term Registry, although it cannot be clearly distillated from this registry how many patients actually had a clear indication for CRT and/or ICD.23
4.3. Mitral regurgitation
In HF patients, the presence of significant MR is a significant predictor for mortality1, 24, 25 and exercise capacity.26 By inclusion, all our patients had at least moderate MR. The described therapeutic interventions resulted in a significant reduction of MR in over half of the patients, consistent with findings recently published by Stolfo et al.27 The relation between clinical effects and MR reduction by medical therapy is not well described in the literature. In contrast, it is well known that improvement of significant MR by CRT is sustained and patients with less residual MR 6 months after CRT have a better survival.28 In this study, it is clearly shown that MR reduction is best related to NYHA class improvement. The potential improvement in MR by HF therapy optimization by a dedicated HF cardiologist may prevent in a large number of HF patients the need for surgical or percutaneous mitral valve interventions.
4.4. Limitations
The major limitations of this study are the retrospective character and the limited number of patients. The latter was mainly caused by our stringent study inclusion criteria, excluding patients in whom adjustment of therapy was started before the first echo in our centre. Also, approximately 20% of patients were deemed to have irreversible HF and referred for heart transplantation. Considering the total cohort of patients, a significant MR reduction in over half of the patients may therefore be an overestimation. On the other hand, approximately 10% of patients was referred back with medical advices thought to be easily implemented by the referring physician, and it may be expected that in these patients, even a larger proportion of patients would have shown improvement in MR. Finally, sacubitril/valsartan was not available at the time of our study. Sacubitril/valsartan has been not only shown to reduce the rate of HF hospitalization and cardiovascular mortality in selected symptomatic patients with HF with an LVEF <35%29 but also to reduce MR severity in patients initially on optimal medical therapy with an ACE‐I/ARB and beta‐blocker and significant secondary MR.30
5. Conclusions
Initiation and up‐titration of recommended HF therapy in patients referred to our tertiary HF outpatient clinic resulted in significant MR reduction in over half of the patients, emphasizing the importance of optimal medical treatment in these very sick cardiac patients with otherwise grave prognosis. MR reduction was best correlated to NYHA improvement.
Conflict of interest
None declared.
de Groot – de Laat, L. E. , Huizer, J. , Lenzen, M. , Spitzer, E. , Ren, B. , Geleijnse, M. L. , and Caliskan, K. (2019) Evolution of mitral regurgitation in patients with heart failure referred to a tertiary heart failure clinic. ESC Heart Failure, 6, 936–943. 10.1002/ehf2.12478.
References
- 1. Rossi A, Dini FL, Faggiano P, Agricola E, Cicoira M, Frattini S, Simioniuc A, Gullace M, Ghio S, Enriquez‐Sarano M, Temporelli PL. Independent prognostic value of functional mitral regurgitation in patients with heart failure. A quantitative analysis of 1256 patients with ischaemic and non‐ischaemic dilated cardiomyopathy. Heart 2011; 97: 1675–1680. [DOI] [PubMed] [Google Scholar]
- 2. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, Falk V, González‐Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GMC, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P, ESC Scientific Document Group . 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2016; 37: 2129–2200. [DOI] [PubMed] [Google Scholar]
- 3. Capomolla S, Febo O, Gnemmi M, Riccardi G, Opasich C, Caporotondi A, Mortara A, Pinna GD, Cobelli F. Beta‐blockade therapy in chronic heart failure: diastolic function and mitral regurgitation improvement by carvedilol. Am Heart J 2000; 139: 596–608. [DOI] [PubMed] [Google Scholar]
- 4. Comin‐Colet J, Sanchez‐Corral MA, Manito N, Gomez‐Hospital JA, Roca J, Fernandez‐Nofrerias E, Valdovinos P, Esplugas E. Effect of carvedilol therapy on functional mitral regurgitation, ventricular remodeling, and contractility in patients with heart failure due to left ventricular systolic dysfunction. Transplant Proc 2002; 34: 177–178. [DOI] [PubMed] [Google Scholar]
- 5. Lowes BD, Gill EA, Abraham WT, Larrain JR, Robertson AD, Bristow MR, Gilbert EM. Effects of carvedilol on left ventricular mass, chamber geometry, and mitral regurgitation in chronic heart failure. Am J Cardiol 1999; 83: 1201–1205. [DOI] [PubMed] [Google Scholar]
- 6. Waagstein F, Stromblad O, Andersson B, Bohm M, Darius M, Delius W, Goss F, Osterziel KJ, Sigmund M, Trenkwalder SP, Wahlqvist I. Increased exercise ejection fraction and reversed remodeling after long‐term treatment with metoprolol in congestive heart failure: a randomized, stratified, double‐blind, placebo‐controlled trial in mild to moderate heart failure due to ischemic or idiopathic dilated cardiomyopathy. Eur J Heart Fail 2003; 5: 679–691. [DOI] [PubMed] [Google Scholar]
- 7. Levine AB, Muller C, Levine TB. Effects of high‐dose lisinopril‐isosorbide dinitrate on severe mitral regurgitation and heart failure remodeling. Am J Cardiol 1998; 82: 1299–1301 A10. [DOI] [PubMed] [Google Scholar]
- 8. Stevenson LW, Bellil D, Grover‐McKay M, Brunken RC, Schwaiger M, Tillisch JH, Schelbert HR. Effects of afterload reduction (diuretics and vasodilators) on left ventricular volume and mitral regurgitation in severe congestive heart failure secondary to ischemic or idiopathic dilated cardiomyopathy. Am J Cardiol 1987; 60: 654–658. [DOI] [PubMed] [Google Scholar]
- 9. St John Sutton M, Ghio S, Plappert T, Tavazzi L, Scelsi L, Daubert C, Abraham WT, Gold MR, Hassager C, Herre JM, Linde C, REsynchronization reVErses Remodeling in Systolic left vEntricular dysfunction (REVERSE) Study Group . Cardiac resynchronization induces major structural and functional reverse remodeling in patients with New York Heart Association class I/II heart failure. Circulation 2009; 120: 1858–1865. [DOI] [PubMed] [Google Scholar]
- 10. St John Sutton MG, Plappert T, Abraham WT, Smith AL, DeLurgio DB, Leon AR, Loh E, Kocovic DZ, Fisher WG, Ellestad M, Messenger J, Kruger K, Hilpisch KE, Hill MR, Multicenter InSync Randomized Clinical Evaluation (MIRACLE) Study Group . Effect of cardiac resynchronization therapy on left ventricular size and function in chronic heart failure. Circulation 2003; 107: 1985–1990. [DOI] [PubMed] [Google Scholar]
- 11. Ypenburg C, Lancellotti P, Tops LF, Bleeker GB, Holman ER, Pierard LA, Schalij MJ, Bax JJ. Acute effects of initiation and withdrawal of cardiac resynchronization therapy on papillary muscle dyssynchrony and mitral regurgitation. J Am Coll Cardiol 2007; 50: 2071–2077. [DOI] [PubMed] [Google Scholar]
- 12. Breithardt OA, Sinha AM, Schwammenthal E, Bidaoui N, Markus KU, Franke A, Stellbrink C. Acute effects of cardiac resynchronization therapy on functional mitral regurgitation in advanced systolic heart failure. J Am Coll Cardiol 2003; 41: 765–770. [DOI] [PubMed] [Google Scholar]
- 13. Onishi T, Onishi T, Marek JJ, Ahmed M, Haberman SC, Oyenuga O, Adelstein E, Schwartzman D, Saba S, Gorcsan J III. Mechanistic features associated with improvement in mitral regurgitation after cardiac resynchronization therapy and their relation to long‐term patient outcome. Circ Heart Fail 2013; 6: 685–693. [DOI] [PubMed] [Google Scholar]
- 14. van Bommel RJ, Marsan NA, Delgado V, Borleffs CJ, van Rijnsoever EP, Schalij MJ, Bax JJ. Cardiac resynchronization therapy as a therapeutic option in patients with moderate‐severe functional mitral regurgitation and high operative risk. Circulation 2011; 124: 912–919. [DOI] [PubMed] [Google Scholar]
- 15. Lancellotti P, Tribouilloy C, Hagendorff A, Popescu BA, Edvardsen T, Pierard LA, Badano L, Zamorano JL, On behalf of the Scientific Document Committee of the European Association of Cardiovascular Imaging: Thor Edvardsen, Oliver Bruder, Bernard Cosyns, Erwan Donal, Raluca Dulgheru, Maurizio Galderisi, Patrizio Lancellotti, Denisa Muraru, Koen Nieman, Rosa S . Recommendations for the echocardiographic assessment of native valvular regurgitation: an executive summary from the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2013; 14: 611–644. [DOI] [PubMed] [Google Scholar]
- 16. Lang RM, Badano LP, Mor‐Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2015; 16: 233–270. [DOI] [PubMed] [Google Scholar]
- 17. Zoghbi WA, Enriquez‐Sarano M, Foster E, Grayburn PA, Kraft CD, Levine RA, Nihoyannopoulos P, Otto CM, Quinones MA, Rakowski H, Stewart WJ, Waggoner A, Weissman NJ, American Society of Echocardiography . Recommendations for evaluation of the severity of native valvular regurgitation with two‐dimensional and Doppler echocardiography. J Am Soc Echocardiogr 2003; 16: 777–802. [DOI] [PubMed] [Google Scholar]
- 18. Group CTS . Effects of enalapril on mortality in severe congestive heart failure. Results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS). N Engl J Med 1987; 316: 1429–1435. [DOI] [PubMed] [Google Scholar]
- 19. A randomized trial of beta‐blockade in heart failure . The Cardiac Insufficiency Bisoprolol Study (CIBIS). CIBIS Investigators and Committees. Circulation 1994; 90: 1765–1773. [DOI] [PubMed] [Google Scholar]
- 20. The Cardiac Insufficiency Bisoprolol Study II (CIBIS‐II): a randomised trial. Lancet 1999; 353: 9–13. [PubMed] [Google Scholar]
- 21. Gonzalez‐Garcia A, Montero Perez‐Barquero M, Formiga F, Gonzalez‐Juanatey JR, Quesada MA, Epelde F, Oropesa R, Díez‐Manglano J, Cerqueiro JM, Manzano L. Has beta‐blocker use increased in patients with heart failure in internal medicine settings? Prognostic implications: RICA registry. Rev Esp Cardiol (Engl Ed) 2014; 67: 196–202. [DOI] [PubMed] [Google Scholar]
- 22. DeVore AD, Mi X, Mentz RJ, Fonarow GC, Van Dyke MK, Maya JF, Hardy NC, Hammill BG, Hernandez AF. Discharge heart rate and beta‐blocker dose in patients hospitalized with heart failure: findings from the OPTIMIZE‐HF registry. Am Heart J 2016; 173: 172–178. [DOI] [PubMed] [Google Scholar]
- 23. Crespo‐Leiro MG, Anker SD, Maggioni AP, Coats AJ, Filippatos G, Ruschitzka F, Ferrari R, Piepoli MF, Delgado Jimenez JF, Metra M, Fonseca C, Hradec J, Amir O, Logeart D, Dahlström U, Merkely B, Drozdz J, Goncalvesova E, Hassanein M, Chioncel O, Lainscak M, Seferovic PM, Tousoulis D, Kavoliuniene A, Fruhwald F, Fazlibegovic E, Temizhan A, Gatzov P, Erglis A, Laroche C, Mebazaa A, on behalf of the Heart Failure Association (HFA) of the European Society of Cardiology (ESC) . European Society of Cardiology Heart Failure Long‐Term Registry (ESC‐HF‐LT): 1‐year follow‐up outcomes and differences across regions. Eur J Heart Fail 2016; 18: 613–625. [DOI] [PubMed] [Google Scholar]
- 24. Grigioni F, Enriquez‐Sarano M, Zehr KJ, Bailey KR, Tajik AJ. Ischemic mitral regurgitation: long‐term outcome and prognostic implications with quantitative Doppler assessment. Circulation 2001; 103: 1759–1764. [DOI] [PubMed] [Google Scholar]
- 25. Lancellotti P, Troisfontaines P, Toussaint AC, Pierard LA. Prognostic importance of exercise‐induced changes in mitral regurgitation in patients with chronic ischemic left ventricular dysfunction. Circulation 2003; 108: 1713–1717. [DOI] [PubMed] [Google Scholar]
- 26. Szymanski C, Levine RA, Tribouilloy C, Zheng H, Handschumacher MD, Tawakol A, Hung J. Impact of mitral regurgitation on exercise capacity and clinical outcomes in patients with ischemic left ventricular dysfunction. Am J Cardiol 2011; 108: 1714–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Stolfo D, Merlo M, Pinamonti B, Poli S, Gigli M, Barbati G, Fabris E, di Lenarda A, Sinagra G. Early improvement of functional mitral regurgitation in patients with idiopathic dilated cardiomyopathy. Am J Cardiol 2015; 115: 1137–1143. [DOI] [PubMed] [Google Scholar]
- 28. Verhaert D, Popovic ZB, De S, Puntawangkoon C, Wolski K, Wilkoff BL, Starling RC, Tang WW, Thomas JD, Griffin BP, Grimm RA. Impact of mitral regurgitation on reverse remodeling and outcome in patients undergoing cardiac resynchronization therapy. Circ Cardiovasc Imaging 2012; 5: 21–26. [DOI] [PubMed] [Google Scholar]
- 29. McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Solomon SD, Swedberg K, Zile MR, PARADIGM‐HF Investigators and Committees . Angiotensin‐neprilysin inhibition versus enalapril in heart failure. N Engl J Med 2014; 371: 993–1004. [DOI] [PubMed] [Google Scholar]
- 30. Kang DH, Park SJ, Shin SH, Hong GR, Lee S, Kim MS, Yun SC, Song JM, Park SW, Kim JJ. Angiotensin receptor neprilysin inhibitor for functional mitral regurgitation. Circulation 2019; 139: 1354–1365. [DOI] [PubMed] [Google Scholar]


